Abstract
While most of this Special Issue is devoted to the testis (which is where most drug and chemically induced toxicity of the male reproductive tract is identified), being able to recognize and understand the potential effects of toxicants on the epididymis is immensely important and an area that is often overlooked. The epididymis is the organ where the post-testicular sperm differentiation occurs, through a complex and still not completely understood sperm maturation process, allowing them to fertilize the oocyte. Also in the epididymis, sperm are stored until ejaculation, while being protected from immunogenic reaction by a blood-epididymis barrier. From a toxicologic perspective the epididymis is inherently complicated as its structure and function can be altered both indirectly and directly. In this review we will discuss the factors that must be considered when attempting to distinguish between indirect and direct epididymal toxicity and highlight what is currently known about mechanisms of epididymal toxicants, using the rat as a reference model. We identify 2 distinguishable signature lesions – one representing androgen deprivation (secondary to Leydig cell toxicity in the testis) and another representing a direct acting toxicant. Other commonly observed alterations will also be shown and discussed. Finally, we point out that many of the key functions of the epididymis can be altered in the absence of a detectable change in tissue structure. Collectively, we hope this will provide pathologists with increased confidence in identification of epididymal toxicity and enable more informed guidance as mechanism of action is considered.
Keywords: androgen, epididymis, histopathology, sperm maturation, toxicity
Abbreviations
- CEMS
Chloroethylmethasulfonate
- CRISP 1
Cysteine-rich secretory protein 1
- DBP
Dibutyl phthalate
- DEHP
Di(2-ethylhexyl) phthalate
- DES
Diethylstilbestrol
- DHT
Dihydrotestosterone
- EDS
Ethane dimethanesulphonate
- ELSPBP 1
Epididymal sperm binding protein 1
- EPI
Epichlorohydrin
- GD
Gestational day
- HFLU
Hydroxyflutamide
- IUI
Intrauterine insemination
- HMG-CoA
3-hydroxy-3-methylglutaryl–coenzyme A
- PAS
Periodic Acid-Schiff
- PND
Postnatal day
- T
Testosterone
- RABP 1 and 2
Retinoic acid binding protein subunits 1 and 2
- SP22
Sperm Protein 22
Signature Phenotype I (Indirect: Androgen Deprivation)
The most notable histological alterations in the epididymis have been documented following complete androgen deprivation subsequent to: 1) castration, 2) implantation of testosterone-estradiol implants, and 3) administration of EDS, a potent Leydig cell toxicant. Other chemicals capable of reducing testosterone biosynthesis by Leydig cells may cause androgen deprivation in the epididymis over time. Significant androgen deprivation results in epithelial apoptosis and reduction in epididymal tubule diameters in all segments of the epididymis. Androgen deprivation also results in decreases in epididymal epithelial cell height in the initial segment and caput. All of these alterations are sustained until androgen levels recover. Finally, another feature of androgen deprivation is the appearance of round spermatids in the lumen of the epididymis (originating from the testis). These cells will have been sloughed from the testis due to the effects of testosterone withdrawal on spermatogenesis. However, since Sertoli cell toxicants may also cause germ cell loss, this should only be used as confirmation if epididymal tubule diameter and epithelial cell height are reduced.
Signature Phenotype II (Direct: Toxicant Action)
Several chemicals have been shown to alter the structure and function of the epididymis directly. Direct toxicity to the epididymal epithelium may result in degeneration, necrosis and exfoliation of principal cells. When this occurs it is generally restricted to a very specific segment of the epididymis, highlighting the importance of examining the entire length of the epididymis In addition, these direct acting epididymal toxicants often alter epithelial cells height, but by increasing, not decreasing the height of the epithelium. These increases in epithelial cell height are most commonly seen in the caput and corpus. It is not uncommon to find that the taller principal cells are paler, contain less supranuclear golgi and/or lipid, and possess a shorter microvillus border. Also, these chemicals often result in a disappearance in clear cells in the cauda epididymis, particularly the proximal cauda. This is well demonstrated in a Periodic Acid-Schiff (PAS) stained section.
Structure and Function of the Epididymis
The epididymis is a single, highly convoluted duct lined by a complex pseudostratified epithelium consisting of multiple cell types, i.e., principal cells, basal cells, clear cells, apical cells, and halo cells.1 The number and appearance of individual cell types varies between segments of the epididymis (i.e., initial segment, caput, corpus, and cauda). Each segment contributes specifically to the luminal microenvironment that is pivotal for testicular sperm to mature by the time they reach the cauda. In the initial segment highly columnar principal cells are responsible for water resorption; ions and small organic molecules are also absorbed. The principal cells in the caput are involved in protein secretion. These proteins adsorb onto the sperm membrane and modify its protein composition. The principal cells in the corpus have abundant lipid in their supranuclear region. These cells may contribute to modification of the lipid component of the sperm plasma membrane. The epithelium in the cauda is much shorter than in the caput/corups segments and clear cells are prevalent. These clear cells phagocytose the cytoplasmic droplets that are shed from the maturing sperm and presumably also phagocytose other luminal debris. In the cauda, excess luminal proteins are resorbed while immobilin is secreted to keep sperm quiescent. Moreover, while not proven, it is likely that the function of the epithelium varies along the length of each histologically distinct segment of the epididymis much like different stages of spermatogenesis occur in a continuum along the length of the seminiferous tubule. This could account for focal lesions within a given epididymal segment.
By the time the sperm reach the proximal cauda in the rat they are capable of fertilization. The post-gonadal process of sperm maturation relies on the microenvironment within the lumen of the epididymis as the infertile sperm entering the epididymis were rendered incapable of protein synthesis during the last stages of spermiogenesis in the testis. Sperm maturation involves a highly orchestrated interaction between the lamina propria surrounding the epididymal epithelium, the epididymal epithelium, the luminal fluid bathing the sperm, and the sperm themselves.2,3 This interaction leads to a luminal microenvironment which facilitates facets of sperm maturation.4-6 Luminal fluid constituents (ions, proteins, lipids) are regulated by secretion and absorption along the length of the epithelium. The process occurs in an orderly fashion, with the acquisition of the capacity for progressive motility preceding the ability to fertilize, which in turn precedes the ability of the fertilized egg to develop normally. One of the most important absorptive events is the uptake of water in the efferent ducts. This results in a significant increase in sperm and protein concentrations by the time the sperm reach the caput segment of the epididymis. This, together with their acquisition of clusterin in the caput sperm, appears to facilitate subsequent migration of the cytoplasmic droplet and increased capacity for motility as sperm transit the epididymal duct.7
The epididymis is highly androgen-dependent with androgen supplied by the testicular fluid entering the duct as well as via the vasculature in the interstitium of the duct. Testosterone (T) and dihydrotestosterone (DHT) are involved in both the maturation and transit of sperm through the duct.8 Androgen-dependent proteins synthesized and secreted by the epididymal epithelium associate with immature sperm in the proximal (i.e., caput) region of the epididymis.9-11 Linking specific epididymal secretions and associated sperm proteins to capacitation and fertilizing ability is problematic as many proteins are normally shed, modified, or un-masked in the female tract. Dacheux & Dacheux7 recently reviewed the luminal fluid and sperm protein literature resulting from the use of new techniques such as genome sequencing, proteomics combined with high-sensitivity mass spectrometry, and gene-knockout approaches. While our knowledge of epididymal region, compartment-specific proteins has increased significantly, the molecular mechanisms involved in both the acquisition of motility and fertilizing ability remain largely a mystery.
Presumably, some of the newly acquired proteins confer progressive motility, while others confer fertilizing ability.12 In the past 3 decades it has been shown that small membranous vesicles secreted by epididymal epithelial cells, i.e., epididymosomes, are involved in this sperm maturation.13-15 Interestingly these epididymosomes have also been shown to transfer a specific sperm binding protein (ELSPBP1) to dead sperm during transit.16 Recently, it was suggested that the cytoplasmic droplets, which are small cytoplasmic residues attached to the sperm after their release fromthe germinal epithelium and which migrates caudally along the sperm during epididymal transit,13 represent a transient organelle that serves as an energy source essential for epididymal sperm maturation.17 These droplets may also serve to store specific proteins of testicular origin prior to their relocalization on the surface of the plasma membrane (e.g., SP22).18
Investigating Toxicity in the Epididymis
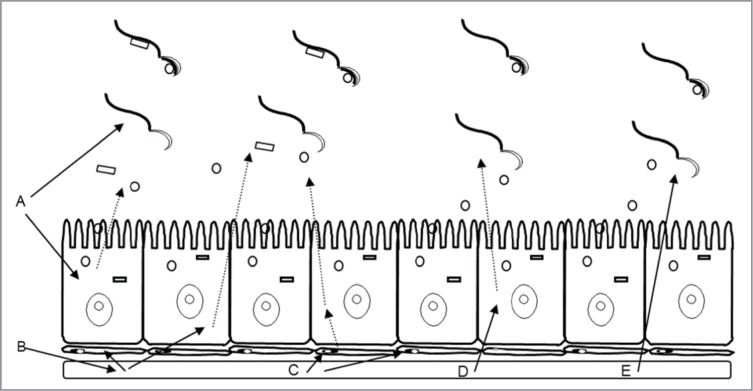
Xenobiotics may alter the processes of sperm maturation by compromising normally occurring sperm membrane changes. As discussed by us in a previous review,19 characterizing toxicity in the epididymis is inherently complicated as its structure and function can be altered both indirectly and directly (Fig. 1). Chemicals that are toxic to the testis can alter the quantity and/or quality of the sperm produced via spermatogenesis and therefore alter the quantity and quality of sperm that enter the epididymis. Toxicant-induced perturbations in testicular fluid (volume or composition) can also alter epididymal epithelial cell function and sperm (Fig. 1A and B). Moreover, chemicals that suppress testosterone production by the Leydig cells will adversely impact the epididymis. Testosterone deprivation reduces the number of qualitatively normal sperm entering the epididymis, as well as causing regression of the epididymal epithelium, and reduces the androgen-dependent facets of sperm maturation. Lastly, chemicals can disrupt the structure and function of the epididymis directly. These direct-acting toxicants can alter the structure and function of the interstitium of the epididymis, the structure and function of the epithelium, and hence the composition of the luminal fluid, and thus the luminal sperm themselves (Fig. 1C–E).
Figure 1.
Schematic depicting multiple direct and indirect means by which a toxicant can perturb the epididymis. A toxicant that alters the sperm or fluid leaving the testis (A); the fluid may in turn alter the epididymal epithelial cell function leading to indirect effects on epididymal sperm. Perturbations in the circulation resulting from a testicular insult (B) might exert direct effects on cells comprising the lamina propria surrounding the epididymis or on the epithelial cells. Altered epithelial cell function could again lead to indirect effects on epididymal sperm. A toxicant in the circulation (C) can exert direct effects on the smooth muscle cells in the lamina propria which can lead to alterations in transit, or indirect effects on sperm mediated through secondary effects on epididymal epithelial cell function. Circulating toxicant can also perturb epididymal epithelial cell function directly (D), and this perturbation could then lead to altered sperm function through compromised secretory activity. Circulating toxicant might also cross the blood–epididymal barrier and exert direct effects on the epididymal sperm thereby compromising their function (E). Adapted from Kempinas, W.G. and Klinefelter, G.R. The Epididymis as a Target for Toxicants. In: Charlene A. McQueen, Comprehensive Toxicology, volume 11, p. 152, 2010. Oxford: Academic Press (with permission).
Remarkably, despite the diversity of the chemicals which perturb the epididymis, there are numerous common manifestations. First, as mentioned above, epididymal toxicants frequently produce epididymis-specific alterations in epididymal sperm numbers (i.e., accelerated transit time). This can occur via suppression of androgen synthesis in the testis (e.g., EDS) or suppression that results from direct inhibition of the androgen receptor in the epididymis (e.g. hydroxyflutamide). Second, we and others20 have observed that several direct acting epididymal toxicants reduce the number of clear cells in the proximal cauda epididymis and even initiate a remodeling of the epithelium in the corpus following exposure to EDS. Third, specific secretory and sperm-associated proteins appear to be sensitive to direct-acting chemicals. The secretion of secretory proteins such as clusterin α and β and proteins B/C (RABP 1 and 2) was reduced following treatment with EDS, as was the sperm biomarker of fertility, SP22.
The following toxicants cause histological alterations in the epididymis of rats: cadmium,21,22 benzimidazole carbonate,23 estradiol,24 diethylstilbestrol (DES),25,26 ethane dimethanesulphonate (EDS),27,28 chloroethylmethasulfonate (CEMS),29 α-chlorodydrin,30,31 ornidazole,32,33 epichlorohydrin (EPI),34,35 6-chloro-6-deoxyglucose,36 flutamide37 and hydroxyflutamide (HFLUT),38 methyl chloride,39-41 cyclophosphamide,42,43 dibutyl phthalate,44 and gossypol.45
To completely study the structure and function of the epididymis following a toxic insult, multiple epididymal segments (e.g., caput/corpus vs cauda) and compartments (e.g sperm vs epithelium) must be evaluated. Thus, to thoroughly “tease out” toxicity, multiple experimental strategies might be employed. Typical initial studies in the adult rat usually involve continuous daily exposure for 70 d to ensure the entire process of spermatogenesis and epididymal sperm maturation is covered. Lesions detected in the epididymis may be suspected as being indirect (i.e., germ cells in the lumen, reduction in epithelial cell height), or direct (i.e., altered epididymal epithelial morphology) at this point. Indirect action on the epididymis can be confirmed by serum and testicular fluid testosterone assay and testicular histology. Confirming direct action on the epididymis relies on our knowledge of epididymal transit time. Since sperm in the caput epididymidis require approximately 4 d to reach the proximal cauda epididymidis under normal conditions,46 sperm that are compromised during their maturational sojourn from the caput to the proximal cauda will most likely be detected in the proximal cauda on day 5.Not only is the proximal cauda epididymidis the site where fertilizing ability is first observed6,8 under normal circumstances, but the sperm in this region are more homogeneous with respect to their residence time compared to those in the distal cauda. Thus, a 5-day exposure protocol is used to study direct effects on the epididymis, diminishing the possibility that testicular factors may be involved.28
Whether alterations in the epididymis are direct or indirect, epididymal sperm quality can be assessed using intrauterine insemination (IUI) (see Kempinas & Klinefelter19 for details).For this, a fixed, optimal number of sperm from the proximal cauda epididymis of rats is inseminated into the uterine horns of receptive females. Klinefelter47 summarizes studies in which significant decreases in fertility either on gestation day 9 (i.e., viable implants / corpora lutea) or day 20 were detected at doses of toxicants acting on the epididymides or testes that would not have resulted in subfertility via natural mating. By using IUI, fertility was itself more sensitive than conventional measures of sperm quality (i.e.% motile sperm,% normal sperm) or sperm quantity (i.e., cauda epididymal reserves). Subsequently, other IUI studies with rats have been conducted to evaluate sperm quality. Exposures which accelerate epididymal transit do compromise sperm quality.48 It was concluded that androgen deprivation accelerated transit and compromised normal facets of sperm maturation in the epididymis. Fernandez et al.49 confirmed these results using diethylstilbestrol (DES) administered with or without exogenous androgen supplementation. Epididymal transit time was accelerated and the fertility of cauda epididymal sperm was compromised in the DES-exposed animals; exogenous androgen ablated these effects.From a histopathology perspective it is important to recognize that when epdidymal sperm transit is accelerated, sperm numbers in the cauda are significantly reduced compared to control. Additionally, if IUI were performed and the histopathologist has knowledge that fertility was decreased one should examine luminal sperm for evidence of sperm clumping via tails and sperm head fusion.
General Considerations on Performing Histopathology in the Epididymis
It is not the aim of this review to present a morphofunctional characterization of the epididymis. There are excellent, comprehensive reviews and book chapters on this subject to which the reader can refer.6,50 In addition, there are excellent, recent reviews on how to collect, process and analyze the epididymis for histopathological purposes.51-55 However, we would like to reinforce some aspects already highlighted by these authors:
Age of the animals used in the experiments. In rats aged 8–9 weeks the epididymis contains increased numbers of degenerating germ cells and has relatively few sperm in the cauda epididymis (Figs. 2 and 3). These features can be mistaken as evidence of testicular toxicity and reduced spermatogenesis. To overcome this problem, rats should be at least 10 weeks of age by the end of the study. On the other hand, one common finding in the epididymis of aged control rats is vacuolization, which can be interpreted as organ toxicity (Fig. 3).
Examine longitudinal sections of the organ for histopathology. Sampling of the epididymis should include all regions (initial segment, caput, corpus, cauda, and junction with the vas deferens). This is best achieved by taking a longitudinal section through the entire organ (Fig. 4). Lesions are often region-specific in the epididymis and sperm density varies significantly depending on location, so consistent and complete sampling is important.
Epididymal histopathology should accompany testicular histopathology since the epididymis can provide important information on recent testicular events.
Luminal contents in the epididymis can alert the pathologist to either indirect (spermatids) or direct (principal, clear cells) toxicity.
Figure 2.
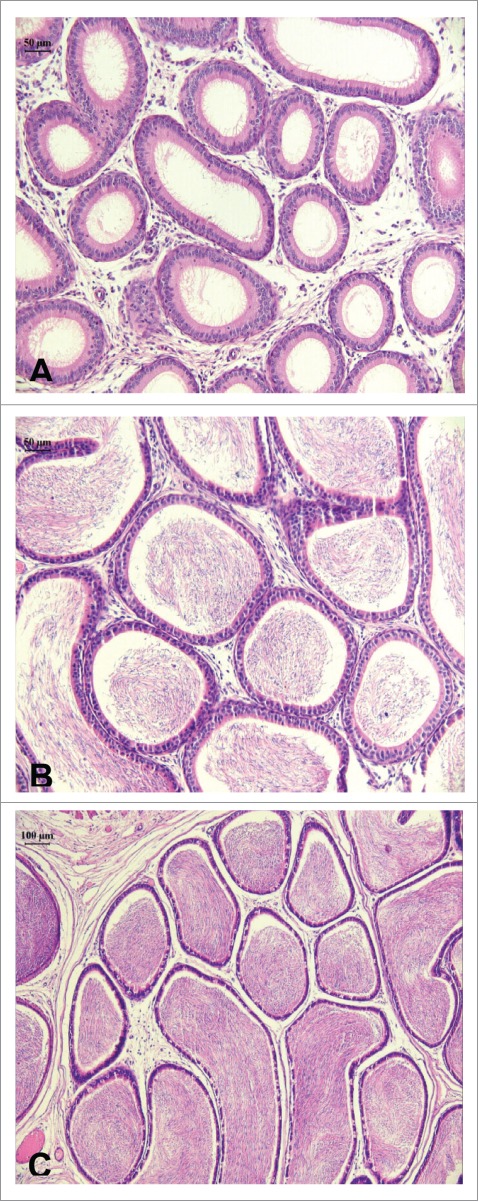
Photomicrographs illustrating longitudinal cuts of the cauda epididymis of rats. (A) (20×) 40 d of age (pre-puberty) in which is observed epididymal duct without germ cells; (B) (20×) 60 d of age (puberty), with the presence of sperm cells in the lumen; (C) (10×) 90 d of age (sexual maturity). Epididymal duct with great quantity of spermatozoids. H&E.
Figure 3.
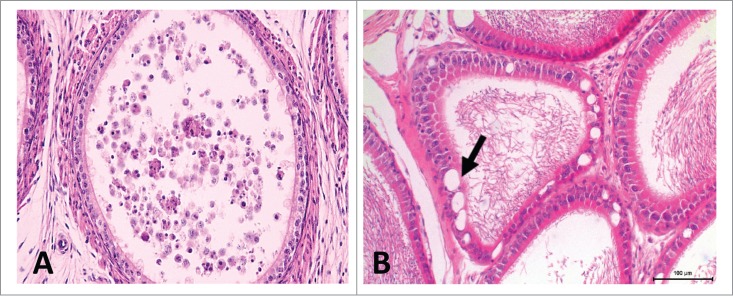
Rat epididymis. (A) Prepubertal rat. Section of the proximal cauda. Notice the large amount of exfoliated germ cells and round bodies in the lumen. (B) Aged rat (9 months old). Section of the proximal cauda, depicting vacuoles (arrow). H&E, 20×.
Figure 4.
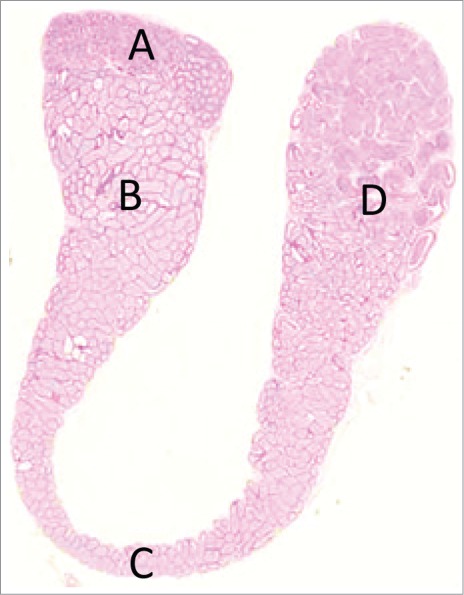
Panoramic view of a longitudinal section of the rat epididymis. (A) Initial segment. (B) Caput. (C) Corpus, (D) Cauda epididymis. H&E.
Indirect Androgen Deprivation
Androgen deprivation during development
Spermatogenesis and steroidogenesis are not yet completely established until puberty, which makes the male genital system more susceptible to the action of chemical agents when the exposure occurs early in life.56,57 Many of these substances interfere with androgen production or signaling and can delay the development of the male genital system, with effects being dependent on the time of exposure.58 Exposure to chemical agents that can alter reproductive function during this period is highly important to reproductive toxicology,59 given that changes in puberty can also alter reproductive function during sexual maturity.60-62
Intrauterine androgen deprivation
Studies in laboratory animals have shown that exposure to high doses of dibutyl phthalate (DBP) during the critical period of male reproductive development, i.e. gestation day (GD) 12–19, results in remarkable phenotypic alterations in normal development.63,64 At adulthood the phenotypes included: cryptorchidism, epididymal agenesis, testicular atrophy with germ cell loss, hypospadias, and absent or smaller seminal vesicles and prostate. These phenotypes can be linked to androgen deprivation during the critical period of sexual differentiation.65 Dams dosed daily with 300 mg/kg/day di(2-ethylhexyl) phthalate (DEHP) from GD 8 until day 17 of lactation also sired pups with malformed epididymis. The aspect of a malformed epididymis after intrauterine exposure to 500 mg/Kg DBP is shown in Figure 5.
Figure 5.
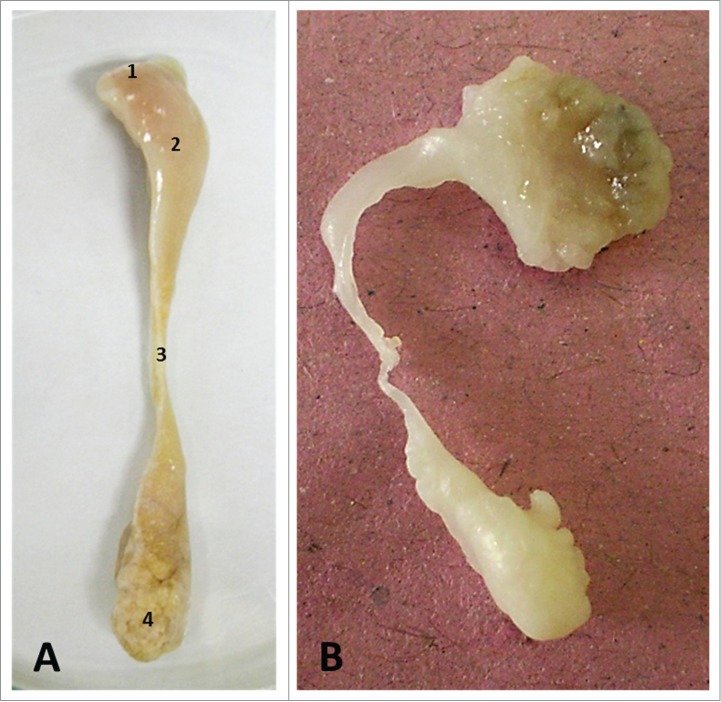
Gross anatomy of the rat epididymis. (A) Normal aspect. (B) Malformed. 1) initial segment, 2) caput, 3) corpus, 4) cauda epididymidis.
Prepubertal androgen deprivation
The effects of antiandrogen exposure during the prepubertal period on the initial sexual development and morphological aspect of the testis and epididymis was analyzed in juvenile rats exposed to 3 or 10 mg/kg/day of rosuvastatin (an HMG-CoA reductase inhibitor decreasing total cholesterol and triglycerides) since PND 21 until puberty onset. In the rosuvastatin-treatedgroups, the results demonstrated a trend toward a decrease in testosterone concentration, but belowthe significance level, as well as delays in both the age of puberty onset and in epididymal development, as shown by the cribriform, less differentiated aspect of the epithelium of the epididymal proximal cauda. There were also testicular alterations, such as seminiferous tubules with acidophilic spermatogonia and spermatocytes, especially in stages IX-XIV, that might be related to delayed puberty and decrease of serum testosterone.66
Androgen deprivation in the adult
The epididymis depends on androgen to maintain normal epithelial architecture and function, which in turn are necessary for normal facets of sperm transport and maturation. The epididymis will undergo atrophy if androgen stimulationis decreased.67 There is an increasing number of chemicals (e.g., ketoconazole, prochloraz, bisphenol A, cadmium chloride, statins) known or suspected to reduce testosterone production significantly by testicular Leydig cells. Continued exposure to any chemical which reduces testosterone significantly is likely to result in some degree of histologic alteration in the androgen-dependent epididymis. Inhibitors of steroid biosynthesis, such as ketoconazole, will result in testicular changes as well as epididymal atrophy, but androgen receptor antagonists such as flutamide or inhibitors of 5a-reductase (which converts testosterone to dihydrotestosterone) cause epididymal atrophy in the absence of any discernable morphological effects on the testis since they are directly compromising androgen dependent function in the epididymis.68 The most notable histological alterations in the epididymis have been documented following complete androgen deprivation subsequent to: 1) castration, 2) implantation of testosterone-estradiol implants, and 3) administration of EDS, a potent Leydig cell toxicant. Castration results in reduction in epididymal tubule diameters of 31%, 14%, 13%, and 43% of control in the initial segment, caput, corpus, and cauda, respectively.69 This effect persists without androgen replacement. Moreover, castration results in decreases in epididymal epithelial cell height in the initial segment and caput, but no decrease in the corpus, and an increase in the cauda. These alterations were also sustained without androgen replacement. This segmental atrophy of the epididymis at the junction between the corpus and cauda is sometimes considered a background finding in rodents, however regression of the epididymal duct/epithelium is a common finding in aged rodents with accompanying degeneration resulting from androgen deprivation.70 Studies using testosterone-estradiol implants to achieve androgen withdrawal also reported the presence of round spermatids in the lumen epididymal duct within 3 weeks.71 As early as 7 d after administration of a single dose of EDS round spermatids can be found in the lumen of the epididymis.72 The number of mature sperm contained in the epididymis is decreased 60% by day 7 and 80% by day 12 following EDS exposure. Moreover, neutrophilic nuclei were evident within the lumen by day 12. Early studies with EDS reported that when males received 5, daily 50 mg/kg i.p. injections and were allowed to mate naturally with untreated females, fertility was reduced 2 to 3 weeks later,27 suggesting epididymal toxicity, which was shown later by,29 both at the structural and functional levels. For their clear role in decreasing testosterone levels, EDS and CEMS are signature chemicals for indirect effects of androgen depletion in the epididymis. However, as we discuss later these chemicals are also direct acting epididymal toxicants.
Whether androgen deprivation is achieved by orchiectomy, ligation, obstructive infertility, and/or chemical deprivation by GnRH-agonists, -antagonists, and/or antiandrogens, the atrophic alterations in the epididymis resulting from insufficient androgen are remarkably similar regardless of species. For instance, the same degree of cellular disorganization, of cytoplasmic atrophy and consequent reduction of epithelial height, especially in the proximal caput region, and loss of stereocilia, and of alterations in size, shape, and arrangement of the nuclei seen in the chimpanzee epididymis were essentially identical to those reported for other species.73 Moreover, the consistency of these histological findings across species lends confidence to the conclusion that the alterations reported do indeed result from androgen withdrawal, rather than from differences in handling, fixation, or the mechanism used to suppress circulating testosterone.
Direct Toxicant Action
Among the first chemicals associated with epididymal toxicity were α-chlorohydrin, methyl chloride,39-41 cyclosphosphamide 42,43and ornidazole.32,33 The antifertility effects of these toxicants occurred within only a few days of dosing and were associated with granuloma formation, alterations in the structure or function of the lamina propria, the epithelial cells and/or the sperm (reviewed in Kempinas & Klinefelter).19
Both EDS and CEMSwere shown to elicit profound structural and functional changes in the epididymis within 4 d of treatment.28,29 Changes which appeared to be unrelated to decreased circulating androgen level or possible alterations in testicular fluid included an epididymis-specific reduction in the number of cauda epididymal sperm, diminution in specific proteins from the profile of proteins in detergent extracts of cauda sperm, and a disappearance in the number of clear cells observed in the cauda epithelium. CEMS exposure resulted in a significantly increased epithelial cell height in both the corpus and proximal cauda. EDS produced a striking remodeling of the corpus epithelium in which principal cells with short microvili were taller, paler, and lacked lipid (Fig. 6). Each of these chemicals resulted in significant reductions in the fertility of proximal cauda epididymal sperm as assessed by in utero insemination. Two other chemicals which reduced the fertility of sperm from the proximal cauda epididymis within 4 d of exposure were epichlorohydrin (EPI) and hydroxyflutamide (HFLUT).48 For this reason EDS, CEMS, EPI and HFLUT are the signature chemicals for direct toxicant action in the epididymis. As discussed earlier, EDS and CEMS may also elicit indirect effects on the epididymis, via androgen depletion. However, only a few alterations, i.e., epididymal weight, some sperm membrane proteins and certain sperm motion parameters, were rescued by maintaining circulating androgen levels.
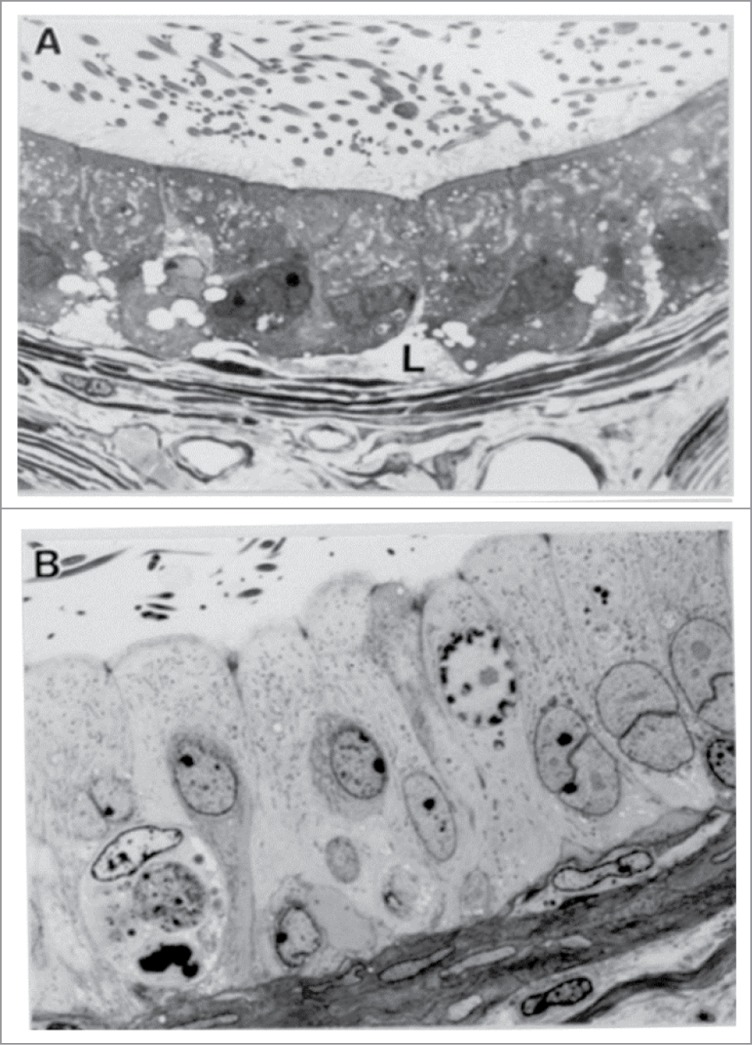
Figure 6.
Light micrographs revealing dramatic changes in the histology of the corpus epididymidis. In the corpus epididymidis of controls (A), the principal cells contain a significant amount of infranuclear lipid (L). In contrast, 4 d after a single injection of EDS (B), these cells are taller, possess a paler cytoplasm without lipid vacuoles, and contain aberrant nuclear figures. From Kempinas, W.G. and Klinefelter, G.R. The Epididymis as a Target for Toxicants. In: Charlene A. McQueen, Comprehensive Toxicology, volume 11, p. 152, 2010. Oxford: Academic Press (with permission).
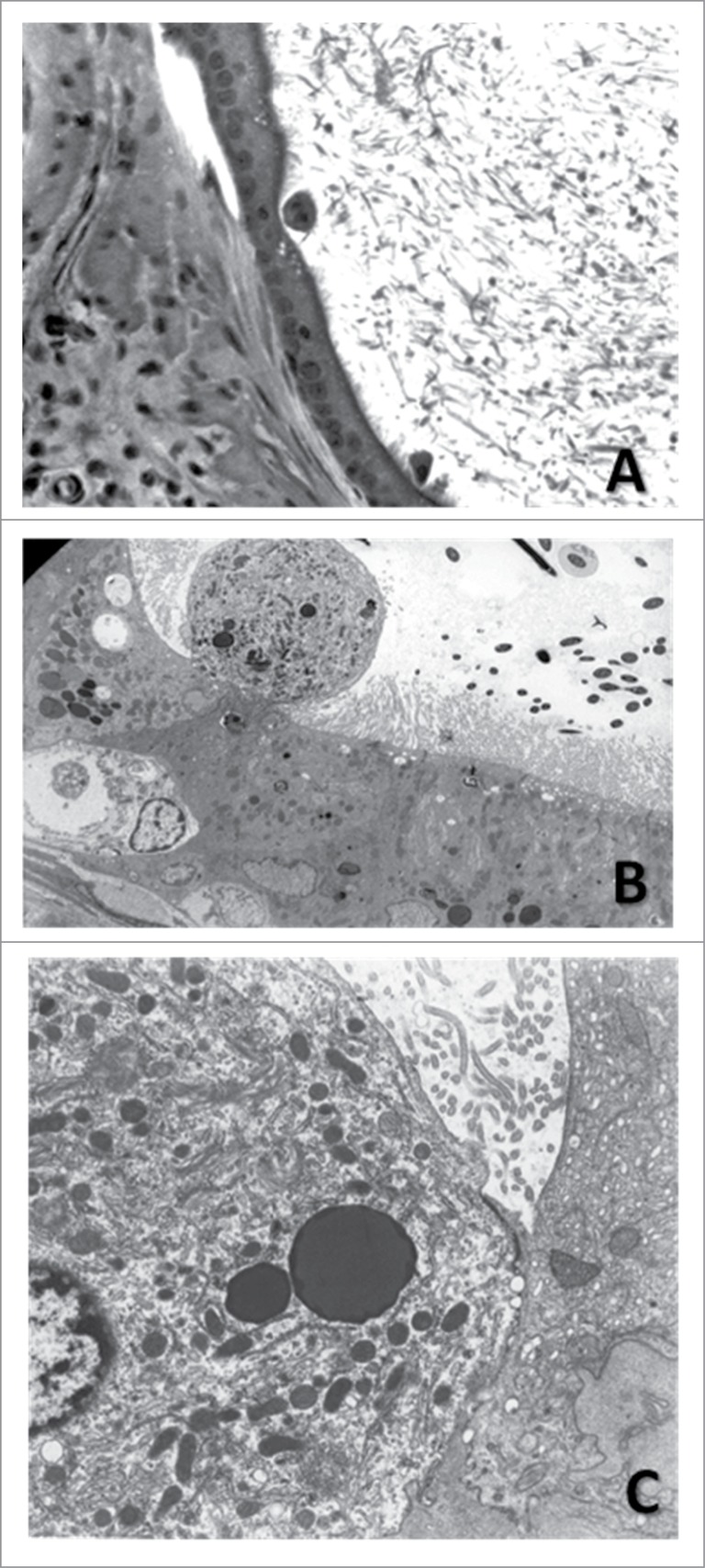
The presence of exfoliated material in the epididymal lumen has been linked to testicular toxicity. Romualdo et al.,74 performing an experiment to investigate epididymal toxicity of gossypol, a compound with antifertility properties extracted from cotton seeds, verified a higher frequency of round structures in the lumen of the cauda epididymidis in the treated rats. That these cells immunostained for protein E, known as cysteine-rich secretory protein (CRISP)-1, a marker of epididymal principal cells, stimulated a subsequent work, in which gossypol-treated rats were hemicastrated 7 d before euthanasia. In theory, after 7 d the round structures should not be observed in regions proximal to distal cauda epididymidis, unless they originate in the epididymis.45 However, these structures were observed, and ultrastructural examination revealed that these represented: 1) principal cells exfoliated from the epididymal epithelium (Fig. 7); 2) epididymal epithelial cell cytoplasm containing degenerating sperm; and 3) degenerating epithelial cells, consisting of vesicles and particles of different sizes, forms and densities. Taken together, the data confirm that gossypol targets the epididymis in addition to the testis, disturbing both the structure and function of this organ, and presumably disrupts sperm maturation.
Figure 7.
Exfoliating principal cell in the cauda epididymidis of a hemicastrated rat treated with gossypol. (A) 20×, H&E. (B) Electron micrograph of the epididymal epithelium showing the exfoliation of a principal cell (2750×). (C) Cell indicated in B in greater detail. Notice the numerous mitochondria and lysosomes characteristic of principal cells (8000×). Adapted from Andrade et al. (2006).
Other Commonly Observed Alterations
Detailed description and illustration of epididymal lesions can be found at.51-55
Debris/germ cells in the epididymal lumen
The lumen of a normal adult rat epididymis contains very few sloughed germ cells or cellular debris. The most common source of cells and cellular debris in the lumen of the epididymis occurs from sloughed germ cells secondary to spermatogenic disturbances or androgen deprivation (causing sloughing of round spermatids) in the testis (Fig. 3). However, as discussed above, some toxicants e.g., gossypol, can result in sloughing of principal cells into the lumen of the epididymis and so it is important to distinguish the origin of the cell debris, since it may be derived from a direct toxicity in the epididymis. In addition, care should be taken not to confuse epididymal apocrine cytoplasmic blebs, originating from the normal process of apocrine secretion by the efferent duct or epididymal epithelium, with the cytoplasm of sloughed testicular germ cells. To confirm the origin of the exfoliated cells, one can ligate the efferent ducts, use androgen replacement or hemi-castrate the animals, or use IHC for epididymis-specific proteins (i.e. CRISP, RABP, Clusterin), or perform electron microscopy.45
Cribriform change
Cribriform change is a hyperplastic alteration in the epithelium. The hyperplastic epithelium often extends to the point of folding on itself and forms pseudoglandular structures (Fig. 8). Cribriform change has been associated with testicular toxicities in rats. The infolding of the epithelium may be a secondary response to lack of sperm cells and testicular fluid, and epididymis atrophy, leading to a disruption of the epididymal microenvironment. It is often anecdotally related to androgen depletion, aging, testicular atrophy, cryptorchidism, and germ cell tumors. In reality, there is no definitive cause of cribriform change in the epididymis. In a study of 167 human samples retrieved following orchiectomy performed for both germ cell and non-germ cell tumors, as well as non-tumor conditions such as inflammation, undescended testis, and cysts, there was a 42% incidence of cribriform hyperplasia in the epididymis; most of these were associated with tumor pathology in the testis.75 However,cribriform hyperplasia should not be mistaken for carcinoma in situ, and thus erroneously connoting a tumor as primary in the epididymis. The frequently focal nature of the atypia and the absence of mitotic activity are useful in distinguishing cribriform hyperplasia from a neoplastic process.
Figure 8.
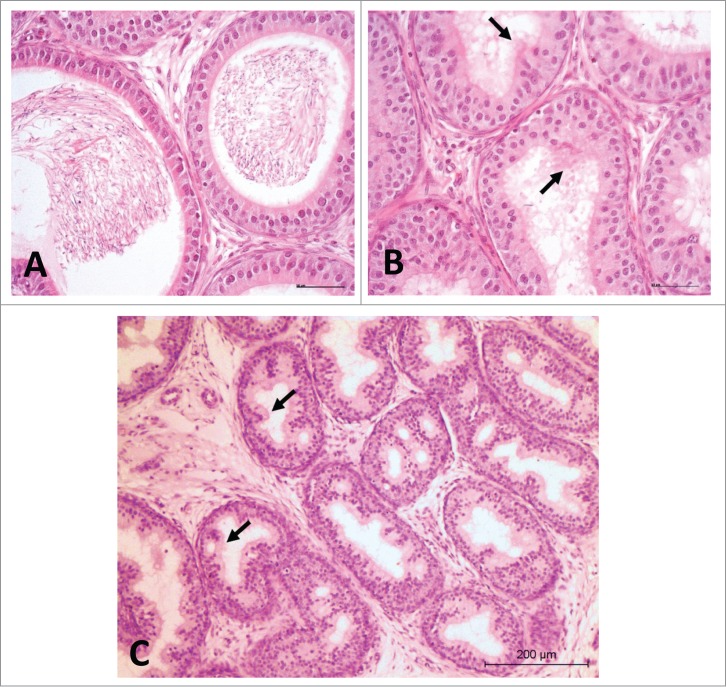
Rat proximal cauda epididymis. H&E. (A) Normal aspect, 20×. (B and C) Cribriform change (arrows); notice the lumen devoid of sperm, 20× and 10×, respectively.
The most prevalent lesion seen in these human samples, intranuclear eosinophilic inclusions, were observed in 73% of all samples. These were not associated with any particular disease state.
Inflammatory infiltrates
Inflammatory infiltrates are commonly observed in the interstitium or in the adjacent adipose tissue of animals (Fig. 9). These infiltrates frequently consist of neutrophils or lymphocytes. Granulomas manifest more significant forms of inflammation (Fig. 10). These can occur anywhere in the epididymis, but are most frequently seen in the distal corpus or caud1. An epididymal spermatic granuloma can be intratubular or extend into the surrounding interstitium. The typical inflammatory population of a granuloma includes macrophages, polymorphonuclear leukocytes, fibroblasts and sperm. The epididymal epithelium will attempt to epithelialize intratubular granulomas. The etiology of the inflammatory changes, including granulomas, is unknown. The most popular belief is that there is a rupture of the epididymal tubule resulting from increased intraluminal pressure, and eruption of the sperm from the break into the interstitium followed by a responsive inflammation which forms a nodule encapsulating the sperm.76 However, it is also plausible to consider that inflammatory changes can be driven by an imbalance in the dynamic equilibrium between immune tolerance and toxicant mediated ‘activation’ of inflammation in the epididymis (also see Gregory and Cyr, this issue). Methyl chloride is an example of a well known chemical provoking sperm granulomas.39-41
Figure 9.
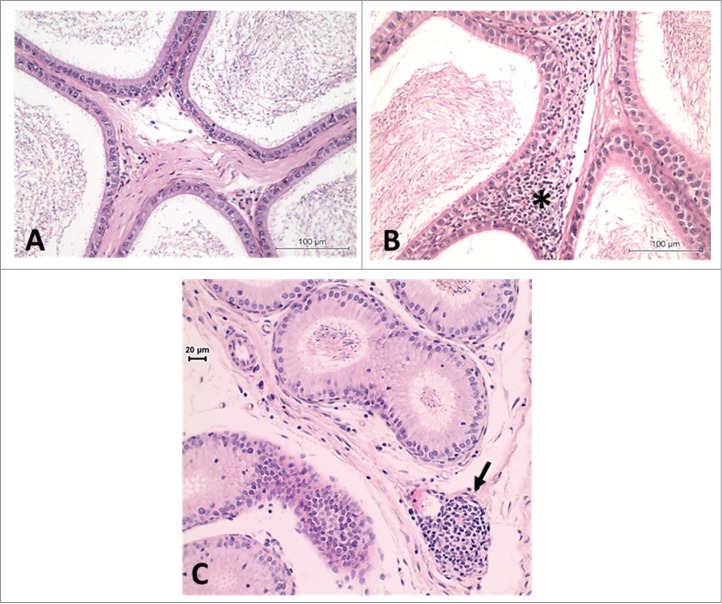
Rat epididymis. (A) Normal aspect of the proximal cauda. (B) Inflammatory infiltrate in the proximal cauda (asterisk). (C) Inflammatory infiltrate in the caput region (arrow). H&E, 20×.
Figure 10.
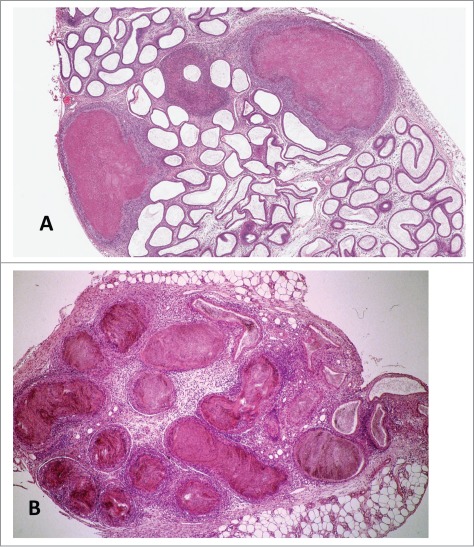
Low power image of (A) distal corpus of the rat epididymis exhibiting multiple sperm granulomas. H&E. (B) Efferent ducts and initial segment exhibiting sperm stasis with leukocyte infiltration in the interstitium and the onset of granuloma formation. H&E.
Epithelial vacuolation
Vacuolation may be diffuse, but more often is localized to a specific segment of the epididymis. Microvacuolation is characterized by small, intracytoplasmic vacuoles often in the apical aspect of the cell (Fig. 2). Macrovacuolation is characterized by the presence of large, clear vacuoles within and between epithelial cells that may disrupt the epithelial alignment and displace the cytoplasm and nucleus to the periphery of the cell. These are often present in the proximal caudal region and are a common age-related change in rats.70,77 However, it is important to recognize that suboptimal fixation and unintended extraction of lipid can result in variable numbers of vacuoles appearing in the resulting sections, particularly in the subcapsular sections of the duct. Vacuolation of the epididymal epithelium may result from a number of degenerative changes including accumulation of fluids, lipids, phospholipids, and glycoproteins. For examples of toxicants producing the phospholipidosis mediated vacuolation (Fig. 11) see the effects of dopamine D3 selective antagonist PNU-17786478 and the antibiotic salinomycin, used in animal husbandry.79
Figure 11.
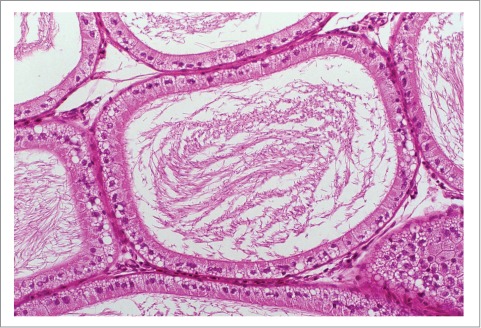
Phospholipidosis in the caput of the rat epididyimis. Note the frothy lipid vacuolization in the supranuclear region of the principal cells.
Increase or decrease in clear cells
Disappearence of the clear cells in the cauda epididymis was reported by Klinefelter et al.,28 after 4 d of treatment with CEMS or EDS. On the other hand, increased numbers of clear cells often accompany increased debris in the lumen (Fig. 12). In these situations the clear cells become more obvious because they contain a lot more lysosomes because they are undergoing a lot more endocytosis, which is just a secondary consequence of all the debris in the lumen. For instance, hyperplasia/hypertrophy of clear cells in the cauda epididymis was reported by de Andrade et al.45 after treatment with gossypol. In this case, clear cell hypertrophy was more evident as there was likely increased endocytosis of exfoliated principal cells. There is morphological evidence that spermiophagy occurs, under normal conditions, by cells lining the excurrent ducts.1 When counting clear cells to determine their relative frequency, refer to Klinefelter et al.28 for methods. In general, these should be indexed to the number of principal cell nuclei; at least 100 principal cells should be counted to obtain a confident assessment of clear cell frequency.
Figure 12.
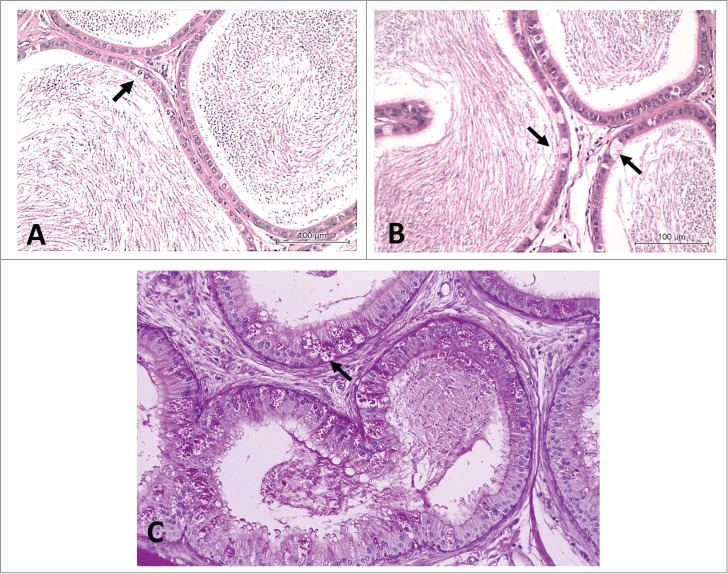
Rat cauda epididymidis. (A) Normal aspect. (B) Hyperplasia of clear cells (arrows). H&E, 20×. (C) Hyperplasia of clear cells. PAS, 40×.
Sperm compaction
Sperm stasis may occur in the efferent ducts as a result of increased fluid resorption or due to impaction of sperm in blind-ending ducts. It may also occur in the cauda epididymis when sperm are not being produced by the testis or removed from the cauda effectively (e.g., obstruction of the vas deferens or significantly decreased epididymal transit time). Sperm stasis generally incites an inflammatory response with time.80 Movement of sperm through the efferent ducts and epididymis is dependent on the correct fluid dynamics (fluid production by the Sertoli cell and fluid reabsorption by the efferent ducts) and also on smooth muscle contraction within the epididymis and vas deferens. Disturbances in either of these processes or obstruction of any part of the duct system can lead to sperm stasis, which in turn often leads to inflammation and/or sperm granulomas (also see Hess, this issue). Sperm stasis can occur in the cauda epididymis from a variety of causes including blockade of adrenergic pathways by α-adrenergic antagonists such as prazosin and guanethidine (for review, see Klinefelter18).
Proliferative lesions of the epididymis
Apart from cribriform change, literature reports of primary proliferative lesions of the epididymis are almost non-existent. Mechanisms favoring metaplasia, such as angiogenic factors, are blocked in the epididymal epithelium. Expression of the main junction-associated proteins (occludins, claudins) is altered in cancerous tissue.81 Tight junctions in the epididymis are the physical component of the blood–epididymis barrier. Stable and persistent tight junctions may be one component of epididymal resistance to cancer.82
Changes in sperm parameters (motility, morphology or density) absent histological alteration
In contrast to testicular toxicants that result in delayed effects on fertility, damage to epididymal sperm will have an almost immediate impact on fertility parameters. It should also be emphasized that toxic effects on sperm can occur in the absence of any histopathological changes in the epididymis or testes and may be detectable only by direct sperm analysis. In recent years many environmental chemicals have been associated with decreased numbers of sperm stored in the cauda epididymis, with little or no reduction in sperm production.38,83,84 This suggests that the epididymis is the functional target of these toxic substances, and that the transit of sperm through the epididymis is accelerated by these exposures38 It is important to note that while transit time is accelerated during androgen deprivation or increased estradiol exposure, we found the direct acting epididymal toxicants, specifically CEMS and HFLUT, accelerated sperm transit when circulating androgen levels were maintained. We speculate that CEMS, like HFLUT, was interfering with an epididymal androgen-dependent function. We also speculate that the acceleration of sperm transit reduces the time available for the processes necessary for the maturation of sperm, which can compromise the function of the resulting sperm.18,49,85
Conclusions
Although toxic effects in the epididymis might be secondary to a testicular lesion, in this chapter we show that direct pathologic effects can occur in the epididymis, and the pathologist must be well trained to interpret the difference.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the co-authors of our articles cited in this review, especially the students. We are also grateful to Cibele dos Santos Borges for her help editing the long list of references and Dr. Dianne Creasy for providing Figures 10, 11 and 12C.
References
- 1. Robaire B, Hermo LL. Efferent Ducts, epididymis and vas deferens: structure, functions, and their regulation. In: Knobil E, Neils JD, eds. The Physiology of Reproduction. Raven Press New York, 1988:999-1080. [Google Scholar]
- 2. Bedford JM. Maturation, Transport, and Fate of Spermatozoa in the Epididymis. Washington: Hamilton Physiological Society; 1975:303-18. [Google Scholar]
- 3. Orgebin-Crist MC, Danzo BJ, Davies J. Endocrine control of the development and maintenance of fertilizing ability in the epididymis. In: Hamilton DW, Greep RO, eds. Handbook of Physiology, Male Reproductive System. Washington: American Physiology Society, 1975:319-38. [Google Scholar]
- 4. Orgebin-Crist MC. Sperm maturation in rabbit epididymis. Nature 1967; 216:816-8;PMID:6074957; http://dx.doi.org/ 10.1038/216816a0 [DOI] [PubMed] [Google Scholar]
- 5. Turner TT. On the epididymis and its role in the development of the fertile ejaculate. J Androl 1995; 16:292-8; PMID:8537245 [PubMed] [Google Scholar]
- 6. Robaire B, Hinton BT, Orgebin-Crist MC. The Epididymis. In: Neill JD, ed. Knobil and Neill´s Physiology of Reproduction. New York: Elsevier, 2006; 1071-148. [Google Scholar]
- 7. Dacheux JL, Dacheux F. New insights into epididymal function in relation to sperm maturation. Reproduction 2014; 147:R27-42; PMID:24218627; http://dx.doi.org/ 10.1530/REP-13-0420 [DOI] [PubMed] [Google Scholar]
- 8. Dyson AL, Orgebin-Crist MC. Effect of hypophysectomy, castration and androgen replacement upon the fertilizing ability of rat epididymal spermatozoa. Endocrinology 1973; 93:391-402; PMID:4718882; http://dx.doi.org/ 10.1210/endo-93-2-391 [DOI] [PubMed] [Google Scholar]
- 9. Klinefelter GR, Hamilton DW. Synthesis and secretion of proteins by perifused caput epididymal tubules, and association of secreted proteins with spermatozoa. Biol Reprod 1985; 33:1017-27; PMID:4084628; http://dx.doi.org/ 10.1095/biolreprod33.4.1017 [DOI] [PubMed] [Google Scholar]
- 10. Moore HDM, Smith CA, Hartman TD. In: Orgebin-Crist MC, Danzo BJ, eds. Cell Biology of the Testis and Epididymis. New York: New York Academy of Sciences, 1987. [Google Scholar]
- 11. Dacheux JL, Dauchex F. Protein secretion in the epididymis. In: Robaire B, Hinton BT, eds. The Epididymis: from Molecules to Clinical Pratice. New York: Kluwer Academic Plenum Publishers, 2002, 152-8. [Google Scholar]
- 12. Orgebin-Crist MC, Jahad N. The maturation of rabbit epididymal spermatozoa in organ culture: inhibition by antiandrogens and inhibitors of ribonucleic acid and protein synthesis. Endocrinology 1978; 103:46-53; PMID:744083; http://dx.doi.org/ 10.1210/endo-103-1-46 [DOI] [PubMed] [Google Scholar]
- 13. Gatti JL, Castella S, Dacheux F, Ecroyd H, Metayer S, Thimon V, Dacheux JL. Post-testicular sperm environment and fertility. Anim Reprod Sci 2004; 82-83:321-39; PMID:15271463; http://dx.doi.org/ 10.1016/j.anireprosci.2004.05.011 [DOI] [PubMed] [Google Scholar]
- 14. Sullivan R, Frenette G, Girouard J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl 2007; 9:483-91; PMID:17589785; http://dx.doi.org/ 10.1111/j.1745-7262.2007.00281.x [DOI] [PubMed] [Google Scholar]
- 15. Sullivan R, Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction 2013; 146:R21-35; PMID:23613619; http://dx.doi.org/ 10.1530/REP-13-0058 [DOI] [PubMed] [Google Scholar]
- 16. D’Amours O, Frenette G, Bordeleau LJ, Allard N, Leclerc P, Blondin P, Sullivan R. Epididymosomes transfer epididymal sperm binding protein 1 (ELSPBP1) to dead spermatozoa during epididymal transit in bovine. Biol Reprod 2012; 87:94; PMID:22875906; http://dx.doi.org/ 10.1095/biolreprod.112.100990 [DOI] [PubMed] [Google Scholar]
- 17. Yuan S, Zheng H, Zheng Z, Yan W. Proteomic analyses reveal a role of cytoplasmic droplets as an energy source during epididymal sperm maturation. PloS One 2013; 8:e77466; PMID:24155961; http://dx.doi.org/ 10.1371/journal.pone.0077466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klinefelter GR, Strader LF, Suarez JD, Roberts NL. Bromochloroacetic acid exerts qualitative effects on rat sperm: implications for a novel biomarker. Toxicol Sci: Off J Soc Toxicol 2002; 68:164-73; PMID:12075119; http://dx.doi.org/ 10.1093/toxsci/68.1.164 [DOI] [PubMed] [Google Scholar]
- 19. Kempinas WG, Klinefelter GR. The Epididymis as a Target for Toxicants. In: McQueen CA, ed. Comprehensive Toxicology. Oxford: Academic Press, 2010; 149-66. [Google Scholar]
- 20. Liu X, Temple-Smith PD, Risbridger GP. Acute effects of ethane dimethane sulfonate (EDS) on the structure of the cauda epididymidis in the rat: selective destruction of clear cells in the proximal cauda region. Reprod, Fert, Dev 1993; 5:295-306; PMID:8272534; http://dx.doi.org/ 10.1071/RD9930295 [DOI] [PubMed] [Google Scholar]
- 21. Mason KE, Young JO. Effects of cadmium upon the excurrent duct system of the rat testis. Anat Rec 1967; 159:311-24; PMID:6082103; http://dx.doi.org/ 10.1002/ar.1091590308 [DOI] [PubMed] [Google Scholar]
- 22. Nagy F. Cadmium-induced alterations in the Siberian hamster epididymis. Arch Androl 1985; 15:91-104; PMID:3833082; http://dx.doi.org/ 10.3109/01485018508986897 [DOI] [PubMed] [Google Scholar]
- 23. Hess RA. Effects of environmental toxicants on the efferent ducts, epididymis and fertility. J Reprod Fert Supp 1998; 53:247-59; PMID:10645284 [PubMed] [Google Scholar]
- 24. Connell CJ, Donjacour A. A morphological study of the epididymides of control and estradiol-treated prepubertal dogs. Biol Reprod 1985; 33:951-69; PMID:4084638; http://dx.doi.org/ 10.1095/biolreprod33.4.951 [DOI] [PubMed] [Google Scholar]
- 25. Wilson TM, Therrien A, Harkness JE. Sperm counts and reproductive tract lesions in male Syrian hamsters exposed in utero to diethylstilbestrol. Lab Anim Sci 1986; 36:41-4; PMID:3959533 [PubMed] [Google Scholar]
- 26. Arai Y, Mori T, Suzuki Y, Bern HA. Long-term effects of perinatal exposure to sex steroids and diethylstilbestrol on the reproductive system of male mammals. Int Rev Cytol 1983; 84:235-68; PMID:6358105; http://dx.doi.org/ 10.1016/S0074-7696(08)61019-0 [DOI] [PubMed] [Google Scholar]
- 27. Jackson H. The effects of alkylating agents on fertility. Brit Med Bull 1964; 20:107-14; PMID:14171898 [DOI] [PubMed] [Google Scholar]
- 28. Klinefelter GR, Laskey JW, Roberts NR, Slott V, Suarez JD. Multiple effects of ethane dimethanesulfonate on the epididymis of adult rats. Toxicol Appl Pharmacol 1990; 105:271-87; PMID:2171157; http://dx.doi.org/ 10.1016/0041-008X(90)90189-2 [DOI] [PubMed] [Google Scholar]
- 29. Klinefelter GR, Laskey JW, Kelce WR, Ferrell J, Roberts NL, Suarez JD, Slott V. Chloroethylmethanesulfonate-induced effects on the epididymis seem unrelated to altered Leydig cell function. Biol Reprod 1994; 51:82-91; PMID:7918878; http://dx.doi.org/ 10.1095/biolreprod51.1.82 [DOI] [PubMed] [Google Scholar]
- 30. Tsunoda Y, Chang MC. Fertilizing ability in vivo and in vitro of spermatozoa of rats and mice treated with alpha-chlorohydrin. J Reprod Fert 1976; 46:401-6; PMID:1255566; http://dx.doi.org/ 10.1530/jrf.0.0460401 [DOI] [PubMed] [Google Scholar]
- 31. Ericsson RJ, Baker VF. Male antifertility compounds: biological properties of U-5897 and U-l5,646. J Reprod Fert 1970; 21:267-73; PMID:5443210; http://dx.doi.org/ 10.1530/jrf.0.0210267 [DOI] [PubMed] [Google Scholar]
- 32. McClain RM, Downing JC. The effect of ornidazole on fertility and epididymal sperm function in rats. Toxicol Appl Pharmacol 1988; 92:488-96; PMID:3353993; http://dx.doi.org/ 10.1016/0041-008X(88)90188-3 [DOI] [PubMed] [Google Scholar]
- 33. Oberlander G, Yeung CH, Cooper TG. Induction of reversible infertility in male rats by oral ornidazole and its effects on sperm motility and epididymal secretions. J Reprod Fert 1994; 100:551-9; PMID:8021876; http://dx.doi.org/ 10.1530/jrf.0.1000551 [DOI] [PubMed] [Google Scholar]
- 34. Jones AR, Davies P, Edwards K, Jackson H. Antifertility effects and metabolism of alpha and epi-chlorhydrins in the rat. Nature 1969; 224:83; PMID:5822916; http://dx.doi.org/ 10.1038/224083a0 [DOI] [PubMed] [Google Scholar]
- 35. Cooper ER, Jones AR, Jackson H. Effects of alpha-chlorohydrin and related compounds on the reproductive organs and fertility of the male rat. J Reprod Fert 1974; 38:379-86; PMID:4833816; http://dx.doi.org/ 10.1530/jrf.0.0380379 [DOI] [PubMed] [Google Scholar]
- 36. Tsang AY, Lee WM, Wong PY. Effects of antifertility drugs on epididymal protein secretion, acquisition of sperm surface proteins and fertility in male rats. Int J Androl 1981; 4:703-12; PMID:7319653; http://dx.doi.org/ 10.1111/j.1365-2605.1981.tb00754.x [DOI] [PubMed] [Google Scholar]
- 37. Dhar JD, Srivastava SR, Setty BS. Flutamide as an androgen antagonist on epididymal function in the rat. Andrologia 1982; 14:55-61; PMID:6461278; http://dx.doi.org/ 10.1111/j.1439-0272.1982.tb03095.x [DOI] [PubMed] [Google Scholar]
- 38. Klinefelter GR, Suarez JD. Toxicant-induced acceleration of epididymal sperm transit: androgen-dependent proteins may be involved. Reprod Toxicol 1997; 11:511-9; PMID:9241671; http://dx.doi.org/ 10.1016/S0890-6238(97)00018-X [DOI] [PubMed] [Google Scholar]
- 39. Working PK, Bus JS, Hamm TE, Jr. Reproductive effects of inhaled methyl chloride in the male Fischer 344 rat. I. Mating performance and dominant lethal assay. Toxicol Appl Pharmacol 1985; 77:133-43; PMID:3966236; http://dx.doi.org/ 10.1016/0041-008X(85)90274-1 [DOI] [PubMed] [Google Scholar]
- 40. Chapin RE, White RD, Morgan KT, Bus JS. Studies of lesions induced in the testis and epididymis of F-344 rats by inhaled methyl chloride. Toxicol Appl Pharmacol 1984; 76:328-43; PMID:6388032; http://dx.doi.org/ 10.1016/0041-008X(84)90014-0 [DOI] [PubMed] [Google Scholar]
- 41. Chellman GJ, Morgan KT, Bus JS, Working PK. Inhibition of methyl chloride toxicity in male F-344 rats by the anti-inflammatory agent BW755C. Toxicol Appl Pharmacol 1986; 85:367-79; PMID:3094195; http://dx.doi.org/ 10.1016/0041-008X(86)90344-3 [DOI] [PubMed] [Google Scholar]
- 42. Qiu J, Hales BF, Robaire B. Adverse effects of cyclophosphamide on progeny outcome can be mediated through post-testicular mechanisms in the rat. Biol Reprod 1992; 46:926-31; PMID:1591348; http://dx.doi.org/ 10.1095/biolreprod46.5.926 [DOI] [PubMed] [Google Scholar]
- 43. Trasler JM, Hermo L, Robaire B. Morphological changes in the testis and epididymis of rats treated with cyclophosphamide: a quantitative approach. Biol Reprod 1988; 38:463-79; PMID:3358980; http://dx.doi.org/ 10.1095/biolreprod38.2.463 [DOI] [PubMed] [Google Scholar]
- 44. Zhou D, Wang H, Zhang J. Di-n-butyl phthalate (DBP) exposure induces oxidative stress in epididymis of adult rats. Toxicol Ind Health 2011; 27:65-71; PMID:20823052; http://dx.doi.org/ 10.1177/0748233710381895 [DOI] [PubMed] [Google Scholar]
- 45. de Andrade SF, Oliva SU, Klinefelter GR, De Grava Kempinas W. Epididymis-specific pathologic disorders in rats exposed to gossypol from weaning through puberty. Toxicol Pathol 2006; 34:730-7; PMID:17162530; http://dx.doi.org/ 10.1080/01926230600932455 [DOI] [PubMed] [Google Scholar]
- 46. Robb GW, Amann RP, Killian GJ. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fert 1978; 54:103-7; PMID:712697; http://dx.doi.org/ 10.1530/jrf.0.0540103 [DOI] [PubMed] [Google Scholar]
- 47. Klinefelter GR. Male infertilty and the environment: a plethora of associations based on a paucity of meaningful data. In: Goldstein M, Schlegel PN, eds. Surgical and Medical Management of Male Infertility. Surgical and Medical Management of Male Infertility: Cambridge University Press, 2011; 249-57. [Google Scholar]
- 48. Klinefelter GR, Laskey JW, Ferrell J, Suarez JD, Roberts NL. Discriminant analysis indicates a single sperm protein (SP22) is predictive of fertility following exposure to epididymal toxicants. J Androl 1997; 18:139-50; PMID:9154508 [PubMed] [Google Scholar]
- 49. Fernandez CD, Porto EM, Arena AC, Kempinas Wde G. Effects of altered epididymal sperm transit time on sperm quality. Int J Androl 2008; 31:427-37; PMID:17822422; http://dx.doi.org/ 10.1111/j.1365-2605.2007.00788.x [DOI] [PubMed] [Google Scholar]
- 50. Robaire B, Hinton BT. The Epididymis: from Molecules to Clinical Pratice. New York: Kluwer Academic Plenum Publishers, 2002. [Google Scholar]
- 51. Creasy DM. Pathogenesis of male reproductive toxicity. Toxicol Pathol 2001; 29:64-76; PMID:11215686; http://dx.doi.org/ 10.1080/019262301301418865 [DOI] [PubMed] [Google Scholar]
- 52. Foley GL. Overview of male reproductive pathology. Toxicol Pathol 2001; 29:49-63; PMID:11215684; http://dx.doi.org/ 10.1080/019262301301418856 [DOI] [PubMed] [Google Scholar]
- 53. Lanning LL, Creasy DM, Chapin RE, Mann PC, Barlow NJ, Regan KS, Goodman DG. Recommended approaches for the evaluation of testicular and epididymal toxicity. Toxicol Pathol 2002; 30:507-20; PMID:12187942; http://dx.doi.org/ 10.1080/016128401750063376 [DOI] [PubMed] [Google Scholar]
- 54. Creasy D, Bube A, de Rijk E, Kandori H, Kuwahara M, Masson R, Nolte T, Reams R, Regan K, Rehm S, et al. . Proliferative and nonproliferative lesions of the rat and mouse male reproductive system. Toxicol Pathol 2012; 40:40S-121S; PMID:22949412; http://dx.doi.org/ 10.1177/0192623312454337 [DOI] [PubMed] [Google Scholar]
- 55. Creasy DM, Chapin RE. Male reproductive system. In: Haschek WM, Rousseaux CG, Wallig MA, eds. Haschek and Rousseaux's Handbook of Toxicologic Pathology, Elsevier Inc Academic Press, 2013; 2493-598. [Google Scholar]
- 56. Johnson L, Welsh THJ, Wilker CE. Anatomy and physiology of the male reproductive system and potential targets of toxicants. In: Boekleheide K, Chapin RE, Hoyer PB, Harris C, eds. Comprehensive Toxicology. New York: Pergamon, 1997; 5-61. [Google Scholar]
- 57. Favareto AP, Fernandez CD, da Silva DA, Anselmo-Franci JA, Kempinas Wde G. Persistent impairment of testicular histology and sperm motility in adult rats treated with Cisplatin at peri-puberty. Basic Clin Pharmacol Toxicol 2011; 109:85-96; PMID:21410649; http://dx.doi.org/ 10.1111/j.1742-7843.2011.00688.x [DOI] [PubMed] [Google Scholar]
- 58. Blystone CR, Furr J, Lambright CS, Howdeshell KL, Ryan BC, Wilson VS, Leblanc GA, Gray LE, Jr. Prochloraz inhibits testosterone production at dosages below those that affect androgen-dependent organ weights or the onset of puberty in the male Sprague Dawley rat. Toxicol Sci: Off J Soc Toxicol 2007; 97:65-74; PMID:17234647; http://dx.doi.org/ 10.1093/toxsci/kfm004 [DOI] [PubMed] [Google Scholar]
- 59. Stoker TE, Laws SC, Guidici DL, Cooper RL. The effect of atrazine on puberty in male wistar rats: an evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol Sci: Off J Soc Toxicol 2000; 58:50-9; PMID:11053540; http://dx.doi.org/ 10.1093/toxsci/58.1.50 [DOI] [PubMed] [Google Scholar]
- 60. Perobelli JE, Alves TR, de Toledo FC, Fernandez CD, Anselmo-Franci JA, Klinefelter GR, Kempinas Wde G. Impairment on sperm quality and fertility of adult rats after antiandrogen exposure during prepuberty. Reprod Toxicol 2012; 33:308-15; PMID:22230644; http://dx.doi.org/ 10.1016/j.reprotox.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 61. Perobelli JE, Patrao MT, Fernandez CD, Sanabria M, Klinefelter GR, Avellar MC, Kempinas WD. Androgen deprivation from pre-puberty to peripuberty interferes in proteins expression in pubertal and adult rat epididymis. Reprod Toxicol 2013; 38:65-71; PMID:23541399; http://dx.doi.org/ 10.1016/j.reprotox.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 62. Mantovani A, Fucic A. Puberty dysregulation and increased risk of disease in adult life: possible modes of action. Reprod Toxicol 2014; 44:15-22; PMID:23791931; http://dx.doi.org/ 10.1016/j.reprotox.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 63. Mylchreest E, Cattley RC, Foster PM. Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: an antiandrogenic mechanism? Toxicol Sci: Off J Soc Toxicol 1998; 43:47-60; PMID:9629619; http://dx.doi.org/ 10.1093/toxsci/43.1.47 [DOI] [PubMed] [Google Scholar]
- 64. Mylchreest E, Wallace DG, Cattley RC, Foster PM. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicol Sci: Off J Soc Toxicol 2000; 55:143-51; PMID:10788569; http://dx.doi.org/ 10.1093/toxsci/55.1.143 [DOI] [PubMed] [Google Scholar]
- 65. Klinefelter GR, Laskey JW, Winnik WM, Suarez JD, Roberts NL, Strader LF, Riffle BW, Veeramachaneni DN. Novel molecular targets associated with testicular dysgenesis induced by gestational exposure to diethylhexyl phthalate in the rat: a role for estradiol. Reproduction 2012; 144:747-61; PMID:23041508; http://dx.doi.org/ 10.1530/REP-12-0266 [DOI] [PubMed] [Google Scholar]
- 66. Leite GA, Rosa Jde L, Sanabria M, Cavariani MM, Franci JA, Pinheiro PF, Kempinas Wde G. Delayed reproductive development in pubertal male rats exposed to the hypolipemiant agent rosuvastatin since prepuberty. Reprod Toxicol 2014; 44:93-103; PMID:24440231; http://dx.doi.org/ 10.1016/j.reprotox.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 67. Fan X, Robaire B. Orchidectomy induces a wave of apoptotic cell death in the epididymis. Endocrinology 1998; 139:2128-36; PMID:9529002 [DOI] [PubMed] [Google Scholar]
- 68. Creasy D. Hormonal mechanisms in male reproductive tract toxicity. In: Harvey PW, Rush KC, Cockburn A, eds. Endocrine and Hormonal Toxicology. New York: Wiley and Sons, 1999:355-406. [Google Scholar]
- 69. Hamzeh M, Robaire B. Effect of testosterone on epithelial cell proliferation in the regressed rat epididymis. J Androl 2009; 30:200-12; PMID:18930902; http://dx.doi.org/ 10.2164/jandrol.108.006171 [DOI] [PubMed] [Google Scholar]
- 70. Serre V, Robaire B. Segment-specific morphological changes in aging Brown Norway rat epididymis. Biol Reprod 1998; 58:497-513; PMID:9475407; http://dx.doi.org/ 10.1095/biolreprod58.2.497 [DOI] [PubMed] [Google Scholar]
- 71. Saito K, O’Donnell L, McLachlan RI, Robertson DM. Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone withdrawal in adult rats. Endocrinology 2000; 141:2779-85; PMID:10919263 [DOI] [PubMed] [Google Scholar]
- 72. Yang ZW, Kong LS, Guo Y, Yin JQ, Mills N. Histological changes of the testis and epididymis in adult rats as a result of Leydig cell destruction after ethane dimethane sulfonate treatment: a morphometric study. Asian J Androl 2006; 8:289-99; PMID:16625278; http://dx.doi.org/ 10.1111/j.1745-7262.2006.00140.x [DOI] [PubMed] [Google Scholar]
- 73. Smithwick EB, Young LG. Histological effects of androgen deprivation on the adult chimpanzee epididymis. Tissue Cell 2001; 33:450-61; PMID:11949781; http://dx.doi.org/ 10.1054/tice.2001.0199 [DOI] [PubMed] [Google Scholar]
- 74. Romualdo GS, Klinefelter GR, de K. Postweaning exposure to gossypol results in epididymis-specific effects throughout puberty and adulthood in rats. J Androl 2002; 23:220-8; PMID:11868815 [PubMed] [Google Scholar]
- 75. Shah VI, Ro JY, Amin MB, Mullick S, Nazeer T, Ayala AG. Histologic variations in the epididymis: findings in 167 orchiectomy specimens. Am J Surg Pathol 1998; 22:990-6; PMID:9706979; http://dx.doi.org/ 10.1097/00000478-199808000-00009 [DOI] [PubMed] [Google Scholar]
- 76. Cooper ER, Jackson H. Chemically induced sperm retention cysts in the rat. J Reprod Fert 1973; 34:445-9; PMID:4741314; http://dx.doi.org/ 10.1530/jrf.0.0340445 [DOI] [PubMed] [Google Scholar]
- 77. Guerra MT, Perobelli JE, Sanabria M, Anselmo-Franci JA, Kempinas Wde G. Long-term effects of perinatal androgenization on reproductive parameters of male rat offspring androgenization and male rat reproduction. Horm Metab Res = Horm- und Stoffwechselforschung = Horm et Metab 2013; 45:586-92; PMID:23549673; http://dx.doi.org/ 10.1055/s-0033-1341434 [DOI] [PubMed] [Google Scholar]
- 78. Rudmann DG, McNerney ME, VanderEide SL, Schemmer JK, Eversole RR, Vonderfecht SL. Epididymal and systemic phospholipidosis in rats and dogs treated with the dopamine D3 selective antagonist PNU-177864. Toxicol Pathol 2004; 32:326-32; PMID:15204974; http://dx.doi.org/ 10.1080/01926230490431754 [DOI] [PubMed] [Google Scholar]
- 79. Ojo OO, Bhadauria S, Rath SK. Dose-dependent adverse effects of salinomycin on male reproductive organs and fertility in mice. PloS One 2013; 8:e69086; PMID:23840907; http://dx.doi.org/ 10.1371/journal.pone.0069086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Radovsky A, Mitsumori K, Chapin RE. Male reproductive tract. In: Maronpot RR, Boorman GA, Gaul BW, eds. Pathology of the Mouse, Reference and Atlas. Vienna: Cache River Press, 1999; 381-407. [Google Scholar]
- 81. Ouban A, Ahmed AA. Claudins in human cancer: a review. Histol Histopathol 2010; 25:83-90; PMID:19924644 [DOI] [PubMed] [Google Scholar]
- 82. Yeung CH, Wang K, Cooper TG. Why are epididymal tumours so rare? Asian J Androl 2012; 14:465-75; PMID:22522502; http://dx.doi.org/ 10.1038/aja.2012.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Goyal HO, Braden TD, Mansour M, Williams CS, Kamaleldin A, Srivastava KK. Diethylstilbestrol-treated adult rats with altered epididymal sperm numbers and sperm motility parameters, but without alterations in sperm production and sperm morphology. Biol Reprod 2001; 64:927-34; PMID:11207210; http://dx.doi.org/ 10.1095/biolreprod64.3.927 [DOI] [PubMed] [Google Scholar]
- 84. Borges CS, Missassi G, Pacini ES, Kiguti LR, Sanabria M, Silva RF, Banzato TP, Perobelli JE, Pupo AS, Kempinas WG. Slimmer or fertile? Pharmacological mechanisms involved in reduced sperm quality and fertility in rats exposed to the anorexigen sibutramine. PloS One 2013; 8:e66091; PMID:23776614; http://dx.doi.org/ 10.1371/journal.pone.0066091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Garcia PV, Barbieri MF, Perobelli JE, Consonni SR, Mesquita Sde F, Kempinas Wde G, Pereira LA. Morphometric-stereological and functional epididymal alterations and a decrease in fertility in rats treated with finasteride and after a 30-day post-treatment recovery period. Fert Steril 2012; 97:1444-51; PMID:22521699; http://dx.doi.org/ 10.1016/j.fertnstert.2012.03.025 [DOI] [PubMed] [Google Scholar]