Abstract
The development of antisocial behavior in youth has been examined with neurobiological theories that suggest adolescents who are less responsive to their environments are less likely to develop empathy in the absence of extant physiological arousal. However, little attention is paid to these individuals’ social context. Individuals with adverse early experiences can also exhibit attenuated physiological arousal. The current investigation examines whether psychopathic symptoms or life stress exposure is associated with cortisol and its diurnal rhythm within 50 incarcerated adolescent boys (14–18 years old). Ten saliva cortisol samples were collected 1–2 weeks after admission to a maximum-security juvenile facility. Hierarchical Linear Modeling distinguished waking cortisol levels, the awakening response (CAR) and the diurnal rhythm. Multiple interviews and self-report measures of CU traits and stressor exposure were collected. Boys with higher levels of CU traits or greater life stress exposure had flat diurnal rhythms and a steeper awakening response in analyses with lifetime stress exposure specifically. Nonetheless, boys who were elevated on both CU traits and prior stress exposure had steeper diurnal rhythms. These results extend neurobiological theories of cortisol and illustrate that boys with the combination of severe stress with CU traits have a unique physiological profile.
Keywords: Cortisol, Context, Incarceration, Adolescence, Stress
Beyond Physiological Hypoarousal: The Role of Context and Callous-Unemotional Traits in Incarcerated Adolescent Males
Callous-unemotional (CU) trait expression has recently been associated with physiological hypoarousal, whereby individuals with higher levels of CU traits are less responsive to their environments and therefore seek out high-intensity situations or display a bold, fearless personality (Raine, 2002; Shirtcliff et al., 2009; van Goozen, Fairchild, Snoek, & Harold, 2007; van Honk, Schutter, Hermans, & Putman, 2003). In parallel, a separate literature suggests physiological hypoarousal, as indexed by the stress hormone cortisol, may be apparent in individuals with extreme early life stress (Miller, Chen, & Zhou, 2007; Weems & Carrion, 2007). Child maltreatment is such an early life stress which can profoundly impact stress physiology (Tarullo & Gunnar, 2006). Emerging theories suggest this confluence of ideas is not a coincidence, but rather stress exposure sets the stage for antisocial behavior (Susman, 2006), in part by reducing empathy and physiological arousal when observing another individual in distress (Shirtcliff, et al., 2009). It remains under-studied whether physiological arousal is apparent within individuals who have both CU traits and prior life stress exposure. The current investigation therefore examines stress-responsive physiology in individuals with severe antisocial behavior: incarcerated adolescent boys. In addition to examining CU traits, we also examine whether prior stressor exposure (e.g., child abuse) moderates the association between CU trait expression and stress physiology. We hypothesize that both CU traits and life stress will impact HPA functioning, exhibited as physiological hypoarousal.
Callous-Unemotional Traits
Callous-Unemotional traits (i.e. lack of empathy, absence of guilt, manipulation of others) have been a fruitful research area for understanding pathways toward persistent violent antisocial behavior. CU traits are a constellation of personality characteristics that have been shown to designate a particular subgroup of antisocial youth consistently more likely to offend into adulthood, employ violence in their criminal acts, use substances earlier (Frick & White, 2008), and eventually account for a substantially greater portion of youth crime compared to youth without CU traits. Youth with high CU traits demonstrate a number of deficits including insufficient fear and negative emotion recognition in comparison with control youth (Blair, Budhani, Colledge, & Scott, 2005; Stevens, Charman, & Blair, 2001), poor passive avoidance (Vitale et al., 2005), and impaired attention to the eyes of attachment figures (Dadds, Jambrak, Pasalich, Hawes, & Brennan, 2011). Youth with high CU traits demonstrate both instrumental and reactive forms of aggression whereas individuals without elevations in CU traits demonstrate solely reactive aggression (Frick et al., 2003). Youth with CU traits also display poor orienting responses to distress (Kimonis, Frick, Munoz, & Aucoin, 2007) and response sets that are overly responsive to rewards versus punishments (Frick, et al., 2003). These findings have led many to conclude CU traits and an associated emotional dysfunction form the stable core of the psychopathy phenotype (Blair, Peschardt, Budhani, Mitchell, & Pine, 2006), which manifests as callous disregard for others, along with a charming, glib interpersonal style, flat affect, and an impulsive and chronically antisocial lifestyle (Hare, 2003). Examining mechanisms underlying CU traits from a neurobiological perspective may advance our understanding of CU trait expression.
In the present study, severe antisocial behavior and a moderate to extreme range of CU traits are targeted by examining adolescent boys incarcerated at a maximum-security correctional treatment facility. Childhood onset problems are 4 to 24 times higher in incarcerated youth compared to the general population (Leve & Chamberlain, 2004; Silverthorn, Frick, & Reynolds, 2001). We focus on boys as range and rates of antisocial behavior are greater in boys than girls (Maughan, Rowe, Messer, Goodman, & Meltzer, 2004; Tracy, Kempf-Leonard, & Abramoske-James, 2009). Finally, we focus on adolescents aged 14–18 as rates of antisocial behavior rise precipitously during adolescence (Moffitt & Caspi, 2001), even within individuals with persistent severe behavior problems (Farrington, Loeber, & Jolliffe, 2008) and these problems can persist across the lifespan (Moffitt, 2006). Beyond the severe range of symptoms expressed within this population, incarcerated youth have another advantage for this type of investigation. The facility established a parallel schedule for each youth in terms of sleeping, eating, taking medications, schoolwork, free-time, and times for saliva collection. This schedule is strictly enforced (as is abstinence from drugs and alcohol), permitting a highly predictable schedule. These are factors which could influence cortisol’s diurnal rhythm if they varied across children; antisocial youth frequently have aberrant bed-times and school schedules (Carskadon, Acebo, Jenni, Dahl, & Spear, 2004; Lindberg et al., 2008). These zeitgebers are unlikely to drive associations with cortisol’s diurnal rhythm in the present study.
A Neurobiological Concept for CU Trait Expression
Recent neurobiological theories for CU traits implicate the Stress Response System (SRS) (Del Giudice, Ellis, & Shirtcliff, 2011; Ellis, Del Giudice, & Shirtcliff, 2012) including the HPA axis in the development of neural, autonomic, and resulting behavioral endophenotypes, initially in the brain (Daversa, 2010; Hawes, Brennan, & Dadds, 2009; Shirtcliff, et al., 2009; Susman, 2006; van Goozen, et al., 2007). Activation of limbic and paralimbic circuitry is associated with instantiation of empathy and empathy-related emotions (Singer, 2006). Structures involved in threat processing and coordination of emotional responses and learning, such as the amygdala, hippocampus and prefrontal cortex (Derryberry & Tucker, 1992; Sterzer, Stadler, Krebs, Kleinschmidt, & Poustka, 2005), appear hypo-aroused in individuals with heightened CU trait expression (Blair, et al., 2006; Kiehl et al., 2001; Marsh et al., 2008). Paralimbic structures such as the anterior cingulate and insula are implicated for their role in detecting an emotion-related mismatch when observing distress in another (Blair, 2007; Kiehl, 2006) even as early as childhood and adolescence (Jones, Laurens, Herba, Barker, & Viding, 2009; Viding, 2004).
Emotion- and stress-responsivity begins in the limbic system, but through both top-down and bottom-up processes, the SRS enhances emotional and social information processing (Nelson, Leibenluft, McClure, & Pine, 2005). Limbic and paralimbic structures provide an error- or threat-detection-signal to activate the hypothalamus to mobilize peripheral physiological resources if a stressor is severe enough. A hormonal cascade continues until the adrenal gland releases glucocorticoids such as cortisol to increase energy mobilization, glucose metabolism and immune function throughout the body and brain. This cascade is adaptive in the face of stressors, helping the individual encode or enhance salient signals in their environment (Del Giudice, et al., 2011).
CU trait expression is posited to be a product of a transactional cycle of hypoarousal in peripheral stress systems and limbic circuitry. Shirtcliff et al. (2009) suggest a substantial contribution of the HPA axis to the development of CU traits, as low cortisol might diminish emotional- or stress-reactions even in social contexts in which stress reactions are appropriate (O’Leary, Loney, & Eckel, 2007). Insufficient mobilization of resources reduces the SRS’s ability to monitor and encode environmental threats (in part through limbic and paralimbic modulation), and leaves the organism vulnerable to further physiological insults in the face of future stressors (Del Giudice, et al., 2011; Ellis, et al., 2012). In the absence of strong SRS activation, we speculate that limbic activity may become further decoupled from SRS functioning insofar as hypoarousal fails to sustain emotion-related limbic and paralimbic activation and concomitant empathic learning (Eisenberg, 2007; van Honk, et al., 2003).
The hypoarousal idea, however, may be complicated given that there are many components to HPA functioning beyond just cortisol levels. Here we focus on waking cortisol levels, the awakening response and diurnal rhythm. Each of these components of the HPA axis have been examined in relation to antisocial behavior or CU traits {von Polier, 2013 #33156;Loney, 2006 #20141;Cima, 2008#20130}, with the least attention thus far putatively paid to the diurnal rhythm. The present study uses extensive repeated measures of cortisol to permit examination of both flexibility and rhythmicity in HPA functioning (Skinner, Shirtcliff, Haggerty, Coe, & Catalano, 2011). Waking cortisol levels are often associated with tonic, basal HPA activity (Shirtcliff, Granger, Booth, & Johnson, 2005). Cortisol increases for 30–45 minutes after waking as the cortisol awakening response (CAR). The CAR may have a role in preparing for the upcoming day (Fries, Dettenborn, & Kirschbaum, 2009), regulating cognitive and immune functioning, and readying of the body as a kind of physiological “jump start” (Clow, Hucklebridge, Stalder, Evans, & Thorn, 2010). The diurnal rhythm is highly stable within individuals (Shirtcliff et al., 2011) and represents a normative pattern of HPA axis activity across a given day. After the CAR, the morning portion of the diurnal rhythm is primarily controlled by the anterior pituitary, under strong genetic influence, and relatively immune from environmental disturbance (Van Hulle, Shirtcliff, Lemery-Chalfant, & Goldsmith, 2012). As the day continues, the diurnal rhythm becomes progressively more responsive to environmental stimuli and concurrent stressor exposure (Bartels, de Geus, Kirschbaum, Sluyter, & Boomsma, 2003; Schreiber et al., 2006; Van Hulle, et al., 2012) including naturally occurring hassles (Adam, 2006; Almeida, McGonagle, & King, 2009; Peeters, Nicholson, & Berkhof, 2003), increasingly allowing the individual to alter HPA functioning to meet current contextual demands; thus, the latter portion of the diurnal rhythm is a strong measure of the flexibility of the HPA axis. Individual differences in this pattern may shed light on the long-term physiological resources of the individual, with a flat slope sometimes considered a measure of HPA dysregulation as it denotes loss of flexibility in the individual’s rhythm. One goal of the present research was to test how the hypoarousal idea applies to cortisol’s diurnal rhythm. Cortisol’s diurnal rhythm is not typically considered a stress reactivity measure, but may still be reflective of environmental perturbations as a component of the SRS.
Exposure to Stress Impacts the HPA axis
Building from a long-established role of cortisol as a stress hormone (Selye, 1950), cortisol increases in response to exposure to acute social stressors (Dickerson & Kemeny, 2004; Gunnar, Talge, & Herrera, 2009). For stressors of longer duration (Essex et al., 2011; Heim, Ehlert, Hanker, & Hellhammer, 1998; Yehuda, 2001) or which affect children early in development (Del Giudice, et al., 2011; Tarullo & Gunnar, 2006), high cortisol may not be consistently observed. Indeed, even in response to acute challenges, cortisol levels appear blunted in some individuals previously exposed to chronic, extreme stress such as child physical abuse (Harkness, Stewart, & Wynne-Edwards, 2011; Kaufman et al., 1997; MacMillan et al., 2009; Ouellet-Morin et al., 2011; Rao, Hammen, Ortiz, Chen, & Poland, 2008). Consequently, chronic stress is capable of exerting a powerful impact across the entire HPA axis, altering basal cortisol levels (Bruce, Fisher, Pears, & Levine, 2009; Cicchetti & Manly, 2001; Shea et al., 2007; Tarullo & Gunnar, 2006), the awakening response (Gonzalez, Jenkins, Steiner, & Fleming, 2009) and the diurnal rhythm (Fisher, Gunnar, Chamberlain, & Reid, 2000; Nicolson, Davis, Kruszewski, & Zautra, 2010; Shirtcliff, Hanson, Rudolph, Strang, & Pollak, under review). The present paper focused on the diurnal rhythm as such effects likely indicate stable stress regulatory challenges (Shirtcliff, et al., 2011) rather than momentary fluctuations from which an individual quickly recovers (Shirtcliff, et al., 2005).
On a practical level, the current study countered some draw-backs of self-reported stressors by focusing on extreme adversity such as physical abuse and utilizing interviews that distinguish the stress and its context from the individual’s emotional response to the stressor; this is important with notions of hypoarousal as such youth are low on correlates of perceived stress (Cohen, Tyrrell, & Smith, 1993), such emotional modulation by anxiety and neuroticism (Frick & White, 2008; Johansson, Andershed, Kerr, & Levander, 2002). We examine whether the youth has experienced physical abuse which is a severe stressor for most (if not all) individuals who experience it. Such measures can be criticized for only capturing the “tip of the iceburg” of stressors. Therefore, we collected youth life stress interviews to capture individualized experiences with stressors and the context in which they occurred (Rudolph & Hammen, 1999). The stressor information is gathered by collecting and evaluating detailed information about the circumstances surrounding the stressor and relying on a team of independent raters to validate stressor impact. The life stress interview distinguishes lifetime events from past-year stress exposure as the timecourse of a stressor may modify its impact, given that distal stressors are more clearly associated with hypoarousal (Miller, et al., 2007; Weems & Carrion, 2007).
Confluence of CU traits and Life Stress Exposure
Some proponents of early concepts searched for biomarkers of antisocial behavior or CU traits (Loney, Butler, Lima, Counts, & Eckel, 2006; Lykken, 1995; Raine, 2002), yet increasingly neurobiological research is developmental in nature and emphasizes that early adversity or stress exposure is a prevailing antecedent to the development of CU traits and antisocial behavior expression (Frick & White, 2008). This is largely based on evidence for substantial overlap between childhood adversity and psychopathy symptoms or antisocial behavior (Luntz & Widom, 1994; Luntz Weiler & Spatz Widom, 1996), even within incarcerated populations in which rates of adversity are high (Marshall & Cooke, 1999).A developmental perspective to neurobiological processes is especially compelling for research focused on understanding CU traits in children and adolescents (Frick & Ellis, 1999), as is the present report. Furthermore, neurobiological ideas are becoming more sophisticated and are leading to the conclusion that the physiological repercussions of early stress exposure can become instantiated in an individual’s physiology and, in turn, operate as a risk factor for antisocial behavior. Van Goozen and colleagues (2007) acknowledge that adverse rearing is common in antisocial youth, but emphasize that these children heighten problems through evocative interactions with parents, peers, siblings and teachers; impaired emotion- or distress-learning apparent in antisocial youth may further exacerbate caustic environments. Susman (2006) emphasizes that environmental stressors begin early, even prenatally, and these stressors exert a specific and persistent effect by attenuating stress responsive physiology; this in turn may manifest across the lifespan as vulnerability for antisocial behavior, but the process begins early enough that evocative reactions from others is unlikely to solely explain environmental risk (Cicchetti & Manly, 2001; Jaffee et al., 2004; Jaffee, Caspi, Moffitt, & Taylor, 2004). Strong genetic influences are apparent for antisocial behavior and CU traits {Viding, 2012 #29982;Viding, 2007 #20327}, suggesting that genetic, personality or other forms of early vulnerabilities may enhance risk for antisocial behavior (Lilienfeld et al., 2012; van Honk, et al., 2003). Proponents of hypoarousal models, such as Susman (2006), acknowledge that severe stress exposure is not the only pathway to hypo-arousal, but that environmental forces may exacerbate genetic vulnerabilities through stress-responsive physiological changes across early development. Shirtcliff and colleagues (2009) specify that HPA hypoarousal acts as a risk factor for antisocial behavior by down-regulating an individual’s ability to encode or respond to distress in others as easily as it reduces an ability to encode stress or distress in oneself, thereby diminishing empathic responses. The HPA accomplishes this neuroregulation largely through direct top-down and bottom-up modulation of brain regions implicated in empathic and social processes. Early adversity is also a pathway to hypoarousal as it allows the individual to become insulated from day to day social stressors, increasing a threshold for HPA activation to only the most salient of signals of danger (Del Giudice, et al., 2011). This viewpoint emphasizes that callousness may stem from early experiences. Adopting an exploitative interpersonal style requires one to be shielded from social rejection and disapproval, so the social informational insulation provided by hypoarousal can later exacerbate antisocial behavior and CU traits.
The present paper sought to empirically examine CU trait expression and life stress as predictors of HPA functioning, indexed by basal waking levels, the CAR and the diurnal rhythm. We hypothesized that CU and life stress would be associated with hypoarousal evident by low cortisol and a blunted diurnal rhythm. We explored the CAR as a marker of HPA functioning, but hypotheses were non-directional as some forms of stress exposure are associated with a blunted CAR (e.g., burnout, fatigue), but other stressors are associated with a heightened CAR especially if the stressor is engaging or active (Chida & Steptoe, 2009; Fries, et al., 2009; Gonzalez, et al., 2009; Thorn, Hucklebridge, Evans, & Clow, 2009; Wust, Federenko, Hellhammer, & Kirschbaum, 2000). One prior study found that callous boys had a blunted CAR(von Polier et al., 2013). We additionally tested whether CU traits and life stress interact, although this hypothesis is exploratory as no prior empirical investigation with incarcerated adolescent boys (to our knowledge) has examined both stress and CU traits with cortisol. The closest study is by Cima and colleagues (2008) who found that cortisol levels were lower within adult psychopathic offenders, but within this hypo-aroused group a history of child abuse was associated with higher cortisol. We examined the interaction of stress and CU traits in a sample of adolescent boys who are incarcerated, recognizing that the context of recent incarceration in a treatment facility (1–2 weeks prior) may be stressful, necessitating adjustment to new rules, expectations, goals, peers, inmates, supervisors, and physical separation from family, peers, and prior lifestyle. Many of the physiological adjustments are expected to have occurred within this period (e.g., adjustment to wake/sleep schedules, meal-times, and other powerful zeitgebers (Adan et al., 2012)); further social adjustments may still be transpiring and it would still take many weeks for treatment effects to culminate in physiological changes. This type of experimental rigor is important within a study of cortisol’s diurnal rhythm in adolescents who often have erratic sleeping patterns (Carskadon, et al., 2004), especially antisocial youth in naturalistic (but not entrained) settings (Lindberg, et al., 2008).
Method
Participants
Participants were 50 male incarcerated adolescents placed at the Mendota Juvenile Treatment Center (MJTC), a program with a unique clinical-correctional hybrid structure. It is operated under the administrative code of the Department of Corrections but housed on the grounds of a State mental health facility with a heavy emphasis on treatment. MJTC receives youth referred by general corrections facilities for extreme behavior problems. This provides for an enriched sampling of severe antisocial behavior. Participants at MJTC whom completed this study range in age from 14 to 18 (M=16.08, SD=1.06) and were predominantly of African-American ethnicity (60%), with a smaller proportion of Caucasian (26%), Hispanic (12%), and Other (2%). Based on chart review of diagnoses and symptom profiles, participants met criteria for Conduct Disorder, though there was a wide range of symptom severity such that mean PCL-total scores were 27 (SD=8.4, N=26 above cutoff of 30, N=10 above 33). Furthermore, psychiatric difficulties are highly prevalent, with 92% and 88% of the sample having at least one comorbid internalizing or externalizing disorder, respectively. Percentages of diagnoses exceed 100% because most adolescents had more than one formal DSM-IV diagnosis. Within externalizing disorders, comorbidity was high, with 62% of the sample having ADHD, 57% having a history of substance use disorder, 31% with PTSD, and 10% with an attachment disorder. Youth were excluded if they had a psychotic episode or thought disorder that would have prevented them from participating in the study. Smoking, caffeine, and illicit drugs were outlawed and not consumed due to correctional facility rules and constant monitoring.
Procedure
The University of New Orleans and MJTC IRB approved the study. Both parental consent and individual assent were obtained for all adolescents before they were able to participate in the current study. There was a small food incentive provided for participation, individuals gained no additional dispensation from the institution for their participation (e.g., additional unit privileges or early release from the institution). Saliva collections were conducted between 1–2 weeks after admission to allow for acclimation to the facility but to minimize possible treatment effects that could result from being in an incarcerated setting with a strong mental health focus. Testing occurred over the course of 3 days, including two days for collecting saliva samples and one for conducting the Psychopathy Checklist Youth Version (PCL-YV) and Life Stress Interview (LSI) interviews as well as the self-report measures of CU traits, physical abuse questionnaires, and demographic information.
Measures
Salivary Cortisol Collection
Salivary cortisol in the participant was collected by research staff members on two days of five samples each day to permit examination of the stability of cortisol’s diurnal rhythm (Shirtcliff & Essex, 2008). Saliva was collected (1) upon waking (range=6:10am to 7:53am, M=7:07am, SD=11 min); (2) 45 minutes later to capture the response to awakening (7:10am to 8:25am, M=7:46am, SD=15 min) (Wust, et al., 2000); (3) before lunch to minimize the influences of mealtimes (range=11:19am to 11:47am, M=11:31am, SD=5 min); (4) before dinner (range 5:10pm to 6:38pm, M=5:33pm, SD=9 minutes); and (5) immediately before bedtime to capture the entire rhythm (range= 9:30pm to 11:55pm, M=10:00pm, SD=16 min). Saliva was collected following published protocols (Schwartz, Granger, Susman, Gunnar, & Laird, 1998) and frozen immediately (−80°C).
Saliva was assayed for cortisol in duplicate using a well-established highly sensitive enzyme immunoassay kit (www.salimetrics.com) by Madison Biodiagnostics (Madison, WI). Mean intra-assay and inter-assay coefficients of variation (CVs) were 3.8% and 7.4%, respectively. Samples were reanalyzed if the CV for the duplicate measurements were ≤20%. To normalize distributions, raw cortisol was log-transformed and extreme values were winsorized (1.07%).
Several control variables were measured by youth self-report in order to best account for salivary hormones based on prior literature (see analyses below). The Daily Diary measured time of awakening, time of collection, medication use, mood, sleep, and daily hassles or uplifts (Shirtcliff, et al., 2005). Sleep times were self-reported and sleep quality (for the prior week) was assessed with the Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). Medication type and frequency of use was self-reported and collected via chart review; medications were then coded according to a published strategy that collapses medications according to the mechanism of action on the HPA axis (Essex, et al., 2011; Schreiber, et al, 2006; Shirtcliff, et al., 2011). The most common medications included neuroleptics (7.5%), mood stabilizers (2.4%), CNS active medications not otherwise specified (2.8%), psychostimulants (2.1%), antidepressants (2.1%) , antihistamines (1.7%) and analgesics (1.7%). Pubertal development was assessed through confidential self-report (Petersen, Crockett, Richards, & Boxer, 1988).
Callous Unemotional Traits
Inventory of Callous Unemotional traits
The ICU (Kimonis et al., 2008) is a 24-item scale designed to measure callous traits in youth, with 11 self-report items specifically measuring callousness (e.g., The feelings of others are unimportant to me; I seem very cold and uncaring to others; I do not care who I hurt to get what I want). The ICU has undergone extensive testing and validation for the measurement of callous traits (Essau, Sasagawa, & Frick, 2006; Roose, Bijttebier, Decoene, Claes, & Frick, 2010). Reliability estimates are excellent (α=83 for the ICU, α=79 for the callous subscale). The ICU was administered in its entirety, but we focus on the Callous subscale because it most closely indexes the aspect of CU traits thought to be linked with hypoarousal. The average ICU Callous score was 10.24, SD=5.17 and the total IC was 29.27, SD=9.98.
Psychopathy Checklist-Youth Version
The PCL-YV (Forth, Kosson, & Hare, 2003) is a 20-item rater-based instrument that involves the administration of semi-structured interview of the youth along with an extensive file review of criminal and social records. Interviewers administered the semi-structured interview and were given complete access to the adolescent’s clinical file. Raters were trained on multiple practice cases; they went through a rigorous training program that involved a full day training on the PCL:YV and conducting “test cases” to practice administration and scoring. Interrater reliability is reported as ICC=.93 for institutional populations (Forth, Kosson, & Hare, 2003). The instrument has been shown to be highly reliable (α=91 for all items, α=81 for the affective subscale) and is among the best validated measures for evaluating psychopathy in adolescents (Corrado, Vincent, Hart, & Cohen, 2004)(Kotler & McMahon, 2010). The average PCL-Affective score was 7.81 (SD=2.09) and the average total PCL was 27, SD=8.4.
Interpersonal Reactivity Index
The IRI (Davis, 1983) is a 28-item self-report instrument, which measures four empathy-related constructs including the 14-item Empathic Concern subscale which most closely reflects opposite of CU traits (e.g., I care for my friends a great deal; When I see someone being taken advantage of, I feel kind of protective toward them; (reversed) Usually I am not extremely concerned when I see someone else in trouble). The IRI has been successfully used with adolescent populations, including justice-involved adolescents (Curwen, 2003; Gini, Albiero, Benelli, & Altoe, 2007). The IRI displays good reliability estimates for all subscales (greater than .70 for all subscales). The average IRI empathic concern score was 16.04, SD=4.45 and the total IRI was 55.6, SD=15.11.
Callous Factor
To capture the latent Callous Unemotional construct incorporating multiple callous measures, the scale most closely resembling CU traits from each measure (ICU, IRI, PCL-YV) was entered into a Principle Component Analysis. A single factor emerged (Eigenvalue=1.45) which explained 48.2% of the total variance and loaded highly on the Callous subscale of the ICU (loading=.75), as well as Empathic Concern from the IRI (loading=−.69) and the Affective subscale of the PCL:YV (loading=.64). Standardized scores were extracted from this principal component analysis to represent an omnibus Callous Factor score for further analyses.
Stress Measures
Child abuse
Two self-report measures of physical abuse exposure, the Childhood Trauma Questionnaire (CTQ) (Bernstein, Ahluvalia, Pogge, & Handelsman, 1997) and the Conflict Tactics Scale (CTS) (Straus, Hamby, Finkelhor, Moore, & Runyan, 1998) were administered, tapping into abuse by the mother and/or father. The physical abuse subscales (r=.78, p<.001) were Z-scored and averaged to form a physical abuse composite across the CTQ and CTS subscales. We focus on Physical Abuse given prior literature that shows its impact on the HPA as a severe life stressor (Carpenter, Shattuck, Tyrka, Geracioti, & Price, 2011; Cicchetti & Rogosch, 2001, 2007). Over 37% of the sample reported physical abuse (PA) by one or both parents on the CTS (CTS PA=6.84, SD=10.32; CTQ PA=8.47, SD=3.63).
Life Stress Interview
The Youth Life Stress Interview (LSI) (Adrian & Hammen, 1993) was administered to the youth to capture subtle individual variation in stressor exposure with a clearly delineated time-course capturing chronic (within the past year) and lifetime stressors. Interviewers were trained through multiple practice interviews and passed a check-out with PhD-level interviewers. The LSI measures chronic stressors across several salient domains including academic, peer, relationship, parent-child and family stress in the past year. The LSI has a set of semi-structured follow-up probes in order to incorporate the context of life events. Later, the interviewer verbally presented the content of the interview to an independent team of 3–7 trained raters, none of whom had met the participant. Language that conveys emotional responses that tap subjective experience of stress was removed prior to rating so that the collective stress codes remained as objective as possible about the impact of stressors. Stressors were rated on a scale of 1 (not at all stressful) through 5 (very severely stressful). Across the 7 chronic stress domains, past-year stress was 3.34 (SD=.60) on a scale of 1 to 5, where 5 is extreme stress.
LSI lifetime stressors were reported by the youth at the end of each interview including specific probes across salient domains for experiences. At the end of the study, these narratives were aggregated and ranked on a 10-point scale by trained raters. The time-course of lifetime stressor exposure ranges from as early as the prenatal period (e.g. teratogen exposure) up until the past year so that it does not overlap with LSI chronic stress in the past year. Intraclass correlations of the LSI across independent rating teams has been shown to indicate high reliability (r(49)=.85, p<.001) (Rudolph & Hammen, 1999). On average, boys ranked 4.7 (SD=2.61) on a scale of 1–10 for the lifetime stress exposure.
Analytic Strategy
Data were cleaned using SPSS v18.0. Due to the regularity of the daily schedule at MJTC, hormone sample and questionnaire missing data were minimal. Out of 500 total possible samples, 443 samples (89%) were obtained in sufficient quantity for assay. Analyses were run using the Hierarchical Linear Modeling (HLM) (Bryk & Raudenbush, 1992) to account for the inherent nesting of samples collected within the individual (level 1) and measures between individuals (level 2). HLM is advantageous as it allows for the simultaneous modeling of waking cortisol levels, the cortisol awakening response, and diurnal slope (Hox, 2002; Snijders & Bosker, 1999). This reduces the total number of models reported in the present report to 6 (to examine the behavioral measures of the callous factor and PCL-Affective and the 3 unique life stress measures of physical abuse, lifetime, and past-year stress). At level 1, cortisol was the outcome and was predicted by several time-varying measures including a dummy variable representing if the sample was the 45-min post waking sample (sample 2) to account for the cortisol awakening response (CAR) (Fries, et al., 2009), β1car=.53, p<.001; a continuous measure of time since waking in hours to capture the diurnal rhythm as cortisol declines across the daytime hours (Ruttle et al., 2011; Van Hulle, et al., 2012), β2TSW=−.13, p<.001; a dummy variable for pre-lunch cortisol (sample 3) to account for lower cortisol at that time than expected, β3sample3=−.23, p<.006; and finally a dummy variable for mid-afternoon cortisol (sample 4) to account for higher cortisol at that time than expected based on the diurnal rhythm, β4sample4=.64, p<.001. After accounting for these predictors, the level-1 intercept represented waking cortisol levels (β0intercept=3.59, p<.001), and 37.8% of the variance in cortisol levels was due to stable, individual differences, χ2(175.87, p<.001).
These level-1 predictors of momentary cortisol levels could then become outcomes-of-interest at level-2 (between-individual differences). First, we determined which control variables were necessary to include. For age (average=16.23, SD=.96), we found that older youth had flatter slopes (Shirtcliff, et al., 2011), γ21=012, p=.041. For BMI (average=26.0, SD=5.14), boys with greater BMIs had higher waking cortisol levels (Dallman et al., 2004), γ01=.006, p=.04. Compared to other ethnicities, white youth (27.5% of the sample) had higher cortisol upon awakening (DeSantis et al., 2007; Skinner, et al., 2011), γ02=38, p=.018, and a trend for lower cortisol around lunchtime, γ31=−.27, p=.07. These variables did not influence any other components of HPA functioning, ps>.10, nor did medication usage, SES, sleep quality, pubertal status or timing, ps>.10. We controlled for age, BMI, and race/ethnicity in all subsequent models. Thereafter, we included the stressor variable, callousness variable, and the interaction between stress*callousness on each component of HPA functioning (see below). Interactions were calculated as continuous scores to reduce the adverse impact of categorizing continuous variables (e.g., generating spurious results and limiting power (MacCallum, Zhang, Preacher, & Rucker, 2002).We then serially removed predictors with ps>.15 to arrive at a parsimonious model (Berk, 1978), with the exception that if the interaction was significant, main effects remained in the model.
Both stress exposure and psychopathy are multi-faceted and we included multiple measures of each to understand their nature. We examined Abuse History, LSI lifetime stress rankings, and LSI chronic stress in the past year to index stressors with different severity and time-courses following Miller (Miller, et al., 2007) or Weems and Carrion (Weems & Carrion, 2007). We considered combining the stress scores further but retained separate models given our conceptualization of the timecourse of stress effects and intercorrelations were modest (abuse with chronic stress, r=.30, p<.05, with lifetime stress, r=.42, p<.01; chronic stress with lifetime stress, r=.20, p>.1). To minimize the number of models, we first used the Callous Factor (CF) to index CU traits, broadly. Given that the PCL-Affective scale is more objective, rater-based stemming from an in-person interview, and relies on collateral information beyond youth self-report, we then specifically examined the PCL-YV affective scale to index CU traits (Neumann, Kosson, Forth, & Hare, 2006; Vincent, Vitacco, Grisso, & Corrado, 2003; Vitacco & Vincent, 2006). CF was correlated with the PCL-Affective scale, r=.64, p<.001. Cross-correlations of stress with CU variables were all non-significant, average r=.005, ps>.05, which allowed the interactions to be calculated without concerns of multicollinearity.
Results
Child Physical Abuse
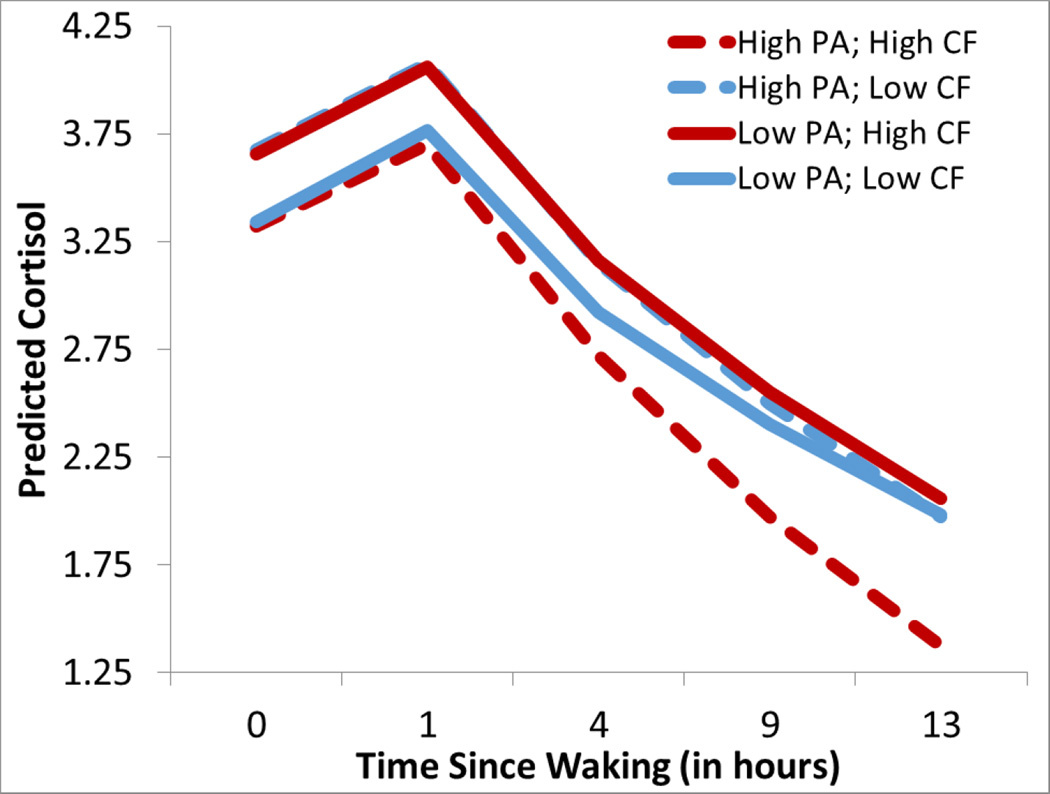
When abuse was included with the callous factor as a predictor of cortisol, we found a main effect of physical abuse on the diurnal rhythm, β=−.016, p<.002, such that abused boys had steeper diurnal rhythms (i.e., sharper declines) after being incarcerated (dashed lines). Further, there was an interaction between abuse and CF scores on waking cortisol levels, β=−.19, p=.04, such that abused boys with high CF scores had low waking cortisol levels as well as boys scoring low on abuse and CF scores. By contrast, boys with either high abuse or high CF scores had elevated waking cortisol. Figure 1 illustrates that boys with elevated CF scores and abuse histories had the lowest cortisol levels in the morning, and ended the day with the lowest cortisol.
Figure 1.
Lines represent predicted scores using Empirical Bayes Estimation of youth who are one standard deviation from the mean, based on the continuous predictor scores within HLM. Predicted cortisol levels in incarcerated adolescent boys who have high physical abuse (PA) (+1SD, dashed lines) as opposed to low PA histories (−1SD, solid lines), and high Callous Unemotional Factor scores (CF, +1SD, red lines) as opposed to low CF scores (−1SD, blue lines). Individuals with high abuse histories and CF scores (red dashed) had low cortisol and steep slopes so they displayed the lowest cortisol across the day. Empirical Bayes are conservative estimates (Bryk & Raudenbush, 1992).
We then specifically examined the affective-facet of the PCL-YV. There was a significant interaction between physical abuse and the PCL-affective scale, β=−.008, p<.041, such that the boys with abuse histories had steep slopes (i.e., sharper declines) if they also had high PCL-affective scores but they had the flattest slopes if they had relatively low PCL scores and thus ended the day with lowest cortisol.1
Lifetime Stress Exposure
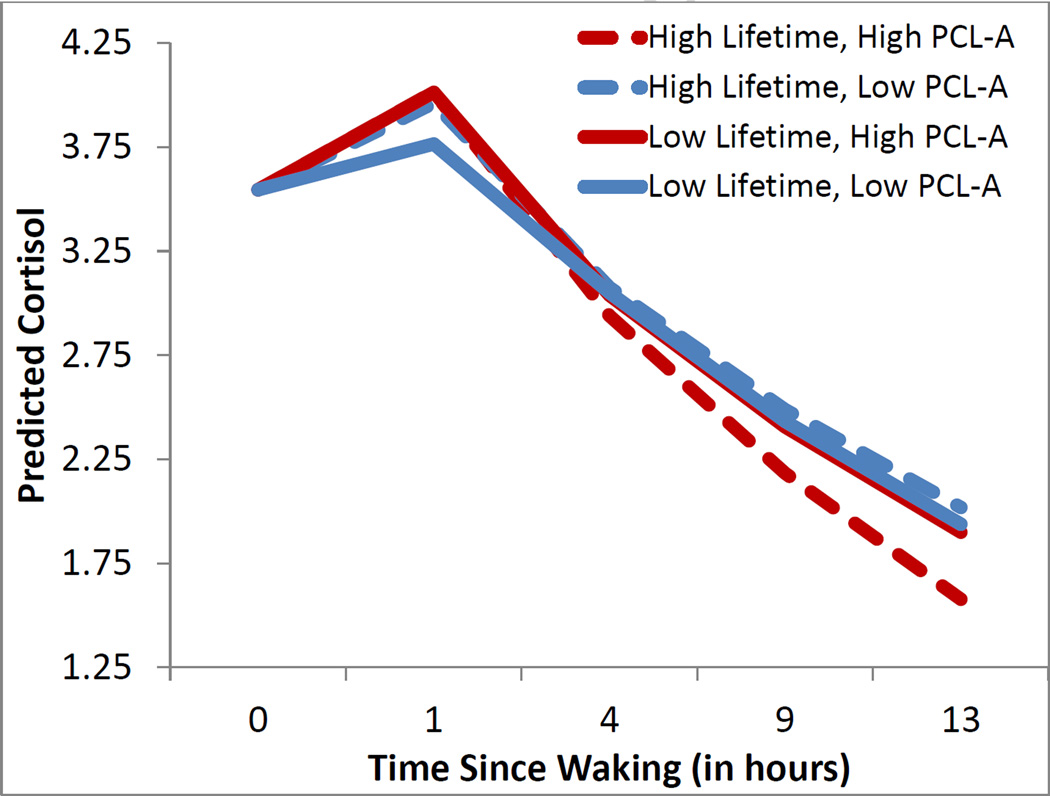
There was no effect of stress or CF on waking cortisol level, ps>.16, but boys with greater stress exposure, β=.056, p<.012, or higher CF scores, β=128, p<.041, had a steeper rise during the cortisol awakening response (CAR).
We then specifically examined the PCL-affective scale, and found that boys with greater PCL scores had a steeper CAR rise, β=.04, p<.007. Regarding the diurnal slope, there was an interaction between lifetime stress and PCL-affective scores, β=−.002, p<.03; Figure 2 illustrates that boys with greater lifetime stress had steep diurnal slopes (i.e., faster drop) only if they also had high PCL-affective scores (red dashed), whereas they had flat slopes if they did not have elevated PCL-affective scores (blue dashed).2
Figure 2.
Boys with high Lifetime stress exposure on the LSI (+1SD, dashed lines) as opposed to low lifetime stress (−1SD, solid lines) are illustrated as well as boys with high PCL:YV Affective scores (+1SD, red lines) as opposed to low PCL:YV Affective scores (−1SD, blue lines). Boys with higher PCL scores had a steep cortisol awakening response (red lines); boys with both elevated PCL and LSI scores (dashed red lines) displayed steep diurnal slopes. Lines represent predicted scores using Empirical Bayes Estimates within HLM for continuous scores one standard deviation from the mean.
Past-Year Chronic Stress
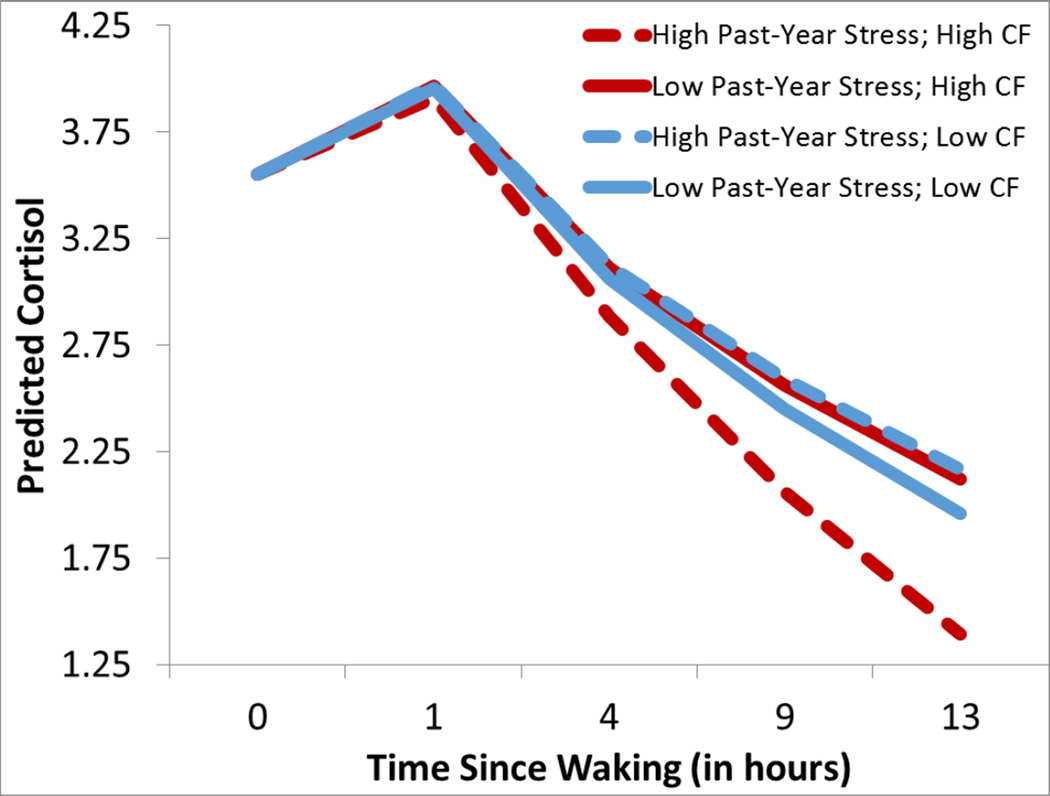
For cortisol’s diurnal rhythm, there was a trend for an effect of past-year stress, β=−.018, p=.06, and a trend for an effect of CF scores, β=−.011, p=.09, which were modified by a significant interaction between past-year stress and callousness, β=−.034, p<.016, such that boys who had elevated stress and CF scores had the steepest diurnal slope (i.e., fast drop across the day), whereas boys who were elevated on only stress or only CF scores had flatter diurnal slopes (see Figure 3).
Figure 3.
Boys with high past-year stress exposure on the LSI (+1SD, dashed lines) as opposed to low lifetime stress (−1SD, solid lines) are illustrated as well as boys with Callous Factor scores (+1SD, red lines) as opposed to low Callous Factor scores (−1SD, blue lines). Boys with both elevated Past-Year Stress and CF scores (dashed red lines) displayed steep diurnal slopes. Lines represent predicted scores using Empirical Bayes Estimates within HLM for continuous scores one standard deviation from the mean.
Focusing on the PCL-affective scale, a trend for an effect of past-year stress on the diurnal slope, β=−.020, p=.056, was qualified by an interaction between stress and PCL-affective scores, β=−.010, p<.019, such that boys who had greater past-year stress and PCL-affective scores had the steepest diurnal slope (i.e., fastest declines), but all the other boys had relatively blunted diurnal rhythms, similar to the effect of lifetime stress exposure3.
Discussion
The findings from the present study involving incarcerated adolescent boys with CU traits and life stress in the moderate to extreme range somewhat support a hypoarousal neurobiological concept for antisocial behavior, but add to this perspective in several ways. First, we examined cortisol’s awakening response and found in some models that steeper CAR increases were associated with lifetime stress exposure and CU traits. Second, we examined cortisol’s diurnal rhythm and typically found that a blunted diurnal rhythm was associated with CU traits or prior life stress, much more so than waking cortisol levels. Third, we found that youth elevated on both CU traits and stress exposure displayed a unique HPA profile, consistently showing steeper diurnal rhythms. Taken together, these findings are largely consistent with the hypoarousal idea (Shirtcliff, et al., 2009; Susman, 2006), but also challenge its conceptualization in several ways.
We found low waking cortisol in boys with high CU traits and an abuse history, but did not simplistically support hypoarousal with any of the other stress or CU measures. This is consistent with Cima and colleagues (2008) insofar as they found that psychopathic symptoms were linked with blunted cortisol levels and the addition of life stress exposure complicated the picture of hypoarousal. Our findings are not consistent with that investigation, however, in that we observed the lowest cortisol within youth with high CU and high stress exposure, whereas Cima and colleagues (2008) found abuse and psychopathy symptoms were linked with elevated cortisol within the psychopathic subgroup.
Our findings are also not consistent in that Cima and colleagues (2008) obtained significant results with overall cortisol levels that hinted toward diurnal cortisol fluctuations across the four samples they obtained. Across each model, we found that cortisol’s diurnal rhythm was the HPA component most consistently associated with CU traits and life stress. Flatter diurnal rhythms have been suggested to indicate that individual is struggling to match their physiological setpoints with the demands of the environment, resulting in a less flexible diurnal rhythm (Skinner, et al., 2011). Yet, we speculate that a flatter diurnal rhythms may also reflect adaptive calibration of the individual’s physiology to the level of challenge in their environment; this is suggestive of a buffering effect (Shirtcliff, 2011) in which a flattened rhythm nonetheless maintains some physiological rhythmicity (Siever & Davis, 1985). This buffering may come at a cost, with the trade-off of maintaining rhythmicity being a less flexible or malleable HPA axis (Del Giudice, et al., 2011; Shirtcliff, et al., under review). Consistent with this, we found that boys with higher CU traits (measured as the callous factor or specifically with the PCL-Affective facet) as well as boys with greater life stress exposure (e.g., physical abuse, lifetime stress, and past-year stress) had flat diurnal rhythms. A stable, flattened diurnal rhythm may indicate that an individual is less “open” to their environment (Skinner, et al., 2011). The practical implications of a less malleable rhythm may include greater difficulties adjusting physiologically to new contexts or day-to-day events. It may take longer for such individuals to change in response to environmental decrements or improvements (i.e., treatment or interventions) (Fisher, et al., 2000; Fisher, Van Ryzin, & Gunnar, 2011).
In contrast, boys elevated on both CU traits and life stress exposure (e.g., physical abuse, lifetime stress, and past-year stress) consistently displayed the steepest diurnal rhythms. This meant that these boys ended the day with the lowest cortisol levels, yet they began the day with normal cortisol levels and awakening responses. We are reticent to conclude or even speculate that high CU and life stress is comparable to an adaptive, flexible or “open” biological profile, for several reasons. First, in an independent investigation, we found that youth with substantiated child physical abuse had a flattened diurnal rhythm (as expected) but that youth with the most severe abuse histories had a somewhat steeper rhythm (Shirtcliff, et al., under review). Relatedly, whereas exposure to one early life stressor was associated with highly variable diurnal rhythms and developmental shifts in HPA functioning across adolescents, those exposed to a combination of multiple early adversities looked remarkably similar to the youth with no early adversity exposure in terms of HPA functioning (Essex, et al., 2011). Thus, it is possible, even at this very high range of adversity, that there is an underlying U-shaped association with HPA functioning (Boyce & Ellis, 2005; Ellis, Essex, & Boyce, 2005). This interpretation fits with Cima and colleagues (2008) whose findings were suggestive of a U-shaped curve in that offenders had both higher and lower cortisol than control participants; moreover, although psychopathic offenders had overall low cortisol, within this group psychopathy symptoms were linked with elevated cortisol.
Another speculation is that there may be something unique about the context of incarceration for which the high CU and high stress boys are uniquely suited; if so, the steep diurnal rhythm would reflect a physiological profile of being “open” to the salient social signals in this environment. Other studies have found that extremely stressed youth undergoing a recent move (e.g., entering foster care) display steeper diurnal rhythms than if they had remained in home setting (Bernard, Butzin-Dozier, Rittenhouse, & Dozier, 2010; Fisher, et al., 2011). The hypo-arousal perspective emphasizes that blunted physiological arousal may be an asset to be shielded from social evaluation and rejection or buffered from the impact of a chaotic environment, but an “open” HPA profile is also expected in youth who are vigilant to signals of danger and threat in their environment (Del Giudice, et al., 2011). These youth may be adept at attending to these new environmental cues and recent changes. Boys had been incarcerated for 1–2 weeks so that youth would likely be physically adjusted to the incarceration (e.g., sleep and diet patterns normalized) but the psychological stress, including interacting with new peers and authority figures, would still be relatively acute. Consistent with this is the observation that in the late afternoon, there was a small cortisol increase superimposed on the steep diurnal rhythm which corresponded to the time of day in which school activities ended and large peer group activities began (captured by the dummy variable for a mid-afternoon cortisol rise). For the most severely antisocial youth, this new peer context may present new opportunities to provoke antisociality, and high cortisol or a steep diurnal rhythm may facilitate such evocative interactions (Raine, 2002; van Goozen, et al., 2007).
Although not as robust as the diurnal slope findings, we also found that the cortisol awakening response was steeper within youth who had were elevated on lifetime stress, greater CU traits or PCL-Affective scores. Some have proposed that the CAR is a process the body uses to ready itself for the day and its potential challenges as a morning “jump start” (Fries, et al., 2009), but the exact function of the CAR remains uncertain. Some forms of stress exposure are associated with a blunted CAR (e.g., burnout, fatigue), but stress exposure is associated with a heightened CAR if the stressor is engaging or active (Chida & Steptoe, 2009; Gonzalez, et al., 2009; Thorn, et al., 2009; Wust, et al., 2000). An intact CAR may help an individual anticipate the upcoming day or recover from inertia (Clow, et al., 2010). One possible speculation for this intact CAR in boys high in CU traits, as opposed to the relatively small CAR among low-CU boys, is that the callous boys have adjusted their neurobiology to fit their stressful context of incarceration within the 1–2 weeks of incarceration. CU traits are often thought to shield an individual from their environment, and in this case it may shield the individual from being overwhelmed or fatigued by their new context and thus capable of displaying a normal CAR. In parallel, boys with elevated CU traits may be highly prepared for the social interactions of the upcoming day and are anticipating or readying themselves for social challenges. Youth with greater lifetime stress exposure prior to incarceration similarly may not experience the context of incarceration as overwhelming or fatiguing (at least in comparison to the daily challenges encountered in their lives prior to incarceration). Despite recent incarceration, such youth may still be capable of preparing themselves for their day. The incarceration setting is stressful, but it is also a departure from the chronic stress present in the home context. Boys without CU traits or the buffering of prior stress exposure may be physiologically struggling to adapt their neurobiology to the context of incarceration. This perspective fits with the adaptive calibration model of stress responsivity which emphasizes that there are trade-offs of adaptation and that individuals will meet the needs or demands of their environment, for better and for worse (Del Giudice, et al., 2011; Ellis, et al., 2012).
Regardless of the explanation, the observation that the low stress, low CU boys displayed an HPA axis profile that looked remarkably similar to the high stress, high CU boys or that the most callous or stressed individuals appeared to display a “normal” CAR has implications for hypoarousal and the potential that low cortisol can become a biomarker for antisocial behavior (Loney, et al., 2006; Lykken, 1995; Raine, 2002). The HPA axis is complex and frequently changes. This is true across the day or in response to awakening and in response to salient social contexts. This biomarker is thereby unlikely to become a useful diagnostic tool, as even these severely antisocial boys did not homogenously display a hypoaroused HPA axis. Heterogeneity is commonly recognized within the literature on CU traits in youth (Frick & Ellis, 1999; Frick, Lilienfeld, Ellis, Loney, & Silverthorn, 1999) and extend to biological measures as well (Kimonis, Frick, Cauffman, Goldweber, & Skeem, 2012). Furthermore, heterogenous findings fit within a broader literature on variations of psychopathy subtypes, including the secondary form that largely overlaps with early life stress exposure (Skeem, Johansson, Andershed, Kerr, & Louden, 2007; Skeem, Poythress, Edens, Lilienfeld, & Cale, 2003). Despite heterogeneity in the sample, cortisol provides a window into the vulnerabilities of an individual within their context. Our results show that hypoarousal may be usefully conceptualized as “environmental openness” as opposed to an unwavering low level of cortisol from waking to bed which we observed within some at-risk youth. When cortisol’s full diurnal rhythm is measured and an individual’s context is considered, the integrative notion of hypoarousal becomes a useful characterization of HPA functioning in youth with CU traits or prior stress exposure. Of interest is whether this pattern persists as youth adjust to the context of incarceration beyond the first few weeks or if similar observations would be discovered in incarcerated adolescent girls.
Despite study strengths such as extensive repeated measures, a severe at-risk population, and well-validated psychological and physiological measures, this investigation has limitations. First, the sample size is small, and may limit our ability to detect robust interactions. The extensive repeated measures within each youth offset some of this reduction in power, but a larger sample size would be helpful for reducing the possibility of type I errors and trend-level effects. Relatedly, a control group would be an advantage although it would be difficult to match on life stress, daily schedule and CU trait expression in non-incarcerated youth. Second, many youth were on medications, as is typical for severely antisocial youth; we were limited to statistically controlling for medications. Third, we allowed for 1–2 weeks for adolescents’ circadian rhythms to be entrained to the strict schedule of the incarceration context since prior studies have shown that rhythms normalize within a few days (Adan, e t al., 2012) and behaviorally, the effects of being in a mental-health oriented facility are observed after two months or more (Caldwell, 2011; Caldwell & Van Rybroek, 2005); nonetheless, we cannot confirm that this set window was ideal for observing HPA effects. Future studies should track the time-course of HPA changes over time. Fourth, this investigation was limited to adolescent boys. Future studies should consider a parallel investigation with incarcerated girls to determine whether they show similar biobehavioral underpinnings. Finally, the present investigation focuses on the HPA axis and the conceptual meaning of hypo-arousal within this population rather than take a more person-centered approach which characterizes or profiles these youth, and their neurobiological patterns, as primary- or secondary-psychopathic, callous, or unemotional. We chose to do so in order to more fully explore policy and practical implications of hypo-arousal within future studies.
In sum, the present study found a general pattern in which incarcerated adolescent boys with greater life stress or CU traits had similar functioning of the HPA axis, and boys elevated on both prior stress and CU traits had a unique HPA profile. The findings illustrate the utility of examining flattened diurnal rhythms as an index of hypoarousal in a sample of severely antisocial youth with a moderate to extreme range of both CU traits and life stress exposure. Just as a developmental perspective helped advance the neurobiological investigation of antisocial behavior, we hope this study illustrates the utility of moving beyond hypoarousal towards a viewpoint in which prior stressors, current context, and behavioral or emotional dispositions together inform our understanding of adolescent behavior problems.
Highlights.
Cortisol and its diurnal rhythm across two days were examined in incarcerated adolescent males.
Callous unemotional traits were associated with a flattened diurnal rhythm and a steeper cortisol awakening response.
Greater life stress exposure including child abuse was associated with a flattened diurnal rhythm.
The combination of CU traits and stressor exposure was linked with steeper diurnal rhythms in the incarceration setting.
Acknowledgments
This study was supported by research support from the Office of Justice Administration (OJA637550) and National Institute of Mental Health (MH093675). Salary support for Dr. Shirtcliff was provided by a K01 Career Development Award (MH077687). We wish to acknowledge Alexander Graf, Susan Bushek and Donald Fluery who provided invaluable help with the research project and the participants and their families who made this research possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Results for PCL-Affective were recalculated controlling for the PCL-Antisocial score in order to establish that CU traits were not simply a proxy for severity of antisocial behavior, following Loney et al (2006). The interaction between abuse and PCL-Affective on the slope remained significant, B=−.008, p=.03, and PCL-Antisocial was not significant, p>9.
For Lifetime stress exposure, the PCL-Antisocial did not predict cortisol or its diurnal rhythm, ps>7, and the interaction between PCL-Affective with lifetime stress exposure on the slope remained robust and significant, B=−.002, p=.014.
As above, we controlled for PCL-antisocial scores (p>.6), in the model where PCL-affective scores and past-year stress were of interest; we found effects of past-year stress (B=−.02, p=.04) and the interaction of past-year stress with PCL-affective (B=−.01, p=.018) persisted and, if anything, were more robust.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: a comprehensive review. Chronobiol Int. 2012;29(9):1153–1175. doi: 10.3109/07420528.2012.719971. [DOI] [PubMed] [Google Scholar]
- Adrian C, Hammen C. Stress exposure and stress generation in children of depressed mothers. J Consult Clin Psychol. 1993;61(2):354–359. doi: 10.1037//0022-006x.61.2.354. [DOI] [PubMed] [Google Scholar]
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography Soc Biol. 2009;55(2):219–237. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, de Geus EJ, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of daytime cortisol levels in children. Behav Genet. 2003;33(4):421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Berk KN. Comparing subset regression procedures. Technometrics. 1978;20(1):1–6. [Google Scholar]
- Bernard K, Butzin-Dozier Z, Rittenhouse J, Dozier M. Cortisol production patterns in young children living with birth parents vs children placed in foster care following involvement of Child Protective Services. Arch Pediatr Adolesc Med. 2010;164(5):438–443. doi: 10.1001/archpediatrics.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Jounral of the American Academy of Child and Adolescent Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci. 2007;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Budhani S, Colledge E, Scott S. Deafness to fear in boys with psychopathic tendencies. J Child Psychol Psychiatry. 2005;46(3):327–336. doi: 10.1111/j.1469-7610.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Peschardt KS, Budhani S, Mitchell DG, Pine DS. The development of psychopathy. J Child Psychol Psychiatry. 2006;47(3-4):262–276. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol Levels in preschool-aged foster children: differential effects of maltreatment type. Dev Psychobiol. 2009;51(1):14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Sage Publications, Inc; 1992. [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Caldwell MF. Treatment-related changes in behavioral outcomes of psychopathy facets in adolescent offenders. Law Hum Behav. 2011;35(4):275–287. doi: 10.1007/s10979-010-9239-z. [DOI] [PubMed] [Google Scholar]
- Caldwell MF, Van Rybroek GJ. Reducing violence in serious juvenile offenders using intensive treatment. Int J Law Psychiatry. 2005;28(6):622–636. doi: 10.1016/j.ijlp.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl) 2011;214(1):367–375. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG, Dahl RE, Spear LP. Adolescent brain development: Vulnerabilities and opportunities. New York: Academy of Sciences; 2004. Regulation of adolescent sleep: Implications for behavior; pp. 276–291. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80(3):265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Manly JT. Operationalizing child maltreatment: developmental processes and outcomes. Dev Psychopathol. 2001;13(4):755–757. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol. 2001;13(3):677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: a multilevel perspective. Dev Psychopathol. 2007;19(3):787–809. doi: 10.1017/S0954579407000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cima M, Smeets T, Jelicic M. Self-reported trauma, cortisol levels, and aggression in psychopathic and non-psychopathic prison inmates. Biol Psychol. 2008;78(1):75–86. doi: 10.1016/j.biopsycho.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev. 2010;35(1):97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, Smith AP. Negative life events, perceived stress, negative affect, and susceptibility to the common cold. J Pers Soc Psychol. 1993;64(1):131–140. doi: 10.1037//0022-3514.64.1.131. [DOI] [PubMed] [Google Scholar]
- Corrado RR, Vincent GM, Hart SD, Cohen IM. Predictive validity of the Psychopathy Checklist: Youth Version for general and violent recidivism. Behav Sci Law. 2004;22(1):5–22. doi: 10.1002/bsl.574. [DOI] [PubMed] [Google Scholar]
- Curwen T. The importance of offense characteristics, victimization history, hostility, and social desirability in assessing empathy of male adolescent sex offenders. Sex Abuse. 2003;15(4):347–364. doi: 10.1177/107906320301500410. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Jambrak J, Pasalich D, Hawes DJ, Brennan J. Impaired attention to the eyes of attachment figures and the developmental origins of psychopathy. J Child Psychol Psychiatry. 2011;52(3):238–245. doi: 10.1111/j.1469-7610.2010.02323.x. [DOI] [PubMed] [Google Scholar]
- Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids--food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145(6):2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- Daversa MT. Early environmental predictors of the affective and interpersonal constructs of psychopathy. Int J Offender Ther Comp Criminol. 2010;54(1):6–21. doi: 10.1177/0306624X08328754. [DOI] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44:113–126. [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Tucker DM. Neural mechanisms of emotion. J Consult Clin Psychol. 1992;60(3):329–338. doi: 10.1037//0022-006x.60.3.329. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health. 2007;41(1):3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Empathy-related responding and prosocial behaviour. Novartis Found Symp. 2007;278:71–80. discussion 80-96, 216-221. [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M, Shirtcliff E. Beyond allostatic load: The stress response system as a mechanism of conditional adaptation. In: Beauchaine TP, Hinshaw SP, editors. Child and Adolescent Psychopathology. 2nd ed. New York: Wiley & Sons; 2012. [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Dev Psychopathol. 2005;17(2):303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Essau CA, Sasagawa S, Frick PJ. Callous-unemotional traits in a community sample of adolescents. Assessment. 2006;13(4):454–469. doi: 10.1177/1073191106287354. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, Armstrong JM. Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Dev Psychopathol. 2011;23(4):1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington DP, Loeber R, Jolliffe D. The age-crime curve in reported offending. In: Loeber R, Farrington DP, Stouthamer-Loeber M, White HR, editors. Violence and serious theft: Development and prediction from childhood to adulthood. New York, NY: Routledge/Taylor & Francis Group; 2008. pp. 77–104. [Google Scholar]
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: impact on children’s behavior, neuroendocrine activity, and foster parent functioning. J Am Acad Child Adolesc Psychiatry. 2000;39(11):1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Van Ryzin MJ, Gunnar MR. Mitigating HPA axis dysregulation associated with placement changes in foster care. Psychoneuroendocrinology. 2011;36(4):531–539. doi: 10.1016/j.psyneuen.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forth AE, Kosson DS, Hare RD. Hare Psychopathy Checklist: Youth Version Technical Manual. Toronto: Multi-health Systems. 2003 [Google Scholar]
- Frick PJ, Cornell AH, Bodin SD, Dane HE, Barry CT, Loney BR. Callous-unemotional traits and developmental pathways to severe conduct problems. Dev Psychol. 2003;39(2):246–260. doi: 10.1037//0012-1649.39.2.246. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Ellis M. Callous-unemotional traits and subtypes of conduct disorder. Clin Child Fam Psychol Rev. 1999;2(3):149–168. doi: 10.1023/a:1021803005547. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Lilienfeld SO, Ellis M, Loney B, Silverthorn P. The association between anxiety and psychopathy dimensions in children. J Abnorm Child Psychol. 1999;27(5):383–392. doi: 10.1023/a:1021928018403. [DOI] [PubMed] [Google Scholar]
- Frick PJ, White SF. Research review: the importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. J Child Psychol Psychiatry. 2008;49(4):359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gini G, Albiero P, Benelli B, Altoe G. Does empathy predict adolescents’ bullying and defending behavior? Aggress Behav. 2007;33(5):467–476. doi: 10.1002/ab.20204. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Jenkins JM, Steiner M, Fleming AS. The relation between early life adversity, cortisol awakening response and diurnal salivary cortisol levels in postpartum women. Psychoneuroendocrinology. 2009;34(1):76–86. doi: 10.1016/j.psyneuen.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34(7):953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD. Hare psychopathy checklist-revised (PCL-R): Second edition, technical manual. Toronto, Canada: Multi-Health Systems; 2003. [Google Scholar]
- Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36(2):173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Hawes DJ, Brennan J, Dadds MR. Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Curr Opin Psychiatry. 2009;22(4):357–362. doi: 10.1097/YCO.0b013e32832bfa6d. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hanker JP, Hellhammer DH. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosom Med. 1998;60(3):309–318. doi: 10.1097/00006842-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Hox J. Multilevel analysis techniques and applications. Lawrence Erlbaum Associates, Publishers; 2002. [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, Polo-Tomas M, Price TS, Taylor A. The limits of child effects: evidence for genetically mediated child effects on corporal punishment but not on physical maltreatment. Dev Psychol. 2004;40(6):1047–1058. doi: 10.1037/0012-1649.40.6.1047. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, Taylor A. Physical maltreatment victim to antisocial child: evidence of an environmentally mediated process. J Abnorm Psychol. 2004;113(1):44–55. doi: 10.1037/0021-843X.113.1.44. [DOI] [PubMed] [Google Scholar]
- Johansson P, Andershed H, Kerr M, Levander S. On the operationalization of psychopathy: further support for a three-faceted personality oriented model. Acta Psychiatr Scand Suppl. 2002;(412):81–85. doi: 10.1034/j.1600-0447.106.s412.18.x. [DOI] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry. 2009;166(1):95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, Ryan ND. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biol Psychiatry. 1997;42(8):669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res. 2006;142(2-3):107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry. 2001;50(9):677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Cauffman E, Goldweber A, Skeem J. Primary and secondary variants of juvenile psychopathy differ in emotional processing. Dev Psychopathol. 2012;24(3):1091–1103. doi: 10.1017/S0954579412000557. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Munoz LC, Aucoin KJ. Can a laboratory measure of emotional processing enhance the statistical prediction of aggression and delinquency in detained adolescents with callous-unemotional traits? J Abnorm Child Psychol. 2007;35(5):773–785. doi: 10.1007/s10802-007-9136-1. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Skeem JL, Marsee MA, Cruise K, Munoz LC, Morris AS. Assessing callous-unemotional traits in adolescent offenders: validation of the Inventory of Callous-Unemotional Traits. Int J Law Psychiatry. 2008;31(3):241–252. doi: 10.1016/j.ijlp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Kotler JC, McMahon RJ. Assessment of child and adolescent psychopathy. In: Salekin RT, lynam DR, editors. Handbook of child and adolescent psychopathy. New York, NY: The Guilford Press; 2010. pp. 79–112. [Google Scholar]
- Leve LD, Chamberlain P. Female juvenile offenders: Defining an early-onset pathway for delinquency. Journal of Child and Family Studies. 2004;13(4):439–452. [Google Scholar]
- Lilienfeld SO, Patrick CJ, Benning SD, Berg J, Sellbom M, Edens JF. The role of fearless dominance in psychopathy: confusions, controversies, and clarifications. Personal Disord. 2012;3(3):327–340. doi: 10.1037/a0026987. [DOI] [PubMed] [Google Scholar]
- Lindberg N, Tani P, Sailas E, Virkkala J, Urrila AS, Virkkunen M. Sleep in conduct-disordered adolescents--a polysomnographic and spectral power analysis study. Psychiatry Res. 2008;159(3):339–345. doi: 10.1016/j.psychres.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Loney BR, Butler MA, Lima EN, Counts CA, Eckel LA. The relation between salivary cortisol, callous-unemotional traits, and conduct problems in an adolescent non-referred sample. J Child Psychol Psychiatry. 2006;47(1):30–36. doi: 10.1111/j.1469-7610.2005.01444.x. [DOI] [PubMed] [Google Scholar]
- Luntz BK, Widom CS. Antisocial personality disorder in abused and neglected children grown up. Am J Psychiatry. 1994;151(5):670–674. doi: 10.1176/ajp.151.5.670. [DOI] [PubMed] [Google Scholar]
- Luntz Weiler B, Spatz Widom C. Psychopathy and violent behaviour in abused and neglected young adults. Criminal Behaviour and Mental Health. 1996;6(3):253–271. [Google Scholar]
- Lykken DT. The antisocial personalities. Hilldale, NJ: Lawrence Erlbaum Associates; 1995. [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7(1):19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biol Psychiatry. 2009;66(1):62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Blair RJ. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165(6):712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Marshall LA, Cooke DJ. The childhood experiences of psychopaths: a retrospective study of familial and societal factors. J Pers Disord. 1999;13(3):211–225. doi: 10.1521/pedi.1999.13.3.211. [DOI] [PubMed] [Google Scholar]
- Maughan B, Rowe R, Messer J, Goodman R, Meltzer H. Conduct disorder and oppositional defiant disorder in a national sample: developmental epidemiology. J Child Psychol Psychiatry. 2004;45(3):609–621. doi: 10.1111/j.1469-7610.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Life-course-persistent versus adolescent-limited antisocial behavior. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Risk, disorder and adaptation. 2 ed. Vol. 3. Hoboken, NJ: John Wiley & Sons Inc; 2006. pp. 570–598. [Google Scholar]
- Moffitt TE, Caspi A. Childhood predictors differentiate life-course persistent and adolescence-limited antisocial pathways among males and females. Dev Psychopathol. 2001;13(2):355–375. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Neumann CS, Kosson DS, Forth AE, Hare RD. Factor structure of the Hare Psychopathy Checklist: Youth Version (PCL: YV) in incarcerated adolescents. Psychol Assess. 2006;18(2):142–154. doi: 10.1037/1040-3590.18.2.142. [DOI] [PubMed] [Google Scholar]
- Nicolson NA, Davis MC, Kruszewski D, Zautra AJ. Childhood maltreatment and diurnal cortisol patterns in women with chronic pain. Psychosom Med. 2010;72(5):471–480. doi: 10.1097/PSY.0b013e3181d9a104. [DOI] [PubMed] [Google Scholar]
- O’Leary MM, Loney BR, Eckel LA. Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology. 2007;32(2):183–191. doi: 10.1016/j.psyneuen.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, Arseneault L. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol Psychiatry. 2011;70(11):1016–1023. doi: 10.1016/j.biopsych.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters F, Nicholson NA, Berkhof J. Cortisol responses to daily events in major depressive disorder. Psychosom Med. 2003;65(5):836–841. doi: 10.1097/01.psy.0000088594.17747.2e. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology. 2002;30(4):311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol Psychiatry. 2008;64(6):521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose A, Bijttebier P, Decoene S, Claes L, Frick PJ. Assessing the affective features of psychopathy in adolescence: a further validation of the inventory of callous and unemotional traits. Assessment. 2010;17(1):44–57. doi: 10.1177/1073191109344153. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Dev. 1999;70(3):660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Fisher DB, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: longitudinal and concurrent associations with cortisol. Horm Behav. 2011;59(1):123–132. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber JE, Shirtcliff E, Van Hulle C, Lemery-Chalfant K, Klein MH, Kalin NH, Goldsmith HH. Environmental influences on family similarity in afternoon cortisol levels: twin and parent-offspring designs. Psychoneuroendocrinology. 2006;31(9):1131–1137. doi: 10.1016/j.psyneuen.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Dev. 1998;69(6):1503–1513. [PubMed] [Google Scholar]
- Selye H. Stress: The physiology and pathology of exposure to stress. Montreal: Acta Medical Publishers; 1950. [Google Scholar]
- Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: preliminary results. Psychoneuroendocrinology. 2007;32(8-10):1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA. Riding the physiological roller-coaster: How life stress alters stress responsive physiological systems and what it means for adolescent health. Paper presented at the Social Development Research Group and School of Social Work; Seattle Washington: University of Washington; 2011. [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev Psychobiol. 2011 doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev Psychobiol. 2008;50(7):691–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Dev Psychopathol. 2005;17(1):167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Hanson JL, Rudolph KD, Strang N, Pollak SD. Cortisol’s Diurnal Rhythm Indexes the Physiological Impact of Child Maltreatment in Adolescents. under review [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C. Neurobiology of empathy and callousness: implications for the development of antisocial behavior. Behav Sci Law. 2009;27(2):137–171. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. Overview: toward a dysregulation hypothesis of depression. Am J Psychiatry. 1985;142(9):1017–1031. doi: 10.1176/ajp.142.9.1017. [DOI] [PubMed] [Google Scholar]
- Silverthorn P, Frick PJ, Reynolds R. Timing of onset and correlates of severe conduct problems in adjudicated girls and boys. Journal of psychopathology and behavioral assessment. 2001;23(3):171–181. [Google Scholar]
- Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev. 2006;30(6):855–863. doi: 10.1016/j.neubiorev.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Skeem J, Johansson P, Andershed H, Kerr M, Louden JE. Two subtypes of psychopathic violent offenders that parallel primary and secondary variants. J Abnorm Psychol. 2007;116(2):395–409. doi: 10.1037/0021-843X.116.2.395. [DOI] [PubMed] [Google Scholar]
- Skeem JL, Poythress N, Edens JF, Lilienfeld SO, Cale EM. Psychopathic personality or personalities? Exploring potential variants of psychopathy and their implications for risk assessment. Aggression and Violent Behavior. 2003;8:513–546. [Google Scholar]
- Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Dev Psychopathol. 2011;23(4):1167–1186. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. Thousand Oaks: Sage Publications; 1999. [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. 2005;57(1):7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Stevens D, Charman T, Blair RJ. Recognition of emotion in facial expressions and vocal tones in children with psychopathic tendencies. J Genet Psychol. 2001;162(2):201–211. doi: 10.1080/00221320109597961. [DOI] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Finkelhor D, Moore DW, Runyan D. Identification of Child Maltreatment With the Parent-Child Conflict Tactics Scales: Development and Psychometric Data for a National Sample of American Parents. Child Abuse and Neglect. 1998;22(4):249–270. doi: 10.1016/s0145-2134(97)00174-9. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neurosci Biobehav Rev. 2006;30(3):376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50(4):632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Thorn L, Hucklebridge F, Evans P, Clow A. The cortisol awakening response, seasonality, stress and arousal: a study of trait and state influences. Psychoneuroendocrinology. 2009;34(3):299–306. doi: 10.1016/j.psyneuen.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Tracy PE, Kempf-Leonard K, Abramoske-James S. Gender differences in delinquency and juvenile justice processing: Evidence from national data. Crime & Delinquency. 2009;55(2):171–215. [Google Scholar]
- van Goozen SH, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychol Bull. 2007;133(1):149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, Hermans EJ, Putman P. Low cortisol levels and the balance between punishment sensitivity and reward dependency. Neuroreport. 2003;14(15):1993–1996. doi: 10.1097/00001756-200310270-00023. [DOI] [PubMed] [Google Scholar]
- Van Hulle CA, Shirtcliff EA, Lemery-Chalfant K, Goldsmith HH. Genetic and environmental influences on individual differences in cortisol level and circadian rhythm in middle childhood. Horm Behav. 2012;62(1):36–42. doi: 10.1016/j.yhbeh.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E. Annotation: understanding the development of psychopathy. J Child Psychol Psychiatry. 2004;45(8):1329–1337. doi: 10.1111/j.1469-7610.2004.00840.x. [DOI] [PubMed] [Google Scholar]
- Vincent GM, Vitacco MJ, Grisso T, Corrado RR. Subtypes of adolescent offenders: affective traits and antisocial behavior patterns. Behav Sci Law. 2003;21(6):695–712. doi: 10.1002/bsl.556. [DOI] [PubMed] [Google Scholar]
- Vitacco MJ, Vincent GM. Understanding the downward extension of psychopathy to youth: implications for risk assessment and juvenile justice. International journal of forensic mental health. 2006;5:29–38. [Google Scholar]
- Vitale JE, Newman JP, Bates JE, Goodnight J, Dodge KA, Pettit GS. Deficient behavioral inhibition and anomalous selective attention in a community sample of adolescents with psychopathic traits and low-anxiety traits. J Abnorm Child Psychol. 2005;33(4):461–470. doi: 10.1007/s10802-005-5727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Polier GG, Herpertz-Dahlmann B, Konrad K, Wiesler K, Rieke J, Heinzel-Gutenbrunner M, Vloet TD. Reduced cortisol in boys with early-onset conduct disorder and callous-unemotional traits. Biomed Res Int. 2013;2013:349530. doi: 10.1155/2013/349530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems CF, Carrion VG. The association between PTSD symptoms and salivary cortisol in youth: the role of time since the trauma. J Trauma Stress. 2007;20(5):903–907. doi: 10.1002/jts.20251. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25(7):707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]