Abstract
Purpose.
Ocular trauma is common in civilian and military populations. Commotio retinae involves acute disruption of photoreceptor outer segments after blunt ocular trauma, with subsequent photoreceptor apoptosis causing permanent visual impairment. The mechanisms of photoreceptor death in commotio retinae have not previously been described, although caspase-dependent death is important in other nontraumatic retinal degenerations. We assessed the role of caspase-9 as a mediator of photoreceptor death in a rat model of ballistic ocular trauma causing commotio retinae.
Methods.
Bilateral commotio retinae was induced in rats by ballistic ocular trauma. Caspase-9 activity was assessed by immunohistochemistry, Western blotting, and bVAD-fmk active caspase capture. Caspase-9 was inhibited by unilateral intravitreal injection of highly specific X-linked inhibitor of apoptosis (IAP) baculoviral IAP repeat 3 (XBIR3) domain linked to the cell transduction peptide penetratin 1 (Pen-1) after ballistic injury, and the affected eyes were compared with control eyes treated with Pen-1 injection alone, and retinal function was assessed by electroretinogram a-wave amplitude and photoreceptor survival by outer nuclear layer thickness.
Results.
Increased levels of cleaved caspase-9 were shown in photoreceptors 5 hours after injury, and catalytically active full-length caspase-9 was isolated from retinas. Photoreceptor death after commotio retinae was reduced by caspase-9 inhibition by using Pen-1–XBIR3, and electroretinographic measurements of photoreceptor function was preserved, providing structural and functional neuroprotection.
Conclusions.
The time course of caspase-9 activation and the neuroprotective effects of inhibition suggest that caspase-9 initiates cell death in a proportion of photoreceptors after blunt ocular trauma and that an intravitreally delivered biologic inhibitor may be an effective translational treatment strategy.
Keywords: apoptosis, caspase-9, commotio retinae, photoreceptors, trauma
After commotio retinae, photoreceptor apoptosis impairs vision. In a translatable severe ocular trauma model with mixed cell death mechanisms, photoreceptor death and reduced retinal function were partially caspase-9 dependent, suggesting caspase-9 inhibition as a translatable therapy for eye injury.
Introduction
Programmed cell death by apoptosis occurs in developing tissues, but in the mature animal, dysregulated apoptosis is induced after injury, and the ensuing cell loss often causes permanent functional impairment.1 Different cells exhibit differential biochemical and cellular responses to injury, and multiple mechanisms of cell death may co-exist in the same tissue.1 Cell death by apoptosis is mediated by initiator caspases belonging to either the intrinsic or extrinsic signaling pathways, both of which converge to activate the executioner caspases-3, -6, and -7. Intracellular damage initiates the intrinsic pathway in which mitochondrial outer membrane permeabilization causes the intermembrane spaces to release cytochrome C, which forms complexes with apoptosis-activating factor-1 (apaf-1), forming an apoptosome. The apoptosome recruits and activates initiator caspase-9, which activates the executioner caspases. Caspase-9 has also been implicated in nonapoptotic, apaf-1-independent programmed cell death in cell culture.2 In the extrinsic pathway, caspase-8 is activated by cell surface “death receptor” ligation and activates the executioner caspases.1
Ocular trauma commonly occurs with military personnel and civilians (civilian lifetime prevalence is 20%).3 The condition of commotio retinae is characterized by photoreceptor damage after blunt ocular trauma and has an incidence of 15% in military and 0.4% in civilian eye injuries,4,5 affecting the macula in 73% of military and 31% of civilian cases.3,6 In cases of macular commotio, 26% of cases stabilize with a visual acuity worse than 6/9, and persistent, visually debilitating, paracentral scotomas are common.6 Visual impairment is caused by photoreceptor degeneration.6,7 In animal models of commotio retinae, photoreceptors die by apoptosis and necrosis.8,9 Thus, regulated apoptotic signaling mediates cell death in a proportion of photoreceptors, implying that antiapoptotic neuroprotective therapies have a potential role in the treatment of commotio retinae.
For example, in animal models of nontraumatic subacute photoreceptor degeneration occurring after retinal detachment, photoreceptor death is correlated with raised levels of caspase-3, -7, and -9, and retinal detachment-induced photoreceptor death is partially reversed when the intrinsic pathway is inhibited by overexpression of the adeno-associated virus (AAV)--transduced X-linked inhibitor of apoptosis protein (XIAP), inhibition of mitochondrial outer membrane permeabilization, and the heat shock protein (HSP70) downregulation of the mTOR pathway.10–12 Extrinsic pathway inhibition by blockade of Fas receptors and tumor necrosis factor-α (TNF-α) and knockout of TNF-α also protects photoreceptors.13,14 Thus, both the intrinsic and extrinsic apoptotic pathways are implicated, and the involvement of caspase-9 is implied but photoreceptor death has not been shown to depend on caspase-9 activity.
Inhibiting apoptosis by neuroprotective therapies improves structural and functional outcomes in pre-clinical studies of acute neuronal injury.1,15 In this study, using a rat model of commotio retinae,8 we demonstrated that caspase-9 initiates photoreceptor death and that inhibition of caspase-9 using the X-linked inhibitor of apoptosis (IAP)-baculoviral IAP repeat 3 domain (XBIR3; a highly specific caspase-9 inhibitor)16 linked to the cell transduction peptide penetratin 1 (Pen-1; PolyPeptide Laboratories, Torrance, CA, USA) prevents photoreceptor death and preserves their function.
Materials and Methods
Animal Care and Procedures
Animal procedures were licensed by the UK Home Office, approved by the University of Birmingham's Biomedical Ethics Review Sub-Committee, and conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Female Lister-hooded rats weighing 170 to 200 g were purchased from Charles River Laboratories (Margate, UK), kept on a 12-hour light–12-hour dark cycle, with a daytime luminance of 80 lux, and fed and watered ad libitum. Surgery and electroretinogram (ERG) recording were performed with the rats under inhalational anesthesia with 2.5% isoflurane in oxygen. Ballistic injury was induced as previously described8: a 0.095 g spherical plastic pellet was fired using compressed air that directly impacted the inferior scleral surface at 20 m/s.
Western Blotting
Groups of test rats and uninjured control rats were killed at 5, 24, and 48 hours post ballistic injury by anesthesia overdose (n = 3 per group, repeated three times); both retinae were removed; protein was extracted in lysis buffer (150 mM NaCl, 20mM Tris, 1 mM EDTA, 0.5 mM EGTA, 1% NP-40, pH 7.4) supplemented with protease inhibitor cocktail (Sigma, Gillingham, UK) denatured by heating to 90°C for 5 minutes and separated on a Tris-glycine gel with 80 μg of protein per lane; and transferred to a polyvinylidene fluoride membrane (Millipore, Watford, UK). Blots were repeated three times. After probing with primary and secondary antibodies, specific protein bands were detected using an enhanced chemiluminescence system (GE Healthcare, Little Chalfont, UK), membranes were read on a digital imaging system (ChemiDoc MP System; Bio-Rad, Hemel Hempstead, UK) for short exposure times (<5 minutes), and exposed to photographic film when longer exposure times were required. Integrated band intensity was measured using the automated gel analysis feature in ImageJ software (http://rsbweb.nih.gov/ij) and is displayed as a percentage of the loading control (α-tubulin) for each protein.
Active Caspase Capture
To capture active caspases, 5 μL of 1 mM bVAD-fmk (MP Biomedicals, Cambridge, UK) in 10% dimethyl sulfoxide/phosphate-buffered saline (PBS) was injected intravitreally into both eyes in groups of test animals (n = 3 per group, repeated 2 times) and intact controls killed at 5 and 48 hours after injury. To capture caspase-9 activated immediately after injury, injuries were induced 2 hours after injection to allow retinal penetration, and all animals were killed 5 hours after injury. To capture active caspase-9 at 48 hours after injury, injuries were induced 43 hours before injection, and all animals were killed 5 hours after injection. Groups of control animals were killed 5 and 7 hours after injection. Retinal protein was extracted in CHAPS lysis buffer (150 mM KCl, 50 mM HEPES, 0.1% CHAPS, pH 8.5). Active caspase was extracted by incubation overnight at 4°C with 2 mg of streptavidin-coated Dynabeads (Invitrogen, Paisley, UK) and then prepared and run as for Western blotting, except that integrated intensity is displayed as arbitrary units.
Immunohistochemistry and TUNEL Assay
At 5 and 48 hours after unilateral ballistic injury, four rats were killed by perfusion with 4% paraformaldehyde in PBS under deep terminal anesthesia; both eyes were removed and cryoprotected in ascending concentrations of sucrose in PBS at 4°C; and anterior segments were removed, and the retinal cup was embedded in OCT and stored at −80°C until required. Sections were cut at 15 μm, using a cryostat (Bright Instruments, Huntingdon, UK) and adhered onto Superfrost (Fisher, Loughborough, UK) coated glass microscope slides. The TUNEL FragEL DNA fragmentation detection kit (Merck, Nottingham, UK) was used according to the manufacturer's instructions, except that proteinase K permeabilization was replaced by immersion in 0.1% Triton X-100 in PBS for 15 minutes.
Cell Culture
To generate a positive control for caspase-8, C6 rat glial tumor cells (Culture Collections, Public Health England, Porton Down, UK) were grown to confluence in supplemented Dulbecco's modified Eagle's medium (DMEM; Sigma) in 2× 175-mL tissue culture flasks. One flask was treated with 100 ng/mL TNF-α (Abcam, Cambridge, UK) 5 hours before protein extraction in ice-cold lysis buffer. Western blotting was performed as described above.
Antibodies
The primary antibodies used were against α-tubulin as a Western blotting loading control (rabbit, polyclonal [Abcam]); caspase-9 to detect full-length (p49) and cleaved (p35–p39) fragments by Western blotting (rabbit, polyclonal; New England Biolabs, Hitchin, UK) and immunohistochemistry (rabbit, polyclonal; Santa Cruz Biotechnology, Dallas, TX, USA); caspase-8 to detect full-length (p55) and cleaved (p18) fragments by Western blotting and immunohistochemistry (rabbit, polyclonal; Santa Cruz Biotechnology); mitochondrial complex IV subunit IV to mark photroreceptor inner segments (Life Technologies, Grand Island, NY, USA). Secondary antibodies used were species-specific peroxidase-conjugated for Western blotting (GE Healthcare) and Alexa Fluor 488 or Texas red-conjugated for immunohistochemistry (Invitrogen).
Caspase Inhibition
Pen-1–XBIR3 was prepared as previously described,17 and 5 μL of 5 μM solution was delivered by unilateral intravitreal injection to inhibit caspase-9 in 8 rats immediately after and at 7 days after bilateral ballistic injury (n = 8 eyes). Contralateral eyes were given control treatment with Pen-1 alone. Eight uninjured rats were killed without intravitreal treatments as intact controls. Rats were killed 14 days after injury, and eyes were processed in the same way as for immunohistochemistry. Pen-1 and XBIR3 were linked by incubating equimolar concentrations at 37°C for 24 hours, and linkage was confirmed by SYPRO Ruby (Sigma) staining of proteins resolved by nonreducing 20% polyacrylamide gel electrophoresis (see Fig. 2J).
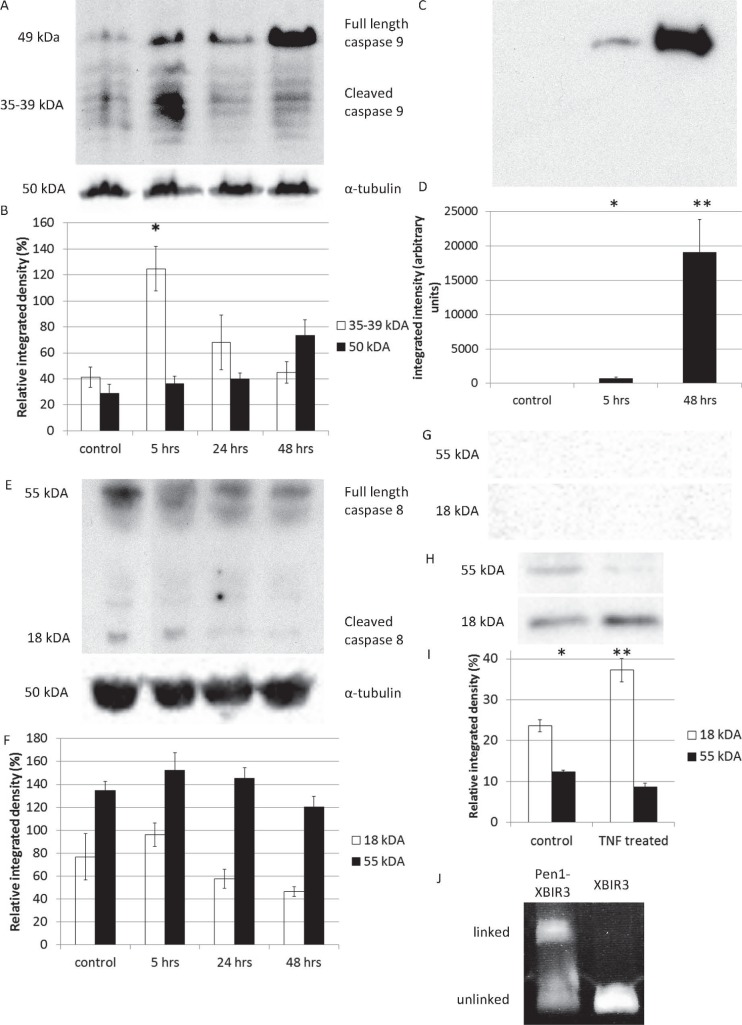
Figure 2.
(A, B) Retinal levels of full-length (p49) and cleaved (p35–p39) caspase-9 are shown after ballistic injury, as assessed by Western blotting showing increased p35–p39 at 5 hours (*P = 0.002) and a nonsignificant increase in p49 at 48 hours (P = 0.136). (C, D) Retinal levels of catalytically active, full-length caspase-9 increased by 5 hours and remained increased at 48 hours after ballistic injury, as detected by bVAD-fmk pull-down assay (*P = 0.047; **P = 0.016). (E, F) Retinal levels of full-length (p55) and cleaved (p18) caspase-8 are shown after ballistic injury, assessed by Western blotting showing no significant change in levels of either p55 (P = 0.246) or p18 (P = 0.103). (G) No catalytically active p55 and p18 caspase-8 were detected by bVAD-fmk pull-down assay (G). (H, I) Caspase-8-positive control. After treatment with TNF-α to induce caspase-8 activity, C6 cells showed increased p18 and reduced p55 levels (*P = 0.026; **P = 0.013). (J) Staining of the of XBIR3 protein, unlinked and linked to Pen-1.
Electroretinography
ERGs were recorded (Hand-Held Multi-species ElectroRetinaGraph; Ocuscience, Kansas City, MO, USA) at 7 and 14 days after injury and in the intact controls and were interpreted using ERGView software (Ocuscience). Animals were dark-adapted overnight and prepared for ERG under dim red light (>630 nm). Scotopic flash ERGs were recorded from −2.5 to +1 log units with respect to standard flash in half-log unit steps, and photopic flash ERGs were recorded with background illumination of 30,000 mcd/m2 over the same range. Dawson, Trick, and Litzkow (DTL) fiber (Unimed Electrode Supplies, Farnham, UK) corneal electrodes with pressure-molded Aclar (Agar Scientific, Stansted, UK) contact lenses were used with needle skin electrodes (Unimed).
Assessment of Photoreceptor Survival
Animals were killed at 14 days, and eyes were processed as for immunohistochemistry. To account for variability in the impact site, seven retinal sections were cut through the optic disc and center of the impact site and at 600, 1,200, and 1,800 μm to either side of this plane. Hematoxylin and eosin (H&E)-stained slides were scanned with a Mirax slide scanner (Zeiss, Cambridge, UK), and the outer nuclear layer (ONL) was manually segmented with Photoshop software (Adobe, San Jose, CA, USA) by a blinded observer. A threshold was imposed on ONL images, and average thickness was calculated for each section by measuring the area of above-threshold pixels and dividing this result by the length of the retinal segment, using ImageJ software as shown in Supplementary Figure S1.
Statistics
Power calculations were performed in G*Power version 3.1.4 software (Kiel University, Kiel, Germany) and indicated that for Western blotting and caspase capture assays, n = 3 animals per time point had 82% power to detect a 1-fold change in caspase-9 levels, assuming a standard deviation of 20% band intensity. Power was calculated for ERG assessment of the effects of caspase-9 inhibition, as this is more variable (and so has a lower power) than ONL thickness measurements; eight animals had a power of 88% to detect a moderate (f = 0.25) effect of treatment (correlation among repeated measures = 0.5 in previous ERG data8).
All statistical analyses were performed with SPSS version 21 software (IBM Co., Armonk, NY, USA). One way repeated measures ANOVA with Tukey post hoc testing and two sample t-tests were used to analyze Western blotting and caspase-capture results. ERG and ONL thickness data were analyzed using generalized estimating equations (type III sum of squares; autoregressive correlation matrix; gamma distribution with log link), and model fit was assessed by plotting residuals and calculating quasilikelihood information criteria.
Results
Activated Caspase-9 Was Present in Photoreceptors After Injury
We have demonstrated photoreceptor apoptosis peripheral to the impact site after ballistic injury.8 However, in the current experiments, TUNEL-positive (TUNEL+) cells were localized in the ONL (Figs. 1A–D) in sections of eyes assayed 2 days after ballistic injury, confirming photoreceptor apoptosis. Using Western blotting, we investigated the expression and processing of caspase-9 in the retina. Compared to uninjured retinae, levels of cleaved caspase-9 were increased at 5 hours and decreased to basal levels by 24 hours after ballistic injury (P = 0.002, repeated measures ANOVA) (Figs. 2A, 2B). Levels of full-length caspase-9 showed a statistically nonsignificant increase by 48 hours after injury (P = 0.136, repeated measures ANOVA) (Figs. 2A, 2B).
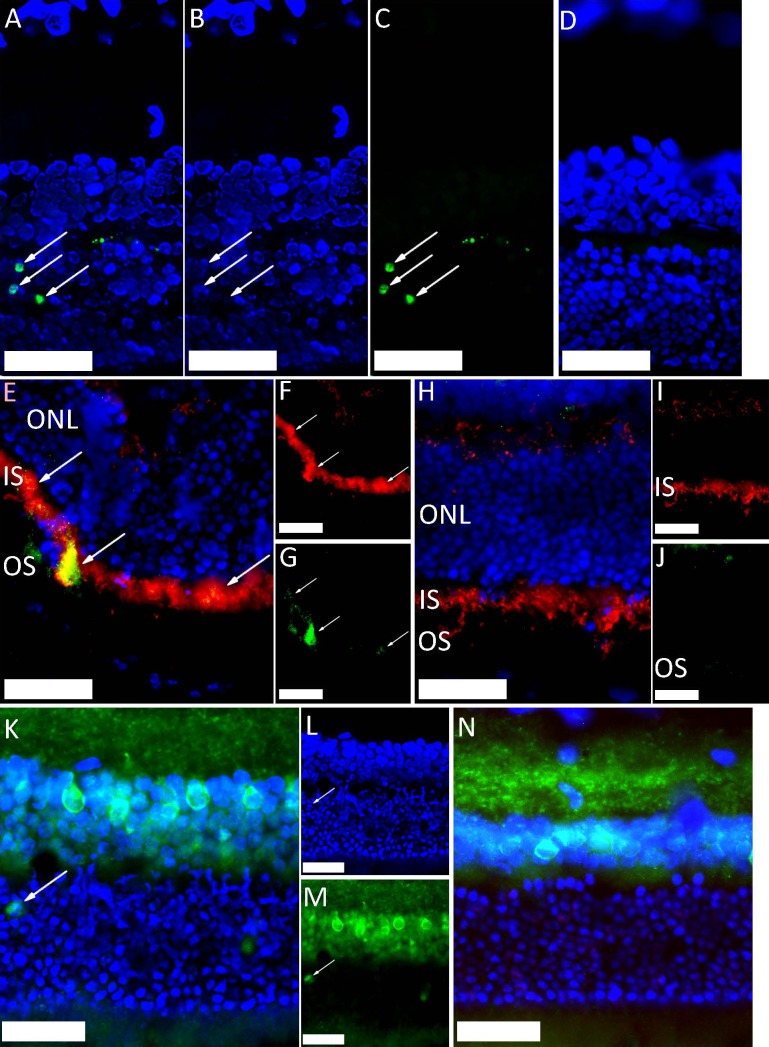
Figure 1.
Immunohistochemical staining of rat outer retinal layers, peripheral to the impact site, after ballistic injury (DAPI-stained nuclei are shown in blue [scale bar: 50 μm]). (A–D) TUNEL-stained photoreceptor nuclei, in green (arrows) at 2 days after injury ([A] blue and green channels [B, C], respectively). TUNEL-negative uninjured control retina (D). (E–J) Caspase-9-stained green (arrows) in photoreceptor inner segments (E, G). Inner segments stained red with anticomplex IV antibody (E, F) at 5 hours after injury. Uninjured control retina (H–J) did not caspase-9 staining. (K–N) Increased caspase-9 levels in photoreceptor cell bodies (arrow) are shown at 2 days in injured retina (K–M). Uninjured control retina did not show caspase-9 staining in the ONL (N).
Caspase-9 is active in both its cleaved and full-length forms; bVAD-fmk caspase-capture isolated higher levels of active full-length caspase-9 from injured than from uninjured retinae (P = 0.004, 1-way ANOVA) at 5 and at 48 hours (Figs. 2C, 2D).
Compared to uninjured control retinae, caspase-9 levels were increased in photoreceptor inner segments peripheral to the impact site in injured animals at 5 hours after injury (Figs. 1E, 1F) and in photoreceptor cell bodies at 48 hours after injury (Figs. 1G–J).
Caspase-8 Was Not Activated After Experimental Commotio Retinae
Western blotting to investigate the expression and processing of caspase-8 showed no significant changes in the levels of either full-length (p55) or cleaved (p18) caspase-8 within 48 hours after ballistic injury (Figs. 2E, 2F). bVAD-fmk caspase-capture did not isolate any caspase-8 after injury, suggesting that the protein present was not catalytically active (Figs. 2G–I). Immunostaining failed to localize any active caspase-8 to photoreceptors (Supplementary Fig. S2).
Caspase-9 Inhibition Induced Structural Neuroprotection
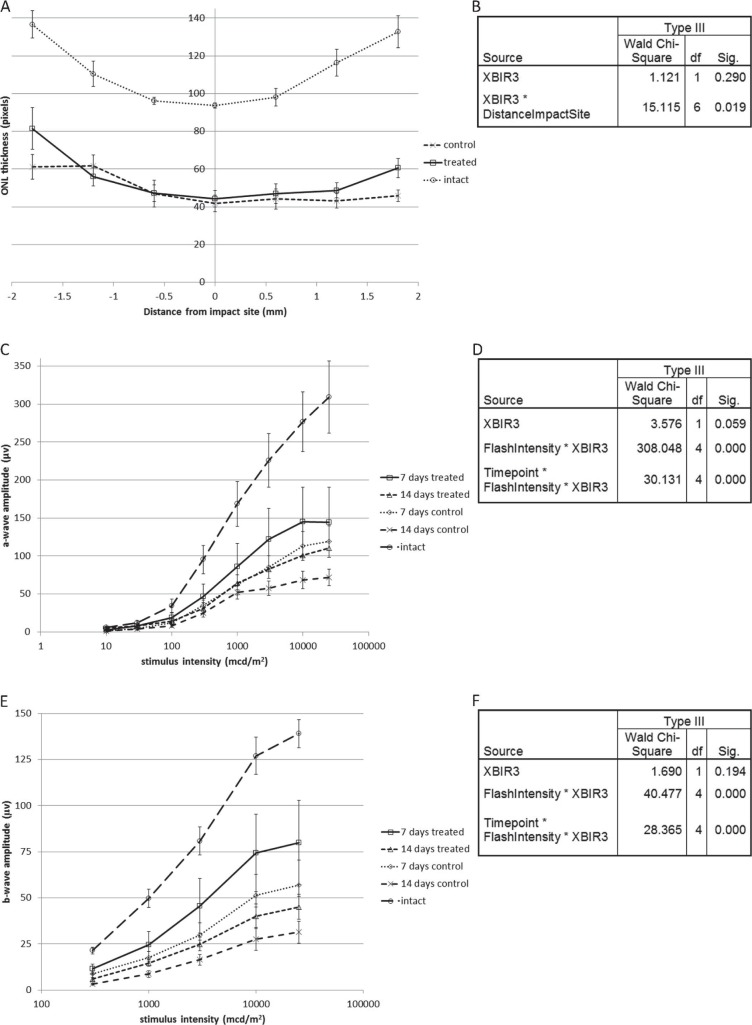
To determine the contribution of caspase-9 to photoreceptor death in commotio retinae, we treated animals with unilateral intravitreal injections of the highly specific cell permeant caspase-9 inhibitor Pen-1–XBIR3 after ballistic injury. Caspase-9 inhibition reduced photoreceptor death, demonstrated by preserved ONL thickness on retinal sections (Figs. 3A, 3B). The effect of treatment on ONL thickness was modeled using generalized estimating equations showing (1) a nonsignificant effect of caspase-9 inhibition across all distances from the impact site (P = 0.29), because there was no effect central to the impact site; (2) an ONL peripheral, but not central, to the impact site in caspase-9-suppressed eyes that was significantly thicker than that in Pen-1-treated eyes (P = 0.019 for the 2-way interaction distance*caspase-9 inhibition), indicating a pro-survival effect of caspase-9 inhibition that increased with increasing distance from the impact site and demonstrating that caspase-9 mediates photoreceptor death after ballistic injury.
Figure 3.
(A) ONL thickness with and without intravitreal Pen-1–XBIR3 is shown as a function of distance from the impact site. ONL thickness in intact controls is shown for comparison. (B) Test of model effects after analysis of ONL thickness, including treatment with Pen-1–XBIR3, distance from the impact site and a term to model their interaction, displaying relevant terms only. (C) Scotopic a-wave amplitude after injury as a function of stimulus intensity with and without intravitreal Pen-1–XBIR3 is shown. Scotopic a-wave amplitude in intact controls is shown for comparison. (D) Test of model effects after analysis of scotopic a-wave amplitude including time after injury (Timepoint), stimulus intensity (FlashIntensity), Pen-1–XBIR3 compared to Pen-1 alone (XBIR3), and terms to model all 2- and 3-way interactions, displaying relevant terms only. (E) Photopic b-wave amplitude after injury as a function of stimulus intensity with and without intravitreal Pen-1–XBIR3 is shown. Photopic b-wave amplitude in intact controls is shown for comparison. (F) Test of model effects is shown after analysis of photopic b-wave amplitude, including time after injury, stimulus intensity, treatment, and terms to model all 2- and 3-way interactions, displaying relevant terms only. Caspase-9 inhibition by Pen-1–XBIR3 increased scotopic a- and photopic b-wave amplitudes with an effect that was more pronounced at higher stimulus intensities and at 14 than at 7 days after injury. Caspase-9 inhibition also increased ONL thickness peripherally but not centrally to the impact site. All data are means ± standard errors of the mean.
Caspase-9 Inhibition Induced Functional Neuroprotection
At 7 and 14 days after ballistic injury and unilateral intravitreal Pen-1–XBIR3 injections, we recorded scotopic (dark-adapted) and photopic (light-adapted) ERGs to assess rod and cone functions, respectively. The effect of treatment on scotopic a-wave amplitude was modeled using generalized estimating equations (Fig. 3D) showing (1) a borderline significant increase in a-wave amplitude after caspase-9 inhibition across all stimulus intensities and time points (P = 0.059); (2) a positive effect of caspase-9 inhibition on a-wave amplitude that increased with stimulus intensity (P < 0.001 for 2-way interaction caspase-9 inhibition*stimulus intensity); and (3) a significant difference in the effect of caspase-9 inhibition between the two time points, with a more pronounced positive effect (less variability) at 14 than at 7 days (P < 0.001 for the 3-way interaction caspase-9 inhibition*stimulus intensity*time). Photopic b-wave analysis showed similar effects (Figs. 3E, 3F); although the main effect of caspase-9 inhibition was not significant (P = 0.194), there was a significant positive effect of caspase-9 inhibition on b-wave amplitude that increased with stimulus intensity (P < 0.001 for 2-way interaction caspase-9 inhibition*stimulus intensity). In summary, compared to eyes injected with intravitreal Pen-1 alone, caspase-9 inhibition improved rod and cone function, demonstrated by increased scotopic a-wave amplitude and photopic b-wave amplitude, and this benefit became more apparent with time after injury (Figs. 3C–F).
Discussion
To our knowledge, this is the first report elucidating the mechanisms of photoreceptor death after blunt ocular trauma, and it highlights a new therapeutic angle in the treatment of this condition. It is also the first demonstration of caspase-9-dependent photoreceptor death. We demonstrate that rod and cone apoptosis after blunt ocular trauma occur through the intrinsic pathway, initiated by caspase-9. Active caspase-trapping assays revealed that caspase-9 is an early mediator of photoreceptor death and its inhibition using a biologic agent reduced photoreceptor death and preserved visual function in both rods and cones, inducing structural and functional neuroprotection that was sustained at 2 weeks post injury.
VAD-fmk is a pan-caspase inhibitor that covalently binds and, in its biotinylated form (bVAD-fmk), isolates caspases-1, -2, -3, -8, and -9 through interaction with immobilized streptavidin.18,19 In this study, we used bVAD-fmk to isolate active caspase-9 within the first 5 hours and at 48 hours after trauma, which was the first demonstration of catalytically active caspase-9 in an ocular trauma model. Levels of active caspase-9 isolated at 48 hours were higher than those isolated at 5 hours, confirming the fact that injection of bVAD-fmk before injury inhibited apoptotic signaling pathways, preventing the establishment of feedback/feed-forward signaling loops that amplified levels of active cell death enzymes. In contrast, by 48 hours after injury, without caspase inhibition for 43 hours post-injury, apoptotic signaling pathways had been activated. The levels of active caspase-9 that were observed to be higher at 48 than at 5 hours cannot therefore be taken to reflect higher caspase-9 activity in vivo, and this comparison would also be confounded by the differing delay between injection and sampling at the two time points. Consistent with the function of caspase-9 as an initiator caspase, the spike in retinal levels of cleaved caspase-9 was recorded 5 hours after injury. No increase in caspase-8 levels or activity was detected. Although we cannot exclude activity at time points not sampled, caspase-8 is an initiator caspase, and activity, where present, would be expected immediately after injury.
Use of peptide pharmacological inhibitors (like VAD-fmk) to study caspase activity is routine. However, overlap in cleavage motifs means that these inhibitors are nonspecific.20 XBIR3, the BIR3 domain of XIAP, is a highly specific inhibitor of caspase-9.16,17
Because 98% of rat photoreceptors are rods and the ONL contains exclusively photoreceptors, ONL thickness reflects rod survival.15 Because there was no sharp demarcation between areas with and without cell death or with apoptosis versus necrosis, the average ONL thickness was measured across the whole retina, assessing the sum effect on photoreceptor survival; however, because sections were cut at various distances from the center of the impact site, region-specific survival was also assessed. Caspase-9 inhibition after blunt ocular trauma most affected ONL thickness peripheral to the impact site, where the highest proportion of apoptotic cells are found, as opposed to central to the impact site, where most cell death is necrotic and therefore less susceptible to modulation by altered caspase activity.8 The significantly thicker ONL in intact controls, compared to those in Pen-1–XBIR3-treated animals, suggests that a large proportion of cell death is necrotic but is also consistent with regulated cell-death and apoptosis pathways being active.
The a-wave is the first negative deflection on the ERG, caused by photoreceptor hyperpolarization, and its amplitude is routinely measured to assess photoreceptor function. Commotio retinae reduces photoreceptor function (demonstrated by reduced a-wave amplitude) out of proportion to the extent of photoreceptor death.8 Scotopic a-wave amplitude in rats reflects rod function and was increased by caspase-9 inhibition, indicating that the increased survival seen on ONL thickness measurements was accompanied by increased rod function.
Photopic ERG were recorded under conditions of light adaptation, bleaching rod photoreceptors, and ensuring that the ERG response was cone-mediated. In rats, the photopic a-waves are small and often undetectable after injury,8 indicating that any variation in b-wave amplitude is attributable to variation in the magnitude of the photoreceptor response. The observed changes in photopic b-wave amplitude, therefore, reflect cone photoreceptor function, which was increased by caspase-9 inhibition, indicating that caspase-9 also mediates cone death after blunt ocular trauma.
The most commonly reported ultrastructural feature of acute commotio retinae is photoreceptor outer segment disruption.7,21 In mild cases of commotio retinae, there is visual recovery, correlated with outer segment regeneration over weeks to months.7,21 The effect of caspase-9 inhibition on cone and rod function was more significant at 14 than at 7 days, because of reduced variability, whereas the amplitudes of scotopic a- and photopic b-waves were reduced at 14 days compared to those at 7 days, suggesting a settling down of death processes and clearance of cellular debris at the later time point. After cessation of caspase-9 inhibition from Pen-1–XBIR3 treatment, photoreceptor death may continue beyond 14 days, although it seems unlikely that such cells would display ERG function.
In conclusion, we show that after ballistic ocular trauma, caspase-9-initiated photoreceptor death in a proportion of photoreceptors and caspase-9 inhibition reduced photoreceptor death with preservation of both rod and cone function.
Acknowledgments
The authors thank Kwang Chear Lee and Lisa Hill (University of Birmingham) for cell culture assistance.
Supported by Ministry of Defence, United Kingdom; Drummond Foundation, United Kingdom; Sir Ian Fraser Foundation; Blind Veterans United Kingdom; NIH (NINDS); and University of Birmingham, United Kingdom.
Disclosure: R.J. Blanch, None; Z. Ahmed, None; A.R. Thompson, None; N. Akpan, None; D.R.J. Snead, None; M. Berry, None; C.M. Troy, None; R.A.H. Scott, None; A. Logan, None
References
- 1. Galluzzi L, Blomgren K, Kroemer G. Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci. 2009; 10: 481–94. [DOI] [PubMed] [Google Scholar]
- 2. Sperandio S, de Belle I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci U S A. 2000; 97: 14376–14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong TY, Klein BE, Klein R. The prevalence and 5-year incidence of ocular trauma. The Beaver Dam eye study. Ophthalmol. 2000; 107: 2196. [DOI] [PubMed] [Google Scholar]
- 4. Weichel ED, Colyer MH, Ludlow SE, Bower KS, Eiseman AS. Combat ocular trauma visual outcomes during operations Iraqi and Enduring Freedom. Ophthalmol. 2008; 115: 2235–2245. [DOI] [PubMed] [Google Scholar]
- 5. Jones NP, Hayward JM, Khaw PT, Claoue CM, Elkington AR. Function of an ophthalmic “accident and emergency” department: results of a six month survey. Br Med J. 1986; 292: 188–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blanch RJ, Good PA, Shah P, Bishop JR, Logan A, Scott RA. Visual outcomes after blunt ocular trauma. Ophthalmol. 2013; 120: 1588–1591. [DOI] [PubMed] [Google Scholar]
- 7. Souza-Santos F, Lavinksy D, Moraes NS, Castro AR, Cardillo JA, Farah ME. Spectral-domain optical coherence tomography in patients with commotio retinae. Retina. 2012; 32: 711–718. [DOI] [PubMed] [Google Scholar]
- 8. Blanch RJ, Ahmed Z, Sik A, et al. Neuroretinal cell death in a murine model of closed globe injury; pathological and functional characterisation. Invest Ophthalmol Vis Sci. 2012; 53: 7220–7226. [DOI] [PubMed] [Google Scholar]
- 9. Sipperley JO, Quigley HA, Gass JD. Traumatic retinopathy in primates: the explanation of commotio retinae. Arch Ophthalmol. 1978; 96: 2267. [DOI] [PubMed] [Google Scholar]
- 10. Zadro-Lamoureux LA, Zacks DN, Baker AN, Zheng QD, Hauswirth WW, Tsilfidis C. XIAP effects on retinal detachment-induced photoreceptor apoptosis [corrected]. Invest Ophthalmol Vis Sci. 2009; 50: 1448–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kayama M, Nakazawa T, Thanos A. Heat shock protein 70 (HSP70) is critical for the photoreceptor stress response after retinal detachment via modulating anti-apoptotic Akt kinase. Am J Pathol. 2011; 178: 1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zacks DN, Hänninen V, Pantcheva M, Ezra E, Grosskreutz C, Miller JW. Caspase activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2003; 44: 1262–1267. [DOI] [PubMed] [Google Scholar]
- 13. Besirli CG, Chinskey ND, Zheng QD, Zacks DN. Inhibition of retinal detachment-induced apoptosis in photoreceptors by a small peptide inhibitor of the fas receptor. Invest Ophthalmol Vis Sci. 2010; 51: 2177–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakazawa T, Kayama M, Ryu M, et al. Tumor necrosis factor-alpha mediates photoreceptor death in a rodent model of retinal detachment. Invest Ophthalmol Vis Sci. 2011; 52: 1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blanch RJ, Ahmed Z, Berry M, Scott RAH, Logan A. Animal models of retinal injury. Invest Ophthalmol Vis Sci. 2012; 53: 2913–2920. [DOI] [PubMed] [Google Scholar]
- 16. Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006; 7: 988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akpan N, Serrano-Saiz E, Zacharia BE, et al. Intranasal delivery of caspase-9 inhibitor reduces caspase-6-dependent axon/neuron loss and improves neurological function after stroke. J Neurosci. 2011; 31: 8894–8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tu S, McStay GP, Boucher LM, Mak T, Beere HM, Green DR. In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nat Cell Biol. 2006; 8: 72–77. [DOI] [PubMed] [Google Scholar]
- 19. Moulin M, Arrigo AP. Caspases activation in hyperthermia-induced stimulation of TRAIL apoptosis. Cell Stress Chaperones. 2008; 13: 313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McStay GP, Salvesen GS, Green DR. Overlapping cleavage motif selectivity of caspases: implications for analysis of apoptotic pathways. Cell Death Differ. 2008; 15: 322–331. [DOI] [PubMed] [Google Scholar]
- 21. Blight R, Hart JC. Structural changes in the outer retinal layers following blunt mechanical non-perforating trauma to the globe: an experimental study. Br J Ophthalmol. 1977; 61: 573. [DOI] [PMC free article] [PubMed] [Google Scholar]