Abstract
Importance of the field
Effective vascularization of thick three-dimensional engineered tissue constructs is a problem in tissue engineering. As in native organs, a tissue-engineered intra-organ vascular tree must be comprised of a network of hierarchically branched vascular segments. Despite this requirement, current tissue-engineering efforts are still focused predominantly on engineering either large-diameter macrovessels or microvascular networks.
Areas covered in this review
We present the emerging concept of organ printing or robotic additive biofabrication of an intra-organ branched vascular tree, based on the ability of vascular tissue spheroids to undergo self-assembly.
What the reader will gain
The feasibility and challenges of this robotic biofabrication approach to intra-organ vascularization for tissue engineering based on organ-printing technology using self-assembling vascular tissue spheroids including clinically relevantly vascular cell sources are analyzed.
Take home message
It is not possible to engineer 3D thick tissue or organ constructs without effective vascularization. An effective intra-organ vascular system cannot be built by the simple connection of large-diameter vessels and microvessels. Successful engineering of functional human organs suitable for surgical implantation will require concomitant engineering of a ‘built in’ intra-organ branched vascular system. Organ printing enables biofabrication of human organ constructs with a ‘built in’ intra-organ branched vascular tree.
Keywords: organ printing, vascularization, vascular tree, tissue spheroids
1. Introduction
“I have learned to use the word ‘impossible’ with the greatest caution”, Wernher von Braun.
1.1 Vascularization as a main impediment in tissue engineering
As described in a number of recent reviews [1–6], vascularization of thick three-dimensional (3D) tissue constructs is still an unsolved problem in tissue engineering despite almost two decades of intensive efforts. To date, neither sophisticated synthetic nor natural solid scaffolds have proved to be an effective solution to the problem of vascularization. Illustrative of great focus and interest in this problem, which is a constant topic for special sessions at tissue engineering meetings, the National Institute of Biomedical Imaging and Bioengineering has identified design, optimization, and formation of vascular networks in engineered tissues as one of the highest priority topics for the NIH’s 2009 Challenge Grants in Health and Science Research [5]. Thus, it is becoming increasingly recognized that the problem of vascularization is one of the main impediments to successful engineering of thick 3D human organs. It is reasonable to argue that the obvious difficulty in solving the problem of vascularization of thick 3D tissue constructs is a manifestation of the intrinsic limitations of solid-scaffold-based ‘top down’ approaches in tissue engineering. If this is the case, then one logical way to overcome this problem is switching to ‘bottom up’ bioassembly approaches such as using layer-by-layer additive biofabrication technologies like organ printing that will enable engineering of ‘built in’ intra-organ hierarchically branched vascular systems that can perfuse 3D tissue and organ constructs [5–7]. Emerging additive biofabrication technologies open new exciting opportunities and, from a theoretical point of view, provide a logically justifiable and feasible approach to address one of the main unsolved problems in tissue engineering.
1.2 Definition of vascularization in tissue engineering
In tissue engineering, effective vascularization is defined as the formation of a network of perfuseable endothelialized vascular channels capable of delivering oxygen and nutrients and removing waste products to ensure viability and function of tissue engineered constructs. Developmental mechanisms of vascular morphogenesis include vasculogenesis (formation new microvessels from non-endothelial cells, usually stem or progenitor cells) [8], angiogenesis (formation of new microvessels from pre-existing endothelial cells) [9] and arteriogenesis (remodeling of arterioles/small vessels into larger ones) [10]. These mechanisms produce precise intra-organ vascular systems in human organs constructed from hierarchically branched vascular segments of different diameters, not simply microvascular networks. Therefore effective vascularization in tissue engineering applications, especially thick 3D organs like the kidney, must include construction of the entire spectrum of hierarchically branched vascular segments of the intra-organ vascular tree in addition to microvascular segments. It is well established that without differentiation of the vascular system into vessel segments specialized for coaxial and transmural transport, it will not function effectively. An effective transportation system includes both highways and small roads; establishment of a high capillary density in tissue-engineered constructs is not sufficient.
1.3 In vitro vascularization: solid-synthetic-scaffold-based approach
In classic solid-scaffold-based tissue engineering approaches, there are three main strategies to enhance vascularization of tissue engineered constructs: designing solid synthetic porous scaffolds that are highly permissive for angiogenic growth, developing effective endothelial cell seeding technology, and loading scaffolds with angiogenic growth factors. The rationale for these strategies has been clearly described in a number of classic reviews [11,12]. However, these strategies have failed to provide effective vascularization of 3D thick tissue constructs, both separately and in combination [1–4,6,13]. Development of effective technologies for in vitro vasculzarization or endothelial cell seeding of solid scaffolds is probably the most promising approach. Using angiogenesis-permissive and even angiogenesis-promoting hydrogels in composite scaffolds is another possible approach. To date, however, ongoing attempts to improve vascularization of thick tissue constructs using scaffold-based approaches have not produced the desired outcome. There are growing doubts that effective vascularization will be ever accomplished using solid-scaffold-based technologies due to the intrinsic limitations of ‘top down’ approaches in tissue engineering [7].
1.4 In vitro vascularization: natural-derived-scaffold-based approach
After two decades of frustrated efforts aimed at solving the problem of vascularization of tissue engineered constructs using solid synthetic scaffolds, a growing number of tissue engineers have turned to a natural or native scaffold approach. Several recent publications in leading journals have highlighted this switch [14,15] The fact that relatively thin, decellularized, naturally-derived scaffolds can be vascularized in vivo and used as scaffolds for tissue engineering organs such as the urinary bladder has been well documented by the pioneering studies of Dr Anthony Atala’s group at Wake Forest [16]. However, Doris Taylor’s recent report that decellularized rodent hearts can be effectively seeded [15] and eventually vascularized raised difficult questions. First, a source of human cardiac decellularized scaffolds is not known. Second, that decellularized human cadaveric hearts can be effectively recellularized is not proven. Finally, the effective reendothelization of a complete intra-organ vascular system remains to be demonstrated. It has however been demonstrated that introduction of laser-machined micropores helps to improve recellularization of natural-scaffold-derived vascular grafts and enhances its vascularization after implantation [17]. How this technology can be adapted to thick 3D decellularized whole organ scaffolds remains to be seen. There is indirect evidence that targeted recruitment of circulating endothelial or endothelial progenitor cells can be accomplished if specific antibodies are immobilized on the preexisting ECM of the intra-organ vascular system located within the decellularized organ scaffold. However, the possibilities of incomplete endothelization and intimal hyperplasia cannot be excluded [18]. While the likelihood of success of this approach is far from guaranteed, the main impediment to clinical application of this approach will be identification of sufficient numbers of high-quality standardized cadaveric human organs. It is also safe to predict that using unfixed animal-derived (xenogenic) organ scaffolds would be even more challenging from a regulatory point of view.
1.5 In vivo vascularization
In vivo vascularization of tissue-engineered constructs is becoming a popular approach in tissue engineering [2,19–22]. There two main approaches to vascularization of tissue engineered constructs in vivo. If the tissue engineered construct is pre-vascularized in vitro then the main goal for in vivo vascularization is connection of the intraconstruct microvasculature of the engineered tissue with the host microvasculature. Due to inherent limitations, this approach would work well only for relatively thin tissue constructs and, even in that case, effective vascularization would require time. The rate of sprouting angiogenesis is around 1.0 mm per week. Therefore constructs would have to be placed in very close apposition to the preexisting host microvasculature or the process must be stimulated by the addition of angiogenic growth factors. Furthermore, newly formed vessels are very fragile and leaky, generating the associated risk of bleeding or edema. Finally, as mentioned above, effective perfusion of thick 3D tissue constructs requires not just microvasculature but also large-diameter segments of the vascular tree. In the second approach, which could be called ‘surgical engineering’, large diameter vessels create sites for implantation of tissue-engineered constructs and angiogenic sprouts from these vessels gradually will invade and vascularize the tissue-engineered construct [20,21]. If the tissue-engineered construct is prevascularized in vitro, this process can be relatively fast. The obvious advantage of the second approach is that the vascularized tissue construct can be surgically dissected together with a large diameter vessel, making it suitable for surgical implantation [19,21]. This could be a very effective approach for certain applications but the associated medical cost, risk and inconvenience associated with growth of tissue constructs in vivo using the human body as a bioreactor (even in the case of subcutaneous growth) limit the practical feasibility of such an approach.
2. Concept of organ printing
2.1 Definition of organ printing
Organ printing is the biomedical application of well-established rapid prototyping technology. It is defined as a layer-by-layer additive biofabrication approach using self-assembling tissue spheroids as building blocks. The fundamental biological and biophysical principle of organ printing technology is tissue fusion driven by surface tension forces or the capacity of closely placed tissue spheroids to fuse into a single entity. Fusion is usually defined as ‘melting together’. This implies that organ printing is in essence a microfluidic technology. There are many rapidly emerging and competing variants of bioprinting technology. The three principal characteristics of organ printing technology that make it unique are the following: i) organ printing is not based on using solid scaffolds – it is a solid-scaffold-free technology; ii) organ printing is based on using self-assembling tissue spheroids or cell aggregates with maximum possible cell density; and iii) organ printing is a direct processing technology where cells and a hydrogel are dispensed simultaneously without intermediate steps such as creation of a synthetic solid supporting scaffold.
2.2 Three main steps in organ printing technology
Using an analogy to rapid prototyping, organ-printing technology can be divided into three main steps: pre-processing, processing and post-processing. Pre-processing means designing an organ ‘blueprint’ or converting computer-aided design (CAD), derived from clinical bioimaging, into a bioprinter friendly standard template library (STL)-like file that represents direct instruction software for printing a patient-specific human organ. Processing is the actual printing or dispensing cells and hydrogel using a bioprinter. It is important to realize that the immediate product of processing is only a printed organ construct. Post-processing will involve transformation of the printed tissue construct into a functional tissue-engineered organ suitable for surgical implantation. The main goal of post-processing is accelerated tissue maturation and achieving a desirable level of construct functionality.
2.3 Five main essential elements of organ printing technology
Using an analogy to the 2D printing process invented by Johannes Guttenberg, we highlight the five most essential elements of organ printing technology. In order to print a book, it is necessary to have written text, a printing press, ink, paper and movable type. Similarly, to print an organ it is necessary to have an organ ‘blueprint’ (CAD in STL file format), a bioprinter (computer-aided robotic automated dispensing device), ‘bioink’ (self-assembling tissue spheroids), ‘biopaper’ (sprayable or dispensible hydrogel), and a cartridge (container for dispensing tissue spheroids). Finally, a bioreactor is essential for post-processing. It can be an integrated but removable part of the bioprinting device or the printed construct could be transferred into bioreactor immediately after completion of the bioprinting process.
2.4 Advantages of organ printing technology
Organ printing is an emerging transformative technology that has the potential for surpassing traditional solid-scaffold-based tissue engineering [6,7,13,23,24]. Organ printing has several advantages: it is an automated approach that offers a pathway for scalable and reproducible mass production of tissue engineered products; it allows the precise simultaneous 3D positioning of several cell types; it enables creation of tissues with a high level of cell density; it can solve the problem of vascularization in thick tissue constructs; and finally, organ printing can be performed in situ [6]. The ultimate goal of organ-printing technology is industrial, scalable robotic biofabrication of complex vascularized functional 3D living human organs suitable for clinical implantation [6].
2.5 Challenges in organ printing technology
Organ printing is a multidisciplinary technology and creating a well-managed multidisciplinary team and a cross-disciplinary research center is an important challenge [6]. Access to biofabrication raw materials such as a broad spectrum of stem or progenitor cells, hydrogels and biomaterials is equally important. Biofabrication research centers must also have sophisticated hardware and software that constitute the organ printing tools including cell sorters, spheroid fabricators, bioprinters, rapid prototyping machines, bioreactors, confocal and multiphoton microscopes and CAD software. In silico biofabrication can enhance and ensure the effective optimization of all steps of the biofabrication process. To that end, utilization of professional expertise in mathematical modeling and computer simulation of all steps of the biofabrication process is critically important. The effective development of organ printing technology will require long-term sustainable financial support. Finally, in order to ensure effective industrial translation and commercialization of organ printing technology, the often-ignored issues of scalability and cost-effectiveness must also be considered as significant intellectual challenges.
3. Designing the intra-organ vascular tree
3.1 Design principles of a vascular tree
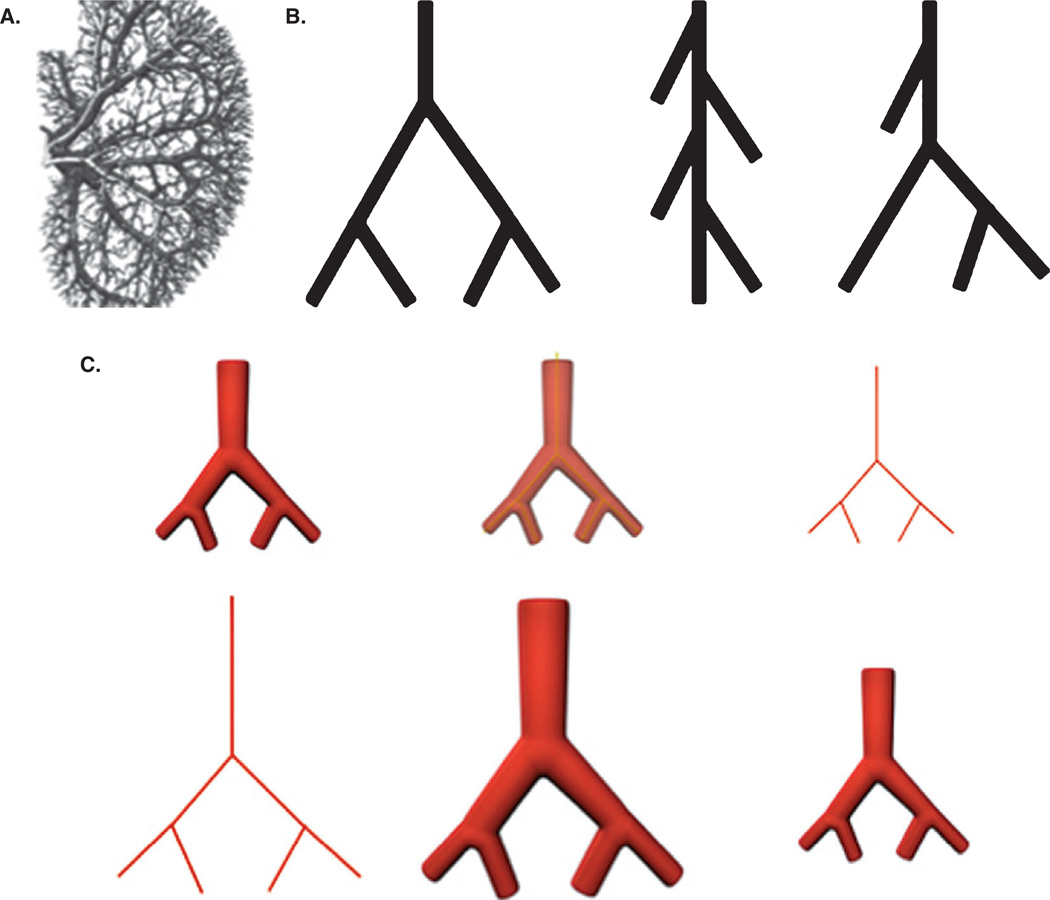
The design principles of an optimal intra-organ branched vascular tree are a well-investigated area. The vascular tree can be classified as a symmetrically and asymmetrically branching tree with intermediate variation (Figure 1A). The main unit of the vascular tree is a ‘Y’ shape branching unit in the case of a dichotomous branching pattern (Figure 1B). Murray described the optimal principles of the vascular tree in the early 20th century [25]. Topological numeric nomenclature as a function of branching order permits a detailed mathematical description of the vascular tree. Branching angle, ‘daughter’ segment diameter and ‘daughter’ segment length, and ratio of ‘maternal’ and ‘daughter’ segment diameters presented as a function of branching order enables the reconstruction of an entire vascular tree (Figure 1C). Thus, the original design of a vascular tree can be reconstructed using quantitative information about branching order.
Figure 1. Design of vascular tree.
A. The complexity of branching pattern of the intraorgan vascular tree is illustrated by this view of the kidney vasculature [30]. B. Classification of branching patterns of a vascular tree. From left to right: symmetrical, asymmetrical and mixed. C. Scheme for designing a patient-specific ‘blueprint’ of a vascular tree: (top, left to right) image acquisition; skeletonization; skeletonized model, (bottom, left to right) enlargement of the skeletonized model using the coefficient of tissue retraction; ‘blueprint’ model; final printed segment of vascular tree.
3.2 Bioimaging of the vascular tree
Modern methods of clinical bioimaging such as MRI, 3D ultrasound and X-Ray angiography permit generation of detailed high-resolution 3D images of the vascular system of human organs in living patients [26–32]. See Figure 1A. For example, it has been shown that the kidney vascular tree consists of 10 – 12 branching orders and includes 10,000 segments [30]. Organospecific organization of vascular trees in human organs is the subject of intensive ongoing research [33–39]. Classical anatomical techniques such as corrosion casts have been combined with high-resolution scanning electron microscopy [40,41]. Thus, the clinical anatomy of intra-organ vascular trees is sufficiently developed for effective use in organ printing technology. Moreover, advanced computer reconstruction techniques enable conversion of these images of intra-organ branched vascular trees to a format that is readable by software used in rapid prototyping.
3.3 Designing the ‘blueprint’ of a vascular tree
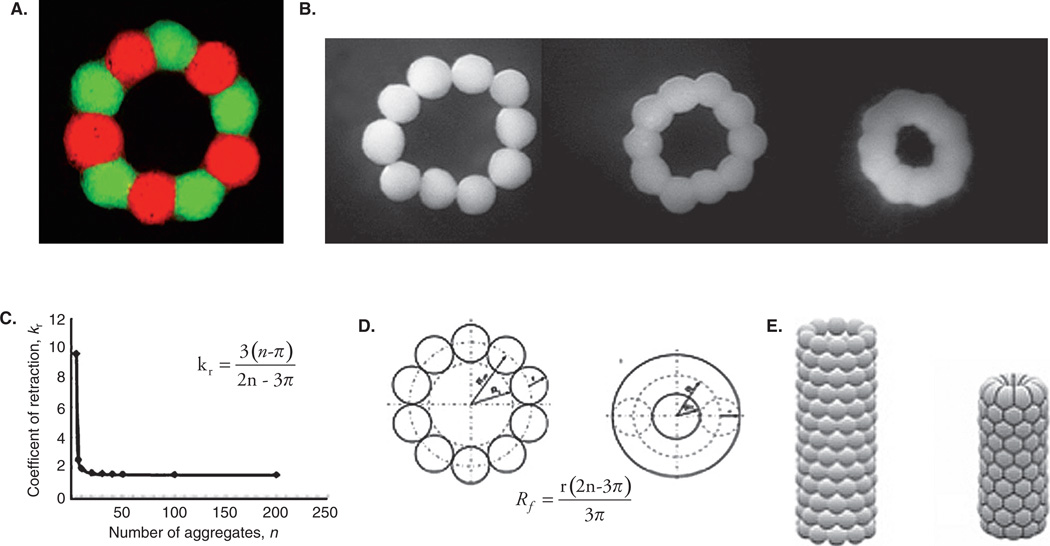
High-resolution imaging of intra-organ vascular trees is only the starting point, not a final ‘blueprint’ for organ printing technology. In a recent paper, we have shown that subsequent to bioprinting, vascular segments undergo retraction resulting in a dramatic two-fold reduction in the internal diameter and length of the printed vascular segment; (Figure 2A, B) [42]. This finding strongly suggests that in order to print vascular segments of desirable diameter, the initial ‘blueprint’ for the bioprinted vascular tree must be twice the final size. Estimation of the post-printing tissue compaction and tissue retraction coefficient is an important prerequisite for designing a ‘blueprint’ for organ printing (Figure 2C, D). It is logical to assume that compaction and retraction properties of different tissues are not the same, thus additional systematic research is necessary to accurately predict the post-printing geometrical evolution of printed tissue and organ constructs. Our experience also demonstrates that employing computer software such as ‘Surface Evolver’ enables prediction of post-printing vascular tissue construct evolution (Figure 2E).
Figure 2. Postprinting evolution of printed vascular constructs.
A. Biofabricated vascular ring formed from 10 vascular tissue spheroids comprised of human vascular smooth muscle cells. Fifty percent of spheroids were fabricated from cells labeled with red vital fluorescent stain and fifty percent were fabricated from cells labeled with green vital fluorescent stain. There is no apparent cell mixing during tissue spheroid fusion. B. Sequential steps in changes to morphology and geometry during tissue fusion in a ring-like tissue construct biofabricated from tissue spheroids comprised of human smooth muscle cells. A dramatic reduction of internal ring diameter is evident while no change in the diameter of the individual tissue spheroids was observed. C. There is a nonlinear relationship between the coefficient of retraction and number of cell aggregates (tissue spheroids). D. Scheme demonstrating calculation of changes in morphology of closely placed tissue spheroids in a ring-like tissue construct into torus-like tissue constructs. The diameter of tissue spheroids is constant during the tissue fusion process while the internal diameter of the ring construct is dramatically reduced. E. Computer simulation (using ‘Surface Evolver’ software) of morphological changes in a tube-like vascular tissue construct during tissue fusion. The initial tube-like vascular tissue construct becomes shorter and narrower. The hexagonal pattern of tissue spheroid packing is clearly demonstrated.
4. Clinically relevant vascular cell sources
4.1 Mature vascular cells
Mature vascular cells were a logical choice during the early stages of vascular tissue engineering (see for review [43–45]). Microvascular endothelial cells isolated from fat tissue have been shown to work well for endothelization of large vessels [46]. Lipoaspiration provides a sufficient amount of autologous endothelial cells. Smooth muscle cells were originally isolated from a small segment of vein dissected from patients [45,47]. Because in vitro propagation of mature smooth muscle cells was not satisfactory, leading researchers in vascular tissue engineering suggested use of telomerized vascular smooth muscle cells [48,49]. However, use of genetically modified cells will face significant regulatory challenges. Related to this, the USA-based vascular tissue engineering company Cytograft Tissue Engineering switched to using fibroblasts instead of vascular smooth muscle cells, based on commercial considerations. Although their tissue engineered vascular grafts have impressive mechanical properties [50,51] and proven clinical utility, the absence of elastin in their tissue engineered vascular graft strongly suggests that fibroblasts may be not the optimal cell source [5].
4.2 Bone-marrow-derived vascular cells
Isolation of bone marrow stem cells is a well-established clinical procedure and FDA-approved cell sorters made harvest a clinical reality. These factors make bone marrow stem cells a very attractive source of autologous vascular cells for vascular tissue engineering [52–57]. However, since yield of plastic stem cells is limited, expansion ex vivo (with its inherent challenges) will probably be necessary, especially in the case of building the vascular system of an entire organ. One of the most elegant outcomes of the application of bone-marrow-derived mesenchymal stem cells to vascular tissue engineering was achieved by Robert Tranquillo’s group at the University of Minnesota [58]. However, their approach required several months of bioreactor-based cultivation to achieve a desirable level of tissue engineered vascular graft functionality and maturation.
4.3 Fat-tissue-derived vascular cells
The original report of isolation of progenitor cells from fat tissue and demonstration of their multipotentiality is one of the most cited papers in the stem cell field [59]. This technology was developed by the US company Cytori Therapeutics in cooperation with the Japanese company Olympus and is now commercialized by GE Healthcare. This clinical cell sorter (Cellutions) is specifically designed for isolation of adipose progenitor cells and is already approved for clinical use in some countries. This sorting device permits isolation of a large amount of progenitor cells from lipoaspirate in one hour. It has been demonstrated that fat-tissue-derived progenitor cells can be differentiated to the smooth muscle lineage using growth factors or other agents [59–62] Our studies support and extend this observation and indicate that it is possible to derive functional smooth muscle cells from fat-tissue-derived progenitor cells (unpublished). Combined with their ease of harvest, the above-mentioned facts strongly suggest that fat-tissue-derived autologous stem cells hold great potential as a clinically relevant cell source for engineering intra-organ vascular trees.
4.4 Embryonic-stem-cell-derived vascular cells
Human embryonic stem cells are a controversial cell source both from an ethical and legal point of view [63]. Although derivation of both endothelial and smooth muscle cells from embryonic stem cells has been reported [64–66] serious scientific, technological and economic challenges remain. Genetic instability has been detected in some embryonic stem cell lineages [67,68]. The presence of allogeneic mitochondrial DNA in the donor cells, even after nuclear transplantation can potentially lead to autoimmune diseases [69,70]. Furthermore, the recent report of recruitment of embryonic stem cells into CNS tumors in a young Israeli patient who underwent ES cell transplantation in Russia as an experimental treatment for ataxia telangiectasia [71] reminds us of long-standing concerns over the detrimental side effects of embryonic stem cell therapy. Of course, the presence of transplanted embryonic cells in the tumors does not mean that embryonic cells were the main effectors of tumor development. It is also important to remember that embryonic stem cells and vascular cells derived from embryonic stem cells are not the same thing. Although endothelial cells and smooth muscle cells can be derived from human embryonic cells, their potential value as a clinically relevant cell source as well as absence of potential immunogenicity and tumorogenicity remains to be proven.
4.5 iPS-cell-derived vascular cells
Recent reports of induced pluripotent stem cells (iPS cells) derived from somatic cells (e.g., fibroblasts) present a source of pluripotent cells that do not raise the legal and ethical concerns raised by ES cells. Derivation of endothelial and smooth muscle cells from iPS cells has recently been reported [72,73]. However, the fact that induction of pluripotential phenotype in these cells requires genetic modification raises legitimate questions about their epigenetic stability. Compared with autologous cells that have not been genetically modified, iPS cells will face more scrutiny from regulatory authorities. It is logical to assume that it will eventually be possible either to induce ES cell-like pluripotency in adult somatic cells without inducing genetic modifications or to identify and isolate embryonic-like pluripotent stem cells in adult human tissues. Recently, the generation of human iPS cells free of vector and transgene sequences has been reported [74]. Moreover, it has recently been demonstrated that it is possible to generate viable progeny from murine IPS cells [75]. Identification, isolation and induced differentiation of pluripotent adult stem cells into vascular cells that are free of transgene sequences will be a very important advance in the fields of stem cell biology, tissue engineering and regenerative medicine.
5. Vascular tissue spheroids as building blocks
5.1 Diversity of vascular tissue spheroids
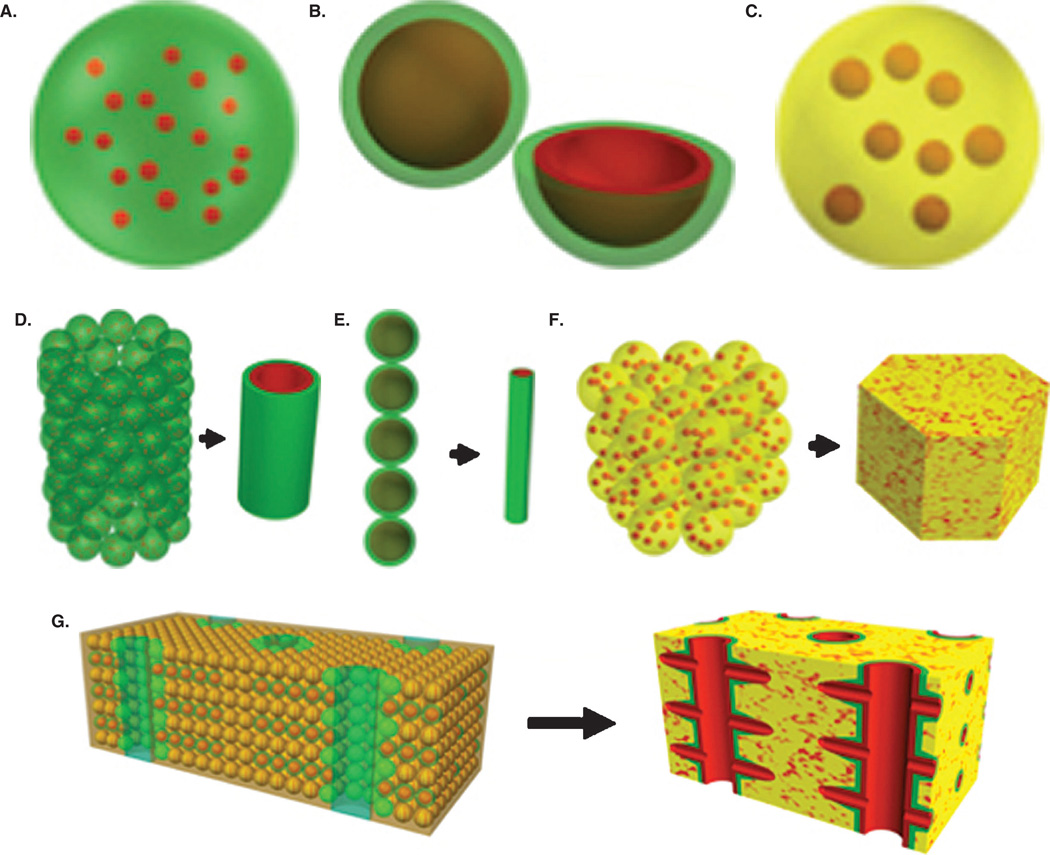
In order to build a complete intra-organ branched vascular tree, we anticipate using three types of vascular tissue spheroids: solid vascular tissue spheroids; lumenized vascular tissue spheroids with a single lumen; and vascularized or multi-lumenal vascular or tissue specific spheroids with well developed internal microvascular networks (Figure 3). Biofabrication of all these type has already been accomplished [7,42]. Such diversity of vascular tissue spheroids is necessary and sufficient to build segments of vascular tree with any requisite diameter. Moreover, at least theoretically, the seamless fusion of different types of vascular tissue spheroids would enable bioprinting of a complete ‘built in’ vascular tree inside of printed organ constructs. Bioprinting of branched segments of a vascular tree using tissue spheroids has recently been reported [7].
Figure 3. Scheme for bioprinting an organ construct with a ‘built in’ intra-organ branched vascular tree.
A. Solid vascular tissue spheroids (red = endothelial cells; green = smooth muscle/pericytic cells). B. Uni-lumenal vascular tissue spheroids with a single lumen (red = endothelial cells; green = smooth muscle/pericytic cells). C. Tissue-specific spheroids with internal microvascular networks (yellow = tissue-specific cells; red = microvasculature). D. Bioprinting of a large-diameter vascular segment using solid vascular tissue spheroids. E. Bioprinting of an intermediate-diameter vascular segment using uni-lumenal vascular tissue spheroids with a single lumen. F. Bioprinting of an organ segment using tissue-specific spheroids with internal microvascular networks G. Bioprinting of a vascularized organ segment using all three types of tissue spheroids.
5.2 Biofabrication of vascular tissue spheroids
Bioprinting of a complete intra-construct/intra-organ branched vascular tree will require millions of tissue spheroids. This will require the development of an effective scalable method of tissue spheroid biofabrication. Although a recent excellent review on this topic [76] demonstrated that there are a variety of different methods of tissue spheroid biofabrication, only small number of them could be considered scalable technologies. Hanging drop culture, the most popular method of tissue spheroid fabrication, is also potentially automatable and scalable [77]. Recent progress in digital microfluidics technology strongly suggests that microfluidics-based large scale biofabrication of tissue spheroids is a feasible goal [78,79]. The high productivity of microfluidics technology (generation of thousands of droplets per second) is especially attractive. Using a sacrificial hydrogel such as a hyaluronan-based hydrogel could accelerate development of corresponding technologies. Emerging large-scale methods of tissue spheroid biofabrication suitable for organ printing must ensure standardization and precise control of tissue spheroid size, shape and diameter. Biofabrication methods must also be flexible enough to fabricate a diversity of tissue spheroids, including solid and lumenized spheroids. Finally, biofabrication methods must not be cytotoxic and must not impede or interfere with post-printing tissue spheroid fusion.
5.3 Vascular tissue spheroid fusion
Tissue fusion is an important biological and biophysical phenomenon that is driven by surface tension forces. Tissue fusion is a ubiquitous event during embryonic development [80]. Thus, tissue fusion represents a fundamental biomimetic principle employed and explored in the emerging technology of organ printing. Fusion of tissue spheroids has been demonstrated [7,42,81,82]. It has also been shown that the degree of tissue cohesion as well as physical (for example, stiffness) and chemical properties (for example, level of Arg– Gly–Asp (RGD) sequences) of the surrounding extracellular matrix may regulate the kinetics of the tissue fusion process [83]. In this context, it is essential to use tissue-fusion-permissive hydrogels that are specifically tailored for application in organ printing technology [6,7,13,84].
5.4 Cell sorting during vascular tissue assembly
Persistent and legitimate concerns about the relatively low resolution of organ printing technology due to use of relatively large diameter tissue spheroids rather than single cells are often cited [85]. The diameter of tissue spheroids used in organ printing technology ranges from 200 to 250 µm while the average size of individual cells employed, for example, in ink jet technologies is in the range of 20 – 30 µm. The large diameter of tissue spheroids does not preclude possession of a very complex and highly organized internal composition and highly controlled spatial cell arrangement, which can be realized even before the bioprinting process. Moreover, Malcolm Steinberg’s differentiation adhesion hypothesis (DAH) [86–88] provides an important tool for controlled and predictable rearrangement of cells even after printing. For example, it has been shown by the Forgacs group (University of Missouri, Columbia, MO), both theoretically and more recently experimentally, that vascular tissue spheroids composed both of smooth muscle and endothelial cells undergo a cell sorting process immediately after printing that results in localization of endothelial cells predominantly to the inner, lumenal portion of printed vascular tubes [42,89,90]. In this case, organ-printing technology is again a biomimetic process that employs the well-known developmental biological principle of cell sorting. Mutually beneficial integration of developmental biology and tissue engineering is an ongoing process [91].
5.5 ‘Built in’ intra-organ vascular tree
It is important to mention that the ultimate goal of bioprinting a branched vascular tree is not just to engineer an isolated vascular tree but rather a branched vascular tree that is ‘built in’ or integral to a bioprinted tissue or organ construct. Simultaneous placement of three types of tissue specific or microvascularized organ specific tissue spheroids (described in section 5.1) is essential for achieving this goal. In another words, sequential layer by layer additive robotic deposition of diverse tissue spheroids, including both vascular tissue spheroids and vascularized organo-specific tissue spheroids, will ensure that bioprinted organ constructs will have a ‘built in’ vascular system.
6. Expert opinion and conclusions
Bioprinting an intra-organ vascular tree is an enormously complex multidisciplinary task. Many difficult challenges remain to be addressed (Table 1). For example, designing a ‘blueprint’ to guide the automated bioprinting of a specific organ or positioning of specific types of tissue spheroids, although technologically feasible, is still an unsolved task. Identification of the optimal autologous cell source is another important prerequisite for organ printing. The optimal cell source should be harvestable in large numbers and have the capacity to be induced to differentiate to the desirable cell lineage and phenotype. Large scale and precise biofabrication of millions of tissue spheroids of desirable composition and size is a hugely challenging task, but application of digital (droplet) microfluidics will enable realization of this goal. Engineering structures with a vascular lumen is also a technological challenge. However, this challenge may be met by placing a sacrificial or removable hydrogel or cells with inducible pro-apoptotic transgenes in the center of the printed vascular segment. Alternatively, application of bubble technology could be used to solve the problem of lumenization. Although fusion of tissue spheroids and separate vascular segments has been demonstrated [7,42] integration of vascular segments into a complete vascular tree is still a challenge. Furthermore, a bioprinted vascular tree cannot be perfused immediately after bioprinting because fusion of tissue spheroids into vascular tubes and segments takes time. Therefore, preserving viability during the time required for vascular tissue self-assembly is one of the major challenges of bioprinting. A specially designed drip-irrigation perfusion bioreactor with a removable porous tube could potentially solve this problem. Organ printing is a microfluidics-based solid scaffold-free technology. The removable porous perfusion tubes in a drip-irrigation perfusion bioreactor could serve as a temporary scaffold. However, the real challenge is designing a novel method of fast transition from a semi-solid like to a solid state using the concept of accelerated tissue maturation [7]. A combination of non-invasive molecular imaging techniques such as multiphoton microscopy for non-destructive visualization of elastin and collagen deposition [92], utilization of cells transfected with reporter genes such as collagen type I and alpha smooth muscle actin labeled with fluorescent proteins [93,94], nanotechnology such as protease-sensitive nanoparticles [95] as well biochemical analysis of matrix remodeling biomarkers in perfusate [96], will permit monitoring in real time of the most essential aspects of vascular tissue maturation. The mechanical properties of the large segments of the vascular tree must be strong enough to permit surgical anastomosis to pre-existing large-diameter host blood vessels. Aspects of optimal biological function of the vascular tree must include maintenance of an uninterrupted continuity of lumen, an athrombogenic endothelial surface, adequate response to vasoactive and endothelial signals, proper material properties and vascular permeability. Thus, bioprinting of a ‘built in’ intra-organ branched vascular system is an extremely challenging yet accomplishable task.
Table 1.
Challenges in bioprinting a branched vascular tree
| Design of blueprint for vascular tree |
| Selection of a clinically relevant vascular cell source |
| Scalable methods for biofabrication of vascular tissue spheroids |
| Engineering a vascular lumen |
| Integration of segments of vascular tree |
| ‘Buying’ time necessary for vascular tissue integration |
| Accelerated vascular tissue maturation |
| Non-invasive biomonitoring of vascular tissue maturation |
| Validation of functionality of the bioprinted vascular tree |
| Surgical integration with preexisting host vasculature |
Faced with these challenges, it is logical to ask whether it is possible to engineer a functional living human organ. This task has been already successfully accomplished for some human tissues and organs: tissue engineered skin, blood vessels, urinary bladders and even tissue engineered fingers are already a clinical reality [16,50,97–99]. Therefore tissue engineering is already fulfilling its promise to become an exciting and increasingly fruitful biomedical field. However, further advancement of the tissue-engineering field must be based on continuous effective adaptation and incorporation of new emerging technologies. The emerging fields of organ printing and robotic biofabrication offer additional or alternative approaches to the use of solid-synthetic- and natural-scaffold-based approaches.
Recent plans to reform health care in the United States strongly indicate that the cost-effectiveness of novel technologies does matter. Moreover, systematic analysis of new product failures strongly suggests that cost-effectiveness must be given serious consideration from the earliest stages of product development [100]. Moreover, as was boldly put by Jonathan Rees “… what needs to be rewarded in medical research is the ability to innovate within a financial envelope. Being able to invent solutions at an affordable price is a design constraint, one that needs to be seen as a challenge. The solution ‘at any price’ is lazy” [101]. Systematic standardization and automation, offered by emerging robotic biofabrication technology, of biofabrication processes like organ printing could make tissue engineering of human organs cost-effective. Another important lesson gleaned from the modern manufacturing practices in engineering of bridges, cars and aircraft is that the successful product must first be fabricated in silico using computer simulation. Design of ‘blueprints’ for organ printing technology using a seamless combination of sophisticated clinical bioimaging, mathematical modeling and computer simulation is nothing more than a manifestation of the global cross-disciplinary manufacturing and engineering trend. Bioengineering with all its biological specifics is still an integral part of 21st century engineering and manufacturing [102]. As an epigraph to this review, we reflect on the words of Dr Wernher von Braun, a recipient of the National Medal of Science for his contributions to rocket physics and astronautics engineering, that ‘nothing is impossible’. It is definitely possible to engineer living human organs, but it is highly improbable to accomplish this task without engineering a ‘built in’ functional intra-organ branched vascular system to perfuse these organs.
Article Highlights.
The vascularization of thick 3D tissue and organ constructs is an unsolved problem in tissue engineering.
An intra-organ branched vascular tree must be comprised of a complex network of hierarchically branched vascular segments of different diameters, not just from large blood vessels and capillaries.
Vascular tissue spheroids can be used as self-assembling building blocks in vascular tissue engineering.
A combination of three types of vascular tissue spheroids will enable bioprinting of an intra-organ vascular tree.
Organ printing or robotic computer-aided layer-by-layer additive biofabrication using self-assembling tissue spheroids enables bioengineering of human organ constructs with a ‘built in’ intra-organ branched vascular tree.
This box summarises key points contained in the article.
Acknowledgements
The authors wish to thank T Trusk and K Brakke for their assistance in assembling the figures.
Footnotes
Declaration of interest
This work was supported by NSF/EPSCOR EPS-0447660 & NSF – FIBR 0526854 (V.M. and R.R.M); NIH-NCRR RR16434-08 (R.P.V. and R.R.M.), AHA 0865325E (R.P. V.), NIH-NCRR 2 P20 RR16461-05A1 (R.R.M.); Foundation Leducq: Mitral 07CVD04 (R.R.M); and NIH-NCRR C06 RR018823, NIH-NCRR C06 RR015455.
Bibliography
- 1.Ogawa R, Oki K, Hyakusoku H. Vascular tissue engineering and vascularized 3D tissue regeneration. Regen Med. 2007;2(5):831–837. doi: 10.2217/17460751.2.5.831. [DOI] [PubMed] [Google Scholar]
- 2.Lokmic Z, Mitchell GM. Engineering the microcirculation. Tissue Eng Part B Rev. 2008;14(1):87–103. doi: 10.1089/teb.2007.0299. [DOI] [PubMed] [Google Scholar]
- 3.Rivron NC, Liu JJ, Rouwkema J, et al. Engineering vascularised tissues in vitro. Eur Cell Mater. 2008;15:27–40. doi: 10.22203/ecm.v015a03. [DOI] [PubMed] [Google Scholar]
- 4.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26(8):434–441. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Mironov V, Kasyanov V. Emergence of clinical vascular tissue engineering. Lancet. 2009;373(9673):1402–1404. doi: 10.1016/S0140-6736(09)60799-6. [DOI] [PubMed] [Google Scholar]
- 6.Mironov V, Kasyanov V, Drake C, Markwald RR. Organ printing: promises and challenges. Regen Med. 2008;3(1):93–103. doi: 10.2217/17460751.3.1.93. [DOI] [PubMed] [Google Scholar]
- 7.Mironov V, Visconti RP, Kasyanov V, et al. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30(12):2164–2174. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 9.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 11.Mooney DJ, Mikos AG. Growing new organs. Sci Am. 1999;280(4):60–65. doi: 10.1038/scientificamerican0499-60. [DOI] [PubMed] [Google Scholar]
- 12.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 13.Mironov V, Reis N, Derby B. Review: bioprinting: a beginning. Tissue Eng. 2006;12(4):631–634. doi: 10.1089/ten.2006.12.631. [DOI] [PubMed] [Google Scholar]
- 14.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 15.Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 16.Atala A, Bauer SB, Soker S, et al. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 17.Kasyanov VA, Hodde J, Hiles MC, et al. Rapid biofabrication of tubular tissue constructs by centrifugal casting in a decellularized natural scaffold with laser-machined micropores. J Mater Sci Mater Med. 2009;20(1):329–337. doi: 10.1007/s10856-008-3590-3. [DOI] [PubMed] [Google Scholar]
- 18.Rotmans JI, Heyligers JM, Verhagen HJ, et al. In vivo cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation. 2005;112(1):12–18. doi: 10.1161/CIRCULATIONAHA.104.504407. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu T, Sekine H, Yang J, et al. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20(6):708–710. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 20.Lokmic Z, Stillaert F, Morrison WA, et al. An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. FASEB J. 2007;21(2):511–522. doi: 10.1096/fj.06-6614com. [DOI] [PubMed] [Google Scholar]
- 21.Morritt AN, Bortolotto SK, Dilley RJ, et al. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115(3):353–360. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- 22.Polykandriotis E, Tjiawi J, Euler S, et al. The venous graft as an effector of early angiogenesis in a fibrin matrix. Microvasc Res. 2008;75(1):25–33. doi: 10.1016/j.mvr.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Mironov V, Boland T, Trusk T, et al. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21(4):157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 24.Jakab K, Neagu A, Mironov V, Forgacs G. Organ printing: fiction or science. Biorheology. 2004;41(3–4):371–375. [PubMed] [Google Scholar]
- 25.Murray CD. The physiological principle of minimum work: I the vascular system and the cost of blood volume. Proc Natl Acad Sci USA. 1926;12(3):207–214. doi: 10.1073/pnas.12.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burrowes KS, Hunter PJ, Tawhai MH. Anatomically based finite element models of the human pulmonary arterial and venous trees including supernumerary vessels. J Appl Physiol. 2005;99(2):731–738. doi: 10.1152/japplphysiol.01033.2004. [DOI] [PubMed] [Google Scholar]
- 27.Dankelman J, Cornelissen AJ, Lagro J, et al. Relation between branching patterns and perfusion in stochastic generated coronary arterial trees. Med Biol Eng Comput. 2007;45(1):25–34. doi: 10.1007/s11517-006-0139-9. [DOI] [PubMed] [Google Scholar]
- 28.Kaimovitz B, Lanir Y, Kassab GS. Large-scale 3-D geometric reconstruction of the porcine coronary arterial vasculature based on detailed anatomical data. Ann Biomed Eng. 2005;33(11):1517–1535. doi: 10.1007/s10439-005-7544-3. [DOI] [PubMed] [Google Scholar]
- 29.Mittal N, Zhou Y, Ung S, et al. A computer reconstruction of the entire coronary arterial tree based on detailed morphometric data. Ann Biomed Eng. 2005;33(8):1015–1026. doi: 10.1007/s10439-005-5758-z. [DOI] [PubMed] [Google Scholar]
- 30.Nordsletten DA, Blackett S, Bentley MD, et al. Structural morphology of renal vasculature. Am J Physiol Heart Circ Physiol. 2006;291(1):H296–H309. doi: 10.1152/ajpheart.00814.2005. [DOI] [PubMed] [Google Scholar]
- 31.Smith NP, Pullan AJ, Hunter PJ. Generation of an anatomically based geometric coronary model. Ann Biomed Eng. 2000;28(1):14–25. doi: 10.1114/1.250. [DOI] [PubMed] [Google Scholar]
- 32.Yu KC, Ritman EL, Higgins WE. System for the analysis and visualization of large 3D anatomical trees. Comput Biol Med. 2007;37(12):1802–1820. doi: 10.1016/j.compbiomed.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamiya A, Togawa T. Optimal branching structure of the vascular tree. Bull Math Biophys. 1972;34(4):431–438. doi: 10.1007/BF02476705. [DOI] [PubMed] [Google Scholar]
- 34.Zamir M. Optimality principles in arterial branching. J Theor Biol. 1976;62(1):227–251. doi: 10.1016/0022-5193(76)90058-8. [DOI] [PubMed] [Google Scholar]
- 35.Karch R, Neumann F, Neumann M, Schreiner W. Staged growth of optimized arterial model trees. Ann Biomed Eng. 2000;28(5):495–511. doi: 10.1114/1.290. [DOI] [PubMed] [Google Scholar]
- 36.Roy S, Silacci P, Stergiopulos N. Biomechanical properties of decellularized porcine common carotid arteries. Am J Physiol Heart Circ Physiol. 2005;289(4):H1567–H1576. doi: 10.1152/ajpheart.00564.2004. [DOI] [PubMed] [Google Scholar]
- 37.Schreiner W, Karch R, Neumann M, et al. Optimized arterial trees supplying hollow organs. Med Eng Phys. 2006;28(5):416–429. doi: 10.1016/j.medengphy.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Schreiner W, Neumann F, Karch R, et al. Shear stress distribution in arterial tree models, generated by constrained constructive optimization. J Theor Biol. 1999;198(1):27–45. doi: 10.1006/jtbi.1999.0898. [DOI] [PubMed] [Google Scholar]
- 39.Assoul N, Flaud P, Chaouat M, et al. Mechanical properties of rat thoracic and abdominal aortas. J Biomech. 2008;41(10):2227–2236. doi: 10.1016/j.jbiomech.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 40.Lagerveld BW, ter Wee RD, de la Rosette JJ, et al. Vascular fluorescence casting and imaging cryomicrotomy for computerized three-dimensional renal arterial reconstruction. BJU Int. 2007;100(2):387–391. doi: 10.1111/j.1464-410X.2007.06914.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee SH, Moon JJ, Miller JS, West JL. Poly (ethylene glycol) hydrogels conjugated with a collagenase-sensitive fluorogenic substrate to visualize collagenase activity during three-dimensional cell migration. Biomaterials. 2007;28(20):3163–3170. doi: 10.1016/j.biomaterials.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Mironov V, Zhang J, Gentile C, et al. Designer ’blueprint’ for vascular trees: morphology evolution of vascular tissue constructs. Virtual Phys Prototyping. 2009;4(2):63–74. [Google Scholar]
- 43.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98(1):25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 44.L’Heureux N, Dusserre N, Marini A, et al. Technology insight: the evolution of tissue-engineered vascular grafts–from research to clinical practice. Nat Clin Pract Cardiovasc Med. 2007;4(7):389–395. doi: 10.1038/ncpcardio0930. [DOI] [PubMed] [Google Scholar]
- 45.L’Heureux N, Paquet S, Labbe R, et al. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12(1):47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 46.Jarrell BE, Williams SK, Stokes G, et al. Use of freshly isolated capillary endothelial cells for the immediate establishment of a monolayer on a vascular graft at surgery. Surgery. 1986;100(2):392–399. [PubMed] [Google Scholar]
- 47.Niklason LE, Gao J, Abbott WM, et al. Functional arteries grown in vitro. Science. 1999;284(5413):489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 48.Klinger RY, Blum JL, Hearn B, et al. Relevance and safety of telomerase for human tissue engineering. Proc Natl Acad Sci USA. 2006;103(8):2500–2505. doi: 10.1073/pnas.0508184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poh M, Boyer M, Solan A, et al. Blood vessels engineered from human cells. Lancet. 2005;365(9477):2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 50.Konig G, McAllister TN, Dusserre N, et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials. 2009;30(8):1542–1550. doi: 10.1016/j.biomaterials.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McAllister TN, Maruszewski M, Garrido SA, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373(9673):1440–1446. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 52.Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111(9):4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB J. 2008;22(6):1635–1648. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kadner A, Hoerstrup SP, Zund G, et al. A new source for cardiovascular tissue engineering: human bone marrow stromal cells. Eur J Cardiothorac Surg. 2002;21(6):1055–1060. doi: 10.1016/s1010-7940(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 55.Matsumura G, Miyagawa-Tomita S, Shin’oka T, et al. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108(14):1729–1734. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 56.Roh JD, Brennan MP, Lopez-Soler RI, et al. Construction of an autologous tissue-engineered venous conduit from bone marrow-derived vascular cells: optimization of cell harvest and seeding techniques. J Pediatr Surg. 2007;42(1):198–202. doi: 10.1016/j.jpedsurg.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 57.Zwaginga JJ, Doevendans P. Stem cell-derived angiogenic/vasculogenic cells: possible therapies for tissue repair and tissue engineering. Clin Exp Pharmacol Physiol. 2003;30(11):900–908. doi: 10.1046/j.1440-1681.2003.03931.x. [DOI] [PubMed] [Google Scholar]
- 58.Ross JJ, Hong Z, Willenbring B, et al. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J Clin Invest. 2006;116(12):3139–3149. doi: 10.1172/JCI28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 60.Jeon ES, Moon HJ, Lee MJ, et al. Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-beta-dependent mechanism. J Cell Sci. 2006;119(Pt 23):4994–5005. doi: 10.1242/jcs.03281. [DOI] [PubMed] [Google Scholar]
- 61.Lee WC, Rubin JP, Marra KG. Regulation of alpha-smooth muscle actin protein expression in adipose-derived stem cells. Cells Tissues Organs. 2006;183(2):80–86. doi: 10.1159/000095512. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez LV, Alfonso Z, Zhang R, et al. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA. 2006;103(32):12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mironov V, Visconti RP, Markwald RR. What is regenerative medicine? Emergence of applied stem cell and developmental biology. Expert Opin Biol Ther. 2004;4(6):773–781. doi: 10.1517/14712598.4.6.773. [DOI] [PubMed] [Google Scholar]
- 64.Ferreira LS, Gerecht S, Shieh HF, et al. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res. 2007;101(3):286–294. doi: 10.1161/CIRCRESAHA.107.150201. [DOI] [PubMed] [Google Scholar]
- 65.Levenberg S, Golub JS, Amit M, et al. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99(7):4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamashita J, Itoh H, Hirashima M, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408(6808):92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 67.Draper JS, Smith K, Gokhale P, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22(1):53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- 68.Pera MF. Unnatural selection of cultured human ES cells? Nat Biotechnol. 2004;22(1):42–43. doi: 10.1038/nbt0104-42. [DOI] [PubMed] [Google Scholar]
- 69.Byrne JA, Pedersen DA, Clepper LL, et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450(7169):497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- 70.Hall VJ, Stojkovic P, Stojkovic M. Using therapeutic cloning to fight human disease: a conundrum or reality? Stem Cells. 2006;24(7):1628–1637. doi: 10.1634/stemcells.2005-0592. [DOI] [PubMed] [Google Scholar]
- 71.Amariglio N, Hirshberg A, Scheithauer BW, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6(2):e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27(3):559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schenke-Layland K, Rhodes KE, Angelis E, et al. Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages. Stem Cells. 2008;26(6):1537–1546. doi: 10.1634/stemcells.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boland MJ, Hazen JL, Nazor KL, et al. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461(7260):91–94. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- 76.Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3(9–10):1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 77.Kelm JM, Fussenegger M. Microscale tissue engineering using gravity-enforced cell assembly. Trends Biotechnol. 2004;22(4):195–202. doi: 10.1016/j.tibtech.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 78.Hsiao AY, Torisawa YS, Tung YC, et al. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials. 2009;30(16):3020–3027. doi: 10.1016/j.biomaterials.2009.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watanabe M, Takagi A. Biological behavior of prostate cancer cells in 3D culture systems. Yakugaku Zasshi. 2008;128(1):37–44. doi: 10.1248/yakushi.128.37. [DOI] [PubMed] [Google Scholar]
- 80.Perez-Pomares JM, Foty RA. Tissue fusion and cell sorting in embryonic development and disease: biomedical implications. Bioessays. 2006;28(8):809–821. doi: 10.1002/bies.20442. [DOI] [PubMed] [Google Scholar]
- 81.Rago AP, Dean DM, Morgan JR. Controlling cell position in complex heterotypic 3D microtissues by tissue fusion. Biotechnol Bioeng. 2009;102(4):1231–1241. doi: 10.1002/bit.22162. [DOI] [PubMed] [Google Scholar]
- 82.Lin RZ, Chu WC, Chiang CC, et al. Magnetic reconstruction of three-dimensional tissues from multicellular spheroids. Tissue Eng Part C Methods. 2008;14(3):197–205. doi: 10.1089/ten.tec.2008.0061. [DOI] [PubMed] [Google Scholar]
- 83.Jakab K, Neagu A, Mironov V, et al. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc Natl Acad Sci USA. 2004;101(9):2864–2869. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fedorovich NE, Alblas J, de Wijn JR, et al. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng. 2007;13(8):1905–1925. doi: 10.1089/ten.2006.0175. [DOI] [PubMed] [Google Scholar]
- 85.Tsang VL, Bhatia SN. Three-dimensional tissue fabrication. Adv Drug Deliv Rev. 2004;56(11):1635–1647. doi: 10.1016/j.addr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 86.Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev. 2007;17(4):281–286. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 87.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278(1):255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 88.Davis GS, Phillips HM, Steinberg MS. Germ-layer surface tensions and "tissue affinities" in Rana pipiens gastrulae: quantitative measurements. Dev Biol. 1997;192(2):630–644. doi: 10.1006/dbio.1997.8741. [DOI] [PubMed] [Google Scholar]
- 89.Jakab K, Norotte C, Damon B, et al. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng Part A. 2008;14(3):413–421. doi: 10.1089/tea.2007.0173. [DOI] [PubMed] [Google Scholar]
- 90.Neagu A, Jakab K, Jamison R, Forgacs G. Role of physical mechanisms in biological self-organization. Phys Rev Lett. 2005;95(17):178104. doi: 10.1103/PhysRevLett.95.178104. [DOI] [PubMed] [Google Scholar]
- 91.Marga F, Neagu A, Kosztin I, Forgacs G. Developmental biology and tissue engineering. Birth Defects Res C Embryo Today. 2007;81(4):320–328. doi: 10.1002/bdrc.20109. [DOI] [PubMed] [Google Scholar]
- 92.Schenke-Layland K. Non-invasive multiphoton imaging of extracellular matrix structures. J Biophotonics. 2008;1(6):451–462. doi: 10.1002/jbio.200810045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dean DM, Rago AP, Morgan JR. Fibroblast elongation and dendritic extensions in constrained versus unconstrained microtissues. Cell Motil Cytoskeleton. 2009;66(3):129–141. doi: 10.1002/cm.20335. [DOI] [PubMed] [Google Scholar]
- 94.Chokalingam K, Juncosa-Melvin N, Hunter SA, et al. Tensile stimulation of murine stem cell-collagen sponge constructs increases collagen type I gene expression and linear stiffness. Tissue Eng Part A. 2009;15(9):2561–2570. doi: 10.1089/ten.tea.2008.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang E, Miller JS, Sun J, et al. Protease-activated quantum dot probes. Biochem Biophys Res Commun. 2005;334(4):1317–1321. doi: 10.1016/j.bbrc.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 96.Zannad F, Rossignol P, Iraqi W. Extracellular matrix fibrotic markers in heart failure. Heart Fail Rev. 2009 doi: 10.1007/s10741-009-9143-0. [DOI] [PubMed] [Google Scholar]
- 97.Nie X, Yang MJ, Deng MJ, et al. Innovative strategies for tissue engineered skin based on multiple growth factors gene transfection. Med Hypotheses. 2009;73(4):516–518. doi: 10.1016/j.mehy.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 98.Kostrominova TY, Calve S, Arruda EM, Larkin LM. Ultrastructure of myotendinous junctions in tendon-skeletal muscle constructs engineered in vitro. Histol Histopathol. 2009;24(5):541–550. doi: 10.14670/hh-24.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Landis WJ, Jacquet R, Lowder E, et al. Tissue engineering models of human digits: effect of periosteum on growth plate cartilage development. Cells Tissues Organs. 2009;189(1–4):241–244. doi: 10.1159/000151432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McAllister TN, Dusserre N, Maruszewski M, L’Heureux N. Cell-based therapeutics from an economic perspective: primed for a commercial success or a research sinkhole? Regen Med. 2008;3(6):925–937. doi: 10.2217/17460751.3.6.925. [DOI] [PubMed] [Google Scholar]
- 101.Rees J. The problem with academic medicine: engineering our way into and out of the mess. PLoS Med. 2005;2(4):e111. doi: 10.1371/journal.pmed.0020111. Published online 26 April 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mironov V, Trusk T, Little S, et al. Biofabrication: a 21st century manufacturing paradigm. Biofabrication. 2009;1(2):1–26. doi: 10.1088/1758-5082/1/2/022001. [DOI] [PubMed] [Google Scholar]