To the Editor
Prevailing clinical experience suggests that allergic patients can become sensitized to new allergen sources in the natural course of their allergic disease and thus expand their IgE reactivity profiles.1 However, there are no experimental studies that have used defined allergen molecules to investigate whether the appearance of allergic reactions against new allergen sources results from true de novo IgE sensitizations against new allergen molecules or from the augmentation of already existing IgE sensitizations passing the threshold required for clinical manifestation.
To address the question of whether and how frequently adult allergic patients and nonallergic subjects have IgE sensitizations to new allergen molecules, we performed a retrospective analysis of serum samples using an allergen microarray that contained 85 different purified allergen molecules (ISAC; Phadia, Uppsala, Sweden).2 The 85 allergens on the chip represented 50 allergen families with experimentally confirmed cross-reactivity (see Fig E1 in this article’s Online Repository at www.jacionline.org). Sera were collected in 1997 and 2007 from 12 adult allergic patients with confirmed allergy to various airborne and food allergens and from 10 subjects with a negative history of allergy. Fig E2 in this article’s Online Repository at www.jacionline.org provides the demographic and clinical characterization (ie, allergen sources, symptoms, and therapies) of the studied subjects. The mean age at first blood sampling was 36 or 32 years (range, 27-58 or 26-50 years), respectively, for the group of allergic and nonallergic subjects.
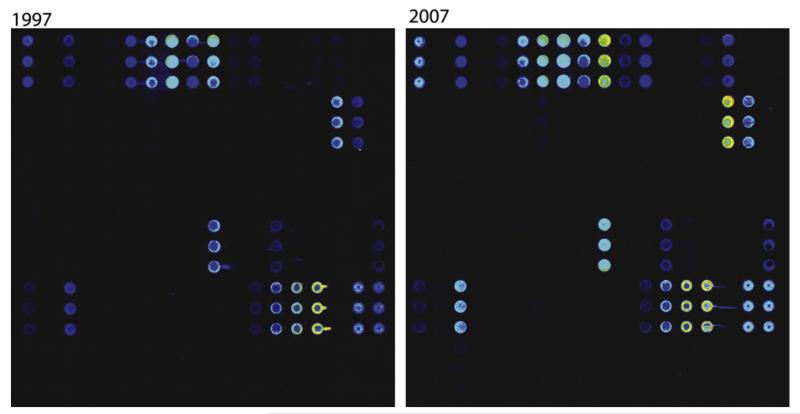
The analysis of sera from the 22 subjects obtained in 1997 and 2007 for IgE reactivity to 85 microarrayed allergens generated 3740 individual IgE test results. Fig 1 shows an example of the IgE reactivity profile of allergic patient A5. The scan images indicate that the patient’s serologic patterns in 1997 and 2007 are very similar. When all IgE test results were analyzed, we found that 42 of the 85 individual allergens that corresponded to 25 of the 50 allergen families were recognized in 1997, 2007, or both (see Fig E1). Fig E3 in this article’s Online Repository at www.jacionline.org displays a detailed analysis of the numbers of allergens and allergen families recognized by each of the sera obtained in 1997 and 2007.
FIG 1.
IgE reactivity profiles in 1997 and 2007 assessed by means of microarray for a representative patient. Scans of the microarrays incubated with serum samples collected in 1997 (left) and 2007 (right) are shown, Allergens are spotted in triplicates.
At first glance, 8 of the 12 allergic patients (patients A2, A3, A4, A6, A5, A7, A8, and A9) and one of the 10 nonallergic subjects (subject N1) seemed to exhibit IgE reactivity to new allergens in 2007. When the allergens were grouped into families of cross-reactive allergens, it turned out that 6 of the 12 allergic patients (patients A2, A3, A5, A7, A8, and A9) and the nonallergic subject (subject N1) mounted IgE antibodies to new allergen families in 2007. Six subjects showed IgE reactivity to 1 additional allergen family, and 1 patient (patient A8) showed IgE reactivities to 3 different allergen families (see Fig E3). The sera from 1997 were retested by means of quantitative IgE CAP measurements to determine whether the newly identified IgE reactivities in serum samples from the 6 allergic patients (patients A2, A3, A5, A7, A8, and A9) and the nonallergic subject (subject N1) in 2007 indeed resulted from a new sensitization or from the boosting of an already existing sensitization (see Fig E4 in this article’s Online Repository at www.jacionline.org). The sample from patient A5 could not be reassessed because no Act d 2 CAP was available. For the other 6 sera, IgE to the respective allergen or allergens could be detected in the 1997 serum samples, indicating that in these cases no de novo sensitizations had occurred (see Fig E4).
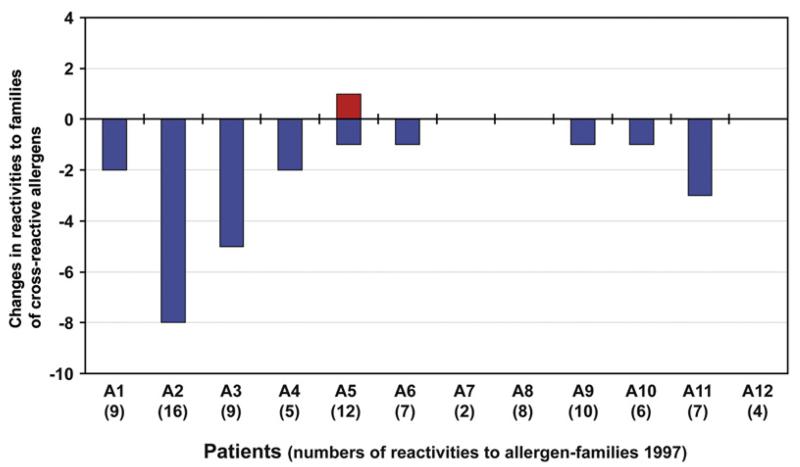
In summary, having analyzed 85 allergens belonging to 50 allergen families, no sensitization to a new allergen family could be detected, except 1 possible sensitization to a single allergen (ie, Act d 2) that could not be reinvestigated (Fig 2). By contrast, IgE reactivities to several allergen families disappeared and were not detectable after 10 years in 2007 (Fig 2).
FIG 2.
Changes in IgE reactivities to families of cross-reactive allergens between 1997 and 2007. Displayed are new (red bar) and lost (blue bars) reactivities to families of cross-reactive allergens (y-axis) for patients A1 to A12 (x-axis) adjusted by CAP results. Numbers of positive allergen families in 1997 are shown in brackets for each patient (x-axis).
Our study thus indicates that de novo sensitizations seem to be a rare event in allergic adults. Possible explanations for the rare occurrence of IgE class-switching in response to new allergens in adults might be the low doses of allergens incorporated on natural allergen exposure, the maturity and thus low plasticity of the immune system in adults, epigenetic modifications controlling responsiveness of allergen-specific B and T cells, the presence of allergen-specific blocking IgG antibodies withdrawing allergen from the system, and/or regulatory cells preventing sensitization.
Yet, it has been demonstrated that exposure to high concentrations of allergens, especially in conjunction with systemic administration or in combination with adjuvants, can lead to the development of new IgE specificities, such as venom allergy in patients with occupational allergy or in the course of allergenspecific immunotherapy (SIT).3-7 In our study only 5 patients had been treated with SIT more than 10 years before the first serum sample was obtained. We can therefore exclude SIT-induced effects on IgE reactivity profiles in our patients.
Data obtained from several cohorts of children have shown that sensitization occurs to common food allergens first and then to respiratory allergens already very early in life.8,9 The youngest patient studied by us was 27 years old in 1997. It therefore remains to be studied whether de novo sensitizations caused by natural allergen exposure can occur earlier in life.
We think that our observation that de novo sensitizations rarely occur in adults is very important because it provides one explanation why SIT is effective for long periods and even after its discontinuation. Furthermore, it provides a good basis for the design of novel allergy vaccines based on recombinant allergen molecules, peptides, and hypoallergenic allergen derivatives.
Supplementary Material
FIG E1. Families of tested allergens and summary of reactivities. Allergens belonging to the same cross-reactive allergen family (left column) were grouped in individual boxes (route of exposure: green, Airborne allergens; orange, food allergens; and yellow, venom allergens). The right column displays the numbers of positive sera in 1997 and 2007. Total numbers of allergen families and allergens recognized in 1997, 2007, or both are shown (bottom line, purple boxes). nsLTP, Nonspecific lipid transfer protein; PR-10, pathogenesis-related protein family 10.
FIG E2. Demographic and clinical characterization of allergic patients (A1-12) and nonallergic subjects (N1-N10). f, Female; m, male. Allergies: a, animals; f, food; g, grass pollen; mi, mites; t, tree pollen; w, weed pollen. Symptoms: as, asthma; c, conjunctivitis; d, dermatitis; oas, oral allergy syndrome; r, rhinitis. Symptomatic therapy: ah, antihistamine; nc, nasal corticosteroid; tc, topical (dermal) corticosteroid. Dashes indicate lack of allergy, symptoms, or therapy.
FIG E3. Detailed analysis of IgE reactivities and their changes for each subject. Numbers of recognized allergen molecules and allergen families in 1997 and 2007, as well as changes in 2007 versus 1997, are shown. Allergens that were negative by means of chip but positive by means of CAP in 1997 are printed in red. PR10, Pathogenesis-related protein family 10.
FIG E4. Results of CAP measurements for sera that were negative to certain allergens in ISAC in 1997 but positive in 2007. kUA/l, Kilounits of antigen-specific IgE per liter.
Acknowledgments
Supported by grants F4605 and F4613 of the Austrian Science Fund (FWF), and research grants from Phadia, Uppsala, Sweden, and Biomay, Vienna, Austria; the Christian Doppler Research Association; and in part by the European Commission’s Seventh Framework Programme under grant agreement 261357.
Footnotes
Disclosure of potential conflict of interest: V. Niederberger and R. Valenta have received research support from the FWF Austrian Science Fund. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Fasce L, Tosca MA, Olcese R, Milanese M, Erba D, Ciprandi G. The natural history of allergy: the development of new sensitizations in asthmatic children. Immunol Lett. 2004;93:45–50. doi: 10.1016/j.imlet.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–6. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- 3.Ball T, Sperr WR, Valent P, Lidholm J, Spitzauer S, Ebner C, et al. Induction of antibody responses to new B cell epitopes indicates vaccination character of allergen immunotherapy. Eur J Immunol. 1999;29:2026–36. doi: 10.1002/(SICI)1521-4141(199906)29:06<2026::AID-IMMU2026>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Moverare R, Elfman L, Vesterinen E, Metso T, Haahtela T. Development of new IgE specificities to allergenic components in birch pollen extract during specific immunotherapy studied with immunoblotting and Pharmacia CAP System. Allergy. 2002;57:423–30. doi: 10.1034/j.1398-9995.2002.13248.x. [DOI] [PubMed] [Google Scholar]
- 5.Green BJ, Cummings KJ, Rittenour WR, Hettick JM, Bledsoe TA, Blachere FM, et al. Occupational sensitization to soy allergens in workers at a processing facility. Clin Exp Allergy. 2011;41:1022–30. doi: 10.1111/j.1365-2222.2011.03756.x. [DOI] [PubMed] [Google Scholar]
- 6.Renstrom A, Olsson M, Hedren M, Johansson SG, van Hage M. Pet shop workers: exposure, sensitization, and work-related symptoms. Allergy. 2011;66:1081–7. doi: 10.1111/j.1398-9995.2011.02591.x. [DOI] [PubMed] [Google Scholar]
- 7.Eich-Wanger C, Muller UR. Bee sting allergy in beekeepers. Clin Exp Allergy. 1998;28:1292–8. doi: 10.1046/j.1365-2222.1998.00411.x. [DOI] [PubMed] [Google Scholar]
- 8.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103:1173–9. doi: 10.1016/s0091-6749(99)70195-8. [DOI] [PubMed] [Google Scholar]
- 9.Niederberger V, Niggemann B, Kraft D, Spitzauer S, Valenta R. Evolution of IgM, IgE and IgG(1-4) antibody responses in early childhood monitored with recombinant allergen components: implications for class switch mechanisms. Eur J Immunol. 2002;32:576–84. doi: 10.1002/1521-4141(200202)32:2<576::AID-IMMU576>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIG E1. Families of tested allergens and summary of reactivities. Allergens belonging to the same cross-reactive allergen family (left column) were grouped in individual boxes (route of exposure: green, Airborne allergens; orange, food allergens; and yellow, venom allergens). The right column displays the numbers of positive sera in 1997 and 2007. Total numbers of allergen families and allergens recognized in 1997, 2007, or both are shown (bottom line, purple boxes). nsLTP, Nonspecific lipid transfer protein; PR-10, pathogenesis-related protein family 10.
FIG E2. Demographic and clinical characterization of allergic patients (A1-12) and nonallergic subjects (N1-N10). f, Female; m, male. Allergies: a, animals; f, food; g, grass pollen; mi, mites; t, tree pollen; w, weed pollen. Symptoms: as, asthma; c, conjunctivitis; d, dermatitis; oas, oral allergy syndrome; r, rhinitis. Symptomatic therapy: ah, antihistamine; nc, nasal corticosteroid; tc, topical (dermal) corticosteroid. Dashes indicate lack of allergy, symptoms, or therapy.
FIG E3. Detailed analysis of IgE reactivities and their changes for each subject. Numbers of recognized allergen molecules and allergen families in 1997 and 2007, as well as changes in 2007 versus 1997, are shown. Allergens that were negative by means of chip but positive by means of CAP in 1997 are printed in red. PR10, Pathogenesis-related protein family 10.
FIG E4. Results of CAP measurements for sera that were negative to certain allergens in ISAC in 1997 but positive in 2007. kUA/l, Kilounits of antigen-specific IgE per liter.