Abstract
Porosomes are the universal secretory portals at the cell plasma membrane, where membrane-bound secretory vesicles transiently dock and fuse to expel intravesicular contents to the outside during cell secretion. In the past decade, the neuronal porosome complex, a 10–15 nm cup-shaped lipoprotein structure has been isolated, its partial composition and 3D contour map determined, and it has been functionally reconstituted into artificial lipid membrane. Here we further determine the composition of the neuronal porosome proteome using immunoisolation and gel filtration chromatography, followed by tandem mass spectrometry. Results from the study demonstrate nearly 40 proteins to constitute the neuronal porosome proteome. Furthermore, interaction of proteins within the porosome and their resulting arrangement is predicted. The association and dissociation of proteins at the porosome following stimulation of cell secretion demonstrates the dynamic nature of the organelle.
Keywords: Neuronal Porosome Proteome, Porosome Dynamics, Cell Secretion, Tandem Mass Spectrometry
Graphical Abstract
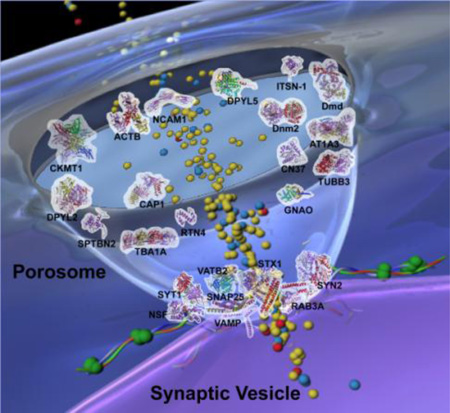
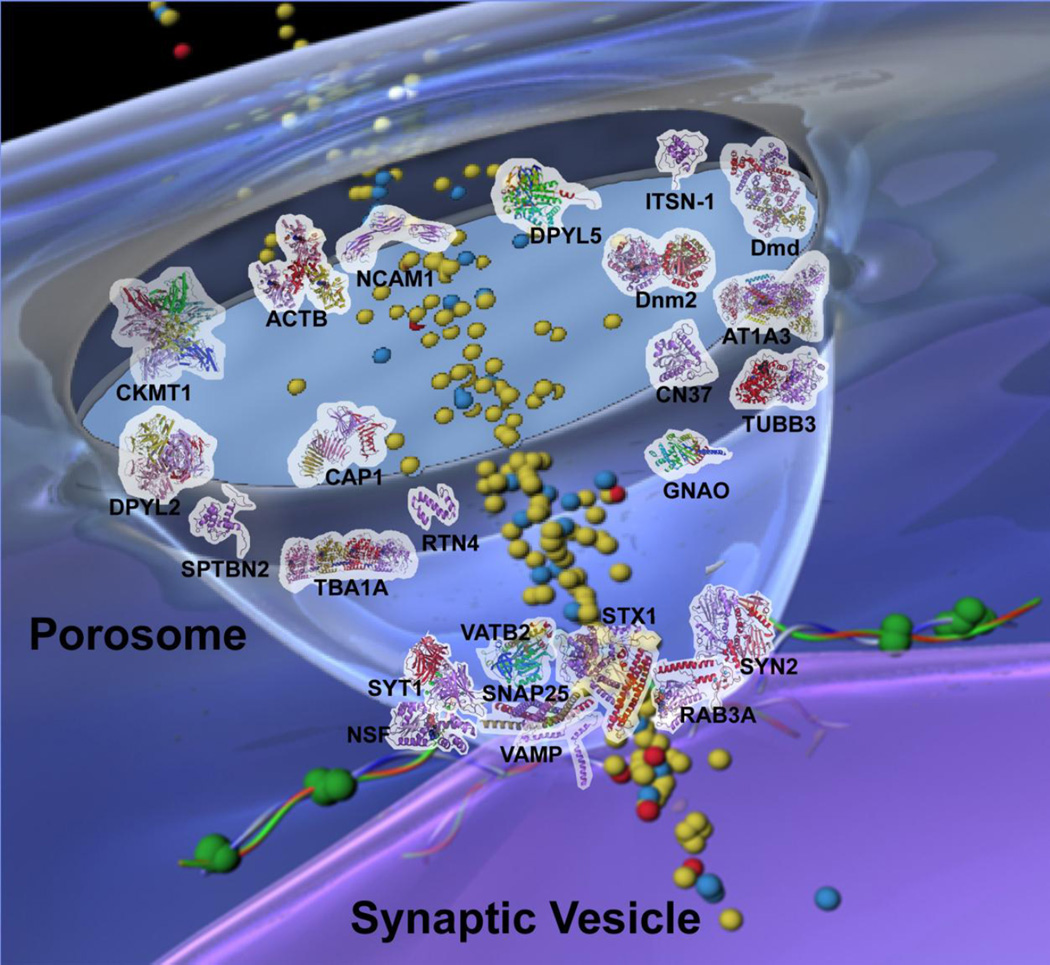
Schematic drawing of neuronal porosome with docked synaptic vesicle. Distribution of key proteins within the 10–15 nm cup-shaped porosome is obtained from experimental results and evidence view of predicted interactions.
1. Introduction
It is well established that the cellular organelle called porosomes, are present at the cell plasma membrane in neurons, exocrine, endocrine, and neuroendocrine cells, where membrane-bound secretory vesicles transiently dock and fuse to expel their contents to the outside during cell secretion [1–7]. Porosomes therefore have been demonstrated to be secretory portals at the plasma membrane in cells [8,9]. The overall morphology (Fig. 1), partial composition, and reconstitution of porosome in exocrine pancreas and in neurons are well documented [2–7], and the 3D contour map of the assembly of proteins within the structure has also been determined [10]. The supramolecular lipoprotein structure of the porosome has been known to include SNAP-23/25, syntaxin, synaptotagmin, the ATPase NSF, cytoskeletal proteins such as actin, α-fodrin, and vimentin, calcium channels β3 and α1c, and chloride ion channels CIC2, CIC3, and their isoforms [3–6]. Recent studies further demonstrate cholesterol to be an integral component of the neuronal porosome complex, required for retaining its biomolecular integrity, and its intramolecular interactions among various constituent proteins [4]. In agreement it has been demonstrated that even in β-cells of the endocrine pancreas, depletion of plasma membrane cholesterol results in the inhibition of insulin secretion [11]. It has also been demonstrated that t-SNAREs and calcium channels interact and are present at the base of the porosome complex [5,12], and actin present at the opening of the structure, enables pore dilation during cell secretion in exocrine and neuroendocrine cells [5,7]. Similarly, the centrally located plug in the neuronal porosome complex regulating the opening and closing of the organelle [13], involving cytoskeletal as well as motor proteins [3].
Fig. 1.
Structure and organization of the neuronal porosome complex at the nerve terminal [33]. (A) Low resolution AFM amplitude image Bar=1mm (A) and high resolution AFM amplitude image Bar=100 nm (B) of isolated rat brain synaptosomes in buffered solution. (C) Electron micrograph of a synaptosome [3], Bar=100 nm. (D) Structure and arrangement of the neuronal porosome complex facing the outside (Fig. D top left), and the arrangement of the reconstituted complex in PC:PS membrane (Fig. D top right). Lower panels are two transmission electron micrographs demonstrating synaptic vesicles (SV) docked at the base of cup-shaped porosome, having a central plug (red arrowhead) [10]. (E) EM, electron density, and 3D contour mapping (Fig. E), provides at the nanoscale, the structure and assembly of proteins within the complex [10]. (F) AFM micrograph of inside-out membrane preparations of isolated synaptosome. Note the porosomes (red arrowheads) to which synaptic vesicles are found docked (blue arrow head) [3]. (G) High-resolution AFM micrograph of a synaptic vesicle docked to a porosome at the cytoplasmic compartment of the presynaptic membrane [3]. (H) AFM measurements (n=15) of porosomes (P, 13.05±0.91) and synaptic vesicles (SV, 40.15±3.14) at the cytoplasmic compartment of the presynaptic membrane [4]. (I) Photon correlation spectroscopy (PCS) on immunoisolated neuronal porosome complex, demonstrating their size to range from 12 to 16 nm [4]. (J) Schematic illustration of a neuronal porosome at the presynaptic membrane, showing the eight ridges connected to the central plug [4].
Similar to porosome isolation from the exocrine pancreas [5,6], the neuronal porosome complex [3] has been immunoisolated from detergent-solubilized synaptosome preparations, using antibody specific to a neuronal t-SNARE. Electrophoretic separation of the immunoisolated neuronal porosome complex, has previously demonstrated the presence of approximately a dozen distinct SYPRO Ruby-stained protein bands [3]. Electrotransfer of the resolved proteins onto nitrocellulose membrane, followed by immunoblot analysis using various antibodies, have further demonstrated the presence of nine proteins, namely SNAP-25, the P/Q-type calcium channel, actin, syntaxin-1, synaptotagmin-1, vimentin, the N-ethylmaleimide-sensitive factor (NSF) [5,6,14], the chloride channel CLC-3, and the alpha subunit of the heterotrimeric GTP-binding Gαo [3]. Some of the identified proteins have been implicated to interact in earlier studies [5,6,15–18], To further test whether the complete porosome complex is immunoisolated, the immunoisolated peparations have also been subjected to both structural and functional reconstitution in artificial lipid membrane [3]. When membrane-reconstituted neuronal porosomes are imaged using high-resolution atomic force microscopy (AFM) [3], they demonstrate the presence of intact porosome complexes similar to what is observed at the presynaptic membrane in the native state in isolated synaptosomes (Fig. 1) [3]. Similarly, to assess the functionality of the reconstituted porosome preparations, an EPC9 electrophysiological bilayer setup has been employed [3]. Membrane capacitance is continually monitored throughout experiments involving reconstitution of the bilayer membrane with porosomes, and following addition of isolated synaptic vesicles to the cis compartment of the bilayer chamber. A large number of synaptic vesicles are demonstrated to fuse at these porosome-reconstituted bilayers, reflected in stepwise increase in membrane capacitance [3]. Also as would be expected of transient fusion of synaptic vesicles at the porosome base, addition of 50 µM ATP results in NSF-induced t-/v-SNARE disassembly and the consequent release of docked synaptic vesicles, resulting in the return of the bilayers membrane capacitance to resting levels [3]. These results are also echoed in studies using intact neurons, demonstrating single synaptic vesicles fusion transiently and successively without loss of vesicle identity [19]. Additionally in neurons, three SNARE pairs were first identified at the porosome base to be involved in neurotransmitter release [20]. In agreement, subsequent studies further demonstrated that neurons require three SNARE pairs for neurotransmitter release [20–23], whereas in pancreatic acinar cells, due to the large size of secretory vesicles and porosomes, several more SNARE pairs would be required to establish the t-/v-SNARE ring complex, for establishing continuity between the secretory vesicle membrane and the porosome base [24,25]. To elucidate the molecular underpinnings of porosome structure-function, the neuronal porosome complex, the smallest and most abundant porosome identified so far, was used in the current study. Porosomes were isolated both immunochemically and using gel filtration chromatography. Results from the study demonstrate the presence of additional proteins constituting the neuronal porosome proteome, and their predicted interactions and arrangement within the complex. Additionally, the association and dissociation of proteins at the porosome complex following stimulation of cell secretion, demonstrates the dynamic nature of the organelle.
2. Materials and Methods
2.1. Synaptosome preparation
Synaptosomes were prepared from rat brains according to published methods [3,4]. For each experiment, Sprague-Dawley rats weighing 100–150 g were euthanized by CO2 inhalation, with all animal procedures preapproved by the Institution Animal Care & Use Committee (IACUC). Whole brain were isolated and placed in ice-cold buffered sucrose solution (5 mM Hepes, pH 7.4, 0.32 M sucrose) supplemented with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The brain tissue was homogenized using 8–10 strokes in a Teflon-glass homogenizer, and the total homogenate was centrifuged for 3 min at 2,500 x g. The resulting supernatant fraction was further centrifuged for 15 min at 14,500 x g, to obtain a pellet. The resultant pellet was resuspended in buffered sucrose solution, and loaded onto a 3–10–23% Percoll gradient. After centrifugation at 28,000 x g for 6 min, the enriched synaptosome fraction at the 10–23% Percoll gradient interface was collected for the study.
2.2. Immunoisolation of the neuronal porosome complex
To isolate the neuronal porosome complex, SNAP-25 specific antibody conjugated to protein A-sepharose® was utilized. Synaptosomes and brain tissue was used for porosome immunoisolation of porosome complexes. For each immunoisolation using synaptosomes, 1 mg of Triton-Lubrol-solubilized synaptosome was used. Protein was estimated by the Bradford method [26]. Similarly for porosome isolation from unstimulated (control) and stimulated (30 mM KCl) brain slices, the tissue was solubilized in Triton/Lubrol solubilization buffer (0.5% Lubrol; 1 mM benzamidine; 5 mM Mg-ATP; 5 mM EDTA; 0.5% Triton X-100, in PBS) supplemented with protease inhibitor mix (Sigma, St. Louis, MO). SNAP-25 antibody conjugated to the protein A- sepharose® was incubated with 1 mg of the solubilized fractions for 1 h at room temperature followed by three washes of 10 vol of wash buffer (500 mM NaCl, 10 mM Tris, 2 mM EDTA, pH 7.5). The immunoprecipitated sample attached to the immunosepharose beads was eluted using low pH buffer (pH 3.5) to obtain the porosome complex. In both porosome preparations from synaptosomes and brain tissue, the eluted sample was neutralized to obtain a final volume of 200 µl. To the 200 µl immunoisolate from brain, 50 µl of 5X Laemmli reducing sample preparation buffer [27] was added and the mixture boiled for 5 min, cooled, and used for SDS-PAGE. Five, 10 and 20 µl of the sample were resolved using 12.5% SDS-PAGE and electrotransferred to 0.2 mm thick nitrocellulose sheets for immunoblot analysis using specific antibodies. Immunoblot analysis was performed using specific antibodies to dynamin, syntaxin-1 (Alomone Labs, Jerusalem, Israel), NSF (Exalpha Biologicals, Boston, MA), and Gαo (Santa Cruz Biotechnology, Santa Cruz, CA). The nitrocellulose membrane was incubated for 1 h at room temperature in blocking buffer (PBST; 5% non-fat milk in PBS containing 0.1% Triton X-100), and immunoblotted for 2 h at room temperature with the primary antibody. The immunoblotted nitrocellulose sheets were washed in PBS containing 0.1% Triton X-100 and were incubated for 1 h at room temperature in horseradish peroxidase-conjugated secondary antibody at a dilution of 1:2000 in PBST. The immunoblots were then washed five times (10ml/wash, 5 min/wash) in the PBST, processed for enhanced chemiluminescence using the Enhanced Chemiluminescence kit (Amersham, Arlington Heights, IL) and photographed using Blue Bio premium autoradiography film. Films were developed in a Kodak X-Omat automatic film processor (Eastman Kodak, Rochester, NY). To identify the different protein bands resolved by SDS-PAGE, fluorescent SYPRO Ruby (Molecular Probes, Eugene, OR) protein staining of the gels was performed.
2.3. Isolation of the neuronal porosome using gel filtration chromatography
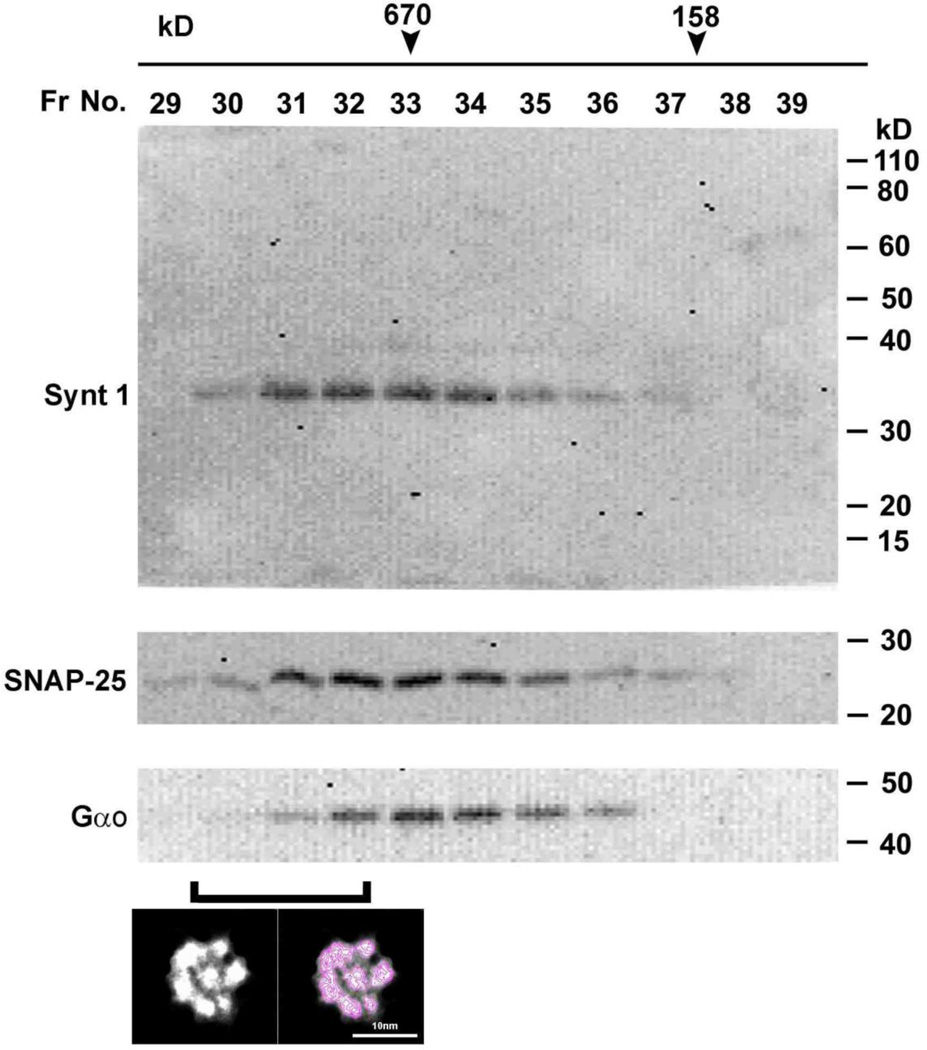
Freshly isolated synaptosome membrane preparations from rat brain was solubilized in ice-cold solubilization buffer (0.5% Lubrol; 1 mM benzamidine; 5 mM Mg-ATP; 5 mM EDTA; 0.5% Triton X-100, in PBS), and 1 mg of total solubilized protein in a volume of 300 µl was loaded onto a calibrated 70 ml G-200 Sephadex gel filtration column. After void volume, 20 fractions each of 1.5 ml were collected and subsequently assayed for the presence of various porosome proteins using immunoblot analysis, and the porosome using negative staining electron microscopy (EM) (Fig. 3).
Fig. 3.
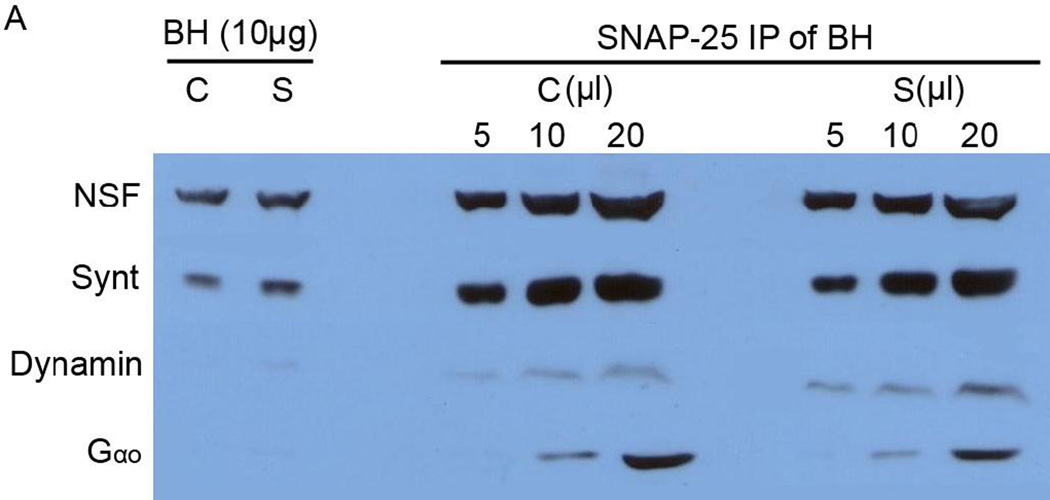
Immunoblot analysis of total rat brain homogenate and SNAP-25 immunoisolated porosomes from control (C) and KCl-stimulated (S) rat brain slices. Approximately equal amounts of NSF, syntaxin-1, dynamin and Gαo-immunoreactivity is seen in both control and stimulated brain tissue. However, the association and dissociation of dynamin and Gαo respectively from the neuronal porosome (5, 10, and 20 µl of SNAP-25 immunoisolates) following stimulation, reflects the dynamic nature of the porosome proteome. Note NSF, syntaxin, dynamin, and Gαo, are greatly enriched in the neuronal porosome complex.
2.4. Negative staining EM
Electron microscopy was performed as previously published [4]. Six micro liter aliquots of porosome (fractions #30–32) samples at 12 µg/ml concentration were adhered to carbon-coated, 400-mesh copper grids previously rendered hydrophilic by glow discharge. The grids were washed with five successive drops of deionized water and then exposed to three successive drops of 2% (w/v) uranyl nitrate for 1.5 min (Ted Pella, Tustin, CA). Images at 80,000x magnification were recorded at defocus of 2.5 and 0.8 mm, respectively on 4K x 4K Gatan UltraScan CCD under low electron dose conditions using a Tacnai 20 electron microscope (Philips Electron Optics/FEI, Eindhoven, The Netherlands) operating at 200 kV (Fig. 5).
Fig. 5.
The elution profile of the neuronal t-SNARE proteins syntaxin-1 (33 kDa) and SNAP-25 (25 kDa) in solubilized synaptosomal membrane preparation resolved on a G-200 sizing column. Note the co-localization of the 44 kDa GTP-binding protein Gαo with t-SNAREs in the fractions. Synaptosomal membrane was solubilized in Triton/Lubrol (1% w/v) PBS, and loaded onto G-200 Sephadex gel filtration column. Fractions 20 to 50 were collected and assayed for t-SNAREs following SDS-PAGE and immunoblot analysis. Note the elution of syntaxin-1, SNAP-25, and Gαo in fractions 30 through 36. As previously demonstrated (Cho et al, 2007), the neuronal porosome appears to be a >650 kDa complex eluted in fractions 30–32.
2.5. Tryptic digestion of isolated neuronal porosomes
In studies using in-solution digestion, purified neuronal porosomes were solubilized in lysis buffer, followed by precipitation in cold acetone as previously described [28]. The resulting protein pellets (25 µg) were solubilized in 8M urea, 0.4% SDS and 250 mM TEAB, a potent buffer system used in our previous studies for membrane and membrane-associated proteins [29–31]. After reduction and cysteine-blocking with 55 mM iodoacetamide, trypsin was added to the diluted sample solution (1:10 w/w) for overnight digestion at 37 °C. In-gel digestion of SYPRO-Ruby stained bands was performed same as previously published [32].
2.6. Matrix-assisted laser desorption ionization (MALDI)
Mass spectrometry was performed using the Applied Biosystems (ABI, Carlsbad, CA) 4700 Proteomics Analyzer (TOF/TOF) in positive ion mode. As previously described [32], a fraction of the tryptic peptides from each gel band was spotted onto a MALDI target plate for mass spectrometric analysis. Peptide mass fingerprints were collected for each well and the four most intense peaks above S/N of 60 were selected for MS/MS analysis. After the MS and MS/MS, spectra were processed using 4700 Exporer™ software (v2.0, Applied Biosystems). The monoisotopic peak lists generated in ABI’s GPS Explorer™ v2.0, was submitted to the GPS Explorer™ v2.0 search tool (based on MASCOT) for protein identities. The Non-redundant Protein Database, NCBInr, was searched using the following parameters for: 0 or 1 missed cleavage by trypsin, carboxyamidomethylation of cysteines as fixed modification, and methionine oxidations, N-terminal protein acetylation, Pyro-glu (N-term E), Pyro-glu (N-term Q) as variable modifications.
2.7. LC-MS/MS analysis and database search
After a detergent removal procedure, tryptic peptides were separated by reverse phase chromatography using Magic C18 column (Michrom, Auburn, CA), followed by ionization with the ADVANCE ion source (Michrom, Auburn, CA), and then analyzed in an LTQ-XL mass spectrometer (Thermo Scientific, Barrington, IL). Six abundant species were fragmented with collision-induced dissociation. Data analysis was performed using Proteome Discoverer 1.1 (Thermo) which incorporated the Mascot algorithm (Matrix Science, Boston, MA). The NCBI database was used against rat protein sequences and a reverse decoy protein database was run simultaneously for false discovery rate (FDR) determination. A duplicate porosome sample was also analyzed by nanoLC-MS/MS. In this case, the tryptic peptides were separated on a reversed-phase C18 column with a 90 min gradient using the Dionex Ultimate™ HPLC system (Thermo Scientific, Barrington, IL). Then the MS and MS/MS spectra were acquired on an Applied Biosystems QSTAR XL mass analyzer using information dependent acquisition mode. A MS scan was performed from m/z 400–1,500 for 1s followed by product ion scans on two most intense multiply charged ions. Peaklists were submitted to Mascot server to search against the NCBInr database for rat sequences with carbamidomethyl (C) used as a fixed modification and oxidation (M), N-acetylation (protein N terminus) as variable modifications. Secondary analysis of both the LTO XL and QSTAR XL were next performed using Scaffold (Proteome Software, Portland, OR). A fixed modification of +57 on cysteine (carbamidomethylation) and variable modifications of +16 on methionine (oxidation) and +42 on protein n-terminus (acetylation) were included in the database search. Minimum protein identification probability was set at ≥95% with 2 unique peptides at 95% minimum peptide identification probability.
3. Results and Discussion
Neuronal communication depends on the fusion of 40–50 nm in diameter membrane-bound synaptic vesicles containing neurotransmitters at the base of porosomes in the presynaptic membrane of the nerve terminal or synaptosomes. Data in Figure 1 are previously published [3,4,10] AFM micrographs, electron micrographs (EM), and photon correlation spectroscopy information on native, and immunoisolated, and lipid membrane-reconstituted neuronal porosome complexes, presented for clarity and for the benefit of the reader new to the field. Figures 1A and 1B are low-resolution AFM micrographs (Figs. 1A, B) of isolated synaptosomes from rat brain tissue. An EM micrograph of isolated synaptosome from an earlier study is presented (Fig. 1C) [3], to illustrate the mushroom-shape of the synaptosome as demonstrated in the AFM micrographs (Figs. 1A, B), and the presence of 40–50 nm in diameter membrane-bound synaptic vesicles within, docked at the base of cup-shaped porosomes in the presynaptic membrane. Figures 1C through 1J from earlier studies [3.4.10] demonstrate the presence of 12–17 nm in diameter cup-shaped neuronal porosomes at the presynaptic membrane. Using SNAP-25-specific antibody, the immunoisolation of neuronal porosomes from Triton/Lubrol-solubilized synaptosome membrane, and demonstration of both structural and functional reconstitution into artificial PC:PS membrane accomplished [3,10]. This structural reconstitution is observed in the Figure 1 AFM micrograph (Fig. 1D right), where all of the characteristic features exhibited in the native porosome structure at the presynaptic membrane are observed following reconstitution (Fig. 1D left). EM, electron density, and 3D contour mapping (Fig. 1E), has further provided at the nanoscale, the structure and assembly of proteins within the neuronal porosome complex [10]. As is clear from both the EM and AFM micrographs, the neuronal porosome exhibits a distinct central plug (Fig. 1D, red arrowhead) that interacts with proteins at the periphery of the structure, conforming to a eight-fold symmetry (Figs. 1D, E). Studies implicate the central plug to be involved in the opening and closing of the porosome to the outside [13]. Furthermore, at the porosome base, where synaptic vesicles dock and transiently fuse (Figs. 1F, G, J), proteins, possibly comprising t-SNAREs, are present in a trimeric ring conformation of three SNARE pairs (Figs. 1E top right) [20]. AFM, in addition to contributing to our understanding of the structure and arrangement of the neuronal porosome complex facing the outside (Figs. 1D top left), and the arrangement of the reconstituted complex in PC:PS membrane (Figs. 1D top right), has also helped in the determination of the porosome structure facing the cytoplasmic compartment of the cell (Figs. 1F, G) [3]. This has been achieved by studying inside-out synaptosome preparations using the AFM (Figs. 1F, G) [3]. These studies reveal the architecture of the 12–15 nm in diameter inverted cup-shaped neuronal porosome structures (Fig. 1F, red arrowhead) arranged in ribbons, some having docked synaptic vesicles (Figs. 1F, blue arrowhead; and 1G, H). Photon correlation spectroscopy (PCS) of the immunoisolated neuronal porosome, has further confirmed the organelle to measure on average, 14–17 nm (Fig. 1I).
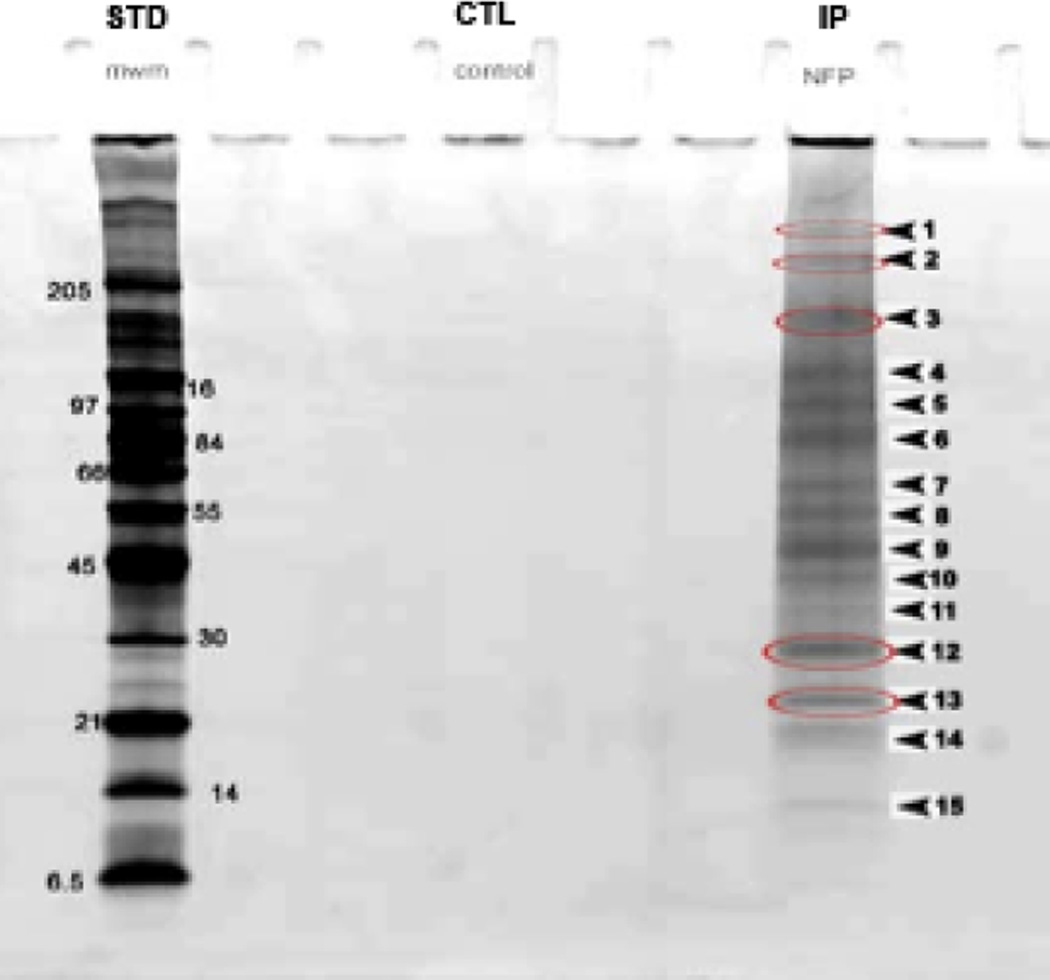
In the current study, to elucidate the molecular underpinnings of the porosome structure-function, the composition of the neuronal porosome complex was further determined using immunoisolation and sizing column gel filtration chromatography, followed by tandem mass spectrometry. Immunoisolated rat brain synaptosomes were resolved using 12.5% SDS-PAGE, followed by fluorescent SYPRO Ruby staining (Fig. 2). Approximately 15 bands were detected, ranging in molecular weights from >205 kDa to >6.5 kDa. Only bands 3–8, yielded new proteins associated with the neuronal porosome complex (Table 1). Nine new proteins namely, tubulin, myosin 7b, spectrin beta chain, creatine kinase, dystrophin, langerin, GTPase activating protein (GAP), intersectin1, and myosin heavy chain 1, were identified. Since mass spectrometry demonstrated the association of intersectin1 with the porosome complex, and since intersectin1 is known to interact with dynamin [34], the association of dynamin with the porosome was hypothesized and confirmed from the results of the study (Fig. 3). Immunoblot analysis of the SNAP-25 immunoisolate from solubilized rat brain homogenate, demonstrate the presence of dynamin and its increased association with the porosome complex following stimulation of neurotransmitter release (Figs. 3, 4). An interesting aspect of the neuronal porosome complex that emerges from the study is the dynamics of its composition during neurotransmitter release process. Immunoblot analysis of the SNAP-25 immunoisolate from solubilized rat brain homogenate from control and KCl-stimulated brain slices, demonstrate the presence of dynamin and its increased association with the neuronal porosome complex following stimulation of neurotransmitter release (Fig. 3). In contrast, a dissociation of Gαo immunoreactivity is demonstrated following neuronal stimulation in the study, since the same blot was used to probe for both antigens: dynamin and Gαo.
Fig. 2.
Photograph of SDS-PAGE of molecular weight standards (left), control (w/o SNAP-25 antibody), and SNAP-25 immunoisolates from solubilized synaptosome preparations. Resolved proteins are stained using fluorescent SYPRO Ruby dye. Note the distinct bands and their relative molecular weights in the SNAP-25 immunoisolate. The band numbers represent the same numbering of the protein digest followed by MALDI-TOF/TOF mass spectrometry presented in Table 1. The bands circled in red were the first to be digested to provide a representation of both higher and lower molecular weight proteins.
Table 1.
MALDI-TOF/TOF results on specific bands in SDS-PAGE-resolved SNAP-25 immunoisolated neuronal porosome complex, obtained using 1% Triton-Lubrol-solubilized rat brain synaptosome preparation.
| Gel Band | Identified Proteins | Accession No. | Confidence Index % (C.I.) |
|---|---|---|---|
| 3 | Tubulin beta chain | 3745822 | C.I. % = 99.83 |
| 4 | Similar to KIAA1512 myosin 7b | 34859107 | C.I. % = 99.39 |
| Similar to Spectrin beta chain (Brain 4) | 34856723 | C.I. % = 98.6 | |
| 5 | Creatine kinase | 31542401 | C.I. % = 99.73 |
| Dystrophin | 18150266 | C.I. % = 70.76 | |
| 6 | Langerin | 17426713 | C.I. % = 60.55 |
| GTPase activating protein (GAP) | 3004867 | C.I. % = 42.98 | |
| Intersectin 1 isoform (ITSN-1) | 47717123 | C.I. % = 52 | |
| 8 | Myosin heavy polypeptide 1 | 7669506 | C.I. % = 21.29 |
Fig. 4.
Schematic drawing depicting the presence and increased association of dynamin with the porosome complex following stimulation of neurotransmitter release. Following stimulation of secretion, synaptic vesicles would dock at the porosome base, develop intravesicular pressure via active transport of water through water channels or aquaporins (AQP) at the vesicle membrane, transiently fuse at the porosome base via SNAREs and calcium, and expel neurotransmitters. After secretion, NSF an ATPase, and dynamin a GTPase, would work synchronously to disassembly t-/v-SNARE complexes and fission the neck of fused vesicles at the porosome base respectively. By this mechanism, partially empty vesicles could go through multiple rounds of docking-fusion-expulsion-dissociation. Unlike protein and peptide containing vesicles, synaptic vesicles have neurotransmitter transporters at the vesicle membrane to rapidly refill vesicles.
Exposure of pancreatic acinar cells [7], growth hormone cells of the pituitary gland [1], or β-cells of the endocrine pancreas [35] to secretagogues, results in the dilation of the porosome opening, and a concomitant increase in cell secretion. Following completion of secretion, porosomes return to their resting size [1,7]. It has been further demonstrated that exposure of cells to cytochalasin B or D, a fungal toxin that inhibits actin polymerization, results in a significant decrease in the size of the porosome opening and a loss in secretion [1,7]. Similarly in neurons, a set of eight protein bundles lining the neuronal porosome cup are present, each connected to a central plug. The vertical movement of the central plug has been implicated for the rapid opening and closing of the porosome to the outside [13]. Studies suggest that the central plug is retracted into the porosome cup in its open conformation, and pushed outward to seal the porosome opening, supporting the hypothesis that the central plug operates as a rapid open-close devise for the complex [13]. These results suggest the involvement of actin and associated proteins such as vimentin [3] and tubulin, spectrin, and dystrophin (current study) in regulating the dynamics of the porosome complex in neurons and possibly in other secretory cells.
It has become increasingly clear that the movement of organelles in cells can be attributed to two groups of motile systems, one based on microtubules, and the other based on actin. Microtubules have been recognized as the “railroad” for movement of organelles over long distances within the cell (several µm), whereas the actin system is responsible for transport over shorter distances, typically from tens to a few hundred nanometers. Thus, microtubule-dependent motors such as kinesin and kinesin-related proteins, and the superfamily of actin-dependent myosin motors, have all been implicated in intracellular organelle transport [36,37]. Myosin motors include the conventional myosin (myosin II) and a large group of unconventional myosins (myosin I, III, V, and VI). In recent years, the prime candidate for secretory vesicle transport in cells has been reported to be the class V myosin motors [38–40]. Myosin V is composed of two heavy chains that dimerise via a coiled-coil motif, located in the stalk region of the heavy chain [41]. The heavy chain contains an amino-terminal actin-binding motor domain [41], followed by a neck region where up to six regulatory light chains can bind. The carboxy-terminus globular domain of the heavy chain is thought to mediate organelle-binding specificity [42]. Interaction between the actin and the microtubule transport system, seems to be a requirement for the correct delivery of intracellular cargo such as secretory vesicles [43–45]. It has been further demonstrated that secretory vesicles in live cells are not free-floating, but tethered to filamentous structures [46]. When membrane-bound secretory vesicles (0.2–1.2 µm in diameter) in live pancreatic acinar cells are trapped at the laser focus of the photonic force microscope and pulled, they are all found tethered to filamentous structures [46]. Mild exposure of cells to nocodazole and cytochalasin B, disrupts the tether. Immunoblot analysis of isolated secretory vesicles, further demonstrates the association of actin, myosin V, and kinesin. These studies demonstrated for the first time that secretory vesicles in live pancreatic acinar cells are tethered and not free-floating, suggesting that following vesicle biogenesis, they are placed on their own railroad track, ready to be transported precisely to their final destination within the cell when required [46]. This makes sense, since precision and regulation are the hallmarks of all cellular process, and therefore would hold true for the transport and localization of subcellular organelles such as secretory vesicles. It is therefore likely, that identification of the association of nebulin, tubulin, spectrin, dystrophin, and myosin, may represent portion of the synaptic vesicle transport and docking machinery at the porosome base. Results from the current study also demonstrates the dynamic nature of the porosome complex in neurons. Dynamin, a GTP-binding protein involved in endocytosis, is found to be present in resting neurons (Fig. 3), suggesting a role in secretion. However, the increased association of dynamin following stimulation of neurotransmission, also clearly supports its role in catalyzing fission of the neck of fused vesicles at the porosome base following expulsion of intravesicular contents. Proteins such as NSF, syntaxin, dynamin, and Gαo, are greatly enriched in the neuronal porosome complex.
When purified rat brain porosomes from two separate experiments using gel filtratio n chromatography (Fig. 5) were analyzed by LC-MS/MS on both LTQ and QSTAR XL, several new proteins constituting the neuronal porosome complex were identified, and the ones that were detected in both experiments are listed in Table 2. Interestingly, plasma membrane calcium-transporting ATPase 1 and 2 are both found to be present in the neuronal porosome complex, suggesting that they may be involved in ATP-mediated expulsion of the extracellular calcium that had entered the cell during stimulation of cell secretion. Similarly, the presence of sodium/potassium-transporting ATPase subunit alpha-3 with the porosome suggests the role of porosome in ATP coupled exchange of sodium and potassium across the plasma membrane (in opposite directions), creating the required electrochemical gradient required for maintaining the membrane resting potential as well as for the regulation of cell volume during secretion. Similarly, 2',3'-cyclic-nucleotide 3'-phosphodiesterase which has been reported to associate with microtubules, and have microtubule-associated protein-like activity, are present in the neuronal porosome. At the porosome, the 2',3'-cyclic-nucleotide 3'-phosphodiesterase can also link tubulin to the cell membrane and participate in regulating the distribution of microtubules in the cytoplasm [47]. Hence, the presence of 2',3'-cyclic-nucleotide 3'-phosphodiesterase at the porosome would therefore be critical to the structural integrity of the complex. Dihydropyrimidinase-related protein present in the porosome proteom is known to play a role in cytoskeletal remodeling and cell polarity [48]. Exactly, how this protein would be involved in neurotransmitter release at the porosome, remains to be established. Similarly, NSF and dynamin at the porosome complex would be required for t-/v-SNARE complex disassembly by NSF, and fission of the established continuity between the lipid vesicle membrane and the porosome base by dynamin, following the transient fusion of synaptic vesicles at the porosome (Fig. 4).
Table 2.
Proteins identified in purified neuronal porosomes in multiple experiments
| Gene symbol |
MW | Protein Name | Found in earlier studies |
|---|---|---|---|
| ACTB | 42 kDa | Actin, cytoplasmic 1 | x, * |
| AT1A3 | 112 kDa | Sodium/potassium-transporting ATPase subunit alpha-3 | |
| AT2B1 | 139 kDa | Plasma membrane calcium-transporting ATPase 1 | |
| AT2B2 | 137 kDa | Plasma membrane calcium-transporting ATPase 2 | |
| BASP1 | 22 kDa | Brain acid soluble protein 1 | |
| CAP1 | 52 kDa | Adenylyl cyclase-associated protein 1 | |
| CN37 | 47 kDa | 2',3'-cyclic-nucleotide 3'-phosphodiesterase | |
| DPYL2 | 62 kDa | Dihydropyrimidinase-related protein 2 | |
| DPYL3 | 62 kDa | Dihydropyrimidinase-related protein 3 | |
| DPYL5 | 62 kDa | Dihydropyrimidinase-related protein 5 | |
| GLNA | 42 kDa | Glutamine synthetase | |
| GNAO | 40 kDa | Guanine nucleotide-binding protein G(o) subunit alpha | x, * |
| NCAM1 | 95 kDa | Neural cell adhesion molecule 1 | |
| NSF | 83 kDa | Vesicle-fusing ATPase | * |
| RAB3A | 25 kDa | Ras-related protein Rab-3A | |
| RTN3 | 102 kDa | Reticulon-3 | |
| RTN4 | 126 kDa | Reticulon-4 | |
| SNP25 | 25 kDa | Synaptosomal-associated protein 25 | x, * |
| STX1A | 33 kDa | Syntaxin-1A | * |
| STX1B | 33 kDa | Syntaxin-1B | * |
| STXB1 | 68 kDa | Syntaxin-binding protein 1 | |
| SYN2 | 63 kDa | Synapsin-2 | |
| SYPH | 33 kDa | Synaptophysin | |
| SYT1 | 47 kDa | Synaptotagmin-1 | * |
| TBA1A | 50 kDa | Tubulin alpha-1A chain | x |
| VAMP1 | 13 kDa | Vesicle-associated membrane protein 1 | |
| VAMP2 | 13 kDa | Vesicle-associated membrane protein 2 | |
| VATB2 | 57 kDa | V-type proton ATPase subunit B, brain isoform |
Purified rat brain porosomes from two separate experiments (Fig. 5, fractions 30–32) were analyzed by LC-MS/MS on both LTQ and QSTAR XL. Only proteins identified in both samples are reported here all of which had protein confidence ≥ 95% with at least two unique peptides each having 95% confidence or above. Proteins also found in earlier immunoisolation studies are marked in a separate column, x indicating proteins identified using MALDI-TOF/TOF;
indicating proteins identified using immunoblot analysis [3].
Since SNAREs are present at the porosome base [6,20,49], and calcium channels have been demonstrated to physically interact with SNAREs at the porosome [12], suggests the presence of both proteins at the porosome base. Similarly, NSF and dynamin would also be present at the porosome base, since they would both be required for the dissociation of the synaptic vesicle from the porosome base following secretion (Fig. 5). Similarly, the centrally located plug in the neuronal porosome complex regulating the opening and closing of the organelle [13] may involve cytoskeletal as well as motor proteins. To further understand the molecular architecture and interaction of proteins within the organelle, known interactions between proteins identified in the porosome were examined (Fig. 6) using the STRING 9.0 database search [50]. STRING 9.0 is a database of known and predicted protein interactions. The interactions include direct (physical) and indirect (functional) associations; derived from four sources: namely, genomic context, high-throughput experiments, conserved co-expression, and from previous knowledge. STRING quantitatively integrates interaction data from these sources for a large number of organisms, and transfers information between these organisms where applicable. The database currently covers 5,214,234 proteins from 1133 organisms. Using the STRING 9.0 database search [50], the STRING maps generated, clearly identifies two clusters of protein-protein interactions in the porosome proteome (Fig. 6). The protein-protein interaction cluster to the left, represent primarily cytoskeletal and signaling proteins, where as the cluster to the right in Fig. is representative of proteins that are primarily involved in membrane fusion. Not surprisingly, this second cluster include both SNAREs, their associated regulatory proteins, as well as calcium channels, suggesting their location to be at the basal compartment of the porosome cup, facing the cytosol (Fig. 7). Heterotrimeric GTP-binging protein and the GTP-binding membrane fission protein dynamin (Dnm2), are present in the left cluster. The presence of dynamine in the left cluster is natural, since it is a microtubule-associated protein. The involvement of Dmn2 in fission of the neck of fused vesicles at the porosome base however, would require their presence at the porosome base. The functional interaction values in the STRING diagram predicted in Fig. 6, represents >99% confidence. Results from the current study, has helped in predicting the possible molecular architecture of the neuronal porosome illustrated in Fig. 7. Future electron crystallography and molecular simulations, will further provide the precise molecular arrangement of proteins within the porosome complex.
Fig. 6.
Schematic drawing depicting the evidence view of predicted interactions between identified proteins within the neuronal porosome proteome and other regulatory proteins. These interactions are generated from inputs of the identified proteins in the neuronal porosome, using STRING 9.0 [50]. STRING 9.0 is a database of known and predicted protein interactions. The interactions include direct (physical) and indirect (functional) associations derived from genomic, high-throughput, conserved co-expression, and earlier knowledge. Note the two clusters of protein-protein interactions identified in the porosome complex. The one cluster to the left, and most likely present at the apical end of the porosome cup are cytoskeletal structure and signalling proteins. The cluster to the right represents proteins that are primarily involved in membrane fusion including SNARE proteins and calcium channels, and therefore their location would be at the basal part of the porosome cup facing the cytosol. Interestingly, heterotrimeric GTP-binging protein and the GTP-binding membrane fission protein dynamin (Dnm2), are present in the left cluster. The presence of dynamin in the left cluster is of little surprise since they are microtubule-associated proteins, and intersectin1 is also known to interact with dynamin. However their involvement in fission of the neck of fused vesicles at the porosome base would require their presence at the base of porosomes. The confidence of the predicted functional interactions shown are >99%.
Fig. 7.
Schematic drawing of the neuronal porosome architecture, associated with a docked synaptic vesicle, and in the process of neurotransmitter release. The possible arrangement of some of the key proteins shown as 3D schematic Richardson diagrams within the cup-shaped neuronal porosome complex, is obtained from experimental results and from the evidence view of predicted interactions using the STRING 9.0 database search. Note most of the structural and signaling proteins are present at the apical domain of the porosome cup in contrast to the membrane fusion proteins and calcium channel found at the base of the organelle.
In summary, results from the present study have further advanced our understanding of the structure-function of the neuronal porosome complex. In addition to previously reported [3] association of actin, Gαo, syntaxin, NSF, synaptophysin, vimentin, calcium and chloride channels, and cholesterol [49], the current study further demonstrates the as sociation of several new proteins, among them dynamin, tubulin, myosin, spectrin, creatine kinase, dystrophin, langerin, GTPase activating protein (GAP), and intersectin1, at the neuronal porosome. The association and dissociation of dynamin and Gαo respectively, from the neuronal porosome during neurotransmitter release, reflects the dynamic nature of the organelle. Furthermore, results from this study helped in predicting the molecular architecture of the neuronal porosome complex, paving the way for future studies to solve the molecular architecture of the organelle.
Highlights.
Neuronal porosome proteome are universal secretory portals in cells.
Here we determine the composition of the neuronal proteome.
Molecular architecture and dynamics of the neuronal porosome is also reported.
Acknowledgements
Financial support from NIH NS-39918 (BPJ) and Wayne State University Startup (XC) is gratefully acknowledged. We thank Dr. Paul Stemmer, Director of the Proteomics Core at Wayne State University, for help in database searches using Mascot and Scaffold.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Jin-Sook Lee was involved in the isolation of the rat neuronal porosome complex from solubilized synaptosomal membrane preparations using gel filtration chromatography and for immunoblot analysis of the fractions. Aleksandar Jeremic was involved in the immunoisolation of the rat neuronal porosome and MALDI-TOF experiments to identify associated proteins. Leah Shin was involved in the stimulation of rat brain slices, and determination of the association of dynamin to porosome following stimulation. Won Jin Cho was involved in AFM imaging of the isolated neuronal porosome complex, and for the design of the schematic drawings. Xuequn Chen was involved in proteomic sample preparation and performing the LC-MS/MS analysis on neuronal porosomes isolated using gel filtration chromatography. Xuequn Chen and Bhanu P. Jena helped in the development and design of experiments, and writing of the manuscript. Conflict-of interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1.Cho SJ, Jeftinija K, Glavaski A, Jeftinija S, Jena BP, Anderson LL. Structure and dynamics of the fusion pores in live GH-secreting cells revealed using atomic force microscopy. Endocrinology. 2002;143:1144–1148. doi: 10.1210/endo.143.3.8773. [DOI] [PubMed] [Google Scholar]
- 2.Cho SJ, Quinn AS, Stromer MH, Dash S, Cho J, Taatjes DJ, et al. Structure and dynamics of the fusion pore in live cells. Cell Biol Int. 2002;26:35–42. doi: 10.1006/cbir.2001.0849. [DOI] [PubMed] [Google Scholar]
- 3.Cho WJ, Jeremic A, Rognlien KT, Zhvania MG, Lazrishvili I, Tamar B, et al. Structure, isolation, composition and reconstitution of the neuronal fusion pore. Cell Biol Int. 2004;28:699–708. doi: 10.1016/j.cellbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Cho WJ, Jeremic A, Jin H, Ren G, Jena BP. Neuronal fusion pore assembly requires membrane cholesterol. Cell Biol Int. 2007;31:1301–1308. doi: 10.1016/j.cellbi.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jena BP, Cho SJ, Jeremic A, Stromer MH, Abu-Hamdah R. Structure and composition of the fusion pore. Biophys J. 2003;84:1–7. doi: 10.1016/S0006-3495(03)74949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeremic A, Kelly M, Cho SJ, Stromer MH, Jena BP. Reconstituted fusion pore. Biophys J. 2003;85:2035–2043. doi: 10.1016/S0006-3495(03)74631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider SW, Sritharan KC, Geibel JP, Oberleithner H, Jena BP. Surface dynamics in living acinar cells imaged by atomic force microscopy: Identification of plasma membrane structures involved in exocytosis. Proc Natl Acad Sci U S A. 1997;94:316–321. doi: 10.1073/pnas.94.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jena BP. Molecular machinery and mechanism of cell secretion. Exp Biol Med. 2005;230:307–319. doi: 10.1177/153537020523000504. [DOI] [PubMed] [Google Scholar]
- 9.Jena BP. Secretion machinery at the cell plasma membrane. Curr Opin Struct Biol. 2007;17:437–443. doi: 10.1016/j.sbi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho WJ, Ren G, Jena BP. EM 3D contour maps provide protein assembly at the nanoscale within the neuronal porosome complex. J Microscopy. 2008;232:106–111. doi: 10.1111/j.1365-2818.2008.02088.x. [DOI] [PubMed] [Google Scholar]
- 11.Vikman J, Jeminez-Felyström J, Nyman P, Thelin J, Eliasson L. Insulin secretion is highly sensitive to desorption of plasma membrane cholesterol. FASEB J. 2009;23:58–67. doi: 10.1096/fj.08-105734. [DOI] [PubMed] [Google Scholar]
- 12.Cho WJ, Jeremic A, Jena BP. Direct interaction between SNAP-23 and L-type calcium channel. J Cellular & Mol Med. 2005;9:380–386. doi: 10.1111/j.1582-4934.2005.tb00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho WJ, Lee J-S, Jena BP. Conformation states of the neuronal porosome complex. Cell Biol Int. 2010;34:1129–1132. doi: 10.1042/CBI20100510. [DOI] [PubMed] [Google Scholar]
- 14.Rothman JE. Mechanism of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 15.Faigle W, Colucci-Guyon E, Louvard D, Amigorena S, Galli T. Vimentin filaments in fibroblasts are a reservoir for SNAP-23, a component of the membrane fusion machinery. Mol Biol Cell. 2000;11:3485–3494. doi: 10.1091/mbc.11.10.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano M, Nogami S, Sato S, Terano A, Shirataki H. Interaction of syntaxin with α-fodrin, a major component of the submembranous cytoskeleton. Biochem Biophys Res Commun. 2001;288:468–475. doi: 10.1006/bbrc.2001.5795. [DOI] [PubMed] [Google Scholar]
- 17.Ohyama A, Komiya Y, Igarashi M. Globular tail of myosin-V is bound to vamp/synaptobrevin. Biochem Biophys Res Commun. 2001;280:988–991. doi: 10.1006/bbrc.2001.4236. [DOI] [PubMed] [Google Scholar]
- 18.Prekereis R, Terrian DM. Brain myosin V is a synaptic vesicle-associated motor protein: evidence for a Ca-C2-dependent interaction with the synaptobrevin-synaptophysin complex. J Cell Biol. 1997;137:1589–1601. doi: 10.1083/jcb.137.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aravanis AM, Pyle JL, Tsien RW. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–647. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- 20.Cho WJ, Shin L, Ren G, Jena BP. Structure of membrane-associated neuronal SNARE complex: Implication in neurotransmitter release. J Cell Mol Med. 2009;13:4161–4165. doi: 10.1111/j.1582-4934.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho WJ, Lee J-S, Ren G, Zhang L, Shin L, Manke CW, Potoff J, Kotaria N, Zhvania MG, Jena BP. Membrane-directed molecular assembly of the neuronal SNARE complex. J Cell Mol Med. 2011;15:31–37. doi: 10.1111/j.1582-4934.2010.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohrmann R, de Wit H, Verhage M, Neher E, Sørensen JB. Fast vesicle fusion in living cells requires at least three SNARE complexes. Science. 2010;330:502–505. doi: 10.1126/science.1193134. [DOI] [PubMed] [Google Scholar]
- 23.Shi L, Shen Q-T, Kiel A, Wang J, Wang H-W, Melia TJ, et al. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science. 2012;335:1355–1359. doi: 10.1126/science.1214984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho S-J, Kelly M, Rognlien KT, Cho JA, Hörber JKH, Jena BP. SNAREs in opposing bilayers interact in a circular array to form conducting pores. Biophys J. 2002;83:2522–2527. doi: 10.1016/s0006-3495(02)75263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho WJ, Jeremic A, Jena BP. Size of supramolecular SNARE complex: membrane-directed self-assembly. J Am Chem Soc. 2005;127:10156–10157. doi: 10.1021/ja052442m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Sans D, Strahler JR, Michailidis G, Ernst SA, Andrews PC, Williams JA. Quantitative proteomics analysis of Rough Endoplasmic Reticulum from normal and acute pancreatitis rat pancreas. J Proteome Res. 2010;9(2):885–896. doi: 10.1021/pr900784c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Ulintz P, Simon E, Williams JA, Andrews PC. Global topology analysis of pancreatic zymogen granule membranes. Molecular & Cellular Proteomics. 2008;7(12):2323–2336. doi: 10.1074/mcp.M700575-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Andrews PC. Quantitative proteomics analysis of pancreatic zymogen granule membrane. In Volume: Membrane Proteomics Methods in Molecular Biology. 2009;527:327–338. doi: 10.1007/978-1-60327-310-7_23. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Simon E, Xiang Y, Kachman M, Andrews PC, Wang Y. Quantitative proteomics analysis of cell cycle regulated Golgi disassembly and reassembly. J Biol Chem. 2010;285(10):7197–7207. doi: 10.1074/jbc.M109.047084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Walker AK, Strahler JR, Simon ES, Tomanicek-Volk SL, Nelson BB, et al. Organellar proteomics: analysis of pancreatic zymogen granule membranes. Molecular & Cellular Proteomics. 2006;5(2):306–312. doi: 10.1074/mcp.M500172-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Jena BP. Porosome: the universal secretory portal in cells. Biomed Rev. 2011;21:1–15. [Google Scholar]
- 34.Evergren E, Gad H, Walther K, Sundborger A, Tomillin N, Shupliakov O. Intersectin is a negative regulator of dynamin recruitment to the synaptic endocytic zone in the central synapse. J Neurosci. 2007;27:379–390. doi: 10.1523/JNEUROSCI.4683-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jena BP. Discovery of the porosome: revealing the molecular mechanism of secretion and membrane fusion in cells. J Cell Mol Med. 2004;8:1–21. doi: 10.1111/j.1582-4934.2004.tb00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuznetsov SA, Langford GM, Weiss DG. Actin-dependent organelle movement in squid axoplasm. Nature. 1992;356:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- 37.Schroer TA, Sheetz MP. Functions of microtubule-based motors. Annu Rev Physiol. 1991;53:629–652. doi: 10.1146/annurev.ph.53.030191.003213. [DOI] [PubMed] [Google Scholar]
- 38.Evans LL, Lee AJ, Bridgman PC, Mooseker MS. Vesicle-associated brain myosin-V can be activated to catalyze actin-based transport. J Cell Sci. 1998;111:2055–2066. doi: 10.1242/jcs.111.14.2055. [DOI] [PubMed] [Google Scholar]
- 39.Rudolf R, Kögel T, Kuznetsov SA, Salm T, Sclicker O, Hellwig A, et al. Myosin Va facilitates the distribution of secretory granules in the F-actin rich cortex of PC12 cells. J Cell Sci. 2003;116:1339–1348. doi: 10.1242/jcs.00317. [DOI] [PubMed] [Google Scholar]
- 40.Varadi A, Tsuboi T, Rutter GA. Myosin Va transports dense core secretory vesicles in pancreatic MIN6 β cells. Mol Biol Cell. 2005;16:2670–2680. doi: 10.1091/mbc.E04-11-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheney RE, O’Shea MK, Heuser JE, Coelho MV, Wolenski JS, Espreafico EM, et al. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 1993;75:13–23. doi: 10.1016/S0092-8674(05)80080-7. [DOI] [PubMed] [Google Scholar]
- 42.Reck-Peterson SL, Provance DW, Jr, Mooseker MS, Mercer JA. Class V myosins. Biochim Biophys Acta. 2000;1496:36–51. doi: 10.1016/s0167-4889(00)00007-0. [DOI] [PubMed] [Google Scholar]
- 43.Rudolf R, Salm T, Rustom A, Gerdes H-H. Dynamics of immature secretory granules. Mol Biol Cell. 2001;12:1353–1365. doi: 10.1091/mbc.12.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirschberg K, Miller CM, Ellenberg J, Presley JF, Siggia ED, Phair RD, et al. Kinetic analysis of secretory protein traffic and characterization of Golgi to plasma membrane transport intermediates in living cells. J Cell Biol. 1998;143(6):1485–1503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manneville JB, Etienne-Manneville S, Skehel P, Carter T, Ogden D, Ferenczi M. Interaction of the actin cytoskeleton with microtubules regulates secretory organelle movement near the plasma membrane in human endothelial cells. J Cell Sci. 2003;116:3927–3938. doi: 10.1242/jcs.00672. [DOI] [PubMed] [Google Scholar]
- 46.Abu-Hamdah R, Cho WJ, Hörber JKH, Jena BP. Secretory Vesicles in Live Cells are not Free-Floating but Tethered to Filamentous Structures: A Study Using Photonic Force Microscopy. Ultramicroscopy. 2006;106:670–673. doi: 10.1016/j.ultramic.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Bifulco M, Laezza C, Stingo S, Wolff J. 2’,3’-Cyclic nucleotide 3’-phosphodiesterase: a membrane-bound, microtubule-associated protein and membrane anchor for tubulin”. Proc Natl Acad Sci U S A. 2002;99:1807–12. doi: 10.1073/pnas.042678799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- 49.Jeremic A, Cho WJ, Jena BP. Cholesterol is critical to the integrity of neuronal porosome/fusion pore. Ultramicroscopy. 2006;106:674–677. doi: 10.1016/j.ultramic.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, Minguez P, et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acid Res. 2011;39:D561–D568. doi: 10.1093/nar/gkq973. [DOI] [PMC free article] [PubMed] [Google Scholar]