The main function of the exocrine pancreas is to produce digestive enzymes, which normally are secreted as inactive zymogens and become activated after reaching the duodenum. Pancreatitis is a relatively common and potentially fatal inflammatory disease of the exocrine pancreas. Its mild forms are self-limited, but severe pancreatitis has 10%–30% mortality. The pathogenesis of pancreatitis remains obscure, and there are no specific treatments. The disease is believed to initiate in acinar cells, the main cell type of the exocrine pancreas. Hallmark responses of acute pancreatitis are the premature, intra-acinar cell activation of trypsinogen (i.e., its conversion from zymogen to active trypsin), vacuole accumulation, inflammation and death of acinar cells through both necrosis and apoptosis.
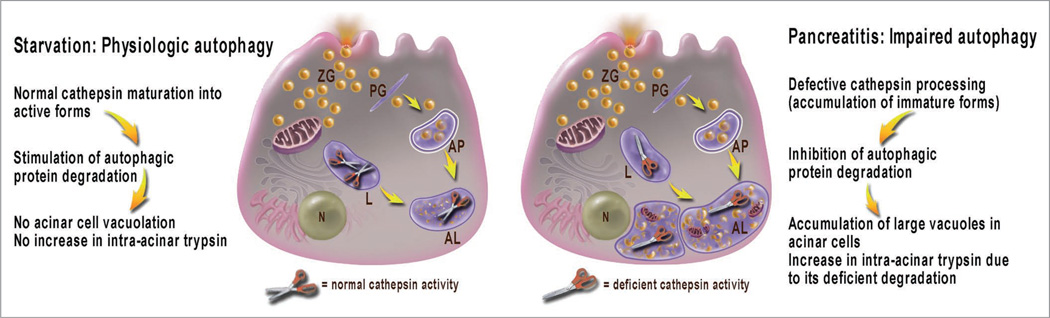
The idea of our study was to compare autophagic responses in experimental acute pancreatitis with physiological autophagy triggered by starvation. We used rodent as well as cell (“in vitro”) models of pancreatitis, in particular those induced by supraphysiological doses of cholecystokinin, the main secretagogue for acinar cells, or its analog, cerulein. We found that macroautophagy (hereafter referred to as autophagy) is impaired in acute pancreatitis, which mediates both acinar cell vacuolation and trypsinogen activation. The results indicate that autophagy progression/resolution (flux) is retarded in pancreatitis due to deficient lysosomal degradation caused by impaired cathepsin processing (Fig. 1).
Figure 1.
Autophagy is induced with both starvation and pancreatitis, but the autophagic flux is retarded in pancreatitis due to inefficient lysosomal degradation resulting from impaired cathepsin processing/maturation. The illustration depicts a pancreatic acinar cell; open and closed scissors represent, respectively, normal or deficient cathepsin activity. AL, autolysosome; AP, autophagosome; L, lysosome; N, nucleus; PG, phagophore; ZG, zymogen granule.
Accumulation of large vacuoles in acinar cells is a long-noted feature of both experimental and human pancreatitis; however, the mechanism of their formation and relation to other pathological responses of pancreatitis remained unclear. We show that these vacuoles have characteristics of autophagic vacuoles (e.g., a double membrane and the presence of LC3-II). Compared with starvation, pancreatitis not only induces many more vacuoles, but they are also strikingly larger. Most contain partially digested material, in particular, zymogen granules (ZG). These features suggested to us that autophagic flux is impaired in pancreatitis.
To prove this we used several approaches. We have measured that the rate of long-lived protein degradation is markedly stimulated in starved acinar cells but it is ~2-fold inhibited in pancreatitis, providing direct evidence for inefficient autophagy. The decrease in autophagic protein degradation is associated with accumulation of LC3 dots in acinar cells. Another manifestation of retarded autophagic flux is accumulation of lysosomal markers, such as Rab7 and LAMP-2, in the ZG-enriched (i.e., heavier) pancreatic subcellular fraction. This is consistent with accumulation of “heavy” autolysosomes (containing incompletely degraded material) due to inefficient lysosomal clearance. In contrast, starvation does not affect the subcellular distribution of lysosomal markers.
We considered two mechanisms that might underlie autophagic flux impairment in pancreatitis. One possibility could be a blockade of autophagosome fusion with lysosomes, which occurs in lysosomal diseases. However, the presence of a great number of vacuoles with partially degraded material in the pancreas of animals with pancreatitis (as well as in human tissue) argues against this possibility. Indeed, colocalization of Rab7 or LAMP-2 with LC3-II increases in models of pancreatitis, which would not occur if autolysosome formation were blocked.
Therefore, we next considered decreased activity of lysosomal hydrolases as a mechanism for retarded autophagic flux in pancreatitis. To this end, we have measured the effects of starvation and pancreatitis on the processing and activities of two major lysosomal hydrolases, cathepsins (Cat) B and L. CatB is relevant for pancreatitis because it proteolytically converts trypsinogen to trypsin; in contrast, until recently there was little known on the role of CatL in pancreatitis. We find that starvation has little effect on pancreatic activities of CatB and CatL and their processing into fully mature, active forms. In contrast, pancreatitis causes a dramatic decrease in enzymatic activities of both cathepsins in the lysosome-enriched pancreatic subcellular fraction, and accumulation of their immature forms. We conclude that defective cathepsin processing and activities mediate the inefficient lysosomal degradation in pancreatitis, resulting in retarded autophagic flux. In fact, pharmacological inhibition of CatB or CatL, or both is sufficient to cause acinar cell vacuolation.
These findings also shed a new light on the mechanism of the pathological, intra-acinar trypsinogen activation, considered a critical initiating event in both human and experimental acute pancreatitis. Our immunofluorescence data show that autophagic vacuoles represent one site of trypsinogen activation, as its marker partially colocalizes with both LC3-II and LAMP-2. Trypsinogen activation in acinar cells is completely blocked by 3-methyladenine; moreover, we notice that a number of dissimilar pharmacological inhibitors of trypsinogen activation are all inhibitors of various stages of autophagy. The link between autophagy and trypsinogen activation has been demonstrated in a recent study on Atg5 null mice from Ken-ichi Yamamura’s group, which proposed enhanced autophagy as the underlying mechanism. However, our data show that it is not the autophagy induction per se but its impairment that is responsible for the pathological manifestations of pancreatitis. Indeed, there is no increase in pancreatic trypsin activity even after prolonged starvation of rats or mice, which greatly stimulates autophagy.
For many years, the paradigm for the pathological trypsinogen activation has been the “colocalization hypothesis” stating that in pancreatitis, CatB becomes “mis-sorted” and thus colocalizes with trypsinogen in some (undefined) ZG-containing compartment(s). However, our immunoblot data show no CatB redistribution from the lysosome-enriched to the ZG-enriched pancreatic subcellular fraction. Instead, our combined immunoblot and enzymatic activity measurements indicate that pancreatitis causes an imbalance between enhanced trypsinogen conversion into trypsin (mediated by CatB) and decreased trypsin degradation (mediated by CatL), resulting in the intra-acinar accumulation of active trypsin. Indeed, a specific CatL inhibitor markedly increases trypsin activity in acinar cells.
Our findings indicate that deficient lysosomal degradation in pancreatitis, rather than “mis-sorting” of CatB or excessive autophagy, is a dominant mechanism for intra-acinar accumulation of active trypsin, thus representing a “paradigm shift.”
In conclusion (Fig. 1), we find that autophagic flux is impaired in acute pancreatitis, mediating two key pathologic responses of this disease: acinar cell vacuolation and the intra-acinar trypsin accumulation. Impaired autophagy, inefficient protein degradation and defective cathepsin maturation render pancreatitis features of a lysosomal disease. Furthermore, the defective autophagy suggests a disordering of the endolysosomal pathway in pancreatitis, which has not been explored and which we hope will be elucidated in the near future.
Abbreviations
- Cat
cathepsin
- ZG
zymogen granule(s)