Summary
Deficiency of acid alpha glucosidase (GAA) causes Pompe disease in which the patients systemically accumulate lysosomal glycogen in muscles and nervous systems, often resulting in infant mortality. Although enzyme replacement therapy (ERT) is effective in treating patients with Pompe disease, formation of antibodies against rhGAA complicates treatment. In this report, we investigated induction of tolerance by oral administration of GAA expressed in chloroplasts. Because full-length GAA could not be expressed, N-terminal 410-amino acids of GAA (as determined by T-cell epitope mapping) were fused with the transmucosal carrier CTB. Tobacco transplastomic lines expressing CTB-GAA were generated through site-specific integration of transgenes into the chloroplast genome. Homoplasmic lines were confirmed by Southern blot analysis. Despite low-level expression of CTB-GAA in chloroplasts, yellow or albino phenotype of transplastomic lines was observed due to binding of GAA to a chloroplast protein that has homology to mannose-6 phosphate receptor. Oral administration of the plant-made CTB-GAA fusion protein even at 330-fold lower dose (1.5 μg) significantly suppressed immunoglobulin formation against GAA in Pompe mice injected with 500 μg rhGAA per dose, with several-fold lower titre of GAA-specific IgG1 and IgG2a. Lyophilization increased CTB-GAA concentration by 30-fold (up to 190 μg per g of freeze-dried leaf material), facilitating long-term storage at room temperature and higher dosage in future investigations. This study provides the first evidence that oral delivery of plant cells is effective in reducing antibody responses in ERT for lysosomal storage disorders facilitating further advances in clinical investigations using plant cell culture system or in vitro propagation.
Keywords: oral tolerance, enzyme replacement therapy, lysosomal storage disorder, bioencapsulation, molecular farming, Pompe disease
Introduction
Genetically modified plant cells are suitable for bioencapsulation and oral delivery of human therapeutic protein drugs and antigens for immune modulation, including oral tolerance induction. However, high levels of transgene expression are required for many therapeutic applications for such an approach. This potential hurdle has been addressed by expression of human therapeutic protein in chloroplasts (Daniell et al., 2009b; Dolgin, 2014). Chloroplasts are highly efficient bioreactors and offer several unique advantages; >10 000 copies of transgenes are expressed in each transformed plant cell; for example, it is possible to produce up to 360 million doses of fully functional anthrax vaccine in one acre of tobacco (Koya et al., 2005) or express human blood proteins up to 70% of total leaf protein in healthy plants (Ruhlman et al., 2010). Maternal inheritance of genetically modified chloroplast genomes and the absence of any reproductive structures offer efficient foreign gene containment and facilitate their safe production in the field (Daniell, 2002, 2007; Daniell et al., 1998). Additionally, chloroplast genetic engineering offers several other unique advantages including lack of gene silencing, position effect due to site-specific transgene integration and multigene engineering in single transformation event (De Cosa et al., 2001; Kumar et al., 2012). Several human blood proteins, vaccine antigens against bacterial, viral and protozoan pathogens and autoantigens have been expressed in transgenic chloroplasts (Daniell et al., 2009b; Kwon et al., 2013a). Many of these proteins have proper post-translational modifications (disulphide bonds, cyclization, lipid modifications) and are fully functional as evaluated by binding assays, cell culture studies, immunoglobulins, antiviral or anticancer activities, pathogen and toxin challenge (Daniell et al., 2009b; Kwon et al., 2013b). In recent studies, several human proteins expressed in chloroplasts are fully functional upon oral delivery including insulin/exendin to treat diabetes (Boyhan and Daniell, 2011; Kwon et al., 2013b), angiotensin converting enzyme/angiotensin for hypertension (Shenoy et al., 2014) or ocular inflammation (Shil et al., 2014) or the removal of plaques from the brain of patients with Alzheimer’s disease (Kohli et al., 2014).
In addition to delivery of functional proteins, high-level expression in chloroplasts combined with bioencapsulation is well suited for tolerogenic delivery of protein antigens (namely for oral tolerance induction). Oral tolerance is defined as the specific suppression of humoral and/or cellular immune responses to an antigen by administration of the same antigen through the oral route. Due to easy administration, antigen specificity, inexpensive production cost and unlimited scalability, plant-derived therapeutic antigen-based oral tolerance is becoming an attractive approach to prevent undesired immune responses that cause a number of diseases (e.g. haemophilia and type 1 diabetes etc.) or that complicate treatment of diseases (Dénes et al., 2013; Kwon et al., 2013a; Ruhlman et al., 2007; Wang et al., 2013). Oral tolerance targets the gut immune system, a natural site for anti-inflammatory and immune regulatory responses, and represents a noninvasive and antigen-specific approach that avoids use of general immune suppressive drugs. Oral tolerance using transgenic plant cells would therefore be ideal to eliminate immune responses such as antibody formation that severely complicate current systemic enzyme replacement therapies (ERTs) for several genetic diseases. However, efficient and cost-effective antigen delivery is required for efficacy and clinical translation.
In an effort to utilize transplastomic technology to address these problems, our laboratories have collaborated and found that oral delivery of coagulation factor VIII or IX, bioencapsulated in plant cells, prevents formation of inhibitory antibodies in replacement therapy for the bleeding disorders haemophilia A (Sherman et al., 2014) and haemophilia B (Verma et al., 2010). We hypothesize that this approach may be widely applicable to inherited protein deficiencies prone to such responses, including lysosomal storage disorders (LSD). Antibody formation is a major hurdle in treatment of Pompe disease, an autosomal recessive LSD caused by mutations in the gene encoding acid-α-glucosidase (GAA, an enzyme required for the degradation of glycogen in lysosomes). GAA deficiency causes a neuromuscular disease that affects skeletal, cardiac and smooth muscles; diaphragm; and motorneurons (DeRuisseau et al., 2009; Falk et al., 2015). ERT with recombinant human GAA (rhGAA) is currently the only clinically available ameliorative therapy (Kishnani et al., 2007, 2010). Infantile-onset patients manifest symptoms as early as 1 month after birth with severe cardiomegaly, troubled feeding, poor muscle tone and respiratory distress. Without ERT, these patients do not survive beyond 2 years of age. Absence of GAA activity in severe disease typically also reflects absence of antigen due to gene deletion or other severe mutations, which in turn also results in a lack of tolerance to infused rhGAA (van Gelder et al., 2015). Consequently, 88% of severe patients have been found to form anti-GAA responses, which not only neutralize therapy but cause immunotoxicities. Currently, the only option to prevent antibody formation is to initiate sustained immune suppression prior to the onset of therapy (Elder et al., 2013). To address this urgent need, we expressed GAA antigen in tobacco chloroplasts and evaluated in GAA-deficient Pompe mouse model, providing the first evidence that oral delivery of transplastomic leaf cells can be effective in reducing antibody responses in ERT for LSDs, even when delivered at very low (330-fold) antigen concentration.
Results
Human acid alpha glucosidase (GAA) expression vector
We first constructed a full length (2859 bp, accession number XM005257194.2) of human acid alpha glucosidase (GAA) chloroplast expression vector pLD-PTD-GAA. However, we could not generate any transplastomic line by bombardment of pLDPTD-GAA vector in spite of repeated trials (~300 bombardments). Therefore, we made another chloroplast expression vector to express a domain containing T-cell epitopes. In our previous investigations, all three identified CD4+ T-cell epitopes of the GAA were mapped around the same region of the GAA protein (amino acids 200th–350th, Nayak et al., 2012). Therefore, we chose the cDNA sequence of the N-terminal 410-amino acid (1st–410th, 1230 bp) of GAA to construct another expression vector. To efficiently deliver the human GAA, the DNA sequence of 410-amino acid N-terminal region was fused in frame with the transmucosal carrier cholera toxin B subunit (CTB) sequence. The CTB-fused shorter version of GAA (CTB-GAA) was then amplified from the vector pLD-PTD-GAA and a CTB-containing plasmid by overlap extension PCR and then cloned into the pLD-ctv vector (Daniell et al., 2004) to create pLD-CTB-GAA (Figure 1a). In the pLD-CTB-GAA chloroplast vector, the CTB-GAA fusion gene is driven by the psbA promoter and 5′ untranslated region (UTR) to increase translation and the transcript was stabilized by the psbA 3′ UTR. The spectinomycin selection marker gene aadA in pLD-CTB-GAA is driven by the tobacco plastid ribosomal operon promoter (Prrn) and the GGAG ribosome binding site was also included to facilitate selection on spectinomycin. In addition, a glycine–proline–glycine–proline (GPGP) hinge was created between the fusion elements to prevent steric hindrance. A furin cleavage site, arginine–arginine–lysine–arginine (RRKR), was also inserted between the fusion elements. The CTB-GAA expression vector was fully sequenced before particle bombardment and expression was evaluated in E. coli.
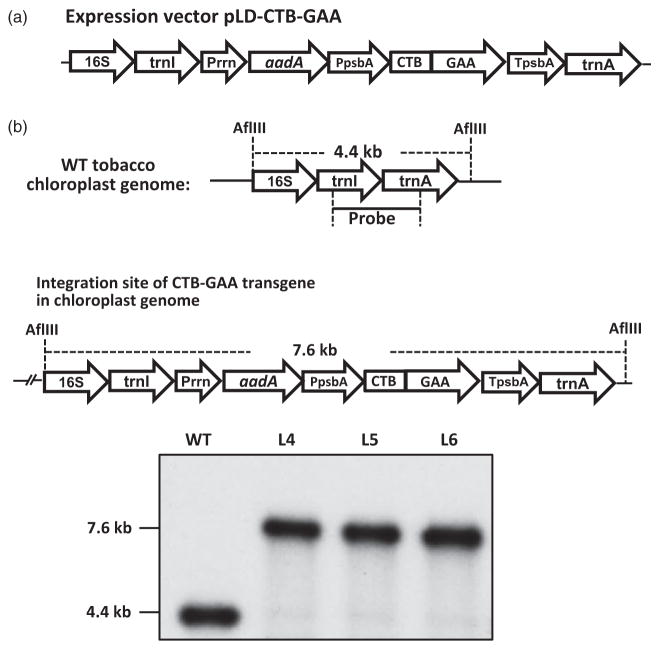
Figure 1.
Schematic diagram of CTB-GAA expression vector and Southern blot analyses of CTB-GAA transplastomic lines. (a) Schematic diagram of CTB-GAA expression vector pLD-CTB-GAA. Homologous chloroplast genome flanking sequences include 16S (16S rRNA), isoleucine tRNA (trnI), alanine tRNA (trnA) genes. A glycine–proline–glycine–proline hinge and furin cleavage site (RRKR) are included between CTB and the GAA sequence. The CTB-GAA expression is regulated by the tobacco psbA UTR and promoter. The selection marker gene aadA (encoding aminoglycoside 3′-adenylyltransferase gene to confer spectinomycin resistance) is driven by a ribosomal RNA operon promoter (Prrn) with GGAG ribosome binding site. (b) Southern blot. Total tobacco DNA (1 μg) was digested with AflIII and probed with 0.81-kb trnI/trnA flanking region fragment. Untransformed line generates a 4.4-kb hybridizing fragment, while transplastomic lines generate 7.6-kb fragment due to site-specific insertion of the transgene cassette. L4, L5 and L6 represent three independent transplastomic lines.
Generation of CTB-GAA transplastomic lines
CTB-GAA transplastomic lines were generated by biolistic particle bombardment of tobacco leaves with the expression vector pLD-CTB-GAA. After selection on spectinomycin-containing media, only three putative transplastomic lines were obtained from 20 bombardments due to potential toxicity of GAA. These transplastomic plants were confirmed by PCR analysis with primer sets 3P/3M, 5P/2M (Verma et al., 2008) and CTB-Fw/GAA-Rv which were designed to anneal specifically to complementary sequences of the chloroplast genome and transgene cassettes. PCR results showed expected sizes of amplicons, 1.65 kb with 3P/3M, 3.70 kb with 5P/2M and 1.54 kb with CTB-Fw/GAA-Rv, indicating site-specific integration of the aadA and CTB-GAA gene cassettes in all three independent transplastomic lines (data not shown). When these PCR-confirmed transplastomic shoots reached 6–7 leaf stage, leaves were cut into small pieces and subjected to second round of selection on spectinomycin and rooting process (3rd round) to generate homoplasmic plants. The site-specific integration of the CTB-GAA gene was further verified by Southern blot analysis probed with the trnI and trnA flanking sequence (Figure 1b). All three independent transplastomic lines (L4, L5 and L6) showed distinct hybridizing fragments with the correct size of 7.6-kb but not the 4.4-kb fragment from wild type in the AflIII-digested total DNA blot (Figure 1b). The total DNA was extracted from plants 4S, 5 and 6K (Figure 2a) that represent transplastomic lines L4, L5 and L6. Both PCR and Southern blot analyses indicated that the homoplasmic plants were generated through site-specific integration of the CTB-GAA expression cassette into the chloroplast genome.
Figure 2.
Phenotype of transplastomic lines expressing CTB-GAA. (a) Phenotypes of control and CTB-GAA plants. WT, untransformed wild-type plant. Plants 4S and 4X were regenerated from line 4; Plant 5 was regenerated from line 5. Plant 6K was regenerated from line 6. (b) Western blot analysis of control and CTB-GAA plants. Plant crude extracts were loaded from WT and CTB-GAA plants at 4 μg (TLP) in each lane; CTB, 10 ng per lane. Antibody titres: anti-CTB rabbit polyclonal antibody 1 : 10 000; anti-GAA rabbit polyclonal antibody 1 : 9000. (c) WT plants and CTB-GAA transplastomic albino plants (2.5 months old).
Phenotype of the transplastomic plants and CTB-GAA expression
Young and newly regenerated shoots derived from the bombarded leaves showed green leaf phenotype (data not shown). However, leaves of the CTB-GAA transplastomic plants after 3rd round of selection turned pale green as they grew larger. Plants grown a longer period of time (~7 weeks) showed unique mosaic-like or yellow leaf phenotype when compared to the normal green leaves of untransformed wild-type (WT) plants (Figure 2a). Plant 4S and 4X were regenerated from leaves of line L4. Plant 5 and plant 6K were regenerated from the leaves of L5 and L6, respectively. To further characterize CTB-GAA transplastomic lines, Western blot analysis was performed. As shown in Figure 2b, CTB-GAA expression was clearly detected in all 3 independent lines L4 (plants 4S, 4X), L5 (plant 5) and L6 (plant 6K) using anti-CTB antibody. To further verify the GAA polypeptide sequence of the CTB-GAA fusion protein expressed in chloroplasts, parallel Western blots were carried out using anti-GAA antibody. Western blots showed the CTB-GAA fusion protein with correct molecular mass of 57.3 kDa (Figure 2b) in all transplastomic lines. In addition, there was no nonspecific cross-reactivity of CTB standard protein (as a negative control) with the anti-GAA antibody although one cross-reactive protein was detected in protein extract from untransformed WT plants (Figure 2b). These results indicated that the CTB-GAA fusion protein was expressed in the chloroplasts of all three transplastomic lines. However, expression of CTB-GAA in tobacco chloroplasts resulted in drastic changes in plant phenotype. When these CTB-GAA plants were grown for a longer period of time (2.5 months), transplastomic lines turned into completely albino plants (Figure 2c). In addition to leaf phenotypic changes, the CTB-GAA plants also showed weaker root system and slower growth when compared with untransformed WT plants. However, the CTB-GAA albino plants were propagated through in vitro culture for more than 1 year through nodal cuttings. Furthermore, the CTB-GAA shoots could also be regenerated from albino leaves of the transplastomic lines. In this way, we were able to scale up production of the CTB-GAA leaf materials for further investigations.
To further investigate why CTB-GAA expression in tobacco chloroplasts resulted in albino phenotype of CTB-GAA transplastomic plants, Western blot assays were performed using anti-human cation-independent mannose 6-phosphate receptor (CI-MPR) polyclonal antibody (Novus Biologicals LLC, Littleton, CO). As shown in Figure 3a, a homologue protein (~60 kDa) of CI-MPR is detectable at low expression level in both leaf extract and purified chloroplast of WT plants. More interestingly, much higher level of this homologue was observed in the leaf extract of CTB-GAA transplastomic plants. The functional full-length GAA is a lysosomal hydrolytic enzyme for degradation of glycogen in human cells. To test whether chloroplast-derived GAA could affect carbohydrate metabolism (although the truncated version of GAA was used in this study), starch staining of tobacco leaves was carried out using Lugol’s iodine solution. As demonstrated in Figure 3b, no significant starch accumulation was detected in the leaves of both CTB-GAA transplastomic and WT plants grown in ½ strength MS medium supplemented with 2% sucrose. The WT leaves growing in potted soil in greenhouse, however, showed high-level starch accumulation (Figure 3b).
Figure 3.
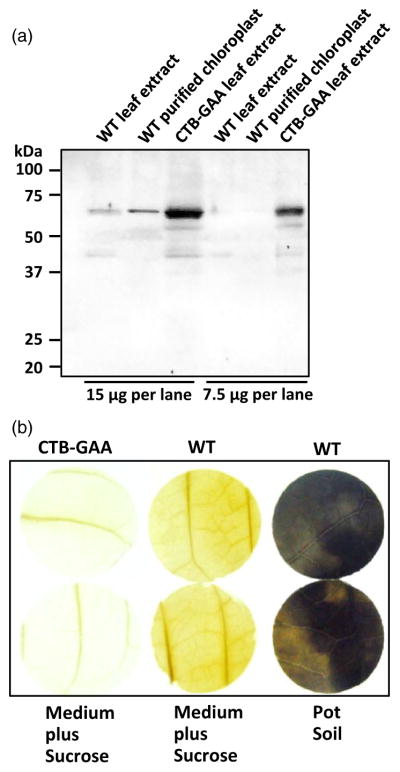
Characterization of CTB-GAA albino plants. (a) Western blot to detect the homologue of human cation-independent mannose 6-phosphate receptor (CI-MPR) in tobacco chloroplasts and leaves. A total of 15 or 7.5 μg of total protein per lane was loaded. Probe: anti-human CI-MPR polyclonal antibody (titre, 1 : 2000). WT, untransformed wild-type sample. (b) Starch staining of CTB-GAA transplastomic and WT leaves. Both CTB-GAA transplastomic leaves (white) and WT leaves (green) grown in culture dishes or potted plants were used for this assay. Three independent experiments were performed, and no detectable starch accumulation was observed in the leaf discs of both CTB-GAA transplastomic and WT plants grown in ½ strength MS medium supplemented with 2% sucrose. Leaf discs of WT plants growing in potted soil in the greenhouse showed high-level synthesis of starch as shown by dark blue staining.
Quantitation and pentamer assembly of CTB-GAA fusion protein in chloroplasts
Quantitation of CTB-GAA fusion proteins in leaf extracts from transplastomic lines was performed by Western blot analysis (Figure 4a). The CTB-GAA plant crude extracts were probed with anti-CTB polyclonal antibody under fully denatured and under reducing conditions. Concentration of the fusion protein was measured by densitometry on Western blots of leaf extracts using known amounts of purified CTB protein as standard. The measured expression level of CTB-GAA protein was between 0.13% and 0.21% of total leaf protein (TLP) varying with different batches of leaf materials or up to 6.38 μg per g of fresh leaves. Lyophilization of fresh leaf materials expressing human therapeutic proteins would facilitate long-term storage at room temperature and eliminate microbes. Moreover, lyophilization also increased the concentration of the therapeutic protein (Kwon et al., 2013b). Therefore, CTB-GAA plant leaf materials were lyophilized. The concentration of CTB-GAA protein in the lyophilized leaf materials was increased ~30-fold and reached up to 190.32 μg per g of dry weight (Figure 4b).
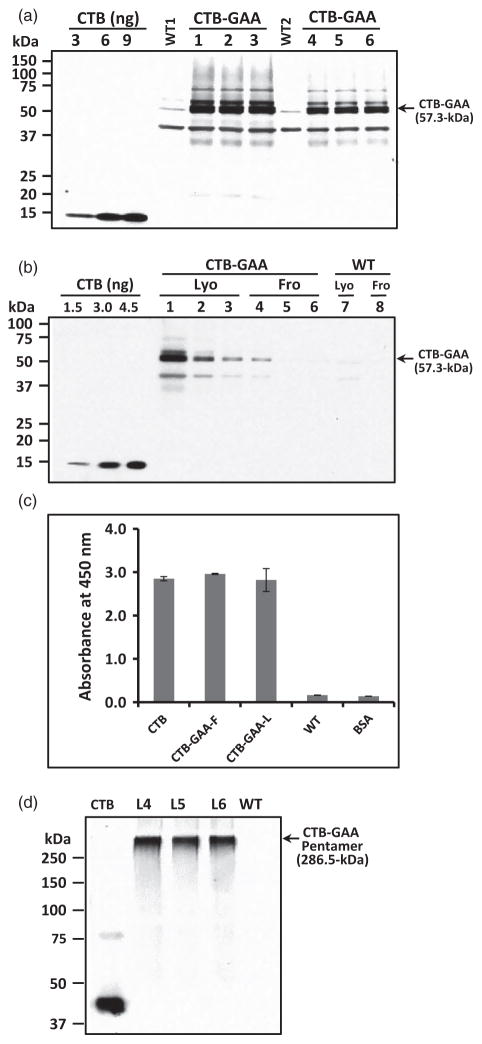
Figure 4.
Quantitation and assembly of CTB-GAA fusion proteins expressed in tobacco chloroplasts. (a) Quantitation of CTB-GAA protein. WT1 and lanes (1, 2, 3), representing protein extracts from independent lines L4 (plants 4S, 4X), L5 (plant 5) and L6 (plant 6K), 12 μg (TLP) per lane; WT2 and lanes (4, 5, 6), 6 μg (TLP) per lane. Probe: anti-CTB rabbit polyclonal antibody (titre 1 : 10 000). (b) Comparison of CTB-GAA concentrations in lyophilized (Lyo) and frozen (Fro) leaf materials. Lanes 1, 2, 3: lyophilized CTB-GAA leaf extracts (DW: 0.125, 0.025, 0.0125 mg). Lanes 4, 5, 6: frozen CTB-GAA leaf extracts (FW: 0.250, 0.050, 0.025 mg). Lane 7: lyophilized WT leaf extract (DW: 0.125 mg). Lane 8: frozen WT leaf extract (FW: 0.250 mg). Probe, anti-CTB rabbit polyclonal antibody (titre 1 : 10 000). (c) GM1 binding assay. CTB, purified CTB protein (5 ng). CTB-GAA-F, frozen CTB-GAA leaf protein extract (100 μg, TLP). CTB-GAA-L, lyophilized CTB-GAA leaf protein extract (100 μg, TLP). WT, lyophilized WT leaf protein extract (100 μg, TLP). BSA, bovine serum albumin (100 μg). (d) Nonreducing gel Western blot analysis. Nonreducing gel Western blot was performed to confirm pentamer assembly of CTB-GAA expressed in chloroplasts. Probe, anti-CTB rabbit polyclonal antibody (titre 1 : 10 000). Protein concentration loaded: CTB, 4 ng per lane; CTB-GAA and WT leaf extracts, 5 μg (TLP) per lane. L4, L5 and L6 represent the three independent transplastomic lines.
Several CTB-fused proteins expressed in chloroplasts (Limaye et al., 2006; Sherman et al., 2014; Verma et al., 2010) have been shown to bind to a plasma membrane receptor (GM1-ganglioside). A pentameric structure is required for binding of CTB fusion to GM1 receptor (Daniell et al., 2001; Tsuji et al., 1995). To evaluate receptor binding ability of CTB-GAA fusion proteins produced in tobacco chloroplasts, GM1-binding ELISA was performed. As shown in Figure 4c, CTB-GAA fusion protein extracts from both frozen leaf samples and freeze-dried leaf materials and purified CTB protein showed strong binding affinity to GM1. Therefore, CTB-GAA fusion protein assembled properly to form pentameric structures within transformed chloroplasts. Lyophilization of CTB-GAA leaf materials maintained the functional pentameric structure. To further confirm the pentamer formation of the chloroplast-derived CTB-GAA fusion proteins, nonreducing gel Western blot was carried out with tobacco leaf extracts using anti-CTB polyclonal antibody. The pentameric structure with expected size of 286.5-kDa was clearly detected in the nonreducing blots (Figure 4d). No cleaved or dissociated CTB-GAA protein was detectable in all three transplastomic lines. Results of both GM1-binding ELISA and nonreducing gel immunoblotting confirm pentamer assembly of CTB-GAA fusion protein expressed in tobacco chloroplasts.
Suppression of anti-GAA formation upon ERT in Pompe mice
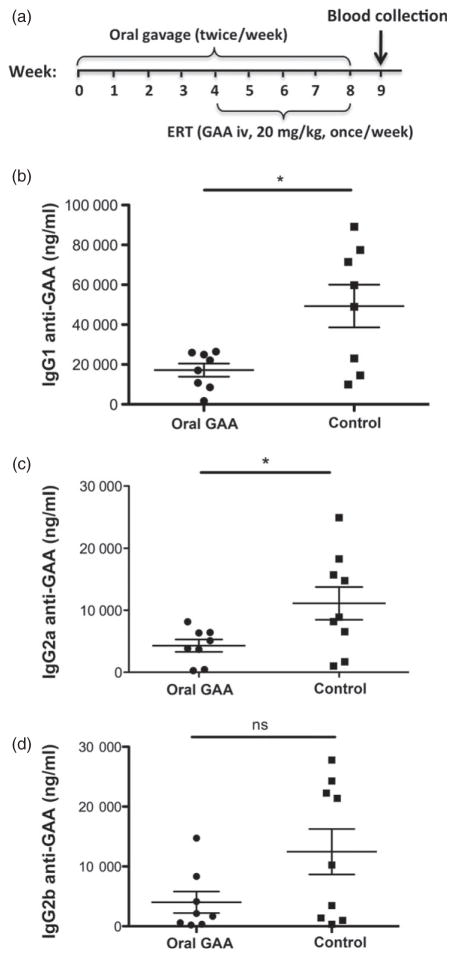
At high doses of 20–40 mg/kg, ERT partially corrects the storage disorder in muscle cells of human patients or experimental animals due to uptake of the enzyme via the mannose-6-phosphate receptor. However, similar to patients with early-onset Pompe disease, Pompe mice lacking GAA expression due to gene deletion form high-titre antibody responses during the course of ERT when they receive high enzyme doses (Nayak et al., 2012 Nayak et al., 2014; Sun et al., 2007). Antibody formation is CD4+ T-helper cell dependent, and chloroplast-made CTB-GAA fusion protein contains the dominant IAb-restricted epitopes presented in the mouse strain used in this study (129SVE Gaa −/− mice). A cohort of these Pompe mice (n = 8) received oral gavages of 250 mg frozen tobacco leaf cells (containing ~1.5 μg CTB-GAA) twice per week for 2 months (Figure 5a). During the second month, ERT was performed by weekly intravenous injections of recombinant human GAA at a typical clinical dose (20 mg/kg, or ~500 μg per mouse). Control Pompe mice (n = 9) received ERT only, which resulted in high-titre anti-GAA formation. Consistent with our previous data, mice predominantly formed IgG1 and only lower titres of IG2a and IgG2b (Figure 5a–d). Importantly, orally tolerized mice showed a significant, on an average threefold lower titre of GAA-specific IgG1 (Figure 5b). In addition, there was a significant further reduction in IgG2a formation (Figure 5c). Although the reduction in IgG2b formation did not reach statistical significance (due to the relatively low titre and greater variability of this subclass in the control mice), the subset of higher titre responders seen in the control mice was absent in tolerized mice (Figure 5d).
Figure 5.
Suppression of antibody formation against GAA by oral administration of bioencapsulated GAA in Pompe mice. (a) Time line and schedule for oral gavages of GAA plant material, challenge by weekly systemic GAA administration, and blood collection. (b) GAA-specific IgG1 titres in plasma of orally tolerized and control mice (systemic GAA delivery only, n = 8–9 per experimental group). Data points are for individual mice. Average titres and standard deviations are also indicated. (c) GAA-specific IgG2a titres in plasma of orally tolerized and control mice. (d) GAA-specific IgG2b titres in plasma of orally tolerized and control mice. * indicated P < 0.05; ns, not significant.
Discussion
In previous research, we advanced the concept of tolerance induction by oral administration of transplastomic leaf cells from initial prevention of destructive T-cell responses in autoimmune disease (in a murine model of type 1 diabetes) to suppression of allo-antibody formation in coagulation factor replacement therapy for haemophilia (Ruhlman et al., 2007; Sherman et al., 2014; Verma et al., 2010). Here, we further expanded the spectrum of application of this promising approach to suppression of antibody formation in ERT for the lysosomal storage disorder (LSD) Pompe disease. These data provide first evidence that the strategy can be successful in the treatment of LSDs, which is severely hindered by these humoral immune responses. Thus, the concept of tolerogenic oral delivery of transplastomic leaf cells is widely, perhaps even universally, applicable to cellular and humeral immune responses that occur in autoimmune disease and rejection of replacement therapy for genetic disease. These new results are encouraging for the development of plant-based oral tolerance for many other inherited protein deficiencies. As more ERTs are being approved for clinical applications, the problem of immune rejection is likely to occur in more diseases (Brooks et al., 2003) and could also emerge as a problem in the newer gene therapies (Byrne et al., 2011; Rogers and Herzog, 2015; Sack et al., 2014).
In this study, we used a truncated form of GAA for chloroplast-derived antigen production of the GAA used for oral tolerance induction. We successfully generated transplastomic tobacco plants which express the 410-amino acid GAA antigen. The GAA can be produced in chloroplasts in truncated form. However, the full-length GAA transplastomic plants could not be generated more likely due to the toxicity of full-length GAA protein. The pale green and albino leaf phenotypes of the partial GAA transplastomic plants indicated that the truncated form of the GAA is still not well tolerated by chloroplasts. The toxicity of GAA protein does not seem to be related to enzymatic activity because the active catalytic site (from F512 to E521) of the GAA (Hermans et al., 1991) is not included in our 410-amino acid GAA sequence. Our study has demonstrated that biological activity is not required for oral tolerance induction. However, the expressed polypeptide needs to include the relevant CD4+ T-cell epitopes for tolerogenic antigen presentation. CD4+ T cells play a critical role in B-cell activation and antibody production in ERT (Nayak et al., 2012). Shorter version of GAA contains all three CD4+ T-cell epitopes presented by the murine MHC II molecule I-Ab. For future translational studies, it will be important to express sufficient GAA amino acid sequence to cover major epitopes in the human population. Because plant-cell-based oral tolerance induces multiple subsets of regulatory T cells (Treg), resulting in active suppression of T-helper cell and antibody responses, it may not be necessary to achieve presentation of all epitopes that may be recognized in a particular patient (Kim et al., 2015; Sherman et al., 2014). In this initial study in the Pompe mouse, we achieve substantial but not complete suppression of anti-GAA formation. Future studies will need to address the level of suppression required to reduce or eliminate immuno-toxicities and improve efficacy of ERT.
In this report, CTB was used as a transmucosal carrier to make a fusion with GAA. CTB is recognized as one of the highly efficient carrier molecules for the induction of oral tolerance (Sun et al., 1994). CTB is composed of five identical 11.5-kDa polypeptide monomers that assemble into a stable pentamer ring (57.5 kDa). The pentamer CTBs bind GM1 ganglioside receptors in the gut epithelial cell membrane and in dendritic cells (DCs) (Kraehenbuhl et al., 1997; Rescigno et al., 2001). It has been demonstrated that CTB is nontoxic when administered to humans (Nashar and Hirst, 1995). In addition, CTB was found to activate both antigen presenting cells (APCs) and Th2 cell responses that are essential for the stimulation of B-cell immunoglobulin responses (Eriksson et al., 2003). The expression level of the truncated GAA protein fused with CTB in the transplastomic leaves is not high. Nonetheless, oral delivery of the CTB-GAA antigen bioencapsulated in plant cells significantly suppressed antibody formation against rhGAA in Pompe mice. The dose that we used for testing the efficacy of oral tolerance in Pompe mice (1.5 μg per gavage) was 10 666-fold lower than the high dose (16 mg) of rhGAA without CTB fusion for oral tolerance induction in wild-type mice (Ohashi et al., 2011), indicating that much more efficient delivery was achieved using CTB as a transmucosal carrier. In comparison with the injected dose (500 μg per mouse) of rhGAA in this study, oral administration of plant-made GAA at 330-fold lower dose can still induce oral tolerance in Pompe mice, demonstrating the high efficacy of oral delivery of bioencapsulated therapeutic proteins. Feeding microgram amounts of potato-made and CTB-conjugated insulin (Arakawa et al., 1998) or tobacco-derived glutamic acid decarboxylase (GAD) and IL-4 (Ma et al., 2004) substantially suppressed autoimmune diabetes in NOD mice. Oral delivery of microgram dose with CTB-fused and tobacco-produced human FVIII or FIX was also effective for induction of oral tolerance to prevent inhibitory antibody formation against FVIII or FIX in haemophilia A or B mice (Sherman et al., 2014; Verma et al., 2010; Wang et al., 2015). The fusion of CTB to insulin generated up to 5000-fold reduction in the amount of autoantigen required than antigen without CTB for immunotolerization (Bergerot et al., 1997).
Recently, we defined the mechanism by which CTB fusion proteins orally delivered via transplastomic plant cells induce immune tolerance (Wang et al., 2015). After uptake by the epithelium of the small intestine, some of the antigen is delivered to DCs, including tolerogenic CD103+ DC. The interaction between systemic antigen delivery during replacement therapy and the tolerogenic oral delivery drives a complex immune regulatory response that increases the frequencies of CD103+ DC, plasmacytoid DC, CD4+ T cells expressing gut homing receptors and LAP+ CD4+ T cells. This response is antigen specific and dependent on the cytokine IL-10, a key anti-inflammatory component of the gut immune system. Adoptive transfer studies revealed that systemic antibody formation is ultimately suppressed by the induction of two distinct subsets of Treg: CD4+CD25+FoxP3+ Treg and CD4+CD25–LAP+ Treg (Sherman et al., 2014; Wang et al., 2015). LAP+ Treg overexpress latency associated peptide (LAP) and are known to potently suppress via a TGF-β-dependent mechanism. We found that LAP+ Treg induced by the plant-cell-based oral tolerance protocol express both TGF-β and IL-10 (Wang et al., 2015). Importantly, low oral antigen doses can prompt this regulated immune response, which explains why we had success with suppression of anti-GAA formation even with feeding low dose plant material.
Prophylactic induction (prior to ERT or commencing with ERT) of immune tolerance is required for infantile patients with Pompe disease to avoid prolonged immune suppression (Elder et al., 2013; Messinger et al., 2012). Our data generated from prophylactic treatment of Pompe mice with the plant-made GAA antigen provide the proof of principle. The present study also suggests that our approach needs further optimization for higher level expression and larger scale production of the GAA antigen. Codon optimization will be one of the strategies to improve the protein expression level in chloroplasts (Daniell et al., 2009a).
In human cells, GAA is transported to lysosomes after binding of mannose 6-phosphorylated glycan on the GAA protein surface to the cation-independent mannose 6-phosphate receptor (CI-MPR) (Maga et al., 2013; Oshima et al., 1988). If the chloroplast-produced GAA binds to the CI-MPR homologue protein in the chloroplast, the homologue-involved metabolic pathway(s) could be adversely affected, eventually resulting in albino plant phenotype. Western blot analysis using anti-human CI-MPR polyclonal antibody detected CI-MPR homologue at low concentration in untransformed chloroplasts and in greater abundance in GAA-expressing chloroplasts (Figure 3a). Greater abundance could be due to binding of GAA to the mannose 6 phosphate receptor or the homologue. In the N-terminal 410-amino acid region of our truncated version of GAA, there are 3 M6P binding sites (Asn140, Asn233 and Asn390) (Hermans et al., 1993; Hoefsloot et al., 1988). This offers one potential explanation for albino plants. Therefore, it is possible to introduce mutations in M6P binding site in order to reduce GAA toxicity and increase GAA expression level. Therefore, an immunogenic GAA lacking M6P binding site could be created in future studies.
In this study, no significant difference of starch synthesis was observed between GAA transplastomic plants and WT plants growing in sucrose-containing medium, suggesting that the truncated GAA expression in tobacco chloroplasts did not interfere starch accumulation. We reason that the undetectable or low-level starch accumulation (as determined by Lugol’s iodine staining method) in both transplastomic and WT leaves growing in sucrose-containing medium was due to exogenous supply of sucrose in the growth medium. Untransformed leaves grown in potted soil showed high-level starch accumulation. Spraying leaves with 50 mM sucrose strongly reduces photosynthesis of sugarcane through the down-regulation of Rubisco abundance and activity, and starch concentration was significantly decreased in leaf and sheath tissues (Lobo et al., 2015).
Chloroplast-expressed GAA protein can be concentrated more than 30-fold after lyophilization when compared to frozen materials. While this study used frozen leaf materials, future studies will use lyophilized plant cells with 30-fold enhanced concentration per dose. Use of lyophilized GAA leaf materials in combination with dose escalation may further enhance the efficacy of inhibitor suppression. The first FDA-approved drug made in plant cells is glucocerebrosidase made in carrot cell culture (Shaaltiel et al., 2007; Walsh, 2014). Therefore, if autotrophic growth is a limitation, plant cell culture may be set up for biomass production and further clinical studies. Alternatively, commercial facilities are available for in vitro propagation. Therefore, GAA-expressing plant cell culture or in vitro grown plants would be ideal for translational studies for Pompe disease.
Experimental procedures
Construction of the CTB-GAA expression vector
The CTB-GAA chimeric gene (1566 bp) was synthesized by overlap extension PCR as described by Heckman and Pease (2007) with the following 4 primers, NdeI-CTB-Fw (5′-TTCATATGACA CCTCAAAATATTACTGATT-3′, the underlined nucleotides represent the start codon of CTB), CTB-GAA-Fw (5′-CCCGGG CCGCGGCGTAAACGC ATGGGAGTGA GGCACCCG-3′, the underlined indicates the start codon of GAA), CTB-GAA-Rv (5′-G CGTTTACGCCGCGGCCCGGGCCCATTTGCCATACTA-3′) and GAA-Rv (5′-AAC TAGTCTAGACTAGGAGTCCATGTAGTCCA GGTCGTTCC-3′, the italics indicates the XbaI restriction site, and the underlined represents the stop codon of GAA). The entire CTB-GAA PCR products were cloned into pZero Blunt TOPO PCR vector (Invitrogen, Carlsbad, CA) to create the pTOPO-CTB-GAA. The CTB-GAA sequence was then verified by sequencing. The chimeric gene of CTB-GAA was isolated with NdeI/XbaI restriction digestion and cloned into a pLD-ctv as reported previously (Daniell et al., 2004) to construct the CTB-GAA expression vector pLDCTB-GAA. The final chloroplast expression vector pLD-CTB-GAA was sequenced and used for transformation of tobacco chloroplasts.
Transformation and characterization of CTB-GAA transplastomic lines
Transformation of tobacco (Nicotiana tabacum cv. Petit Havana) chloroplasts with the expression vector pLD-CTB-GAA and PCR analysis of the transplastomic lines were performed according to the previously published protocols (Verma et al., 2008). The CTB-GAA transplastomic tobacco lines were selected and regenerated on spectinomycin (500 mg/L) selection media. Site-specific integration of the transgenes including the aadA selection cassette and the CTB-GAA expression cassette was first confirmed by PCR analysis with two primer sets 3P/3M and 5P/2M (Verma et al., 2008). The 3rd primer pair CTB-Fw (5′-CA TATGACACCTCAAAATATTACTGATT-3′) and GAA-Rv (the same primer used for overlap extension PCR) was also used to identify the integration of CTB-GAA expression cassette. Southern blot analysis was carried out essentially as described by Kumar and Daniell (2004). Total tobacco DNA (1 μg) was digested with AflIII, separated on a 0.8% agarose gel and then transferred to a nylon membrane. A 0.81-kb DNA probe was isolated from pUC-CT plasmid with BamHI and BglII digestion and labeled with 32P. After labeling, membrane hybridization was carried out by following the QUICK-HYB hybridization protocol (Stratagene, La Jolla, CA).
Preparation of intact chloroplasts
Chloroplasts from young (1.5 months old) tobacco leaves were isolated using the sucrose gradient method as described by Saski et al. (2007) based on Jansen et al. (2005) protocol with the following modifications. About 10 g of leaf tissue was homogenized in isolation buffer using prechilled tissue blender. The homogenate was filtered using three layers of Miracloth (EMD Chemicals, Inc. San Diego, CA). The filtrate was centrifuged at 1,000 × g for 15 min at 4°C. After resuspension of the pellets in 6 mL ice cold wash buffer, 1.5 mL of suspension was loaded onto the sucrose gradient consisting of 8 mL of 52% sucrose in the lower phase and 3.5 mL of 30% sucrose in the upper phase. Following centrifugation of the sucrose gradient at 55,000 × g (SW-41 rotor, Beckman) for 60 min at 4°C, the chloroplast band was removed with a Pasteur pipette. The collected chloroplasts were diluted with 10 volumes of wash buffer. After centrifugation at 1,500 × g for 15 min at 4°C, purified chloroplast pellets were lysed with plant protein extraction buffer (Verma et al., 2008) without sucrose for Western blot analysis.
Visualization of starch in tobacco Leaves
Starch staining of tobacco leaves was performed as reported previously (Chen et al., 2005). In brief, leaf discs were collected from similar age (~2 months old) of GAA transplastomic and WT tobacco plants. The collected leaf discs were blanched with 80% ethanol and then stained with Lugol’s solution (Sigma-Aldrich, St. Louis, MO). After destaining with double-distilled water, the stained leaf discs were visualized and photographed.
Reducing and nonreducing gel Western blot analysis
In order to test the expression of CTB-GAA in the transplastomic plants and to quantitate the concentration of CTB-GAA fusion protein in the harvested leaf materials, reducing PAGE gel under complete denature condition and Western blot analysis were performed according to the methods of Verma et al. (2008). Total leaf proteins were extracted from both frozen leaves and lyophilized leaf materials and then separated on 10% SDS–PAGE gel. Western blot analyses were carried out with rabbit anti-CTB polyclonal antibody (GeneWay, San Diego, CA) and rabbit anti-GAA polyclonal antibody separately. To analyse the pentameric structure of the CTB-GAA fusion proteins expressed in tobacco chloroplasts, nonreducing gel/Western blot analysis was also performed. CTB-GAA proteins were extracted in an extraction buffer without DTT or β-mercaptoethanol (BME). The sample loading buffer did not contain the reducing agent either. Gel running, protein transfer and detection of the blots were carried out by following the procedures as described by Verma et al. (2008).
GM1 binding assay
To test the ability of the tobacco chloroplast-derived CTB-GAA to bind to the GM1 receptor (GM1-ganglioside), a CTB–GM1 binding assay was performed according to the method of Ruhlman et al. (2007). Tobacco protein extracts were prepared in an extraction buffer without DTT or BME (200 mM Tris–HCl, pH8.0, 100 mM NaCl, 200 mM sucrose, 10 mM EDTA, 0.05% Tween-20, 2 mM PMSF and protease inhibitor cocktail). A total of 100 μg of leaf proteins were loaded in the wells of 96-well plate. CTB as a positive control and BSA as a negative control were also added for this GM1 binding assay. Rabbit anti-CTB primary antibody and HRP-conjugated donkey anti-rabbit secondary antibody were used to detect the binding activity which was read on a plate reader (Dynex Technologies, Chantilly, VA) at 450 nm.
Lyophilization
Crumbled and frozen tobacco leaves expressing CTB-GAA fusion proteins were transported to the lyophilizer (Genesis 35XL, SP Scientific.) on dry ice and treated at –40, –30, –20, –15, –10, –5 and 25°C for a total of 72 h under vacuum 400 mTorr. The lyophilized leaf materials were ground in a coffee grinder (Hamilton Beach, Southern Pines, NC) at maximum speed for three times (each time, pulse on 6 s and off 90 s). The fine powder was stored under moisture-free condition at room temperature with silica gels.
Animal studies
Pompe mice with targeted deletion of Gaa (on 129SVE genetic background, equal numbers of male and female 8-week old mice) were given oral gavage of frozen tobacco leaf cells twice per week for 2 months as published in the study by Sherman et al. (2014); Verma et al. (2010). The dose was 250 mg plant material containing ~1.5 μg CTB-GAA fusion antigen. During the second month of the regimen, mice were given 4 weekly intravenous (tail vein) injections of recombinant human GAA (Myozyme, Genzyme, Cambridge, MA) at a dose of 20 mg/kg. Blood samples were collected 1 week after the last injection, and GAA-specific antibody titres were measured in plasma by subclass-specific ELISA as published in the study by Nayak et al. (2012). Unpaired Student’s t-test was used to compare experimental groups, and P < 0.05 was considered a statistically significant difference.
Acknowledgments
This work was supported by NIH grants R01 HL107904 [to H.D. and R.W.H.], R01 HL109442 [to H.D., K.W.L. and R.W.H.].
Footnotes
Author contributions
JS and AS performed experiments. All authors participated in data analysis. JS, RWH and HD wrote the manuscript. HD and RWH conceived and supervised the study and designed experiments.
Conflict of interest
Henry Daniell is an inventor in several US and international patents on chloroplast transformation technology to produce vaccines and biopharmaceuticals.
References
- Arakawa T, Yu J, Chong DK, Hough J, Engen PC, Langridge WH. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat Biotechnol. 1998;16:934–938. doi: 10.1038/nbt1098-934. [DOI] [PubMed] [Google Scholar]
- Bergerot I, Ploix C, Petersen J, Moulin V, Rask C, Fabien N, Lindblad M, Mayer A, Czerkinsky C, Holmgren J, Thivolet C. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:4610–4614. doi: 10.1073/pnas.94.9.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyhan D, Daniell H. Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant Biotechnol J. 2011;9:585–598. doi: 10.1111/j.1467-7652.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DA, Kakavanos R, Hopwood JJ. Significance of immune response to enzyme-replacement therapy for patients with a lysosomal storage disorder. Trends Mol Med. 2003;9:450–453. doi: 10.1016/j.molmed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Byrne BJ, Falk DJ, Pacak CA, Nayak S, Herzog RW, Elder ME, Collins SW, Conlon TJ, Clement N, Cleaver BD, Cloutier DA, Porvasnik SL, Islam S, Elmallah MK, Martin A, Smith BK, Fuller DD, Lawson LA, Mah CS. Pompe disease gene therapy. Hum Mol Genet. 2011;20:R61–R68. doi: 10.1093/hmg/ddr174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Hajirezaei M, Börnke F. Differential expression of sucrose-phosphate synthase isoenzymes in tobacco reflects their functional specialization during dark-governed starch mobilization in source leaves. Plant Physiol. 2005;139:1163–1174. doi: 10.1104/pp.105.069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H. Molecular strategies for gene containment in transgenic crops. Nat Biotechnol. 2002;20:581–586. doi: 10.1038/nbt0602-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H. Transgene containment by maternal inheritance: effective or elusive? Proc Natl Acad Sci USA. 2007;104:6879–6880. doi: 10.1073/pnas.0702219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Datta R, Varma S, Gray S, Lee SB. Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat Biotechnol. 1998;16:345–348. doi: 10.1038/nbt0498-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Lee SB, Panchal T, Wiebe PO. Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J Mol Biol. 2001;31:1001–1009. doi: 10.1006/jmbi.2001.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Ruiz ON, Dhingra A. Chloroplast genetic engineering to improve agronomic traits. Methods Mol Biol. 2004;286:111–137. doi: 10.1385/1-59259-827-7:111. [DOI] [PubMed] [Google Scholar]
- Daniell H, Ruiz G, Denes B, Sandberg L, Langridge W. Optimization of codon composition and regulatory elements for expression of human insulin like growth factor-1 in transgenic chloroplasts and evaluation of structural identity and function. BMC Biotechnol. 2009a;9:33. doi: 10.1186/1472-6750-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Singh ND, Mason H, Streatfield SJ. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009b;14:669–679. doi: 10.1016/j.tplants.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cosa B, Moar W, Lee SB, Miller M, Daniell H. Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat Biotechnol. 2001;19:71–74. doi: 10.1038/83559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dénes B, Fodor I, Langridge WHR. Persistent suppression of Type 1 Diabetes by a multicomponent vaccine containing a cholera toxin B subunit-autoantigen fusion protein and Complete Freund’s Adjuvant. Clin Dev Immunol. 2013:Article ID 578786. doi: 10.1155/2013/578786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRuisseau LR, Fuller DD, Qiu K, DeRuisseau KC, Donnelly WH, Mah C, Reier PJ, Byrne BJ. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci USA. 2009;106:9419–9424. doi: 10.1073/pnas.0902534106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin E. Immunology-Oral solutions. Nature. 2014;515:S166–S167. doi: 10.1038/515S166a. [DOI] [PubMed] [Google Scholar]
- Elder ME, Sushrusha NS, Collins SW, Lawson LA, Kelley JS, Herzog RW, Modica RF, Lew J, Lawrence RM, Byrne BJ. B-Cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in Infantile-Onset Pompe disease. J Pediatr. 2013;163:847–854. doi: 10.1016/j.jpeds.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K, Fredriksson M, Nordström I, Holmgren J. Cholera toxin and its B subunit Promote dendritic cell vaccination with different influences on Th1 and Th2 development. Infect Immun. 2003;71:1740–1747. doi: 10.1128/IAI.71.4.1740-1747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DJ, Todd AG, Lee S, Soustek MS, ElMallah MK, Fuller DD, Notterpek L, Byrne BJ. Peripheral nerve and neuromuscular junction pathology in Pompe disease. Hum Mol Genet. 2015;24:625–636. doi: 10.1093/hmg/ddu476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder CM, Hoogeveen-Westerveld M, Kroos MA, Plug I, van der Ploeg AT, Reuser AJJ. Enzyme therapy and immune response in relation to CRIM status: the Dutch experience in classic infantile Pompe disease. J Inherit Metab Dis. 2015;38:305–314. doi: 10.1007/s10545-014-9707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman KL, Pease L. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc. 2007;2:924–932. doi: 10.1038/nprot.2007.132. [DOI] [PubMed] [Google Scholar]
- Hermans MMP, Marian A, Kroos MA, van Beeurnens J, Ben A, Oostra BA, Reuser AJJ. Human Lysosomal a-Glucosidase –characterization of the catalytic site. J Biol Chem. 1991;266:13507–13512. [PubMed] [Google Scholar]
- Hermans MMP, Wisselaar HA, Kroos MA, Oostra BA, Reuser AJJ. Human lysosomal a-glucosidase: functional characterization of the glycosylation sites. Biochem J. 1993;289:681–686. doi: 10.1042/bj2890681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefsloot LH, Hoogeveen-Westerveld M, Kroos MA, van Beeumen J, Reuser JA, Oostra BA. Primary structure and processing of lysosomal a-glucosidase; homology with the intestinal sucrase isomaltase complex. EMBO J. 1988;7:1697–1704. doi: 10.1002/j.1460-2075.1988.tb02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RK, Raubeson LA, Boore JL, dePamphilis CW, Chumley TW, Haberle RC, Wyman SK, Alverson AJ, Peery R, Herman SJ, Fourcade HM, Kuehl JV, McNeal JR, Leebens-Mack J, Cui L. Methods for obtaining and analyzing chloroplast genome sequences. Methods Enzymol. 2005;395:348–384. doi: 10.1016/S0076-6879(05)95020-9. [DOI] [PubMed] [Google Scholar]
- Kim YC, Zhang AH, Su Y, Rieder SA, Rossi RJ, Ettinger RA, Pratt KP, Shevach EM, Scott DW. Engineered antigen-specific human regulatory T cel ls: immunosuppression of FVIII-specific T- and B-cell responses. Blood. 2015;125:1107–1115. doi: 10.1182/blood-2014-04-566786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, Leslie N, Levine J, Spencer C, Mcdonald M, Li J, Dumontier J, Halberthal M, Chien YH, Hopkin R, Vijayaraghavan S, Gruskin D, Bartholomew D, Van Der PA, Clancy JP, Parini R, Morin G, Beck M, De La Gastine GS, Jokic M, Thurberg B, Richards S, Bali D, Davison M, Worden MA, Chen YT, Wraith JE. Recombinant human acid alpha-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Goldenberg PC, Dearmey SL, Heller J, Benjamin D, Young S, Bali D, Smith SA, Li JS, Mandel H, Koeberl D, Rosenberg A, Chen YT. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli N, Westerveld DR, Ayache AC, Verma A, Shil P, Prasad T, Zhu P, Chan SL, Li Q, Daniell H. Oral delivery of bioencapsulated proteins across blood–brain and blood–retinal barriers. Mol Ther. 2014;22:535–546. doi: 10.1038/mt.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya V, Moayeri M, Leppla SH, Daniell H. Plant-based vaccine: mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect Immun. 2005;73:8266–8274. doi: 10.1128/IAI.73.12.8266-8274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenbuhl JP, Hopkins SA, Kernéis S, Pringault E. Antigen sampling by epithelial tissues: implication for vaccine design. Behring Inst Mitt. 1997;98:24–32. [PubMed] [Google Scholar]
- Kumar S, Hahn FM, Baidoo E, Kahlon TS, Wood DF, McMahan CM, Cornish K, Keasling JD, Daniell H, Whalen MC. Remodeling the isoprenoid pathway in tobacco by expressing the cytoplasmic mevalonate pathway in chloroplasts. Metab Eng. 2012;14:19–28. doi: 10.1016/j.ymben.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Daniell H. Engineering the chloroplast genome for hyperexpression of human therapeutic proteins and vaccine antigens. Methods Mol Biol. 2004;267:365–383. doi: 10.1385/1-59259-774-2:365. [DOI] [PubMed] [Google Scholar]
- Kwon KC, Verma D, Singh ND, Herzog R, Daniell H. Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Deliv Rev. 2013a;65:782–799. doi: 10.1016/j.addr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KC, Nityanandam R, James S, New JS, Daniell H. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotechnol J. 2013b;11:77–86. doi: 10.1111/pbi.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye A, Koya V, Samsam M, Daniell H. Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. FASEB J. 2006;20:959–961. doi: 10.1096/fj.05-5134fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo AKM, Martins MO, Neto MCL, Machado EC, Ribeiro RV, Silveira JAG. Exogenous sucrose supply changes sugar metabolism and reduces photosynthesis of sugarcane through the down-regulation of Rubisco abundance and activity. J Plant Physiol. 2015;179:113–121. doi: 10.1016/j.jplph.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Ma S, Huang Y, Yin Z, Menassa R, Brandle JE, Jevnikar AM. Induction of oral tolerance to prevent diabetes with transgenic plants requires glutamic acid decarboxylase (GAD) and IL-4. Proc Natl Acad Sci USA. 2004;101:5680–5685. doi: 10.1073/pnas.0307420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga JA, Zhou J, Kambampati R, Peng S, Wang X, Bohnsack RN, Thomm A, Golata S, Tom P, Dahms NM, Byrne BJ, LeBowitz JH. Glycosylation-independent lysosomal targeting of acid α-glucosidase enhances muscle glycogen clearance in Pompe mice. J Biol Chem. 2013;288:1428–1438. doi: 10.1074/jbc.M112.438663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger YH, Mendelsohn NJ, Rhead W, Dimmock D, Hershkovitz E, Michael Champion M, Jones SA, Olson R, White A, Wells C, Bali D, Case LE, Young SP, Rosenberg AS, Kishnani PS. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet Med. 2012;14:135–142. doi: 10.1038/gim.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashar TO, Hirst TR. Immunoregulatory role of H-2 and intra-H-2 alleles on antibody responses to recombinant preparations of B-subunits of Escherichia coli heat-labile enterotoxin (rEtxB) and cholera toxin (rCtxB) Vaccine. 1995;13:803–810. doi: 10.1016/0264-410x(94)00077-z. [DOI] [PubMed] [Google Scholar]
- Nayak S, Sivakumar R, Cao O, Daniell H, Byrne BJ, Herzog RW. Mapping the T helper cell response to acid α-glucosidase in Pompe mice. Mol Genet Metab. 2012;106:189–195. doi: 10.1016/j.ymgme.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S, Doerfler PA, Porvasnik SL, Cloutier DD, Khanna R, Valenzano KJ, Herzog RW, Byrne BJ. Immune responses and hypercoagulation in ERT for Pompe disease are mutation and rhGAA dose dependent. PLoS One. 2014;9:e98336. doi: 10.1371/journal.pone.0098336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Iizuka S, Shimada Y, Eto Y, Ida H, Hachimura S, Kobayashi H. Oral administration of recombinant human acid α-glucosidase reduces specific antibody formation against enzyme in mouse. Mol Genet Metab. 2011;103:98–100. doi: 10.1016/j.ymgme.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Oshima A, Nolan CM, Kyle JW, Grubb JH, Sly WS. The human cation-independent mannose 6-phosphate receptor. Cloning and sequence of the full-length cDNA and expression of functional receptor in COS cells. J Biol Chem. 1988;263:2553–2562. [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Rogers GL, Herzog RW. Gene therapy for hemophilia. Front Biosci (Landmark Ed) 2015;20:556–603. doi: 10.2741/4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H. Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts-oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol J. 2007;5:495–510. doi: 10.1111/j.1467-7652.2007.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlman T, Verma D, Samson N, Daniell H. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol. 2010;152:2088–2104. doi: 10.1104/pp.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack BK, Herzog RW, Terhorst C, Markusic DM. Development of gene transfer for induction of antigen-specific tolerance. Mol Ther Methods Clin Dev. 2014;1:14013. doi: 10.1038/mtm.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saski C, Lee SB, Fjellheim S, Guda C, Jansen RK, Luo H, Tomkins J, Rognli OA, Daniell H, Clarke JL. Complete chloroplast genome sequences of Hordeum vulgare, Sorghum bicolor and Agrostis stolonifera, and comparative analyses with other grass genomes. Theor Appl Genet. 2007;115:571–590. doi: 10.1007/s00122-007-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaltiel Y, Bartfeld D, Hashmueli S, Baum G, Brill-Almon E, Galili G, Dym O, Boldin-Adamsky SA, Israel Silman I, Sussman JL, Futerman AH, Aviezer D. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol J. 2007;5:579–590. doi: 10.1111/j.1467-7652.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- Shenoy V, Kwon KC, Rathinasabapathy A, Lin S, Jin G, Song C, Shil P, Nair A, Qi Y, Li Q, Francis J, Katovich MJ, Daniell H, Raizad MK. Oral delivery of Angiotensin-Converting Enzyme2 and Angiotensin-(1–7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension. 2014;64:1248–1259. doi: 10.1161/HYPERTENSIONAHA.114.03871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman A, Su J, Lin S, Wang X, Herzog RW, Daniell H. Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood. 2014;124:1659–1668. doi: 10.1182/blood-2013-10-528737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shil PK, Kwon KC, Zhu P, Verma A, Daniell H, Li Q. Oral Delivery of ACE2/Ang-(1–7) bioencapsulated in plant cells protects against experimental uveitis and autoimmune uveoretinitis. Mol Ther. 2014;22:2069–2082. doi: 10.1038/mt.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JB, Holmgren J, Czerkinsky C. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci USA. 1994;91:10795–10799. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Bird A, Young SP, Kishnani PS, Chen YT, Koeberl DD. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am J Hum Genet. 2007;81:1042–1049. doi: 10.1086/522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Watanabe K, Miyama A. Monomer of the B subunit of heat-labile enterotoxin from enterotoxigenic Escherichia coli has little ability to bind to GM1 ganglioside compared to its coligenoid. Microbiol Immunol. 1995;39:817–819. doi: 10.1111/j.1348-0421.1995.tb03262.x. [DOI] [PubMed] [Google Scholar]
- Verma D, Samson NP, Koya V, Daniell H. A protocol for expression of foreign genes in chloroplasts. Nat Protoc. 2008;3:739–758. doi: 10.1038/nprot.2007.522. [DOI] [PubMed] [Google Scholar]
- Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, Daniell H. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci USA. 2010;107:7101–7106. doi: 10.1073/pnas.0912181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh G. Biopharmaceutical benchmarks. Nat Biotechnol. 2014;32:992–1000. doi: 10.1038/nbt.3040. [DOI] [PubMed] [Google Scholar]
- Wang X, Sherman A, Liao G, Kam W, Leong KW, Daniell H, Terhorst C, Herzog RW. Mechanism of oral tolerance induction to therapeutic proteins. Adv Drug Deliv Rev. 2013;65:759–773. doi: 10.1016/j.addr.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su J, Sherman A, Rogers GL, Liao G, Hoffman BE, Leong KW, Terhorst C, Daniell H, Herzog RW. Plant-based oral tolerance for hemophilia results from a complex immune regulatory mechanism including LAP+CD4+ T cells. Blood. 2015;125:2418–2427. doi: 10.1182/blood-2014-08-597070. [DOI] [PMC free article] [PubMed] [Google Scholar]