Abstract
Despite advances in chemotherapy, radiotherapy and targeted drug development, cancer remains a disease of high morbidity and mortality. The treatment of human cancer patients with chemotherapy has become commonplace and accepted over the past 100 years. In recent years, and with a similar incidence of cancer to people, the use of cancer chemotherapy drugs in veterinary patients such as the dog has also become accepted clinical practice. The poor predictability of tumour responses to cancer chemotherapy drugs in rodent models means that the standard drug development pathway is costly, both in terms of money and time, leading to many drugs failing in Phase I and II clinical trials. This has led to the suggestion that naturally occurring cancers in pet dogs may offer an alternative model system to inform rational drug development in human oncology. In this review, we will explore the species variation in tumour responses to conventional chemotherapy and highlight our understanding of the differences in pharmacodynamics, pharmacokinetics and pharmacogenomics between humans and dogs. Finally, we explore the potential hurdles that need to be overcome to gain the greatest value from comparative oncology studies.
Keywords: chemotherapy, human, canine, dog, comparative oncology
1. Introduction
The German chemist, Paul Ehrlich, who devised the term ‘chemotherapy’, was also one of the first people to document the utility of animal models in screening chemicals for their potential anti-cancer activity. Chemotherapy is now widely known as the use of chemicals to treat disease and it has been widely employed to treat cancer since the beginning of the twentieth century. Despite pessimism about the utility of chemotherapy to cure cancer, the success of combination chemotherapy in curing paediatric acute leukaemia and diffuse large B-cell lymphoma in the 1960s spurred the formation of medical oncology as a specialty. Since the 1970s, extensive development of anti-cancer drug screening programmes occurred with animal models supporting early assessment of therapeutic and toxicity potential. These animal models are often the final investigation in the development of drug candidates for human clinical trials following promising in vitro activity.
Animal models are diverse and often distinguished on the basis of the origin of the tumour—namely, spontaneous or inducible tumour development versus tumours that are transplanted. While human tumour xenografts have been the most extensively used model to predict anti-tumour efficacy, several studies have indicated variable correlation between xenograft models and clinical activity [1–10]. Mouse models tend to suffer from a number of limitations that impact their predictive behaviour for human tumour types; while not an exhaustive list, some primary reasons for failure of xenograft models include biological differences between species (for example, telomerase is active in almost all murine cells contrary to in human cells), altered downstream signalling in the mouse compared with known human cancer signalling pathways such as Ras, altered metabolism of and sensitivity to DNA-damaging agents, variations in immune competency and altered tumour microenvironment [1,11–14]. The progression of mouse models from syngeneic to sophisticated genetically engineered mouse (GEM) models and recently to non-germline GEM models has vastly improved current understanding of cancer biology but has modestly improved throughput, expense and the practicality of preclinical drug testing [1,13–15]. This latter aspect is predominantly due to the complexity of cancer, as it is difficult to design an animal model that preserves the same genotypic and phenotypic characteristics of the tumours from which they were derived [12,16,17].
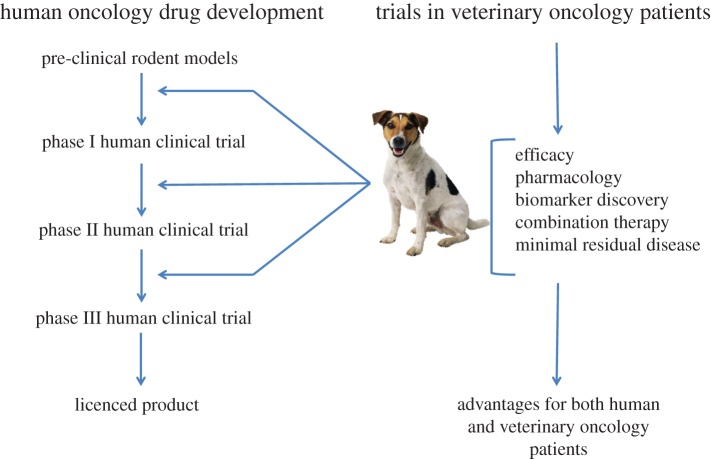
Preclinical studies of the anti-tumour activity of novel or modified existing chemotherapy agents requires the use of a model system capable of answering specific questions regarding the drug's efficacy. A failure to consider an appropriate animal model may contribute to the failure of a compound to achieve approval for use or hinder further investigation of new drugs. The integration of comparative oncology approaches using pet dogs with spontaneously occurring tumours as models in drug development pathways has garnered much attention in recent years due to numerous clinical and molecular similarities between common canine and human cancers [18–21] (figure 1). Whilst implementing a combined use model, wherein information from mouse and large animal models both provide input into rational development of chemotherapy drugs, it is important to address interspecies similarities and differences in drug metabolism, physiology, absorption and distribution. Given that in some clinical scenarios, even human beings are not predictive models of cancer in distinct human groups, consideration must also be given to emerging inter- and intra-species pharmacogenetic differences in order to maximize the human predictive potential of data obtained from various screening systems [11,22].
Figure 1.
A unique collaboration between human oncology drug development and trials in veterinary oncology patients has the potential to increase the speed with which new human drugs reach the clinic. (Online version in colour.)
Tumour responsiveness to various chemotherapy drugs is dependent on a multitude of factors that go far beyond simple dosage and frequency of chemotherapy administration. Responsiveness is also dependent on tumour histology, growth rate, tumour heterogeneity and mechanisms of drug resistance. Tumour sensitivity is therefore a dynamic and complicated issue, particularly when acquired resistance is taken into account. Additional factors such as immunologic responses to tumour antigens, serum protein content and natural barriers (e.g. the blood-brain barrier) also play integral roles in chemotherapy distribution to tumour and normal tissues. This article is not intended to provide an overview of all mechanisms of tumour response, but rather aims to summarize some of the challenges and opportunities that present when considering animal models in predicting tumour response to chemotherapy. A general overview of chemotherapy indications and evidence for clinical use are presented using comparative human and canine histologies; the question as to whether or not there are significant differences in tumour response between species is also posed. Subsequently, species differences in pharmacokinetic, pharmacodynamics, pharmacogenomics and immunologic factors are also discussed. This article focuses on increasing interest in the dog as a model for comparative oncology approaches but also references common laboratory species that continue to serve as the mainstay for chemotherapy research. The purpose of the review is to stimulate discussion on the underlying question as to whether an appreciation of interspecies differences can alert researchers and clinicians to deviations in tumour response to chemotherapy between species.
2. What is the rationale for chemotherapy?
The overall objective of chemotherapy is to reduce the population of tumour cells to zero in order to effect a cure. Following studies of murine leukaemia, the fractional cell kill hypothesis was generally accepted as a method by which to approximate tumour response; specifically, the hypothesis stated that a given drug concentration applied for a defined time period will kill a constant fraction of the cell population, regardless of the initial, absolute number of tumour cells [23]. This provided the rationale for many chemotherapy protocols, particularly leukaemia and lymphoma protocols, as the outcome of chemotherapy is dependent on the drug dosage and the number and frequency of drug administrations. Indeed, both human and canine non-Hodgkin's lymphoma (NHL) are treated primarily with multidrug chemotherapy with remission rates approaching 90% in both species [24,25]. When applied to solid tumours, multiple confounding factors wreak havoc with the fractional cell kill hypothesis, such as decelerating growth with a small fraction of cycling cells, intrinsic resistance (as postulated with tumour stem cells), impaired tumour vascularity and variations within the tumour heterogeneity and the tumour microenvironment. Selection pressure following chemotherapy treatment provides an entirely unique tumour population to work with, also invalidating the fractional cell kill model [26–28]. While acknowledging that chemotherapy alone rarely induces a durable response for solid tumours, adjuvant systemic treatment has made an impact on several solid tumour histologies in humans [29,30]. Post-operative adjuvant therapy, when indicated, generally significantly reduces the risk of recurrence compared with surgery alone although the degree of benefit varies from conservative to remarkable. The benefit of adjuvant chemotherapy has been recognized in many tumour histologies in veterinary patients as well, even though the principles of chemotherapy are altered with one of the primary goals centring on the maintenance of good-to-excellent quality-of-life measures [31–34].
3. Comparative aspects of chemotherapy indications for selected tumours
(a). Non-Hodgkin's lymphoma
The principles of treatment for NHL in humans are complex and must take into account patient factors, subtype of lymphoma, stage and biologic behaviour of the disease [35]. However, multidrug chemotherapy protocols, and often CHOP-based (Cyclophosphamide, Hydroxydaunorubicin, Oncovin, Prednisone) protocols, form the mainstay of treatment for high-grade, multifocal follicular lymphoma and diffuse large B-cell lymphoma in humans, the two most common lymphomas to affect adults in the United States [35]. While the inclusion of rituximab (R) to chemotherapy combinations has improved response rates and outcome, response rates to R-CHOP are approximately 85–90% and 70–80%, respectively, with median progression-free survival of approximately 5–6 years and 3–4 years, respectively [35–38]. While histological classification is not routinely sought following diagnosis of canine NHL, the majority are diffuse large-cell lymphoma and are subsequently treated with CHOP-based chemotherapy [24,39–41]. Remission rates vary depending on several factors but average 80–95% with CHOP-based therapy, historically providing median survival times of approximately 11–12 months with 25% of dogs alive at 2 years [24]. As many pet owners choose to proceed with rescue chemotherapy following first relapse, median survival times for dogs tend to be greater than 1 year [42].
(b). Sarcomas
Sarcomas are biologically complex mesenchymal tumours requiring multi-disciplinary management due to the variation in location and behaviour. In osteosarcoma (OSA) of the extremity, the most common primary malignant bone cancer in people primarily affecting young children or adolescents, treatment prior to 1970 centred on amputation, with most patients experiencing a 5-year survival rate of only 20–30% due to distant metastatic disease [25,43,44]. While surgical and radiation therapy approaches have been consistently instrumental in controlling localized disease, the therapeutic benefit of chemotherapy at delaying or reducing metastasis was first observed over 40 years ago [30,45]. Subsequent randomized controlled clinical trials provided the evidence needed to justify incorporating chemotherapy into standard therapeutic protocols [25,44,46,47]. Since the addition of cisplatin-based adjuvant therapy in the 1980s, adjuvant or neoadjuvant chemotherapy protocols have provided 5-year survival rates of 60–70% [25,46,47]. While the survival benefit of chemotherapy has been well established in human OSA, the use of adjuvant chemotherapy for soft tissue sarcomas (STS), a vastly heterogeneous group of tumours, has taken a more circuitous path. Several factors are predictive of outcome in human STS, including tissue of origin, histological grade, tumour size, anatomic site, degree of invasion or depth into underlying tissues and patient performance score [29,48–51]. While surgery with or without radiation therapy is the mainstay of treatment for loco-regional control of STS, many patients—approximately 50% of patients with high-grade STS—will develop and succumb to recurrent and/or metastatic disease. Despite the recognition of many prognostic factors predictive of disease recrudescence, adjuvant chemotherapy has failed to unequivocally provide clinical benefit in patients with high-risk disease. The standard first-line treatment tends to be single agent doxorubicin, although some literature suggests there is added overall clinical benefit in some patients to the addition of ifosfamide despite the associated increase in toxicity [52,53]. In a landmark meta-analysis of individual patient data, data analysis from 1568 patients from 14 clinical studies with a median follow-up of 9.4 years demonstrated evidence that adjuvant chemotherapy significantly improved local and distant recurrence-free intervals; however, there was no significant benefit in overall survival [54]. Subsequent meta-analyses confirmed the suggestion that doxorubicin-based chemotherapy improved recurrence-free survival at 10 years with a non-significant trend towards improved survival [52,55]. However, most current guidelines suggest that patient selection is paramount in order to justify the chemotherapy-associated toxicities associated with treatment [29,52,53].
Veterinary studies, despite being less numerous and under-powered in comparison to human studies, have predominantly paralleled the findings in support of adjuvant therapy for sarcomas. Because of salient clinical and molecular similarities between OSA in humans and dogs, canine appendicular OSA has been considered a valid model for human OSA [18,56–59]. Similar to humans, amputation alone yields a poor prognosis with most dogs euthanized within four to five months due to metastatic disease [60–62]. The addition of adjuvant chemotherapy to amputation or limb-sparing surgery has demonstrated clear benefit in the disease-free survival and overall survival, similar to human OSA [60–65]. Various protocols have been assessed with platinum agents or doxorubicin forming the basis for most protocols; to date, there has been no clear benefit with the use of combination chemotherapy but rather single agent carboplatin or doxorubicin are most often used due to ease of administration, acceptable toxicity profile and comparable outcomes [60–66]. Unfortunately, while there is clear short-term benefit to adjuvant chemotherapy, long-term survival for canine OSA remains poor with 1-year survival estimated at approximately 35–45% [66]. Paralleling the case in human OSA, few improvements in outcome have been made in the last 20 years with local control with peri-operative chemotherapy remaining the standard approach for optimal outcome. While there has been a clear benefit to the use of chemotherapy in canine OSA, the utility of chemotherapy following local control for canine high-grade STS is unclear. Similar to human studies, there is no evidence to support a significant role for chemotherapy in the management of low-risk disease and surgery with or without radiation therapy is considered the standard-of-care. Doxorubicin alone and doxorubicin-based protocols have shown the most promise with advanced measurable STS and therefore are most often elected for dogs at risk for metastasis [67,68]. However, in a report of 39 dogs with high-grade STS, there was no improvement in either disease-free interval or overall survival in dogs treated with surgery and doxorubicin compared with surgery alone [69]. This report was small and included uncommon (visceral) STS in the analysis thus results may have represented type II error; nonetheless, use of chemotherapy for high-grade STS remains controversial.
4. Is there a variation in response to chemotherapy in dogs versus humans?
Given the similar trends in response and indications for chemotherapy in some veterinary patients, as illustrated above, some (often pet owners) query the seeming lack of equivalent response to chemotherapy in their pet dogs. For example, why do dogs with lymphoma benefit from 11 to 12 months of survival with chemotherapy, whereas humans often achieve 3–4 years? The domestic dog is an interesting study of age-specific mortality evolution, possibly associated with selection for body size. Body size in dogs varies by almost two orders of magnitude and a longevity factor of two; this implies that, on average, small breed dogs die at approximately 10–15 years, while large breed dogs die at approximately 5–8 years [70,71]. While paradoxical to the common notion that there is a positive relationship between lifespan and body size, as is obvious when considering survival of a rat (5 years) compared with a whale (often more than 100 years), there is an inverse relationship in occasional species such as humans and dogs [72–74]. The commonly touted ‘7-year rule’ that defines 1 ‘human year’ as equivalent to 7 ‘dog years’ is mythical, with research suggesting that dogs indeed age faster than humans and that after 2 years of age for a dog (equivalent to approx. 24 human years), each year of a dog's life is equivalent to approximately 4–5 human years [75]. Extrapolating from this, comparable remission durations could be deemed reasonable with a 10–11 month remission on CHOP comparable with approximately 4 years of remission in a human. Using similar extrapolation, a dog with OSA may only achieve one-quarter of the remission duration of a paediatric human patient with OSA, suggesting either differences in tumour response, drug sensitivity or biologic behaviour as most dogs die of metastatic disease rather than co-morbid factors associated with age. It must be acknowledged, however, that given the vast variation in breed size, it would seem impossible to develop a single factor to account for translating ‘dog years’ to ‘human years’ [71]. Given that lifespan in dogs is inversely related to body size, breed and intra-breed variability needs to be worked into an appropriate model. The biologic basis for the inverse relationship between size and lifespan is not understood although some investigators have suggested that the insulin-like growth factor 1 (IGF-1) signalling cascade plays a role, as smaller dogs have lower levels of IGF-1 compared with large breed dogs [76–78]. While small breed dogs with OSA are postulated to have a better prognosis than large breed dogs treated with local control and chemotherapy, this potential difference could be explained by the size-to-lifespan relationship. A recent report evaluating 26 small breed dogs with appendicular OSA treated with surgery and chemotherapy indicated the median survival time was 415 days, longer than reports including dogs of all sizes (predominantly large breed dogs) [60–66,79]. However, recognizing that small breed dogs take a longer time to ‘age’, 415 days in a small breed dog may be comparable to 330 days in a large breed dog [70,76]. The more accepted hypothesis, however, is that there is a relative difference in dosing of chemotherapy in small breed dogs, with large breed dogs receiving a lower dosing than small breed dogs. Alternatively, the biologic behaviour of OSA in small breed dogs is truly altered in comparison to large breed dogs, as suggested by lower mitotic indices and grade [79].
With respect to comparative aspects of STS, there is the suggestion of a small yet consistent improved recurrence-free interval in humans with the use of adjuvant doxorubicin-based chemotherapy following local control; this has not been realized in dogs, although only one small study has addressed the issue. The disease-free survival for human STS increased from 45 to 55% at 10 years with the use of adjuvant chemotherapy but there was an insignificant improvement in overall survival; the study was not powered to detect a small change (less than 4-year improvement) in survival [54]. Given the relationship of dog aging to human aging and presuming equivalent STS response to doxorubicin, it is possible that doxorubicin only induced an undetectable short (months) improvement in disease-free interval [69].
5. What impact do dose and dose intensity have on tumour response in dogs versus humans?
Chemotherapy drugs are considered some of the most dangerous within the medical arsenal due to their narrow therapeutic index and the desire to use them near their maximally tolerated dose. Most chemotherapy drugs are currently dosed in both companion animals and humans on the basis of the patient's body surface area (BSA), which tends to correlate poorly with drug pharmacokinetics [80,81]. BSA is proportional to both blood volume and glomerular filtration rate (GFR), despite neither contributing to chemotherapy efficacy or toxicity as much as liver function or other metabolic variations [82–85]. Interestingly, BSA was initially derived as a mathematical approach to estimating tolerable starting doses in humans for phase I trials based on preclinical data in animals; BSA dosing essentially normalizes the maximum tolerated dose of many chemotherapy drugs in humans, dogs, rats and mice [83–86]. There has been no clear relationship between pharmacokinetic parameters and BSA for common chemotherapy drugs, and in people, up to 20-fold variation in pharmacokinetics is routinely observed in patients receiving BSA-calculated doses [82]. In dogs, there has been empirical evidence that smaller dogs experience increased toxicity compared with larger dogs when administered chemotherapy dosed based on BSA [87–90]. For non-metabolized drugs, the use of BSA may be effective, but when tumour effects and side effects are based on complex systems such as metabolism and genetics, there are too many size-independent factors that can affect a generalized BSA approach to dosing [83,91]. It is important to perform studies that relate drug exposure to tumour response in species commonly used in drug development, whether looking at animal models as predictive of efficacy or toxicity. Generally speaking, these data are lacking in companion animals for many drugs despite the recognition that there are many limitations to BSA dosing of chemotherapy in dogs [92,93]. One pivotal study in cats demonstrated a clear relationship between drug exposure and neutrophil nadir, clearance and GFR, permitting calculation of individual animal dosing [94,95]. A recent study in dogs attempted to develop a simple strategy for measuring doxorubicin exposure in dogs in order to improve the study of the correlation between pharmacokinetics and both toxicity and tumour response [96]. It is not yet clear if pharmacokinetic-based dosing improves outcome in companion animals, but several studies in humans have demonstrated beneficial effects both in terms of reducing toxicity and improving disease-free intervals for various chemotherapy drugs and protocols [97–102]. In a phase II study of metastatic colorectal cancer, both efficacy and tolerability of pharmacokinetic-adjusted fluorouracil as part of a multidrug protocol were higher than BSA dosing [103]. An earlier phase III study comparing pharmacokinetically adjusted fluorouracil to conventional dosing in metastatic colorectal cancer patients demonstrated that personalized dosing improved the response rate, decreased severe toxicity and led to a trend in improved survival [102]. Importantly, the mean fluorouracil dose was higher in the phase III trial with personalized dosing, which was also the group with decreased occurrence of severe toxicity [102]. As quality-of-life measures are important when considering any chemotherapy regimen, efforts to improve outcome while decreasing toxicity are paramount to advancing cancer care.
Despite efforts to investigate drug exposure in companion animals and the assumptions made to define the relationship between chemotherapy drug exposure and effect, conflicting results exist in the veterinary literature. In canine lymphoma, one study showed that dogs that developed grade III or IV neutropenia after chemotherapy demonstrated improved survival, leading to the suggestion that neutropenia was associated with more optimal drug exposure [104]. A separate study showed similar results: dogs requiring dose delays and dosage reductions during chemotherapy for lymphoma demonstrated improved outcomes compared with those without adjustments [105].
6. What role do interspecies differences in pharmacokinetics, pharmacodynamics and pharmacogenomics play in tumour response to chemotherapy?
The pharmacologic treatment of cancer, regardless of human or pet origin, is a challenging endeavour, as medical oncologists must choose and use drugs with relatively narrow efficacy profiles while being aware of serious toxicities and while monitoring tumour response. Clinical pharmacology is defined as the study of drugs, and the application of clinical pharmacology attempts to predict and explain variable drug actions and interactions. Chemosensitivity depends heavily on factors such as drug uptake into the cell, interaction within the cell and the cellular response to damage; exposure of tumour cells to chemotherapy effects is heavily dependent on pharmacologic effects. As the quantitative study of drug absorption, distribution, elimination and drug interactions, pharmacokinetics is often termed ‘what the body does to the drug’ and plays an integral role early in clinical study design [106]. Many methods of scaling have been developed to predict pharmacokinetic parameters from animals to humans, however little research has addressed scaling within different animal species. In humans, clearance is considered the most important pharmacokinetic parameter as it is directly linked to area under the curve [107]. Clearance of any drug from the body involves multiple organ systems and there are several allometric models that can be used to predict clearance in humans from animals (and vice versa). There are several excellent reviews highlighting numerous interspecies differences in drug pharmacokinetics, with an emphasis on drug development and the use of preclinical models [108–110]. Contrary to pharmacokinetics, pharmacodynamics, as the study of the drug dose and kinetics in relation to clinical effects, is often redefined simply as ‘what the drug does to the body’. Pharmacodynamic differences across species are often reported as differences in toxicity profiles for a specific drug in question. Quite prominently lacking in the veterinary literature is information on pharmacogenomic differences across species, in spite of the fact that the field of pharmacogenomics has erupted as a major area of advancement in humans. Pharmacogenomics, or the study of the role genetics plays in drug response, offers a host of additional reasons for altered responses to drugs such as those used in chemotherapy and an integrative systems pharmacology approach including pharmacokinetics, pharmacodynamics and pharmacogenomics is now proposed as an ideal method to approach drug regimen design [80,81,111].
6-Mercaptopurine (6-MP) is a core purine antimetabolite chemotherapy drug in maintenance protocols in childhood acute lymphoblastic leukaemia. 6-MP is inactive and undergoes activation to form 6-thioguanine (6-TG), which exerts cytotoxicity by incorporation into DNA and RNA, which is linked to cytotoxicity. 6-MP is cleared by either oxidation to the inactive 6-thiouric acid by xanthine oxidase or by S-methylation by thiopurine methyltransferase (TPMT) to yield 6-methyl mercaptopurine [80,112]. Haematopoietic cells do not have xanthine oxidase activity, thus leaving TPMT as the primary mechanism of metabolism [80,113]. In the absence of TPMT, 6-MP is metabolized by haematopoietic cells to produce high levels of 6-TG, causing profound haematologic toxicity. It is now recognized that there is significant variability in red blood cell TPMT activity in humans, with approximately 11% encoding for a nucleotide polymorphism associated with low TPMT activity [114,115]. This recognition has altered current practice paradigms as myelosuppression following treatment is directly related to TPMT phenotype. Greater than 60–65% of human patients experiencing extreme toxicity have TPMT deficiency, most of which can be detected by genetic testing for TPMT*2, TPMT*3A and TPMT*3C alleles [80,116–118]. Clinical guidelines incorporating this pharmacogenetic information for 6-MP in leukaemia are now recommended in order to manage both efficacy and toxicity [116–118]. Although 6-MP is not widely used in veterinary oncology, its prodrug azathioprine is commonly prescribed for various diseases. Dogs have variable red blood cell TPMT levels and while some breed tendencies were noted (Labrador Retrievers with high TPMT activity and Cocker Spaniels with low activity), there was a considerable range (ninefold) of activity across dogs [119]. Cats are recognized as being extremely sensitive to azathioprine and have lower red blood cell TPMT activity compared with dogs and humans [120,121]. Additional research in companion animals and other non-human species will build on preliminary research and explore the functional and clinical impact of TPMT polymorphisms to help identify altered drug responses, ultimately improving animal models in drug development [119,120,122].
While the TPMT story provides the best example of applied pharmacogenetics in human oncology, much work needs to be done to identify the impact of other known genetic and metabolic differences across species. It is widely recognized that cats can respond vastly differently to several drugs compared with other companion animals although the underlying reasons are not always clear. Generally speaking, drugs that are metabolized via conjugation are cleared slowly in cats compared with dogs and humans due to a lack of many conjugation enzymes besides TPMT, including UDP-glucuronosyltransferase (UGT) enzymes and N-acetyltransferase 2 (NAT2). The human UGT family consists of 19 different isoforms that are primarily expressed in liver, kidney and intestinal mucosa, which are primary sites of drug metabolism, thus highlighting a substantial difference between cats and humans, making drug dosing and response comparisons inherently difficult [123,124]. N-acetylation of amines in humans occurs via N-acetyltransferase enzymes NAT1 and NAT2 activity; dogs and related canids are deficient in NAT genes, emphasizing another primary difference in metabolism among companion animal species and humans [125]. Interestingly, NATs have been widely studied in humans due to their importance in xenobiotic metabolism while NAT polymorphism has been linked to population differences in drug metabolism [126] (figure 1).
There are many other examples of altered parameters affecting drug absorption and protein binding, drug delivery to the target tissue and toxicities that are beyond the scope of this review; table 1 provides additional examples of variables that may affect response to chemotherapy. Despite the overwhelming range of potential factors that can influence drug efficacy and tumour response, it is remarkable that correlations can be made across species in support of the field of comparative oncology.
Table 1.
Selected examples of species differences in drug pharmacokinetics, pharmacodynamics and pharmacogenomics that may influence response to cancer chemotherapy or targeted drug therapy.
| parameter | species | feature/example |
|---|---|---|
| serum albumin binding | variable | canine and human albumin site II binding were very similar while albumin derived from rabbits, rats and cows were markedly different [127] |
| plasma protein binding | variable | total plasma protein content was highest in the dog compared with mouse, rat, rabbit, monkey and human [128] |
| protein binding affinity: alendronate | dog versus rats | alendronate demonstrated little binding in the dog as opposed to high binding in the rat [129] |
| immune response: macrophages | humans versus dogs, rats and mice | pulmonary alveolar macrophages in humans have highest phagocytic ability compared with rat, mouse or dogs suggesting some targeted drugs (liposomes) may effect species differences in response [109,130] |
| immune response: hypersensitivity and anaphylactic responses [109] | humans | shock organs include lung, larynx and vasculature |
| dog | shock organ is classically considered the liver but includes all splanchnic circulation | |
| rat | shock organs include liver and intestine | |
| mouse | shock organs include vasculature and intestine | |
| immune response: opsonization via complement proteins and immunoglobulins—Cremophor EL and polysorbate 80 | dog | dogs display a much greater hypersensitivity to both Cremophor EL and polysorbate 80 compared with other species such as mice and pigs [131,132] |
| immune response: opsonization of liposomes—liposome encapsulated doxorubicin | rats versus dogs, pigs and humans | rats were markedly less sensitive to liposomal phospholipids compared with dogs, pigs and humans [131] |
| drug absorption from interstitial tissue | variable | macromolecules absorbed via capillaries in rats, whereas macromolecules often dependent on lymphatic absorption in dogs, sheep and humans [108,109,133,134] |
| drug delivery to tumour: colloidal osmotic pressure | variable | interstitial fluid pressure at the periphery of a tumour likely differs significantly between dogs, cats, rats and humans [135–137] |
| drug delivery to tumour: transport across blood-brain barrier | variable | active transporter expression is highly variable between humans, rodents, cows, pigs and dogs. Despite P-glycoprotein (ABCB1A) homology across species, significant differences in substrate recognition and transport efficiency have been noted between human and mouse [138–141] |
| breed-related physiologic differences: Sighthounds | dogs | lower volume of distribution of lipophilic compounds in Sighthounds compared with other breeds [142] |
| breed-related physiologic differences: dog size | dogs | gastrointestinal transit, fecal quality, intestinal permeability and GFR related to body size in dogs [143,144] |
| breed-related metabolic differences: CYP2D15 (similar to human CYP2D6) | Beagle dog versus humans | polymorphisms in CYP2D15 greatly affected metabolism of celecoxib across purebred Beagles [145] |
| breed-related pharmacogenetic differences | dogs—namely herding breeds (Collie, Australian shepherd, long-haired Whippet, Shetland Sheepdog, Old English Sheepdog, White Swiss Shepherd) [146,147] | polymorphisms in ABCB1 altered response associated with P-glycoprotein substrates [148–150] |
7. Concluding remarks
Therapeutic response of a particular cancer to chemotherapy is very difficult to predict across the species. The use of rodent models to dissect the biology of cancer has proved invaluable in supporting the exponential growth of our understanding of this disease but rodents still prove to be poor models for predicting therapeutic responses leading to an incredibly costly linear drug development pathway. Naturally occurring cancer in dogs has been suggested as an alternative therapeutic model system that could prove to be more cost effective, with greater predictability and potentially allowing enormous savings in drug development costs. However, as we have seen, even with natural models we need to have a greater understanding of pharmacodynamics, pharmacokinetics and pharmacogenomics in natural models such as the dog. Publication of the canine genome and the development of a toolbox of reagents to study canine pharmacology and cancer biology will help to underpin progress in this area. However, to gain the optimal clinical benefit from comparative studies, we need to:
— obtain a greater understanding of the comparative biology of cancer between dogs and humans;
— develop a toolbox of reagents that can be used to dissect the biology of cancer in both species and underpinned by genomic, proteomic, metabolomic studies with appropriate bioinformatics;
— gain wider acceptance among the medical and scientific community that we must use the best model for a particular biological question. While rodent models have many benefits, they are not necessarily the best models for rational drug development;
— gain wider acceptance from the approvals agencies (FDA/EMEA) that studies done in species other than the mouse may offer greater predictability of use of a drug in people; and
— conduct well-designed, statistically appropriate studies in veterinary patients.
As a final consideration, it may be important to adopt a more holistic systems biology approach to cancer chemotherapy across the species. In this approach, the whole patient and networks are considered rather than a ‘reductionist’ type study where ‘cause and effect’ are the only parameters. Reductionism focuses on the disease rather than on the individualization of treatment or on a multidimensional use of drugs. To this notion of reductionism, the true benefit of chemotherapy for many human solid tumours has been brought into question by some researchers who suggest that chemotherapy neither improves survival nor provides a higher quality of life [91,151–153]. While chemotherapy for some cancers has decreased tumour size, tumour response comes at the expense of an increased risk of chemotherapy-induced neoplasia and an adversely affected lifestyle. At least for the foreseeable future, for the veterinary oncologist, and irrespective of apparent differences in tumour responses across species, the focus for veterinary patients such as the dog is maintaining or improving an excellent quality-of-life, as perceived by the owner and/or attending veterinary clinician.
Authors' contributions
J.L. drafted the preliminary manuscript and provided the relevant review materials. D.C. helped draft the manuscript and provide pertinent comparisons and critiques. D.A. coordinated the review and helped draft the manuscript. All authors gave final approval for publication.
Conflict of interests
All authors (J.L., D.C. and D.A.) are employed by the University of Edinburgh. We have no personal financial or competing interests.
References
- 1.Langdon SP. 2012. Animal modeling of cancer pathology and studying tumor response to therapy. Curr. Drug Targets 13, 1535–1547. ( 10.2174/138945012803530152) [DOI] [PubMed] [Google Scholar]
- 2.Bailey MJ, Gazet JC, Smith IE, Steel GG. 1980. Chemotherapy of human breast-carcinoma xenografts. Br. J. Cancer 42, 530–536. ( 10.1038/bjc.1980.276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Favre R, Marotia L, Drancourt M, Jaquemier J, Delpero JR, Guerinel G, Carcassone Y. 1986. 6-day subrenal capsule assay (SRCA) as a predictor of the response of advanced cancers to chemotherapy. Eur. J. Cancer Clin. Oncol. 22, 1171–1178. ( 10.1016/0277-5379(86)90318-4) [DOI] [PubMed] [Google Scholar]
- 4.Fiebig HH, Maier A, Burger AM. 2004. Clonogenic assay with established human tumour xenografts: correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur. J. Cancer 40, 802–820. ( 10.1016/j.ejca.2004.01.009) [DOI] [PubMed] [Google Scholar]
- 5.Inoue K, Fujimoto S, Ogawa M. 1983. Antitumor efficacy of seventeen anticancer drugs in human breast cancer xenograft (MX-1) transplanted in nude mice. Cancer Chemother. Pharmacol. 10, 182–186. ( 10.1007/BF00255758) [DOI] [PubMed] [Google Scholar]
- 6.Johnson JI, et al. 2001. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br. J. Cancer 84, 1424–1431. ( 10.1054/bjoc.2001.1796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattern J, Bak M, Hahn EW, Volm M. 1988. Human tumor xenografts as model for drug testing. Cancer Metastasis Rev. 7, 263–284. ( 10.1007/BF00047755) [DOI] [PubMed] [Google Scholar]
- 8.Taetle R, Rosen F, Abramson I, Venditti J, Howell S. 1987. Use of nude mouse xenografts as preclinical drug screens: in vivo activity of established chemotherapeutic agents against melanoma and ovarian carcinoma xenografts. Cancer Treat. Rep. 71, 297–304. [PubMed] [Google Scholar]
- 9.Voskoglou-Nomikos T, Pater JL, Seymour L. 2003. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin. Cancer Res. 9, 4227–4239. [PubMed] [Google Scholar]
- 10.Peterson JK, Houghton PJ. 2004. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur. J. Cancer 40, 837–844. ( 10.1016/j.ejca.2004.01.003) [DOI] [PubMed] [Google Scholar]
- 11.Gordon IK, Khanna C. 2010. Modeling opportunities in comparative oncology for drug development. ILAR 51, 214–220. ( 10.1093/ilar.51.3.214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rangarajan A, Weinberg RA. 2003. Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat. Rev. Cancer 3, 952–959. ( 10.1038/nrc1235) [DOI] [PubMed] [Google Scholar]
- 13.Frese KK, Tuveson DA. 2007. Maximizing mouse cancer models. Nat. Rev. Cancer 7, 645–658. ( 10.1038/nrc2192) [DOI] [PubMed] [Google Scholar]
- 14.Talmadge JE, Singh RK, Fidler IJ, Raz A. 2007. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am. J. Pathol. 170, 793–804. ( 10.2353/ajpath.2007.060929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyer J, Kwong LN, Lowe SW, Chin L. 2010. Non-germline genetically engineered mouse models for translational cancer research. Nat. Rev. Cancer 10, 470–480. ( 10.1038/nrc2877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. 2000. The hallmarks of cancer. Cell 100, 57–70. ( 10.1016/S0092-8674(00)81683-9) [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 18.Mueller F, Fuchs B, Kaser-Hotz B. 2007. Comparative biology of human and canine osteosarcoma. Anticancer Res. 27, 155–164. [PubMed] [Google Scholar]
- 19.Paoloni M, Khanna C. 2008. Translation of new cancer treatments from pet dogs to humans. Nat. Rev. Cancer 8, 147–156. ( 10.1038/nrc2273) [DOI] [PubMed] [Google Scholar]
- 20.Porrello A, Cardelli P, Spugnini EP. 2006. Oncology of companion animals as a model for humans. An overview of tumor histotypes. J. Exp. Clin. Cancer Res. 25, 97–105. [PubMed] [Google Scholar]
- 21.Vail DM, MacEwen EG. 2000. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 18, 781–792. ( 10.3109/07357900009012210) [DOI] [PubMed] [Google Scholar]
- 22.Fleischer S, Sharkey M, Mealey K, Ostrander EA, Martinez M. 2008. Pharmacogenetic and metabolic differences between dog breeds: their impact on canine medicine and the use of the dog as a preclinical animal model. AAPS J. 10, 110–119. ( 10.1208/s12248-008-9011-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skipper HE, Schabel FM, Jr, Mellett LB, Montgomery JA, Wilkoff LJ, Lloyd HH, Brockman RW. 1970. Implications of biochemical, cytokinetic, pharmacologic, and toxicologic relationships in the design of optimal therapeutic schedules. Cancer Chemother. Rep. Part 1 54, 431–450. [PubMed] [Google Scholar]
- 24.Vail DM, Pinkerton ME, Young KM. 2013. Hematopoietic tumors. In Small animal clinical oncology (eds Withrow SJ, Vail DM, Page RL.), pp. 608–678, 5th edn St Louis, MO: Saunders. [Google Scholar]
- 25.Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M, Smith FO, Reaman GH. 2010. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J. Clin. Oncol. 28, 2625–2634. ( 10.1200/JCO.2009.27.0421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deininger MW, Druker BJ. 2004. SRCircumventing imatinib resistance. Cancer Cell 6, 108–110. ( 10.1016/j.ccr.2004.08.006) [DOI] [PubMed] [Google Scholar]
- 27.Gerrie AS, Power MM, Shepherd JD, Savage KJ, Sehn LH, Connors JM. 2014. Chemoresistance can be overcome with high-dose chemotherapy and autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Ann. Oncol. 25, 2218–2223. ( 10.1093/annonc/mdu387) [DOI] [PubMed] [Google Scholar]
- 28.Maxwell SA, Mousavi-Fard S. 2013. Non-Hodgkin's B-cell lymphoma: advances in molecular strategies targeting drug resistance. Exp. Biol. Med. 238, 971–990. ( 10.1177/1535370213498985) [DOI] [PubMed] [Google Scholar]
- 29.Kirkwood JM, Tarhini A, Sparano JA, Patel P, Schiller JH, Vergo MT, Benson AB, III, Tawbi H. 2013. Comparative clinical benefits of systemic adjuvant therapy for paradigm solid tumors. Cancer Treatment Rev. 39, 27–43. ( 10.1016/j.ctrv.2012.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen G, Marcove RC, Huvos AG, Caparros BI, Lane JM, Nirenberg A, Cacavio A, Groshen S. 1983. Primary osteogenic sarcoma: eight-year experience with adjuvant chemotherapy. J. Cancer Res. Clin. Oncol. 106, 55–67. ( 10.1007/BF00625054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gustafson DL PR. 2013. Cancer chemotherapy. In Small animal clinical oncology (eds Withrow SJ, Vail DM, Page RL.), pp. 157–179, 5th edn St Louis, MO: Saunders. [Google Scholar]
- 32.Thamm DH, Vail DM. 2007. Aftershocks of cancer chemotherapy: managing adverse effects. J. Am. Anim. Hosp. Assoc. 43, 1–7. ( 10.5326/0430001) [DOI] [PubMed] [Google Scholar]
- 33.Bowles DB, Robson MC, Galloway PE, Walker L. 2010. Owner's perception of carboplatin in conjunction with other palliative treatments for cancer therapy. J. Small Anim. Pract. 51, 104–112. ( 10.1111/j.1748-5827.2009.00891.x) [DOI] [PubMed] [Google Scholar]
- 34.Bronden LB, Rutteman GR, Flagstad A, Teske E. 2003. Study of dog and cat owners’ perceptions of medical treatment for cancer. Vet. Rec. 152, 77–80. ( 10.1136/vr.152.3.77) [DOI] [PubMed] [Google Scholar]
- 35.Friedberg JW MP, Rimsza L, Fisher RI. 2011. Non-Hodgkin lymphomas. In Cancer principles and practice of oncology (eds DeVita VJ, Lawrence TS, Rosenberg SA.), pp. 1855–1893, 9th edn Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- 36.Nastoupil LJ, et al. 2014. Comparison of the effectiveness of frontline chemoimmunotherapy regimens for follicular lymphoma used in the United States. Leuk. Lymphoma 2014, 1–28. ( 10.3109/10428194.2014.953144) [DOI] [PubMed] [Google Scholar]
- 37.Jung SH, et al. 2014. Weekly rituximab consolidation following four cycles of R-CHOP induction chemotherapy in very elderly patients with diffuse large B-cell lymphoma: Consortium for improving survival of lymphoma study (CISL). Eur. J. Haematol. ( 10.1111/ejh.12459) [DOI] [PubMed] [Google Scholar]
- 38.Plosker GL, Figgitt DP. 2003. Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs 63, 803–843. ( 10.2165/00003495-200363080-00005) [DOI] [PubMed] [Google Scholar]
- 39.Sueiro FA, Alessi AC, Vassallo J. 2004. Canine lymphomas: a morphological and immunohistochemical study of 55 cases, with observations on p53 immunoexpression. J. Comp. Pathol. 131, 207–213. ( 10.1016/j.jcpa.2004.04.002) [DOI] [PubMed] [Google Scholar]
- 40.Valli VE, et al. 2011. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet. Pathol. 48, 198–211. ( 10.1177/0300985810379428) [DOI] [PubMed] [Google Scholar]
- 41.Vezzali E, Parodi AL, Marcato PS, Bettini G. 2010. Histopathologic classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO. Vet. Comp. Oncol. 8, 38–49. ( 10.1111/j.1476-5829.2009.00201.x) [DOI] [PubMed] [Google Scholar]
- 42.Flory AB, Rassnick KM, Erb HN, Garrett LD, Northrup NC, Selting KA, Phillips BS, Locke JE, Chretin JD. 2011. Evaluation of factors associated with second remission in dogs with lymphoma undergoing retreatment with a cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy protocol: 95 cases (2000–2007). J. Am. Vet. Med. Assoc. 238, 501–506. ( 10.2460/javma.238.4.501) [DOI] [PubMed] [Google Scholar]
- 43.Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A, Angeles C, Menendez LR. 2012. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012, 704872 ( 10.1155/2012/704872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcove R, Applewhite A, Vlamis V, Rosen G. 1992. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan–Kettering experience. J. Clin. Oncol. 10, 5–15. [DOI] [PubMed] [Google Scholar]
- 45.Cortes EP, Holland JF, Wang JJ, Sinks LF, Blom J, Senn H, Bank A, Glidewell O. 1974. Amputation and adriamycin in primary osteosarcoma. N. Engl. J. Med. 291, 998–1000. ( 10.1056/NEJM197411072911903) [DOI] [PubMed] [Google Scholar]
- 46.Link MP, et al. 1986. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N. Engl. J. Med. 314, 1600–1606. ( 10.1056/NEJM198606193142502) [DOI] [PubMed] [Google Scholar]
- 47.Winkler K, et al. 1988. Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J. Clin. Oncol. 6, 329–337. [DOI] [PubMed] [Google Scholar]
- 48.Sleijfer S, Ouali M, van Glabbeke M, Krarup-Hansen A, Rodenhuis S, Le Cesne A, Hogendoorn PCW, Verweij J, Blay J-Y. 2010. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur. J. Cancer 46, 72–83. ( 10.1016/j.ejca.2009.09.022) [DOI] [PubMed] [Google Scholar]
- 49.Patrikidou A, Domont J, Cioffi A, Le Cesne A. 2011. Treating soft tissue sarcomas with adjuvant chemotherapy. Curr. Treat. Options Oncol. 12, 21–31. ( 10.1007/s11864-011-0145-5) [DOI] [PubMed] [Google Scholar]
- 50.Gronchi A, Casali PG. 2013. Adjuvant therapy for high-risk soft tissue sarcoma in the adult. Curr. Treat. Options Oncol. 14, 415–424. ( 10.1007/s11864-013-0243-7) [DOI] [PubMed] [Google Scholar]
- 51.Scurr M. 2011. Histology-driven chemotherapy in soft tissue sarcomas. Curr. Treat. Options Oncol. 12, 32–45. ( 10.1007/s11864-011-0140-x) [DOI] [PubMed] [Google Scholar]
- 52.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. 2008. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 113, 573–581. ( 10.1002/cncr.23592) [DOI] [PubMed] [Google Scholar]
- 53.Verma S, Younus J, Stys-Norman D, Haynes AE, Blackstein M. 2008. Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat. Rev. 34, 339–347. ( 10.1016/j.ctrv.2008.01.005) [DOI] [PubMed] [Google Scholar]
- 54.Collaboration SM-A. 1997. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. (Sarcoma meta-analysis collaboration.) Lancet 350, 1647–1654. ( 10.1016/S0140-6736(97)08165-8) [DOI] [PubMed] [Google Scholar]
- 55.Sarcoma Meta-analysis Collaboration (SMAC). 2000. Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst. Rev. 2000(4): CD001419 Available at http://www.ncbi.nlm.nih.gov/pubmed/11034717. [DOI] [PubMed] [Google Scholar]
- 56.Paoloni M, et al. 2009. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics 10, 625 ( 10.1186/1471-2164-10-625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fenger JM, London CA, Kisseberth WC. 2014. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J. 55, 69–85. ( 10.1093/ilar/ilu009) [DOI] [PubMed] [Google Scholar]
- 58.Withrow SJ, Wilkins RM. 2010. Cross talk from pets to people: translational osteosarcoma treatments. ILAR J. 51, 208–213. ( 10.1093/ilar.51.3.208) [DOI] [PubMed] [Google Scholar]
- 59.Rankin KS, Starkey M, Lunec J, Gerrand CH, Murphy S, Biswas S. 2012. Of dogs and men: comparative biology as a tool for the discovery of novel biomarkers and drug development targets in osteosarcoma. Pediatr. Blood Cancer 58, 327–333. ( 10.1002/pbc.23341) [DOI] [PubMed] [Google Scholar]
- 60.Thompson JP, Fugent MJ. 1992. Evaluation of survival times after limb amputation, with and without subsequent administration of cisplatin, for treatment of appendicular osteosarcoma in dogs: 30 cases (1979–1990). J. Am. Vet. Med. Assoc. 200, 531–533. [PubMed] [Google Scholar]
- 61.Spodnick GJ, et al. 1992. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988). J. Am. Vet. Med. Assoc. 200, 995–999. [PubMed] [Google Scholar]
- 62.Mauldin GN, Matus RE, Withrow SJ, Patnaik AK. 1988. Canine osteosarcoma. Treatment by amputation versus amputation and adjuvant chemotherapy using doxorubicin and cisplatin. J. Vet. Intern. Med. 2, 177–180. ( 10.1111/j.1939-1676.1988.tb00313.x) [DOI] [PubMed] [Google Scholar]
- 63.Selmic LE, Burton JH, Thamm DH, Withrow SJ, Lana SE. 2014. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J. Vet. Intern. Med. 28, 554–563. ( 10.1111/jvim.12313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips B, Powers BE, Dernell WS, Straw RC, Khanna C, Hogge GS, Vail DM. 2009. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J. Am. Anim. Hosp. Assoc. 45, 33–38. ( 10.5326/0450033) [DOI] [PubMed] [Google Scholar]
- 65.Bacon NJ, Ehrhart NP, Dernell WS, Lafferty M, Withrow SJ. 2008. Use of alternating administration of carboplatin and doxorubicin in dogs with microscopic metastases after amputation for appendicular osteosarcoma: 50 cases (1999–2006). J. Am. Vet. Med. Assoc. 232, 1504–1510. ( 10.2460/javma.232.10.1504) [DOI] [PubMed] [Google Scholar]
- 66.Ehrhart NP, Ryan SP, Fan TM. 2013. Tumors of the skeletal system. In Small animal clinical oncology (eds Withrow SJ, Vail DM, Page RL.), pp. 463–503, 5th edn St Louis, MO: Saunders. [Google Scholar]
- 67.Ogilvie GK, Reynolds HA, Richardson RC, Withrow SJ, Norris AM, Henderson RA, Klausner JS, Fowler JD, McCaw D. 1989. Phase II evaluation of doxorubicin for treatment of various canine neoplasms. J. Am. Vet. Med. Assoc. 195, 1580–1583. [PubMed] [Google Scholar]
- 68.Liptak JM FL. 2013. Soft tissue sarcomas. In Small animal clinical oncology (eds Withrow SJ, Vail DM, Page RL.), pp. 356–380, 5th edn St Louis, MO: Saunders. [Google Scholar]
- 69.Selting KA PB, Thompson LJ, Mittleman E, Tyler JW, Lafferty MH, Withrow SJ. 2005. Outcome of dogs with high-grade soft tissue sarcomas treated with and without adjuvant doxorubicin chemotherapy: 39 cases (1996–2004). J. Am. Vet. Med. Assoc. 227, 1442–1448. ( 10.2460/javma.2005.227.1442) [DOI] [PubMed] [Google Scholar]
- 70.Kraus C, Pavard S, Promislow DE. 2013. The size-life span trade-off decomposed: why large dogs die young. Am. Nat. 181, 492–505. ( 10.1086/669665) [DOI] [PubMed] [Google Scholar]
- 71.Patronek GJ, Waters DJ, Glickman LT. 1997. Comparative longevity of pet dogs and humans: implications for gerontology research. J. Gerontol. A Biol. Sci. Med. Sci. 52, B171–B178. ( 10.1093/gerona/52A.3.B171) [DOI] [PubMed] [Google Scholar]
- 72.de Magalhaes JP, Costa J. 2009. A database of vertebrate longevity records and their relation to other life-history traits. J. Evol. Biol. 22, 1770–1774. ( 10.1111/j.1420-9101.2009.01783.x) [DOI] [PubMed] [Google Scholar]
- 73.Caulin AF, Maley CC. 2011. Peto's paradox: evolution's prescription for cancer prevention. Trends Ecol. Evol. 26, 175–182. ( 10.1016/j.tree.2011.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Samaras TT, Storms LH. 1992. Impact of height and weight on life span. Bull. World Health Organ. 70, 259–267. [PMC free article] [PubMed] [Google Scholar]
- 75.Lebeau A. 1956. L'age du chien et celui de l'homme. Essai de statistique sur la mortality canine. Bull. Acad. Vet. Fr. 26, 229–232. [Google Scholar]
- 76.Greer KA, Hughes LM, Masternak MM. 2011. Connecting serum IGF-1, body size, and age in the domestic dog. Age 33, 475–483. ( 10.1007/s11357-010-9182-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sutter NB, et al. 2007. A single IGF1 allele is a major determinant of small size in dogs. Science 316, 112–115. ( 10.1126/science.1137045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rimbault M, Beale HC, Schoenebeck JJ, Hoopes BC, Allen JJ, Kilroy-Glynn P, Wayne RK, Sutter NB, Ostrander EA. 2013. Derived variants at six genes explain nearly half of size reduction in dog breeds. Genome Res. 23, 1985–1995. ( 10.1101/gr.157339.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amsellem PM, Selmic LE, Wypij JM, Bacon NJ, Culp WT, Ehrhart NP, Powers BE, Stryhn H, Farese JP. 2014. Appendicular osteosarcoma in small-breed dogs: 51 cases (1986–2011). J. Am. Vet. Med. Assoc. 245, 203–210. ( 10.2460/javma.245.2.203) [DOI] [PubMed] [Google Scholar]
- 80.Walko CM, Ikediobi O. 2012. Pharmacogenomic applications in oncology. J. Pharm. Pract. 25, 439–446. ( 10.1177/0897190012448308) [DOI] [PubMed] [Google Scholar]
- 81.Walko CM, McLeod H. 2009. Pharmacogenomic progress in individualized dosing of key drugs for cancer patients. Nat. Clin. Pract. Oncol. 6, 153–162. ( 10.1038/ncponc1303) [DOI] [PubMed] [Google Scholar]
- 82.Gurney H. 1996. Dose calculation of anticancer drugs: a review of the current practice and introduction of an alternative. J. Clin. Oncol. 14, 2590–2611. [DOI] [PubMed] [Google Scholar]
- 83.Gao B, Klumpen HJ, Gurney H. 2008. Dose calculation of anticancer drugs. Expert Opin. Drug Metab. Toxicol. 4, 1307–1319. ( 10.1517/17425255.4.10.1307) [DOI] [PubMed] [Google Scholar]
- 84.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. 1966. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. Rep. 1 50, 219–244. [PubMed] [Google Scholar]
- 85.Goldsmith MA, Slavik M, Carter SK. 1975. Quantitative prediction of drug toxicity in humans from toxicology in small and large animals. Cancer Res. 35, 1354–1364. [PubMed] [Google Scholar]
- 86.Pinkel D. 1958. The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res. 18, 853–856. [PubMed] [Google Scholar]
- 87.Page RL, Macy DW, Thrall DE, Dewhirst MW, Allen SL, Heidner GL, Sim DA, McGee ML, Gillette EL. 1988. Unexpected toxicity associated with use of body surface area for dosing melphalan in the dog. Cancer Res. 48, 288–290. [PubMed] [Google Scholar]
- 88.Arrington KA, Legendre AM, Tabeling GS, Frazier DL. 1994. Comparison of body surface area-based and weight-based dosage protocols for doxorubicin administration in dogs. Am. J. Vet. Res. 55, 1587–1592. [PubMed] [Google Scholar]
- 89.Ogilvie GK, Moore AS, Curtis CR. 1989. Evaluation of cisplatin-induced emesis in dogs with malignant neoplasia: 115 cases (1984–1987). J. Am. Vet. Med. Assoc. 195, 1399–1403. [PubMed] [Google Scholar]
- 90.Ogilvie GK, et al. 1989. Acute and short-term toxicoses associated with the administration of doxorubicin to dogs with malignant tumors. J. Am. Vet. Med. Assoc. 195, 1584–1587. [PubMed] [Google Scholar]
- 91.Greek R, Rice MJ. 2012. Animal models and conserved processes. Theor. Biol. Med. Model. 9, 40 ( 10.1186/1742-4682-9-40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Price GS, Frazier DL. 1998. Use of body surface area (BSA)-based dosages to calculate chemotherapeutic drug dose in dogs: I. Potential problems with current BSA formulae. J. Vet. Intern. Med. 12, 267–271. ( 10.1111/j.1939-1676.1998.tb02121.x) [DOI] [PubMed] [Google Scholar]
- 93.Frazier DL, Price GS. 1998. Use of body surface area to calculate chemotherapeutic drug dose in dogs: II. Limitations imposed by pharmacokinetic factors. J. Vet. Intern. Med. 12, 272–278. ( 10.1111/j.1939-1676.1998.tb02122.x) [DOI] [PubMed] [Google Scholar]
- 94.Bailey DB, Rassnick KM, Dykes NL, Pendyala L. 2009. Phase I evaluation of carboplatin by use of a dosing strategy based on a targeted area under the platinum concentration-versus-time curve and individual glomerular filtration rate in cats with tumors. Am. J. Vet. Res. 70, 770–776. ( 10.2460/ajvr.70.6.770) [DOI] [PubMed] [Google Scholar]
- 95.Bailey DB, Rassnick KM, Erb HN, Dykes NL, Hoopes PJ, Page RL. 2004. Effect of glomerular filtration rate on clearance and myelotoxicity of carboplatin in cats with tumors. Am. J. Vet. Res. 65, 1502–1507. ( 10.2460/ajvr.2004.65.1502) [DOI] [PubMed] [Google Scholar]
- 96.Wittenburg LA, Thamm DH, Gustafson DL. 2014. Development of a limited-sampling model for prediction of doxorubicin exposure in dogs. Vet. Comp. Oncol. 12, 114–119. ( 10.1111/j.1476-5829.2012.00340.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Engels FK, Loos WJ, van der Bol JM, de Bruijn P, Mathijssen RH, Verweij J, Mathot RAA. 2011. Therapeutic drug monitoring for the individualization of docetaxel dosing: a randomized pharmacokinetic study. Clin. Cancer Res. 17, 353–362. ( 10.1158/1078-0432.CCR-10-1636) [DOI] [PubMed] [Google Scholar]
- 98.Kline CL, et al. 2014. Personalized dosing via pharmacokinetic monitoring of 5-fluorouracil might reduce toxicity in early- or late-stage colorectal cancer patients treated with infusional 5-fluorouracil-based chemotherapy regimens. Clin. Colorectal Cancer 13, 119–126. ( 10.1016/j.clcc.2013.11.001) [DOI] [PubMed] [Google Scholar]
- 99.Rousseau A, Marquet P. 2002. Application of pharmacokinetic modelling to the routine therapeutic drug monitoring of anticancer drugs. Fundam. Clin. Pharmacol. 16, 253–262. ( 10.1046/j.1472-8206.2002.00086.x) [DOI] [PubMed] [Google Scholar]
- 100.Salas S, et al. 2006. Therapeutic drug monitoring for dose individualization of Cisplatin in testicular cancer patients based upon total platinum measurement in plasma. Ther. Drug Monit. 28, 532–539. ( 10.1097/00007691-200608000-00008) [DOI] [PubMed] [Google Scholar]
- 101.Mercier C, et al. 2006. Dose individualization of carboplatin after a 120-hour infusion schedule: higher dose intensity but fewer toxicities. Ther. Drug Monit. 28, 212–218. ( 10.1097/01.ftd.0000198646.32128.ef) [DOI] [PubMed] [Google Scholar]
- 102.Gamelin E, et al. 2008. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 2099–2105. ( 10.1200/JCO.2007.13.3934) [DOI] [PubMed] [Google Scholar]
- 103.Capitain O, Asevoaia A, Boisdron-Celle M, Poirier AL, Morel A, Gamelin E. 2012. Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: a phase II, proof-of-concept study. Clin. Colorectal Cancer 11, 263–267. ( 10.1016/j.clcc.2012.05.004) [DOI] [PubMed] [Google Scholar]
- 104.Vaughan A, Johnson JL, Williams LE. 2007. Impact of chemotherapeutic dose intensity and hematologic toxicity on first remission duration in dogs with lymphoma treated with a chemoradiotherapy protocol. J. Vet. Intern. Med. 21, 1332–1339. ( 10.1111/j.1939-1676.2007.tb01956.x) [DOI] [PubMed] [Google Scholar]
- 105.Sorenmo K, Overley B, Krick E, Ferrara T, LaBlanc A, Shofer F. 2010. Outcome and toxicity associated with a dose-intensified, maintenance-free CHOP-based chemotherapy protocol in canine lymphoma: 130 cases. Vet. Comp. Oncol. 8, 196–208. ( 10.1111/j.1476-5829.2010.00222.x) [DOI] [PubMed] [Google Scholar]
- 106.Ratain MJ, Mick R. 1996. Principles of pharmacokinetics and pharmacodynamics. In Principles of antineoplastic drug development and pharmacology basic and clinical oncology (eds Schilsky RL, Milano GA, Ratain MJ.), pp. 123–142. New York, NY: Marcel Dekker. [Google Scholar]
- 107.Mahmood I. 2007. Application of allometric principles for the prediction of pharmacokinetics in human and veterinary drug development. Adv. Drug Deliv. Rev. 59, 1177–1192. ( 10.1016/j.addr.2007.05.015) [DOI] [PubMed] [Google Scholar]
- 108.Martinez MN. 2009. Interspecies differences in physiology and pharmacology: extrapolating preclinical data to human populations. In Preclinical drug development (eds Rogge MC, Taft D.), pp. 35–70. Boca Raton, FL: Taylor and Francis Group. [Google Scholar]
- 109.Martinez MN. 2011. Factors influencing the use and interpretation of animal models in the development of parenteral drug delivery systems. AAPS J. 13, 632–649. ( 10.1208/s12248-011-9303-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martignoni M, Groothuis GM, de Kanter R. 2006. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2, 875–894. ( 10.1517/17425255.2.6.875) [DOI] [PubMed] [Google Scholar]
- 111.Gumbo T. 2008. Integrating pharmacokinetics, pharmacodynamics and pharmacogenomics to predict outcomes in antibacterial therapy. Curr. Opin. Drug Discov. Dev. 11, 32–42. [PubMed] [Google Scholar]
- 112.Elion GB. 1989. The purine path to chemotherapy. Science 244, 41–47. ( 10.1126/science.2649979) [DOI] [PubMed] [Google Scholar]
- 113.Parks DA, Granger DN. 1986. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol. Scand. Suppl. 548, 87–99. [PubMed] [Google Scholar]
- 114.McLeod HL, Relling MV, Liu Q, Pui CH, Evans WE. 1995. Polymorphic thiopurine methyltransferase in erythrocytes is indicative of activity in leukemic blasts from children with acute lymphoblastic leukemia. Blood 85, 1897–1902. [PubMed] [Google Scholar]
- 115.Weinshilboum RM, Sladek SL. 1980. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am. J. Hum. Genet. 32, 651–662. [PMC free article] [PubMed] [Google Scholar]
- 116.Lopez-Lopez E, Gutierrez-Camino A, Bilbao-Aldaiturriaga N, Pombar-Gomez M, Martin-Guerrero I, Garcia-Orad A. 2014. Pharmacogenetics of childhood acute lymphoblastic leukemia. Pharmacogenomics 15, 1383–1398. ( 10.2217/pgs.14.106) [DOI] [PubMed] [Google Scholar]
- 117.Swen JJ, et al. 2011. Pharmacogenetics: from bench to byte--an update of guidelines. Clin. Pharmacol. Ther. 89, 662–673. ( 10.1038/clpt.2011.34) [DOI] [PubMed] [Google Scholar]
- 118.Relling MV, et al. 2011. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin. Pharmacol. Ther. 89, 387–391. ( 10.1038/clpt.2010.320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salavaggione OE, Kidd L, Prondzinski JL, Szumlanski CL, Pankratz VS, Wang L, Trepanier L, Weinshilboum RM. 2002. Canine red blood cell thiopurine S-methyltransferase: companion animal pharmacogenetics. Pharmacogenetics 12, 713–724. ( 10.1097/00008571-200212000-00005) [DOI] [PubMed] [Google Scholar]
- 120.Salavaggione OE, Yang C, Kidd LB, Thomae BA, Pankratz VS, Trepanier LA, Weinshilboum RM. 2004. Cat red blood cell thiopurine S-methyltransferase: companion animal pharmacogenetics. J. Pharmacol. Exp. Ther. 308, 617–626. ( 10.1124/jpet.103.059055) [DOI] [PubMed] [Google Scholar]
- 121.Court MH. 2013. Feline drug metabolism and disposition: pharmacokinetic evidence for species differences and molecular mechanisms. Vet. Clin. N. Am. Small Anim. Pract. 43, 1039–1054. ( 10.1016/j.cvsm.2013.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Salavaggione OE, Wang L, Wiepert M, Yee VC, Weinshilboum RM. 2005. Thiopurine S-methyltransferase pharmacogenetics: variant allele functional and comparative genomics. Pharmacogen. Genomics 15, 801–815. ( 10.1097/01.fpc.0000174788.69991.6b) [DOI] [PubMed] [Google Scholar]
- 123.Court MH, Zhang X, Ding X, Yee KK, Hesse LM, Finel M. 2012. Quantitative distribution of mRNAs encoding the 19 human UDP-glucuronosyltransferase enzymes in 26 adult and 3 fetal tissues. Xenobiotica 42, 266–277. ( 10.3109/00498254.2011.618954) [DOI] [PubMed] [Google Scholar]
- 124.Court MH, Greenblatt DJ. 1997. Molecular basis for deficient acetaminophen glucuronidation in cats. An interspecies comparison of enzyme kinetics in liver microsomes. Biochem. Pharmacol. 53, 1041–1047. ( 10.1016/S0006-2952(97)00072-5) [DOI] [PubMed] [Google Scholar]
- 125.Trepanier LA, Ray K, Winand NJ, Spielberg SP, Cribb AE. 1997. Cytosolic arylamine N-acetyltransferase (NAT) deficiency in the dog and other canids due to an absence of NAT genes. Biochem. Pharmacol. 54, 73–80. ( 10.1016/S0006-2952(97)00140-8) [DOI] [PubMed] [Google Scholar]
- 126.Sim E, Abuhammad A, Ryan A. 2014. Arylamine N-acetyltransferases: from drug metabolism and pharmacogenetics to drug discovery. Br. J. Pharmacol. 171, 2705–2725. ( 10.1111/bph.12598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kosa T, Maruyama T, Otagiri M. 1997. Species differences of serum albumins: I. Drug binding sites. Pharm. Res. 14, 1607–1612. ( 10.1023/A:1012138604016) [DOI] [PubMed] [Google Scholar]
- 128.Davies B, Morris T. 1993. Physiological parameters in laboratory animals and humans. Pharm. Res. 10, 1093–1095. ( 10.1023/A:1018943613122) [DOI] [PubMed] [Google Scholar]
- 129.Lin JH, Chen IW, deLuna FA. 1994. Nonlinear kinetics of alendronate. Plasma protein binding and bone uptake. Drug Metab. Dispos. 22, 400–405. [PubMed] [Google Scholar]
- 130.Haley PJ. 2003. Species differences in the structure and function of the immune system. Toxicology 188, 49–71. ( 10.1016/S0300-483X(03)00043-X) [DOI] [PubMed] [Google Scholar]
- 131.Szebeni J, Alving CR, Rosivall L, Bunger R, Baranyi L, Bedocs P, Tóth M, Barenholz Y. 2007. Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. J. Liposome Res. 17, 107–117. ( 10.1080/08982100701375118) [DOI] [PubMed] [Google Scholar]
- 132.Poirier VJ, Hershey AE, Burgess KE, Phillips B, Turek MM, Forrest LJ, Beaver L, Vail DM. 2004. Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. J. Vet. Intern. Med. 18, 219–222. ( 10.1111/j.1939-1676.2004.tb00164.x) [DOI] [PubMed] [Google Scholar]
- 133.Trevaskis NL, Caliph SM, Nguyen G, Tso P, Charman WN, Porter CJ. 2013. A mouse model to evaluate the impact of species, sex, and lipid load on lymphatic drug transport. Pharm. Res. 30, 3254–3270. ( 10.1007/s11095-013-1000-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Porter CJ, Edwards GA, Charman SA. 2001. Lymphatic transport of proteins after s.c. injection: implications of animal model selection. Adv. Drug Deliv. Rev. 50, 157–171. ( 10.1016/S0169-409X(01)00153-3) [DOI] [PubMed] [Google Scholar]
- 135.Jain RK. 1987. Transport of molecules in the tumor interstitium: a review. Cancer Res. 47, 3039–3051. [PubMed] [Google Scholar]
- 136.Netti PA, et al. 1999. Enhancement of fluid filtration across tumor vessels: implication for delivery of macromolecules. Proc. Natl Acad. Sci. USA 96, 3137–3142. ( 10.1073/pnas.96.6.3137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thomas LA, Brown SA. 1992. Relationship between colloid osmotic pressure and plasma protein concentration in cattle, horses, dogs, and cats. Am. J. Vet. Res. 53, 2241–2244. [PubMed] [Google Scholar]
- 138.Gerhart DZ, Leino RL, Borson ND, Taylor WE, Gronlund KM, McCall AL, Drewes LR. 1995. Localization of glucose transporter GLUT 3 in brain: comparison of rodent and dog using species-specific carboxyl-terminal antisera. Neuroscience 66, 237–246. ( 10.1016/0306-4522(94)00544-F) [DOI] [PubMed] [Google Scholar]
- 139.Westerhout J, Danhof M, De Lange EC. 2011. Preclinical prediction of human brain target site concentrations: considerations in extrapolating to the clinical setting. J. Pharm. Sci. 100, 3577–3593. ( 10.1002/jps.22604) [DOI] [PubMed] [Google Scholar]
- 140.Borst P, Schinkel AH. 2013. P-glycoprotein ABCB1: a major player in drug handling by mammals. J. Clin. Invest. 123, 4131–4133. ( 10.1172/JCI70430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Syvanen S, Lindhe O, Palner M, Kornum BR, Rahman O, Langstrom B, Knudsen GM, Hammarlund-Udenaes M. 2009. Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug Metab. Dispos. 37, 635–643. ( 10.1124/dmd.108.024745) [DOI] [PubMed] [Google Scholar]
- 142.Hay Kraus BL, Greenblatt DJ, Venkatakrishnan K, Court MH. 2000. Evidence for propofol hydroxylation by cytochrome P4502B11 in canine liver microsomes: breed and gender differences. Xenobiotica 30, 575–588. ( 10.1080/004982500406417) [DOI] [PubMed] [Google Scholar]
- 143.Hernot DC, Biourge VC, Martin LJ, Dumon HJ, Nguyen PG. 2005. Relationship between total transit time and faecal quality in adult dogs differing in body size. J. Anim. Physiol. Anim. Nutr. 89, 189–193. ( 10.1111/j.1439-0396.2005.00544.x) [DOI] [PubMed] [Google Scholar]
- 144.Randell SC, Hill RC, Scott KC, Omori M, Burrows CF. 2001. Intestinal permeability testing using lactulose and rhamnose: a comparison between clinically normal cats and dogs and between dogs of different breeds. Res. Vet. Sci. 71, 45–49. ( 10.1053/rvsc.2001.0483) [DOI] [PubMed] [Google Scholar]
- 145.Paulson SK, Engel L, Reitz B, Bolten S, Burton EG, Maziasz TJ, Yan B, Schoenhard GL. 1999. Evidence for polymorphism in the canine metabolism of the cyclooxygenase 2 inhibitor, celecoxib. Drug Metab. Dispos. 27, 1133–1142. [PubMed] [Google Scholar]
- 146.Gramer I, Leidolf R, Doring B, Klintzsch S, Kramer EM, Yalcin E, Petzinger E, Geyer J. 2011. Breed distribution of the nt230(del4) MDR1 mutation in dogs. Vet. J. 189, 67–71. ( 10.1016/j.tvjl.2010.06.012) [DOI] [PubMed] [Google Scholar]
- 147.Mealey KL, Meurs KM. 2008. Breed distribution of the ABCB1–1Delta (multidrug sensitivity) polymorphism among dogs undergoing ABCB1 genotyping. J. Am. Vet. Med. Assoc. 233, 921–924. ( 10.2460/javma.233.6.921) [DOI] [PubMed] [Google Scholar]
- 148.Mealey KL. 2008. Canine ABCB1 and macrocyclic lactones: heartworm prevention and pharmacogenetics. Vet. Parasitol. 158, 215–222. ( 10.1016/j.vetpar.2008.09.009) [DOI] [PubMed] [Google Scholar]
- 149.Mealey KL, Fidel J, Gay JM, Impellizeri JA, Clifford CA, Bergman PJ. 2008. ABCB1–1Δ polymorphism can predict hematologic toxicity in dogs treated with vincristine. J. Vet. Intern. Med. 22, 996–1000. ( 10.1111/j.1939-1676.2008.0122.x) [DOI] [PubMed] [Google Scholar]
- 150.Munana KR, Nettifee-Osborne JA, Bergman RL, Jr, Mealey KL. 2012. Association between ABCB1 genotype and seizure outcome in Collies with epilepsy. J. Vet. Intern. Med. 26, 1358–1364. ( 10.1111/j.1939-1676.2012.01006.x) [DOI] [PubMed] [Google Scholar]
- 151.Heng HH. 2008. The conflict between complex systems and reductionism. JAMA 300, 1580–1581. ( 10.1001/jama.300.13.1580) [DOI] [PubMed] [Google Scholar]
- 152.Bear HD. 2003. Earlier chemotherapy for breast cancer: perhaps too late but still useful. Ann. Surg. Oncol. 10, 334–335. ( 10.1245/ASO.2003.02.023) [DOI] [PubMed] [Google Scholar]
- 153.Mittra I. 2007. The disconnection between tumor response and survival. Nat. Clin. Pract. Oncol. 4, 203 ( 10.1038/ncponc0772) [DOI] [PubMed] [Google Scholar]