Abstract
Cirrhosis occurs as a result of various chronic liver injuries, which may be caused by viral infections, alcohol abuse and the administration of drugs and chemicals. Recently, bone marrow cells (BMCs), hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) have been used for developing treatments for cirrhosis. Clinical trials have investigated the therapeutic potential of BMCs, HSCs and MSCs for the treatment of cirrhosis based on their potential to differentiate into hepatocytes. Although the therapeutic mechanisms of BMC, HSC and MSC treatments are still not fully characterized, the evidence thus far has indicated that the potential therapeutic mechanisms of MSCs are clearer than those of BMCs or HSCs with respect to liver regenerative medicine. MSCs suppress inflammatory responses, reduce hepatocyte apoptosis, increase hepatocyte regeneration, reverse liver fibrosis and enhance liver functionality. This paper summarizes the clinical studies that have used BMCs, HSCs and MSCs in patients with liver failure or cirrhosis. We also present the potential therapeutic mechanisms of BMCs, HSCs and MSCs for the improvement of liver function.
Keywords: Cirrhosis, Cell therapy, Bone marrow cells, Mesenchymal stem cells, Hematopoietic stem cells, Immune-modulation, Trophic factors, Anti-fibrosis
Core tip: Mesenchymal stem cells (MSCs) are considered to be a potential therapeutic agent for the treatment of cirrhosis because of their potential to differentiate into hepatocytes, their immune-modulatory properties and their ability to secrete trophic factors. Nevertheless, several issues, including the fibrogenic potential of MSCs and their ability to promote pre-existing tumor cell growth, must be carefully considered.
INTRODUCTION
Cirrhosis is the end stage of chronic liver disease, which may lead to severe hepatic dysfunction and even life-threatening conditions. Currently, liver transplantation is the only curative remedy for end-stage cirrhosis[1], but this treatment is associated with many problems, such as donor shortage, surgical complications, immunological rejection and high medical costs. In 2000, Theise et al[2] reported that Y chromosome-positive hepatocytes were observed in autopsied women who had received therapeutic bone marrow transplantations from male donors, which implies the existence of pluripotent stem cells among bone marrow cells. Since then, attention has been focused on bone marrow cells as a cell source for liver regenerative therapies[3-9]. As of December 16, 2014, more than 216 clinical trials had been registered or were in proof when ClinicalTrials.gov was searched for the terms “stem cells AND liver diseases”. Bone marrow contains both hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs), which can differentiate into hepatocyte-like cells both in vitro and in vivo. Recently, MSCs have also been isolated from adipose tissue, umbilical cord blood, peripheral blood, brain, lung, liver, dermis, and skeletal muscle[10-15]. This paper summarizes the clinical studies that have used unsorted bone marrow cells (BMCs), HSCs and MSCs in patients with liver failure or cirrhosis and presents the potential therapeutic mechanisms proposed for these cell types.
CELL THERAPY FOR CIRRHOSIS
Cell therapies for liver disease can be broadly classified into bio-artificial liver devices that contain hepatocytes or the direct infusion of cells. Bio-artificial liver devices have been used in patients with acute liver failure. Regarding direct infusion, cells such as primary hepatocytes, BMCs, HSCs and MSCs have all been used. Of these cell types, the transplantation of hepatocytes has been shown to be unsuitable because human hepatocytes are difficult to obtain and because it is difficult to maintain their viability and function when they are cryo-preserved or cultured in vitro. Therefore, bone marrow-derived cells (i.e., BMCs, HSCs and MSCs) appear to be viable alternatives. Because stem cells present among BMCs are known to differentiate into hepatocytes, clinical studies have used both unsorted BMCs and stem cells (i.e., HSCs and MSCs) isolated from BMCs.
Unsorted autologous BMC infusion
The infusion of autologous BMCs into the hepatic artery or the peripheral veins of patients with cirrhosis has been reported to be safe and feasible. Moreover, the infusion of autologous BMCs increases the serum albumin level and improves liver function[16-23]. Terai et al[24] reported that the infusion of BMCs repopulated the damaged liver and differentiated into albumin-producing hepatocytes in a mouse model of chronic liver injury induced by continuous administration of carbon tetrachloride (CCl4). These authors also showed that the infusion of BMCs elevated the levels of matrix metalloproteinase (MMP)-2, MMP-9 and MMP-14, reduced liver fibrosis and improved the survival rate[25-28]. Currently, MMPs are considered beneficial factors associated with a reduction in liver fibrosis, and several reports have indicated that adenoviral delivery of MMPs into the liver ameliorates experimental cirrhosis[29-31]. However, the therapeutic mechanism of BMC infusion remains controversial. Several studies have suggested that hematopoietic cells among BMCs may generate hepatocyte-like cells, whereas others have hypothesized that they act primarily by fusion with hepatocytes or through a paracrine effect[32-35]. To generate a greater beneficial effect of BMC infusion, granulocyte-colony-stimulating factor (G-CSF) has been used to mobilize BMCs (monitored by the number of CD34+ cells) into the peripheral blood[36].
HSC transplantation
HSCs can be isolated from the bone marrow or peripheral blood after the administration of G-CSF, which induces the mobilization of CD34+ cells into the peripheral blood. HSCs express hematopoietic markers[37-39], such as c-kit, Sca-1, Thy-1, CD34, CD45 and CD133, and can differentiate into hepatocytes. Gordon and colleagues reported that the infusion of CD34+ cells isolated from the peripheral blood after the administration of G-CSF via the hepatic artery or portal vein improves the level of serum albumin[40]. Moreover, Pai et al[41] reported that the autologous infusion of expanded mobilized CD34+ cells improves the level of serum albumin and the Child-Pugh score. However, the therapeutic mechanism by which HSC infusion ameliorates liver damage remains unclear, although some authors have proposed that infused HSCs can differentiate into hepatocytes through cell fusion or through a paracrine effect[32-35].
MSC transplantation
More recently, clinical studies using bone marrow-derived MSCs have been conducted. MSCs have several advantages over other cell types, such as their relatively simple acquisition and strong proliferative capacity. Moreover, a sufficient number of MSCs required for clinical trials may be expanded ex vivo, without a loss of differentiation or proliferation potential, and then cryo-preserved until needed. Therefore, MSCs can be injected repeatedly without a concomitant loss of their viability and function. In one study, autologous bone marrow-derived MSCs were infused through the peripheral veins of 4 patients with decompensated cirrhosis. No side effects were observed, and the Mayo End-Stage Liver Disease (MELD) score was improved in half of the patients. Furthermore, the quality of life for all four patients improved by the end of the follow-up period[42]. Kharaziha et al[43] also reported improved liver function in patients with cirrhosis who were injected with autologous MSCs via the peripheral or portal veins. In another study, Jang et al[44] demonstrated the beneficial effects of transplanting autologous bone marrow MSCs for the treatment of alcoholic cirrhosis. MSCs (5 × 107 cells) were injected into the hepatic artery twice at weeks 4 and 8. According to the Laennec fibrosis system, histological improvement was observed in 6 of the 11 patients (54.5%).
We conducted a systematic review to evaluate the safety, feasibility and effects of MSC therapy in patients with liver disease and to explore possible future directions (Table 1). We searched the OVID-Medline, EMBASE and Cochrane library databases for studies published through November 2014 to identify studies in which MSC therapy was administered to patients with liver disease. The main search strategy combined the terms that indicated MSC and liver disease. The methodological quality of the studies was assessed with the SIGN (Scottish Intercollegiate Guidelines Network) checklist. Two authors independently extracted the studies with predefined data fields and included indicators of study quality. Of the 568 studies identified, 14 were eligible for inclusion. These studies evaluated a mean sample size of 32 patients and a mean follow-up of 11.6 mo. The publication year of the studies ranged from 2007 to 2014. The majority of the study designs were small single-cohort studies, clinical trials, or case control studies. Overall, the study quality was moderate or poor. Most of the studies used bone marrow-derived MSCs, and 3 used umbilical cord-derived cells. The majority of the studies used the peripheral route, two used the hepatic artery, one used the portal vein, and one used the intrasplenic route for cell delivery. One study compared the administration of cells by intrasplenic injection and by peripheral administration, whereas another investigation compared the intrasplenic and intrahepatic administration of cells. Although marked heterogeneity was observed among studies with respect to the injection dose, cell source, delivery route and study design, MSC therapy was shown to be safe and feasible. The majority of analyzed studies demonstrated improved liver function, which was measured by biochemical outcome, changes in liver function or associated prognostic indicators. The bilirubin, albumin, aspartate aminotransferase [AST, serum glutamic oxaloacetic transaminase (SGOT)), alanine aminotransferase [ALT, serum glutamic pyruvic transaminase (SGPT)], MELD, Child-Pugh and histological scores (Laennec system) demonstrated a statistically significant improvement in 9/10, 11/11, 7/8, 9/9, 8/8, 4/4 and 1/1 studies, respectively. Additionally, critical adverse events or complications were not observed in any of the studies. Hence, although MSC therapy is a much-needed possibility for treating liver disease, further robust clinical trials and evidence regarding the preferred source of cells, dose and route of delivery are required.
Table 1.
Summary of the clinical studies that have used mesenchymal stem cells in patients with cirrhosis
| Ref. | Publication year | Country | Study design | Mean age (yr) range (mean ± SD) | Sample size | Follow-up (mo) | Cell source | Injection route | Dose (cells/kg) | Main outcome | Side effects or complications |
| Amin et al[83] | 2013 | Egypt | Cohort | 42-60 (51.3 ± 6.2) | n = 20 (M:F = 14:6) | 6 | Bone marrow | Intrasplenic | A mean of 10 × 106 | I | None |
| Jang et al[44] | 2014 | South Korea | Clinical trials | 37-60 (50 ± 8) | n = 11 (M:F = 10:1) | 45 | Bone marrow | Hepatic artery | 5 × 106 | I | None |
| Kharaziha et al[43] | 2009 | Sweden | Cohort | 38-67 (55.63) | n = 8 (M:F = 4:4) | 6 | Bone marrow | Portal vein (n = 6) or Peripheral vein (n = 2) | 3 × 107-5 × 107 | I | None |
| Mohamadnejad et al[84] | 2013 | Iran | RCT | MSC 43.1 ± 17.6Placebo 34.6 ± 13.8 | n = 25 (M:F = 13:12) MSC (n = 14) Placebo (n = 11) | 12 | Bone marrow | Peripheral vein | Median of 1.95 × 108 (range: 1.2-2.95) | I | None |
| Mohamadnejad et al[42] | 2007 | Iran | Case series | 34-56 (47.3) | n = 4 (M:F = 1:3) | 12 | Bone marrow | Peripheral vein | (5.2 ± 0.63) × 109 | I | None |
| Salama et al[85] | 2014 | Egypt | RCT | (1) MSC 50.27 ± 6.05(2) Control 50.9 ± 7.23 | n = 40 (M:F = 33:7) (1) MSC (n = 20) (2) Control (n = 20) | 6 | Bone marrow | Peripheral vein | 1 × 106 | I | None |
| Wang et al[86] | 2013 | China | Clinical trial | 33-58 (40) | n = 7 (M:F=1:6) | 12 | Umbilical cord | Peripheral vein | 0.5 × 106 | NR | None |
| Zhang et al[87] | 2012 | China | Case control | (1) MSC: 48(2) Control: 47 | n = 45 (M:F = 40:5) (1) MSC (n = 30) (2) Control (n = 15) | 12 | Umbilical cord | Peripheral vein | 0.5 × 106 | I | None |
| Wang et al[88] | 2014 | China | Cohort | 30-60(50) | n = 10 (M:F=1:9) | 12 | Bone marrow | Peripheral vein | I | None | |
| El-Ansary et al[89] | 2010 | Egypt | RCT | Intrasplenic 48.50 ± 11.09Peripheral 50.83 ± 6.88 | n = 12 (M:F = 8:4) intrasplenic (n = 6) peripheral (n = 6) | 6 | Bone marrow | IntrasplenicPeripheral vein | 1 × 108/5 mL | I | None |
| Shi et al[90] | 2012 | China | Case control | (1) MSC 24-59(2) Control 26-62 | n = 43 (M:F = 35:8) MSC (n = 24) Control (n = 21) | 18 | Umbilical cord | Peripheral vein | 0.5 × 106 | I | None |
| Peng et al[91] | 2011 | China | Case control | MSC 42.19 ± 10.80Control 42.22 ± 11.37 | n = 158 (M:F=149:9) MSC (n = 53) Control (n = 105) | 45 (195 wk) | Bone marrow | Hepatic artery | 3.4 × 108-3.8 × 108 | I | None |
| El-Ansary et al[92] | 2012 | Egypt | Case control | MSC 48.0 ± 7.4Control 51.6 ± 7.2 | n = 25 (M:F=19:6) MSC (n = 15) Control (n = 10) | 6 | Bone marrow | Peripheral vein | 1 × 106 | I | None |
| Amer et al[23] | 2011 | Egypt | RCT | MSC 50.5 ± 4.1Control (45-55) ± 3.6 | n = 40 (M:F=33:7) MSC (n = 20) Control (n = 20) | 6 | Bone marrow | 1) Intrasplenic2) Intrahepatic | 2 × 107 | I | None |
I: Improved; NR: Not reported; None: Not observed.
POSSIBLE THERAPEUTIC MECHANISMS OF BMCS, HSCS AND MSCS FOR CIRRHOSIS
Although cell therapy for cirrhosis has demonstrated that BMCs, HSCs and MSCs can improve liver function and deliver beneficial effects in terms of liver regeneration, the therapeutic mechanisms responsible for these effects are still far from being fully characterized. Several mechanisms by which these cells might contribute to liver regeneration have been proposed, including their differentiation into hepatocytes, their fusion with endogenous hepatocytes and a proliferative paracrine effect on hepatocytes[32,34,45-47].
Unsorted autologous BMCs
Terai et al[46] demonstrated that BMCs could differentiate into albumin-producing hepatocytes in a mouse model of chronic liver injury. These authors also showed that the infusion of BMCs caused an elevation in the levels of MMP-2, MMP-9 and MMP-14, which was associated with reduced liver fibrosis[25,26]. Furthermore, MSCs present among cultured BMCs may represent candidates for treating cirrhosis[17]. However, Thomas et al[48] reported that macrophages among BMCs were beneficial for repairing cirrhosis. Indeed, macrophages, cells of hematopoietic origin, are known to play a critical role in the regulation of liver fibrosis in murine models[48,49]. Furthermore, the intravenous administration of autologous BMCs caused hepatic homing of the injected cells, which suggests that this easy, safe route may represent an adequate option for BMC infusion in patients with cirrhosis[25,50].
HSCs
Although HSCs can differentiate into hepatocyte-like cells under specified culture conditions in vitro or in animals with liver injury[24,51-54], controversy still exists concerning the therapeutic mechanisms by which HSCs contribute to hepatocyte regeneration or liver repair. Some authors have proposed that conversion to hepatocytes may occur via cell fusion in vivo[32,33,55]. Other possible explanations for target organ regeneration and improvements in function include the activation of endogenous hepatic progenitor cells and the release of vascular endothelial growth factor (VEGF), which would increase the blood supply to the cells and aid in the repair of the damaged tissue[33,56]. It has also been suggested that HSCs may act in a regenerative capacity simply through upregulating the expression of the B-cell leukemia/lymphoma-2 gene (Bcl-2), which would suppress apoptosis[57,58], and downregulating immune responses in the diseased organ via the interleukin-6 (IL-6) pathway[59].
MSCs
Recently, several studies have emphasized the critical importance of cell types other than hepatocytes, such as macrophages, hepatic stellate cells and lymphocytes, in the regulation of liver regeneration[60-62]. Moreover, unbalanced immune cell populations or infiltration of the liver by immune cells can disrupt the immune-privileged state of the liver, which may cause liver injury or fibrosis. Accumulating evidence indicates that the therapeutic mechanisms of MSC treatments are better defined than those of BMCs or HSCs in terms of liver regeneration (Figure 1). For instance, MSCs can differentiate into hepatocyte-like cells both in vitro and in vivo[63-65] and can secrete trophic factors, including growth factors, cytokines and chemokines, which promote the regeneration of the impaired liver. Trophic factors expressed by MSCs are known not only to reduce the inflammation, apoptosis and fibrosis of damaged tissues but also to stimulate angiogenesis and tissue regeneration[66-68]. Moreover, trophic factors [i.e., IL-10, hepatocyte growth factor (HGF), transforming growth factor-beta 3 (TGF-β3) and tumor necrosis factor alpha (TNF-α)] secreted by MSCs inhibit the proliferation of hepatic stellate cells and decrease collagen synthesis[69,70]. In addition, MSCs have immune-modulatory properties and are able to migrate to damaged tissues. MSCs can also express various soluble factors, such as nitric oxide (NO), prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), IL-6, IL-10 and human leukocyte antigen G (HLA-G). These soluble factors regulate the proliferation and functions of a variety of immune cells, and they have also been shown to induce regulatory T (Treg) cells[71]. The beneficial effects of autologous bone marrow MSC transplantation in patients with cirrhosis are listed in Table 1.
Figure 1.
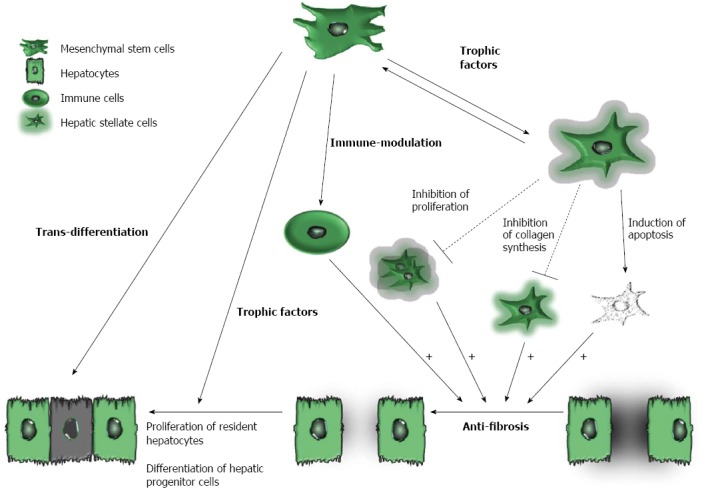
Potential role of mesenchymal stem cells in cirrhosis. Potential protective mechanisms of mesenchymal stem cells (MSCs) include the following: (1) trans-differentiation into hepatocyte-like cells; (2) suppression of immune reactions; (3) secretion of trophic factors to suppress activated hepatic stellate cells and to increase the proliferation of both resident hepatocytes and hepatic progenitor cells; and (4) anti-fibrotic action that results from the regulation of activated hepatic stellate cells and immune cells. Solid lines and dashed lines indicate stimulatory and inhibitory modifications, respectively. The + sign represents tentative stimulatory effects. The shadows represent extracellular matrix (ECM) that is secreted from hepatic stellate cells.
FUTURE PERSPECTIVES
For patients with cirrhosis, autologous BMCs, HSCs or MSCs may be excellent candidates for use in cell therapies to stimulate the production of functional hepatocytes that can replenish diminished liver function. However, it is uncertain whether these cells can differentiate into hepatocytes in vivo at a clinically useful and stable level because the trans-differentiation of BMCs, HSCs or MSCs into hepatocytes has not been commonly observed in animal models[72]. In addition, it is difficult to distinguish trans-differentiated hepatocytes from resident hepatocytes in patients. Nevertheless, the beneficial effects of these cells have been reported in patients with cirrhosis. Several critical issues in clinical trials require further investigation, such as the optimal cell type for infusion, the optimal therapeutic timing, the most effective number of cells, the best route of administration and the optimal period or number of injections. Before these issues can be solved, we must consider what types of cells among the bone marrow cells are primarily responsible for liver regeneration. Accumulating evidence has revealed that resident stem cells (i.e., HSCs and MSCs) among the bone marrow cells may differentiate into hepatocytes and improve liver function. Of the different types of stem cells, MSCs can be easily isolated due to their plastic and adhesive properties, and they can be expanded to a sufficient cell number for clinical application without a loss of stemness. In addition, the length of survival of the engrafted cells is important to achieve a sustained efficacy. To detect the infused cells in many pre-clinical animal studies, human cells have been observed by immunohistochemical analysis using human-specific markers[73-75]; however, for clinical translation, more sophisticated techniques will be required to identify and follow the fates of the injected cells. For instance, infused cells can be labeled with superparamagnetic iron oxide nanoparticles or reporter genes, which may allow them to be traced with advanced imaging technologies[76-80]. Because nanoparticles or reporter genes can modify the properties of cells, biomarkers specific to the injected cells, as well as other identifiers that do not cause cell damage, must be developed, even if the development of such tools requires an extended period of time.
In addition, the transplantation of BMCs, HSCs and MSCs is considered safe and has been widely tested in clinical trials in individuals with liver diseases with encouraging results. However, the development of cell therapy for cirrhosis requires larger clinical studies to obtain meaningful insights into the safety and clinical efficacy of cell infusion[81,82]. This is especially true in the case of MSC transplantation, for which reports on the efficacy of MSCs are controversial and are dependent on the research group; however, we expect that issues concerning the efficacy of these cells will be resolved in the near future by a large multicenter randomized clinical trial that is currently being conducted in Korea. Moreover, multicenter international clinical studies on the safety and efficacy of MSC treatments for cirrhosis can help clinicians reach a consensus regarding the treatment of liver fibrosis, which will ultimately improve the prognosis of patients.
CONCLUSION
Cell therapy with autologous BMCs, HSCs or MSCs has been suggested as an effective strategy for patients with cirrhosis. Accumulating evidence has revealed that the potential therapeutic mechanisms of MSC treatments are better defined than those of BMCs or HSCs in terms of liver regenerative medicine. Furthermore, MSCs are considered a potential agent for treating cirrhosis because of their potential to differentiate into hepatocytes, as well as their immune-modulatory properties and their ability to secrete trophic factors. Nevertheless, several issues, including those that involve the fibrogenic potential of MSCs and their ability to promote pre-existing tumor cell growth, must be carefully considered.
Footnotes
Supported by The Yonsei University Future-leading Research Initiative of 2014.
Conflict-of-interest statement: The authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 7, 2015
First decision: May 18, 2015
Article in press: August 31, 2015
P- Reviewer: Kollmann D, Linard C, Varga G, Zhang Q S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Fallowfield JA, Iredale JP. Targeted treatments for cirrhosis. Expert Opin Ther Targets. 2004;8:423–435. doi: 10.1517/14728222.8.5.423. [DOI] [PubMed] [Google Scholar]
- 2.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 3.Houlihan DD, Newsome PN. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology. 2008;135:438–450. doi: 10.1053/j.gastro.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 4.Souza BS, Nogueira RC, de Oliveira SA, de Freitas LA, Lyra LG, Ribeiro dos Santos R, Lyra AC, Soares MB. Current status of stem cell therapy for liver diseases. Cell Transplant. 2009;18:1261–1279. doi: 10.3727/096368909X470522. [DOI] [PubMed] [Google Scholar]
- 5.Bird TG, Lorenzini S, Forbes SJ. Activation of stem cells in hepatic diseases. Cell Tissue Res. 2008;331:283–300. doi: 10.1007/s00441-007-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peggins JO, McMahon TF, Beierschmitt WP, Weiner M. Comparison of hepatic and renal metabolism of acetaminophen in male and female miniature swine. Drug Metab Dispos. 1987;15:270–273. [PubMed] [Google Scholar]
- 7.Muraca M. Evolving concepts in cell therapy of liver disease and current clinical perspectives. Dig Liver Dis. 2011;43:180–187. doi: 10.1016/j.dld.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Stutchfield BM, Forbes SJ, Wigmore SJ. Prospects for stem cell transplantation in the treatment of hepatic disease. Liver Transpl. 2010;16:827–836. doi: 10.1002/lt.22083. [DOI] [PubMed] [Google Scholar]
- 9.Moon KM, Kim G, Baik SK, Choi E, Kim MY, Kim HA, Cho MY, Shin SY, Kim JM, Park HJ, et al. Ultrasonographic scoring system score versus liver stiffness measurement in prediction of cirrhosis. Clin Mol Hepatol. 2013;19:389–398. doi: 10.3350/cmh.2013.19.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 11.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 12.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 14.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HK, Kim YJ, Chung WJ, Kim SS, Shim JJ, Choi MS, Kim do Y, Jun DW, Um SH, Park SJ, et al. Clinical outcomes of transjugular intrahepatic portosystemic shunt for portal hypertension: Korean multicenter real-practice data. Clin Mol Hepatol. 2014;20:18–27. doi: 10.3350/cmh.2014.20.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito T, Tomita K, Haga H, Okumoto K, Ueno Y. Bone marrow cell-based regenerative therapy for liver cirrhosis. World J Methodol. 2013;3:65–69. doi: 10.5662/wjm.v3.i4.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terai S, Takami T, Yamamoto N, Fujisawa K, Ishikawa T, Urata Y, Tanimoto H, Iwamoto T, Mizunaga Y, Matsuda T, et al. Status and prospects of liver cirrhosis treatment by using bone marrow-derived cells and mesenchymal cells. Tissue Eng Part B Rev. 2014;20:206–210. doi: 10.1089/ten.TEB.2013.0527. [DOI] [PubMed] [Google Scholar]
- 18.Terai S, Tanimoto H, Maeda M, Zaitsu J, Hisanaga T, Iwamoto T, Fujisawa K, Mizunaga Y, Matsumoto T, Urata Y, et al. Timeline for development of autologous bone marrow infusion (ABMi) therapy and perspective for future stem cell therapy. J Gastroenterol. 2012;47:491–497. doi: 10.1007/s00535-012-0580-5. [DOI] [PubMed] [Google Scholar]
- 19.Takami T, Terai S, Sakaida I. Stem cell therapy in chronic liver disease. Curr Opin Gastroenterol. 2012;28:203–208. doi: 10.1097/MOG.0b013e3283521d6a. [DOI] [PubMed] [Google Scholar]
- 20.Lyra AC, Soares MB, da Silva LF, Fortes MF, Silva AG, Mota AC, Oliveira SA, Braga EL, de Carvalho WA, Genser B, et al. Feasibility and safety of autologous bone marrow mononuclear cell transplantation in patients with advanced chronic liver disease. World J Gastroenterol. 2007;13:1067–1073. doi: 10.3748/wjg.v13.i7.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyra AC, Soares MB, da Silva LF, Braga EL, Oliveira SA, Fortes MF, Silva AG, Brustolim D, Genser B, Dos Santos RR, et al. Infusion of autologous bone marrow mononuclear cells through hepatic artery results in a short-term improvement of liver function in patients with chronic liver disease: a pilot randomized controlled study. Eur J Gastroenterol Hepatol. 2010;22:33–42. doi: 10.1097/MEG.0b013e32832eb69a. [DOI] [PubMed] [Google Scholar]
- 22.Saito T, Okumoto K, Haga H, Nishise Y, Ishii R, Sato C, Watanabe H, Okada A, Ikeda M, Togashi H, et al. Potential therapeutic application of intravenous autologous bone marrow infusion in patients with alcoholic liver cirrhosis. Stem Cells Dev. 2011;20:1503–1510. doi: 10.1089/scd.2011.0074. [DOI] [PubMed] [Google Scholar]
- 23.Amer ME, El-Sayed SZ, El-Kheir WA, Gabr H, Gomaa AA, El-Noomani N, Hegazy M. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011;23:936–941. doi: 10.1097/MEG.0b013e3283488b00. [DOI] [PubMed] [Google Scholar]
- 24.Terai S, Sakaida I, Yamamoto N, Omori K, Watanabe T, Ohata S, Katada T, Miyamoto K, Shinoda K, Nishina H, et al. An in vivo model for monitoring trans-differentiation of bone marrow cells into functional hepatocytes. J Biochem. 2003;134:551–558. doi: 10.1093/jb/mvg173. [DOI] [PubMed] [Google Scholar]
- 25.Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 26.Terai S, Sakaida I, Nishina H, Okita K. Lesson from the GFP/CCl4 model--translational research project: the development of cell therapy using autologous bone marrow cells in patients with liver cirrhosis. J Hepatobiliary Pancreat Surg. 2005;12:203–207. doi: 10.1007/s00534-005-0977-0. [DOI] [PubMed] [Google Scholar]
- 27.Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83–88. doi: 10.1097/01.tp.0000128326.95294.14. [DOI] [PubMed] [Google Scholar]
- 28.Sakaida I, Terai S, Nishina H, Okita K. Development of cell therapy using autologous bone marrow cells for liver cirrhosis. Med Mol Morphol. 2005;38:197–202. doi: 10.1007/s00795-005-0298-z. [DOI] [PubMed] [Google Scholar]
- 29.Iimuro Y, Nishio T, Morimoto T, Nitta T, Stefanovic B, Choi SK, Brenner DA, Yamaoka Y. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445–458. doi: 10.1053/gast.2003.50063. [DOI] [PubMed] [Google Scholar]
- 30.Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, Watanabe T, Okano H, Matsuzaki Y, Shiota G, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213–222. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- 31.Siller-López F, Sandoval A, Salgado S, Salazar A, Bueno M, Garcia J, Vera J, Gálvez J, Hernández I, Ramos M, et al. Treatment with human metalloproteinase-8 gene delivery ameliorates experimental rat liver cirrhosis. Gastroenterology. 2004;126:1122–133; discussion 949. doi: 10.1053/j.gastro.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 32.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 34.Quintana-Bustamante O, Alvarez-Barrientos A, Kofman AV, Fabregat I, Bueren JA, Theise ND, Segovia JC. Hematopoietic mobilization in mice increases the presence of bone marrow-derived hepatocytes via in vivo cell fusion. Hepatology. 2006;43:108–116. doi: 10.1002/hep.21005. [DOI] [PubMed] [Google Scholar]
- 35.Thorgeirsson SS, Grisham JW. Hematopoietic cells as hepatocyte stem cells: a critical review of the evidence. Hepatology. 2006;43:2–8. doi: 10.1002/hep.21015. [DOI] [PubMed] [Google Scholar]
- 36.Gaia S, Smedile A, Omedè P, Olivero A, Sanavio F, Balzola F, Ottobrelli A, Abate ML, Marzano A, Rizzetto M, et al. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol. 2006;45:13–19. doi: 10.1016/j.jhep.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Fujio K, Evarts RP, Hu Z, Marsden ER, Thorgeirsson SS. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab Invest. 1994;70:511–516. [PubMed] [Google Scholar]
- 38.Hines IN, Kremer M, Isayama F, Perry AW, Milton RJ, Black AL, Byrd CL, Wheeler MD. Impaired liver regeneration and increased oval cell numbers following T cell-mediated hepatitis. Hepatology. 2007;46:229–241. doi: 10.1002/hep.21674. [DOI] [PubMed] [Google Scholar]
- 39.Petersen BE, Grossbard B, Hatch H, Pi L, Deng J, Scott EW. Mouse A6-positive hepatic oval cells also express several hematopoietic stem cell markers. Hepatology. 2003;37:632–640. doi: 10.1053/jhep.2003.50104. [DOI] [PubMed] [Google Scholar]
- 40.Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, M’Hamdi H, Thalji T, Welsh JP, Marley SB, et al. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822–1830. doi: 10.1634/stemcells.2005-0629. [DOI] [PubMed] [Google Scholar]
- 41.Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, Tait P, Scott M, Marley SB, Jestice K, et al. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952–1958. doi: 10.1111/j.1572-0241.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- 42.Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, Baharvand H, Ghavamzadeh A, Malekzadeh R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10:459–466. [PubMed] [Google Scholar]
- 43.Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, Telkabadi M, Atashi A, Honardoost M, Zali MR, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199–1205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 44.Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW, Cho MY, Park HJ, Park SY, Kim BR, Kim JW, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. 2014;34:33–41. doi: 10.1111/liv.12218. [DOI] [PubMed] [Google Scholar]
- 45.Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 46.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- 47.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- 48.Thomas JA, Pope C, Wojtacha D, Robson AJ, Gordon-Walker TT, Hartland S, Ramachandran P, Van Deemter M, Hume DA, Iredale JP, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003–2015. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- 49.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292–2298. doi: 10.1634/stemcells.2005-0542. [DOI] [PubMed] [Google Scholar]
- 51.Fiegel HC, Lioznov MV, Cortes-Dericks L, Lange C, Kluth D, Fehse B, Zander AR. Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003;21:98–104. doi: 10.1634/stemcells.21-1-98. [DOI] [PubMed] [Google Scholar]
- 52.Almeida-Porada G, Porada CD, Chamberlain J, Torabi A, Zanjani ED. Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood. 2004;104:2582–2590. doi: 10.1182/blood-2004-01-0259. [DOI] [PubMed] [Google Scholar]
- 53.Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 54.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 55.Levicar N, Pai M, Habib NA, Tait P, Jiao LR, Marley SB, Davis J, Dazzi F, Smadja C, Jensen SL, et al. Long-term clinical results of autologous infusion of mobilized adult bone marrow derived CD34+ cells in patients with chronic liver disease. Cell Prolif. 2008;41 Suppl 1:115–125. doi: 10.1111/j.1365-2184.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nikeghbalian S, Pournasr B, Aghdami N, Rasekhi A, Geramizadeh B, Hosseini Asl SM, Ramzi M, Kakaei F, Namiri M, Malekzadeh R, et al. Autologous transplantation of bone marrow-derived mononuclear and CD133(+) cells in patients with decompensated cirrhosis. Arch Iran Med. 2011;14:12–17. [PubMed] [Google Scholar]
- 57.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 58.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 59.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Pretreatment with adult progenitor cells improves recovery and decreases native myocardial proinflammatory signaling after ischemia. Shock. 2006;25:454–459. doi: 10.1097/01.shk.0000209536.68682.90. [DOI] [PubMed] [Google Scholar]
- 60.Meijer C, Wiezer MJ, Diehl AM, Schouten HJ, Schouten HJ, Meijer S, van Rooijen N, van Lambalgen AA, Dijkstra CD, van Leeuwen PA. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66–77. doi: 10.1034/j.1600-0676.2000.020001066.x. [DOI] [PubMed] [Google Scholar]
- 61.Atzori L, Poli G, Perra A. Hepatic stellate cell: a star cell in the liver. Int J Biochem Cell Biol. 2009;41:1639–1642. doi: 10.1016/j.biocel.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Tumanov AV, Koroleva EP, Christiansen PA, Khan MA, Ruddy MJ, Burnette B, Papa S, Franzoso G, Nedospasov SA, Fu YX, et al. T cell-derived lymphotoxin regulates liver regeneration. Gastroenterology. 2009;136:694–704.e4. doi: 10.1053/j.gastro.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kia R, Sison RL, Heslop J, Kitteringham NR, Hanley N, Mills JS, Park BK, Goldring CE. Stem cell-derived hepatocytes as a predictive model for drug-induced liver injury: are we there yet? Br J Clin Pharmacol. 2013;75:885–896. doi: 10.1111/j.1365-2125.2012.04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shu SN, Wei L, Wang JH, Zhan YT, Chen HS, Wang Y. Hepatic differentiation capability of rat bone marrow-derived mesenchymal stem cells and hematopoietic stem cells. World J Gastroenterol. 2004;10:2818–2822. doi: 10.3748/wjg.v10.i19.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie. 2013;95:2196–2211. doi: 10.1016/j.biochi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 67.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–C682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 68.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 69.Parekkadan B, van Poll D, Megeed Z, Kobayashi N, Tilles AW, Berthiaume F, Yarmush ML. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;363:247–252. doi: 10.1016/j.bbrc.2007.05.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Bian C, Liao L, Zhu Y, Li J, Zeng L, Zhao RC. Inhibition of hepatic stellate cells proliferation by mesenchymal stem cells and the possible mechanisms. Hepatol Res. 2009;39:1219–1228. doi: 10.1111/j.1872-034X.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 71.Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014;54:1418–1437. doi: 10.1111/trf.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dai LJ, Li HY, Guan LX, Ritchie G, Zhou JX. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res. 2009;2:16–25. doi: 10.1016/j.scr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 73.Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatsos VE. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J Comp Neurol. 2009;514:297–309. doi: 10.1002/cne.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan J, Xu L, Welsh AM, Hatfield G, Hazel T, Johe K, Koliatsos VE. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raore B, Federici T, Taub J, Wu MC, Riley J, Franz CK, Kliem MA, Snyder B, Feldman EL, Johe K, et al. Cervical multilevel intraspinal stem cell therapy: assessment of surgical risks in Gottingen minipigs. Spine (Phila Pa 1976) 2011;36:E164–E171. doi: 10.1097/BRS.0b013e3181d77a47. [DOI] [PubMed] [Google Scholar]
- 76.Wang F, Dennis JE, Awadallah A, Solchaga LA, Molter J, Kuang Y, Salem N, Lin Y, Tian H, Kolthammer JA, et al. Transcriptional profiling of human mesenchymal stem cells transduced with reporter genes for imaging. Physiol Genomics. 2009;37:23–34. doi: 10.1152/physiolgenomics.00300.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yaghoubi SS, Campbell DO, Radu CG, Czernin J. Positron emission tomography reporter genes and reporter probes: gene and cell therapy applications. Theranostics. 2012;2:374–391. doi: 10.7150/thno.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang SJ, Wu JC. Comparison of imaging techniques for tracking cardiac stem cell therapy. J Nucl Med. 2007;48:1916–1919. doi: 10.2967/jnumed.107.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neri M, Maderna C, Cavazzin C, Deidda-Vigoriti V, Politi LS, Scotti G, Marzola P, Sbarbati A, Vescovi AL, Gritti A. Efficient in vitro labeling of human neural precursor cells with superparamagnetic iron oxide particles: relevance for in vivo cell tracking. Stem Cells. 2008;26:505–516. doi: 10.1634/stemcells.2007-0251. [DOI] [PubMed] [Google Scholar]
- 80.Hu SL, Zhang JQ, Hu X, Hu R, Luo HS, Li F, Xia YZ, Li JT, Lin JK, Zhu G, et al. In vitro labeling of human umbilical cord mesenchymal stem cells with superparamagnetic iron oxide nanoparticles. J Cell Biochem. 2009;108:529–535. doi: 10.1002/jcb.22283. [DOI] [PubMed] [Google Scholar]
- 81.Healy BC, Schoenfeld D. Comparison of analysis approaches for phase III clinical trials in amyotrophic lateral sclerosis. Muscle Nerve. 2012;46:506–511. doi: 10.1002/mus.23392. [DOI] [PubMed] [Google Scholar]
- 82.Gladman M, Cudkowicz M, Zinman L. Enhancing clinical trials in neurodegenerative disorders: lessons from amyotrophic lateral sclerosis. Curr Opin Neurol. 2012;25:735–742. doi: 10.1097/WCO.0b013e32835a309d. [DOI] [PubMed] [Google Scholar]
- 83.Amin MA, Sabry D, Rashed LA, Aref WM, el-Ghobary MA, Farhan MS, Fouad HA, Youssef YA. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant. 2013;27:607–612. doi: 10.1111/ctr.12179. [DOI] [PubMed] [Google Scholar]
- 84.Mohamadnejad M, Alimoghaddam K, Bagheri M, Ashrafi M, Abdollahzadeh L, Akhlaghpoor S, Bashtar M, Ghavamzadeh A, Malekzadeh R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33:1490–1496. doi: 10.1111/liv.12228. [DOI] [PubMed] [Google Scholar]
- 85.Salama H, Zekri AR, Medhat E, Al Alim SA, Ahmed OS, Bahnassy AA, Lotfy MM, Ahmed R, Musa S. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther. 2014;5:70. doi: 10.1186/scrt459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang L, Li J, Liu H, Li Y, Fu J, Sun Y, Xu R, Lin H, Wang S, Lv S, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28 Suppl 1:85–92. doi: 10.1111/jgh.12029. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, Chen L, Lv S, Li Y, Yu S, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27 Suppl 2:112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 88.Wang L, Han Q, Chen H, Wang K, Shan GL, Kong F, Yang YJ, Li YZ, Zhang X, Dong F, et al. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev. 2014;23:2482–2489. doi: 10.1089/scd.2013.0500. [DOI] [PubMed] [Google Scholar]
- 89.El-Ansary M, Mogawer Sh, Abdel-Aziz I, Abdel-Hamid S. Phase I Trial: Mesenchymal Stem Cells Transplantation in End Stage Liver Disease. J Am Sci. 2010;6:135–144. [Google Scholar]
- 90.Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725–731. doi: 10.5966/sctm.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 92.El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, Wahdan M. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. 2012;8:972–981. doi: 10.1007/s12015-011-9322-y. [DOI] [PubMed] [Google Scholar]


