Abstract
Refrigerated food processing facilities are specific man-made niches likely to harbor cold-tolerant bacteria. To characterize this type of microbiota and study the link between processing plant and product microbiomes, we followed and compared microbiota associated with the raw materials and processing stages of a vacuum-packaged, cooked sausage product affected by a prolonged quality fluctuation with occasional spoilage manifestations during shelf life. A total of 195 samples were subjected to culturing and amplicon sequence analyses. Abundant mesophilic psychrotrophs were detected within the microbiomes throughout the different compartments of the production plant environment. However, each of the main genera of food safety and quality interest, e.g., Leuconostoc, Brochothrix, and Yersinia, had their own characteristic patterns of contamination. Bacteria from the genus Leuconostoc, commonly causing spoilage of cold-stored, modified-atmosphere-packaged foods, were detected in high abundance (up to >98%) in the sausages studied. The same operational taxonomic units (OTUs) were, however, detected in lower abundances in raw meat and emulsion (average relative abundance of 2% ± 5%), as well as on the processing plant surfaces (<4%). A completely different abundance profile was found for OTUs phylogenetically close to the species Yersinia pseudotuberculosis. These OTUs were detected in high abundance (up to 28%) on the processing plant surfaces but to a lesser extent (<1%) in raw meat, sausage emulsion, and sausages. The fact that Yersinia-like OTUs were found on the surfaces of a high-hygiene packaging compartment raises food safety concerns related to their resilient existence on surfaces.
INTRODUCTION
Refrigeration is used throughout the modern food chain to ensure the safety and quality of perishable products. Chilling has also been extended to food processing facilities to ensure that food manufacture complies with the legislative requirements governing maximum food temperature. Perishable food is usually packaged under a carbon dioxide-containing modified atmosphere to suppress bacterial growth. These modern food manufacturing practices have changed the order of prevalence of food-borne bacteria. Instead of aerobic Gram-negative bacteria, (facultatively) anaerobic Gram-positive bacteria prevail in modified-atmosphere-packaged (MAP) foods (1). In addition to refrigeration, the daily cleaning and sanitizing procedures used at the processing facilities might lead to the resilience of certain microbes and thus persistent contamination.
Hygiene in a food processing facility is monitored through an internal control procedure, including hazard analysis of critical control points. The manufacturer is responsible for guaranteeing the safety and quality of its products during the shelf life set for each respective product. In routine hygiene monitoring, the microbiological quality of the raw materials, cleanliness of the surfaces, and quality of the end products are included in these internal control protocols. Bacterial contamination within food processing facilities has been addressed in scientific studies using different molecular typing techniques enabling bacterial contamination to be tracked and traced during food manufacture (1–5). These approaches have involved massive sets of culturing, followed by the application of molecular typing methods on purified bacterial cultures.
To date, only one (6) study dealing with contamination of a meat processing environment has been conducted using 16S rRNA amplicon sequencing. Compared to studies of the microbiota in other man-made environments, such as shopping malls (7), homes (8), and hospitals (9), microbiota associated with food processing facilities have not been analyzed to the same extent using comprehensive microbial ecology approaches. When we were consulted to overcome a processing hygiene problem in a large-scale meat processing plant, we decided to approach this challenge by conducting a large-scale 16S rRNA gene amplicon sequencing study. Vacuum-packaged (VP) sausages of the manufacturer concerned had shown variable sensory spoilage changes, i.e., slime or opaque drip formation and loosening of the vacuum.
This paper presents the bacterial community composition in various processing stages of a man-made food manufacture niche. To characterize the processing plant and product microbiomes and to address the persistent hygiene problem, a total of 195 samples were taken from the processing plant environment, raw materials, and the end products. Alongside the amplicon analyses, we applied cultivation techniques and identified the main species of spoilage-causing lactic acid bacteria (LAB) using a database employing numerical analysis of 16S and 23S rRNA gene restriction patterns. We also defined the abundances and contamination patterns of some relevant cold-tolerant bacteria associated with safety and spoilage risks of vacuum-packaged, cooked meat products.
MATERIALS AND METHODS
Study design and sampling.
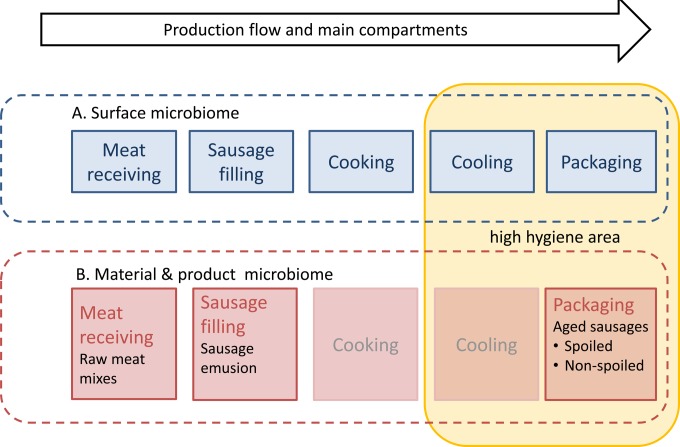
The processing environment was sampled twice, first in October 2012 after the spoiled products had been received from customers and then a second time in early December 2012. Samples were taken both times early in the morning from cleaned surfaces before production started and during ongoing production in the afternoon (Table 1). Sampling covered both the compartments where the raw meat was handled and the packaging areas with high-hygiene practices (Table 1 and Fig. 1). Altogether, 101 samples were taken during the two sampling rounds. We also analyzed sausages, which were produced during the two visits to the processing plant, at the end of their shelf life.
TABLE 1.
Sampling sites, conducted measurements, and outcome of DNA- and cultivation-based analysesa
| Site | Sampling location | Material | First sampling |
Second sampling |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| During production |
Before production |
During production |
Before production |
|||||||||||
| Sample name | PCR resulte | Cell abundance/platec | Sample name | PCR result | Cell abundance/plate | Sample name | PCR result | Cell abundance/plate | Sample name | PCR result | Cell abundance/plate | |||
| MRUb | Emulsion transport belt | Plastic | Fa1 | • | ++ | Fa69 | • | + | Fa109 | − | − | |||
| Emulsion transport belt | Plastic | Fa111 | − | − | ||||||||||
| Meat grinder | Metal | Fa2 | − | +++ | Fa75 | • | +++ | Fa112 | − | + | ||||
| Emulsion blender | Metal | Fa3 | − | +++ | Fa74 | − | + | Fa113 | − | − | ||||
| Emulsion storage silo door | Plastic | Fa4 | • | − | Fa70 | − | + | |||||||
| Emulsion storage silo door | Metal | Fa5 | • | − | Fa71 | − | − | Fa110 | − | − | ||||
| Emulsion storage silo door | Metal | Fa6 | • | − | Fa72 | − | − | |||||||
| Emulsion storage silo door | Metal | Fa73 | − | − | ||||||||||
| Air | NAd | Fa7 | NA | + | Fa46 | NA | − | Fa68 | NA | ++ | Fa108 | NA | − | |
| Tube on the floor | Plastic | Fa49 | − | +++ | ||||||||||
| Floor | Concrete | Fa50 | − | +++ | ||||||||||
| Cutting board | Plastic | Fa47 | − | +++ | ||||||||||
| Sausage-filling machinery | Filling machine | Plastic | Fa8 | • | ++ | Fa33 | − | − | Fa77 | • | +++ | Fa117 | • | − |
| Emulsion storage silo | Metal | Fa9 | • | +++ | Fa76 | − | ++ | |||||||
| Emulsion storage silo | Metal | Fa10 | • | + | ||||||||||
| Tube to move emulsion | Metal | Fa45 | • | + | ||||||||||
| Filling machine | Metal | Fa11 | • | +++ | Fa41 | − | ++ | Fa119 | − | +++ | ||||
| Filling machine | Metal | Fa42 | • | ++ | Fa118 | • | +++ | |||||||
| Air | NA | Fa12 | NA | + | − | − | − | |||||||
| Filling machine vacuum | Metal | Fa43 | • | − | Fa120 | − | + | |||||||
| Filling machine | Metal | Fa121 | − | − | ||||||||||
| Sausage string hanging cart | Metal | Fa122 | − | − | ||||||||||
| Packaging compartment | Link cutting drum | Plastic | Fa13 | • | +++ | Fa28 | • | + | Fa80 | − | − | Fa93 | − | − |
| Link cutting drum | Metal | Fa26 | − | − | Fa97 | − | − | |||||||
| Link cutting drum | Metal | Fa27 | • | − | ||||||||||
| Transport belt from drum | Plastic | Fa14 | • | + | Fa29 | • | − | Fa79 | − | − | Fa95 | − | − | |
| Transport belt from drum | Metal | Fa30 | • | − | Fa81 | − | − | Fa99 | − | − | ||||
| Air | NA | Fa18 | NA | − | Fa25 | NA | − | Fa78 | NA | − | Fa96 | NA | − | |
| Air | NA | Fa19 | NA | − | Fa88 | NA | − | Fa101 | NA | − | ||||
| Air | NA | Fa87 | NA | − | Fa103 | NA | − | |||||||
| Air | NA | Fa92 | NA | − | ||||||||||
| Cooling cabinet wall | Metal | Fa20 | − | − | Fa89 | − | − | Fa102 | • | − | ||||
| Packaging tray | Metal | Fa21 | • | − | Fa32 | • | − | Fa85 | − | − | Fa98 | − | − | |
| Pit from transport belt to packaging tray | Metal | Fa22 | • | + | Fa31 | • | − | Fa84 | − | ++ | ||||
| Transport cart for sausage hanging sticks | Metal | Fa23 | • | − | Fa40 | • | − | Fa83 | − | − | Fa100 | − | − | |
| Sausage string hanging cart | Metal | Fa24 | • | − | Fa90 | − | − | Fa104 | − | − | ||||
| Transport belt from drum | Plastic | Fa94 | − | − | ||||||||||
| Knife storage | Metal | Fa34 | − | − | ||||||||||
| Sausage string hanging cart | Metal | Fa105 | − | ++ | ||||||||||
| Sausage string hanging stick | Metal | Fa82 | − | − | − | − | − | |||||||
| Pit from transport belt to packaging tray | Metal | Fa86 | − | − | − | − | − | |||||||
| Wall | Metal | Fa91 | − | − | Fa106 | − | − | |||||||
The numerical data for raw materials and cooked products can be found in the supplemental material.
MRU, meat receiving unit.
In surface microbiome samples, the numbers of colonies on MRS-A swabs are identified as follows: −, none detected; +, 1 to 10; ++, 11 to 100; +++, >100.
NA, not applicable.
•, PCR successful; −, no PCR product.
FIG 1.
Schematic picture of the processing plant (blue) and the raw material flow (red) in corresponding process stages. No material samples were taken from the cooking and cooling compartments.
In addition to the surface and sausage samples, 12 production lots from raw materials, sausage emulsion, and subsequently manufactured and cooked products were sampled during a period of 5 months after the first processing plant visit. This was done to identify the spoilage microbiota in raw materials in addition to the ones on surfaces and to understand the contamination patterns during sausage production. These production lots were sampled in a time-dependent manner according to production flow. The raw materials and sausage emulsion were analyzed within 24 h of sampling in the processing plant, and the sausages at the end of a 26-day shelf life (±1 day). All samples were subjected to cultivation, DNA extraction, and PCR. Quantitative cultivation through a dilution series of the raw meat and products was done to analyze both LAB (de Man, Rogosa, and Sharpe [MRS] agar plates; Oxoid, Hampshire, United Kingdom) and total microbial (plate count agar [PCA]) levels. Surface samples were taken with sterile cotton swabs from the processing line surfaces that the raw materials, the emulsion used in sausage production, and the sausages were in contact with (detailed information is provided in Table 1). These samples were swabbed semiquantitatively from 5- by 5-cm surface areas when possible. In some of the surfaces it was not possible to sample a 5- by 5-cm square area due to the shape of the equipment. In addition to the microbiome analyses, the sausages were evaluated for spoilage changes by a three-person expert panel judging the formation of sensory changes visually at the end of shelf life. The changes evaluated included formation of slime or drip, color of the slime or drip (clear to opaque), and potential off-odor rated on a scale of 1 to 5.
Microbiological analysis and identification of spoilage LAB. (i) Processing line surfaces and air.
The 5- by 5-cm swabs taken from surfaces (see above) were directly streaked onto MRS plates containing amphotericin (MRS-A) as an antifungal agent (Sigma-Aldrich Co., St. Louis, MO, USA) to culture LAB. An Anderson sampler was used to sample 283 liters of air from each room into MRS-A plates both during and before production (Table 1). MRS was chosen as it is known to be selective for LAB, and the goal of the cultivation-based approach was to identify the specific spoilage organisms to the species level.
(ii) Raw materials and sausage emulsion.
Bacterial levels in raw materials were analyzed quantitatively. Serial 10-fold dilution series were made from 22 g of each material with 198 ml of peptone-salt buffer (0.1% peptone, 0.9% NaCl) and homogenized by using a stomacher at medium power for 1 min (Stomacher 400; Seward, West Sussex, United Kingdom), followed by cultivation onto MRS plates (LAB). In addition, plate count agar (PCA) was used to quantify the total cultivable microbes from the raw materials and cooked sausages.
The PCA plates were incubated at 25°C for 3 to 5 days before colony enumeration. The MRS-A and MRS plates were incubated in jars made anaerobic by a commercial atmosphere generation system (AnaeroGen; Oxoid) at 25°C for 5 days, after which the CFU were calculated, and 5 to 10 colonies were randomly collected for ribotyping-based species identification (5). A total of 222 individual colonies from the MRS-A plates sampled from the processing plant surfaces were selected and purified by subculturing on MRS agar, and 175 (78%) of these were ribotyped. Similarly, 103 LAB colonies from material samples grown on MRS agar were selected for pure cultures, and 74 of these (72%) were identified using ribotyping.
LAB species identification.
LAB species identification was conducted as described by Vihavainen et al. (2). In brief, selected colonies were grown in MRS broth, and DNA was extracted using the guanidium thiocyanate method complemented with mutanolysin and lysozyme (5). The DNA was sheared with HindIII, and the resulting fragments were separated by gel electrophoresis. The DNA fragments were transferred onto a nylon membrane using a vacuum blotting device. The fragments containing 16S or 23S rRNA genes were detected with a digoxigenin-labeled oligonucleotide probe mixture, Oligomix5 (10). The HindIII ribopatterns were compared to the corresponding patterns in the previously established LAB database of the Department of Food and Environmental Hygiene, University of Helsinki, Helsinki, Finland, with patterns from all relevant food-associated LAB in the genera Aerococcus, Carnobacterium, Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, Streptococcus, and Weissella (2). For numerical analyses, the ribopatterns were normalized based on the mobility of standards, and a similarity matrix was created using the BioNumerics (version 5.10) software package (Applied Maths, Sint-Martens-Latem, Belgium). The similarity between all pairs was expressed by Dice coefficient correlation, and clustering using the unweighted-pair group method using arithmetic averages (UPGMA) was used to construct the dendrogram. Based on the use of internal controls in the database, a pattern optimization and a band position tolerance of 0.5 and 1.5, respectively, were allowed. The isolates were identified based on the locations of type and reference strains within the clusters.
DNA-based 16S rRNA gene amplicon analysis.
Samples for DNA extraction were taken from the processing plant using nylon swabs (FLOQSwapos; Copan Flock Technologies, Brescia, Italy) dipped in SDS-NaCl buffer (0.15 M NaCl, 0.1% Tween 20) prior to swabbing a 5- by 5-cm area (11). The swabs were stored in 500 μl of the SDS-NaCl buffer and frozen at −20°C until DNA extraction. The raw material, emulsion, and end-of-shelf-life sausages were diluted into a peptone-salt buffer and mixed in a stomacher for 30 s at a low setting. Both a 10−1 and 1:1 dilution were used in DNA extraction. In order to separate the mammalian and bacterial cells, two-stage centrifugation was used. First, the supernatants from homogenization were centrifuged in 15-ml conical tubes at 200 relative centrifugal force (RCF) for 3 min. The bacterial cells from the supernatant were pelleted by centrifuging 10 ml of the supernatant from the first centrifugation at 10,000 RCF for 3 min.
DNA was extracted from the swabs and from the pelleted bacterial cells using GES (guanidium thiocyanate, 100 mM EDTA, and 0.5% [wt/vol] Sarkosyl) and phenol-chloroform extraction. First, the swabs together with the storage buffer were transferred to a FastPrep lysing matrix E tube (MP Biomedicals, Santa Ana, CA, USA), and 500 μl of denaturing buffer (4 M guanidium thiocyanate, 25 mM sodium citrate, 0.5% sarcosyl, 0.1 M 2-mercaptoethanol) was added, followed by 500 μl of phenol-chloroform-isoamyl alcohol (pH 8.8; Sigma-Aldrich). For the bacterial cell pellets, denaturing buffer and phenol-chloroform-isoamyl alcohol were added to the tubes with the pellets, vortexed, and transferred to the lysing matrix tubes. The lysing matrix tubes were bead beaten for 40 s at 5.5 m/s in a FastPrep-24 instrument (MP Biomedicals), after which the tubes were incubated on ice for 5 min and centrifuged for 10 min at 13,000 RCF (Eppendorf, Hamburg, Germany). Chloroform (500 μl) was added to the upper layer, and the tubes were centrifuged for 10 min after vortexing. The nucleic acids in the upper layer were precipitated with a 1/10 volume of 3 M sodium acetate, 1 μl of GlycoBlue (Invitrogen, Carlsbad, CA, USA), and 3× ethanol. The pellet was washed with 70% ethanol and eluted to 50 μl of sterile nuclease-free water.
To characterize the microbiome, the V1 to V3 area of the 16S rRNA gene was amplified through PCR with primers 8f and 518r (12). The PCR consisted of 1× Phusion GC buffer, 200 μM deoxynucleoside triphosphate (dNTP) mix, 0.2 μM each primer, 2.5% dimethyl sulfoxide (DMSO), and 50 to 250 ng of community DNA. After the PCR mix was heated to 98°C, 1 U of Phusion polymerase (Life Technologies, Carlsbad, CA, USA) was added to the reaction mixture. The PCR program was as follows: denaturation at 98°C for 30 s and 20 cycles of 98°C for 10 s, 65°C for 30 s, and 72°C for 10 s, followed by 72°C for 5 min and cooling to 4°C. The PCR products were purified with 0.9× Ampure beads (Beckman Coulter, Pasadena, CA, USA) and eluted to 40 μl of 0.1× Tris-EDTA (TE) buffer. The bar codes and sequencing adapter were added to the amplified PCR fragments in a second PCR with a 1× Phusion GC buffer, 200 μM dNTP mix, 0.05 μM each primer, 2.5% DMSO, and approximately 50 ng of purified PCR product. Again, the Phusion polymerase (1 U) was added after the reaction mixture was heated to 98°C. The reverse primer had sequencing adapter A attached, and the forward primer included sequencing adapter B with a sample-specific bar code (8 bp). Three replicate PCRs were done per sample, and the products were pooled prior to purification. PCR product purification and sequencing were conducted at the Institute of Biotechnology, University of Helsinki, using the Roche 454 Titanium FLX protocol.
Sequences were analyzed with QIIME (13). First, the sequence reads were filtered for quality, and reads with a length of less than 200 bp, containing ambiguous bases, with a quality score below 30 (q30) or mismatches in the primer sequence were discarded, and the remaining reads were assigned to samples based on the sample specific bar code. OTUs were picked using the uclust (14) algorithm with 97% similarity, and the representative sequence read from each OTU was assigned to taxonomy with BLAST (15) against the Greengenes database (version 13.8.2014) (16). Chloroplast OTUs of plant origin were removed by name (“Streptophyta”) based on Greengenes taxonomic classifications (average 2.3% of sequences in each sample ±7.0% standard deviation [SD]). The OTU sequences were aligned with PyNast (17), and a phylogenetic tree was built from the filtered alignments with FastTree (18). Alpha and beta diversity metrics were calculated from the rarified OTU table. Rarefaction was done to the lowest number of sequence reads used in analysis (sample Fa43 from filling machinery; 2,706 reads), with QIIME and R (19) using the Vegan package, version 1.8-2 (20). The statistical analysis was conducted with STAMP, version 2.00 (21), using two-sided Fisher's exact test with the Storey false discovery rate (FDR) correction.
Microarray data accession number.
The 16S rRNA gene sequences were deposited in the Sequence Read Archive (SRA) of the NCBI under BioProject number PRJNA293141. In order to characterize the clustering of sequences assigned as Yersinia, these sequence reads were aligned with PyNast (17) with selected reference sequences. The subsequent phylogenetic analysis was conducted with FastTree (18).
RESULTS
Results from cultivation and the outcome of DNA extraction of the surface samples from the processing plant are shown in Table 1. Figure 2 and Table S1 in the supplemental material present the results of similar analyses from 94 samples of raw materials and cooked sausages manufactured from them. Thirty, 63, and 9 samples yielded PCR products from the processing plant, raw material, and products, respectively, and these were subsequently sequenced. 16S rRNA analysis produced a total of 766,686 sequence reads. Since sequencing produced a varying number of good-quality reads from each sample (average, 4,819 ± 1,742 reads; in total, 443,335 reads), the data were rarified to 2,707, the lowest number of reads in samples, before diversity analysis.
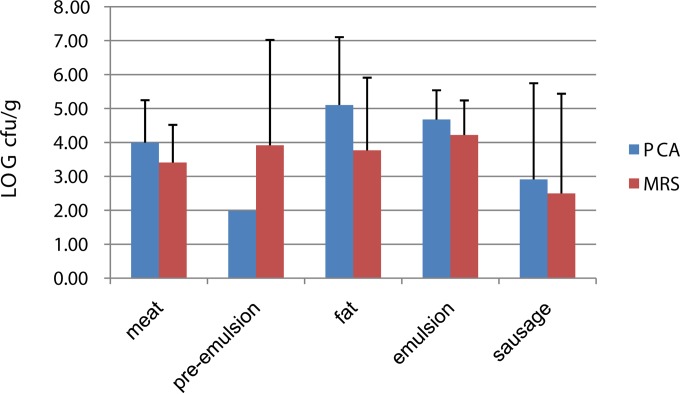
FIG 2.
Bacterial cell numbers (log CFU/g) on PCA and MRS plates in raw materials and cooked products.
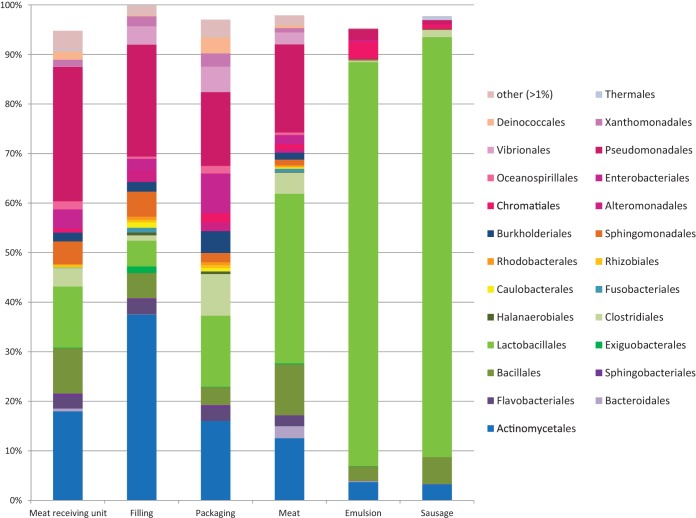
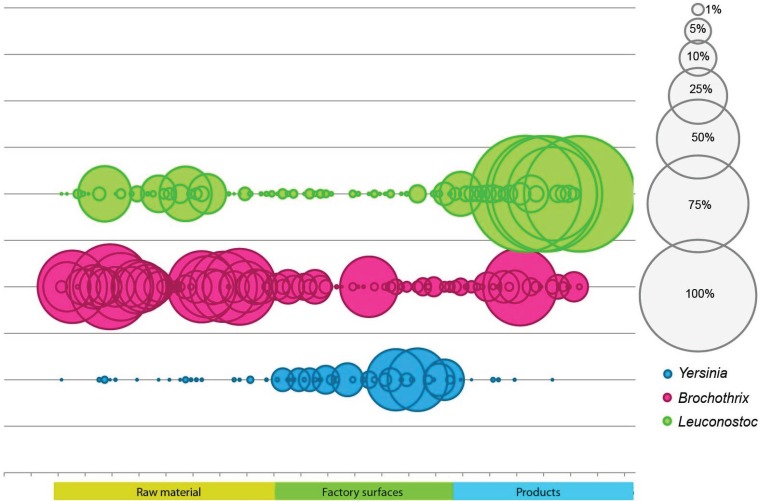
16S rRNA gene sequencing showed the bacterial community in the sausages to be comprised mainly of Firmicutes (Fig. 3) and, in detail, of the genus Leuconostoc (Fig. 4A). The OTUs classified as leuconostocs were detected in low relative abundance (2% ± 5%) in raw meat and emulsion, as well as on the processing plant surfaces (<4% relative abundance) (Fig. 5). In the processing plant, surface samples with Leuconostoc levels of >2% of the sequence reads were obtained from the high-hygiene compartment where the sausages were packaged. Despite the low abundance on the processing plant surfaces and in raw materials, in the cooked sausages the Leuconostoc sequence abundance increased up to >98% (Fig. 5), with an average of 40% relative abundance (Fig. 4A).
FIG 3.
Order-level classification of the sequence reads by main compartment or material. Other, minor orders with abundance of <1%.
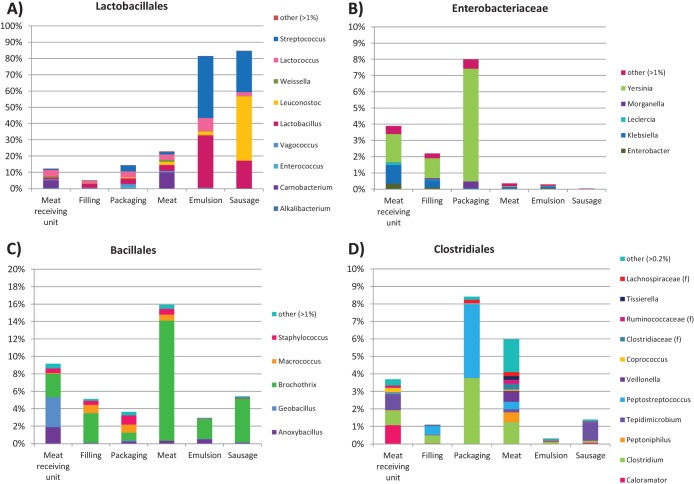
FIG 4.
Relative abundances of sequence reads assigned as indicated. f, family-level classification.
FIG 5.
Relative abundance of Leuconostoc, Yersinia, and Brochothrix in raw materials, on processing plant surfaces, and in cooked sausages. Sphere size corresponds to relative abundance.
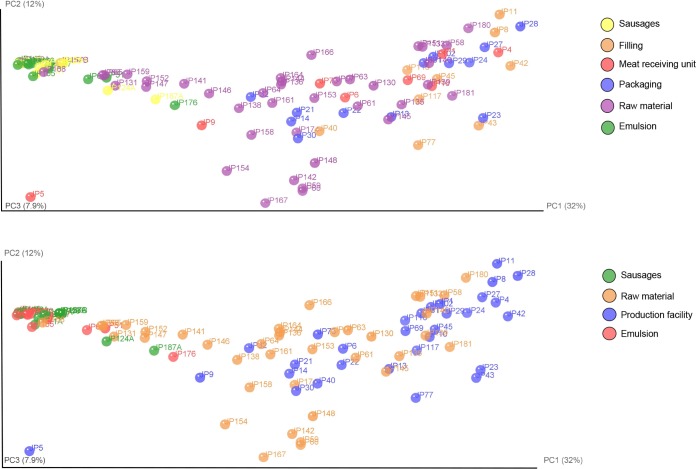
Bacterial communities in the compartments dealing with raw products were mainly composed of bacteria from the orders Pseudomonadales, Actinomycetales, Bacillales, and Lactobacillales (Fig. 3). In the high-hygiene packaging area, the main bacterial orders were Actinomycetales, Pseudomonadales, Lactobacillales, Clostridiales, and Enterobacteriales. Detected microbial diversity was between 53 and 393 OTUs (average, 216 ± 78 OTUs) throughout the processing plant, and there was no substantial difference in diversities between the main compartments (see Table S1 in the supplemental material). Although phylogenetic assignment of OTUs showed that the microbiomes of different processing compartments differed, the processing plant samples did not show site-specific clustering in the UniFrac analysis (Fig. 6). Similar OTUs were found throughout the facility from both standard and high-hygiene compartments although differences in the relative abundances were detected. Based on the UniFrac results, the processing plant samples clustered together with the raw meat samples, whereas the sausage emulsion samples were more similar to the samples from cooked sausages (Fig. 6).
FIG 6.
UniFrac analyses of the microbiomes of raw materials, sausage emulsion, and cooked sausages for facility compartments and material source (top panel) and source alone (bottom panel).
As the UniFrac analysis indicated, the microbial community profile of the raw materials (meat cut selections as well as rind and fat) used for the sausage manufacture was similar to that in the meat receiving unit (Fig. 3). The raw materials contained a significantly (P ≤ 0.001) higher proportion than the meat receiving unit of bacterial sequences from the genera Pseudomonas, Brochothrix, Carnobacterium, Arthrobacter, and Lactobacillus. In the meat receiving unit, Psychrobacter and Rhodococcus were found to be more abundant (P ≤ 0.001). The bacterial communities in the sausage emulsion and sausages at the end of the shelf life were composed mainly of Lactobacillales with lower relative abundance of gammaproteobacterial orders and Actinomycetales (Fig. 3; see also Fig. S1 in the supplemental material). However, at the genus level, the abundance of leuconostocs was found to be significantly (P ≤ 0.001) higher in the sausages than in the sausage emulsion. In sausage emulsion, bacteria from the genera of Streptococcus, Lactococcus, and Lactobacillus were more abundant (Fig. 4A).
OTUs classified as Yersinia were observed in rather high abundance (up to 28%) on the processing plant surfaces but to a lesser extent in the raw meat, sausage emulsion, and cooked sausages (relative abundance of <1%) (Fig. 4B and 5). The largest phylogenetic cluster was similar to that of the pathogenic Yersinia pseudotuberculosis (see Fig. S2 in the supplemental material), with one OTU comprising a total of 3,438 sequences (OTU denovo2230). When the abundance of this OTU on the processing line was studied, the highest relative abundance was found from the packaging area, with relative abundance of over 27% in two samples. The relative abundance in raw meat was low, below 1% (see Table S2 in the supplemental material). The other Enterobacteriaceae found from the sequenced OTUs included representatives from the genera of Klebsiella (0.02% to 1.2%), Enterobacter (0.1% to 0.3%), and Morganella (0.001% to 0.3%), but representatives from the genus Yersinia were by the far most abundant (Fig. 4B).
More detailed analysis of sequences assigned as Bacillales (Fig. 4C) revealed the Listeriaceae to be the main bacterial family. However, the genus Brochothrix was found to be the most abundant genus in the raw meat (Fig. 4C and 5), whereas bacteria from the genus Listeria were rare and detected only in low abundance (<0.01%) in all samples. Thus, the risk of Listeria monocytogenes contamination appeared low. Sequences assigned as Clostridiales were found throughout the processing plant and from the raw material and sausages. However, there were differences among the abundant genera in this order: surface samples harbored Peptostreptococcus, Clostridium, and Tepidimicrobium. The meat receiving unit and raw meat had similar profiles with large diversity (Fig. 4D), and the clostridia found in the cooked sausages consisted mainly of Tepidimicrobium.
Differences were observed in the surface sample microbiotas before and after daily cleaning procedures. The highest Leuconostoc levels detected (up to 3.3%) were in samples taken after daily cleaning before the production started from the high-hygiene packaging compartment. In the samples taken during production, the relative abundance did not reach 1%. Similarly, the highest Yersinia and Clostridia abundances were found in the high-hygiene compartment before production. In the surface samples taken during production, the Proteobacteria levels were the highest (27% ± 26.7%).
On the processing plant surfaces, the highest LAB levels based on the MRS-A swabs (>100 colonies) (Table 1) were in the samples from the meat receiving unit of the plant during and before production at both of the sampling times. The number of colonies in the sausage-filling machinery ranged from 0 to >100 both before and during production. Intriguingly, the high-hygiene packaging compartment had LAB levels ranging from low (>10) to high (>100) both during production and before production was started. The high LAB levels were found during production from the device separating the sausages from a string (sample Fa13), and lower levels were found from the transport belt and from the pit from the transport belt to the packaging tray (Table 1). Before production, the detectable bacterial growth was found in sample Fa28 from the same cutting drum blade.
In the raw materials and cooked sausages, both LAB (MRS) and total microbial (PCA) levels varied among different lots (Fig. 2). In raw meat and in the rind and associated fat, the total microbial levels (PCA) were 4.0 ± 1.3 and 5.1 ± 2 log CFU/g, respectively. In emulsion, the log CFU/g for total microbes was 4.7 ± 0.9, decreasing to 2.9 ± 2.8 in cooked sausages. The LAB log CFU/g levels on MRS plates varied from 3.4 to 3.8 (±1.1 to 3.1) in raw meat, increasing to 4.2 (±1.0) in emulsion. Based on cultivation, the LAB levels in the sausages at the end of shelf life varied from 0.0 to 9.7 log CFU/g (see Table S3 in the supplemental material). In 8 of the 14 batches, the sausages were found to have clear drip around them at the end of shelf life, and in these eight production lots the LAB levels were <10,000 CFU/g, except in two lots, where the levels had reached log 5.4 CFU/g (see Table S4 in the supplemental material). In two of the lots, the drip around the sausages was mildly turbid and contained 0.0 to 8.0 log CFU/g LAB cells. Four of the lots had high (7.4 to 9.7 log CFU/g) LAB levels, and the packages contained gray and turbid drip on the last day of shelf life. Additionally, in one of these the vacuum was loosened.
A total of 175 colonies from processing plant surfaces and 74 from material samples were ribotyped. The majority (41% from processing microbiome and 72% from materials) of these colonies represented species of the genus Leuconostoc (see Table S5 and Fig. S3 in the supplemental material). Additionally, bacterial cells were found in 3 of the 15 air samples (Table 1). The LAB species identified were Leuconostoc mesenteroides, Leuconostoc pseudomesenteroides, Leuconostoc gelidum subsp. gasicomitatum, Lactobacillus curvatus, Lactococcus lactis, and Brochothrix sp. (see Table S5 in the supplemental material).
DISCUSSION
The finding that bacteria from the phylum Firmicutes dominated the cooked sausage microbiome was expected since LAB are common spoilage organisms in MAP meat products (1). However, we did not anticipate that they would dominate the sausage emulsion microbiome as well (Fig. 3) since Firmicutes were not abundant in the raw materials. It is thus likely that the emulsification step with harsh mechanical mixing and the addition of salt and nitrite led to the selection of the Gram-positive bacteria. Exploration of the main genera (Fig. 3 and 4) within the processing plant 16S rRNA gene amplicons showed that all of the abundantly found genera contained mesophilic psychrotrophs. This is understandable as approximately 80% of the earth is cold, and most of the psychrotolerant microbes (optimal temperatures between 20°C and 25°C) on earth live in fluctuating temperatures (22). The surface samples had high levels of Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria. Bacteria from these phyla have been detected also in previous indoor microbiome studies (23–25). Intriguingly, of these phyla, Proteobacteria, Bacteroidetes, and Firmicutes comprise known cold-tolerant food spoilage bacteria (Fig. 3). Furthermore, the orders Pseudomonadales and Actinomycetales have been found in a butchery environment, beef carcass, and aerobically stored beef products (6). These microbes are thus likely to tolerate conditions such as the recurrent cleaning and sanitizing procedures prevailing in the processing plants.
There were dramatic differences between the 16S rRNA microbiomes of raw materials, emulsion, surfaces, and sausages (Fig. 3). Firmicutes were found in high abundance in all raw materials and sausage lots studied (Fig. 3; see also Fig. S1 in the supplemental material). The similarity of the emulsion and cooked sausage microbiomes was visualized by the UniFrac distances (Fig. 6), where, apart from the processing plant samples and most of the raw meat samples, the emulsion and sausage samples clustered together. The few raw meat samples clustering with the emulsion and sausages were from assorted meat chunks and chopped meat. The processing plant surface samples did not cluster based on the sampling location in the present study. Similar results were seen in a cooking center microbiome study (24), where the sampling location did not affect the clustering, and consequently samples from the same location had different microbiomes.
Leuconostoc spoilage is common in cold-stored, modified-atmosphere-packaged (MAP) foods (1, 2, 26), and representatives from this genus have been causing severe food spoilage cases in Finland since 1996 (4, 27, 28). Recently, the same phenomenon has been detected in refrigerated ready-to-eat (RTE) foods, as well as in meat and vegetable products in Central Europe (29), although L. gelidum has not been detected abundantly in raw materials and production facility surfaces (30). Some L. gelidum strains have also been found to adhere well to food contact surfaces (31). In the present study, OTUs from the genus Leuconostoc prevailed in the products at the end of shelf life, as determined by both cultivation-dependent and -independent approaches. Nevertheless, the abundance on the surfaces based on the sequence data was low (<4% relative abundance), and thus the high abundance of leuconostocs in the product at the end of shelf life can be considered to be due to the efficient growth ability this species has on vacuum-packaged sausages (Fig. 2; see also Table S3 in the supplemental material).
These leuconostocs can evidently outcompete the other bacteria present in packaged foods. This is supported by growth ability in cold temperatures and low-oxygen MAP (28) and the ability to adhere to surfaces (31), which may help the bacteria tolerate the washes and disinfectant treatments. Similarly, several contaminating Leuconostoc carnosum strains were recovered from a meat processing environment, but only a single strain was found to prevail in the spoiled ham produced at that plant (28). The predominance of this spoilage strain was concluded to be due to its physiological characteristics improving adaptation and competence in cooked, VP ham. The LAB identified by culturing from the production plant surfaces were considered to be Leuconostoc gelidum subsp. gasicomitatum and subsp. gelidum, Lactobacillus sakei, and Carnobacterium maltaromaticum (see Table S5 and Fig. S3 in the supplemental material), all known as food spoilage bacteria (1). The same species were retrieved using culturing also in both the raw materials and in the cooked sausages, supporting the view that they originated in the raw material (see Table S5).
The bacterial richness in the processing plant varied from 53 to 393 OTUs (see Table S1 in the supplemental material), showing much lower diversity in this controlled environment than in nature, as in temperate and cold soils microbial richness has been found to range from 338 to 725 OTUs (32). Some of the processing plant samples were interesting when the focus was on the estimated microbial diversity. The sample from a cart used for transporting sausages from the filling stage through cooking to the packaging had the highest Chao1 diversity values. This is the only device shared between the regular and high-hygiene compartments of the processing plant and could have acted as a vehicle for contamination. The microbial growth on the MRS-A swabs from this device was not detectable (Table 1), indicating the viable microbiota to be other than LAB, which is supported by the amplicon sequencing (Fig. 3 and 4). Other interesting sites in the packaging area included the conveyor belt and the packaging tray, where the number of observed OTUs was higher, 313 and 301, respectively, than in the packaging area as a whole. The first sample had 1 to 10 colonies in the MRS-A plate, showing viable LAB in the site.
The high abundance of Yersinia bacteria in the processing plant surfaces was unexpected. Yersinia is usually associated with raw pork meat (33), and especially Yersinia enterocolitica and Yersinia pseudotuberculosis have been detected in both pigs and surface samples from pig farms (34). As the OTUs from this genus were detected in only a few of the meat assortments, it can be considered that they had adhered well to the processing plant surfaces and might have formed biofilms. This is supported by the finding of these OTUs both before and after daily cleaning procedures. The fact that Yersinia-like OTUs were found from the packaging compartment raises concerns regarding food safety, but the sausage cooking and storage conditions seemingly prevented the growth of these species since they were not found in the cooked sausages. Yersinia bacteria are known to have from 5 to 6 copies of rRNA operons, and this could bias the results. However, the other main groups have several copies of the ribosomal operons (Brochothrix with 9 and Leuconostoc with 4). Thus, this bias can be considered not to have an impact on the results.
Like the Enterobacteriales order, to which Yersinia belongs, the other main bacterial orders dominating the processing plant surfaces and raw meat were not observed in the sausage emulsion or cooked sausages, based on the 16S amplicons. Also L. monocytogenes, a typical biofilm-forming bacterium in food processing plants with hygiene problems (35), was not among the sequenced OTUs. However, Brochothrix thermosphacta, a bacterium from the same family as Listeria monocytogenes, was detected in the raw meat (Fig. 5) but not in the cooked products on the last day of shelf life. This meat spoilage bacterium is commonly associated with the spoilage of fresh meats (1) and is capable of growing under both aerobic and anaerobic conditions (36). However, under anaerobic conditions, B. thermosphacta has not been observed at the end of the shelf life (37) since during microbial succession the abundance of LAB increases.
To conclude, the microbiota in this food processing plant had a different characteristic microbiome from the one grown in the cooked sausages during product shelf life. The main bacterial phyla colonizing this processing plant, the raw materials, and cooked sausages comprised known food-spoilage-causing psychrotrophs. Despite the different hygiene practices, microbiota in the standard and high-hygiene compartments did not differ drastically (Fig. 3 and 4). Only the genera of Yersinia and Peptostreptococcus were significantly more abundant in the high-hygiene compartments. The microbes on the processing plant surfaces can be occasional contaminants, or, as in the case of Yersinia, they may have become resilient. Nevertheless, the main genera observed with food safety and quality interest had their own characteristic patterns of contamination, suggesting different food safety risk profiles. Yersinia bacteria were detected on the surfaces but not in the cooked sausages, yet in the raw materials, Brochothrix was one of the most abundant genera, whereas Leuconostoc was scarcely present in the surfaces and raw materials. Based on the observed abundance patterns, the leuconostocs do not cause spoilage of the products due to high initial contamination levels since they were not abundant in the raw materials or on surfaces but are evidently able to grow during microbial succession under the packaging and storage conditions used. This shows that there is a need to develop total enumeration-based hygiene monitoring tools toward more specific ones. The abundance patterns revealed here increased our understanding of different contamination patterns of psychrotrophic processing plant microbiota and their implications for food spoilage and safety.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the Academy of Finland (Centre of Excellence Program 2008–2013 in Microbial Food Safety and GROWTHREG, grant number 267623).
We thank Henna Niinivirta and Erja Merivirta for skillful technical assistance. We thank the meat processing company for giving us access to the facility and providing the material samples.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02228-15.
REFERENCES
- 1.Doulgeraki AI, Ercolini D, Villani F, Nychas GJ. 2012. Spoilage microbiota associated to the storage of raw meat in different conditions. Int J Food Microbiol 157:130–141. doi: 10.1016/j.ijfoodmicro.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Vihavainen E, Lundström H-S, Susiluoto T, Koort J, Paulin L, Auvinen P, Björkroth KJ. 2007. Role of broiler carcasses and processing plant air in contamination of modified-atmosphere-packaged broiler products with psychrotrophic lactic acid bacteria. Appl Environ Microbiol 73:1136–1145. doi: 10.1128/AEM.01644-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pothakos V, Snauwaert C, De Vos P, Huys G, Devlieghere F. 2014. Monitoring psychrotrophic lactic acid bacteria contamination in a ready-to-eat vegetable salad production environment. Int J Food Microbiol 185:7–16. doi: 10.1016/j.ijfoodmicro.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Björkroth KJ, Korkeala HJ. 1997. Use of rRNA gene restriction patterns to evaluate lactic acid bacterium contamination of vacuum-packaged sliced cooked whole-meat product in a meat processing plant. Appl Environ Microbiol 63:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Björkroth J, Korkeala H. 1996. rRNA gene restriction patterns as a characterization tool for Lactobacillus sake strains producing ropy slime. Int J Food Microbiol 30:293–302. doi: 10.1016/0168-1605(96)00955-5. [DOI] [PubMed] [Google Scholar]
- 6.De Filippis F, La Storia A, Villani F, Ercolini D. 2013. Exploring the sources of bacterial spoilers in beefsteaks by culture-independent high-throughput sequencing. PLoS One 8:e70222. doi: 10.1371/journal.pone.0070222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tringe SG, Zhang T, Liu X, Yu Y, Lee WH, Yap J, Yao F, Suan ST, Ing SK, Haynes M, Rohwer F, Wei CL, Tan P, Bristow J, Rubin EM, Ruan Y. 2008. The airborne metagenome in an indoor urban environment. PLoS One 3:e1862. doi: 10.1371/journal.pone.0001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lax S, Smith DP, Hampton-Marcell J, Owens SM, Handley KM, Scott NM, Gibbons SM, Larsen P, Shogan BD, Weiss S, Metcalf JL, Ursell LK, Vazquez-Baeza Y, Van Treuren W, Hasan NA, Gibson MK, Colwell R, Dantas G, Knight R, Gilbert JA. 2014. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 345:1048–1052. doi: 10.1126/science.1254529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramos T, Dedesko S, Siegel JA, Gilbert JA, Stephens B. 2015. Spatial and temporal variations in indoor environmental conditions, human occupancy, and operational characteristics in a new hospital building. PLoS One 10:e0118207. doi: 10.1371/journal.pone.0118207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regnault B, Grimont F, Grimont PA. 1997. Universal ribotyping method using a chemically labelled oligonucleotide probe mixture. Res Microbiol 148:649–659. doi: 10.1016/S0923-2508(99)80064-3. [DOI] [PubMed] [Google Scholar]
- 11.Hanski I, von Hertzen L, Fyhrquist N, Koskinen K, Torppa K, Laatikainen T, Karisola P, Auvinen P, Paulin L, Makela MJ, Vartiainen E, Kosunen TU, Alenius H, Haahtela T. 2012. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci U S A 109:8334–8339. doi: 10.1073/pnas.1205624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards U, Rogall T, Blocker H, Emde M, Bottger EC. 1989. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res 17:7843–7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 15.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 16.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. 2010. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price MN, Dehal PS, Arkin AP. 2009. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Development Core Team. 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 20.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H. 2006. Vegan: community ecology package. R package version 1.8-2. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 21.Parks DH, Beiko RG. 2010. Identifying biologically relevant differences between metagenomic communities. Bioinformatics 26:715–721. doi: 10.1093/bioinformatics/btq041. [DOI] [PubMed] [Google Scholar]
- 22.De Maayer P, Anderson D, Cary C, Cowan DA. 2014. Some like it cold: understanding the survival strategies of psychrophiles. EMBO Rep 15:508–517. doi: 10.1002/embr.201338170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flores GE, Bates ST, Caporaso JG, Lauber CL, Leff JW, Knight R, Fierer N. 2013. Diversity, distribution and sources of bacteria in residential kitchens. Environ Microbiol 15:588–596. doi: 10.1111/1462-2920.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stellato G, La Storia A, Cirillo T, Ercolini D. 2015. Bacterial biogeographical patterns in a cooking center for hospital foodservice. Int J Food Microbiol 193:99–108. doi: 10.1016/j.ijfoodmicro.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Rintala H, Pitkäranta M, Toivola M, Paulin L, Nevalainen A. 2008. Diversity and seasonal dynamics of bacterial community in indoor environment. BMC Microbiol 8:56. doi: 10.1186/1471-2180-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pothakos V, Taminiau B, Huys G, Nezer C, Daube G, Devlieghere F. 2014. Psychrotrophic lactic acid bacteria associated with production batch recalls and sporadic cases of early spoilage in Belgium between 2010 and 2014. Int J Food Microbiol 191:157–163. doi: 10.1016/j.ijfoodmicro.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Björkroth KJ, Geisen R, Schillinger U, Weiss N, De Vos P, Holzapfel WH, Korkeala HJ, Vandamme P. 2000. Characterization of Leuconostoc gasicomitatum sp. nov., associated with spoiled raw tomato-marinated broiler meat strips packaged under modified-atmosphere conditions. Appl Environ Microbiol 66:3764–3772. doi: 10.1128/AEM.66.9.3764-3772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Björkroth KJ, Vandamme P, Korkeala HJ. 1998. Identification and characterization of Leuconostoc carnosum, associated with production and spoilage of vacuum-packaged, sliced, cooked ham. Appl Environ Microbiol 64:3313–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pothakos V, Nyambi C, Zhang BY, Papastergiadis A, De Meulenaer B, Devlieghere F. 2014. Spoilage potential of psychrotrophic lactic acid bacteria (LAB) species: Leuconostoc gelidum subsp. gasicomitatum and Lactococcus piscium, on sweet bell pepper (SBP) simulation medium under different gas compositions. Int J Food Microbiol 178:120–129. doi: 10.1016/j.ijfoodmicro.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Pothakos V, Stellato G, Ercolini D, Devlieghere F. 2015. Processing environment and ingredients are both sources of Leuconostoc gelidum, which emerges as major spoiler in ready-to-eat meals. Appl Environ Microbiol 81:3529–3541. doi: 10.1128/AEM.03941-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pothakos V, Aulia YA, Van der Linden I, Uyttendaele M, Devlieghere F. 2015. Exploring the strain-specific attachment of Leuconostoc gelidum subsp. gasicomitatum on food contact surfaces. Int J Food Microbiol 199:41–46. doi: 10.1016/j.ijfoodmicro.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Chu H, Fierer N, Lauber CL, Caporaso JG, Knight R, Grogan P. 2010. Soil bacterial diversity in the Arctic is not fundamentally different from that found in other biomes. Environ Microbiol 12:2998–3006. doi: 10.1111/j.1462-2920.2010.02277.x. [DOI] [PubMed] [Google Scholar]
- 33.Fredriksson-Ahomaa M. 2012. Isolation of enteropathogenic Yersinia from non-human sources. Adv Exp Med Biol 954:97–105. doi: 10.1007/978-1-4614-3561-7_12. [DOI] [PubMed] [Google Scholar]
- 34.Laukkanen-Ninios R, Fredriksson-Ahomaa M. 2012. Epidemiology, virulence genes, and reservoirs of enteropathogenic Yersinia species, p 269–287. In Faruque SM. (ed), Foodborne and waterborne bacterial pathogens. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 35.Lunden JM, Autio TJ, Sjoberg AM, Korkeala HJ. 2003. Persistent and nonpersistent Listeria monocytogenes contamination in meat and poultry processing plants. J Food Prot 66:2062–2069. [DOI] [PubMed] [Google Scholar]
- 36.Ercolini D, Russo F, Torrieri E, Masi P, Villani F. 2006. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl Environ Microbiol 72:4663–4671. doi: 10.1128/AEM.00468-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Y, Gao F, Xu XL, Suc Y, Ye KP, Zhou GH. 2010. Changes in the bacterial communities of vacuum-packaged pork during chilled storage analyzed by PCR-DGGE. Meat Sci 86:889–895. doi: 10.1016/j.meatsci.2010.05.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.