Abstract
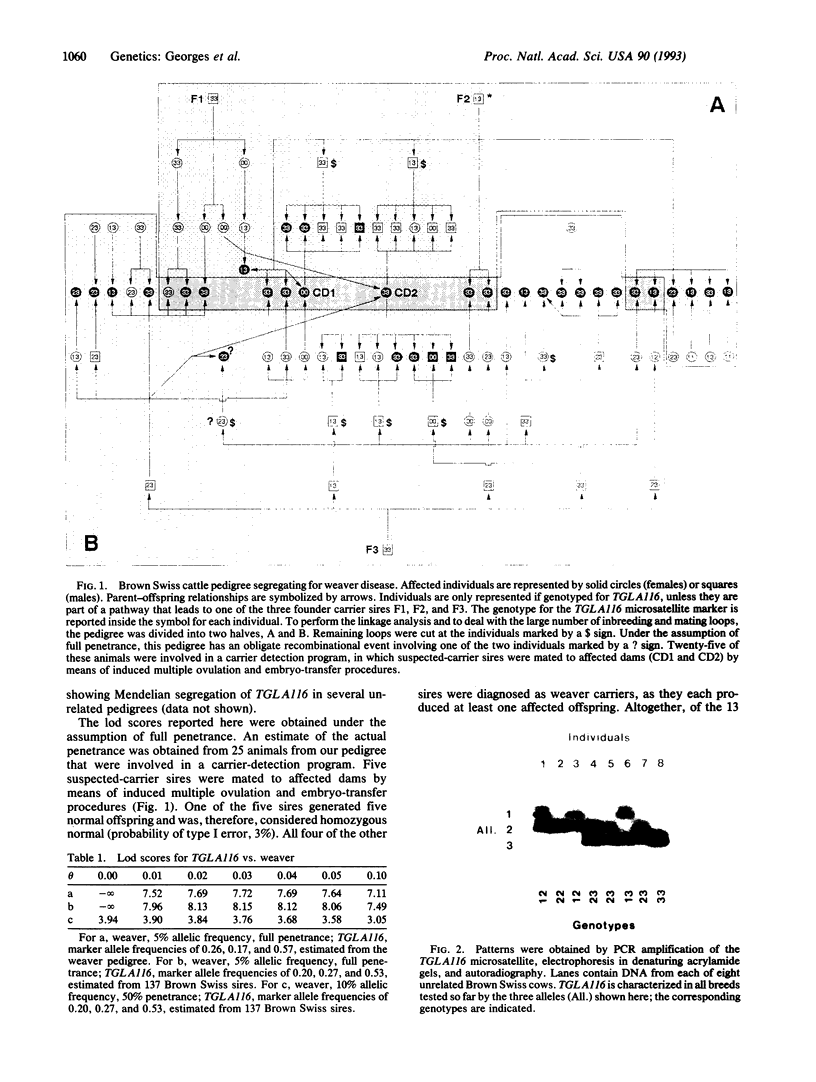
A genetic disease in cattle, progressive degenerative myeloencephalopathy (weaver disease), is associated with increased milk production. This association could result from population stratification, from a pleiotropic effect of a single gene, or from linkage disequilibrium between the gene causing weaver disease and a quantitative trait locus (QTL) for milk production. To test these hypotheses, we performed an extensive linkage study in a bovine pedigree segregating for the weaver condition and identified a microsatellite locus (TGLA116) closely linked to the weaver gene (zmax, 8.15; theta, 0.03). TGLA116 and, by extension, the weaver locus were assigned to bovine synteny group 13. This microsatellite can be used to identify weaver carriers, to select against this genetic defect, and to study the effect of the corresponding chromosomal region on milk production in Brown Swiss and other breeds of cattle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheim N., Strange C., Erlich H. Use of pooled DNA samples to detect linkage disequilibrium of polymorphic restriction fragments and human disease: studies of the HLA class II loci. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6970–6974. doi: 10.1073/pnas.82.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalet C., Corpet F. Statistical decision rules concerning synteny or independence between markers. Cytogenet Cell Genet. 1986;43(3-4):132–139. doi: 10.1159/000132311. [DOI] [PubMed] [Google Scholar]
- Dietz A. B., Neibergs H. L., Womack J. E. Assignment of eight loci to bovine syntenic groups by use of PCR: extension of a comparative gene map. Mamm Genome. 1992;3(2):106–111. doi: 10.1007/BF00431254. [DOI] [PubMed] [Google Scholar]
- Georges M., Gunawardana A., Threadgill D. W., Lathrop M., Olsaker I., Mishra A., Sargeant L. L., Schoeberlein A., Steele M. R., Terry C. Characterization of a set of variable number of tandem repeat markers conserved in bovidae. Genomics. 1991 Sep;11(1):24–32. doi: 10.1016/0888-7543(91)90098-y. [DOI] [PubMed] [Google Scholar]
- Hoeschele I., Meinert T. R. Association of genetic defects with yield and type traits: the weaver locus effect on yield. J Dairy Sci. 1990 Sep;73(9):2503–2515. doi: 10.3168/jds.S0022-0302(90)78936-9. [DOI] [PubMed] [Google Scholar]
- Lander E. S., Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989 Jan;121(1):185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K., Sobel E. A random walk method for computing genetic location scores. Am J Hum Genet. 1991 Dec;49(6):1320–1334. [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet. 1984 Mar;36(2):460–465. [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipold H. W., Blaugh B., Huston K., Edgerly C. G., Hibbs C. M. Weaver syndrome in Brown Swiss cattle: clinical signs & pathology. Vet Med Small Anim Clin. 1973 Jun;68(6):645–647. [PubMed] [Google Scholar]
- Li L., Teale A., Bensaid A., Dunlap S., Dietz A. B., Womack J. E. Somatic cell mapping of T-cell receptor CD3 complex and CD8 genes in cattle. Immunogenetics. 1992;36(4):224–229. doi: 10.1007/BF00215052. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Phillips M. S. Malignant hyperthermia. Science. 1992 May 8;256(5058):789–794. doi: 10.1126/science.1589759. [DOI] [PubMed] [Google Scholar]
- Rudolph J. A., Spier S. J., Byrns G., Rojas C. V., Bernoco D., Hoffman E. P. Periodic paralysis in quarter horses: a sodium channel mutation disseminated by selective breeding. Nat Genet. 1992 Oct;2(2):144–147. doi: 10.1038/ng1092-144. [DOI] [PubMed] [Google Scholar]
- Steele M. R., Georges M. Generation of bovine multisite haplotypes using random cosmid clones. Genomics. 1991 Aug;10(4):889–904. doi: 10.1016/0888-7543(91)90177-g. [DOI] [PubMed] [Google Scholar]
- Stuart L. D., Leipold H. W. Lesions in bovine progressive degenerative myeloencephalopathy ("Weaver") of Brown Swiss cattle. Vet Pathol. 1985 Jan;22(1):13–23. doi: 10.1177/030098588502200103. [DOI] [PubMed] [Google Scholar]
- Thomas D. C. Fitting genetic data using Gibbs sampling: an application to nevus counts in 38 Utah kindreds. Cytogenet Cell Genet. 1992;59(2-3):228–230. doi: 10.1159/000133255. [DOI] [PubMed] [Google Scholar]
- Womack J. E., Moll Y. D. Gene map of the cow: conservation of linkage with mouse and man. J Hered. 1986 Jan-Feb;77(1):2–7. doi: 10.1093/oxfordjournals.jhered.a110160. [DOI] [PubMed] [Google Scholar]




