Abstract
In most bony fishes, testes are paired elongated organs that are attached to the dorsal wall of the body by a mesorchium. Histological examination of teleost testes, and also in all vertebrates, shows that the testes are formed of germ cells and somatic cells, comprising the germinal and interstitial compartments. Both compartments are separated by a basement membrane. The germ cells may be spermatogonia, meiotic spermatocytes and haploid spermatids that differentiate into spermatozoa. The process of spermatogenesis includes a sequence of morphological and physiological changes of germ cells that begin with the differentiation of spermatogonia that become meiotic spermatocytes. After the second meiotic division, through a process of spermiogenesis, these differentiate into spermatozoa. Spermatogonia associate with Sertoli cells to form spermatocysts or cysts. The cyst is the unit of spermatogenic function, composed of a cohort of isogenic germ cells surrounded by encompassing Sertoli cells. The teleost testis is organized morphologically into 3 types of testis: 1) tubular testis type, present in lower bony fishes as salmonids, cyprinids and lepisosteids; 2) unrestricted spermatogonial testis type, found in neoteleosts except Atherinomorpha; and 3) restricted spermatogonial testis type, characteristic of all Atherinomorpha. The morphology of the testicular germinal epithelium changes during the annual reproductive cycle, reflecting reproductive seasonality.
Keywords: bony fishes, restricted testis type, spermatogenesis, testicular structure, tubular testis type, unrestricted testis type
Abbreviations
- FSH
follicule-stimulating hormone
- LH
luteinizing hormone
- 11-KT
11-ketotestosterone
- PCNA
proliferating cell nuclear antigen
Introduction
In most bony fishes, testes are paired elongated organs, attached to the dorsal wall of the body by a mesorchium. But, in some species there is a single testis, as in Tomeurus gracilis (Poeciliidae)1 or a partially fused testis, as in the perch, Perca flavescens and in the goodeids such as Goodea atripinnis. There may be a totally fused testis, as in the guppy, Poecilia reticulata.2 When paired, both testes join caudally, converging in a central deferent duct system that is open to the exterior throughout the urogenital pore.
The testes change morphologically during the annual reproductive cycle, passing through 5 phases: Regressed, Early Maturation, Mid Maturation, Late Maturation, and Regression (Fig. 1). These phases reflect reproductive seasonality when the testis grows, spermatogenesis is activated, spermiation occurs and spermatozoa fill the deferent duct system during the spawning season. During the Regression phase, spermatogenesis is intensely reduced and during the Regressed phase, diploid spermatogonia become the only germ cells in the testes which may divide mitotically, preparing for the next initiation of spermatogenesis and, consequently, the restitution of spermatozoa production in the testis.2-5
Figure 1.
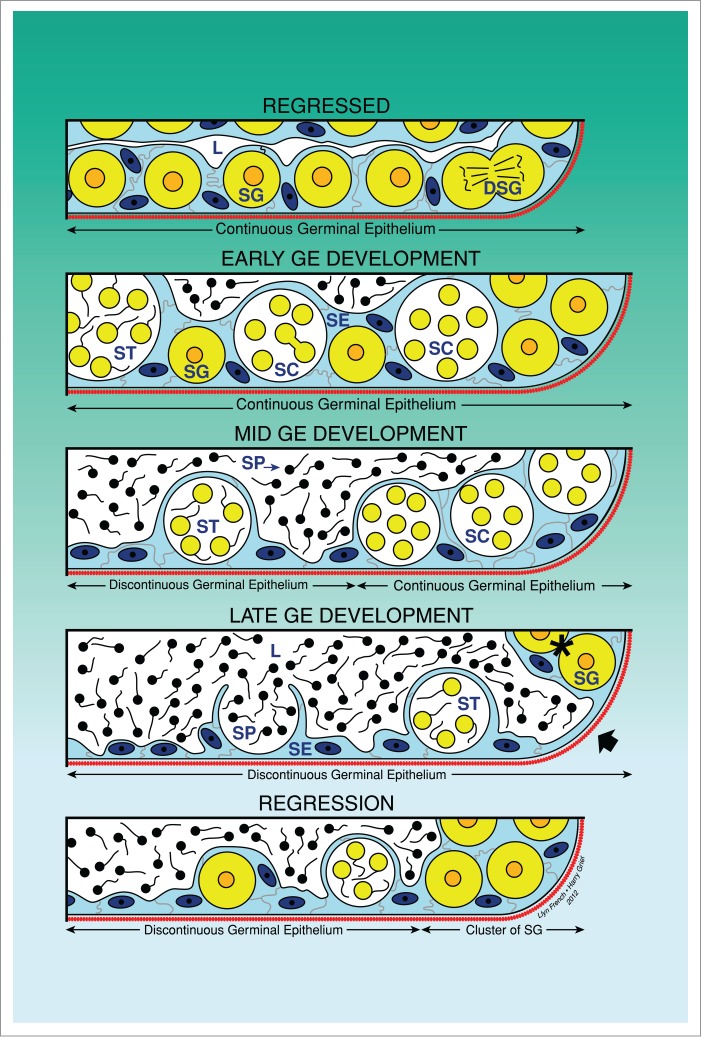
Unrestricted spermatogonial testis. Schematic drawing illustrating the changes of the continuity and discontinuity of the germinal epithelium (GE) during the annual reproductive cycle. The continuity is observed when spermatogonia and/or cysts with germinal cells are continuous along the lobular lumen. During spermiation, the discontinuity is observed when there are portions where there are no germinal cells, only Sertoli cells. The 5 phases of the reproductive cycle are illustrated: Regressed, Early GE Development, Mid GE Development, Late GE Development and Regression. Lobular lumen (L), Sertoli cells (SE), spermatogonia (SG), dividing spermatogonia (DSG), spermatocytes (SC), spermatids (ST), spermatozoa (SP).
Histological examination of bony fishes testes shows that these organs are composed of both germinal and interstitial compartments that are always separated by a basement membrane; therefore, the cells in one compartment do not intermix with the cells of the other compartment.4
The germinal compartment is composed of the germinal epithelium, being formed by 2 cell types: germ cells and somatic epithelial cells, the latter being Sertoli cells. They are homologous among all vertebrates, based on the common origin from the germinal ridge.6 As with all epithelia, Sertoli cells rest upon a supporting layer, the basement membrane, which separates the epithelium from the subjacent interstitium. The germinal cells are between Sertoli cells or surrounded by them, but not in contact with the basement membrane. Germ cells include all the stages of differentiation of the spermatogenic cells. Spermatogenesis is initiated with the mitotic proliferation of spermatogonia, proceeds through 2 divisions of meiosis, and concludes with spermiogenesis, during which the haploid spermatids transform into spermatozoa.5 Spermatogenesis in bony fishes has similarities to those observed in other vertebrates, but also having clear differences. Spermatogenesis proceeds within a cystic structure (Fig. 1) in which all germ cells develop as a synchronous clone that is surrounded by Sertoli cells.2,7 The cystic type of spermatogenesis is typical of fishes and amphibians.8,9 Cyst formation is initiated when cytoplasmic extensions of Sertoli cells surround a spermatogonium. During spermatogenesis the number of germ cells increases greatly within the cyst, and the number of Sertoli cells per cyst also increases due to Sertoli cell proliferation.10
During spermatogenesis in all bony fishes, spermatogonia are associated in the germinal compartment with Sertoli cells by processes that join laterally. These processes completely envelop the germ cells throughout spermatogenesis, isolating them from contact with both the basement membrane, and the lobular lumen. Initially, Sertoli cell processes surround individual spermatogonia. After mitotic divisions of spermatogonia, Sertoli cell processes surround an isogenic clone of spermatogonia, enclosing them within a cyst, or spermatocyst, in which meiosis and spermiogenesis occur. Sertoli cells provide nutrients and a permeability barrier during spermatogenesis.7 This barrier results from the formation of desmosomes and tight junctions between adjacent Sertoli cells.11 Sertoli cells also phagocytise residual bodies of developing spermatids.12,13
The interstitial compartment is composed of connective tissue and Leydig cells. The connective tissue contains fibroblasts, immunological cells such as macrophages and lymphocytes, collagen fibers, myoid cells, blood vessels and nerve fibers. The steroidogenic Leydig cells are located especially near the blood vessels and produce testosterone.2,11,14 In some species, such as poeciliids and goodeids, the Leydig cells are abundant around the efferent ducts, as it is described in Poecilia latipinna.8,15
Testis Types of Bony Fishes
The structure of the testicular germinal epithelium varies among bony fishes. Basal taxa have a tubular testis and derived teleosts, have a lobular testis Lobular testis is divided into 2 types, which is, based on the distribution of spermatogonia. In non-atherinomorph neoteleosts, the testis corresponds to an unrestricted lobular testis, where spermatogonia may be distributed throughout the length of the lobules. By contrast, atherinomorph fishes possess a restricted lobular testis in which lobules show an orderly progression of developing germ cells, with spermatogonia confined to the testicular periphery.4,7,16 An ample revision of diverse species, according to these 3 testis types, is presented.16
Tubular Testis Type
In this testis type (Fig. 2), the tubules do not terminate at the testis periphery, but they form loops at the periphery which double back to the efferent ducts, forming a highly branched structure or forming anastomoses of the tubules where they may branch and rejoin (Figs. 2A,B). Germ cells develop as synchronous clones around the lobules and spermatozoa are liberated to the lumen of the tubules (Figs. 2C,D). This testis type is present in lower fishes, as salmonids, cyprinids and lepisosteids. As examples, tubular testes were described in the gar, Lepisosteus platyrhinchus (Lepisosteidae), Ictalurus natalis (Siluridae), Opisthonema oglinum and Dorosoma petense (Clupeidae)7; Black Tetra, Gimnocorhymbus conirostris (Pimelodidae),17 the Rainbow Trout, Oncorhynchus mykiss (Salmonidae), Esox niger (Esocidae)7and the Tarpon, Megalops atlanticus (Elopidae); Gymnotus carapo.18
Figure 2.
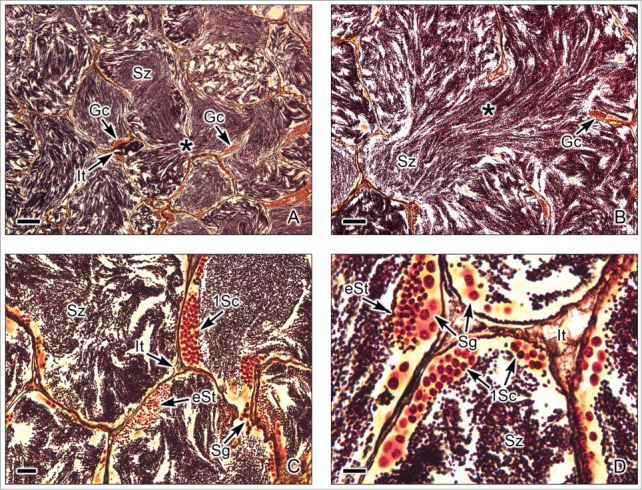
Tubular testis of the Gar Lepisosteus osseous. (A,B). Abundant spermatozoa (Sz) are observed within highly branched tubules (*). Interstitial tissue (It) is between the tubules. Germinal cells (Gc) are seen, adjacent to the basement membrane of the tubule wall. (C,D). The germinal cells are seen during early stages of spermatogenesis, as spermatogonia (Sg), primary spermatocytes (1Sc) and early spermatids (eSt). The primary spermatocytes and early spermatids are seen in cysts, forming synchronous groups of cells. The lumen of the tubules contains abundant spermatozoa (Sz). Interstitial tissue (It) between the lobules. Argentic-reticuline impregnation technique. (A,B): Bars = 100 μm; C: Bar = 20 μm; D: Bar = 10 μm.
Unrestricted Spermatogonial Testis Type
This testis type (Figs. 3-6) is found in neoteleosts, except for the atherinomorphs. In this type of testis, the germinal compartment is formed of lobules that extend to the periphery of the testis, terminating blindly (Figs. 3A, 5A). It is characterized by the occurrence of spermatogonia along the lengths of the lobules. The lobules near the periphery contained abundant cysts with germinal cells (Figs. 3B,D, 4A-E, 5B-C), meanwhile, the lobules near the efferent ducts contained less cysts with germinal cells (Figs. 3C, 5D). During spermiation, the spermatozoa are released into the lobular lumen (Figs. 3-6) that is in continuity with that of the efferent ducts (Figs. 3A, 4F, 5A). During the active spermatogenesis, cysts in different stages are seen all along the lobules (Figs. 3E, 4A-E, 5A-C, 6A-D). As examples, Unrestricted lobular testes were described in higher teleosts, such as Perca flavescens (Percidae),17 the Common Snook, Centropomus undecimalis (Centropomidae),19 the Cobia, Rachycentron canadum (Rachycentridae)20; Oreochromis mossambicus and Cichlasoma dimerus (Cichlidae).21
Figure 3.
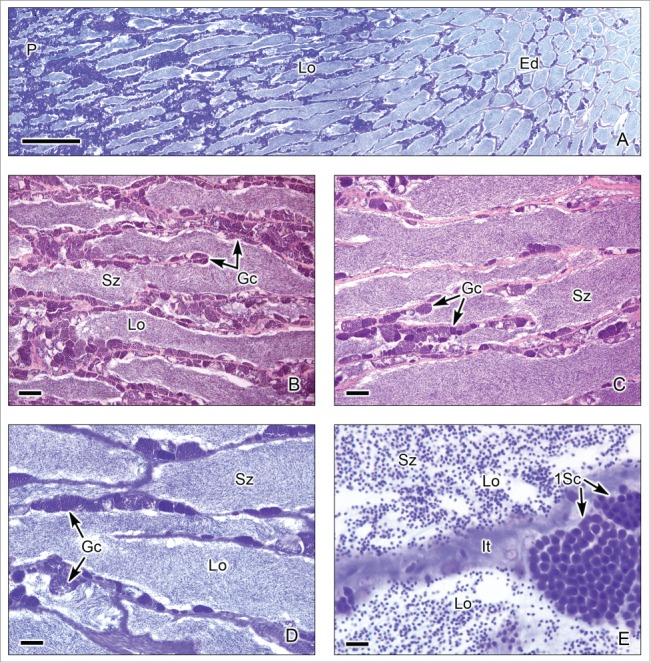
Unrestricted lobular testis of the Red Drum Scianops ocellatus. (A). Longitudinal section of the testis, from the periphery (P) to the efferent ducts (Ed). Testicular lobules (Lo) run parallel and illustrate the typical Mid germinal epithelium development Phase during the annual reproductive cycle. (B). Section near the periphery of the testis containing continuous cysts with synchronous germinal cells (Gc) along the wall of the lobules (Lo), a continuous germinal epithelium, and spermatozoa (Sz) in the lumen. (C,D). Sections near the efferent ducts where the cysts of germinal cells (Gc) are discontinuous, compared with these seen near the periphery. The lumen of the lobules (Lo) contains abundant spermatozoa (Sz). €. Interstitial tissue (It) between the lobules (Lo). There are abundant spermatozoa (Sz) in the lobular lumen. Cysts with primary spermatocytes (1Sc) adjacent to the lobule wall are seen. (A,D,E): Toluidine blue. (B,C): Hematoxylin-eosin. (A): Bar = 1mm; (B,C): Bars = 100 μm; (D): Bar = 50 μm; (E): Bar = 10 μm.
Figure 4.
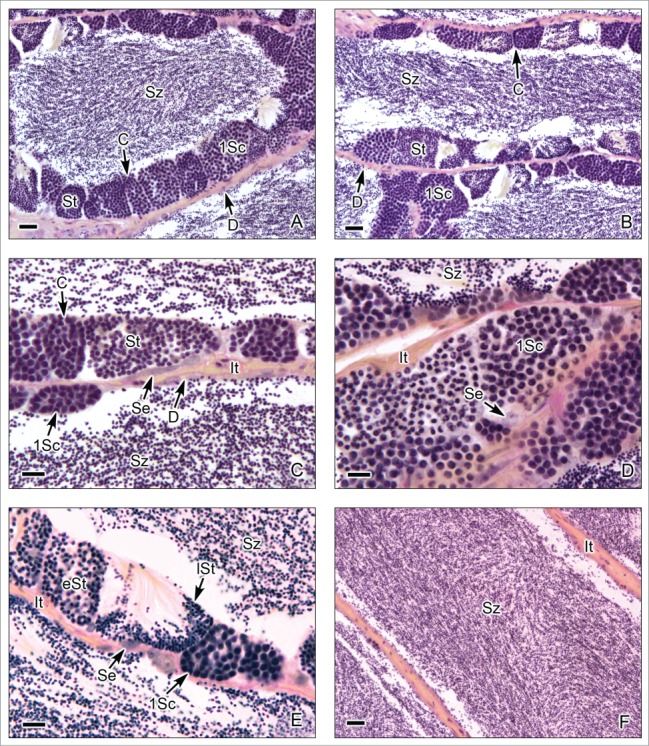
Unrestricted lobular testis of the Red Drum Scianops ocellatus. (A-C). The germinal compartments are lobules with continuous (C) and discontinuous (D) germinal epithelium along the lobular wall. The germinal epithelium contains Sertoli cells (Se) and cysts with primary spermatocytes (1Sc) and spermatids (St). Around the lobules, the interstitial compartment (It) is formed by connective tissue. There are abundant spermatozoa (Sz) in the lobular lumen. (D,E). Cysts with primary spermatocytes (1Sc), early spermatids (eSt) and late spermatids (lSt). Sertoli cells (Se) are observed next to cysts. There are abundant spermatozoa (Sz) in the lobular lumen. (F). Lobules near the efferent ducts with abundant spermatozoa (Sz). In this region of the lobules there is no germinal epithelium as all of the germ cells matured and were released as spermatozoa. The interstitial tissue (It) borders the lobules. Hematoxylin-eosin. (A,B,F): Bars = 20 μm; (C-D): Bars = 10 μm.
Figure 5.
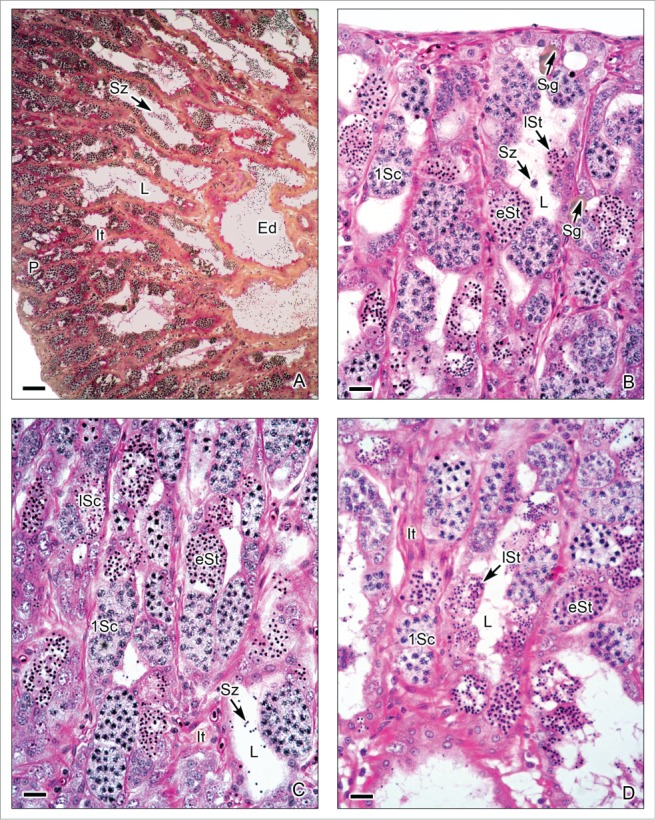
Unrestricted lobular testis of Oreochromis mossambicus. (A). Longitudinal section of the testis, from the periphery (P) to the efferent ducts (Ed). At the early season of spermatogenesis, there are numerous cysts with germinal cells in different stages of development at the periphery of the testis, but the lobular lumen (L) contains, until now, few spermatozoa (Sz). Interstitial tissue (It) between the lobules. (B-D). Testicular lobules containing spermatogonia (Sg) near the lobular wall, and cysts with primary spermatocytes (1Sc), early spermatids (eSt) and late spermatids (lSt). The interstitial tissue (It) contains capillaries. (A): Metanil-yellow-PAS-Hematoxylin; (B-D): Hematoxylin-eosin. (A): Bar = 100 μm, (B-D): Bar = 20 μm.
Figure 6.
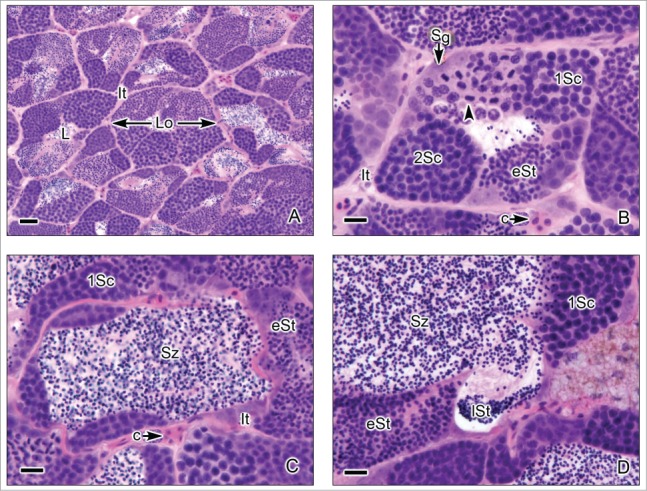
Unrestricted lobular testis of the common snook, Centropomus undecimalis. (A). Transverse sections of the testicular lobules. The lobules (Lo) are formed by cysts of synchronous germinal cells and a central lumen (L) which contains abundant spermatozoa (Sz). Interstitial tissue (It) is between the lobules. (B-D). At the margin of the lobules spermatogonia (Sg) are observed. There are cysts with primary spermatocytes (1Sc), primary spermatocytes during metaphase of the first division of meiosis (arrow head), secondary spermatocytes (2Sc), early spermatids (eSt) and late spermatids (lSt). Around the lobules, it is observed the interstitial tissue (It) where capillaries (c) are evident. Hematoxylin-eosin. (A): Bar = 50 μm; (B-D): Bars = 20 μm.
Restricted Spermatogonial Testis Type
This testis type (Figs. 7–9) is found in all Atherinomorpha (Atheriniformes, Beloniformes and Cyprinodontiformes). The restriction of spermatogonia to the termini of the lobules supports the monophyly of that group.16 In this testis type, similar to that mentioned for the unrestricted type, the germinal compartment extends to the periphery of the testis and terminates blindly (Figs. 7A,B, 8E,F, 9A,B). During spermatogenesis, the cysts migrate toward the efferent duct system, as germ cells mature inside the cyst (Figs. 7A-C, 8A,B, 9A-D). The restricted distribution of spermatogonia (Figs. 7C,D, 8E, 9B-E) occurs, as examples, in the species of the Family Hemiramphidae,22 in the viviparous species Xenotoca eiseni, Goodea atripinnis and Ilyodon white (Goodeidae),4,23 in the cyprinodontid, Fundulus grandis (Fundulidae),13 and in the also viviparous species of Poeciliidae, as Poecilia latipinna, P. reticulata, Gambusia affinis, Heterandria formosa17,23 and Phalloceros caudimaculatus.24 Spermatozoa are packaged in naked bundles, or spermatozeugmata (Figs. 8A-D, 9F), seen in the testis ducts.1 Interestingly, sperm flagella course over the surface of spermatozeugmata in the species of Goodeidae (Figs. 8C,D), while sperm nuclei form the surfaces of spermatozeugmata in species of Poeciliidae (Fig. 9F).6,25 This is a reflection on the fact that spermatid nuclei associate with Sertoli cells in poeciliids during spermiogenesis, while sperm flagella are associated with Sertoli cells during spermiogenesis in goodeids.
Figure 7 (See previous page).
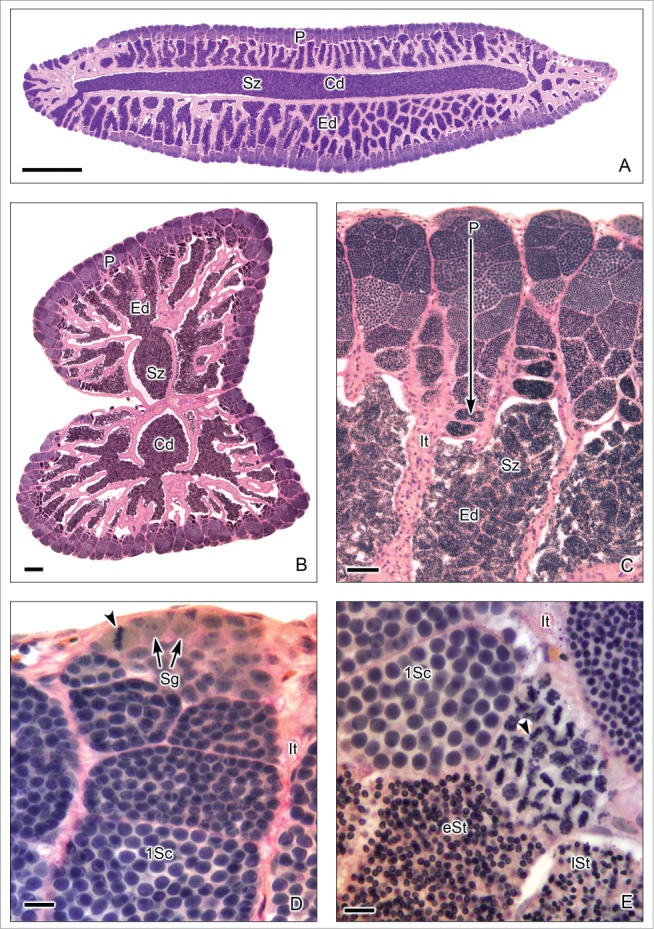
Restricted lobular testis of the goodeid Goodea atripinnis. (A). Longitudinal section of the testis. (B). Transverse section of both testes. Spermatogenesis occurs at the periphery (P) or distal part of the testis lobules. Sperm are released, into a net of efferent ducts (Ed) which communicate with the central efferent duct (Cd). Efferent and central ducts are full of spermatozoa (Sz). (C). The lobules contain spermatocysts with synchronous germinal cells at different stages of spermatogenesis. Cysts at the later stages of spermatogenesis are located progressively closer (large arrow) to the efferent ducts (Ed) which contain abundant spermatozoa (Sz), organized in bundles called spermatozeugmata. Lobules do not have a central lumen. The interstitial tissue (It) is around the lobules. (D). Spermatogonia (Sg) are restricted to the periphery of the testis. A spermatogonium in mitotic metaphase is observed (arrow head). Cysts containing primary spermatocytes (1Sc) are seen. Interstitial tissue (It). (E). Cysts containing primary spermatocytes (1Sc), primary spermatocytes during metaphase of the first division of meiosis (arrow head), early spermatids (eSt) and late spermatids (lSt) are seen. Interstitial tissue (It). Hematoxylin-eosin. (A): Bar = 1mm; (B): Bar = 200 μm; (C): Bar = 50 μm; (D-E): Bars = 10 μm.
Figure 8.
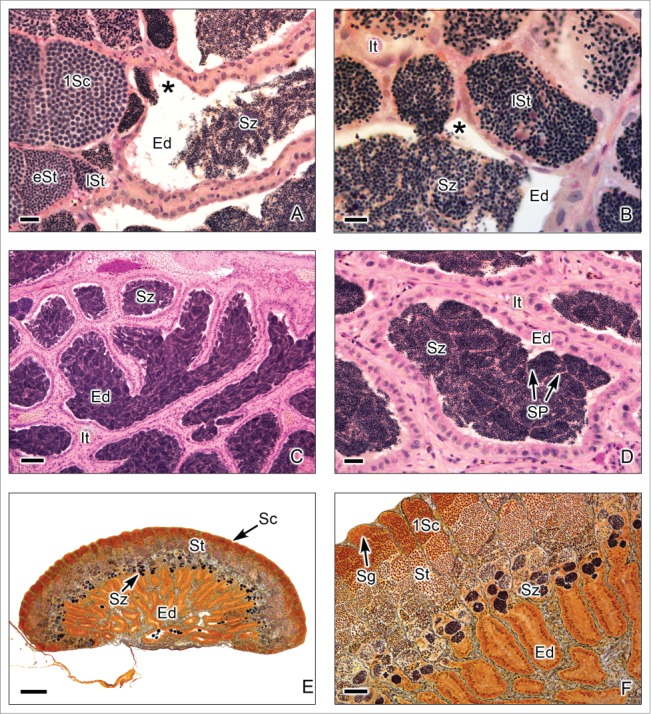
Restricted lobular testis of the goodeids Goodea atripinnis (A-D) and Xenotoca eiseni (E-F). (A,B). Spermiation occurs in the testicular lobules near the efferent ducts (Ed). The lobules contain primary spermatocytes (1Sc), primary spermatocytes during metaphase of the first division of meiosis (arrow head), early spermatids (eSt) and late spermatids (lSt). The spermatozoa are moving from the cyst (*) to the lumen of the efferent duct. Interstitial tissue (It). (C,D). Efferent ducts (Ed) with abundant spermatozoa (Sz). The spermatozoa are situated in groups forming spermatozeugmata (SP). Interstitial tissue (It). (E,F). Longitudinal section of the testis during early reproductive season, when the spermatogenic activity is initiated. The lobules contain abundant cysts with primary spermatocytes (1Sc) and spermatids (St), but the spermatozoa are scarce, and the efferent ducts (Ed) do not contain yet spermatozoa. (A-D): Hematoxylin-eosin; (E-F): Argentic-reticuline impregnation technique (A): Bar = 20 μm; (B): Bar = 10 μm; (C): Bar = 100 μm; (D): Bars = 20 μm (E): Bar = 200 μm; (F): Bar = 50 μm.
Figure 9.
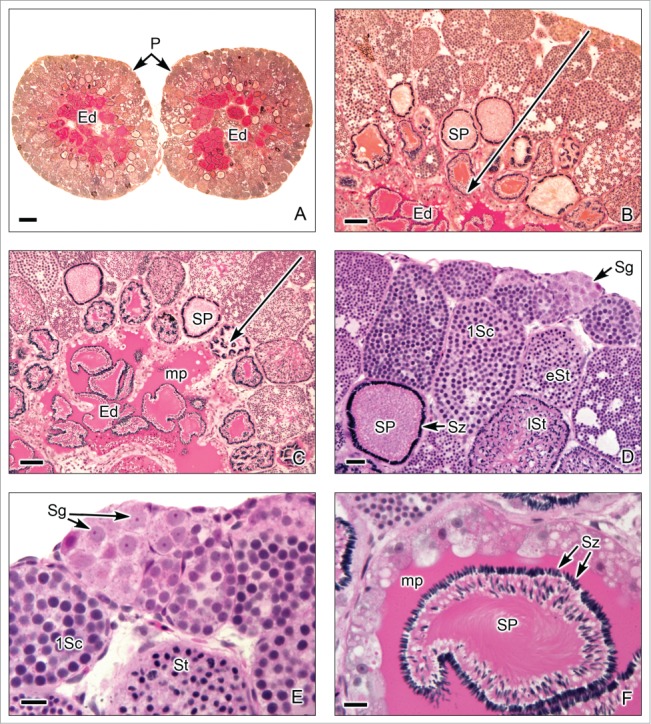
Restricted lobular testis of the poeciliid Poecilia latipinna. (A). Transverse sections of both testes. Similar to that described in goodeid testis, spermatogenesis occurs at the periphery (P) or distal part of the testis lobules. Sperm are released, into a net of efferent ducts (Ed). (B,C). The germ cells in spermatogenesis are within cysts, the later stages (large arrow) are progressively closer to the efferent ducts (Ed). The spermatozoa are located in groups of spermatozeugmata (SP). A characteristic of the spermatozeugmata in poeciliids is that the heads of the spermatozoa are oriented toward the Sertoli cells. In the efferent ducts, the spermatozeugmata are surrounded by a PAS+ mucoprotein complex (mp). (D,E). Lobules at the testicular periphery with cysts of germ cells during several stages of spermatogenesis, as spermatogonia (Sg), restricted at the periphery, primary spermatocytes (1Sc), early spermatids (eSt), late spermatids (lSt), and spermatozoa (Sz) in spermatozeugmata. (F). In the efferent ducts (Ed), the spermatozeugmata (SP) are surrounded by the PAS+ mucoprotein complex (mp). The nuclei of the spermatozoa are oriented toward the Sertoli cells. A,B: PAS; C-F: Hematoxylin-eosin. A: Bar = 200 μm. B,C: Bar = 50 μm. D: Bar = 20 μm. E,F: Bar = 10 μm.
Spermatocysts line the walls of tubules (in tubular testis) (Figs. 2A-D) and lobules (in unrestricted spermatogonial testis) (Figs. 3–6) and sperm collect in the lumina of either tubules or lobules except in Atherinomorpha (in restricted spermatogonial testis) (Figs. 7–9) where the lobules lack lumina because spermatocysts completely extend across the entire interiors of the lobules.
The differences between tubular and lobular testis types are significant regarding their systematic implications, distinguishing between gonad types in lower and higher fishes.16
Germ Cells During Spermatogenesis
Spermatogenesis includes a sequence of morphological and physiological changes in the germ cells as they differentiate from spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids and spermatozoa. Several authors described spermatogenesis in fishes and the morphology of the germ cells during this process.2,4,5,26
Spermatogonia A and B
In bony fishes, a reserve of spermatogonia is present throughout the year in the testis, localized in the germinal epithelium. These spermatogonia have been called spermatogonia A. They are diploid stem cells that proliferate by mitotic divisions (Fig. 7D), increasing the number of germ cells within the germinal compartment (Figs. 2D, 5B, 6B, 7D, 9D,E). Spermatogonia A are the largest germ cells in the germ line with diameters of 12–16 μm. These cells contain light granular cytoplasm and spherical nuclei in a central position which present delicate granular diploid chromatin and one or 2 nucleoli. Spermatogonia B are considered to be committed to differentiation when they are surrounded by Sertoli cell processes, thus being enclosed within a spermatocyst. They are spherical cells, smaller than the spermatogonia A, having diameters of 9–12 μm. As in spermatogonia A, spermatogonia B have a spherical nucleus with diploid chromosomes and one or 2 nucleoli. They replicate their chromosomes and enter in meiosis, becoming primary spermatocytes.
Primary and secondary spermatocytes
As occurs in all male vertebrates, meiosis consists of 2 consecutive cell divisions that result in 4 haploid spermatids. Primary spermatocytes are spherical cells, with diameters of 8–12 μm (Figs. 2D, 3E, 4A-E, 5B-D, 6B-D, 7D,E, 8A, 9D,E), similar in size to spermatogonia B. The nucleus of primary spermatocytes has replicated homologous chromosomes which are in different stages of meiotic prophase I: leptotene (fine reticular replicated, homologous chromosomes), zygotene (homologous chromosomes in synapsis), pachytene (paired homologous chromosomes condensed and occurring crossing-over) and diplotene (separation of homologous, replicated chromosomes that remain attached at the chiasmata). Cysts with primary spermatocytes in pachytene of meiotic prophase I (Figs. 6B, 7E) are relatively abundant, whereas those in leptotene, zygotene, and especially in diplotene stage are rarely seen. The primary spermatocytes enter metaphase I, anaphase I, and telophase I, resulting in 2 secondary spermatocytes when the reduction of duplicated homologous chromosomes occurs and, consequently, the chromosome number is halved with only a single set of chromosomes. Then, the nucleus of secondary spermatocytes contains the haploid number of chromosomes, but duplicated. Secondary spermatocytes (Fig. 6B), are small cells with diameters of 4–7 μm. They are spherical in shape with spherical nucleus and filamentous chromosomes, ready for immediately entry in the second division of meiosis, advancing to metaphase II, anaphase II, and telophase II, resulting in 2 spermatids of each secondary spermatocytes. Therefore, they are seen less frequently than primary spermatocytes as they divide rapidly, after a short interphase between the 2 divisions of meiosis.
Spermatids and spermatozoa
Early, haploid spermatids (Figs. 2C,D, 4A-C, 5B-D, 6B,D, 7E, 8A,B, 9E) are cells with diameters of 2–4 μm, are spherical in shape and have a spherical nucleus. They do not divide any more but undergo morphological transformation, becoming spermatozoa through the process called spermiogenesis. This process is characterized by significant morphological changes associated with the formation of the sperm head and its condensed nucleus, with midpiece, and flagellum. As spermiogenesis proceeds, the nuclei of spermatids become smaller while the chromatin shows increasing degrees of condensation (Figs. 2C-D, 3E, 4A-F, 5C, 6C,D, 8A,B, 9F). During spermiogenesis, spermatozoa become motile by the development of a flagellum, retain mitochondria that become localized within the sperm midpiece, and void excess cytoplasm as a residual body which is phagocytized by Sertoli cells. The acrosome is absent in the spermatozoa of most bony fishes, but it is found in the sturgeons (Acipenseridae, Chondrostei) as Acipenser persicus27 and Acipenser gueldenstaedtii.28 The structure and size of spermatozoa vary considerably in complexity among teleost species. Fish spermatozoa are classified according to the external or internal mode of fertilization into 2 forms, aquasperm and introsperm, respectively.29 The aquasperm is isodiametric with a globular head, while the introsperm is anisodiametric with an elongate head; both types of spermatozoa have one flagellum formed by the typical 9+2 microtubule;30-32 or some species have 2 flagella, both with the same structure, as described in species of the teleost families Cetopsidae, Aspredinidae, and Nematogenyidae,33 or in the species Satanoperca jurupari (Cichlidae).34 The spermatozoa of the neotropical teleosts have rounded heads, a characteristic of fish that externally fertilize their eggs, for example, Oryzias latipes (Adrianichthyidae), Rivulus marmoratus (Rivulidae), Hemiramphus balao (Hemiramphidae). On the other hand, the spermatozoa of viviparous poeciliids, that have internal fertilization, have an elongated head, for example, Poecilia reticulata, P. latipinna, Gambusia affinis, Xiphophorus helleri (Poeciliidae).35 In several species of teleosts which are internal fertilizers, the spermatozoa are arranged into packets which are formed in the spermatocysts and released during spermiation into the efferent duct system. These sperm packets are termed spermatophores, when they are covered by a capsule. When these packets are unencapsulated or naked they are known as spermatozeugmata.7,22,25,36,37 The formation of spermatozeugmata always involves the association of Sertoli cells with the spermatozoa. In the Poeciliidae, the sperm nuclei associate with Sertoli cells, and spermatozeugmata have outwardly-directed sperm nuclei (Figs. 9C,D,F). In the Goodeidae, the spermatid flagella associate with the Sertoli cells (Figs. 8C,D), and sperm flagella cover the surface of the spermatozeugmata.6 The presence of secretions in the efferent ducts as PAS-positive material has been described in testes of teleosts which produce sperm packets. These secretions have been found accompanying spermatozeugmata in species of the families Poeciliidae8 (Figs. 9A-C,F), Hemirhamphidae22 and Characidae.37 At the end of the spermiogenesis, during the spermiation, the cysts open and the spermatozoa move to the efferent ducts (Figs. 8A,B).
Regulation of Spermatogenesis
Fish spermatogenesis, from spermatogonial stem-cell renewal to spermatozoa, as in all vertebrates, is a complex process, regulated by the neuroendocrine system. Several reviews described neuroendocrine regulation of fish spermatogenesis.26,38-43 Spermatogenesis occurs in the special biochemical environment defined by the Sertoli cells that are associated with the germ cells. The Sertoli cells surround the germ cells, communicate with them, and separate them from the rest of the somatic tissues by a permeability barrier.7 These interactions between Sertoli and germ cells are controlled by the neuroendocrine axis: hypothalamus – hypophysis – testis. Have differing roles in spermatogenesis: the gonadotropins, follicule-stimulating hormone (FSH) during early stages of spermatogenesis and luteinizing hormone (LH) during late stages of spermatogenesis.43 Sertoli cells express receptors for sex steroids and follicle-stimulated hormone (FSH), mediating the hormone's effect to the germ cells, testosterone which acts via feedback mechanisms to FSH-dependent steroidogenesis and the importance of LH for the regulation of androgen production.39 The steroids, under the control of FSH, interact in this system in different ways: low doses of estrogens (E2) stimulate the spermatogonial –stem cell-proliferation; 11-ketotestosterone (11-KT) controls the initiation of meiosis; progestins, under the control of LH, are involved in spermiogenesis and spermiation.38,39,43
Annual Reproductive Cycles
Most bony fishes have well-defined annual reproductive cycles. During the annual reproductive cycle, spermatogenesis may be divided into 1) early spermatogenesis, when scarce free spermatozoa are in the lumina (Figs. 8E,F; 2) middle spermatogenesis (Figs. 5A-D), when a small population of free spermatozoa is present in the lumina of some tubules; and 3) late spermatogenesis (Figs. 2–4, 6, 7, 9), when most of the tubules and the efferent duct are full of free spermatozoa.4,44
During early spermatogenesis, Sertoli cells, spermatogonia A and B, and spermatocytes proliferate. In contrast, during late spermatogenesis, the number of proliferative spermatogonia and Sertoli cells decrease. The morphology of the testicular germinal epithelium changes during the annual reproductive cycle, reflecting reproductive seasonality (Fig. 1). It has long been assumed that when fish were not spawning, the gonads were in a resting stage, but, rather than resting, dividing spermatogonia are active during this stage, as it was described in the testes of the swamp eel, Synbranchus marmoratus, during the so-called resting period when the fish was not reproducing.45 These authors also used an immunocytochemical technique to detect proliferating cell nuclear antigen (PCNA) which labeled both spermatogonia and Sertoli cells during the resting stage, indicating that these cells may divide. The gonads of bony fishes never rest. Thus, the frequently used term “resting stage” does not apply to fish gonads and should no longer be used, as is indicated by newly developed information on cell cycles and accumulating evidence that fish Sertoli cells do divide.4
The determination of mature reproductive stage based in germ cells and alterations of germinal epithelium is an important tool for characterization of reproductive biology in teleosts.8,18
Histological examination of testis structure in the Common Snook, Centropomus undecimalis19,46 initially led to the definition of continuous and discontinuous germinal epithelia (Figs. 1, 4A-C) during the annual reproductive cycle. However, since the initial description the terminology has been updated4 and now reflects upon these changes taking place in the germinal epithelium. Hence, the initial terminology Early Maturation, Mid maturation and Late maturation correspond to Early germinal epithelium development, Mid germinal epithelium development and Late germinal epithelium development. As the Regressed Phase ends, the fish testis begins to develop, spermatogonial divisions and meiosis are initiated and lobules elongate. There is a continuous population of Sertoli cells, in association with germ cells, along the lengths of lobules. That is, the germinal epithelium is continuous between the testis ducts and the testis periphery as it is composed of a “continuous” population of Sertoli cells and germ cells, and the meiotic germ cells are within spermatocysts. This is the Early germinal epithelium development Phase in the annual reproductive cycle. As development continues, however, the germinal epithelium remains continuous distally in the growing, distal lobule termini, near the edge of the testis. However, it becomes discontinuous proximally near the testis ducts where germ cells mature, becoming sperm that are released into the lumen of the lobules. Therefore, as sperm mature and are released, newly formed spermatocysts do not replace those from which sperm were released. The presence of a discontinuous germinal epithelium proximally and a continuous germinal epithelium distally designates the Mid germinal epithelium development Phase during the annual reproductive cycle. Then, a discontinuous germinal epithelium is observed to extend to the testis periphery, and this defines the Late germinal epithelium development Phase of the annual reproductive cycle,46 basic changes illustrated in Figure 1.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank the histology staff of the Florida Fish and Wildlife Research Institute, Noretta Perry, and Yvonne Waters; and of the Facultad de Ciencias, Universidad Nacional Autónoma de México, to Gabino De la Rosa-Cruz and Adriana García-Alarcón, for their great quality in histological processing. We are grateful to Lynne French for the accurate drawing of the Fig. 1, to Ana Isabel Bieler Antolin for the helpful technical assistance with digital photography; and José Antonio Hernández Gómez for the excellent digital preparation of histological figures.
References
- 1. Parenti LR, Lo Nostro FL, Grier HJ. Reproductive histology of Tomeurus gracilis Eigenmann, 1909 (Teleostei: Atherinomorpha: Poeciliidae) with comments on evolution of viviparity in atherinomorph fishes. J Morphol 2010; 1(11):1388–1406; PMID:20862693; http://dx.doi.org/ 10.1002/jmor.10886 [DOI] [PubMed] [Google Scholar]
- 2. Billard R. Spermatogenesis and spermatology of some teleost fish species. Reprod Nutr Develop 1986; 26:877-920; http://dx.doi.org/ 10.1051/rnd:19860601 [DOI] [Google Scholar]
- 3. Pudney J. Spermatogenesis in nonmammalian vertebrates. Microsc Res Techniq 1995; 32:459-497; PMID:8605396 [DOI] [PubMed] [Google Scholar]
- 4. Grier HJ, Uribe MC. Chapter 4. Male Reproductive System: Testis, Spermatogenesis and Testicular Cycles. In: Barrie Jamieson. (Editor). Reproductive Biology and Phylogeny of Fish (Agnatha and Osteichthyes). Science Publishers, Inc. Enfield. NH, USA; Plymouth, UK: 2009. pp:119-142. [Google Scholar]
- 5. Schulz RW, França LR, Lareyre JJ, Legac F, Chiarini-Garcias H, Nobrega RH, Miura T. Spermatogenesis in Fish. Gen Comp Endocrinol 2010; 165(3):390-411; PMID:19348807; http://dx.doi.org/ 10.1016/j.ygcen.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 6. Grier HJ, Uribe MC, Parenti LR, De La Rosa-Cruz G. Fecundity, the germinal epithelium, and folliculogenesis in viviparous fishes. InUribe MC, Grier HJ. (Eds). Viviparous Fishes. New Life Publications Homestead, FL, USA: 2005. Pp:191-126. [Google Scholar]
- 7. Grier HJ. Comparative organization of Sertoli cells including the Sertoli cell barrier. In Russell LD, Griswald MD. (Eds), (The Sertoli Cell. Cache River Press, Clearwater, FL: 1993. Pp:703-739. [Google Scholar]
- 8. Grier HJ. Cellular organization of the testis and spermatogenesis in fishes. Am Zool. 1981; 21:345-357. [Google Scholar]
- 9. Uribe MC. Spermatogenesis and Male Reproductive System. Urodela. In: Ogielska M. (Editor). Reproduction of Amphibians. Science Publishers, Inc Enfield, Chapter 3 2009. Pp:100-124. [Google Scholar]
- 10. Schulz RW, Menting S, Bogerd J, França LR, Vilela DAR, Godinho HP. Sertoli cell proliferation in the adult testis—evidence from two fish species belonging to different orders. Biol Reprod 2005; 73:891-898; PMID:16000552; http://dx.doi.org/ 10.1095/biolreprod.105.039891 [DOI] [PubMed] [Google Scholar]
- 11. Grier HJ, Linton JR. Ultrastructural indentification of the Sertoli cell in the testis of the Northern Pike, Esox lucius. Am J Anat 1977; 149:282-288; PMID:879048 [DOI] [PubMed] [Google Scholar]
- 12. Grier HJ, Hurk Van Den R, Billard R. Cytological identification of cell types in the testis of Esox lucius and E. niger. Cell Tissue Res 1989; 257:491-496 [Google Scholar]
- 13. Patiño R, Takashima F. Gonads. In Takashima F, Hibiya T. (Eds). An Atlas of Fish Histology, Normal and Pathological Features. Kodanska Ltd/ Gustav Fisher Verlag; Tokyo/Stuttgart/New York: 1995. Pp:128-153. [Google Scholar]
- 14. Fawcett DW, Jensh RP. Epithelium. Concise Histology. 2nd Edition Arnold Publishers, London: 2002. Pp:29-41. [Google Scholar]
- 15. Hurk van den R. The localization of steroidogenesis in the testis of oviparous and viviparous teleosts. Proceedings Koninklijke Nederlandse Akademie Wetensch Series C 76. 1973. 270-279. [Google Scholar]
- 16. Parenti LR, Grier HJ. Evolution and phylogeny of gonad morphology in bony fishes. Integ Comp Biol 2004; 44:333-348; PMID:21676719; http://dx.doi.org/ 10.1093/icb/44.5.333 [DOI] [PubMed] [Google Scholar]
- 17. Grier HJ, Linton JR, Leatherland F, Devlaming VL. Structural evidence for two difference testicular types in teleost fishes. Am J Anat. 1980; 159:331-345; PMID:7211713; http://dx.doi.org/ 10.1002/aja.1001590307 [DOI] [PubMed] [Google Scholar]
- 18. Vergilio CS, Moreira RV, Carvalho Cevmelo EJT. Characterization of mature testis and sperm morphology of Gymnotus carapo (Gymnotidae, Teleostei) from the southeast of Brazil. Acta Zool 2012; 94:364-370 [Google Scholar]
- 19. Taylor RG, Grier HJ, Whittington JA. Spawning rhythms of common snook in Florida. J Fish Biol 1998. 53:502-520; http://dx.doi.org/ 10.1111/j.1095-8649.1998.tb00998.x [DOI] [Google Scholar]
- 20. Brown-Peterson N, Grier HJ, Overstreet R. Annual changes in the germinal epithelium determine reproductive classes in male cobia, Rachycentron canadum. J Fish Biol 2002. 60:178-202 [Google Scholar]
- 21. Rey Vázquez G, Dacuña RH, Meijide FJ, Guerrero G. Spermatogenesis and changes in testicular structure during the reproductive cycle in Cichlasoma dimerus Teleostei, Perciformes). Acta Zool. 2012. 93:338-350 [Google Scholar]
- 22. Downing AL, Burns JR. Testis morphology and spermatozeugma formation in three genera of viviparous halfbeaks: Nomorhamphus, Dermogenys, and Hemirhamphodon (Teleostei: Hemirhamphidae). J Morphol. 1995. 225:329-343; http://dx.doi.org/ 10.1002/jmor.1052250305 [DOI] [PubMed] [Google Scholar]
- 23. Uribe MC, Grier HJ, Mejía-Roa V, Yánez-García N, García-Alarcón A, De La Rosa-Cruz G, Aguilar-Morales M. Functional structure of the testis and spermatogenesis of goodeids and poeciliids. In: Uribe MC. and Grier HJ. (Editors) Viviparous Fishes II. New Life Publications. Homestead, FL. USA: 2010. Pp:151-172. [Google Scholar]
- 24. Vicentini CA, Muñoz ME, Bastos Franceschini, Vicentini I. Ultrastructural Aspects of Spermatogenesis in Phalloceros audimaculatus (Teleostei, Poeciliidae). Int J Morphol 2010; 28(3):951-956. [Google Scholar]
- 25. Grier HJ, Fitzsimmons JM, Linton JR. Structure and ultrastructure of the testis and sperm formation in goodeid teleosts. J Morphol 1978; 156:419-438; PMID:671556; http://dx.doi.org/ 10.1002/jmor.1051560306 [DOI] [PubMed] [Google Scholar]
- 26. Weltzien FA, Taranger GL, Karlsen O, Norberg B. Spermatogenesis and related plasma androgen levels in Atlantic halibut (Hippoglossus hippoglossus L.). Comp Biochem Phys 2002; Part A 132:567-575; PMID:12044766 [DOI] [PubMed] [Google Scholar]
- 27. Hatef A, Alavi SMH, Novein SB, Poorbagher H, Alipour AR, Pourkazemi M, Linhart O. Morphology and fine structure of Acipenser persicus (Acipenseridae, Chondrostei) spermatozoon: Inter-species comparison in Acipenseriformes. Anim Reprod Sci 2011; 123(1-2):81-88; PMID:21144681; http://dx.doi.org/ 10.1016/j.anireprosci.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 28. Hatef A, Alavi SMH, Rodina M, Linhart O. Morphology and fine structure of the Russian sturgeon, Acipenser gueldenstaedtii (Acipenseridae, Chondrostei) spermatozoa. J Appl Ichthyol 2012; 28(6):978-983. [Google Scholar]
- 29. Jamieson BGM. Fish Evolution and Systematics: Evidence from Spermatozoa. Cambridge University Press, Cambridge: 1991. 319 . [Google Scholar]
- 30. Martins YS, De Moura DF, Santos GB, Rizzo E, Bazzoli N. Comparative folliculogenesis and spermatogenesis of four teleost fish from a Reservoir in south-eastern Brazil. Acta Zool 2010; 91:466-473; http://dx.doi.org/ 10.1111/j.1463-6395.2009.00437.x [DOI] [Google Scholar]
- 31. Fishelson L, Baldwin CC, Hastings PA. Gonad morphology, gametogenesis, and reproductive modes in fishes of the tribe Starksiini (Teleostei, Blenniiformes). J Morphol 2013; 274(5):496-511.; PMID:23293058; http://dx.doi.org/ 10.1002/jmor.20110 [DOI] [PubMed] [Google Scholar]
- 32. Papah MB, Kisia SM, Ojoo RO, Makanya AN, Wood CM, Kavembe GD, Maina JN, Johannsson OE, Bergman HL, Laurent P, et al. Morphological evaluation of spermatogenesis in Lake Magadi tilapia (Alcolapia grahami): a fish living on the edge. Tissue Cell 2013; 45:371-382; PMID:23916093; http://dx.doi.org/ 10.1016/j.tice.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 33. Spadella MA, Oliveira C, Quagio-Grassiotto I. Occurrence of biflagellate spermatozoa in the Siluriformes families Cetopsidae, Aspredinidae, and Nematogenyidae (Teleostei: Ostariophysi). Zoomorphology 2006; 125:108-118; http://dx.doi.org/ 10.1007/s00435-006-0018-9 [DOI] [Google Scholar]
- 34. Matos E, Santos MNS, Azevedo C. Biflagellate spermatozoon structure of the hermaphrodite fish Satanoperca jurupari (Heckel, 1840) (Teleostei, Cichlidae) from the Amazon River. Braz J Biol 2002. 62(4B):847-852; PMID:12659036 [DOI] [PubMed] [Google Scholar]
- 35. Jamieson BGM. Ultrastructure of spermatozoa: Acanthopterygii: Mugilomorpha and Atherinomorpha. In: Jamieson B. (Ed). “Reproductive Biology and Phylogeny of Fish (Agnatha and Osteichthyes)”. Science Publishers, Inc Enfield. NH, USA; Plymouth, UK: 2009. pp:447-501 [Google Scholar]
- 36. Gardiner DM. The origin and fate of spermatophores in the viviparous teleost Cymatogaster aggregata (Perciformes: Embiotocidae). J Morphol 1978; 155:157-172. [DOI] [PubMed] [Google Scholar]
- 37. Pecio A, Lahnsteiner F, Rafinski J. Ultrastructure of the epithelial cells in the aspermatogenic part of the testis in Mimagoniates barberi (Teleostei: Characidae: Glandulocaudinae) and the role of their secretions in spermatozeugmata formation. Ann Anat 2001; 183:427-435; PMID:11677808 [DOI] [PubMed] [Google Scholar]
- 38. Nagahama Y. Endocrine regulation of gametogenesis in fish. Int J Dev Biol 1994; 38(2):217-29; PMID:7981031 [PubMed] [Google Scholar]
- 39. Schulz RW, Miura T. Spermatogenesis and its endocrine regulation. Fish Physiol Biochem 2002. 26:43-56; http://dx.doi.org/ 10.1023/A:1023303427191 [DOI] [Google Scholar]
- 40. Miura T, Miura CI. Molecular control mechanisms of fish spermatogenesis. Fish Physiol Biochem 2003. 28:181-186; http://dx.doi.org/ 10.1023/B:FISH.0000030522.71779.47 [DOI] [Google Scholar]
- 41. Miura T, Higuchi M, Ozaki Y, Ohta T, Miura C. Progestin is an essential factor for the initiation of the meiosis in spermatogenetic cells of the eel. Dev Biol 2006; 103(19):7333-7338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ozaki Y, Higuchi M, Miura C, Yamaguchi S, Tozawa Y, Miura T. Roles of 11β-hydroxysteroid dehydrogenase in fish spermatogenesis. Endocrinology 2006; 147(11):5139-5146; PMID:16887910; http://dx.doi.org/ 10.1210/en.2006-0391 [DOI] [PubMed] [Google Scholar]
- 43. Knapp R, Carlisle SL. Testicular function and hormonal regulation in fishes. In: Norris DO, Lopez KH. (Eds). Volume 1: Fishes, Hormones and Reproduction in Vertebrates. Ac Press; 2011. Pp:43-63 [Google Scholar]
- 44. Chavez-Pozo E, Mulero V, Meseguer J, Ayala AG. An overview of cell renewal in the testis throughout the reproductive cycle of a seasonal breeding teleost, the Gilthead Seabream (Sparus aurata L.). Biol Reprod 2005; 72:593-601; PMID:15548730 [DOI] [PubMed] [Google Scholar]
- 45. Nostro F Lo. Grier HJ, Andreone L, Guerrero GA. Involvement of the gonadal germinal epithelium during sex reversal and seasonal testicular cycling in the protogynous swamp eel, Synbranchus marmoratus Bloch, 1795. (Teleostei, Synbranchidae). J Morphol 2003; 258:107-126; PMID:12740902 [DOI] [PubMed] [Google Scholar]
- 46. Grier HJ, Taylor RG. Testicular maturation and regression in the common snook. J Fish Biol 1998; 53:521-542; http://dx.doi.org/ 10.1111/j.1095-8649.1998.tb00999.x [DOI] [Google Scholar]


