Abstract
Neuronal communication relies on chemical synaptic transmission for information transfer and processing. Chemical neurotransmission is initiated by synaptic vesicle fusion with the presynaptic active zone resulting in release of neurotransmitters. Classical models have assumed that all synaptic vesicles within a synapse have the same potential to fuse under different functional contexts. In this model, functional differences among synaptic vesicle populations are ascribed to their spatial distribution in the synapse with respect to the active zone. Emerging evidence suggests, however, that synaptic vesicles are not a homogenous population of organelles, and they possess intrinsic molecular differences and differential interaction partners. Recent studies have reported a diverse array of synaptic molecules that selectively regulate synaptic vesicles' ability to fuse synchronously and asynchronously in response to action potentials or spontaneously irrespective of action potentials. Here we discuss these molecular mediators of vesicle pool heterogeneity that are found on the synaptic vesicle membrane, on the presynaptic plasma membrane, or within the cytosol and consider some of the functional consequences of this diversity. This emerging molecular framework presents novel avenues to probe synaptic function and uncover how synaptic vesicle pools impact neuronal signaling.
Keywords: vesicle pools, spontaneous neurotransmission, asynchronous neurotransmission, synchronous neurotransmission, vesicle exocytosis, synaptic vesicle, synaptic transmission, neurotransmitter release, SNARE proteins
Introduction
Synaptic connections between neurons are vitally important for information transfer throughout the nervous system. At chemical synapses action potentials cause calcium influx into the presynaptic terminal, which typically triggers the fusion of synaptic vesicles with the presynaptic active zone membrane and the release of neurotransmitter; however, neurotransmitter can also be released in the absence of action potentials. The neurotransmitter then travels across the synaptic cleft to bind postsynaptic receptors, which subsequently send an excitatory or inhibitory signal to the postsynaptic neuron. It is becoming clear that synaptic vesicle trafficking and exocytosis require many interwoven molecular components that have yet to be fully untangled (1). Many molecular mediators of trafficking and exocytosis have been identified, but the contributions of each molecule to various types of exocytosis and the resulting effects on information flow remain incompletely elucidated.
Historically synaptic vesicles have been divided into a number of “pools” based on the contexts under which they travel to the presynaptic membrane and exocytose. For example, vesicles have been categorized as part of the readily releasable pool, the recycling pool, the reserve pool, or the resting pool depending on their relative rates of trafficking in response to stimulation (2-5). Readily releasable vesicles are generally assumed to be both docked and primed at the presynaptic active zone membrane and probabilistically fuse in response to a single action potential while the recycling pool consists of all vesicles that are recruited for release during strong or prolonged stimulation. Vesicles in the reserve pool, in contrast, are extremely reluctant to traffic to the presynaptic membrane for fusion, and their function in neuronal signaling under physiological conditions remains unclear (2). This pool of vesicles, therefore, has also been referred to as the “resting pool” (3, 5). Few molecular mediators that differentiate vesicles into these various pools have been identified; instead, these pools remain defined by their functional properties and, in some cases, ultrastructurally defined distances between vesicles and the active zone (2, 3, 5-7, but see 8).
Recent emphasis has been placed on defining vesicle pools by their molecular components and the temporal relationship of vesicle release to the arrival of presynaptic action potentials (Figure 1). This classification divides vesicles into those that are released synchronously with the arrival of an action potential, those that are released asynchronously in the wake of an action potential, and those that are released spontaneously in the absence of action potentials (9-11). In addition to functional studies demonstrating these forms of release, molecular evidence suggests that a concurrent molecular framework underlies this vesicle pool classification (11-14). Here we will describe key molecular players that sort vesicles into functionally distinct pools and discuss potential implications for nervous system function.
Figure 1. Presynaptic vesicle pools.
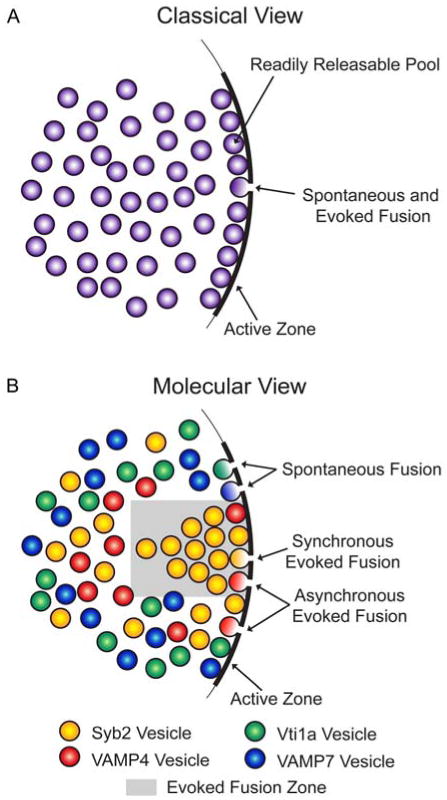
A. Classically vesicles have been considered a homogenous population within the presynaptic terminal. Under this model synchronous evoked, asynchronous evoked, and spontaneous fusion events arise from a single population of presynaptic vesicles, and other factors besides vesicle heterogeneity (e.g. distance from the active zone) determine whether a vesicle is in the readily releasable, recycling, reserve, or resting pools. B. Recently molecules like vesicular SNARE proteins have been hypothesized to confer heterogeneity to presynaptic vesicle populations. For example, synaptobrevin 2 (syb2) vesicles mainly drive synchronous evoked release, vesicle-associated membrane protein 4 (VAMP4) vesicles primarily drive asynchronous evoked release, and vps10p tail interactor 1a (vti1a) and vesicle-associated membrane protein 7 (VAMP7) vesicles mostly drive spontaneous release. Some studies have also shown that neurotransmitter released from evoked and spontaneous vesicles activates separate populations of postsynaptic receptors, so fusion sites for these vesicle pools may be spatially segregated along the active zone.
Functional Segregation of Vesicle Pools
Researchers have been measuring postsynaptic responses to presynaptic activity since the mid-twentieth century (15), although synaptic vesicles themselves were not yet discovered (16, 17). In response to electrical stimulation, vesicles release neurotransmitters that, in turn, induce large, synchronous currents in the postsynaptic cell that can be measured using electrophysiological techniques (11). These “evoked” synchronous events often precede asynchronous currents that, although induced by stimulation, are produced by delayed vesicle fusion events (11, 18). In contrast small, so-called “miniature” postsynaptic events, first detected in 1952 (19), still occur in the absence of action potential stimulation and represent small numbers of vesicles fusing with the presynaptic membrane (10, 11). These miniature events exhibit a seemingly random temporal pattern and have been, therefore, referred to as spontaneous events despite their sensitivity to presynaptic calcium signaling (20).
These three forms of vesicle release – synchronous evoked, asynchronous evoked, and spontaneous – can be studied separately even when an experimental preparation is capable of all forms of release, but distinguishing the behavior of individual synapses and vesicles has not been possible until recently. For example early work using the neuromuscular junction preparation disagreed on whether spontaneously released vesicles and vesicles released in response to stimulation resided at the same release sites (21-24), but now it is clear that, although some synapses favor one form of release over the other, many boutons produce both types of release (25-29). The ability to tease apart how these types of release are modulated and produced has become paramount to understanding synaptic transmission in the nervous system.
Many studies have described differential modulation of each form of fusion, suggesting that these vesicle pools are molecularly distinct (14, 30). It has been difficult to distinguish asynchronous and synchronous evoked release with a functional assay, besides observing their respective timing in response to action potentials, because these two pools may share vesicles and activate the same postsynaptic receptor populations (9, 26, 31, 32), but vesicles released spontaneously often can be modulated independently from those that are released in response to stimulation. For example, Drosophila shibire mutants undergo temperature-sensitive inhibition of neurotransmission and recover evoked neurotransmission more easily than spontaneous neurotransmission, with functional recovery correlating with different stages of presynaptic ultrastructural recovery (33). The Gi/o protein-coupled GABAB receptor agonist baclofen inhibits evoked excitatory, spontaneous excitatory, and evoked inhibitory events but not spontaneous inhibitory events in hippocampal slice cultures and cerebellar slices (34, 35). Additionally cadmium, a calcium channel blocker, inhibits evoked but not spontaneous neurotransmission in some preparations, suggesting that spontaneous and evoked events are differentially dependent upon calcium influx (34). Application of nitrosonium donors to cultured hippocampal neurons simultaneously inhibits evoked excitatory neurotransmission while enhancing spontaneous excitatory neurotransmission (36). Similarly a recent study in rat hindbrain slices demonstrated that Gi/o protein-coupled cannabinoid receptor 1 activation selectively inhibits evoked but not spontaneous neurotransmission while TRPV1 receptor activation inhibits evoked neurotransmission and facilitates spontaneous neurotransmission (37, 38). These studies make it clear that spontaneous and evoked neurotransmission are functionally separable under particular experimental contexts.
The differential modulation of these forms of vesicle release suggests that they originate from distinct vesicle pools. Investigators have tagged spontaneously recycled and stimulation-evoked vesicles independently with fluorescent probes or dyes and shown that the release properties of these tagged vesicles are substantially different (39-42), although other studies have detected complete overlap between the pools (43-46). Blocking excitatory postsynaptic receptors activated by evoked neurotransmission does not inhibit receptors activated by spontaneous events and vice versa (26), suggesting that vesicles from different pools fuse to the plasma membrane at distinct locations, allowing their neurotransmitter molecules to bind separate postsynaptic receptors. To determine whether these vesicle pools are truly distinct, it has become necessary to molecularly dissect vesicle trafficking pathways. Proteins involved in endocytosis likely contribute to this vesicle sorting (47-49), and vesicle biogenesis mechanisms have been reviewed in this Traffic review series and elsewhere (50-52). This review, however, will focus on how vesicles are differentially trafficked to the plasma membrane for exocytosis.
During exocytosis, vesicular soluble NSF attachment protein receptor (v-SNARE) proteins bind to target membrane SNARE (t-SNARE) proteins to form a complex that allows the vesicular and plasma membranes to fuse. Canonically the v-SNARE synaptobrevin 2 (syb2; also known as vesicle-associated membrane protein 2 or VAMP2) binds to t-SNAREs syntaxin 1 and synaptosomal-associated protein of 25 kDa (SNAP-25) to bring the membranes together for fusion and release of neurotransmitter, a process that is catalyzed by calcium binding to the canonical calcium sensor synaptotagmin 1 (syt1) (53). Increasing the number of SNARE proteins facilitates vesicle fusion synchrony in vitro, but only a small number of SNARE proteins is required for fusion to occur (54, 55), so many cooperative factors modulate the synchrony of exocytosis. A variety of synaptic proteins have been identified that regulate fusion, including proteins located in the vesicle membrane, the plasma membrane, and the cytosolic space between the membranes (Figure 2) (53). Molecular, genetic, and functional studies are now identifying which of these proteins sort vesicles into synchronous, asynchronous, and spontaneous vesicle pools.
Figure 2. Molecules contributing to presynaptic vesicle pool heterogeneity.

Vesicles are shuttled to the presynaptic active zone for fusion and release of neurotransmitter synchronously in response to the arrival of an action potential, asynchronously after an action potential, or spontaneously in the absence of action potentials. Vesicular proteins (a) confer heterogeneity to synaptic vesicle populations while cytosolic and plasma membrane molecules (b) coordinate with vesicular proteins to determine the vesicle's ultimate fusion parameters.
Vesicle-associated Proteins that Segregate Vesicle Pools
The vesicle membrane in particular contains a variety of proteins important for vesicle exocytosis (56-58). Evidence suggests that some v-SNAREs, calcium sensors, and other vesicular proteins participate in trafficking vesicles to segregated vesicle pools for release (Figure 2), so vesicles may have distinct molecular identities germane to their functional properties.
Vesicular SNAREs
There are multiple v-SNAREs that participate in synaptic vesicle trafficking and fusion with the plasma membrane (Figure 1; Table 1), but the canonical v-SNARE syb2 is the primary v-SNARE responsible for synchronous evoked vesicle release. Syb2 is one of the most abundant vesicle proteins (56, 58), so it is unsurprising that it drives the majority of neurotransmission. In cultured mouse hippocampal neurons deficient in syb2, evoked neurotransmission is all but abolished while spontaneous neurotransmission persists, although at a decreased event frequency (59, 60). This phenotype is also observed in Drosophila neurons deficient in the synaptobrevin-like protein n-syb (61-65). Similarly, vesicles deficient in syb2 recycle styryl dye with kinetics resembling control vesicles during spontaneous neurotransmission (39), suggesting that only spontaneous neurotransmission remains. Tetanus toxin, which cleaves syb2 (66), severely impairs evoked neurotransmission while decreasing but not eliminating spontaneous neurotransmission (61, 67-69). Additionally, vesicles tagged with a membrane probe during stimulation selectively co-label with antibody against syb2 compared to vesicles tagged during spontaneous neurotransmission (70), suggesting that syb2 is found in a higher proportion of evoked vesicles than spontaneously recycling vesicles. Recently, studies using mutated syb2 have found that juxtamembrane and transmembrane regions of the protein control the strength of evoked neurotransmission and its balance with spontaneous neurotransmission (71-77). Altogether, these studies suggest that syb2 primarily traffics vesicles to the plasma membrane for release during evoked synchronous neurotransmission. It is possible that the small number of syb2-containing vesicles that is released spontaneously interacts stochastically with the spontaneous release machinery, but these vesicles could also contain a combination of additional vesicular proteins that is distinct from vesicles that swiftly respond to stimulation.
Table 1. Vesicular SNAREs that segregate vesicle pools.
| Molecule | Manipulation | Experimental System | Synchronous Fusion | Asynchronous Fusion | Spontaneous Fusion | References |
|---|---|---|---|---|---|---|
| Synaptobrevin 2/n-synaptobrevin | KO/null mutation | Cultured mouse hippocampal neurons; Drosophila NMJ | ↓↓ | ↓ | ↓ | (59-64, 71) |
| Juxtamembrane region mutations | Cultured mouse hippocampal neurons | ↓ | ↓ | ↓ | (71, 74) | |
| TMR mutations | Cultured mouse cortical neurons | ↓ | ↓ | = | (77) | |
| Tetanus toxin | Drosophila NMJ; Crayfish NMJ; Torpedo electric organ | ↓↓ | ↓ | ↓ | (61, 67-69) | |
| Synaptobrevin 1 | KO/null mutation | Mouse NMJ | ↓ | ↑ | ↓ | (82) |
| VAMP4 | KD | Cultured inhibitory mouse hippocampal neurons | ↓ | ↓↓ | ↓ | (90) |
| Vti1a | KD | Cultured rat hippocampal neurons | = | ND | ↓ | (97) |
| N-terminal deletion mutation | Cultured rat hippocampal neurons | = | ND | ↑ | (97) | |
| VAMP7 | reelin | Cultured rat hippocampal neurons | = | ND | ↑ | (111) |
| KD + reelin | Cultured rat hippocampal neurons | ND | ND | = | (111) |
Abbreviations: knockdown (KD), knockout (KO), neuromuscular junction (NMJ), not determined (ND), transmembrane region (TMR), vesicle-associated membrane protein (VAMP), vps10p tail interactor 1a (vti1a), abolished or nearly abolished (↓↓), significantly reduced (↓), similar to control (=), significantly increased (↑).
Synaptobrevin 1 (syb1, also known as VAMP1) is a v-SNARE related to syb2 that also promotes synchronous vesicle fusion. Syb1 is expressed on synaptic vesicles and is found widely throughout the nervous system, but syb1 expresses at relatively different levels than syb2 in most brain regions and is more highly expressed than syb2 peripherally (56, 58, 78-81). Null mutations in mouse syb1 cause both spontaneous and synchronous evoked release to decrease with a marked increase in asynchronous release at the neuromuscular junction (82). In cultured, autaptic hippocampal neurons from syb2 knockout mice, heterologous syb1 expression rescues synchronous evoked release to a greater degree than spontaneous release (83). Although syb1 promotes both evoked and spontaneous fusion, these results suggest that it functions primarily to synchronize evoked fusion events.
In contrast to syb2 and syb1, which facilitate synchronous evoked release, v-SNARE VAMP4 facilitates asynchronous evoked release. VAMP4 is better known for its trafficking role in endosomes and the trans-Golgi network (84-88), but it also localizes to synaptic vesicles (84, 89, 90), although less abundantly than syb2 (58). Knockdown of VAMP4 in cultured hippocampal neurons reduces the asynchronous phase of inhibitory postsynaptic currents after strong stimulation, and slow calcium chelation inhibits VAMP4-, but not syb2-, mediated neurotransmission when these proteins are overexpressed in syb2-deficient neurons (90). Additionally, vesicles expressing pH-sensitive fluorescent tags fused to VAMP4 are more reluctant to release in response to stimulation than vesicles expressing tagged syb2 (90). Although VAMP4 also drives a small amount of synchronous evoked and spontaneous neurotransmission (70, 90), the majority of release maintained by VAMP4-containing vesicles occurs in a desynchronized manner.
Recently it was discovered that the v-SNARE vps10p tail interactor 1a (vti1a) participates in spontaneous vesicle recycling. Vti1a localizes mostly to the Golgi apparatus (85, 88, 89), but at least one splice variant has been identified in synaptic vesicles (58, 91, 92). Vti1a deficiency does not produce global protein trafficking or developmental deficits (93, 94), but loss of vti1a in adrenal chromaffin cells reduces the number of dense core vesicles available for hormone secretion (95). Vti1a knockdown in cultured hippocampal neurons reduces the frequency of spontaneous neurotransmission events measured via electrophysiology, and fluorescently tagged vti1a-expressing vesicles do not traffic in response to stimulation as effectively as tagged syb2-expressing vesicles (70, 96, 97), suggesting that this decrease in neurotransmission is not caused solely by vesicle depletion. Spontaneously fused vti1a-containing vesicles are also trafficked independently of syb2-containing vesicles within the same synaptic bouton, further suggesting that these two v-SNAREs drive different pools of vesicles (97). Vti1a-containing synaptic vesicles, therefore, appear to traffic spontaneously to the plasma membrane for fusion.
VAMP7 (also known as tetanus toxin-insensitive VAMP, or TI-VAMP) is another v-SNARE that participates in protein trafficking but also drives spontaneous synaptic transmission (98). VAMP7 is widely expressed and localizes mainly to endosomes, lysosomes, and somatodendritic compartments (91, 99, 100), a pattern that is driven by its longin domain (101, 102). VAMP7 also promotes neurite outgrowth during development (103-105) and is enriched in synaptic vesicles of mature hippocampal mossy fiber terminals (106, 107). VAMP7 knockout mice, however, develop normally and express only subtle behavioral phenotypes (108, 109). When VAMP7 is tagged with a pH-sensitive pHluorin and overexpressed in cultured hippocampal neurons, electrical stimulation induces less vesicle fusion than when syb2-pHluorin is used (97, 110). Also, a much smaller fraction of VAMP7-containing vesicles than syb2-containing vesicles participates in the stimulation-driven recycling pool (97, 110). Recently reelin, a glycoprotein previously shown to signal postsynaptically, was found to drive presynaptic spontaneous vesicle fusion by recruiting VAMP7-containing vesicles but not syb2-, VAMP4-, or vti1a-containing vesicles for exocytosis (111). VAMP7-contanting synaptic vesicles, therefore, can be recruited for action potential-independent fusion events.
Vesicle-Associated Calcium Sensors
Calcium sensors are proteins that bind calcium and subsequently catalyze vesicle fusion (112, 113). There are multiple calcium sensors that contribute to specific forms of neurotransmission (Table 2), but one of the most-studied is syt1, which promotes synchronous evoked vesicle fusion. Syt1 is the most abundant synaptotagmin at mammalian synapses (56-58) and is widely expressed in the rodent brain (114-117). In cultured neurons from syt1 knockout mice, synchronous evoked release measured via electrophysiology is abolished while asynchronous release and the frequency of spontaneous events are increased (118-121), an effect that is also seen in Drosophila neurons deficient for syt1 (122-125). Vesicles tagged during evoked neurotransmission are also enriched with syt1 compared to vesicles tagged during spontaneous neurotransmission (70), confirming syt1's propensity for synchronously fused vesicles. Interestingly, some inhibitory neurons in hippocampal slice culture and excitatory autaptic neurons in dissociated culture are less affected by syt1 deficiency (126, 127), suggesting that syt1 is not the primary calcium sensor for all synchronous release even within the same brain region. Many studies have been performed to determine how the C2A and C2B domains of syt1 bind calcium and differentially modulate synchronous, asynchronous, and spontaneous release (128-138), but there is an emerging consensus around the notion that these domains cooperate to promote synchronous fusion and clamp delayed fusion (139-144). Overall, these studies suggest that vesicles containing syt1 are likely to be trafficked to the plasma membrane for synchronous evoked release.
Table 2. Calcium sensors that segregate vesicle pools.
| Molecule | Manipulation | Experimental System | Synchronous Fusion | Asynchronous Fusion | Spontaneous Fusion | References |
|---|---|---|---|---|---|---|
| Synaptotagmin 1 | KO/null mutation | Autaptic/low density mouse hippocampal neurons | ↓↓ or ↓ | ↑ | = | (118, 119, 127, 132, 133) |
| Cultured mouse hippocampal or cortical neurons | ↓↓ or ↓ | ↑ or = | ↑ | (120, 121, 127, 140, 150) | ||
| Cultured mouse striatal neurons | ↓ | ND | ND | (121) | ||
| Organotypic mouse hippocampal slice cultures | ↓↓ or ↓ | = | ↑ | (126) | ||
| Drosophila NMJ | ↓↓ or ↓ | ↑ | = or ↑ | (122-124, 131, 134, 141) | ||
| Acute ablation | Drosophila NMJ | ↓ | ND | = | (125) | |
| C2A domain mutations | Autaptic mouse hippocampal neurons | ↓ or = | ND | = | (129, 132) | |
| Cultured mouse cortical neurons; acute mouse cortical slices | ↓ or ↑ | ND | ↓ or ↑ | (139, 140) | ||
| Drosophila NMJ | = or ↓ | = or ↑ | = or ↑ | (136, 141-143) | ||
| C2B domain mutations | Autaptic mouse hippocampal neurons | = or ↓ or ↓↓ | = or ↑ | ND | (133, 137) | |
| Cultured mouse cortical neurons | ↓↓ | ND | ↑ | (139, 140) | ||
| Drosophila NMJ | ↓↓ or ↓ | = or ↑ | = or ↑ | (130, 131, 134, 135, 141-143) | ||
| C2A and C2B domain mutation + WT protein | Drosophila NMJ | ↑ | ND | = | (143) | |
| C2A and C2B domain mutation + KO/null mutation | Drosophila NMJ | ↓↓ | ND | ↑ | (143) | |
| Juxtamembrane region mutation | Cultured mouse cortical neurons | ↓ | ND | = | (140) | |
| Linker mutation between C2 domains | Cultured mouse hippocampal neurons | ↓ | ND | = | (144) | |
| Synaptotagmin 2 | KO/null mutation | Mouse NMJ; mouse calyx of Held | ↓ | ↑ | ↑ | (145, 151, 152) |
| Cultured mouse dorsal neostriatum neurons | = | ND | ND | (145) | ||
| KD | Zebrafish NMJ | ↓ | = | ↑ | (149) | |
| Point mutation | Mouse calyx of Held | ↓ | ↑ | ↑ | (150) | |
| Mouse NMJ | ND | ND | ↑ | (150) | ||
| C2A domain mutations | Autaptic mouse hippocampal neurons | = | ND | ND | (132) | |
| C2B domain mutations | Mouse calyx of Held | = or ↓ | = | = or ↑ | (152) | |
| Lentiviral expression + synaptotagmin 1 KO | Cultured mouse cortical neurons | = | ND | ND | (121) | |
| Synaptotagmin 7 | KO/null mutation | Cultured mouse hippocampal or inhibitory cortical neurons | = | = or ↓ | = | (190, 203, 204) |
| KO/null mutation + synaptotagmin 1 KO | Cultured mouse inhibitory cortical neurons | ↓↓ | ↑ | ND | (203) | |
| KO/null mutation + SNAP-25 KO + SNAP-23 lentiviral expression | Autaptic excitatory mouse hippocampal neurons | ↓ | ↑ | ↑ | (190) | |
| KD | Zebrafish NMJ | = | ↓ | = | (149) | |
| Drosophila NMJ | = | ND | ND | (155) | ||
| Cultured mouse hippocampal neurons | = | = | = | (204) | ||
| Acute mouse hippocampal slices | = | ND | ND | (204) | ||
| Cultured hippocampal neurons from synaptotagmin 1 KO mouse | ↓ | ↓ | = | (204) | ||
| Acute mouse hippocampal slices with synaptotagmin 1 KD | = | ↓ | ND | (204) | ||
| C2A domain mutations | Cultured hippocampal neurons from synaptotagmin 1 KO mouse | ↓ | ↓ | = | (204) | |
| C2B domain mutations | Cultured hippocampal neurons from synaptotagmin 1 KO mouse | = | = | ↓ | (204) | |
| C2A and C2B domain mutations | Cultured mouse hippocampal neurons with synaptotagmin 1 KD | ND | ↓ | ND | (204) | |
| Cultured hippocampal neurons from synaptotagmin 1 KO mouse | ↓ | ↓ | = | (204) | ||
| Lentiviral expression | Cultured hippocampal neurons from WT mouse | ND | ND | = | (204) | |
| Cultured hippocampal neurons from synaptotagmin 1 KO mouse | = | ↑ | ↓ | (204) | ||
| Lentiviral expression + synaptotagmin 1 KO | Cultured mouse cortical neurons | ↓ | = | ND | (121, 203) | |
| Synaptotagmin 9 | Conditional KO | Cultured mouse striatal neurons | ↓ | = | ND | (121) |
| Lentiviral expression + synaptotagmin 1 KO | Cultured mouse cortical neurons | = | ↑ | ND | (121) | |
| Synaptotagmin 12 | Lentiviral expression | Cultured mouse cortical neurons | = | = | ↑ | (156) |
| Linker mutation between TMR and C2 domains | Cultured mouse cortical neurons | ND | ND | = | (156) | |
| Doc2 proteins | KO or KD of doc2α | Cultured mouse hippocampal neurons; acute mouse hippocampal slices | = | ↓ | ND | (226, 228) |
| C2A and C2B domain mutations of doc2α | Cultured hippocampal neurons from synaptotagmin 1 KO mouse | ND | ↑ | ND | (228) | |
| KO of doc2β | Acute mouse cerebellar slices | ND | ND | ↓ | (227) | |
| Cultured hippocampal neurons from synaptotagmin 1 KO mouse | = | = | ND | (228) | ||
| Double KO of doc2α and doc2β | Autaptic mouse hippocampal neurons; mouse calyx of Held | = | = | ↓ | (227) | |
| Lentiviral expression of doc2α or doc2β | Cultured mouse hippocampal neurons | = | ↑ | ND | (228) | |
| Quadruple KD of doc2α, doc2β, doc2γ, and rabphilin | Cultured mouse cortical neurons | = | = | ↓ | (229) | |
| Quadruple KD of doc2α, doc2β, doc2γ, and rabphilin | Cultured cortical neurons from synaptotagmin 1 KO mouse | ND | = | = | (229) | |
| C2 domain mutation of doc2β + quadruple KD of doc2α, doc2β, doc2γ, and rabphilin | Cultured mouse cortical neurons | ND | ND | = | (229) |
Abbreviations: double C2 domain (doc2), knockdown (KD), knockout (KO), neuromuscular junction (NMJ), not determined (ND), transmembrane region (TMR), wild type (WT), abolished or nearly abolished (↓↓), significantly reduced (↓), similar to control (=), significantly increased (↑).
Syt2 is another calcium sensor for synchronous evoked vesicle fusion. Syt2 is expressed in rodent brain less abundantly than syt1 and in relatively different regions (58, 114, 115, 117, 145). It also exhibits calcium-dependent binding to phospholipids and both calcium-dependent and calcium-independent binding to t-SNAREs via its C2 domains (146-148). Syt2 knockout or knockdown reduces evoked but increases spontaneous neurotransmission at the neuromuscular junction (145, 149), and Syt2 expression in cortical syt1 knockout neurons rescues synchronous evoked neurotransmission (121). In calyx of Held synapses, which normally express syt2 rather than syt1, synchronous evoked neurotransmission is reduced when syt2 is knocked out or mutated (150-152). Syt2, therefore, promotes evoked synchronous release and clamps spontaneous release in a similar manner as syt1, but these two sensors are differentially expressed throughout the nervous system.
Other synaptotagmins also coordinate vesicle trafficking and fusion. Of the multiple known synaptotagmins expressed in brain tissue (117, 153), however, only a small minority have been shown both to localize to synaptic vesicle membranes and to differentially modulate these three forms of vesicle fusion. In addition to syt1 and syt2, syt9 and syt12 appear to meet these criteria. Syt9 expression in cortical syt1 knockout neurons rescues synchronous evoked neurotransmission, and syt9 deficiency in striatal neurons reduces synchronous evoked responses while leaving asynchronous fusion events unaffected (121). Overexpression of syt12, one of the few synaptotagmins that does not exhibit calcium-dependent binding to its targets (146, 154, 155), increases the frequency of inhibitory spontaneous events in cultured cortical neurons in a protein kinase A-dependent manner but does not alter synchronous or asynchronous evoked responses (156). The synaptotagmin protein family, therefore, exhibits diversity in its effects on neurotransmission.
Other Vesicle-associated Proteins
In addition to v-SNAREs and calcium sensors, other vesicular proteins also have reported roles in guiding vesicles to segregated release pools. For example, mouse hippocampal neurons deficient in rab3a, a GTP-binding protein, exhibit subtly increased evoked but normal spontaneous release while rab3a-deficient neuromuscular junctions produce reduced spontaneous yet normal evoked release (157-159). Synapsin 1, in contrast, differentially alters inhibitory and excitatory evoked neurotransmission without changing spontaneous neurotransmission (160, 161). Additionally inhibitory hippocampal neurons from synapsin 2 knockout mice display increased synchronous evoked and decreased asynchronous evoked and spontaneous neurotransmission (162). These proteins, therefore, may alter the likelihood that vesicles will release synchronously.
Plasma Membrane-associated Proteins that Segregate Vesicle Pools
Vesicular proteins interact with target membrane proteins to complete vesicle trafficking to the plasma membrane for subsequent fusion (53). The target membrane contains a variety of molecules (57, 58), including t-SNAREs and calcium channels, that affect vesicle release properties and, therefore, vesicle pool membership (Figure 2; Table 2; Table 3).
Table 3. Target membrane SNAREs that segregate vesicle pools.
| Molecule | Manipulation | Experimental System | Synchronous Fusion | Asynchronous Fusion | Spontaneous Fusion | References |
|---|---|---|---|---|---|---|
| t-SNAREs: Syntaxin 1 | KO/null mutation of syntaxin 1A | Drosophila NMJ | ↓↓ | ↓ | ↓↓ | (61, 64, 166) |
| KO of syntaxin 1B | Cultured mouse hippocampal neurons | = | ND | ↓ | (170) | |
| Double KO of syntaxin 1A and syntaxin 1B | Autaptic mouse cortical neurons | ↓ | ↑ | = or ↓ | (170) | |
| KD of syntaxin 1B | Cultured cortical neurons from syntaxin 1A KO mouse | ↓ | ↓ | ↓ | (169) | |
| SNARE motif mutation | Drosophila NMJ | ↓↓ | ↑ | ↓ | (167) | |
| Inhibitory peptide against SNARE motif | Cultured rat hippocampal neurons | ↓ | ND | ND | (168) | |
| N-terminal deletion mutation of syntaxin 1A + syntaxin 1B KD | Cultured cortical neurons from syntaxin 1A KO mouse | ↓ | ↓ | ↓ | (169) | |
| Habc domain deletion mutation of syntaxin 1A + syntaxin 1B KD | Cultured cortical neurons from syntaxin 1A KO mouse | = | = | ↓ | (169) | |
| Linker mutation between SNARE motif and TMR of syntaxin 1A + syntaxin 1B KD | Cultured mouse cortical neurons | ↓ | ↓ | = | (77) | |
| TMR mutation + syntaxin 1B KD | Cultured mouse cortical neurons | = | = | = | (77) | |
| TMR mutation + linker mutation between SNARE motif & TMR + syntaxin 1B KD | Cultured mouse cortical neurons | ↓ | ↓ | ↑ | (77) | |
| Syntaxin 13 | Inhibitory peptide | Cultured rat hippocampal neurons | ↓ | ND | ↑ | (96) |
| SNAP-25 | KO/null mutation | Cultured mouse hippocampal, inhibitory cortical, or striatal neurons | ↓↓ | ND | ↓ | (182-185) |
| Mouse NMJ | ↓↓ | ND | ↑ | (182) | ||
| KO/null mutation heterozygote | Cultured mouse excitatory hippocampal neurons | ↑ | ND | = | (186) | |
| Cultured mouse inhibitory hippocampal neurons | ↓ | ND | = | (186) | ||
| Temperature-sensitive mutation | Drosophila NMJ at 22°C | ↑ | ND | ↑ | (181) | |
| Drosophila NMJ at 37°C | ↓ | ND | = | (181) | ||
| Central α-helix mutation | Autaptic mouse hippocampal neurons | = | = | ↑ | (187) | |
| C-terminal deletion mutation | Autaptic mouse hippocampal neurons | ↓↓ | = | ↓↓ | (187) | |
| N-terminal deletion mutation | Autaptic mouse hippocampal neurons | = | ↓ | ↑ | (187) | |
| Botulinum toxin A | Organotypic rat hippocampal slice cultures | ↓ | ND | ↓ | (180) | |
| SNAP-23 | Lentiviral expression + SNAP-25 KO | Autaptic excitatory mouse hippocampal neurons | ↓ | = | ↑ | (190) |
| Cultured mouse hippocampal neurons | ↓ | ↑ | = | (185) | ||
| Lentiviral expression + double KO of SNAP-25 and synaptotagmin 7 | Autaptic excitatory mouse hippocampal neurons | ↓↓ | ↑ | ↑ | (190) |
Abbreviations: knockdown (KD), knockout (KO), neuromuscular junction (NMJ), not determined (ND), transmembrane region (TMR), abolished or nearly abolished (↓↓), significantly reduced (↓), similar to control (=), significantly increased (↑).
Target Membrane SNAREs
Syntaxin 1 is one of two canonical t-SNAREs recruited during neurotransmission. Syntaxin 1 is highly expressed throughout the nervous system, especially at presynaptic terminals (58, 163). This membrane-anchored protein dimerizes with the other canonical t-SNARE SNAP-25 before binding to v-SNAREs, and this dimer is capable of binding to VAMP4 and VAMP7, although less efficiently than syb2 (90, 164, 165). Syntaxin 1 knockout in Drosophila neuromuscular junctions abolishes all forms of neurotransmission except for rare asynchronous fusion events (61, 64, 166), but syntaxin 1 hypomorphs exhibit small evoked responses while retaining spontaneous neurotransmission (166). Interference with binding sites on syntaxin 1A's H3 domain, which destabilizes SNARE complex formation, in Drosophila neuromuscular junction or mammalian hippocampal neurons severely impairs evoked neurotransmission with smaller effects on spontaneous neurotransmission (167, 168). Additionally, expression of syntaxin 1 mutants with residues added between the transmembrane domain and the SNARE binding domain reduces evoked but not spontaneous fusion (77). Knockdown of syntaxin 1 in cultured cortical neurons impairs all neurotransmission, but interestingly overexpression of syntaxin 1 with a mutated Habc domain, which alters syntaxin 1's ability to bind Munc18-1, in syntaxin 1 knockdown neurons rescues inhibitory synchronous evoked neurotransmission but not spontaneous event frequency (169). Similarly hippocampal neurons deficient in syntaxin 1B exhibit decreased excitatory and inhibitory spontaneous event frequencies but normal excitatory and inhibitory synchronous evoked responses (170). Syntaxin 1A and 1B double knockout neurons from cortex, however, display impaired synchronous evoked and spontaneous neurotransmission but increased asynchronous evoked release (170). These studies suggest that syntaxin 1 is vital for neurotransmission, but different regions of the molecule or its different isoforms may be responsible for modulating each form of neurotransmission and, therefore, for guiding vesicles from different pools to the membrane for fusion.
Another syntaxin with the potential to differentially modulate these forms of neurotransmission is syntaxin 13. Syntaxin 13 is expressed in brain and is usually localized to endosomes (171). When syntaxin 13 is tagged with pHluorin and overexpressed in hippocampal neurons, it traffics to presynaptic terminals and participates in synaptic vesicle recycling (96). Co-expressing syntaxin 13-pHluorin with a soluble fragment of syntaxin 13, which acts dominant-negatively, decreases exocytosis after a short electrical stimulus train but increases exocytosis after longer stimulus trains and during a period of spontaneous neurotransmission (96). Although it is unclear whether syntaxin 13 participates directly in neurotransmission under physiological conditions, it is possible that this molecule promotes synchronous fusion events and clamps delayed and spontaneous events.
SNAP-25, the canonical t-SNARE that dimerizes with syntaxin 1, is also important for neurotransmission. SNAP-25 is almost exclusively expressed in the brain (172). It does not contain a transmembrane domain, but it is anchored to the membrane through palmitoylated cysteine residues and its interaction with syntaxin 1 (173-175). Inhibiting the C-terminus of SNAP-25 by introducing mutations or applying exogenous peptides reduces vesicular secretion from chromaffin cells (176, 177). Botulinum neurotoxin A, which cleaves SNAP-25 (178, 179), reduces both action potential-dependent and spontaneous neurotransmission in neurons from hippocampal slice culture (180). Temperature-sensitive SNAP-25 Drosophila mutants, however, exhibit smaller synchronous evoked response amplitudes but similar spontaneous event frequency changes at destabilizing temperatures when compared to wild type flies (181). Homozygous knockout of SNAP-25 in mouse, which is embryonically lethal, abolishes evoked muscle contractions at embryonic neuromuscular junctions while maintaining spontaneous neurotransmission (182). In cortical slices from embryonic SNAP-25 knockout mice, inhibitory evoked responses are absent while some spontaneous neurotransmission remains (183). Similarly, in cultured neurons from SNAP-25 knockout mice, both excitatory and inhibitory evoked responses are abolished while a small amount of excitatory and inhibitory spontaneous neurotransmission remains (184), and these deficits are rescued by genetic reintroduction of SNAP-25 (185). A recent study has also reported that excitatory evoked neurotransmission is increased in SNAP-25 heterozygous neurons while inhibitory evoked responses are decreased and spontaneous neurotransmission is normal (186), suggesting that heterozygosity unmasks functions of SNAP-25 that are undetected in knockout neurons. C-terminal deletions in SNAP-25 severely impair synchronous and spontaneous forms of release, but interestingly N-terminal deletions and central mutations unclamp spontaneous release without altering synchronous evoked release, suggesting that different regions of the protein differentially modulate these forms of neurotransmission (187). Altogether, these studies suggest that SNAP-25 is essential for evoked neurotransmission but less vital for spontaneous neurotransmission.
SNAP-23 may also play a role in differentially promoting particular forms of neurotransmission. SNAP-23, which is structurally related to SNAP-25, is expressed in a wide variety of tissues, including the brain (172). SNAP-23-dependent vesicle docking occurs at lower calcium concentrations than SNAP-25-dependent docking in vitro, and SNAP-23 has a lower affinity for syt1 than SNAP-25 (188). Chromaffin cells from SNAP-25 knockout mice exhibit increased sustained release when SNAP-23 is overexpressed but stimulated bursts when SNAP-25 is reintroduced (189). Similarly, SNAP-25 knockout neurons overexpressing SNAP-23 exhibit excitatory and inhibitory asynchronous vesicle fusion, which contrasts with the rescue of synchronous evoked neurotransmission by SNAP-25 (185, 190). Although it is unclear whether SNAP-23 modulates physiological neurotransmission, it may function to promote asynchronous neurotransmission under defined contexts.
Plasma Membrane Calcium Sensors
Syt7, in contrast to syt1, syt2, and syt9, does not promote synchronous vesicle fusion and is not clearly present in synaptic vesicle membranes (191). It is widely expressed in the rodent brain (117, 192) and is found in lysosomes and at synapses (193-197), where it is much less abundant than syt1 (58). Although syt7 is reportedly found in large dense core vesicles of PC12 cells (197-199), electron microscopy studies of hippocampal neurons suggest that it localizes to the plasma membrane at synapses rather than to synaptic vesicles (192, 200). Syt7 exhibits a higher calcium affinity than syt1 (146, 201, 202), and its slow disassembly kinetics suggest that it is better suited for coordinating delayed rather than synchronous fusion events (148). This is consistent with syt7's ability to bind SNAP-23, the t-SNARE involved in asynchronous fusion events (188). Interestingly, syt7 has multiple splice variants that differentially modulate vesicle endocytosis (192, 200). Syt7 knockdown in neuromuscular junctions of zebrafish and Drosophila has no effect on synchronous evoked release as measured via electrophysiology, but the asynchronous component of release is reduced in zebrafish (149, 155). Inhibitory cortical neurons from syt7 knockout mice, however, do not display consistent deficits in evoked or spontaneous neurotransmission (203). In contrast inhibitory hippocampal syt1 knockout neurons, which lack synchronous release, display markedly reduced asynchronous release when syt7 is knocked down (204). Additionally inhibitory spontaneous neurotransmission in wild type and syt1 knockout hippocampal neurons is not affected by syt7 knockdown, but overexpression of syt7 in syt1 knockout neurons reduces spontaneous neurotransmission (204). These data suggest that syt7 promotes asynchronous evoked release and possibly clamps spontaneous fusion, but these phenotypes are not apparent in mammalian neurons expressing an abundance of syt1.
Calcium Channels
Calcium channels also seem to be important for determining the temporal dynamics of vesicle fusion. Presynaptic calcium channels are known to interact directly with presynaptic release machinery, and this close interaction facilitates the coupling of stimulation-evoked calcium entry and vesicle fusion (205, 206). Calcium influx through voltage-gated calcium channels like N-type, P/Q-type, and R-type channels also mediates a portion of spontaneous fusion events (207). For example, cholecystokinin-expressing interneurons that synapse onto hippocampal dentate granule neurons exhibit higher levels of spontaneous neurotransmission than parvalbumin-expressing interneurons, and this effect has been linked to different relative amounts of N-type and P/Q-type calcium channels (208). It is becoming clear, however, that spontaneous and asynchronous vesicle pools do not always depend on these voltage-gated calcium channels for release (209). Blocking calcium channels with cadmium in cerebellar slices does not reduce inhibitory spontaneous event frequencies (210), and application of peptides that prevent calcium channel binding to SNARE proteins reduces evoked but increases asynchronous release in superior cervical ganglion neurons (211). Similarly, mutations in the Drosophila gene fuseless, which prevent calcium channel recruitment to presynaptic terminals of the neuromuscular junction, cause reduced evoked excitatory junctional currents but increased frequency and amplitude of spontaneous events (212). Phorbol ester-induced potentiation of evoked release in cultured hippocampal neurons does not appear to depend on L-type calcium channels while the phorbol ester-induced increase in spontaneous event frequency does (213, 214). Interestingly, excitatory spontaneous neurotransmission in cultured neocortical neurons appears to depend on a G protein-coupled calcium-sensing receptor while inhibitory spontaneous neurotransmission in the same preparation depends on nanodomain calcium entry from voltage-gated calcium channels (215, 216). These studies provide evidence that vesicle release from different pools can be guided by differential dependence on calcium influx from calcium channels located in the plasma membrane.
Cytosolic Proteins that Segregate Vesicle Pools
Synapses contain many soluble molecules that interact with vesicular and plasma membrane proteins and are important for modulating vesicle fusion (53, 58). Some of these proteins, as discussed below, also appear to be important for maintaining segregated vesicle pools (Figure 2; Table 2; Table 4).
Table 4. Cytosolic active zone proteins that segregate vesicle pools.
| Molecule | Manipulation | Experimental System | Synchronous Fusion | Asynchronous Fusion | Spontaneous Fusion | References |
|---|---|---|---|---|---|---|
| Munc13 | KO/null mutation of Munc13-1 | Autaptic or cultured mouse excitatory hippocampal neurons | ↓↓ | ND | ↓ | (233, 234) |
| Autaptic mouse inhibitory hippocampal neurons | = | ND | ND | (233) | ||
| C1 domain mutation of Munc13-1 | Autaptic mouse excitatory hippocampal neurons | = | = | ↑ | (236) | |
| KO or acute ablation of unc13-long | Caenorhabditis elegans NMJ | ↓ | = | ↓ | (238, 239) | |
| C2A domain deletion mutation of unc13-long | Caenorhabditis elegans NMJ | ↓ | ↑ | ↓ | (239) | |
| N-terminal deletion mutation of unc13-long | Caenorhabditis elegans NMJ | = | ↑ | ↓ | (239) | |
| Acute ablation of unc13-long with N-terminal deletion mutation | Caenorhabditis elegans NMJ | = | ↓ | = | (239) | |
| KO/null mutation of Munc13-2 | Autaptic mouse excitatory or inhibitory hippocampal neurons; mouse calyx of Held | = | ND | = | (233, 240) | |
| Double KO of Munc13-1 and 2 | Autaptic mouse excitatory or inhibitory hippocampal neurons | ↓↓ | ↓↓ | ↓↓ | (233) | |
| Mouse NMJ | ↓ | ND | ↑ | (235) | ||
| KO/null mutation of unc13-short | Caenorhabditis elegans NMJ | ↓ | ↓ | = | (238) | |
| Double KO of Unc13-long and unc13-short | Caenorhabditis elegans NMJ | ↓↓ | ND | ↓↓ | (238, 239) | |
| KO/null mutation of Munc13-3 | Mouse calyx of Held | = | ND | ND | (240) | |
| Double KO of Munc13-2 and 3 | Mouse calyx of Held | = | ↓ | ND | (240) | |
| Dominant negative Munc13-1 + double KO of Munc13-2 and 3 | Mouse calyx of Held | ↓ | ND | ND | (240) | |
| Rim1α | KO/null mutation | Autaptic mouse excitatory hippocampal neurons; acute mouse hippocampal slices | ↓ | ↓ | = | (246, 247) |
| Caenorhabditis elegans NMJ | ↓ | ND | ↓ | (248) | ||
| Tomosyn | KO/null mutation | Acute mouse hippocampal slices | ↑ | ND | ND | (256) |
| Caenorhabditis elegans NMJ | = or ↑ | ↑ | = | (238, 254, 255) | ||
| Complexins | KO/null mutation of complexin 1 or complexin | Autaptic mouse excitatory hippocampal neurons | = | ND | = | (273) |
| Mouse spiral ganglion-bushy cell auditory synapse | ↓ | ↑ | ↓ | (267) | ||
| Drosophila NMJ; Caenorhabditis elegans NMJ | ↓ | ↑ | ↑ | (261, 266, 268, 269, 272, 282) | ||
| SNARE motif mutations of complexin 1 or complexin | Autaptic mouse excitatory hippocampal neurons; cultured mouse cortical or olfactory bulb neurons; Caenorhabditis elegans NMJ | ↓ | ND | ↑ | (264, 268, 270, 274) | |
| Drosophila NMJ | ↓ | ND | = | (272) | ||
| Inhibitory peptide against complexin SNARE motif | Zebrafish retinal bipolar cells | = | ND | ↑ | (271) | |
| Accessory α-helix mutations of complexin 1 or complexin | Autaptic mouse hippocampal neurons; cultured mouse cortical or olfactory bulb neurons; Drosophila NMJ | = | = | = or ↓ or ↑ | (262, 263, 270, 272, 279) | |
| C-terminal truncation mutation of complexin 1 or complexin | Cultured mouse cortical neurons | = | = | = or ↑ | (262, 265) | |
| Drosophila NMJ; Caenorhabditis elegans NMJ | ↓ | ND | ↑ | (261, 268) | ||
| C-terminal point mutations of complexin 1 or complexin | Cultured mouse cortical neurons; Drosophila NMJ | = | = | = or ↑ | (265, 282) | |
| C-terminal membrane attachment mutation of complexin 1 | Cultured mouse cortical neurons | = | ND | ↑ | (262) | |
| N-terminal truncation mutation of complexin 1 or complexin | Autaptic mouse excitatory hippocampal neurons | ↓ | ND | ND | (274) | |
| Cultured mouse cortical neurons | ↓ | ND | = | (264) | ||
| Cultured mouse olfactory bulb neurons | ↓ | ND | ↑ | (270) | ||
| Drosophila NMJ | = | ND | ↑ | (272) | ||
| N-terminal truncation + SNARE motif mutation of complexin 1 | Autaptic mouse excitatory hippocampal neurons | ↓ | ND | ND | (274) | |
| N-terminal + accessory α-helix deletion mutations of complexin 1 | Autaptic mouse excitatory hippocampal neurons | ↓ | ND | ND | (274) | |
| Cultured mouse cortical neurons | ↓ | ND | ↑ | (264) | ||
| Cultured mouse olfactory bulb neurons | ↓ | ND | = | (270) | ||
| KO/null mutation of complexin 2 | Autaptic mouse excitatory hippocampal neurons | = | = | = | (273, 274) | |
| Double KO of complexins 1 and 2 | Autaptic mouse excitatory hippocampal neurons | ↓ | = | = or ↓ | (273, 275) (274) | |
| Autaptic mouse inhibitory striatal neurons | ↓ | ND | = | (275) | ||
| Cultured mouse cortical neurons | ↓ | ND | ↑ | (262) | ||
| Cultured mouse olfactory bulb neurons | ↓ | ND | ↓ | (262) | ||
| Double KD of complexins 1 and 2 | Cultured mouse cortical neurons | ↓ | = or ↓ | ↑ | (262-265) | |
| Cultured mouse olfactory bulb neurons | ↓ | ND | ↑ | (270) (262) | ||
| KO of complexin 3 | Autaptic mouse excitatory hippocampal or inhibitory striatal neurons | = | ND | = | (275) | |
| Double KO of complexins 2 and 3 | Autaptic mouse excitatory hippocampal or inhibitory striatal neurons | = | ND | = | (275) | |
| Triple KO of complexins 1-3 | Autaptic mouse excitatory hippocampal neurons | ↓ | ND | = or ↓ | (275, 279) | |
| Autaptic mouse inhibitory striatal neurons | ↓ | ND | = | (275) |
Abbreviations: knockdown (KD), knockout (KO), neuromuscular junction (NMJ), not determined (ND), abolished or nearly abolished (↓↓), significantly reduced (↓), similar to control (=), significantly increased (↑).
Cytosolic Calcium Sensors
Potential cytosolic calcium sensors include the double C2 domain (doc2) protein family, which contains three proteins structurally similar to syt1: doc2α, doc2β, and doc2γ. Doc2 proteins have two calcium-binding C2 domains and translocate to the plasma membrane after calcium influx to interact with the Munc13-1 priming protein, t-SNAREs, or the membrane itself (217-225). Excitatory hippocampal neurons and inhibitory cerebellar neurons deficient in doc2α or both doc2α and doc2β exhibit normal synchronous evoked neurotransmission measured via electrophysiology (226, 227), but the frequency of spontaneous neurotransmission is reduced in doc2α/doc2β double knockout neurons (227). Additionally, asynchronous neurotransmission measured in the presence or absence of syt1 is decreased in excitatory hippocampal neurons with doc2α, but not doc2β, knocked down (228). Knocking down all three doc2 proteins along with the related protein rabphilin reduces spontaneous neurotransmission in excitatory and inhibitory cortical neurons but does not alter asynchronous release in inhibitory neurons (229). The precise role of doc2 proteins as calcium sensors in synaptic transmission is debated, but these proteins likely promote spontaneous and possibly asynchronous neurotransmission.
Active Zone Proteins
Biochemical priming of vesicles after docking at the presynaptic active zone is vitally important for vesicle fusion (53). One such priming protein, Munc13-1, is presynaptically localized and is perinatally lethal when knocked out in mice (58, 230, 231). Munc13-1 has also been proposed to function as a calcium sensor at the presynaptic terminal (232). Cultured Munc13-1 knockout neurons from hippocampus exhibit impaired evoked excitatory neurotransmission but relatively spared spontaneous event frequencies (233, 234). In mice doubly deficient for Munc13-1 and the related protein Munc13-2, neuromuscular junction synapses exhibit decreased evoked response amplitudes and increased spontaneous event frequencies (235). Knocking in a Munc13-1 mutation that cannot bind phorbol esters onto a Munc13-2 knockout background produces murine hippocampal neurons with higher basal spontaneous event frequencies without altering evoked response amplitudes, suggesting that vesicle pools can be differentially modulated by the phorbol ester binding region of Munc13-1 (236). In Caenorhabditis elegans, neurons express two forms of unc13: a long form that is analogous to mammalian Munc13-1 and ubMunc13-2 and a short form that is analogous to mammalian bMunc13-2 and Munc13-3 (237). The long form of C. elegans unc13 localizes closer to active zones than the short form, and expression of the long form in a double deficiency background rescues evoked synchronous release while expression of the short form rescues asynchronous evoked release, with either form rescuing spontaneous event frequency (238).
Similarly, removing the N-terminal C2A domain of the long form of unc13 impairs synchronous evoked and spontaneous release, and acute ablation of a diffusely localized unc13 isoform selectively impairs asynchronous release (239). This is consistent with mammalian calyx of Held, in which Munc13-2 and Munc13-3 double knockout produces reduced asynchronous release, but introducing a dominant-negative Munc13-1 into the double knockout background reduces overall evoked response amplitudes (240). Overall, Munc13-1 promotes synchronous evoked vesicle fusion while other Munc13 isoforms facilitate asynchronous fusion events.
Munc13-1 is known to interact with additional proteins in the presynaptic terminal like Rim1. Rim1α is found near active zones (241, 242), and knocking out Rim1α in mice is perinatally lethal when combined with knockout of the related protein Rim2α (243). Double knockout of Rim1α and Rim2α reduces both evoked and spontaneous release in neuromuscular junctions, hippocampal neurons, and calyx of Held synapses, putatively due to reduced vesicle docking near calcium channels (243-245). Munc13-1 levels are decreased in Rim1α single knockout mouse neurons (246), and hippocampal autaptic as well as slice neurons from Rim1α knockout mice exhibit reduced excitatory evoked neurotransmission, with a relatively more reduced asynchronous component, and normal spontaneous event frequency (246, 247). Neuromuscular junction synapses from unc10/Rim1 C. elegans mutants also express decreased evoked responses but instead exhibit decreased spontaneous event frequencies (248). Rim1α, therefore, appears to promote synchronous evoked release in general but also asynchronous evoked and spontaneous release in particular contexts.
Tomosyn, another soluble active zone protein, is a SNARE protein that differentially affects vesicle fusion events. In vitro studies suggest that tomosyn binds to t-SNAREs to create a SNARE complex that clamps membrane fusion until fusion-promoting v-SNAREs displace it (249-253). Tomosyn inhibits both synchronous and asynchronous evoked responses in C. elegans neuromuscular junction and murine excitatory synapses without changing the spontaneous event frequency, and some of these effects seem to occur through clamping unc13/Munc13 activity (254-256). Tomosyn, therefore, appears to selectively modulate evoked fusion events.
Complexins
Complexins are another family of proteins that differentially modulate synchronous, asynchronous, and spontaneous forms of neurotransmission. Complexin I and complexin II are soluble proteins expressed in the nervous system both presynaptically and postsynaptically (58, 257-259). Complexin III is also widely expressed in the nervous system, albeit at lower levels than complexin I and complexin II, but complexin IV is restricted to ribbon synapses of the retina (260). Complexin III and complexin IV, unlike complexin I and complexin II, are anchored to membranes by their C-terminal domains (260-262). In cultured cortical neurons from mice doubly deficient in complexin I and complexin II, excitatory synchronous evoked response amplitudes measured via electrophysiology are reduced while excitatory spontaneous event frequencies are increased (262-265). Complexin deficiency in Drosophila neuromuscular junction, C. elegans neuromuscular junction, mouse auditory pathway, mouse olfactory bulb, and zebrafish retinal bipolar cells also produces these effects on neurotransmission (266-272). Interestingly autaptic hippocampal neurons deficient in both complexin I and complexin II exhibit reduced EPSC amplitudes but no increase in spontaneous event frequency (273-275). Complexin deficiency may also increase the relative amount of asynchronous release present in the evoked response (263, 267, 269, 273), even though complexin does not bind VAMP4-containing SNARE complexes that select for asynchronous release (90). Various in vitro vesicle fusion assays and complexin mutants have been employed to determine the biochemical basis of complexin's differential effects. The central α-helix of the protein seems to interact with SNARE machinery to dock and clamp vesicles from synchronous and spontaneous pools to await calcium influx (263, 264, 270, 274, 276-278) while the negative electrical charges on the accessory α-helix function to clamp neurotransmission (263, 264, 270, 272, 274, 279). The N-terminus promotes fusion events normally suppressed by the accessory α-helix (264, 270, 274, 280), and the C-terminus, which differs between the soluble complexins I and II and the membrane-anchored complexins III and IV, promotes docking of vesicles while clamping spontaneous release (261, 262, 265, 272, 281, 282). Overall complexins seem to promote synchronous evoked vesicle fusion, while in some circumstances they also clamp spontaneous release.
Membrane Lipid Components that Modulate Vesicle Pools
There are additional molecules found in membranes that modulate the form of fusion a vesicle undergoes (Figure 2). For example, cholesterol content at the presynaptic terminal appears to promote synchronous evoked release. At crayfish and frog neuromuscular junction and in cultured hippocampal neurons, pretreating neurons with methyl-β-cyclodextrin to remove cholesterol inhibits evoked vesicle fusion as measured by electrophysiological and vesicle labeling techniques (283-285). Despite this reduction in evoked release, removing cholesterol from membranes increases the frequency of spontaneous fusion events (283-286). The effects of cholesterol on evoked, but not spontaneous, events are absent in hippocampal syb2 knockout neurons (284). Because syb2 is preferentially expressed in vesicles that fuse synchronously in response to stimulation (70, 97), this further suggests that cholesterol differentially affects vesicles belonging to different vesicle pools.
Functional Consequences of Segregated Vesicle Pools
The presence of molecularly and functionally distinct vesicle pools suggests that each form of neurotransmission has differential downstream effects on neuronal function. Information transfer throughout the nervous system has been classically assumed to depend on synchronous evoked release, due to its temporal precision, while asynchronous evoked and spontaneous release have been thought to modulate the information carried by the synchronous evoked signal (11). A recent study, however, showed that reducing synchronous evoked release in hippocampus, entorhinal cortex, or prefrontal cortex by knocking down syt1 had only subtle effects on fear conditioning memory tests in mice (287), suggesting that asynchronous release is sufficient to deliver the majority of the informational signal. Spontaneous neurotransmission, in turn, modulates action potential generation in postsynaptic neurons (288-290) and homeostatically scales postsynaptic receptor levels (291, 292), which is thought to occur via activation of postsynaptic signaling cascades that are distinct from those activated by evoked release (10, 14). Inhibiting spontaneous neurotransmission also appears to be the mechanism by which glutamate receptor blockers induce fast-acting antidepressant behaviors in rodents (293-295), suggesting that spontaneous neurotransmission indeed exerts potent effects on neurotransmission. Each form of neurotransmission, therefore, works to refine the signal being propagated through the circuit. Because of these distinct contributions to neurotransmission, determining the molecular basis of different vesicle pools is important for understanding nervous system function.
Conclusions
Evidence suggests that a complex array of presynaptic factors guides synaptic vesicles toward different modes of release. Even though classical vesicle pools have been defined by their release characteristics or ultrastructural localization (2-6), a molecular framework is now emerging where distinct vesicle populations can be functionally and molecularly distinguished (11, 12). Vesicular membrane proteins like v-SNAREs and calcium sensors give each vesicle a molecular identity while soluble factors like Munc13-1 or doc2 and plasma membrane proteins like t-SNAREs guide vesicles through segregated, though likely partially overlapping, fusion pathways. These molecularly defined vesicle pools are unlikely to be a static property of the synapse, however, due to activity-dependent rearrangements that arise from bulk vesicle endocytosis, lipid mixing, and exogenous factors that recruit particular vesicles under defined contexts. It is becoming increasingly clear that synaptic vesicle populations and their interaction partners exhibit greater heterogeneity than previously appreciated, and future work is needed to clarify the molecular and functional distinctions between synaptic vesicle pools.
Synopsis.
In this review, Crawford and Kavalali discuss the various molecular components required to traffic synaptic vesicles to the presynaptic plasma membrane for synchronous evoked, asynchronous evoked, or spontaneous fusion. It is becoming clear that synaptic vesicles exhibit distinct molecular identities and a variety of molecules interact with vesicles to modulate their exocytosis in relation to neuronal action potentials. These distinct forms of neurotransmission, in turn, are critical for proper information transfer and synaptic signaling homeostasis throughout the nervous system.
Acknowledgments
The authors were supported by National Institutes of Health grants F32MH102915 (D.C.C.) and MH066198 (E.T.K.) and by the Brain and Behavior Research Foundation (E.T.K.).
References
- 1.Kasai H, Takahashi N, Tokumaru H. Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol Rev. 2012;92(4):1915–64. doi: 10.1152/physrev.00007.2012. [DOI] [PubMed] [Google Scholar]
- 2.Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6(1):57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- 3.Sudhof TC. The synaptic vesicle cycle revisited. Neuron. 2000;28(2):317–20. doi: 10.1016/s0896-6273(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 4.Denker A, Rizzoli SO. Synaptic vesicle pools: an update. Frontiers in synaptic neuroscience. 2010;2:135. doi: 10.3389/fnsyn.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alabi AA, Tsien RW. Synaptic vesicle pools and dynamics. Cold Spring Harb Perspect Biol. 2012;4(8):a013680. doi: 10.1101/cshperspect.a013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci. 2001;4(4):391–5. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- 7.Marra V, Burden JJ, Thorpe JR, Smith IT, Smith SL, Hausser M, et al. A preferentially segregated recycling vesicle pool of limited size supports neurotransmission in native central synapses. Neuron. 2012;76(3):579–89. doi: 10.1016/j.neuron.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzoli SO, Betz WJ. The structural organization of the readily releasable pool of synaptic vesicles. Science. 2004;303(5666):2037–9. doi: 10.1126/science.1094682. [DOI] [PubMed] [Google Scholar]
- 9.Sakaba T. Roles of the fast-releasing and the slowly releasing vesicles in synaptic transmission at the calyx of held. J Neurosci. 2006;26(22):5863–71. doi: 10.1523/JNEUROSCI.0182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kavalali ET, Chung C, Khvotchev M, Leitz J, Nosyreva E, Raingo J, et al. Spontaneous neurotransmission: an independent pathway for neuronal signaling? Physiology (Bethesda) 2011;26(1):45–53. doi: 10.1152/physiol.00040.2010. [DOI] [PubMed] [Google Scholar]
- 11.Kaeser PS, Regehr WG. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu Rev Physiol. 2014;76:333–63. doi: 10.1146/annurev-physiol-021113-170338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez DM, Kavalali ET. The role of non-canonical SNAREs in synaptic vesicle recycling. Cellular logistics. 2012;2(1):20–7. doi: 10.4161/cl.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung C, Raingo J. Vesicle dynamics: how synaptic proteins regulate different modes of neurotransmission. J Neurochem. 2013;126(2):146–54. doi: 10.1111/jnc.12245. [DOI] [PubMed] [Google Scholar]
- 14.Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci. 2014;16(1):5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- 15.Fatt P, Katz B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951;115(3):320–70. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Robertis ED, Bennett HS. Some features of the submicroscopic morphology of synapses in frog and earthworm. J Biophys Biochem Cytol. 1955;1(1):47–58. doi: 10.1083/jcb.1.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palay SL. Synapses in the central nervous system. J Biophys Biochem Cytol. 1956;2(4 Suppl):193–202. doi: 10.1083/jcb.2.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett EF, Stevens CF. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117(1):109–28. [PMC free article] [PubMed] [Google Scholar]
- 20.Glitsch MD. Spontaneous neurotransmitter release and Ca2+--how spontaneous is spontaneous neurotransmitter release? Cell Calcium. 2008;43(1):9–15. doi: 10.1016/j.ceca.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Colmeus C, Gomez S, Molgo J, Thesleff S. Discrepancies between spontaneous and evoked synaptic potentials at normal, regenerating and botulinum toxin poisoned mammalian neuromuscular junctions. Proceedings of the Royal Society of London Series B, Containing papers of a Biological character Royal Society. 1982;215(1198):63–74. doi: 10.1098/rspb.1982.0028. [DOI] [PubMed] [Google Scholar]
- 22.Cherki-Vakil R, Ginsburg S, Meiri H. The difference in shape of spontaneous and uniquantal evoked synaptic potentials in frog muscle. J Physiol. 1995;482(Pt 3):641–50. doi: 10.1113/jphysiol.1995.sp020546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zefirov A, Benish T, Fatkullin N, Cheranov S, Khazipov R. Localization of active zones. Nature. 1995;376(6539):393–4. doi: 10.1038/376393b0. [DOI] [PubMed] [Google Scholar]
- 24.Van der Kloot W. Spontaneous and uniquantal-evoked endplate currents in normal frogs are indistinguishable. J Physiol. 1996;492(Pt 1):155–62. doi: 10.1113/jphysiol.1996.sp021297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macleod GT, Gan J, Bennett MR. Vesicle-associated proteins and quantal release at single active zones of amphibian (Bufo marinus) motor-nerve terminals. J Neurophysiol. 1999;82(3):1133–46. doi: 10.1152/jn.1999.82.3.1133. [DOI] [PubMed] [Google Scholar]
- 26.Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, et al. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci. 2008;28(40):10151–66. doi: 10.1523/JNEUROSCI.2432-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melom JE, Akbergenova Y, Gavornik JP, Littleton JT. Spontaneous and evoked release are independently regulated at individual active zones. J Neurosci. 2013;33(44):17253–63. doi: 10.1523/JNEUROSCI.3334-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peled ES, Newman ZL, Isacoff EY. Evoked and spontaneous transmission favored by distinct sets of synapses. Curr Biol. 2014;24(5):484–93. doi: 10.1016/j.cub.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leitz J, Kavalali E. Fast retrieval and autonomous regulation of single spontaneously recycling synaptic vesicles. eLife. 2014;3 doi: 10.7554/eLife.03658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez DM, Kavalali ET. Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr Opin Neurobiol. 2011;21(2):275–82. doi: 10.1016/j.conb.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otsu Y, Murphy TH. Optical postsynaptic measurement of vesicle release rates for hippocampal synapses undergoing asynchronous release during train stimulation. J Neurosci. 2004;24(41):9076–86. doi: 10.1523/JNEUROSCI.2060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otsu Y, Shahrezaei V, Li B, Raymond LA, Delaney KR, Murphy TH. Competition between phasic and asynchronous release for recovered synaptic vesicles at developing hippocampal autaptic synapses. J Neurosci. 2004;24(2):420–33. doi: 10.1523/JNEUROSCI.4452-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenig JH, Ikeda K. Contribution of active zone subpopulation of vesicles to evoked and spontaneous release. J Neurophysiol. 1999;81(4):1495–505. doi: 10.1152/jn.1999.81.4.1495. [DOI] [PubMed] [Google Scholar]
- 34.Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9(5):919–27. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- 35.Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16(5):1623–33. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan ZH, Segal MM, Lipton SA. Nitric oxide-related species inhibit evoked neurotransmission but enhance spontaneous miniature synaptic currents in central neuronal cultures. Proc Natl Acad Sci U S A. 1996;93(26):15423–8. doi: 10.1073/pnas.93.26.15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J Neurosci. 2010;30(43):14470–5. doi: 10.1523/JNEUROSCI.2557-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fawley JA, Hofmann ME, Andresen MC. Cannabinoid 1 and transient receptor potential vanilloid 1 receptors discretely modulate evoked glutamate separately from spontaneous glutamate transmission. J Neurosci. 2014;34(24):8324–32. doi: 10.1523/JNEUROSCI.0315-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45(4):563–73. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 40.Mathew SS, Pozzo-Miller L, Hablitz JJ. Kainate modulates presynaptic GABA release from two vesicle pools. J Neurosci. 2008;28(3):725–31. doi: 10.1523/JNEUROSCI.3625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci. 2009;12(6):751–8. doi: 10.1038/nn.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sara Y, Bal M, Adachi M, Monteggia LM, Kavalali ET. Use-dependent AMPA receptor block reveals segregation of spontaneous and evoked glutamatergic neurotransmission. J Neurosci. 2011;31(14):5378–82. doi: 10.1523/JNEUROSCI.5234-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prange O, Murphy TH. Correlation of miniature synaptic activity and evoked release probability in cultures of cortical neurons. J Neurosci. 1999;19(15):6427–38. doi: 10.1523/JNEUROSCI.19-15-06427.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groemer TW, Klingauf J. Synaptic vesicles recycling spontaneously and during activity belong to the same vesicle pool. Nat Neurosci. 2007;10(2):145–7. doi: 10.1038/nn1831. [DOI] [PubMed] [Google Scholar]
- 45.Hua Y, Sinha R, Martineau M, Kahms M, Klingauf J. A common origin of synaptic vesicles undergoing evoked and spontaneous fusion. Nat Neurosci. 2010;13(12):1451–3. doi: 10.1038/nn.2695. [DOI] [PubMed] [Google Scholar]
- 46.Wilhelm BG, Groemer TW, Rizzoli SO. The same synaptic vesicles drive active and spontaneous release. Nat Neurosci. 2010;13(12):1454–6. doi: 10.1038/nn.2690. [DOI] [PubMed] [Google Scholar]
- 47.Voglmaier SM, Edwards RH. Do different endocytic pathways make different synaptic vesicles? Curr Opin Neurobiol. 2007;17(3):374–80. doi: 10.1016/j.conb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Chandrasekar I, Huettner JE, Turney SG, Bridgman PC. Myosin II regulates activity dependent compensatory endocytosis at central synapses. J Neurosci. 2013;33(41):16131–45. doi: 10.1523/JNEUROSCI.2229-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evstratova A, Chamberland S, Faundez V, Toth K. Vesicles derived via AP-3-dependent recycling contribute to asynchronous release and influence information transfer. Nat Commun. 2014;5:5530. doi: 10.1038/ncomms6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kokotos AC, Cousin MA. Synaptic Vesicle Generation from Central Nerve Terminal Endosomes. Traffic. 2014 doi: 10.1111/tra.12235. [DOI] [PubMed] [Google Scholar]
- 51.Rizzoli SO. Synaptic vesicle recycling: steps and principles. EMBO J. 2014;33(8):788–822. doi: 10.1002/embj.201386357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan JR, Comstra HS, Cohen M, Faundez V. Presynaptic membrane retrieval and endosome biology: defining molecularly heterogeneous synaptic vesicles. Cold Spring Harb Perspect Biol. 2013;5(10):a016915. doi: 10.1101/cshperspect.a016915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sudhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80(3):675–90. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohrmann R, de Wit H, Verhage M, Neher E, Sorensen JB. Fast vesicle fusion in living cells requires at least three SNARE complexes. Science. 2010;330(6003):502–5. doi: 10.1126/science.1193134. [DOI] [PubMed] [Google Scholar]
- 55.van den Bogaart G, Holt MG, Bunt G, Riedel D, Wouters FS, Jahn R. One SNARE complex is sufficient for membrane fusion. Nat Struct Mol Biol. 2010;17(3):358–64. doi: 10.1038/nsmb.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127(4):831–46. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 57.Weingarten J, Lassek M, Mueller BF, Rohmer M, Lunger I, Baeumlisberger D, et al. The proteome of the presynaptic active zone from mouse brain. Mol Cell Neurosci. 2014;59:106–18. doi: 10.1016/j.mcn.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Wilhelm BG, Mandad S, Truckenbrodt S, Krohnert K, Schafer C, Rammner B, et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science. 2014;344(6187):1023–8. doi: 10.1126/science.1252884. [DOI] [PubMed] [Google Scholar]
- 59.Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, et al. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294(5544):1117–22. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 60.Deak F, Schoch S, Liu X, Sudhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 2004;6(11):1102–8. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- 61.Broadie K, Prokop A, Bellen HJ, O'Kane CJ, Schulze KL, Sweeney ST. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron. 1995;15(3):663–73. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 62.Deitcher DL, Ueda A, Stewart BA, Burgess RW, Kidokoro Y, Schwarz TL. Distinct requirements for evoked and spontaneous release of neurotransmitter are revealed by mutations in the Drosophila gene neuronal-synaptobrevin. J Neurosci. 1998;18(6):2028–39. doi: 10.1523/JNEUROSCI.18-06-02028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshihara M, Ueda A, Zhang D, Deitcher DL, Schwarz TL, Kidokoro Y. Selective effects of neuronal-synaptobrevin mutations on transmitter release evoked by sustained versus transient Ca2+ increases and by cAMP. J Neurosci. 1999;19(7):2432–41. doi: 10.1523/JNEUROSCI.19-07-02432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saitoe M, Schwarz TL, Umbach JA, Gundersen CB, Kidokoro Y. Absence of junctional glutamate receptor clusters in Drosophila mutants lacking spontaneous transmitter release. Science. 2001;293(5529):514–7. doi: 10.1126/science.1061270. [DOI] [PubMed] [Google Scholar]
- 65.Kidokoro Y. Roles of SNARE proteins and synaptotagmin I in synaptic transmission: studies at the Drosophila neuromuscular synapse. Neuro-Signals. 2003;12(1):13–30. doi: 10.1159/000068912. [DOI] [PubMed] [Google Scholar]
- 66.Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359(6398):832–5. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 67.Herreros J, Miralles FX, Solsona C, Bizzini B, Blasi J, Marsal J. Tetanus toxin inhibits spontaneous quantal release and cleaves VAMP/synaptobrevin. Brain Res. 1995;699(2):165–70. doi: 10.1016/0006-8993(95)00739-d. [DOI] [PubMed] [Google Scholar]
- 68.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14(2):341–51. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 69.Hua SY, Raciborska DA, Trimble WS, Charlton MP. Different VAMP/synaptobrevin complexes for spontaneous and evoked transmitter release at the crayfish neuromuscular junction. J Neurophysiol. 1998;80(6):3233–46. doi: 10.1152/jn.1998.80.6.3233. [DOI] [PubMed] [Google Scholar]
- 70.Revelo NH, Kamin D, Truckenbrodt S, Wong AB, Reuter-Jessen K, Reisinger E, et al. A new probe for super-resolution imaging of membranes elucidates trafficking pathways. J Cell Biol. 2014;205(4):591–606. doi: 10.1083/jcb.201402066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deak F, Shin OH, Kavalali ET, Sudhof TC. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J Neurosci. 2006;26(25):6668–76. doi: 10.1523/JNEUROSCI.5272-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kesavan J, Borisovska M, Bruns D. v-SNARE actions during Ca(2+)-triggered exocytosis. Cell. 2007;131(2):351–63. doi: 10.1016/j.cell.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 73.Fdez E, Martinez-Salvador M, Beard M, Woodman P, Hilfiker S. Transmembrane-domain determinants for SNARE-mediated membrane fusion. J Cell Sci. 2010;123(Pt 14):2473–80. doi: 10.1242/jcs.061325. [DOI] [PubMed] [Google Scholar]
- 74.Guzman RE, Schwarz YN, Rettig J, Bruns D. SNARE force synchronizes synaptic vesicle fusion and controls the kinetics of quantal synaptic transmission. J Neurosci. 2010;30(31):10272–81. doi: 10.1523/JNEUROSCI.1551-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ngatchou AN, Kisler K, Fang Q, Walter AM, Zhao Y, Bruns D, et al. Role of the synaptobrevin C terminus in fusion pore formation. Proc Natl Acad Sci U S A. 2010;107(43):18463–8. doi: 10.1073/pnas.1006727107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fang Q, Zhao Y, Lindau M. Juxtamembrane tryptophans of synaptobrevin 2 control the process of membrane fusion. FEBS Lett. 2013;587(1):67–72. doi: 10.1016/j.febslet.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou P, Bacaj T, Yang X, Pang ZP, Sudhof TC. Lipid-anchored SNAREs lacking transmembrane regions fully support membrane fusion during neurotransmitter release. Neuron. 2013;80(2):470–83. doi: 10.1016/j.neuron.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baumert M, Maycox PR, Navone F, De Camilli P, Jahn R. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989;8(2):379–84. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elferink LA, Trimble WS, Scheller RH. Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J Biol Chem. 1989;264(19):11061–4. [PubMed] [Google Scholar]
- 80.Trimble WS, Gray TS, Elferink LA, Wilson MC, Scheller RH. Distinct patterns of expression of two VAMP genes within the rat brain. J Neurosci. 1990;10(4):1380–7. doi: 10.1523/JNEUROSCI.10-04-01380.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raptis A, Torrejon-Escribano B, Gomez de Aranda I, Blasi J. Distribution of synaptobrevin/VAMP 1 and 2 in rat brain. Journal of chemical neuroanatomy. 2005;30(4):201–11. doi: 10.1016/j.jchemneu.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Sugiura Y, Lin W. The role of synaptobrevin1/VAMP1 in Ca2+-triggered neurotransmitter release at the mouse neuromuscular junction. J Physiol. 2011;589(Pt 7):1603–18. doi: 10.1113/jphysiol.2010.201939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zimmermann J, Trimbuch T, Rosenmund C. Synaptobrevin 1 mediates vesicle priming and evoked release in a subpopulation of hippocampal neurons. J Neurophysiol. 2014;112(6):1559–65. doi: 10.1152/jn.00340.2014. [DOI] [PubMed] [Google Scholar]
- 84.Steegmaier M, Klumperman J, Foletti DL, Yoo JS, Scheller RH. Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol Biol Cell. 1999;10(6):1957–72. doi: 10.1091/mbc.10.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, et al. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol. 2002;156(4):653–64. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feldmann A, Winterstein C, White R, Trotter J, Kramer-Albers EM. Comprehensive analysis of expression, subcellular localization, and cognate pairing of SNARE proteins in oligodendrocytes. J Neurosci Res. 2009;87(8):1760–72. doi: 10.1002/jnr.22020. [DOI] [PubMed] [Google Scholar]
- 87.Park JJ, Gondre-Lewis MC, Eiden LE, Loh YP. A distinct trans-Golgi network subcompartment for sorting of synaptic and granule proteins in neurons and neuroendocrine cells. J Cell Sci. 2011;124(Pt 5):735–44. doi: 10.1242/jcs.076372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shitara A, Shibui T, Okayama M, Arakawa T, Mizoguchi I, Sakakura Y, et al. VAMP4 is required to maintain the ribbon structure of the Golgi apparatus. Mol Cell Biochem. 2013;380(1-2):11–21. doi: 10.1007/s11010-013-1652-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kreykenbohm V, Wenzel D, Antonin W, Atlachkine V, von Mollard GF. The SNAREs vti1a and vti1b have distinct localization and SNARE complex partners. European journal of cell biology. 2002;81(5):273–80. doi: 10.1078/0171-9335-00247. [DOI] [PubMed] [Google Scholar]
- 90.Raingo J, Khvotchev M, Liu P, Darios F, Li YC, Ramirez DM, et al. VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission. Nat Neurosci. 2012;15(5):738–45. doi: 10.1038/nn.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, et al. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273(17):10317–24. doi: 10.1074/jbc.273.17.10317. [DOI] [PubMed] [Google Scholar]
- 92.Antonin W, Riedel D, von Mollard GF. The SNARE Vti1a-beta is localized to small synaptic vesicles and participates in a novel SNARE complex. J Neurosci. 2000;20(15):5724–32. doi: 10.1523/JNEUROSCI.20-15-05724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bethani I, Werner A, Kadian C, Geumann U, Jahn R, Rizzoli SO. Endosomal fusion upon SNARE knockdown is maintained by residual SNARE activity and enhanced docking. Traffic. 2009;10(10):1543–59. doi: 10.1111/j.1600-0854.2009.00959.x. [DOI] [PubMed] [Google Scholar]
- 94.Kunwar AJ, Rickmann M, Backofen B, Browski SM, Rosenbusch J, Schoning S, et al. Lack of the endosomal SNAREs vti1a and vti1b led to significant impairments in neuronal development. Proc Natl Acad Sci U S A. 2011;108(6):2575–80. doi: 10.1073/pnas.1013891108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walter AM, Kurps J, de Wit H, Schoning S, Toft-Bertelsen TL, Lauks J, et al. The SNARE protein vti1a functions in dense-core vesicle biogenesis. EMBO J. 2014;33(15):1681–97. doi: 10.15252/embj.201387549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoopmann P, Punge A, Barysch SV, Westphal V, Buckers J, Opazo F, et al. Endosomal sorting of readily releasable synaptic vesicles. Proc Natl Acad Sci U S A. 2010;107(44):19055–60. doi: 10.1073/pnas.1007037107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramirez DM, Khvotchev M, Trauterman B, Kavalali ET. Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission. Neuron. 2012;73(1):121–34. doi: 10.1016/j.neuron.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y, Tang BL. SNAREs in neurons--beyond synaptic vesicle exocytosis (Review) Molecular membrane biology. 2006;23(5):377–84. doi: 10.1080/09687860600776734. [DOI] [PubMed] [Google Scholar]
- 99.Coco S, Raposo G, Martinez S, Fontaine JJ, Takamori S, Zahraoui A, et al. Subcellular localization of tetanus neurotoxin-insensitive vesicle-associated membrane protein (VAMP)/VAMP7 in neuronal cells: evidence for a novel membrane compartment. J Neurosci. 1999;19(22):9803–12. doi: 10.1523/JNEUROSCI.19-22-09803.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chaineau M, Danglot L, Galli T. Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett. 2009;583(23):3817–26. doi: 10.1016/j.febslet.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 101.Martinez-Arca S, Rudge R, Vacca M, Raposo G, Camonis J, Proux-Gillardeaux V, et al. A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc Natl Acad Sci U S A. 2003;100(15):9011–6. doi: 10.1073/pnas.1431910100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Proux-Gillardeaux V, Raposo G, Irinopoulou T, Galli T. Expression of the Longin domain of TI-VAMP impairs lysosomal secretion and epithelial cell migration. Biology of the cell/under the auspices of the European Cell Biology Organization. 2007;99(5):261–71. doi: 10.1042/BC20060097. [DOI] [PubMed] [Google Scholar]
- 103.Martinez-Arca S, Alberts P, Zahraoui A, Louvard D, Galli T. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J Cell Biol. 2000;149(4):889–900. doi: 10.1083/jcb.149.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martinez-Arca S, Coco S, Mainguy G, Schenk U, Alberts P, Bouille P, et al. A common exocytotic mechanism mediates axonal and dendritic outgrowth. J Neurosci. 2001;21(11):3830–8. doi: 10.1523/JNEUROSCI.21-11-03830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alberts P, Rudge R, Irinopoulou T, Danglot L, Gauthier-Rouviere C, Galli T. Cdc42 and actin control polarized expression of TI-VAMP vesicles to neuronal growth cones and their fusion with the plasma membrane. Mol Biol Cell. 2006;17(3):1194–203. doi: 10.1091/mbc.E05-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muzerelle A, Alberts P, Martinez-Arca S, Jeannequin O, Lafaye P, Mazie JC, et al. Tetanus neurotoxin-insensitive vesicle-associated membrane protein localizes to a presynaptic membrane compartment in selected terminal subsets of the rat brain. Neuroscience. 2003;122(1):59–75. doi: 10.1016/s0306-4522(03)00567-0. [DOI] [PubMed] [Google Scholar]
- 107.Scheuber A, Rudge R, Danglot L, Raposo G, Binz T, Poncer JC, et al. Loss of AP-3 function affects spontaneous and evoked release at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2006;103(44):16562–7. doi: 10.1073/pnas.0603511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sato M, Yoshimura S, Hirai R, Goto A, Kunii M, Atik N, et al. The role of VAMP7/TI-VAMP in cell polarity and lysosomal exocytosis in vivo. Traffic. 2011;12(10):1383–93. doi: 10.1111/j.1600-0854.2011.01247.x. [DOI] [PubMed] [Google Scholar]
- 109.Danglot L, Zylbersztejn K, Petkovic M, Gauberti M, Meziane H, Combe R, et al. Absence of TI-VAMP/Vamp7 leads to increased anxiety in mice. J Neurosci. 2012;32(6):1962–8. doi: 10.1523/JNEUROSCI.4436-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hua Z, Leal-Ortiz S, Foss SM, Waites CL, Garner CC, Voglmaier SM, et al. v-SNARE composition distinguishes synaptic vesicle pools. Neuron. 2011;71(3):474–87. doi: 10.1016/j.neuron.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bal M, Leitz J, Reese AL, Ramirez DM, Durakoglugil M, Herz J, et al. Reelin mobilizes a VAMP7-dependent synaptic vesicle pool and selectively augments spontaneous neurotransmission. Neuron. 2013;80(4):934–46. doi: 10.1016/j.neuron.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Current opinion in cell biology. 2010;22(4):496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walter AM, Groffen AJ, Sorensen JB, Verhage M. Multiple Ca2+ sensors in secretion: teammates, competitors or autocrats? Trends Neurosci. 2011;34(9):487–97. doi: 10.1016/j.tins.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 114.Ullrich B, Li C, Zhang JZ, McMahon H, Anderson RG, Geppert M, et al. Functional properties of multiple synaptotagmins in brain. Neuron. 1994;13(6):1281–91. doi: 10.1016/0896-6273(94)90415-4. [DOI] [PubMed] [Google Scholar]
- 115.Marqueze B, Boudier JA, Mizuta M, Inagaki N, Seino S, Seagar M. Cellular localization of synaptotagmin I, II, and III mRNAs in the central nervous system and pituitary and adrenal glands of the rat. J Neurosci. 1995;15(7 Pt 1):4906–17. doi: 10.1523/JNEUROSCI.15-07-04906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xi D, Chin H, Gainer H. Analysis of synaptotagmin I-IV messenger RNA expression and developmental regulation in the rat hypothalamus and pituitary. Neuroscience. 1999;88(2):425–35. doi: 10.1016/s0306-4522(98)00234-6. [DOI] [PubMed] [Google Scholar]
- 117.Mittelsteadt T, Seifert G, Alvarez-Baron E, Steinhauser C, Becker AJ, Schoch S. Differential mRNA expression patterns of the synaptotagmin gene family in the rodent brain. J Comp Neurol. 2009;512(4):514–28. doi: 10.1002/cne.21908. [DOI] [PubMed] [Google Scholar]
- 118.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, et al. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79(4):717–27. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 119.Nishiki T, Augustine GJ. Synaptotagmin I synchronizes transmitter release in mouse hippocampal neurons. J Neurosci. 2004;24(27):6127–32. doi: 10.1523/JNEUROSCI.1563-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maximov A, Sudhof TC. Autonomous function of synaptotagmin 1 in triggering synchronous release independent of asynchronous release. Neuron. 2005;48(4):547–54. doi: 10.1016/j.neuron.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 121.Xu J, Mashimo T, Sudhof TC. Synaptotagmin-1, -2, and -9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54(4):567–81. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 122.Littleton JT, Stern M, Schulze K, Perin M, Bellen HJ. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993;74(6):1125–34. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- 123.Broadie K, Bellen HJ, DiAntonio A, Littleton JT, Schwarz TL. Absence of synaptotagmin disrupts excitation-secretion coupling during synaptic transmission. Proc Natl Acad Sci U S A. 1994;91(22):10727–31. doi: 10.1073/pnas.91.22.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Littleton JT, Stern M, Perin M, Bellen HJ. Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc Natl Acad Sci U S A. 1994;91(23):10888–92. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marek KW, Davis GW. Transgenically encoded protein photoinactivation (FlAsH-FALI): acute inactivation of synaptotagmin I. Neuron. 2002;36(5):805–13. doi: 10.1016/s0896-6273(02)01068-1. [DOI] [PubMed] [Google Scholar]
- 126.Kerr AM, Reisinger E, Jonas P. Differential dependence of phasic transmitter release on synaptotagmin 1 at GABAergic and glutamatergic hippocampal synapses. Proc Natl Acad Sci U S A. 2008;105(40):15581–6. doi: 10.1073/pnas.0800621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu H, Dean C, Arthur CP, Dong M, Chapman ER. Autapses and networks of hippocampal neurons exhibit distinct synaptic transmission phenotypes in the absence of synaptotagmin I. J Neurosci. 2009;29(23):7395–403. doi: 10.1523/JNEUROSCI.1341-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Popoli M, Venegoni A, Buffa L, Racagni G. Ca2+/phospholipid-binding and syntaxin-binding of native synaptotagmin I. Life sciences. 1997;61(7):711–21. doi: 10.1016/s0024-3205(97)00536-5. [DOI] [PubMed] [Google Scholar]
- 129.Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410(6824):41–9. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 130.Mackler JM, Reist NE. Mutations in the second C2 domain of synaptotagmin disrupt synaptic transmission at Drosophila neuromuscular junctions. J Comp Neurol. 2001;436(1):4–16. [PubMed] [Google Scholar]
- 131.Yoshihara M, Littleton JT. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 2002;36(5):897–908. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
- 132.Stevens CF, Sullivan JM. The synaptotagmin C2A domain is part of the calcium sensor controlling fast synaptic transmission. Neuron. 2003;39(2):299–308. doi: 10.1016/s0896-6273(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 133.Nishiki T, Augustine GJ. Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J Neurosci. 2004;24(39):8542–50. doi: 10.1523/JNEUROSCI.2545-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Okamoto T, Tamura T, Suzuki K, Kidokoro Y. External Ca2+ dependency of synaptic transmission in drosophila synaptotagmin I mutants. J Neurophysiol. 2005;94(2):1574–86. doi: 10.1152/jn.00205.2005. [DOI] [PubMed] [Google Scholar]
- 135.Tamura T, Hou J, Reist NE, Kidokoro Y. Nerve-evoked synchronous release and high K+ -induced quantal events are regulated separately by synaptotagmin I at Drosophila neuromuscular junctions. J Neurophysiol. 2007;97(1):540–9. doi: 10.1152/jn.00905.2006. [DOI] [PubMed] [Google Scholar]
- 136.Mace KE, Biela LM, Sares AG, Reist NE. Synaptotagmin I stabilizes synaptic vesicles via its C(2)A polylysine motif. Genesis. 2009;47(5):337–45. doi: 10.1002/dvg.20502. [DOI] [PubMed] [Google Scholar]
- 137.Xue M, Ma C, Craig TK, Rosenmund C, Rizo J. The Janus-faced nature of the C(2)B domain is fundamental for synaptotagmin-1 function. Nat Struct Mol Biol. 2008;15(11):1160–8. doi: 10.1038/nsmb.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chiang CW, Chen YC, Lu JC, Hsiao YT, Chang CW, Huang PC, et al. Synaptotagmin I regulates patterned spontaneous activity in the developing rat retina via calcium binding to the C2AB domains. PLoS One. 2012;7(10):e47465. doi: 10.1371/journal.pone.0047465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shin OH, Xu J, Rizo J, Sudhof TC. Differential but convergent functions of Ca2+ binding to synaptotagmin-1 C2 domains mediate neurotransmitter release. Proc Natl Acad Sci U S A. 2009;106(38):16469–74. doi: 10.1073/pnas.0908798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu J, Pang ZP, Shin OH, Sudhof TC. Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nat Neurosci. 2009;12(6):759–66. doi: 10.1038/nn.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yoshihara M, Guan Z, Littleton JT. Differential regulation of synchronous versus asynchronous neurotransmitter release by the C2 domains of synaptotagmin 1. Proc Natl Acad Sci U S A. 2010;107(33):14869–74. doi: 10.1073/pnas.1000606107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paddock BE, Wang Z, Biela LM, Chen K, Getzy MD, Striegel A, et al. Membrane penetration by synaptotagmin is required for coupling calcium binding to vesicle fusion in vivo. J Neurosci. 2011;31(6):2248–57. doi: 10.1523/JNEUROSCI.3153-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee J, Guan Z, Akbergenova Y, Littleton JT. Genetic analysis of synaptotagmin C2 domain specificity in regulating spontaneous and evoked neurotransmitter release. J Neurosci. 2013;33(1):187–200. doi: 10.1523/JNEUROSCI.3214-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu H, Bai H, Xue R, Takahashi H, Edwardson JM, Chapman ER. Linker mutations reveal the complexity of synaptotagmin 1 action during synaptic transmission. Nat Neurosci. 2014;17(5):670–7. doi: 10.1038/nn.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, Dickey BF, et al. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26(52):13493–504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li C, Ullrich B, Zhang JZ, Anderson RG, Brose N, Sudhof TC. Ca(2+)-dependent and -independent activities of neural and non-neural synaptotagmins. Nature. 1995;375(6532):594–9. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 147.Rickman C, Archer DA, Meunier FA, Craxton M, Fukuda M, Burgoyne RD, et al. Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J Biol Chem. 2004;279(13):12574–9. doi: 10.1074/jbc.M310710200. [DOI] [PubMed] [Google Scholar]
- 148.Hui E, Bai J, Wang P, Sugimori M, Llinas RR, Chapman ER. Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis. Proc Natl Acad Sci U S A. 2005;102(14):5210–4. doi: 10.1073/pnas.0500941102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wen H, Linhoff MW, McGinley MJ, Li GL, Corson GM, Mandel G, et al. Distinct roles for two synaptotagmin isoforms in synchronous and asynchronous transmitter release at zebrafish neuromuscular junction. Proc Natl Acad Sci U S A. 2010;107(31):13906–11. doi: 10.1073/pnas.1008598107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pang ZP, Sun J, Rizo J, Maximov A, Sudhof TC. Genetic analysis of synaptotagmin 2 in spontaneous and Ca2+-triggered neurotransmitter release. EMBO J. 2006;25(10):2039–50. doi: 10.1038/sj.emboj.7601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Sudhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450(7170):676–82. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kochubey O, Schneggenburger R. Synaptotagmin increases the dynamic range of synapses by driving Ca(2)+-evoked release and by clamping a near-linear remaining Ca(2)+ sensor. Neuron. 2011;69(4):736–48. doi: 10.1016/j.neuron.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 153.Dean C, Dunning FM, Liu H, Bomba-Warczak E, Martens H, Bharat V, et al. Axonal and dendritic synaptotagmin isoforms revealed by a pHluorin-syt functional screen. Mol Biol Cell. 2012;23(9):1715–27. doi: 10.1091/mbc.E11-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sudhof TC, Rizo J. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron. 1996;17(3):379–88. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 155.Saraswati S, Adolfsen B, Littleton JT. Characterization of the role of the Synaptotagmin family as calcium sensors in facilitation and asynchronous neurotransmitter release. Proc Natl Acad Sci U S A. 2007;104(35):14122–7. doi: 10.1073/pnas.0706711104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Maximov A, Shin OH, Liu X, Sudhof TC. Synaptotagmin-12, a synaptic vesicle phosphoprotein that modulates spontaneous neurotransmitter release. J Cell Biol. 2007;176(1):113–24. doi: 10.1083/jcb.200607021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, et al. The role of Rab3A in neurotransmitter release. Nature. 1994;369(6480):493–7. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- 158.Geppert M, Goda Y, Stevens CF, Sudhof TC. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387(6635):810–4. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- 159.Sons MS, Plomp JJ. Rab3A deletion selectively reduces spontaneous neurotransmitter release at the mouse neuromuscular synapse. Brain Res. 2006;1089(1):126–34. doi: 10.1016/j.brainres.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 160.Menegon A, Bonanomi D, Albertinazzi C, Lotti F, Ferrari G, Kao HT, et al. Protein kinase A-mediated synapsin I phosphorylation is a central modulator of Ca2+-dependent synaptic activity. J Neurosci. 2006;26(45):11670–81. doi: 10.1523/JNEUROSCI.3321-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chiappalone M, Casagrande S, Tedesco M, Valtorta F, Baldelli P, Martinoia S, et al. Opposite changes in glutamatergic and GABAergic transmission underlie the diffuse hyperexcitability of synapsin I-deficient cortical networks. Cereb Cortex. 2009;19(6):1422–39. doi: 10.1093/cercor/bhn182. [DOI] [PubMed] [Google Scholar]
- 162.Medrihan L, Cesca F, Raimondi A, Lignani G, Baldelli P, Benfenati F. Synapsin II desynchronizes neurotransmitter release at inhibitory synapses by interacting with presynaptic calcium channels. Nat Commun. 2013;4:1512. doi: 10.1038/ncomms2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Inoue A, Akagawa K. Neuron specific expression of a membrane protein, HPC-1: tissue distribution, and cellular and subcellular localization of immunoreactivity and mRNA. Brain Res Mol Brain Res. 1993;19(1-2):121–8. doi: 10.1016/0169-328x(93)90156-j. [DOI] [PubMed] [Google Scholar]
- 164.Martinez-Arca S, Proux-Gillardeaux V, Alberts P, Louvard D, Galli T. Ectopic expression of syntaxin 1 in the ER redirects TI-VAMP- and cellubrevin-containing vesicles. J Cell Sci. 2003;116(Pt 13):2805–16. doi: 10.1242/jcs.00467. [DOI] [PubMed] [Google Scholar]
- 165.Vites O, Florin EL, Jahn R. Docking of liposomes to planar surfaces mediated by trans-SNARE complexes. Biophys J. 2008;95(3):1295–302. doi: 10.1529/biophysj.108.129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schulze KL, Broadie K, Perin MS, Bellen HJ. Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell. 1995;80(2):311–20. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- 167.Fergestad T, Wu MN, Schulze KL, Lloyd TE, Bellen HJ, Broadie K. Targeted mutations in the syntaxin H3 domain specifically disrupt SNARE complex function in synaptic transmission. J Neurosci. 2001;21(23):9142–50. doi: 10.1523/JNEUROSCI.21-23-09142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mishima T, Fujiwara T, Akagawa K. Reduction of neurotransmitter release by the exogenous H3 domain peptide of HPC-1/syntaxin 1A in cultured rat hippocampal neurons. Neurosci Lett. 2002;329(3):273–6. doi: 10.1016/s0304-3940(02)00662-6. [DOI] [PubMed] [Google Scholar]
- 169.Zhou P, Pang ZP, Yang X, Zhang Y, Rosenmund C, Bacaj T, et al. Syntaxin-1 N-peptide and Habc-domain perform distinct essential functions in synaptic vesicle fusion. EMBO J. 2013;32(1):159–71. doi: 10.1038/emboj.2012.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mishima T, Fujiwara T, Sanada M, Kofuji T, Kanai-Azuma M, Akagawa K. Syntaxin 1B, but not syntaxin 1A, is necessary for the regulation of synaptic vesicle exocytosis and of the readily releasable pool at central synapses. PLoS One. 2014;9(2):e90004. doi: 10.1371/journal.pone.0090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tang BL, Tan AE, Lim LK, Lee SS, Low DY, Hong W. Syntaxin 12, a member of the syntaxin family localized to the endosome. J Biol Chem. 1998;273(12):6944–50. doi: 10.1074/jbc.273.12.6944. [DOI] [PubMed] [Google Scholar]
- 172.Ravichandran V, Chawla A, Roche PA. Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem. 1996;271(23):13300–3. doi: 10.1074/jbc.271.23.13300. [DOI] [PubMed] [Google Scholar]
- 173.Hess DT, Slater TM, Wilson MC, Skene JH. The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J Neurosci. 1992;12(12):4634–41. doi: 10.1523/JNEUROSCI.12-12-04634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, et al. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20(9):2202–13. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rickman C, Meunier FA, Binz T, Davletov B. High affinity interaction of syntaxin and SNAP-25 on the plasma membrane is abolished by botulinum toxin E. J Biol Chem. 2004;279(1):644–51. doi: 10.1074/jbc.M310879200. [DOI] [PubMed] [Google Scholar]
- 176.Gutierrez LM, Canaves JM, Ferrer-Montiel AV, Reig JA, Montal M, Viniegra S. A peptide that mimics the carboxy-terminal domain of SNAP-25 blocks Ca(2+)-dependent exocytosis in chromaffin cells. FEBS Lett. 1995;372(1):39–43. doi: 10.1016/0014-5793(95)00944-5. [DOI] [PubMed] [Google Scholar]
- 177.Sorensen JB, Wiederhold K, Muller EM, Milosevic I, Nagy G, de Groot BL, et al. Sequential N- to C-terminal SNARE complex assembly drives priming and fusion of secretory vesicles. EMBO J. 2006;25(5):955–66. doi: 10.1038/sj.emboj.7601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365(6442):160–3. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- 179.Schiavo G, Rossetto O, Catsicas S, Polverino de Laureto P, DasGupta BR, Benfenati F, et al. Identification of the nerve terminal targets of botulinum neurotoxin serotypes A, D, and E. J Biol Chem. 1993;268(32):23784–7. [PubMed] [Google Scholar]
- 180.Capogna M, McKinney RA, O'Connor V, Gahwiler BH, Thompson SM. Ca2+ or Sr2+ partially rescues synaptic transmission in hippocampal cultures treated with botulinum toxin A and C, but not tetanus toxin. J Neurosci. 1997;17(19):7190–202. doi: 10.1523/JNEUROSCI.17-19-07190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rao SS, Stewart BA, Rivlin PK, Vilinsky I, Watson BO, Lang C, et al. Two distinct effects on neurotransmission in a temperature-sensitive SNAP-25 mutant. EMBO J. 2001;20(23):6761–71. doi: 10.1093/emboj/20.23.6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5(1):19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- 183.Tafoya LC, Mameli M, Miyashita T, Guzowski JF, Valenzuela CF, Wilson MC. Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. J Neurosci. 2006;26(30):7826–38. doi: 10.1523/JNEUROSCI.1866-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bronk P, Deak F, Wilson MC, Liu X, Sudhof TC, Kavalali ET. Differential effects of SNAP-25 deletion on Ca2+ -dependent and Ca2+ -independent neurotransmission. J Neurophysiol. 2007;98(2):794–806. doi: 10.1152/jn.00226.2007. [DOI] [PubMed] [Google Scholar]
- 185.Delgado-Martinez I, Nehring RB, Sorensen JB. Differential abilities of SNAP-25 homologs to support neuronal function. J Neurosci. 2007;27(35):9380–91. doi: 10.1523/JNEUROSCI.5092-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Antonucci F, Corradini I, Morini R, Fossati G, Menna E, Pozzi D, et al. Reduced SNAP-25 alters short-term plasticity at developing glutamatergic synapses. EMBO reports. 2013;14(7):645–51. doi: 10.1038/embor.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Weber JP, Reim K, Sorensen JB. Opposing functions of two sub-domains of the SNARE-complex in neurotransmission. EMBO J. 2010;29(15):2477–90. doi: 10.1038/emboj.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chieregatti E, Chicka MC, Chapman ER, Baldini G. SNAP-23 functions in docking/fusion of granules at low Ca2+ Mol Biol Cell. 2004;15(4):1918–30. doi: 10.1091/mbc.E03-09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sorensen JB, Nagy G, Varoqueaux F, Nehring RB, Brose N, Wilson MC, et al. Differential control of the releasable vesicle pools by SNAP-25 splice variants and SNAP-23. Cell. 2003;114(1):75–86. doi: 10.1016/s0092-8674(03)00477-x. [DOI] [PubMed] [Google Scholar]
- 190.Weber JP, Toft-Bertelsen TL, Mohrmann R, Delgado-Martinez I, Sorensen JB. Synaptotagmin-7 Is an Asynchronous Calcium Sensor for Synaptic Transmission in Neurons Expressing SNAP-23. PLoS One. 2014;9(11):e114033. doi: 10.1371/journal.pone.0114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Andrews NW, Chakrabarti S. There's more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII. Trends in cell biology. 2005;15(11):626–31. doi: 10.1016/j.tcb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 192.Sugita S, Han W, Butz S, Liu X, Fernandez-Chacon R, Lao Y, et al. Synaptotagmin VII as a plasma membrane Ca(2+) sensor in exocytosis. Neuron. 2001;30(2):459–73. doi: 10.1016/s0896-6273(01)00290-2. [DOI] [PubMed] [Google Scholar]
- 193.Rao SK, Huynh C, Proux-Gillardeaux V, Galli T, Andrews NW. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J Biol Chem. 2004;279(19):20471–9. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]
- 194.Arantes RM, Andrews NW. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J Neurosci. 2006;26(17):4630–7. doi: 10.1523/JNEUROSCI.0009-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Czibener C, Sherer NM, Becker SM, Pypaert M, Hui E, Chapman ER, et al. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J Cell Biol. 2006;174(7):997–1007. doi: 10.1083/jcb.200605004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Monterrat C, Grise F, Benassy MN, Hemar A, Lang J. The calcium-sensing protein synaptotagmin 7 is expressed on different endosomal compartments in endocrine, neuroendocrine cells or neurons but not on large dense core vesicles. Histochemistry and cell biology. 2007;127(6):625–32. doi: 10.1007/s00418-007-0271-0. [DOI] [PubMed] [Google Scholar]
- 197.Fukuda M, Kanno E, Satoh M, Saegusa C, Yamamoto A. Synaptotagmin VII is targeted to dense-core vesicles and regulates their Ca2+ -dependent exocytosis in PC12 cells. J Biol Chem. 2004;279(50):52677–84. doi: 10.1074/jbc.M409241200. [DOI] [PubMed] [Google Scholar]
- 198.Fukuda M, Ogata Y, Saegusa C, Kanno E, Mikoshiba K. Alternative splicing isoforms of synaptotagmin VII in the mouse, rat and human. Biochem J. 2002;365(Pt 1):173–80. doi: 10.1042/BJ20011877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tsuboi T, Fukuda M. Synaptotagmin VII modulates the kinetics of dense-core vesicle exocytosis in PC12 cells. Genes to cells: devoted to molecular & cellular mechanisms. 2007;12(4):511–9. doi: 10.1111/j.1365-2443.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 200.Virmani T, Han W, Liu X, Sudhof TC, Kavalali ET. Synaptotagmin 7 splice variants differentially regulate synaptic vesicle recycling. EMBO J. 2003;22(20):5347–57. doi: 10.1093/emboj/cdg514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang P, Chicka MC, Bhalla A, Richards DA, Chapman ER. Synaptotagmin VII is targeted to secretory organelles in PC12 cells, where it functions as a high-affinity calcium sensor. Mol Cell Biol. 2005;25(19):8693–702. doi: 10.1128/MCB.25.19.8693-8702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Brandt DS, Coffman MD, Falke JJ, Knight JD. Hydrophobic contributions to the membrane docking of synaptotagmin 7 C2A domain: mechanistic contrast between isoforms 1 and 7. Biochemistry. 2012;51(39):7654–64. doi: 10.1021/bi3007115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Maximov A, Lao Y, Li H, Chen X, Rizo J, Sorensen JB, et al. Genetic analysis of synaptotagmin-7 function in synaptic vesicle exocytosis. Proc Natl Acad Sci U S A. 2008;105(10):3986–91. doi: 10.1073/pnas.0712372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bacaj T, Wu D, Yang X, Morishita W, Zhou P, Xu W, et al. Synaptotagmin-1 and synaptotagmin-7 trigger synchronous and asynchronous phases of neurotransmitter release. Neuron. 2013;80(4):947–59. doi: 10.1016/j.neuron.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Atlas D. Functional and physical coupling of voltage-sensitive calcium channels with exocytotic proteins: ramifications for the secretion mechanism. J Neurochem. 2001;77(4):972–85. doi: 10.1046/j.1471-4159.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- 206.Catterall WA. Interactions of presynaptic Ca2+ channels and snare proteins in neurotransmitter release. Ann N Y Acad Sci. 1999;868:144–59. doi: 10.1111/j.1749-6632.1999.tb11284.x. [DOI] [PubMed] [Google Scholar]
- 207.Ermolyuk YS, Alder FG, Surges R, Pavlov IY, Timofeeva Y, Kullmann DM, et al. Differential triggering of spontaneous glutamate release by P/Q-, N- and R-type Ca2+ channels. Nat Neurosci. 2013;16(12):1754–63. doi: 10.1038/nn.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Goswami SP, Bucurenciu I, Jonas P. Miniature IPSCs in hippocampal granule cells are triggered by voltage-gated Ca2+ channels via microdomain coupling. J Neurosci. 2012;32(41):14294–304. doi: 10.1523/JNEUROSCI.6104-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Smith SM, Chen W, Vyleta NP, Williams C, Lee CH, Phillips C, et al. Calcium regulation of spontaneous and asynchronous neurotransmitter release. Cell Calcium. 2012;52(3-4):226–33. doi: 10.1016/j.ceca.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Llano I, Gerschenfeld HM. Inhibitory synaptic currents in stellate cells of rat cerebellar slices. J Physiol. 1993;468:177–200. doi: 10.1113/jphysiol.1993.sp019766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mochida S, Sheng ZH, Baker C, Kobayashi H, Catterall WA. Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron. 1996;17(4):781–8. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 212.Long AA, Kim E, Leung HT, Woodruff E, 3rd, An L, Doerge RW, et al. Presynaptic calcium channel localization and calcium-dependent synaptic vesicle exocytosis regulated by the Fuseless protein. J Neurosci. 2008;28(14):3668–82. doi: 10.1523/JNEUROSCI.5553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Waters J, Smith SJ. Phorbol esters potentiate evoked and spontaneous release by different presynaptic mechanisms. J Neurosci. 2000;20(21):7863–70. doi: 10.1523/JNEUROSCI.20-21-07863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lou X, Scheuss V, Schneggenburger R. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature. 2005;435(7041):497–501. doi: 10.1038/nature03568. [DOI] [PubMed] [Google Scholar]
- 215.Vyleta NP, Smith SM. Spontaneous glutamate release is independent of calcium influx and tonically activated by the calcium-sensing receptor. J Neurosci. 2011;31(12):4593–606. doi: 10.1523/JNEUROSCI.6398-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Williams C, Chen W, Lee CH, Yaeger D, Vyleta NP, Smith SM. Coactivation of multiple tightly coupled calcium channels triggers spontaneous release of GABA. Nat Neurosci. 2012;15(9):1195–7. doi: 10.1038/nn.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Orita S, Sasaki T, Naito A, Komuro R, Ohtsuka T, Maeda M, et al. Doc2: a novel brain protein having two repeated C2-like domains. Biochem Biophys Res Commun. 1995;206(2):439–48. doi: 10.1006/bbrc.1995.1062. [DOI] [PubMed] [Google Scholar]
- 218.Orita S, Naito A, Sakaguchi G, Maeda M, Igarashi H, Sasaki T, et al. Physical and functional interactions of Doc2 and Munc13 in Ca2+-dependent exocytotic machinery. J Biol Chem. 1997;272(26):16081–4. doi: 10.1074/jbc.272.26.16081. [DOI] [PubMed] [Google Scholar]
- 219.Mochida S, Orita S, Sakaguchi G, Sasaki T, Takai Y. Role of the Doc2 alpha-Munc13-1 interaction in the neurotransmitter release process. Proc Natl Acad Sci U S A. 1998;95(19):11418–22. doi: 10.1073/pnas.95.19.11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sakaguchi G, Orita S, Maeda M, Igarashi H, Takai Y. Molecular cloning of an isoform of Doc2 having two C2-like domains. Biochem Biophys Res Commun. 1995;217(3):1053–61. doi: 10.1006/bbrc.1995.2876. [DOI] [PubMed] [Google Scholar]
- 221.Fukuda M, Mikoshiba K. Doc2gamma, a third isoform of double C2 protein, lacking calcium-dependent phospholipid binding activity. Biochem Biophys Res Commun. 2000;276(2):626–32. doi: 10.1006/bbrc.2000.3520. [DOI] [PubMed] [Google Scholar]
- 222.Groffen AJ, Friedrich R, Brian EC, Ashery U, Verhage M. DOC2A and DOC2B are sensors for neuronal activity with unique calcium-dependent and kinetic properties. J Neurochem. 2006;97(3):818–33. doi: 10.1111/j.1471-4159.2006.03755.x. [DOI] [PubMed] [Google Scholar]
- 223.Friedrich R, Groffen AJ, Connell E, van Weering JR, Gutman O, Henis YI, et al. DOC2B acts as a calcium switch and enhances vesicle fusion. J Neurosci. 2008;28(27):6794–806. doi: 10.1523/JNEUROSCI.0538-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Friedrich R, Gottfried I, Ashery U. Munc13-1 Translocates to the Plasma Membrane in a Doc2B- and Calcium-Dependent Manner. Frontiers in endocrinology. 2013;4:119. doi: 10.3389/fendo.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gaffaney JD, Xue R, Chapman ER. Mutations that disrupt Ca(2)(+)-binding activity endow Doc2beta with novel functional properties during synaptic transmission. Mol Biol Cell. 2014;25(4):481–94. doi: 10.1091/mbc.E13-10-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sakaguchi G, Manabe T, Kobayashi K, Orita S, Sasaki T, Naito A, et al. Doc2alpha is an activity-dependent modulator of excitatory synaptic transmission. Eur J Neurosci. 1999;11(12):4262–8. doi: 10.1046/j.1460-9568.1999.00855.x. [DOI] [PubMed] [Google Scholar]
- 227.Groffen AJ, Martens S, Diez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327(5973):1614–8. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yao J, Gaffaney JD, Kwon SE, Chapman ER. Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell. 2011;147(3):666–77. doi: 10.1016/j.cell.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pang ZP, Bacaj T, Yang X, Zhou P, Xu W, Sudhof TC. Doc2 supports spontaneous synaptic transmission by a Ca(2+)-independent mechanism. Neuron. 2011;70(2):244–51. doi: 10.1016/j.neuron.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Brose N, Hofmann K, Hata Y, Sudhof TC. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem. 1995;270(42):25273–80. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- 231.Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400(6743):457–61. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- 232.Junge HJ, Rhee JS, Jahn O, Varoqueaux F, Spiess J, Waxham MN, et al. Calmodulin and Munc13 form a Ca2+ sensor/effector complex that controls short-term synaptic plasticity. Cell. 2004;118(3):389–401. doi: 10.1016/j.cell.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 233.Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99(13):9037–42. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Deak F, Liu X, Khvotchev M, Li G, Kavalali ET, Sugita S, et al. Alpha-latrotoxin stimulates a novel pathway of Ca2+-dependent synaptic exocytosis independent of the classical synaptic fusion machinery. J Neurosci. 2009;29(27):8639–48. doi: 10.1523/JNEUROSCI.0898-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Varoqueaux F, Sons MS, Plomp JJ, Brose N. Aberrant morphology and residual transmitter release at the Munc13-deficient mouse neuromuscular synapse. Mol Cell Biol. 2005;25(14):5973–84. doi: 10.1128/MCB.25.14.5973-5984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Basu J, Betz A, Brose N, Rosenmund C. Munc13-1 C1 domain activation lowers the energy barrier for synaptic vesicle fusion. J Neurosci. 2007;27(5):1200–10. doi: 10.1523/JNEUROSCI.4908-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Brose N, Rosenmund C, Rettig J. Regulation of transmitter release by Unc-13 and its homologues. Curr Opin Neurobiol. 2000;10(3):303–11. doi: 10.1016/s0959-4388(00)00105-7. [DOI] [PubMed] [Google Scholar]
- 238.Hu Z, Tong XJ, Kaplan JM. UNC-13L, UNC-13S, and Tomosyn form a protein code for fast and slow neurotransmitter release in Caenorhabditis elegans. eLife. 2013;2:e00967. doi: 10.7554/eLife.00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhou K, Stawicki TM, Goncharov A, Jin Y. Position of UNC-13 in the active zone regulates synaptic vesicle release probability and release kinetics. eLife. 2013;2:e01180. doi: 10.7554/eLife.01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen Z, Cooper B, Kalla S, Varoqueaux F, Young SM., Jr The Munc13 proteins differentially regulate readily releasable pool dynamics and calcium-dependent recovery at a central synapse. J Neurosci. 2013;33(19):8336–51. doi: 10.1523/JNEUROSCI.5128-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388(6642):593–8. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 242.Weimer RM, Gracheva EO, Meyrignac O, Miller KG, Richmond JE, Bessereau JL. UNC-13 and UNC-10/rim localize synaptic vesicles to specific membrane domains. J Neurosci. 2006;26(31):8040–7. doi: 10.1523/JNEUROSCI.2350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Schoch S, Mittelstaedt T, Kaeser PS, Padgett D, Feldmann N, Chevaleyre V, et al. Redundant functions of RIM1alpha and RIM2alpha in Ca(2+)-triggered neurotransmitter release. EMBO J. 2006;25(24):5852–63. doi: 10.1038/sj.emboj.7601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Han Y, Kaeser PS, Sudhof TC, Schneggenburger R. RIM determines Ca(2)+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69(2):304–16. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144(2):282–95. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, et al. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415(6869):321–6. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- 247.Calakos N, Schoch S, Sudhof TC, Malenka RC. Multiple roles for the active zone protein RIM1alpha in late stages of neurotransmitter release. Neuron. 2004;42(6):889–96. doi: 10.1016/j.neuron.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 248.Gracheva EO, Hadwiger G, Nonet ML, Richmond JE. Direct interactions between C. elegans RAB-3 and Rim provide a mechanism to target vesicles to the presynaptic density. Neurosci Lett. 2008;444(2):137–42. doi: 10.1016/j.neulet.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Masuda ES, Huang BC, Fisher JM, Luo Y, Scheller RH. Tomosyn binds t-SNARE proteins via a VAMP-like coiled coil. Neuron. 1998;21(3):479–80. doi: 10.1016/s0896-6273(00)80559-0. [DOI] [PubMed] [Google Scholar]
- 250.Hatsuzawa K, Lang T, Fasshauer D, Bruns D, Jahn R. The R-SNARE motif of tomosyn forms SNARE core complexes with syntaxin 1 and SNAP-25 and down-regulates exocytosis. J Biol Chem. 2003;278(33):31159–66. doi: 10.1074/jbc.M305500200. [DOI] [PubMed] [Google Scholar]
- 251.Yamamoto Y, Fujikura K, Sakaue M, Okimura K, Kobayashi Y, Nakamura T, et al. The tail domain of tomosyn controls membrane fusion through tomosyn displacement by VAMP2. Biochem Biophys Res Commun. 2010;399(1):24–30. doi: 10.1016/j.bbrc.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 252.Bielopolski N, Lam AD, Bar-On D, Sauer M, Stuenkel EL, Ashery U. Differential interaction of tomosyn with syntaxin and SNAP25 depends on domains in the WD40 beta-propeller core and determines its inhibitory activity. J Biol Chem. 2014;289(24):17087–99. doi: 10.1074/jbc.M113.515296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yu H, Rathore SS, Gulbranson DR, Shen J. The N- and C-terminal domains of tomosyn play distinct roles in soluble N-ethylmaleimide-sensitive factor attachment protein receptor binding and fusion regulation. J Biol Chem. 2014;289(37):25571–80. doi: 10.1074/jbc.M114.591487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gracheva EO, Burdina AO, Holgado AM, Berthelot-Grosjean M, Ackley BD, Hadwiger G, et al. Tomosyn inhibits synaptic vesicle priming in Caenorhabditis elegans. PLoS Biol. 2006;4(8):e261. doi: 10.1371/journal.pbio.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.McEwen JM, Madison JM, Dybbs M, Kaplan JM. Antagonistic regulation of synaptic vesicle priming by Tomosyn and UNC-13. Neuron. 2006;51(3):303–15. doi: 10.1016/j.neuron.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 256.Sakisaka T, Yamamoto Y, Mochida S, Nakamura M, Nishikawa K, Ishizaki H, et al. Dual inhibition of SNARE complex formation by tomosyn ensures controlled neurotransmitter release. J Cell Biol. 2008;183(2):323–37. doi: 10.1083/jcb.200805150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.McMahon HT, Missler M, Li C, Sudhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83(1):111–9. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 258.Takahashi S, Yamamoto H, Matsuda Z, Ogawa M, Yagyu K, Taniguchi T, et al. Identification of two highly homologous presynaptic proteins distinctly localized at the dendritic and somatic synapses. FEBS Lett. 1995;368(3):455–60. doi: 10.1016/0014-5793(95)00713-j. [DOI] [PubMed] [Google Scholar]
- 259.Ono S, Baux G, Sekiguchi M, Fossier P, Morel NF, Nihonmatsu I, et al. Regulatory roles of complexins in neurotransmitter release from mature presynaptic nerve terminals. Eur J Neurosci. 1998;10(6):2143–52. doi: 10.1046/j.1460-9568.1998.00225.x. [DOI] [PubMed] [Google Scholar]
- 260.Reim K, Wegmeyer H, Brandstatter JH, Xue M, Rosenmund C, Dresbach T, et al. Structurally and functionally unique complexins at retinal ribbon synapses. J Cell Biol. 2005;169(4):669–80. doi: 10.1083/jcb.200502115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Buhl LK, Jorquera RA, Akbergenova Y, Huntwork-Rodriguez S, Volfson D, Littleton JT. Differential regulation of evoked and spontaneous neurotransmitter release by C-terminal modifications of complexin. Mol Cell Neurosci. 2013;52:161–72. doi: 10.1016/j.mcn.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yang X, Cao P, Sudhof TC. Deconstructing complexin function in activating and clamping Ca2+-triggered exocytosis by comparing knockout and knockdown phenotypes. Proc Natl Acad Sci U S A. 2013;110(51):20777–82. doi: 10.1073/pnas.1321367110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yang X, Kaeser-Woo YJ, Pang ZP, Xu W, Sudhof TC. Complexin clamps asynchronous release by blocking a secondary Ca(2+) sensor via its accessory alpha helix. Neuron. 2010;68(5):907–20. doi: 10.1016/j.neuron.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323(5913):516–21. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kaeser-Woo YJ, Yang X, Sudhof TC. C-terminal complexin sequence is selectively required for clamping and priming but not for Ca2+ triggering of synaptic exocytosis. J Neurosci. 2012;32(8):2877–85. doi: 10.1523/JNEUROSCI.3360-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat Neurosci. 2007;10(10):1235–7. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- 267.Strenzke N, Chanda S, Kopp-Scheinpflug C, Khimich D, Reim K, Bulankina AV, et al. Complexin-I is required for high-fidelity transmission at the endbulb of Held auditory synapse. J Neurosci. 2009;29(25):7991–8004. doi: 10.1523/JNEUROSCI.0632-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Martin JA, Hu Z, Fenz KM, Fernandez J, Dittman JS. Complexin has opposite effects on two modes of synaptic vesicle fusion. Curr Biol. 2011;21(2):97–105. doi: 10.1016/j.cub.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jorquera RA, Huntwork-Rodriguez S, Akbergenova Y, Cho RW, Littleton JT. Complexin controls spontaneous and evoked neurotransmitter release by regulating the timing and properties of synaptotagmin activity. J Neurosci. 2012;32(50):18234–45. doi: 10.1523/JNEUROSCI.3212-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Cao P, Yang X, Sudhof TC. Complexin activates exocytosis of distinct secretory vesicles controlled by different synaptotagmins. J Neurosci. 2013;33(4):1714–27. doi: 10.1523/JNEUROSCI.4087-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Vaithianathan T, Zanazzi G, Henry D, Akmentin W, Matthews G. Stabilization of spontaneous neurotransmitter release at ribbon synapses by ribbon-specific subtypes of complexin. J Neurosci. 2013;33(19):8216–26. doi: 10.1523/JNEUROSCI.1280-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Cho RW, Kummel D, Li F, Baguley SW, Coleman J, Rothman JE, et al. Genetic analysis of the Complexin trans-clamping model for cross-linking SNARE complexes in vivo. Proc Natl Acad Sci U S A. 2014;111(28):10317–22. doi: 10.1073/pnas.1409311111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, et al. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104(1):71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 274.Xue M, Reim K, Chen X, Chao HT, Deng H, Rizo J, et al. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat Struct Mol Biol. 2007;14(10):949–58. doi: 10.1038/nsmb1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Xue M, Stradomska A, Chen H, Brose N, Zhang W, Rosenmund C, et al. Complexins facilitate neurotransmitter release at excitatory and inhibitory synapses in mammalian central nervous system. Proc Natl Acad Sci U S A. 2008;105(22):7875–80. doi: 10.1073/pnas.0803012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313(5787):676–80. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- 277.Schaub JR, Lu X, Doneske B, Shin YK, McNew JA. Hemifusion arrest by complexin is relieved by Ca2+-synaptotagmin I. Nat Struct Mol Biol. 2006;13(8):748–50. doi: 10.1038/nsmb1124. [DOI] [PubMed] [Google Scholar]
- 278.Malsam J, Parisotto D, Bharat TA, Scheutzow A, Krause JM, Briggs JA, et al. Complexin arrests a pool of docked vesicles for fast Ca2+-dependent release. EMBO J. 2012;31(15):3270–81. doi: 10.1038/emboj.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Trimbuch T, Xu J, Flaherty D, Tomchick DR, Rizo J, Rosenmund C. Re-examining how complexin inhibits neurotransmitter release. eLife. 2014;3:e02391. doi: 10.7554/eLife.02391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Xue M, Craig TK, Xu J, Chao HT, Rizo J, Rosenmund C. Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nat Struct Mol Biol. 2010;17(5):568–75. doi: 10.1038/nsmb.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Diao J, Cipriano DJ, Zhao M, Zhang Y, Shah S, Padolina MS, et al. Complexin-1 enhances the on-rate of vesicle docking via simultaneous SNARE and membrane interactions. J Am Chem Soc. 2013;135(41):15274–7. doi: 10.1021/ja407392n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Iyer J, Wahlmark CJ, Kuser-Ahnert GA, Kawasaki F. Molecular mechanisms of COMPLEXIN fusion clamp function in synaptic exocytosis revealed in a new Drosophila mutant. Mol Cell Neurosci. 2013;56:244–54. doi: 10.1016/j.mcn.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zamir O, Charlton MP. Cholesterol and synaptic transmitter release at crayfish neuromuscular junctions. J Physiol. 2006;571(Pt 1):83–99. doi: 10.1113/jphysiol.2005.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wasser CR, Ertunc M, Liu X, Kavalali ET. Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J Physiol. 2007;579(Pt 2):413–29. doi: 10.1113/jphysiol.2006.123133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Rodrigues HA, Lima RF, Fonseca MD, Amaral EA, Martinelli PM, Naves LA, et al. Membrane cholesterol regulates different modes of synaptic vesicle release and retrieval at the frog neuromuscular junction. Eur J Neurosci. 2013 doi: 10.1111/ejn.12300. [DOI] [PubMed] [Google Scholar]
- 286.Petrov AM, Yakovleva AA, Zefirov AL. Role of membrane cholesterol in spontaneous exocytosis at frog neuromuscular synapses: reactive oxygen species-calcium interplay. J Physiol. 2014;592(Pt 22):4995–5009. doi: 10.1113/jphysiol.2014.279695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Xu W, Morishita W, Buckmaster PS, Pang ZP, Malenka RC, Sudhof TC. Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron. 2012;73(5):990–1001. doi: 10.1016/j.neuron.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Otmakhov N, Shirke AM, Malinow R. Measuring the impact of probabilistic transmission on neuronal output. Neuron. 1993;10(6):1101–11. doi: 10.1016/0896-6273(93)90058-y. [DOI] [PubMed] [Google Scholar]
- 289.Carter AG, Regehr WG. Quantal events shape cerebellar interneuron firing. Nat Neurosci. 2002;5(12):1309–18. doi: 10.1038/nn970. [DOI] [PubMed] [Google Scholar]
- 290.Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38(6):929–39. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- 291.Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52(4):663–77. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125(4):785–99. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 293.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33(16):6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A. 2014;111(23):8649–54. doi: 10.1073/pnas.1323920111. [DOI] [PMC free article] [PubMed] [Google Scholar]


