Abstract
Botrytis cinerea is one of the most important pathogens worldwide, causing gray mold on a large variety of crops. Botrytis pseudocinerea has been found previously to occur together with B. cinerea in low abundance in vineyards and strawberry fields. Here, we report B. pseudocinerea to be common and sometimes dominant over B. cinerea on several fruit and vegetable crops in Germany. On apples with calyx end rot and on oilseed rape, it was the major gray mold species. Abundance of B. pseudocinerea was often negatively correlated with fungicide treatments. On cultivated strawberries, it was frequently found in spring but was largely displaced by B. cinerea following fungicide applications. Whereas B. cinerea strains with multiple-fungicide resistance were common in these fields, B. pseudocinerea almost never developed resistance to any fungicide even though resistance mutations occurred at similar frequencies in both species under laboratory conditions. The absence of resistance to quinone outside inhibitors in B. pseudocinerea was correlated with an intron in cytB preventing the major G143A resistance mutation. Our work indicates that B. pseudocinerea has a wide host range similar to that of B. cinerea and that it can become an important gray mold pathogen on cultivated plants.
INTRODUCTION
Botrytis cinerea Pers.:Fr. is one of the most important plant pathogens worldwide, causing gray mold rot on far more than 200 plant species, including cultivated fruits, vegetables, and ornamental flowers. In addition to its wide host range, typical features of B. cinerea are a necrotrophic, macerating mode of infection, abundant production of asexual macroconidia on the surface of colonized host tissue, and formation of melanized sclerotia for long-term survival and sexual reproduction. Control of gray mold often relies on the use of fungicides although the efficacy of such treatments is threatened worldwide by the abundance of fungicide-resistant B. cinerea field strains (1). Based on gene sequence comparisons, the genus Botrytis has been divided into two phylogenetically separate clades (2). Clade 1 includes B. cinerea and B. pseudocinerea as well as the host-specific species B. fabae, B. calthae, and B. sinoviticola; all of these infect only dicot plants. Clade 2 is phylogenetically more diverse and currently comprises 23 host-specific Botrytis species that infect predominantly monocots (2–4).
B. cinerea shows considerable variability in mycelial growth phenotype, conidiation, and sclerotium formation. Numerous studies have also documented a high degree of genetic variability within the species. Based on the analysis of PCR-restricted fragment length polymorphism (RFLP) patterns, fungicide resistance, and the detection of transposable elements, Giraud et al. provided evidence of the existence of genetically distinct groups within B. cinerea (5, 6). In particular, the presence or absence of the long terminal repeat retrotransposon Boty (7) and of the DNA transposon Flipper (8) was used to divide isolates into different transposon types, e.g., transposa (containing both transposons) and vacuma (containing no transposon). The value of this feature for genetic classification turned out to be low, but evidence has been obtained that transposa and vacuma types of B. cinerea show different adaptations to several host plants and seasonal changes in their relative frequencies (9, 10). A subgroup of B. cinerea vacuma strains, called group I, was found to be clearly distinct from transposa and other vacuma strains (group II) on the basis of genetic markers and sequence data (11, 12). These and other data confirmed group I as a separate lineage distinct from that of B. cinerea, and it was defined as a new species called B. pseudocinerea (13). Phenotypic differentiation of B. pseudocinerea from B. cinerea is possible based on its natural resistance to fenhexamid and its hypersensitivity to several other fungicides (11, 13). Remarkably, the phylogenetic distance between B. cinerea and B. pseudocinerea is larger than that between B. cinerea and the host-specific species B. fabae (2, 3).
Apart from the separation of B. pseudocinerea, the identification of distinct lineages of B. cinerea and correlations between biological traits and genetic patterns has been difficult. Population genetic studies have provided evidence of the existence of genetic clusters within B. cinerea populations from different host plants with a reduced but still existing genetic exchange (14, 15). In German strawberry fields, a variant of B. cinerea, called Botrytis group S (subsequently referred to as B. cinerea S), showed significant divergence from previously described B. cinerea strains (in the following called B. cinerea N, for normal genotype) in several genes (16). In addition, strains of B. cinerea S showed a higher frequency of multiple-fungicide resistance than B. cinerea N in German soft fruit fields (16).
B. pseudocinerea has been discovered in vineyards as a minor gray mold pathogen species that occurs in sympatry with the dominant B. cinerea (12). It was more frequently isolated from flowers in spring and early summer and only rarely from ripe berries (13, 17). B. pseudocinerea has also been observed on several other plant species, indicating that it has a rather wide host range (12, 18, 19). The goal of this study was to compare the frequencies of occurrence of B. pseudocinerea and B. cinerea in order to estimate their contributions to gray mold disease on several cultivated plants. Furthermore, available genetic and phenotypic markers were optimized and evaluated for their ability to differentiate B. pseudocinerea from B. cinerea and other Botrytis species. B. pseudocinerea was found to be a major cause of gray mold in some crop species although it often appeared to be replaced by B. cinerea after fungicide treatments. In striking contrast to B. cinerea, B. pseudocinerea strains were sensitive to fungicides even if the strains were isolated from plants subjected to repeated fungicide treatments. A partial explanation of this is the presence of an intron in the cytB gene which prevents the major mutation leading to quinone outside inhibitor (QoI) resistance.
MATERIALS AND METHODS
Examination of Botrytis strains for fungicide resistance.
Field strains were isolated and cultivated as described previously (16). Fungicide sensitivity or resistance of gray mold isolates was determined for seven major classes of site-specific fungicides against B. cinerea, represented by fenhexamid, boscalid, cyprodinil, fludioxonil, azoxystrobin (QoI), iprodione, and carbendazim. Currently, the most commonly used fungicides against gray mold in Europe are Switch (a mixture of fludioxonil and cyprodinil), Teldor (fenhexamid), and Signum (a mixture of boscalid and the QoI fungicide pyraclostrobin). Iprodione was last registered in fruit production in 2007 but continues to be used in vegetable production, and carbendazim or other β-tubulin inhibitors have not been used in German horticulture since about 1975 (strawberries) or 2007 (pome fruits). Fungicide resistance tests were performed on agar plates containing discriminatory fungicide concentrations (liter−1) of 10 mg of fenhexamid, 0.2 mg of fludioxonil, 16 mg of cyprodinil, 5 mg of carbendazim, 25 mg of iprodione, 3 mg of boscalid, or 25 of mg azoxystrobin (19). For testing the HydR1 phenotype of B. pseudocinerea, 5-mm discs were cut from agar plates containing Gamborg B5 (GB5; Sigma-Aldrich) medium and inoculated with 10 μl of a suspension of 105 conidia liter−1. After 16 h of incubation, the discs with the germinated conidia were inverted and placed onto 0.5% sucrose agar plates containing 5 mg of fenhexamid liter−1. Mycelium growth was evaluated 3 days after incubation. For quantitative determination of fenpropidin sensitivity, conidia were inoculated in 100 μl of 0.5% sucrose at a concentration of 105 conidia ml−1 in microwell plates. After 2 days of incubation, mycelial growth was evaluated. To distinguish between B. pseudocinerea and B. cinerea by their fenpropidin sensitivity, 105 conidia ml−1 (in water) was applied in 5-μl droplets onto 0.5% sucrose agar plates containing 1 mg fenpropidin liter−1. Colony growth was evaluated after 3 days.
Generation of fungicide-resistant Botrytis mutants.
To obtain boscalid-resistant mutants of B. cinerea B05.10 and B. pseudocinerea VD110 (13) and carbendazim-resistant mutants of strain VD110, a UV treatment was performed prior to selection. For this purpose, 107 conidia from 12-day-old sporulating cultures were spread on yeast, Bacto peptone, and acetate (YBA) plates containing 3 mg of boscalid liter−1 or on HA plates containing 3 mg carbendazim liter−1 (16). UV irradiation was performed in a Gene Linker UV chamber (Bio-Rad Laboratories, Hercules, CA) at 120 mJ cm−2 for 18 s, which resulted in 70 to 80% lethality (nongerminating conidia on nonselective HA medium). Following incubation for 14 days at 20°C, resistant colonies were purified for further analysis. Screening for the resistance mutation was performed by a combination of primer-introduced restriction analysis PCR (PIRA-PCR) and sequencing.
PCR-based differentiation of Botrytis species and B. cinerea groups.
For differentiation between B. cinerea N, B. cinerea S, and B. pseudocinerea isolates, total DNA was used as the template for PCR with primer pairs BcinN-in-F/BcinN-in-R detecting an 18-bp indel, Mrr1-spez-F/Mrr1-spez-R detecting a 21-bp indel, and g2944_137_F/g2944_273_R detecting a B. pseudocinerea-specific 24-bp deletion (see Fig. S1A in the supplemental material). For identification of B. paeoniae isolates, DNA was amplified with primers HSP60_fw/HSP60_rev, followed by digestion with either BglII (restriction site present only in B. paeoniae and all of 19 other Botrytis clade 2 species tested) or AccI (restriction site present in all Botrytis clade 1 species including B. cinerea and B. pseudocinerea) (see Fig. S1B). For identification of B. fabae, DNA was amplified with primers C729_for/C729_rev, detecting a B. fabae-specific 122-bp deletion (20) (see Fig. S1C). For differentiation between B. pseudocinerea groups A and B, DNA was amplified with primers ms547_685_R/ms547_201_F, followed by digestion with EarI (see Fig. S1D). All primers used in this study are listed in Table S3 in the supplemental material.
Phylogenetic analysis and statistics.
For phylogenetic analysis, the tree-building algorithm for neighbor joining provided by the SeaView software was applied, using Kimura's two-parameter model for distance calculation (21). Differences in relative frequencies of B. pseudocinerea organisms in Botrytis field populations were tested for significance with Fisher's exact test. For analysis of accumulation of fungicide resistances in B. cinerea strains by fungicide treatments, a two-sided t test was used.
Nucleotide sequence accession numbers.
The sequences of the following genes used for phylogenetic studies have been deposited in GenBank (accession number): for B. pseudocinerea strain VD110 (13), tubA (KR030048), g3pdh (KR030052), ms547 (KR030050), and rps3 (KR054110); for B. pseudocinerea group B strain D11_T_B41, tubA (KR030047), g3pdh (KR030051), and ms547 (KR030049); for B. cinerea S strain G09_S33, tubA (KT335260) and g3pdh (KT335261); for B. fabae strain 11002 (16), tubA (KR054109) and g3pdh (KR030053); and for B. calthae strain MUCL2830 (16), tubA (KR054108). Sequences of additional B. pseudocinerea strains were obtained from Johnston et al. (17).
RESULTS
Differentiation of B. pseudocinerea and two B. cinerea genotypes.
Botrytis isolates were recovered from gray mold-infected tissues of nine crop plant species. To distinguish between the two B. cinerea genotypes N and S and B. pseudocinerea, the sizes of PCR fragments generated with two primer pairs flanking 18-bp and 21-bp insertion-deletions (indels) in mrr1 were determined (16) (see Fig. S1 in the supplemental material). A few B. cinerea strains yielded fragments with the same size as those of B. pseudocinerea strains; these were found to be genetically distinct from B. cinerea N and B. cinerea S and are provisionally referred to as B. cinerea X, based on sequencing data (C. Plesken, S. Rupp, and M. Hahn, unpublished data). A method previously described for the identification of B. pseudocinerea by PCR-RFLP of a sequence in B. cinerea hch (for het-c homolog) (11) was unable to differentiate B. pseudocinerea from B. calthae (19). For unambiguous and facilitated differentiation, a PCR was developed that detected a B. pseudocinerea-specific 24-bp deletion in the homologue of the B. cinerea gene BC1G_07159, discovered by genome sequencing (Plesken et al., unpublished data) (Table 1). All of 54 analyzed B. pseudocinerea isolates yielded consistent results with both PCR methods, whereas none of >500 B. cinerea strains examined (including genotypes N, S, and X) had the 24-bp deletion (see Fig. S1A in the supplemental material).
TABLE 1.
Frequencies of UV-induced fungicide-resistant mutants of B. pseudocinerea and B. cinerea
| Strain | Fungicide used for selection | Expt no. | Mutation rate (no. of colonies) | Resistance mutation profile |
||
|---|---|---|---|---|---|---|
| No. of mutants tested | Identified mutation in target gene (no. of mutants)a | No. of unknown mutationsb | ||||
| B. pseudocinerea VD110 | Boscalid | 1 | 5.8 × 10−5 (58) | 13 | P225L (12) | 1 |
| 2 | 5.5 × 10−5 (55) | 10 | P225L (10) | |||
| Carbendazim | 1 | 2.7 × 10−5 (27) | 5 | F200Y (5) | ||
| 2 | 2.6 × 10−5 (26) | 5 | F200Y (5) | |||
| B. cinerea B05.10 | Boscalid | 1 | 1.6 × 10−5 (16) | 3 | P225L (3) | |
| 2 | 4.8 × 10−5 (48) | 10 | P225L (8) | 2 | ||
Target genes were tubA for carbendazim resistance and sdhB for boscalid resistance.
These mutations occurred outside sdhB.
B. pseudocinerea is common and sometimes dominant over B. cinerea on several crop plants.
In this study, the occurrence of B. pseudocinerea and B. cinerea and their fungicide resistance patterns were investigated on nine crop plant species, including five fruits, two vegetables, one ornamental flower, and oilseed rape. The results are summarized in Fig. 1 and Table S1 in the supplemental material. Almost all B. pseudocinerea isolates analyzed in this study were sensitive to all fungicides tested, except for a low-level natural resistance to fenhexamid (HydR1 phenotype [see below]).
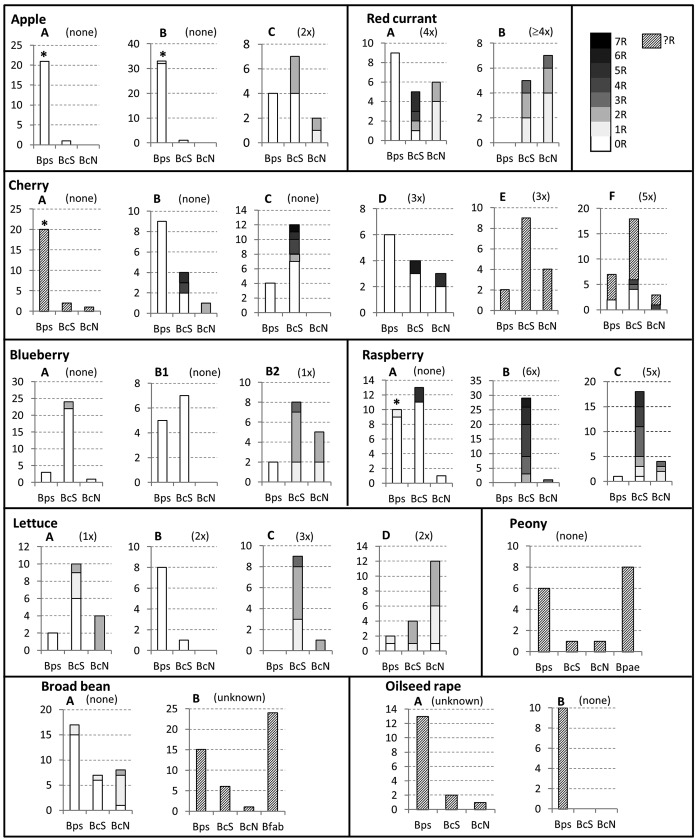
FIG 1.
Genetic diversity and fungicide resistance frequencies of Botrytis species and B. cinerea genotypes isolated from fields or orchards of different host plants. Each diagram shows the isolates from one field or orchard (A, B, C, etc.). Frequencies of resistance (0R, 1R, 2R, etc.) against up to seven fungicides are indicated by different shadings of gray according to the legend on the figure. Detailed information of the strains is provided in Table S1 in the supplemental material. Bps, B. pseudocinerea; BcS, B. cinerea S; BcN, B. cinerea N; Bpae, B. paeoniae; Bfab, B. fabae. R, fungicide resistance not determined. Significantly higher frequencies of B. pseudocinerea among Botrytis isolates from untreated versus fungicide-treated fields are indicated by asterisks (Fisher's exact test, P = 0.01). The “×” values above the panels indicate the frequencies of fungicide sprayings in each of the fields.
On apples showing symptoms of calyx end rot, the large majority of isolates from two organically managed orchards (A and B) belonged to B. pseudocinerea. In orchard C, which had received two QoI fungicide treatments, the relative frequency of B. pseudocinerea was significantly lower, and several of the B. cinerea isolates were resistant to QoI (and boscalid). Isolates from sweet cherries were obtained from three untreated and three fungicide-treated orchards (see Table S1 in the supplemental material). The share of B. pseudocinerea isolates among all gray mold isolates appeared to be higher in the untreated than in the fungicide-treated cherry orchards, but this was significant only for orchard A (Fisher's exact test, P < 0.01). In all cherry orchards, B. cinerea S was prevalent over B. cinerea N. Isolates from highbush blueberry fields yielded B. pseudocinerea in lower numbers than B. cinerea. Field B was untreated in 2012 (field B1, Grethem) but sprayed with QoI plus boscalid in 2014 (field B2, Grethem) (see Table S1 in the supplemental material), which strikingly correlated with an enrichment of B. cinerea strains with resistance to these fungicides. On intensively treated red currants, B. pseudocinerea was detected in only one of two fields examined. Isolates from raspberries were examined in three fields. Two fields (A and B) were adjacent to each other, separated only by a hedgerow (<10 m). Field A had been organically cultivated for at least 3 years prior to sampling, whereas field B, as well as field C, had been subjected to intensive fungicide treatments during the same period. Whereas most of the strains from untreated field A were fungicide sensitive and nearly half of them belonged to B. pseudocinerea, strains from the treated fields B and C were predominantly multiresistant B. cinerea S strains. Two 5-fold-resistant isolates from field A were considered immigrants from the adjacent fungicide-treated plot. In four lettuce fields treated with iprodione alone or iprodione plus QoI, variable frequencies of B. pseudocinerea and the two B. cinerea genotypes were identified.
Botrytis blight, a common disease of garden peony (Paeonia spp.), is known to be caused by the host-specific species B. paeoniae and by B. cinerea. One of the isolates (D12-pae14) was confirmed as B. paeoniae by analysis of the sequence of g3pdh, which was identical to that of B. paeoniae strain 0003 (2) (GenBank accession number AJ705027). For the identification of other B. paeoniae isolates, a PCR-RFLP was developed, based on a BglII restriction site present in hsp60 of B. paeoniae and all other sequenced species of Botrytis clade 2, but not in the clade 1 species B. cinerea and B. pseudocinerea, and on an AccI site present in hsp60 of B. cinerea and B. pseudocinerea but not B. paeoniae (see Fig. S1B in the supplemental material). In a peony field cultured without fungicide treatments, isolates from rotten stem bases were identified as eight B. paeoniae, six B. pseudocinerea, and two B. cinerea strains. Chocolate spots is a common disease on broad beans, reported to be caused by the host-specific species B. fabae or B. fabiopsis and by B. cinerea (22). For identification of B. fabae, a PCR that detects a B. fabae-specific 122-bp deletion in B. cinerea BC1G_06014 was used (20) (see Fig. S1C in the supplemental material). B. pseudocinerea comprised half of all fungal isolates in field A but was detected only once in field B. In contrast, B. fabae was dominant in field B but not found among 34 isolates in the other field.
Botrytis isolates from oilseed rape (Brassica napus) were recovered from a field (field A, Lauterecken) (see Table S1 in the supplemental material) in western Germany. An additional 16 strains isolated between 1986 and 2012 in 12 fields in northern Germany (B, diverse sites) (see Table S1) were provided by colleagues. Except for three B. cinerea strains, all other strains were identified as B. pseudocinerea, indicating that it is the dominant gray mold species on B. napus in Germany.
Seasonal changes in the abundance of B. cinerea and B. pseudocinerea in strawberry fields.
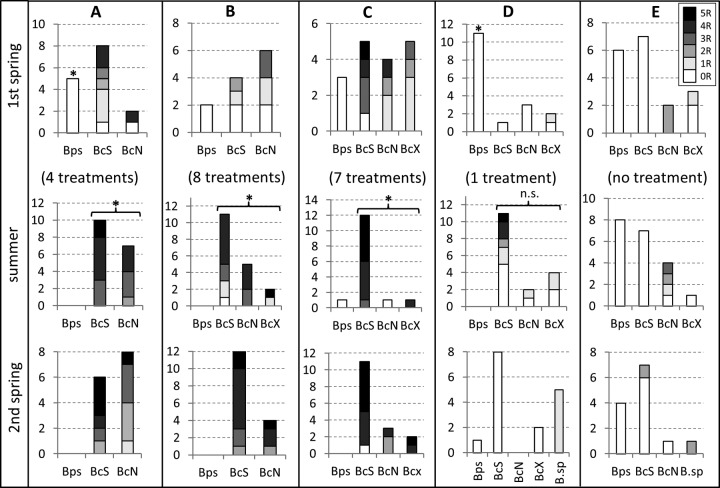
In order to characterize the dynamics of gray mold populations in response to different fungicide treatments, commercial strawberry fields planted in 2011 (four fields) and 2012 (one field) were sampled in spring and summer of the following year and again in spring of the third year (i.e., 2012 to 2013 and 2013 to 2014, respectively) (Fig. 2). Fields A to C were heavily sprayed with fungicides during flowering, field D was sprayed only once, and field E was untreated in 2013. In the first spring, preceding the fungicide treatments, B. pseudocinerea was found together with B. cinerea in all fields on rotten leaves, stems, and a few fruit mummies. In the following summer, only B. cinerea (except one B. pseudocinerea isolate in field C) could be isolated from infected fruits on the fungicide-treated fields, and fungicide resistance frequencies in the B. cinerea isolates had significantly increased. In the second spring, B. pseudocinerea remained absent or rare in fungicide-treated fields. In contrast, B. pseudocinerea was found at similar frequencies in all samplings of the untreated field D. Genetic characterization of the strawberry gray mold population revealed a frequent occurrence of B. cinerea genotype X, in addition to B. cinerea N and B. cinerea S. Furthermore, a few isolates from two fields were found to be genetically different from any of the described Botrytis species; these are under investigation (Plesken et al., unpublished data).
FIG 2.
Seasonal variations of B. pseudocinerea (Bps), B. cinerea S (BcS), B. cinerea N (BcN), B. cinerea X (BcX), and Botrytis spp. (B.sp.) and fungicide resistance frequencies in five commercial strawberry fields. Fungal isolates were collected in spring 2012, summer 2012, and spring 2013 (fields A to D) and in spring 2013, summer 2013, and spring 2014 (field E). Frequencies of resistance against up to five fungicides (excluding iprodione and carbendazim) are indicated by increasingly dark shading, similar to the scheme used in Fig. 1, according to the legend on the figure. Significantly higher frequencies of B. pseudocinerea among Botrytis isolates in the first spring compared to those of the summer after fungicide treatments (Fisher's exact test, P = 0.01) and significant increases in the accumulation of fungicide resistances in B. cinerea isolates after the treatments (t test, P = 0.01) are indicated by asterisks. ns, not significant. If B. cinerea X (BcX) and Botrytis spp. were not found, they are not shown in the diagrams of the respective fields.
Differentiation of B. pseudocinerea and B. cinerea by their natural fungicide sensitivities.
To confirm HydR1-type fenhexamid resistance as a phenotypic marker for B. pseudocinerea, strains were tested for mycelium growth on 0.5% sucrose agar plates with 5 mg of fenhexamid ml−1. All of 61 tested B. pseudocinerea strains, except for one strain belonging to B. pseudocinerea group B (see below), formed colonies on this medium, whereas no growth was observed for 23 fenhexamid-sensitive B. cinerea strains (see Fig. S2 in the supplemental material). In a test for hypersensitivity to fenpropidin as another phenotypic marker of B. pseudocinerea (23), an analysis of eight B. pseudocinerea and five B. cinerea strains confirmed a >20-fold difference in sensitivity between the two species (see Table S2). On 0.5% sucrose agar containing 1 mg of fenpropidin ml−1, conidia of 21 additional B. cinerea isolates formed a mycelium after 3 days, whereas growth of 29 of 30 B. pseudocinerea isolates was strongly suppressed (see Fig. S2). The only exception, again, was B. pseudocinerea group B strain D11_T_B41, which showed weak but significant growth on 1 mg of fenpropidin ml−1 (see below).
Genetic differentiation of B. pseudocinerea.
Twenty-three B. pseudocinerea strains from two fields were examined for their two mating type loci, revealing five strains each of MAT1-1 and MAT1-2 from a broad bean field and eight strains of MAT1-1 and five strains of MAT1-2 from an apple orchard (see Table S1 in the supplemental material). These findings are evidence for natural occurrence of sexual recombination in B. pseudocinerea. An analysis of 34 B. pseudocinerea strains from different fields revealed the presence of Boty in 23 strains and of Flipper in 11 strains; of these, 9 strains harbored both transposable elements (see Fig. S3 in the supplemental material). Gene sequencing of B. pseudocinerea strains from vineyards in New Zealand has revealed the existence of two genetic groups, only one of which has previously been described in Europe (17). Based on published sequences of ms547, a PCR-RFLP screen was developed for differentiation of the two groups (see Fig. S1D). From a total of 185 strains tested, two were assigned to the second B. pseudocinerea group, referred to as group B (see Table S1). For strain D11_T_B41, sequencing of tubA, gapdh, and ms547 clearly confirmed its membership in group B (Fig. 3).
FIG 3.
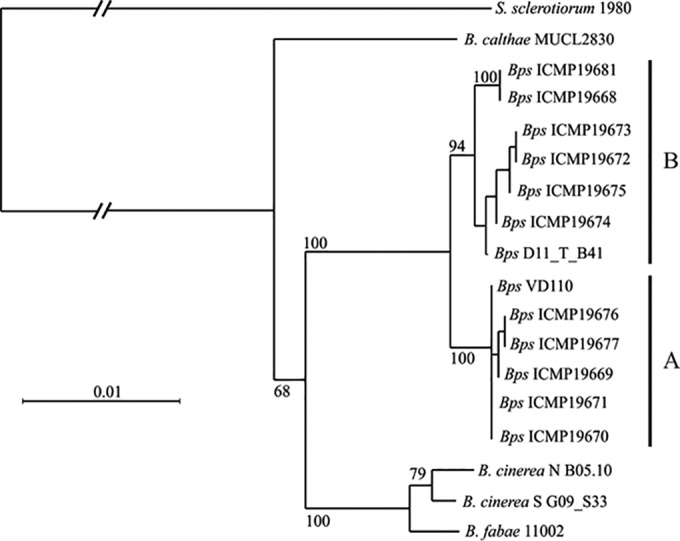
Neighbor-joining tree of B. pseudocinerea group A and group B strains and other Botrytis clade 1 species (B. calthae used as an outgroup), based on the combined sequences of g3pdh, tubA, and ms547, with 1,000 bootstrap replicates. Bootstrap values of >70 are shown. Bps, B. pseudocinerea; S. sclerotiorum, Sclerotinia sclerotiorum.
B. pseudocinerea strains contain an intron in cytB that prevents the G143A mutation.
Since QoI resistance was not observed in any of our B. pseudocinerea isolates, 128 strains of this species were tested by PCR for the presence of the 1,204-bp intron in cytB, which has been shown to prevent the most effective QoI resistance mutation, G143A (24–26). All B. pseudocinerea strains except four contained the intron. To test the hypothesis that the strains lacking the intron might have acquired their mitochondrial DNA from B. cinerea, the 1,782-bp coding region of the gene encoding mitochondrial ribosomal protein S3 (rps3) was analyzed. The rps3 sequences were found to be identical in B. cinerea and B. pseudocinerea strain VD110, except for a 15-bp insertion-deletion (indel). Screening of more strains by PCR, however, revealed that the indel could not distinguish the mitochondrial DNA of these two species (see Fig. S4 in the supplemental material).
In vitro selection of fungicide resistance in B. cinerea and B. pseudocinerea.
Among 165 B. pseudocinerea strains screened for fungicide resistance, all were sensitive to the seven major site-specific fungicides used against Botrytis, except for four strains with resistance to carbendazim. We therefore tested whether B. pseudocinerea has a lower mutation rate of fungicide resistance than B. cinerea under experimental conditions. UV mutagenesis was performed with spores of a B. cinerea strain and a B. pseudocinerea strain, and the frequencies of resistant mutants were determined. Similar rates of resistance mutations against the succinate dehydrogenase inhibitor boscalid were observed for both strains and against the β-tubulin inhibitor carbendazim for B. pseudocinerea, with B. cinerea B05.10 being resistant to carbendazim (Table 1). We analyzed the resistant strains by a combination of primer-induced restriction analysis (PIRA)-PCR for the most common mutations in the target genes and sequencing of these genes, namely, tubA (encoding β-tubulin) for carbendazim and sdhB (encoding succinate dehydrogenase subunit B) for boscalid. Boscalid-resistant mutants of both species carried the P225L mutation (CCC → CTC) in 33 cases and unknown mutations outside sdhB in only 3 cases. All of 10 B. pseudocinerea mutants resistant to carbendazim carried the F200Y (TTC → TAC) mutation. In contrast, three carbendazim-resistant B. pseudocinerea field isolates had the more common E198A mutation.
DISCUSSION
We have developed a PCR based on a B. pseudocinerea-specific 24-bp deletion for rapid identification of this species and its differentiation from B. cinerea, which is easier to perform than the previously published PCR-RFLP with hch (11). B. pseudocinerea had been found in previous studies as a minor species together with the dominant B. cinerea in vineyards and strawberry fields and in low numbers on clover, pea, dog rose, blackberry, blueberry, tomato, oilseed rape, and Caltha palustris (12, 13, 16–19, 27, 28). Our study provides clear evidence that B. pseudocinerea can be a significant gray mold pathogen, being observed in major proportions relative to B. cinerea on six fruit, two vegetable, and one ornamental flower plant species as well as on oilseed rape. On several of these plants, B. pseudocinerea has not been reported before. On apples, gray mold lesions appeared either as calyx end rot during the season, which is initiated by Botrytis infections of flower organs (29, 30), or as postharvest decay. The observed dominance of B. pseudocinerea over B. cinerea on apple was confirmed by a phenotypic (but not molecular) analysis of 62 isolates from six organically managed orchards and 167 isolates from 14 fungicide-treated orchards (2011-2014 harvests), revealing that 79% and 51%, respectively, of these isolates showed the characteristic combination of an HydR1-type response to fenhexamid coupled with the absence of resistance to other fungicides (R. W. S. Weber, unpublished data). These data suggest that B. pseudocinerea is the dominant blossom end rot pathogen of apple in northern Germany. In contrast, Greek Botrytis isolates from apples with storage rot belonged exclusively to B. cinerea (10). On raspberries, B. pseudocinerea was found at high frequencies only in an organic orchard (field A) but appeared to be largely absent from two intensively treated fields. This pattern was supported by a phenotypic analysis of 123 Botrytis isolates from seven additional intensively treated raspberry fields in 2012 to 2014. The large majority of these isolates were resistant against at least one fungicide, but only four fulfilled the phenotypic criteria for B. pseudocinerea (Weber, unpublished data).
Chocolate spot disease has been reported to be caused by the host-specific species B. fabae and B. fabiopsis and by the generalist B. cinerea (22, 31). Our study shows that B. pseudocinerea can also be a major pathogen of Vicia faba. Similarly, on rotting stem bases of peony, the host-specific B. paeoniae was found together with B. pseudocinerea and B. cinerea. These observations are in line with a recent report that stem and leaf infections on C. palustris are caused not only by the host-specific B. calthae but also by the generalists B. cinerea and B. pseudocinerea (19). A similar cooccurrence of different Botrytis spp. has been described for onion fields in China, which were found to be infected by B. porri, B. sinoallii, B. squamosa, B. byssoidea, and B. cinerea (32). On oilseed rape, gray mold can be a major disease under wet and cool weather conditions, for example, in winter rape. Our data, based on samples taken from many sites in Germany, indicate that B. pseudocinerea rather than B. cinerea is the dominant gray mold pathogen of oilseed rape. This is in line with a study by Fekete et al. (18), who found that all eight isolates in a field in Hungary belonged to B. pseudocinerea.
Together with previous studies, our work demonstrates that B. pseudocinerea grows on at least a dozen host species belonging to six plant families and has a wide host range similar to that of B. cinerea but in contrast to that of all other described Botrytis species. Since our analysis was focused on northern Germany, the occurrence of B. pseudocinerea in other geographical locations remains to be characterized. Findings of B. pseudocinerea in Hungary (18), Greece (G. Karaoglanidis, personal communication), New Zealand (17), California (27), and China (28) confirm that the species occurs worldwide.
Seasonal variations in the occurrence of the two gray mold species were investigated in strawberry fields in response to fungicide treatments. While B. pseudocinerea was observed on overwintering plant parts in the first spring together with the dominating B. cinerea, it all but disappeared from fields after the first use of fungicides at flowering but continued to be present throughout the samplings in an organically managed strawberry field. Due to small sample sizes, statistical significance of the apparent suppression of B. pseudocinerea by fungicides was demonstrated in only two of four fields. In French and New Zealand vineyards, B. pseudocinerea has been found more frequently on vegetative tissues and flowers than on mature grapes. This was attributed to a higher saprotrophic competitiveness and preferred colonization of flowers but lower virulence on the berries than that of B. cinerea (12, 13, 17). Our data only partly support these conclusions. An alternative explanation is that the preferential occurrence of B. pseudocinerea in spring and its disappearance in summer may be caused by fungicide treatments. In the vineyard studies, no spray schedules were provided, and therefore a similar suppressive effect of fungicide treatments cannot be discounted. The abundance of B. pseudocinerea in natural habitats not subjected to fungicides may thus be higher than that observed in cultivated plants. Among the various factors that govern the occurrence of the two Botrytis species, host plant genotypes may also contribute. However, we have no data supporting any differential susceptibility of different strawberry cultivars to either species.
Considering the accumulation of multiple fungicide resistance mutations in B. cinerea strains, the virtual absence of resistance, including multidrug resistance (MDR)-based partial resistance, in B. pseudocinerea even in strongly fungicide-treated fields was surprising. A partial explanation was provided by the observation that all but four of 128 B. pseudocinerea strains tested had an intron in cytB preventing the G143A resistance mutation against QoI fungicides. This confirms data from Leroux et al. (33) but is in striking contrast to the high frequency of QoI-resistant B. cinerea strains worldwide (16, 26, 34). Because QoI fungicides are applied at least once per season in most German horticultural crops under integrated pest management, their use could selectively suppress B. pseudocinerea. Further analysis of a B. pseudocinerea strain lacking the intron revealed no clear evidence of whether it might have obtained its mitochondrial DNA from B. cinerea. Sequencing and PCR analysis of cytB (data not shown) and of the mitochondrial rps3 coding region revealed high sequence similarities, which did not allow us to distinguish between the mitochondrial genomes of B. cinerea and B. pseudocinerea.
The lack of resistance in B. pseudocinerea to non-QoI fungicides remains unexplained. Attempts to induce resistance mutations by UV mutagenesis resulted in similar mutation rates for B. pseudocinerea and B. cinerea. However, most of the boscalid-resistant mutants had a base change (CCC → CTC) which resulted in a P225L substitution in sdhB. This substitution has not been found in boscalid-resistant field strains of B. cinerea, which contained H272R, H272Y, H272L, N230I, and P225F substitutions instead (35, 36; S. Rupp, unpublished data). Induced selection of carbendazim resistance in B. pseudocinerea resulted in the preferential occurrence of the F200Y mutation in β-tubulin, which is less common than the E198A mutation in field strains of B. cinerea (37). UV-induced mutations can be different from spontaneous mutations occurring in the field (38). Therefore, the biological relevance of mutagenesis data for interpreting the situation in the field is questionable. Similar to B. cinerea, B. pseudocinerea shows abundant conidiation and efficient aerial spore dissemination, a short reproduction cycle, and the ability to infect different developmental stages of a variety of host plants. It therefore remains puzzling why B. pseudocinerea, in contrast to the high-risk pathogen B. cinerea, behaves as a low-risk organism with respect to the development of fungicide resistance in the field.
In a previous study (16) we discovered two distinguishable genetic groups, referred to as B. cinerea N and B. cinerea S (Botrytis group S). In Germany, B. cinerea S strains were almost absent from vineyards but often dominated the B. cinerea populations in strawberry fields, where they showed higher frequencies of fungicide resistance as well as a stronger variant of the efflux-based MDR1 resistance phenotype (16). In contrast, B. cinerea N was found to be prevalent and more resistant than the S genotype in Greek strawberry fields (39). In this study, B. cinerea S was generally prevalent over B. cinerea N on fruits, except for red currants. No clear dominance of either group was evident on the nonfruit plants, often because of the low overall numbers of B. cinerea isolates that could be obtained.
B. pseudocinerea and B. cinerea are indistinguishable in various growth parameters, except for differences in their natural fungicide sensitivities (13, 18). The HydR1 phenotype of B. pseudocinerea (23) has been correlated with a reduced affinity of fenhexamid to its target enzyme, 3-ketoreductase, and with its ability to detoxify fenhexamid by an inducible cytochrome P450 monooxygenase (40; S. Fillinger, personal communication). HydR1 was confirmed for 61 tested B. pseudocinerea isolates, except for a single fenhexamid-sensitive group B strain. In Hungary, four B. pseudocinerea isolates have been reported to be fenhexamid sensitive, confirming that HydR1 is not an unambiguous marker (18). In agreement with an earlier report (23), we confirmed that B. pseudocinerea strains were >20-fold more sensitive than B. cinerea to fenpropidin, except for the fenhexamid-sensitive group B strain. In New Zealand, B. pseudocinerea strains preselected for fenhexamid resistance belonged to group A and group B, showing that group B isolates can have the HydR1 phenotype (17). We have optimized discriminatory growth tests on agar medium containing fenhexamid and fenpropidin as a convenient preliminary tool to distinguish between B. pseudocinerea and B. cinerea strains. However, for unambiguous identification, DNA-based tests remain essential.
Sexual reproduction has been described for B. cinerea and B. pseudocinerea, but fruiting bodies (apothecia) are only rarely observed in nature. Nevertheless, population genetic studies (41) and the observation of similar frequencies of the mating type alleles MAT1-1 and MAT1-2 (42) have demonstrated that sexual recombination must occur regularly. The existence of both mating types in B. pseudocinerea isolates from the same fields confirms previous suggestions that this species performs sexual reproduction (13).
Although B. pseudocinerea strains have originally been described to be of the vacuma type, i.e., lacking both Boty and Flipper transposable elements (6), later studies revealed that this species has a variable transposon content (13, 17, 18). This was confirmed by the present study. A weakness of transposon analysis is the difficulty of reproducibly detecting Boty and Flipper sequences because of their sequence and copy number variability. Depending on the method (dot blot hybridization or PCR) and the experimental conditions for PCR, results can be variable (43). The occurrence of two genetic groups of B. pseudocinerea, first described in New Zealand (17), can now be extended to Germany, even if we have found only two group B isolates so far. It remains to be analyzed whether these two groups show different physiological characters and host preferences.
Supplementary Material
ACKNOWLEDGMENTS
We thank our colleagues Andreas von Tiedemann and Christine Struck for providing isolates from oilseed rape. We are grateful to Andreas Düker, Sandra Bergstein, Sibylle Rumsey, Katharina Stephenson, and Anne Sexauer for help with characterization of isolates.
This work was supported by funds of the Federal Ministry of Food and Agriculture based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food under the innovation support program (FKZ 2814705711).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01719-15.
REFERENCES
- 1.Hahn M. 2014. The rising threat of fungicide resistance in plant-pathogenic fungi: Botrytis as a case study. J Chem Biol 7:133–141. doi: 10.1007/s12154-014-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staats M, van Baarlen P, van Kan JAL. 2005. Molecular phylogeny of the plant-pathogenic genus Botrytis and the evolution of host specificity. Mol Biol Evol 22:333–346. [DOI] [PubMed] [Google Scholar]
- 3.Hyde KD, Nilsson RH, Alias SA, Ariyawansa HA, Blair JE, Cai L, de Cock AWAM, Dissanayake AJ, Glockling SL, Goonasekara ID, Gorczak M, Hahn M, Jayawardena RS, van Kan JAL, Laurence MH, Lévesque CA, Li X, Liu J-K, Maharachchikumbura SSN, Manamgoda DS, Martin FN, McKenzie EHC, McTaggart AR, Mortimer PE, Nair PVR, Pawłowska J, Rintoul TL, Shivas RG, Spies CFJ, Summerell BA, Taylor PWJ, Terhem RB, Udayanga D, Vaghefi N, Walther G, Wilk M, Wrzosek M, Xu J-C, Yan J, Zhou N. 2014. One stop shop: backbones trees for important phytopathogenic genera: I 2014. Fungal Divers 67:21–125. doi: 10.1007/s13225-014-0298-1. [DOI] [Google Scholar]
- 4.Zhou J, Zhang J, Wang XD, Yang L, Jiang DH, Li GQ, Hsiang T, Zhuang WY. 2014. Morphological and phylogenetic identification of Botrytis sinoviticola, a novel cryptic species causing gray mold disease of table grapes (Vitis vinifera) in China. Mycologia 106:43–56. doi: 10.3852/13-032. [DOI] [PubMed] [Google Scholar]
- 5.Giraud T, Fortini D, Levis C, Leroux P, Brygoo Y. 1997. RFLP markers show genetic recombination in Botryotinia fuckeliana (Botrytis cinerea) and transposable elements reveal two sympatric species. Mol Biol Evol 14:1177–1185. doi: 10.1093/oxfordjournals.molbev.a025727. [DOI] [PubMed] [Google Scholar]
- 6.Giraud T, Fortini D, Levis C, Lamarque C, Leroux P, LoBuglio Y, Brygoo Y. 1999. Two sibling species of the Botrytis cinerea complex, transposa and vacuma, are found in sympatry on numerous host plants. Phytopathology 89:967–973. doi: 10.1094/PHYTO.1999.89.10.967. [DOI] [PubMed] [Google Scholar]
- 7.Diolez A, Marches F, Fortini D, Brygoo Y. 1995. Boty, a long-terminal-repeat retroelement in the phytopathogenic fungus Botrytis cinerea. Appl Environ Microbiol 61:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levis C, Fortini D, Brygoo Y. 1997. Flipper, a mobile Fot1-like transposable element in Botrytis cinerea. Mol Gen Genet 254:674–680. doi: 10.1007/s004380050465. [DOI] [PubMed] [Google Scholar]
- 9.Martinez F, Dubos B, Fermaud M. 2005. The role of saprotrophy and virulence in the population dynamics of Botrytis cinerea in vineyards. Phytopathology 95:692–700. doi: 10.1094/PHYTO-95-0692. [DOI] [PubMed] [Google Scholar]
- 10.Samuel S, Veloukas T, Papavasileiou A, Karaoglanidis GS. 2012. Differences in frequency of transposable elements present in Botrytis cinerea populations from several hosts in Greece. Plant Dis 96:1286–1290. doi: 10.1094/PDIS-01-12-0103-RE. [DOI] [PubMed] [Google Scholar]
- 11.Fournier E, Levis C, Fortini D, Leroux P, Giraud T, Brygoo Y. 2003. Characterization of Bc-hch, the Botrytis cinerea homolog of the Neurospora crassa het-c vegetative incompatibility locus, and its use as a population marker. Mycologia 95:251–261. doi: 10.2307/3762036. [DOI] [PubMed] [Google Scholar]
- 12.Fournier E, Giraud T, Albertini C, Brygoo Y. 2005. Partition of the Botrytis cinerea complex in France using multiple gene genealogies. Mycologia 97:1251–1267. doi: 10.3852/mycologia.97.6.1251. [DOI] [PubMed] [Google Scholar]
- 13.Walker AS, Gautier AL, Confais J, Martinho D, Viaud M, Le Pêcheur P, Dupont J, Fournier E. 2011. Botrytis pseudocinerea, a new cryptic species causing grey mould in French vineyards in sympatry with Botrytis cinerea. Phytopathology 101:1433–1445. doi: 10.1094/PHYTO-04-11-0104. [DOI] [PubMed] [Google Scholar]
- 14.Fournier E, Giraud T. 2008. Sympatric genetic differentiation of a generalist pathogenic fungus Botrytis cinerea, on two different host plants, grapevine and bramble. J Evol Biol 21:122–132. [DOI] [PubMed] [Google Scholar]
- 15.Walker AS, Micoud A, Rémuson F, Grosman J, Gredt M, Leroux P. 2013. French vineyards provide information that opens ways for effective resistance management of Botrytis cinerea (grey mould). Pest Manag Sci 69:667–678. doi: 10.1002/ps.3506. [DOI] [PubMed] [Google Scholar]
- 16.Leroch M, Plesken C, Weber RWS, Kauff F, Scalliet G, Hahn M. 2013. Gray mold populations in German strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Appl Environ Microbiol 79:159–167. doi: 10.1128/AEM.02655-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston PR, Hoksbergen K, Park D, Beever RE. 2014. Genetic diversity of Botrytis in New Zealand vineyards and the significance of its seasonal and regional variation. Plant Pathol 63:888–898. doi: 10.1111/ppa.12143. [DOI] [Google Scholar]
- 18.Fekete E, Fekete E, Irinyi L, Karaffa L, Árnyasi M, Asadollahi M, Sándor E. 2012. Genetic diversity of a Botrytis cinerea cryptic species complex in Hungary. Microbiol Res 167:283–291. doi: 10.1016/j.micres.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Plesken C, Westrich LD, Hahn M. 2015. Genetic and phenotypic characterization of Botrytis calthae. Plant Pathol 64:128–134. doi: 10.1111/ppa.12240. [DOI] [Google Scholar]
- 20.Rigotti R, Gindro L, Richter H, Viret O. 2002. Characterization of molecular markers for specific and sensitive detection of Botrytis cinerea Pers.: Fr. in strawberry (Fragaria × ananassa Duch.) using PCR. FEMS Microbiol Lett 209:169–174. doi: 10.1111/j.1574-6968.2002.tb11127.x. [DOI] [PubMed] [Google Scholar]
- 21.Gouy M, Guindon S, Gascuel O. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Wu MD, Li GQ, Yang L, Yu L, Jiang DH, Huang HC, Zhuang WY. 2010. Botrytis fabiopsis, a new species causing chocolate spot of broad bean in central China. Mycologia 102:1114–1126. doi: 10.3852/09-217. [DOI] [PubMed] [Google Scholar]
- 23.Leroux P, Chapeland F, Desbrosses D, Gredt M. 1999. Patterns of cross-resistance to fungicides in Botryotinia fuckeliana (Botrytis cinerea) isolates from French vineyards. Crop Prot 18:687–697. doi: 10.1016/S0261-2194(99)00074-5. [DOI] [Google Scholar]
- 24.Grasso V, Palermo S, Sierotzki H, Garibaldi A, Gisi U. 2006. Cytochrome b gene structure and consequences for resistance to Qo inhibitor fungicides in plant pathogens. Pest Manag Sci 62:465–472. doi: 10.1002/ps.1236. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, Ding L, Michailides TJ, Li H, Ma Z. 2009. Molecular characterization of field azoxystrobin-resistant isolates of Botrytis cinerea. Pest Biochem Physiol 93:72–76. doi: 10.1016/j.pestbp.2008.11.004. [DOI] [Google Scholar]
- 26.Samuel S, Papayiannis LC, Leroch M, Veloukas T, Hahn M, Karaoglanidis GS. 2011. Evaluation of the incidence of the G143A mutation and cytB intron presence in the cytochrome bc-1 gene conferring QoI resistance in Botrytis cinerea populations from several hosts. Pest Manag Sci 67:1029–1036. doi: 10.1002/ps.2226. [DOI] [PubMed] [Google Scholar]
- 27.Saito S, Michailides TJ, Xiao CL. 2014. First report of Botrytis pseudocinerea causing gray mold on blueberry in North America. Plant Dis 98:1743. doi: 10.1094/PDIS-06-14-0573-PDN. [DOI] [PubMed] [Google Scholar]
- 28.Li N, Zhang J, Yang L, Wu MD, Li GQ. 2015. First report of Botrytis pseudocinerea causing gray mold on tomato (Lycopersicon esculentum) in central China. Plant Dis 99:283. doi: 10.1094/PDIS-03-14-0256-PDN. [DOI] [PubMed] [Google Scholar]
- 29.Tronsmo A, Raa J. 1977. Life cycle of the dry eye rot pathogen Botrytis cinerea Pers. on apple. Phytopathol Zeitschr 89:203–207. [Google Scholar]
- 30.Weber RWS, Dralle N. 2013. Fungi associated with blossom-end rot of apples in Germany. Eur J Hort Sci 78:97–105. [Google Scholar]
- 31.Harrison JG. 1988. The biology of Botrytis spp. on Vicia beans and chocolate spot disease—a review. Plant Pathol 37:168–201. doi: 10.1111/j.1365-3059.1988.tb02064.x. [DOI] [Google Scholar]
- 32.Zhang J, Zhang L, Li GQ, Yang L, Jiang DH, Zhuang WY, Huang HC. 2010. Botrytis sinoallii: a new species of the grey mould pathogen on allium crops in China. Mycoscience 51:421–431. [Google Scholar]
- 33.Leroux P, Gredt M, Leroch M, Walker AS. 2010. Exploring mechanisms of resistance to respiratory inhibitors in field strains of Botrytis cinerea, the causal agent of gray mold. Appl Environ Microbiol 76:6615–6630. doi: 10.1128/AEM.00931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernández-Ortuño D, Chen F, Schnabel G. 2012. Resistance to pyraclostrobin and boscalid in Botrytis cinerea isolates from strawberry fields in the Carolinas. Plant Dis 96:1198–1203. doi: 10.1094/PDIS-12-11-1049-RE. [DOI] [PubMed] [Google Scholar]
- 35.Veloukas T, Leroch M, Hahn M, Karaoglanidis GS. 2011. Detection and molecular characterization of boscalid-resistant Botrytis cinerea isolates from strawberry. Plant Dis 95:1302–1307. doi: 10.1094/PDIS-04-11-0317. [DOI] [PubMed] [Google Scholar]
- 36.Amiri A, Heath SM, Peres NA. 2014. Resistance to fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from strawberry. Plant Dis 98:532–539. doi: 10.1094/PDIS-07-13-0753-RE. [DOI] [PubMed] [Google Scholar]
- 37.Yarden O, Katan T. 1993. Mutations leading to substitutions at amino acids 198 and 200 of beta-tubulin that correlate with benomyl-resistance phenotypes of field strains of Botrytis cinerea. Phytopathology 83:1478–1483. doi: 10.1094/Phyto-83-1478. [DOI] [Google Scholar]
- 38.Brent KJ, Hollomon DW. 1998. Fungicide resistance: the assessment of risk. Fungicide Resistance Action Committee monograph no. 2. Global Crop Protection Federation, Brussels, Belgium. [Google Scholar]
- 39.Konstantinou S, Veloukas T, Leroch M, Menexes G, Hahn M, Karaoglanidis G. 2015. Population structure, fungicide resistance profile, and sdhB mutation frequency of Botrytis cinerea from strawberry and greenhouse-grown tomato in Greece. Plant Dis 99:240–248. doi: 10.1094/PDIS-04-14-0373-RE. [DOI] [PubMed] [Google Scholar]
- 40.Leroux P, Fritz R, Debieu D, Albertini C, Lanen C, Bach J, Gredt M, Chapeland F. 2002. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Manag Sci 58:876–888. doi: 10.1002/ps.566. [DOI] [PubMed] [Google Scholar]
- 41.Walker AS, Gladieux P, Decognet V, Fermaud M, Confais J, Roudet J, Bardin M, Bout A, C Nicot P, Poncet C, Fournier E. 2015. Population structure and temporal maintenance of the multihost fungal pathogen Botrytis cinerea: causes and implications for disease management. Environ Microbiol 17:1261–1274. doi: 10.1111/1462-2920.12563. [DOI] [PubMed] [Google Scholar]
- 42.Faretra F, Pollastro S. 1993. Genetics of sexual compatibility and resistance to benzimidazole and dicarboximide fungicides in isolates of Botryotinia fuckeliana (Botrytis cinerea) from nine countries. Plant Pathol 42:48–57. doi: 10.1111/j.1365-3059.1993.tb02933.x. [DOI] [Google Scholar]
- 43.Muñoz G, Hinrichsen P, Brygoo Y, Giraud T. 2002. Genetic characterization of Botrytis cinerea populations in Chile. Mycol Res 106:594–601. doi: 10.1017/S0953756202005981. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.