Abstract
Lung cancer remains a challenging disease. It is responsible for the high cancer mortality rates in the US and worldwide. Elucidation of the molecular mechanisms operative in lung cancer is an important first step in developing effective therapies. Accumulating evidence over the last 2 decades suggests a critical role for Signal Transducer and Activator of Transcription 3 (STAT3) as a point of convergence for various signaling pathways that are dysregulated in the disease. In this review, we discuss possible molecular mechanisms involving STAT3 in lung tumorigenesis based on recent literature. We consider possible roles of STAT3 in cancer cell proliferation and survival, in the tumor immune environment, and in epigenetic regulation and interaction of STAT3 with other transcription factors. We also discuss the potential role of STAT3 in tumor suppression, which complicates strategies of targeting STAT3 in cancer therapy.
Keywords: U-STAT, Non-canonical, immune evasion, miRNA, stem cell, tumor suppressor
Abbreviations
- ALDH
aldehyde dehydrogenase
- CSC
cancer stem cell
- DNMT1
DNA methyl transferase 1
- EGFR
Epidermal growth factor receptor
- ETC
electron transport chain
- HIF1-a
hypoxia-inducible factor1-alpha
- HP1
- heterochromatin protein 1
- iNOS
inducible nitric oxide synthase
- JAK
Janus kinase
- MAPK
Mitogen-activated protein kinase
- miRNA
micro RNA
- NSCLC
non-small cell lung cancer
- PIAS
protein inhibitors of activated STAT
- SH2
Src homology 2
- SOCS
suppressors of cytokine signaling
- STAT3
Signal Transducer and Activator of Transcription 3
- STAT3-C
Constitutively activated STAT3
- U-STAT
unphosphorylated STAT
Introduction
Lung cancer is the most lethal cancer worldwide. Based on 2013 figures from the World Health Organization, it accounts for 1.37 million deaths annually, which are more deaths than from the next 3 most common cancers, i.e., colon, breast and pancreatic cancer, combined.1 Over half of the people with lung cancer die within one year of being diagnosed mainly due to the late stage of detection and the scarceness of late-stage treatment options. About 80% of patients with non-small cell lung cancer (NSCLC), a class of lung cancer that constitutes 80–85% of the cases, develop stage IV disease.2 Not surprisingly then, the 5-year survival rate for lung cancer patients is only 16.6%, which is much lower than for many other common cancers such as colon (64.2%), breast (89.2%) and prostate (99.2%).3
Figure 1.
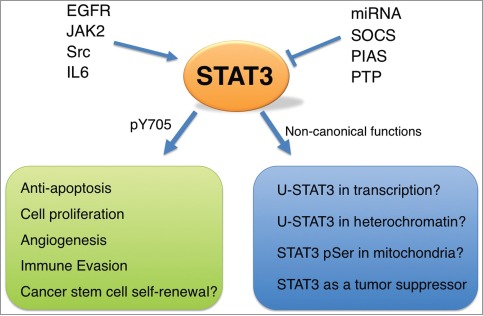
Role of STAT3 in lung cancer. In lung cancer cells, STAT3 can be activated by EGFR, JAK2, Src, IL6, or others. Negative regulators of STAT3 include SOCS, PIAS, Protein tyrosine phosphatases, and miRNAs. In the canonical pathway, activated STAT3 is phosphorylated at Tyr705 and functions as a transcription factor, inducing downstream target genes that are important for cell proliferation, induction of angiogenesis, prevention of apoptosis, evasion of host immune surveillance, or cancer stem cell self-renewal. Potential non-canonical functions of STAT3 may also operate in lung cancer cells. See text for more details.
Chemotherapy remains the principal treatment for lung cancer patients. However, the platinum-based chemotherapeutics (e.g., cisplatin) often result in detrimental side effects due to their toxicity toward normal tissues.4,5 In order to more precisely attack malignant tissues in the lung and to provide patients with “targeted therapy,” one must be able to interfere with the function of a specific molecule that has a central role in cancer cells. Without a doubt, designing such a specific drug requires an understanding of the precise molecular mechanism that underlies the involvement of the targeted molecule in that particular cancer. STAT3 may be one of the key oncogenic drivers in NSCLC.6-14 However, several studies have found that STAT3 also plays a role in tumor suppression,15-19 which should be taken into consideration in cancer therapies based on STAT3 inhibition. In this review, we focus on the molecular function of STAT3. We discuss how this transcription factor and its upstream effectors and downstream targets may be involved in lung cancer and how STAT3 may affect several characteristic hallmarks of various pulmonary tumors including NSCLC (Fig. 1). Moreover, we discuss the crosstalk between STAT3 and other transcription factors such as p53 and NF-κB.
The JAK-STAT Signaling Pathway
The Janus kinase (JAK) and STAT pathway is involved in mediating cytokine signals. In mammals, there are 4 JAKs (JAK1, JAK2, JAK3, and Tyrosine kinase 2 or TyK2) and 7 STATs (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B and STAT6). In the canonical pathway, JAK-STAT signaling starts with the binding of a cytokine (e.g., IL-6) to its specific cell-surface receptor (e.g., gp130), leading the tyrosine phosphorylation of the receptor-associated JAK protein. The phosphorylated (activated) form of JAK kinase, in turn, cross-phosphorylates tyrosine residues on the cytoplasmic tail of the receptor, resulting in the recruitment of latent STAT protein to the receptor and a single phosphorylation of STAT C-terminus around amino acid 700 (Tyr705 in STAT3) by JAK.20,21 In addition to cytokines, several growth factors (e.g., EGF) activate STATs. The growth factors interact with cell-surface receptors that either have intrinsic tyrosine kinase activity (RTKs like EGFR) or are able to recruit a non-receptor tyrosine kinase (NRTK such as Src) to the activation site.22 Activated STATs then hetero- or homo-dimerize via reciprocal SH2 domain–phosphor-tyrosine interactions, are released from the receptor, and translocate into the nucleus, where they modulate the transcription of a wide range of genes in both developing and adult tissues. For example, STAT3 directly binds to the promoters of Fas, cyclin E1, HGF, VEGF, TGF-β, hypoxia-inducible factor1-α (HIF1-α), Akt, c-Myc, c-Jun, c-Fos, matrix metalloproteinase 2 and the anti-apoptotic genes BclxL, Mcl1 and surviving.23
As with other signaling pathways, STATs are under positive and negative regulatory controls. With respect to positive feedback, treatment with one cytokine (e.g., IFN-α) augments the reaction to the next cytokine (e.g., IFN-γ). Regarding negative feedback, STATs are regulated by suppressors of cytokine signaling (SOCS), by protein inhibitors of activated STAT (PIAS), and by protein phosphatases.24 Furthermore, a naturally occurring truncated form of STAT3, which lacks the transactivation domain, can also act as a dominant negative inhibitor.25 Finally, STAT3 has been shown to undergo degradation through proteasome-mediated ubiquitination.26,27
MAP kinases (ERK, JNK, and p38MAPK) regulate STATs as well by governing a second phosphorylation event on a conserved serine residue (Ser727 in STAT3), resulting in a fully activated transcription factor.28 Protein kinase C and cyclin-dependent kinase 5 are also able to mediate this serine phosphorylation.29
Besides phosphorylation, reversible lysine acetylation has been revealed as an additional regulator of STAT activity.30 STAT acetylation within the SH2 domain (Lys685 in STAT3) is thought to support the conformational change that is required for STAT dimerization.31 Interestingly, acetylated STATs seem to attract the methylation machinery, in turn. For example, acetylated STAT3 has been reported to mediate methylation of tumor-suppressor gene promoters.32
Aberrant Activation of STAT3 in Lung Cancer
Constitutive activation of STAT3 is a common feature in NSCLC, and has also been proposed to play an important role in tumor resistance to conventional and targeted small-molecule therapies.8-13 Normally, activated STAT protein has a short lifespan, since STAT can be dephosphorylated quickly by protein phosphatases. However, in many primary cancers and cancer cell lines, including lung cancers, STATs (STAT3 and STAT5) are persistently active.8,9,11,33-35 Persistent STAT activation can be caused by either the increased expression of receptors and kinases (in both an autocrine and paracrine manners) or reduced activity of negative regulators, such as SOCS, protein phosphatases (e.g., SHP-1 and SHP- 2), and PIAS.24 There are also naturally occurring mutations in STATs that make them constitutively active,36 though these mutations are rare. One recent study has found a significant enrichment of mutations in the JAK/STAT pathway in NSCLC.37
STAT3, originally identified as an acute phase response factor to IL-6, seems to be associated more frequently than other STATs with tumor formation.7,23,33,38 Indeed, an engineered form of STAT3 (STAT3-C), which spontaneously dimerizes, can cause malignant transformation of immortablized fibroblasts, which form tumors in nude mice,39 and over-expression of STAT3-C in mice alveolar type II epithelial cells promotes chronic inflammation and causes spontaneous lung bronchoalveolar adenocarcinoma.40 It has also been shown that STAT3 is required for cell transformation downstream of activated Src,41 and that persistently activated STAT3 can cause malignant transformation of cultured fibroblast cells upon spontaneous immortalization.42
A significant body of evidence points to the importance of upstream regulators of STAT3 in lung cancer. For example, IL-6 is up-regulated in approximately 40% of lung cancer patients.43 EGFR is also over-expressed in NSCLC cells.44 and Src is over-activated in many cases of human lung tumors.45 It is therefore not surprising that over 50% of NSCLC primary tumors and cell lines contain high levels of activated STAT39,46,47 Activating mutations in the EGFR kinase domain also make a large contribution to STAT3-mediated tumorigenesis in lung cancer cells.7-9,48,49 An autocrine mechanism involving IL-6 seems likely in this case.8
Besides EGFR, Src and JAK are candidate tyrosine kinases that are thought to be responsible for the constitutively activated STAT3 detected in lung tumors6,11,50 In NSCLC A549 cells, the activation of STAT3 by growth factors and IL-6 has been shown to require Src kinase activity.6 There is also a reciprocal synergy between Src and EGFR in which the Src protein that is activated by EGF mediates the phosphorylation of a tyrosine residue on EGFR (Y845 in the mutant EGFR and Y869 in the full-length EGFR), leading to the enhancement of EGFR kinase activity.51
Prevention of Apoptosis by STAT3
STAT3 enables cancer cells to resist radiation and cytotoxic drugs through inactivation of both extrinsic and intrinsic apoptotic pathways. STAT3 activation causes down-regulation of Fas, a major extracellular apoptotic ligand.52 STAT3 also acts jointly with a noncanonical NF-κB signaling pathway to increase the proteolytic processing of NF-κB p100 to NF-κB p50, which is an anti-apoptotic protein.53 Regarding intrinsic apoptosis, STAT3 promotes the expression of Mcl-1 and Bcl-xL, 2 Bcl-2 proteins that prevent release of cytochrome c from the mitochondria.54,55 STAT3 also enhances the level of survivin, which directly binds to the pro-apoptotic protein caspase 9 and inhibits its activity in multiple cancers.56 In addition, a modified version of STAT3 with increased DNA binding affinity, STAT3-C is simultaneously able to induce higher levels of survivin and lower levels of the pro-apoptotic protein Bax.57
In several NSCLC cell lines, blocking of STAT3 activity induces apoptosis.6,58 In line with these data, STAT3 has been shown to prolong the survival of human PC-13 large-cell lung carcinoma cells upon serum withdrawal.59 Additionally, in HeLa cervical cancer cells, STAT3 binds to the promoter of Skp2, a cell-cycle factor, and induces its expression.60 Skp2 plays an anti-apoptotic role, and is highly expressed in many cancers. Interestingly, Skp2 correlates with a poor prognosis in NSCLC,61 but whether STAT3 has a direct role in Skp2-mediated survival of lung cancer cells is not yet clear.
Of note, STAT3 can inhibit apoptosis in normal lung cells as well. For example, the overexpression of STAT3-C in pulmonary epithelium protects the cells from inflammation and lung injury caused by hyperoxia.62 STAT3 also protects human lung fibroblasts and human bronchial epithelia cells from apoptosis upon exposure to cigarette smoke, leading to the survival of cells with DNA damage.63
Promoting Cell Proliferation and Angiogenesis by STAT3
Constitutive activation of STAT3 correlates with enhanced cell proliferation, metastasis, and angiogenesis in multiple cancers including NSCLC.7,14 This effect of STAT3 is mediated through its ability to induce the expression of several growth-promoting genes such as c-Myc, Pim-1, and cyclin D1, or gene promoting angiogenesis, such as VEGF and bFGF.14,64 The c-Myc protein is the major regulator of the transition from G1 to S and the inducer of the cdc25A gene, whose product regulates the activity of cyclin-dependent kinases.65 Binding of STAT3 to the c-Myc promoter is essential for the induction of c-Myc transcription upon IL-6 treatment and Src activation.38 Pim-1, which is also a transcriptional target of STAT3, cooperates with c-Myc to promote STAT3-depencent cell cycle progression.66 Moreover, in fibroblast cell lines, a constitutively active form of STAT3 up-regulates cyclin D1 expression at the transcriptional level.39 while the ablation of STAT3 in head and neck carcinoma cell lines represses cyclin D1 promoter activity, and also cyclin D1 mRNA levels.67
STAT3 and the Evasion of Anti-Tumor Immunity
One of the key hallmarks of cancer is the ability to escape immune surveillance. STAT3 is a negative regulator of both inflammatory and T cell responses under normal physiological conditions.68-71 In tumor cells, STAT3 reduces the production of pro-inflammatory cytokines and chemokines, which in turn results in lower levels of pro-inflammatory signals (e.g., IL-12) within immune cells.70,72,73
STAT3 promotes the expression in cancer cells of immune-suppressive genes whose products (e.g., IL-10) not only inhibit dendritic cell maturation but also induce STAT3 signaling in dendritic cells and other immune cells, including both T cells and cells mediating innate immune responses.74 In this way, STAT3 keeps a stable feed-forward loop going between tumor cells and tumor-interacting immune cells. Indeed, many tumor cells that contain constitutively activated STAT3 lose their STAT3 phosphorylation once they are in culture without neighboring immune cells. This probably explains why silencing of STAT3 often inhibits tumor growth more effectively in an in vivo environment than in in vitro cultures. STAT3 activation also prevents dendritic cells from activating naive T cells.73 In addition, activated STAT3 leads to the local accumulation of immunosuppressive cells such as regulatory T cells, Th17 cells, and myeloid-derived immunosuppressive cells.74
STAT3 signaling contributes significantly to the evasion of antitumor immunity in lung cancer. Increased numbers of macrophages, lymphocytes, and neutrophils surround lung tumors in mice carrying an epithelial cell–specific STAT3 deletion.75 In addition, the levels of antitumor inflammatory mediators such as TNF-α and IFN-γ are enhanced in the lung tumors of the STAT3-deleted mice. This might be due to the higher expression levels of chemokines (e.g., RANTES and CXCL10) in tumor cells in the absence of STAT3. The lack of STAT3 also can reduce the expression of MHC class I antigens in lung cancer cells, leading to elevated cytotoxic activity of natural killers cells, which in turn stimulates an adaptive immune response.75 Hypoxia is also known to reduce tumor susceptibility to cytotoxic T lymphocytes. Targeting STAT3 leads to the downregulation of hypoxia-inducible factor 1-α (HIF1-α), and a significant restoration of NSCLC cell susceptibility to cytotoxic T lymphocyte-mediated killing under hypoxic conditions.76
STAT3-MicroRNA regulatory circuits in lung cancer
Many miRNAs are transcriptionally regulated by STAT3, either directly or indirectly, in tumors as well as normal cells. For example, STAT3 interacts directly with the promoters of miR-21 and miR-199a-2 in myeloma cells and cardiomyocytes, respectively.77 The STAT3/miR-21/PTEN/NF-κB circuit is also part of a positive feedback loop in breast cancer.78 A case in which STAT3 plays an indirect role is its binding to the promoter of the Lin-28 gene, which encodes a miRNA processing protein. The STAT3-mediated up-regulation of Lin-28 subsequently leads to a reduction of the let-7 family of miRNAs, the upregulation of the high-mobility group A protein 2 (HMGA2), which is a let-7 target, and the epithelial-mesenchymal transition in breast cancer cells.79
Conversely, several miRNAs are known to interact with the 3′-UTR of STAT3 mRNA and thereby negatively regulate STAT3 at the protein level. Lung branching morphogenesis is one of the cases in which the regulatory circuit between STAT3 and miRNAs plays a pivotal role during development. Here, miR-17, miR-20a, and miR-106b, which are important in modulating the levels and distribution of E-Cadherin, do so via their down-regulation of STAT3 and MAPK14 protein concentrations.80
In NSCLC cell lines, miR-21 inhibition can restrain cell invasion.81 In line with this observation, STAT3-induced miR-21 is important for an epigenetic switch between the non-transformed and transformed states in many cells, including lung cancer cell lines.78 In lung adenocarcinoma cells from patients who have never smoked, activating mutations in the EGFR kinase domain seem to enhance induction of miR-21 expression and anti-apoptotic activity, although whether this EGFR/miR-21 circuit operates via STAT3 has not yet been investigated.82 STAT3 also mediates transcription of miR-92a in lung cancer cells, where miR-92a directly targets RECK, a matrix metalloproteinase inhibitor whose expression is strongly associated with invasiveness.83
STAT3 and Other Transcription Factors
During tumorigenesis, the transcriptional regulation of certain genes (e.g., the anti-apoptotic gene Bcl-2, the signal inhibitory gene SOCS3, and the chemokine gene CXCL2) requires cooperation between STAT3 and NF-κB.84 Sometimes this means that the 2 transcription factors bind to neighboring sites within a regulatory region of the shared target gene.85 Other times, for example in the case of the inducible nitric oxide synthase (iNOS) gene, STAT3 and NF-κB physically interact to modulate each other's transcriptional activity.86 Interestingly, transcription of iNOS is also dependent on the direct interaction between STAT3 and nuclear EGFR, which acts as a transcription factor in a variety of highly proliferative cells.87 Finally, either STAT3 or NF-κB transcription may up-regulate a protein (e.g., IL-6), which in turn induces the activity of the other transcription factor.88
The tumor suppressor p53 transcriptionally activates genes that are involved in G1 cell growth arrest and apoptosis, particularly upon DNA damage.89,90 STAT3 has been shown to bind to the promoter of p53, inhibiting its expression and thereby down-regulating p53-reponsive genes in fibroblasts.91 In osteosarcoma cells, the STAT3 effect seems to be indirect because here STAT3 binds to the p53-RELA complex, allowing it to interact with the miR-21 promoter.92 On the other hand, STAT3 can be one of the downstream effectors of p53. This is the case in prostate and breast cancer cell lines where p53 over-expression leads to a significant reduction in the tyrosine phosphorylation and DNA binding activity of STAT3-C.93 Whether p53 has a direct effect on STAT3 is not yet clear.
STAT3 in Lung Cancer Stem Cells
Multiple cancers, including lung cancer, have a minor population of cancer stem cells (CSCs) that have a large capacity for repopulation.94,95 Similarly to normal stem cells, CSCs can self-renew and differentiate into heterogeneous cell populations. CSCs are derived from both normal stem cells and differentiated progenitor cells, and exhibit several peculiar properties such as high telomerase activity, quiescence, and a high degree of invasiveness. CSCs are thought to be the major reason for the intrinsic resistance of tumors to therapy (both chemotherapy and radiotherapy) and for tumor relapse after therapy-induced remission.96,97
Both NSCLC and SCLC cell lines contain CSCs.98 Lung CSCs express the CD133, CD166, and CD44 cell surface markers, and show elevated aldehyde dehydrogenase-1 (ALDH) activity. It should be noted that CSCs cannot be isolated by means of a single marker, and a combination of markers is often used to identify these cells in lung carcinomas.99 The CD133- or ALDH-positive cells isolated from NSCLC patient samples contain higher levels of the activated form of STAT3.100 Treatment of these CD133-positive cells with a specific JAK-STAT inhibitor leads to a downregulation of their self-renewal capability as well as a lowering of their resistance to multiple cancer drugs.100,101 The mechanism underlying STAT3 function in lung CSCs remains to be investigated.
Noncanonical Functions of STAT3 and Epigenetic Regulation
In the canonical JAK/STAT pathway, latent STAT resides in the cytoplasm. However, many STAT proteins, including STAT3, are found to shuttle in and out of the nucleus independently of their phosphorylation status.21,102,103 In Drosophila, where there is only one member of the STAT family, it turns out that the unphosphorylated Drosophila STAT (U-STAT92E) binds to heterochromatin protein 1 (HP1) to promote the heterochromatin stability and protect Drosophila cells from DNA damage.104-106 Similarly, human U-STAT5A interacts with HP1α to stabilize heterochromatin.107 Moreover, U-STAT5A and HP1α downregulate many cancer genes in common in a colon cancer cell line and act as tumor suppressors in a mouse xenograft model.107 It will be interesting to see whether STAT3 plays a similar role in regulating heterochromatin and genome integrity.
Regardless of heterochromatin regulation, there is evidence that STAT3 can modulate the epigenetic status of cancer cells. This effect has been attributed to the transcriptional regulation of STAT3 target genes that encode modifiers of chromatin. Indeed, it has been shown that in pluripotent stem cells, DNA methyl transferase 1 (DNMT1) is one of the transcriptional targets of STAT3.108 In addition, STAT3 also interacts with epigenetic regulatory factors, bringing them to transcriptional regulatory sites. For example, STAT3 causes gene silencing by recruiting DNMT1 to the promoter of the SHP-1 tyrosine phosphatase in malignant T lymphocytes.109 In lung carcinoma, STAT3 brings the histone deacetylase HDAC5 to the PTPN13 promoter.110 STAT3 can also play a role in epigenetic de-repression. For example, STAT3 targets the p300 histone acetyl transferase to the promoter of Skp2 in cervical cancer cells.60
The acetylation status of STAT3 seems to have a large impact on the epigenetic silencing activity of the protein. Mutation of Lys685 of STAT3 results in elevated expression of several tumor suppressor genes such as SHP-1 and p53, whose transcription is normally inhibited by STAT3.91,109 One possible explanation is that the lack of acetylation of the Lys685 residue disrupts the interaction between STAT3 and methyl transferases and thereby results in fewer methyl groups at the transcription sites. Indeed, targeting of STAT3 acetylation with small-molecule drugs can reverse the aberrant methylation of CpG islands at several tumor-suppressor gene promoters in cancer cell lines.32
STAT3 is also found in the mitochondria and to drive oncogenic transformation via a sustained alteration in mitochondrial oxidative phosphorylation that is typically observed in cancer cells.111 It has been shown that mitochondrial STAT3 associates with components of the electron transport chain (ETC) in mitochondria and controls ETC activity, and that the function of mitochondrial STAT3 depends on phosphorylation of Ser727, but not Tyr705.112 Whether STAT3 plays a direct role in mitochondrial oxidative phosphorylation, however, is debated.113 It would be interesting to learn if mitochondrial STAT3 plays any role in lung carcinogenesis.
In the nucleus, it has been shown that U-STAT3 can bind to DNA and drive the expression of several genes whose transcriptional activity is not normally regulated by the phosphorylated form of STAT3 protein.85 Thus, U-STAT3 and phospho-STAT3 seem to regulate different sets of genes, which lead to oncogenesis via different routes.85 Since lung cancers of various types show high expression levels of STAT3, it would be helpful to know whether U-STAT3 makes a contribution to tumorigenesis in the lung.
STAT3 as a Therapeutic Target for Lung Cancer Treatment
The research on JAK inhibitors for use in cancer therapy began in 1996.114 After the discovery of a JAK2 mutation in myeloproliferative disorders, several JAK inhibitors were developed. For example, the JAK2 inhibitor AZD1480 blocks STAT3 activation in several solid tumor cell lines, including several small and non-small cell lung cancer cell lines, and suppresses the growth cancer xenografts harboring persistent STAT3 activation.115-117 In NSCLC cells, IL-6 neutralizing antibody has been shown to inhibit tumor growth in a mouse xenograft model by suppressing JAK1/STAT3 signaling.50 Moreover, the JAK1/2 inhibitor ruxolitinib significantly slowed down the growth of xenografted NSCLC cells in nude mice.11,118 Introducing sh/siRNA targeting STAT3 into cancer cell lines has also been shown to inhibit tumor growth when implanted in immunodeficient mice.115
By the use of murine animal models and primary cell lines, it has been demonstrated that targeting STAT3 is an effective way to inhibit lung cancer growth.119 Finding potent inhibitors of STAT3 tyr705 phosphorylation is the subject of an increasing number of studies in the field. In addition to tyr705, phosphorylation of STAT3 ser727 makes a significant contribution to the progression of many types of cancers.120 Thus, it is likely that inhibitors of STAT3 ser727 phosphorylation will also become useful in the future. Targeting STAT3 dimerization and DNA-binding activity has gained much attention as well.121 Different strategies for designing inhibitors of STAT3 have been reviewed elsewhere.122,123
Role of STAT3 as a Tumor Suppressor
Despite considerable effort, none of the anti-STAT3 agents, ranging from small-molecule inhibitors to oligonucleotide-based drugs have so far passed clinical trials, emphasizing the need for a better understanding of the properties of STAT3 (phosphorylated and unphosphorylated) in order to allow for a more rationale design of future inhibitors with better therapeutic properties. It also necessitates a better understanding of the biological functions of STAT3 in normal animal physiology and in diseases.
A potential factor that complicates the targeting of STAT3 for cancer therapy is that STAT3 has also been reported to function as a tumor suppressor, depending on the mutational context or the stage of the cancer.15-19 For instance, STAT3 plays a critical anti-proliferative and tumor suppressive role in PTEN-deficient glioblastoma cells.15,124 Conditional deletion of STAT3 in mouse lung epithelial cells increases carcinogen or oncogenic K-Ras-induced tumorigenesis.18 Likewise, conditional deletion of STAT3 in intestinal epithelial cells of Apc(Min/+) mice promotes tumorigenesis.17 In addition, knocking down STAT3 in thyroid cancer cell lines increases their tumor growth as xenografts.19 Thus, in some situations, inhibiting STAT3 could promote cancer progression. It has been shown that U-STAT5A inhibits colon cancer growth by promoting heterochromatin formation.107 It remains to be determined whether the reported tumor suppressive function of STAT3 is due to pSTAT3 or U-STAT3. Perhaps in malignancies such as lung cancer, U-STAT3 plays a role similar to that of U-STAT5A in colon cancer.
Conclusion
Within a normal cellular context, the JAK-STAT pathway operates downstream of cytokine signaling and drives cellular proliferation and survival. Various cancers hijack this pathway in order to counter apoptosis and continue abnormal proliferation. In the past few decades, various aspects of the signaling processes that utilize STAT3 have been worked out in lung cancer models in vivo and in vitro. However, with regard to STAT3, there are still questions that are probably worthy of investigation within the context of lung cancer. Among the JAK-STAT pathway components, STAT3 seem to be particularly vulnerable to activating mutations that promote cell proliferation. As with many other cancers, various oncogenic pathways in lung cancer converge on STAT3 and critically depend on activated STAT3. Therefore, STAT3 seems to be a promising target for chemotherapy in the fight against lung cancer and in the quest for longer disease-free survival, if not cure. However, targeting STAT3 may be quite complicated, given that STAT3 also has tumor suppressor functions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported, in part, by grants from the National Institutes of Health and the University of California Cancer Research Coordinating Committee (to W.X.L.), and by a grant from the Swedish Cancer Society (to N.S.)
References
- 1. World Health Organization Cancer. fact sheet no 297. US N Inst Health Nat Cancer Inst SEER Cancer Statistics Rev, 1975-2010, 2013. [Google Scholar]
- 2. American Cancer Society Cancer facts and figures. 2014. [Google Scholar]
- 3. Centers for Disease Control and Prevention National Center for Health Statistics. CDC WONDER On-line database, compiled from compressed mortality file 1999-2010 series 20 No. 2P, 2013. [Google Scholar]
- 4. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Eastern cooperative oncology G. comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. New Eng J Med 2002; 346:92-8; PMID:11784875; http://dx.doi.org/ 10.1056/NEJMoa011954 [DOI] [PubMed] [Google Scholar]
- 5. Milosavljevic N, Duranton C, Djerbi N, Puech PH, Gounon P, Lagadic-Gossmann D, Dimanche-Boitrel MT, Rauch C, Tauc M, Counillon L, et al. Nongenomic effects of cisplatin: acute inhibition of mechanosensitive transporters and channels without actin remodeling. Cancer Res 2010; 70:7514-22; PMID:20841472; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-1253 [DOI] [PubMed] [Google Scholar]
- 6. Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene 2003; 22:4150-65; PMID:12833138; http://dx.doi.org/ 10.1038/sj.onc.1206479 [DOI] [PubMed] [Google Scholar]
- 7. Alvarez JV, Greulich H, Sellers WR, Meyerson M, Frank DA. Signal transducer and activator of transcription 3 is required for the oncogenic effects of non-small-cell lung cancer-associated mutations of the epidermal growth factor receptor. Cancer Res 2006; 66:3162-8; PMID:16540667; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-3757 [DOI] [PubMed] [Google Scholar]
- 8. Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest 2007; 117:3846-56; PMID:18060032; http://dx.doi.org/ 10.1172/JCI31871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haura EB, Zheng Z, Song L, Cantor A, Bepler G. Activated epidermal growth factor receptor-stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res 2005; 11:8288-94; PMID:16322287; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-0827 [DOI] [PubMed] [Google Scholar]
- 10. Zimmer S, Kahl P, Buhl TM, Steiner S, Wardelmann E, Merkelbach-Bruse S, Buettner R, Heukamp LC. Epidermal growth factor receptor mutations in non-small cell lung cancer influence downstream akt, MAPK and stat3 signaling. J Cancer Res Clin Oncol 2009; 135:723-30; PMID:19002495; http://dx.doi.org/ 10.1007/s00432-008-0509-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Looyenga BD, Hutchings D, Cherni I, Kingsley C, Weiss GJ, MacKeigan JP. STAT3 Is activated by JAK2 independent of key oncogenic driver mutations in non-small cell lung carcinoma. PLoS ONE 2012; 7; PMID:22319590; http://dx.doi.org/ 10.1371/journal.pone.0030820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yin Z, Zhang Y, Li Y, Lv T, Liu J, Wang X. Prognostic significance of STAT3 expression and its correlation with chemoresistance of non-small cell lung cancer cells. Acta Histochem 2012; 114:151-8; PMID:21549414; http://dx.doi.org/ 10.1016/j.acthis.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 13. You S, Li R, Park D, Xie M, Sica GL, Cao Y, Xiao ZQ, Deng X. Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol Cancer Ther 2014; 13:606-16; PMID:24362463; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harada D, Takigawa N, Kiura K. The role of STAT3 in non-small cell lung cancer. Cancers 2014; 6:708-22; PMID:24675568; http://dx.doi.org/ 10.3390/cancers6020708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de la Iglesia N, Konopka G, Puram SV, Chan JA, Bachoo RM, You MJ, Levy DE, Depinho RA, Bonni A. Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes Dev 2008; 22:449-62; PMID:18258752; http://dx.doi.org/ 10.1101/gad.1606508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee J, Kim JC, Lee SE, Quinley C, Kim H, Herdman S, Corr M, Raz E. Signal transducer and activator of transcription 3 (STAT3) protein suppresses adenoma-to-carcinoma transition in apcmin/+ mice via regulation of snail-1 (SNAI) protein stability. J Biol Chem 2012; 287:18182-9; PMID:22496368; http://dx.doi.org/ 10.1074/jbc.M111.328831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Musteanu M, Blaas L, Mair M, Schlederer M, Bilban M, Tauber S, Esterbauer H, Mueller M, Casanova E, Kenner L, et al. Stat3 is a negative regulator of intestinal tumor progression in apc(min) mice. Gastroenterol 2010; 138:1003-11 e1-5; PMID:19962983; http://dx.doi.org/ 10.1053/j.gastro.2009.11.049 [DOI] [PubMed] [Google Scholar]
- 18. Zhou J, Qu Z, Yan S, Sun F, Whitsett JA, Shapiro SD, Xiao G. Differential roles of STAT3 in the initiation and growth of lung cancer. Oncogene 2014; PMID:25284582; http://dx.doi.org/ 10.1038/onc.2014.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Couto JP, Daly L, Almeida A, Knauf JA, Fagin JA, Sobrinho-Simoes M, Lima J, Maximo V, Soares P, Lyden D, et al. STAT3 negatively regulates thyroid tumorigenesis. Proc Nat Acad Sci U S A 2012; 109:E2361-70; PMID:22891351; http://dx.doi.org/ 10.1073/pnas.1201232109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Darnell JE. STATs and gene regulation. Science 1997; 277:1630-5; PMID:9287210; http://dx.doi.org/ 10.1126/science.277.5332.1630 [DOI] [PubMed] [Google Scholar]
- 21. Li WX. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol 2008; 18:545-51; PMID:18848449; http://dx.doi.org/ 10.1016/j.tcb.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silvennoinen O, Schindler C, Schlessinger J, Levy DE. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science 1993; 261:1736-9; PMID:8378775; http://dx.doi.org/ 10.1126/science.8378775 [DOI] [PubMed] [Google Scholar]
- 23. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9:798-809; PMID:19851315; http://dx.doi.org/ 10.1038/nrc2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levy DE, Darnell JE. STATs: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002; 3:651-62; PMID:12209125; http://dx.doi.org/ 10.1038/nrm909 [DOI] [PubMed] [Google Scholar]
- 25. Yoo JY, Huso DL, Nathans D, Desiderio S. Specific ablation of stat3beta distorts the pattern of stat3-responsive gene expression and impairs recovery from endotoxic shock. Cell 2002; 108:331-44; PMID:11853668; http://dx.doi.org/ 10.1016/S0092-8674(02)00636-0 [DOI] [PubMed] [Google Scholar]
- 26. Daino H, Matsumura I, Takada K, Odajima J, Tanaka H, Ueda S, Shibayama H, Ikeda H, Hibi M, Machii T, et al. Induction of apoptosis by extracellular ubiquitin in human hematopoietic cells: possible involvement of STAT3 degradation by proteasome pathway in interleukin 6-dependent hematopoietic cells. Blood 2000; 95:2577-85; PMID:10753837 [PubMed] [Google Scholar]
- 27. Ulane CM, Kentsis A, Cruz CD, Parisien JP, Schneider KL, Horvath CM. Composition and assembly of STAT-targeting ubiquitin ligase complexes: paramyxovirus V protein carboxyl terminus is an oligomerization domain. J Virol 2005; 79:10180-9; PMID:16051811; http://dx.doi.org/ 10.1128/JVI.79.16.10180-10189.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene 2000; 19:2628-37; PMID:10851062; http://dx.doi.org/ 10.1038/sj.onc.1203481 [DOI] [PubMed] [Google Scholar]
- 29. Fu AK, Fu WY, Ng AK, Chien WW, Ng YP, Wang JH, Ip NY. Cyclin-dependent kinase 5 phosphorylates signal transducer and activator of transcription 3 and regulates its transcriptional activity. Proc Nat Acad Sci U S A 2004; 101:6728-33; PMID:15096606; http://dx.doi.org/ 10.1073/pnas.0307606100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wieczorek M, Ginter T, Brand P, Heinzel T, Kramer OH. Acetylation modulates the STAT signaling code. Cytokine Growth Factor Rev 2012; 23:293-305; PMID:22795479; http://dx.doi.org/ 10.1016/j.cytogfr.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 31. Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 2005; 307:269-73; PMID:15653507; http://dx.doi.org/ 10.1126/science.1105166 [DOI] [PubMed] [Google Scholar]
- 32. Lee H, Zhang P, Herrmann A, Yang C, Xin H, Wang Z, Hoon DS, Forman SJ, Jove R, Riggs AD, et al. Acetylated STAT3 is crucial for methylation of tumor-suppressor gene promoters and inhibition by resveratrol results in demethylation. Proc Nat Acad Sci U S A 2012; 109:7765-9; PMID:22547799; http://dx.doi.org/ 10.1073/pnas.1205132109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bromberg J. Stat proteins and oncogenesis. J Clin Invest 2002; 109:1139-42; PMID:11994401; http://dx.doi.org/ 10.1172/JCI0215617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levy DE, Lee C-K. What does Stat3 do? J Clin Invest 2002; 109:1143-8; PMID:11994402; http://dx.doi.org/ 10.1172/JCI0215650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu H, Jove R. The STATs of cancer–new molecular targets come of age. Nat Rev Cancer 2004; 4:97-105; PMID:14964307; http://dx.doi.org/ 10.1038/nrc1275 [DOI] [PubMed] [Google Scholar]
- 36. Koskela HL, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmaki H, Andersson EI, Lagstrom S, Clemente MJ, Olson T, Jalkanen SE, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Eng J Med 2012; 366:1905-13; PMID:22591296; http://dx.doi.org/ 10.1056/NEJMoa1114885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, Maher CA, Fulton R, Fulton L, Wallis J, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012; 150:1121-34; PMID:22980976; http://dx.doi.org/ 10.1016/j.cell.2012.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, Irby R, Yeatman T, Courtneidge SA, Jove R. Stat3-mediated myc expression is required for src transformation and PDGF-induced mitogenesis. Proc Nat Acad Sci U S A 2001; 98:7319-24; PMID:11404481; http://dx.doi.org/ 10.1073/pnas.131568898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG,Albanese C, Darnell JE, Jr. Stat3 as an oncogene. Cell 1999; 98:295-303; PMID:10458605; http://dx.doi.org/ 10.1016/S0092-8674(00)81959-5 [DOI] [PubMed] [Google Scholar]
- 40. Li Y, Du H, Qin Y, Roberts J, Cummings OW, Yan C. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res 2007; 67:8494-503; PMID:17875688; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0647 [DOI] [PubMed] [Google Scholar]
- 41. Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE Jr. Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol 1998; 18:2553-8; PMID:9566875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Demaria M, Misale S, Giorgi C, Miano V, Camporeale A, Campisi J, Pinton P, Poli V. STAT3 can serve as a hit in the process of malignant transformation of primary cells. Cell Death Differ 2012; 19:1390-7; PMID:22402588; http://dx.doi.org/ 10.1038/cdd.2012.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yanagawa H, Sone S, Takahashi Y, Haku T, Yano S, Shinohara T, Ogura T. Serum levels of interleukin 6 in patients with lung cancer. Br J Cancer 1995; 71:1095-8; PMID:7734307; http://dx.doi.org/ 10.1038/bjc.1995.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rusch V, Baselga J, Cordon-Cardo C, Orazem J, Zaman M, Hoda S, McIntosh J, Kurie J, Dmitrovsky E. Differential expression of the epidermal growth factor receptor and its ligands in primary non-small cell lung cancers and adjacent benign lung. Cancer Res 1993; 53:2379-85; PMID:7683573 [PubMed] [Google Scholar]
- 45. Mazurenko NN, Kogan EA, Zborovskaya IB, Kisseljov FL. Expression of pp60c-src in human small cell and non-small cell lung carcinomas. Europ J Cancer 1992; 28:372-7; PMID:1375484; http://dx.doi.org/ 10.1016/S0959-8049(05)80056-5 [DOI] [PubMed] [Google Scholar]
- 46. Mukohara T, Kudoh S, Yamauchi S, Kimura T, Yoshimura N, Kanazawa H, Hirata K, Wanibuchi H, Fukushima S, Inoue K, et al. Expression of epidermal growth factor receptor (EGFR) and downstream-activated peptides in surgically excised non-small-cell lung cancer (NSCLC). Lung Cancer 2003; 41:123-30; PMID:12871775; http://dx.doi.org/ 10.1016/S0169-5002(03)00225-3 [DOI] [PubMed] [Google Scholar]
- 47. Seki Y, Suzuki N, Imaizumi M, Iwamoto T, Usami N, Ueda Y, Hamaguchi M. STAT3 and MAPK in human lung cancer tissues and suppression of oncogenic growth by JAB and dominant negative STAT3. Int J Oncol 2004; 24:931-4; PMID:15010832 [PubMed] [Google Scholar]
- 48. Greulich H, Chen TH, Feng W, Janne PA, Alvarez JV, Zappaterra M, Bulmer SE, Frank DA, Hahn WC, Sellers WR, et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med 2005; 2:e313; PMID:16187797; http://dx.doi.org/ 10.1371/journal.pmed.0020313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park OK, Schaefer TS, Nathans D. In vitro activation of stat3 by epidermal growth factor receptor kinase. Proc Nat Acad Sci USA 1996; 93:13704-8; PMID:8942998; http://dx.doi.org/ 10.1073/pnas.93.24.13704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Song L, Rawal B, Nemeth JA, Haura EB. JAK1 activates STAT3 activity in non-small-cell lung cancer cells and IL-6 neutralizing antibodies can suppress JAK1-STAT3 signaling. Mol Cancer Ther 2011; 10:481-94; PMID:21216930; http://dx.doi.org/ 10.1158/1535-7163.MCT-10-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on tyr845 and tyr1101 is associated with modulation of receptor function. J Biol Chem 1999; 274:8335-43; PMID:10075741; http://dx.doi.org/ 10.1074/jbc.274.12.8335 [DOI] [PubMed] [Google Scholar]
- 52. Ivanov VN, Bhoumik A, Krasilnikov M, Raz R, Owen-Schaub LB, Levy D, Horvath CM, Ronai Z. Cooperation between STAT3 and c-jun suppresses fas transcription. Mol Cell 2001; 7:517-28; PMID:11463377; http://dx.doi.org/ 10.1016/S1097-2765(01)00199-X [DOI] [PubMed] [Google Scholar]
- 53. Nadiminty N, Lou W, Lee SO, Lin X, Trump DL, Gao AC. Stat3 activation of NF-{kappa}B p100 processing involves CBP/p300-mediated acetylation. Proc Nat Acad Sci U S A 2006; 103:7264-9; PMID:16651533; http://dx.doi.org/ 10.1073/pnas.0509808103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernández-Luna JL, Nuñez G, et al. Constitutive activation of stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 1999; 10:105-15; PMID:10023775; http://dx.doi.org/ 10.1016/S1074-7613(00)80011-4 [DOI] [PubMed] [Google Scholar]
- 55. Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang JM, Yang-Yen HF, Karras J, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest 2001; 107:351-62; PMID:11160159; http://dx.doi.org/ 10.1172/JCI9940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Diaz N, Minton S, Cox C, Bowman T, Gritsko T, Garcia R, Eweis I, Wloch M, Livingston S, Seijo E, et al. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin Cancer Res 2006; 12:20-8; PMID:16397019; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-1749 [DOI] [PubMed] [Google Scholar]
- 57. Shen Y, Devgan G, Darnell JE, Jr., Bromberg JF. Constitutively activated stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated stat1. Proc Nat Acad Sci USA 2001; 98:1543-8; PMID:11171987; http://dx.doi.org/ 10.1073/pnas.98.4.1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu J, Kern JA. Neuregulin-1 activates the JAK-STAT pathway and regulates lung epithelial cell proliferation. Am J Resp Cell Mol Biol 2002; 27:306-13; PMID:12204892; http://dx.doi.org/ 10.1165/rcmb.4850 [DOI] [PubMed] [Google Scholar]
- 59. Akca H, Tani M, Hishida T, Matsumoto S, Yokota J. Activation of the AKT and STAT3 pathways and prolonged survival by a mutant EGFR in human lung cancer cells. Lung Cancer 2006; 54:25-33; PMID:16872715; http://dx.doi.org/ 10.1016/j.lungcan.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 60. Huang H, Zhao W, Yang D. Stat3 induces oncogenic Skp2 expression in human cervical carcinoma cells. Biochem Biophysical Res Commun 2012; 418:186-90; PMID:22252296; http://dx.doi.org/ 10.1016/j.bbrc.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 61. Salon C, Merdzhanova G, Brambilla C, Brambilla E, Gazzeri S, Eymin B. E2F-1, Skp2 and cyclin E oncoproteins are upregulated and directly correlated in high-grade neuroendocrine lung tumors. Oncogene 2007; 26:6927-36; PMID:17471231; http://dx.doi.org/ 10.1038/sj.onc.1210499 [DOI] [PubMed] [Google Scholar]
- 62. Lian X, Qin Y, Hossain SA, Yang L, White A, Xu H, Shipley JM, Li T, Senior RM, Du H, et al. Overexpression of stat3C in pulmonary epithelium protects against hyperoxic lung injury. J Immunol 2005; 174:7250-6; PMID:15905571; http://dx.doi.org/ 10.4049/jimmunol.174.11.7250 [DOI] [PubMed] [Google Scholar]
- 63. Liu X. STAT3 activation inhibits human bronchial epithelial cell apoptosis in response to cigarette smoke exposure. Biochem Biophysical Res Commun 2007; 353:121-6; PMID:17173857; http://dx.doi.org/ 10.1016/j.bbrc.2006.11.147 [DOI] [PubMed] [Google Scholar]
- 64. Zhao M, Gao FH, Wang JY, Liu F, Yuan HH, Zhang WY, Jiang B. JAK2/STAT3 signaling pathway activation mediates tumor angiogenesis by upregulation of VEGF and bFGF in non-small-cell lung cancer. Lung Cancer 2011; 73:366-74; PMID:21333372; http://dx.doi.org/ 10.1016/j.lungcan.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 65. Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature 1996; 382:511-7; PMID:8700224; http://dx.doi.org/ 10.1038/382511a0 [DOI] [PubMed] [Google Scholar]
- 66. Shirogane T, Fukada T, Muller JM, Shima DT, Hibi M, Hirano T. Synergistic roles for pim-1 and c-myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity 1999; 11:709-19; PMID:10626893; http://dx.doi.org/ 10.1016/S1074-7613(00)80145-4 [DOI] [PubMed] [Google Scholar]
- 67. Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res 2002; 62:3351-5; PMID:12067972 [PubMed] [Google Scholar]
- 68. Cheng F, Wang HW, Cuenca A, Huang M, Ghansah T, Brayer J, Kerr WG, Takeda K, Akira S, Schoenberger SP, et al. A critical role for stat3 signaling in immune tolerance. Immunity 2003; 19:425-36; PMID:14499117; http://dx.doi.org/ 10.1016/S1074-7613(03)00232-2 [DOI] [PubMed] [Google Scholar]
- 69. Hackenmiller R, Kim J, Feldman RA, Simon MC. Abnormal stat activation, hematopoietic homeostasis, and innate immunity in c-fes-/- mice. Immunity 2000; 13:397-407; PMID:11021537; http://dx.doi.org/ 10.1016/S1074-7613(00)00039-X [DOI] [PubMed] [Google Scholar]
- 70. Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of stat3 in macrophages and neutrophils. Immunity 1999; 10:39-49; PMID:10023769; http://dx.doi.org/ 10.1016/S1074-7613(00)80005-9 [DOI] [PubMed] [Google Scholar]
- 71. Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, Kano A, Iwamoto Y, Li E, Craft JE, et al. STAT3 deletion during hematopoiesis causes crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Nat Acad Sci U S A 2003; 100:1879-84; PMID:12571365; http://dx.doi.org/ 10.1073/pnas.0237137100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by stat3 signaling in the tumor microenvironment. Cancer Cell 2009; 15:114-23; PMID:19185846; http://dx.doi.org/ 10.1016/j.ccr.2008.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, et al. Regulation of the innate and adaptive immune responses by stat-3 signaling in tumor cells. Nat Med 2004; 10:48-54; PMID:14702634; http://dx.doi.org/ 10.1038/nm976 [DOI] [PubMed] [Google Scholar]
- 74. Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mule J, Kerr WG, et al. Inhibiting stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med 2005; 11:1314-21; PMID:16288283; http://dx.doi.org/ 10.1038/nm1325 [DOI] [PubMed] [Google Scholar]
- 75. Ihara S, Kida H, Arase H, Tripathi LP, Chen YA, Kimura T, Yoshida M, Kashiwa Y, Hirata H, Fukamizu R, et al. Inhibitory roles of signal transducer and activator of transcription 3 in antitumor immunity during carcinogen-induced lung tumorigenesis. Cancer Res 2012; 72:2990-9; PMID:22659452; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-4062 [DOI] [PubMed] [Google Scholar]
- 76. Noman MZ, Buart S, Van Pelt J, Richon C, Hasmim M, Leleu N, Suchorska WM, Jalil A, Lecluse Y, El Hage F, et al. The cooperative induction of hypoxia-inducible factor-1 α and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. J Immunol 2009; 182:3510-21; PMID:19265129; http://dx.doi.org/ 10.4049/jimmunol.0800854 [DOI] [PubMed] [Google Scholar]
- 77. Loffler D, Brocke-Heidrich K, Pfeifer G, Stocsits C, Hackermuller J, Kretzschmar AK, Burger R, Gramatzki M, Blumert C, Bauer K, et al. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood 2007; 110:1330-3; PMID:17496199; http://dx.doi.org/ 10.1182/blood-2007-03-081133 [DOI] [PubMed] [Google Scholar]
- 78. Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell 2010; 39:493-506; PMID:20797623; http://dx.doi.org/ 10.1016/j.molcel.2010.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guo L, Chen C, Shi M, Wang F, Chen X, Diao D, Hu M, Yu M, Qian L, Guo N. Stat3-coordinated lin-28-let-7-HMGA2 and miR-200-ZEB1 circuits initiate and maintain oncostatin M-driven epithelial-mesenchymal transition. Oncogene 2013; 32:5272-82; PMID:23318420; http://dx.doi.org/ 10.1038/onc.2012.573 [DOI] [PubMed] [Google Scholar]
- 80. Carraro G, El-Hashash A, Guidolin D, Tiozzo C, Turcatel G, Young BM, De Langhe SP, Bellusci S, Shi W, Parnigotto PP, et al. miR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-Cadherin distribution. Dev Biol 2009; 333:238-50; PMID:19559694; http://dx.doi.org/ 10.1016/j.ydbio.2009.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin Chim Acta 2010; 411:846-52; PMID:20223231; http://dx.doi.org/ 10.1016/j.cca.2010.02.074 [DOI] [PubMed] [Google Scholar]
- 82. Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc N Acad Sci U S A 2009; 106:12085-90; PMID:19597153; http://dx.doi.org/ 10.1073/pnas.0905234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lin HY, Chiang CH, Hung WC. STAT3 upregulates miR-92a to inhibit RECK expression and to promote invasiveness of lung cancer cells. Br J Cancer 2013; 109:731-8; PMID:23820254; http://dx.doi.org/ 10.1038/bjc.2013.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev 2010; 21:11-9; PMID:20018552; http://dx.doi.org/ 10.1016/j.cytogfr.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev 2007; 21:1396-408; PMID:17510282; http://dx.doi.org/ 10.1101/gad.1553707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yu Z, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J 2002; 367:97-105; PMID:12057007; http://dx.doi.org/ 10.1042/BJ20020588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, Bartholomeusz G, Shih JY, Hung MC. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell 2005; 7:575-89; PMID:15950906; http://dx.doi.org/ 10.1016/j.ccr.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 88. Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell 2008; 13:7-9; PMID:18167335; http://dx.doi.org/ 10.1016/j.ccr.2007.12.020 [DOI] [PubMed] [Google Scholar]
- 89. Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A 1992; 89:7491-5; PMID:1323840; http://dx.doi.org/ 10.1073/pnas.89.16.7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 1993; 362:847-9; PMID:8479522; http://dx.doi.org/ 10.1038/362847a0 [DOI] [PubMed] [Google Scholar]
- 91. Niu G, Wright KL, Ma Y, Wright GM, Huang M, Irby R, Briggs J, Karras J, Cress WD, Pardoll D, et al. Role of Stat3 in regulating p53 expression and function. Mol Cell Biol 2005; 25:7432-40; PMID:16107692; http://dx.doi.org/ 10.1128/MCB.25.17.7432-7440.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Choy MK, Movassagh M, Siggens L, Vujic A, Goddard M, Sanchez A, Perkins N, Figg N, Bennett M, Carroll J, et al. High-throughput sequencing identifies STAT3 as the DNA-associated factor for p53-NF-kappaB-complex-dependent gene expression in human heart failure. Genome Med 2010; 2:37; PMID:20546595; http://dx.doi.org/ 10.1186/gm158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lin J, Tang H, Jin X, Jia G, Hsieh JT. p53 regulates stat3 phosphorylation and DNA binding activity in human prostate cancer cells expressing constitutively active stat3. Oncogene 2002; 21:3082-8; PMID:12082540; http://dx.doi.org/ 10.1038/sj.onc.1205426 [DOI] [PubMed] [Google Scholar]
- 94. Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994; 367:645-8; PMID:7509044; http://dx.doi.org/ 10.1038/367645a0 [DOI] [PubMed] [Google Scholar]
- 95. Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008; 8:755-68; PMID:18784658; http://dx.doi.org/ 10.1038/nrc2499 [DOI] [PubMed] [Google Scholar]
- 96. Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444:756-60; PMID:17051156; http://dx.doi.org/ 10.1038/nature05236 [DOI] [PubMed] [Google Scholar]
- 97. Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl A S U S A 2009; 106:16281-6; PMID:19805294; http://dx.doi.org/ 10.1073/pnas.0905653106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 2007; 15:504-14; PMID:18049477; http://dx.doi.org/ 10.1038/sj.cdd.4402283 [DOI] [PubMed] [Google Scholar]
- 99. O’Flaherty JD, Barr M, Fennell D, Richard D, Reynolds J, O’Leary J, O’Byrne K. The cancer stem-cell hypothesis: its emerging role in lung cancer biology and its relevance for future therapy. J Thorac Oncol 2012; 7:1880-90 10.097/JTO.0b013e31826bfbc6; PMID:23154562; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 100. Shao C, Sullivan JP, Girard L, Augustyn A, Yenerall P, Rodriguez-Canales J, Liu H, Behrens C, Shay JW, Wistuba II, et al. Essential role of aldehyde dehydrogenase 1A3 for the maintenance of non-small cell lung cancer stem cells is associated with the STAT3 pathway. Clin Cancer Res 2014; 20:4154-66; PMID:24907115; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hsu HS, Huang PI, Chang YL, Tzao C, Chen YW, Shih HC, Hung SC, Chen YC, Tseng LM, Chiou SH. Cucurbitacin I inhibits tumorigenic ability and enhances radiochemosensitivity in nonsmall cell lung cancer-derived CD133-positive cells. Cancer 2011; 117:2970-85; PMID:21225866; http://dx.doi.org/ 10.1002/cncr.25869 [DOI] [PubMed] [Google Scholar]
- 102. Iyer J, Reich NC. Constitutive nuclear import of latent and activated STAT5a by its coiled coil domain. Faseb J 2008; 22:391-400; PMID:17846080; http://dx.doi.org/ 10.1096/fj.07-8965com [DOI] [PubMed] [Google Scholar]
- 103. Liu L, McBride KM, Reich NC. STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-alpha3. Proc Natl Acad Sci U S A 2005; 102:8150-5; PMID:15919823; http://dx.doi.org/ 10.1073/pnas.0501643102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shi S, Larson K, Guo D, Lim SJ, Dutta P, Yan SJ, Li WX. Drosophila STAT is required for directly maintaining HP1 localization and heterochromatin stability. Nat Cell Biol 2008; 10:489-96; PMID:18344984; http://dx.doi.org/ 10.1038/ncb1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Silver-Morse L, Li WX. JAK-STAT in heterochromatin and genome stability. Jak-Stat 2013; 2:e26090; PMID:24069569; http://dx.doi.org/ 10.4161/jkst.26090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yan SJ, Lim SJ, Shi S, Dutta P, Li WX. Unphosphorylated STAT and heterochromatin protect genome stability. FASEB J 2011; 25:232-41; PMID:20847228; http://dx.doi.org/ 10.1096/fj.10-169367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hu X, Dutta P, Tsurumi A, Li J, Wang J, Land H, Li WX. Unphosphorylated STAT5A stabilizes heterochromatin and suppresses tumor growth. Proc Natl Acad Sci USA 2013; 110:10213-8; PMID:23733954; http://dx.doi.org/ 10.1073/pnas.1221243110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tang Y, Luo Y, Jiang Z, Ma Y, Lin CJ, Kim C, Carter MG, Amano T, Park J, Kish S, et al. Jak/Stat3 signaling promotes somatic cell reprogramming by epigenetic regulation. Stem Cells 2012; 30:2645-56; PMID:22968989; http://dx.doi.org/ 10.1002/stem.1225 [DOI] [PubMed] [Google Scholar]
- 109. Zhang Q, Wang HY, Marzec M, Raghunath PN, Nagasawa T, Wasik MA. STAT3- and DNA methyltransferase 1-mediated epigenetic silencing of SHP-1 tyrosine phosphatase tumor suppressor gene in malignant T lymphocytes. Proc Natl Acad Sci U S A 2005; 102:6948-53; PMID:15870198; http://dx.doi.org/ 10.1073/pnas.0501959102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Han XJ, Xue L, Gong L, Zhu SJ, Yao L, Wang SM, Lan M, Zhang W, Li YH. Stat3 inhibits PTPN13 expression in squamous cell lung carcinoma through recruitment of HDAC5. BioMed Res Int 2013; 2013:468963; PMID:24191246; http://dx.doi.org/ 10.1155/2013/468963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports ras-dependent oncogenic transformation. Science 2009; 324:1713-6; PMID:19556508; http://dx.doi.org/ 10.1126/science.1171721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, et al. Function of mitochondrial stat3 in cellular respiration. Science 2009; 323:793-7; PMID:19131594; http://dx.doi.org/ 10.1126/science.1164551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Phillips D, Reilley MJ, Aponte AM, Wang G, Boja E, Gucek M, Balaban RS. Stoichiometry of STAT3 and mitochondrial proteins: Implications for the regulation of oxidative phosphorylation by protein-protein interactions. J Biol Chem 2010; 285:23532-6; PMID:20558729; http://dx.doi.org/ 10.1074/jbc.C110.152652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature 1996; 379:645-8; PMID:8628398; http://dx.doi.org/ 10.1038/379645a0 [DOI] [PubMed] [Google Scholar]
- 115. Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, et al. The JAK2 inhibitor AZD1480 potently blocks stat3 signaling and oncogenesis in solid tumors. Cancer Cell 2009; 16:487-97; PMID:19962667; http://dx.doi.org/ 10.1016/j.ccr.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lee JH, Park KS, Alberobello AT, Kallakury B, Weng MT, Wang Y, Giaccone G. The janus kinases inhibitor AZD1480 attenuates growth of small cell lung cancers in vitro and in vivo. Clin Cancer Res 2013; 19:6777-86; PMID:24158701; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Xin H, Herrmann A, Reckamp K, Zhang W, Pal S, Hedvat M, Zhang C, Liang W, Scuto A, Weng S, et al. Antiangiogenic and antimetastatic activity of JAK inhibitor AZD1480. Cancer Res 2011; 71:6601-10; PMID:21920898; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hu Y, Hong Y, Xu Y, Liu P, Guo DH, Chen Y. Inhibition of the JAK/STAT pathway with ruxolitinib overcomes cisplatin resistance in non-small-cell lung cancer NSCLC. Apoptosis 2014; 19:1627-36; PMID:25213670; http://dx.doi.org/ 10.1007/s10495-014-1030-z [DOI] [PubMed] [Google Scholar]
- 119. Zhang X, Zhang J, Wang L, Wei H, Tian Z. Therapeutic effects of STAT3 decoy oligodeoxynucleotide on human lung cancer in xenograft mice. BMC Cancer 2007; 7:149; PMID:17683579; http://dx.doi.org/ 10.1186/1471-2407-7-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hazan-Halevy I, Harris D, Liu Z, Liu J, Li P, Chen X, Shanker S, Ferrajoli A, Keating MJ, Estrov Z. STAT3 is constitutively phosphorylated on serine 727 residues, binds DNA, and activates transcription in CLL cells. Blood 2010; 115:2852-63; PMID:20154216; http://dx.doi.org/ 10.1182/blood-2009-10-230060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, et al. Selective chemical probe inhibitor of stat3, identified through structure-based virtual screening, induces antitumor activity. Proc N Acad Sci U S A 2007; 104:7391-6; PMID:17463090; http://dx.doi.org/ 10.1073/pnas.0609757104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fagard R, Metelev V, Souissi I, Baran-Marszak F. STAT3 inhibitors for cancer therapy: have all roads been explored? Jak-Stat 2013; 2:e22882; PMID:24058788; http://dx.doi.org/ 10.4161/jkst.22882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhao M, Jiang B, Gao FH. Small molecule inhibitors of STAT3 for cancer therapy. Curr Med Chem 2011; 18:4012-8; PMID:21824090; http://dx.doi.org/ 10.2174/092986711796957284 [DOI] [PubMed] [Google Scholar]
- 124. de la Iglesia N, Konopka G, Lim KL, Nutt CL, Bromberg JF, Frank DA, Mischel PS, Louis DN, Bonni A. Deregulation of a STAT3-interleukin 8 signaling pathway promotes human glioblastoma cell proliferation and invasiveness. J Neurosci 2008; 28:5870-8; PMID:18524891; http://dx.doi.org/ 10.1523/JNEUROSCI.5385-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]


