Abstract
Cardiovascular disease is a leading cause of death and reduced quality of life worldwide. Arterial vessels are a primary target for endothelial dysfunction and atherosclerosis, which is accompanied or even driven by increased oxidative stress. Recent research in this field identified different sources of reactive oxygen and nitrogen species contributing to the pathogenesis of endothelial dysfunction. According to lessons from the past, improvement of endothelial function and prevention of cardiovascular disease by systemic, unspecific, oral antioxidant therapy are obviously too simplistic an approach. Source- and cell organelle-specific antioxidants as well as activators of intrinsic antioxidant defense systems might be more promising. Since basic research demonstrated the contribution of different inflammatory cells to vascular oxidative stress and clinical trials identified chronic inflammatory disorders as risk factors for cardiovascular events, atherosclerosis and cardiovascular disease are closely associated with inflammation. Therefore, modulation of the inflammatory response is a new and promising approach in the therapy of cardiovascular disease. Classical anti-inflammatory therapeutic compounds, but also established drugs with pleiotropic immunomodulatory abilities, demonstrated protective effects in various models of cardiovascular disease. However, results from ongoing clinical trials are needed to further evaluate the value of immunomodulation for the treatment of cardiovascular disease.
Keywords: cardiovascular disease, endothelial dysfunction, oxidative stress, inflammation, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide analogs
1. Oxidative Stress in Cardiovascular Disease and Inflammation
1.1. Introduction
The majority of cardiovascular diseases are accompanied by an imbalance between the formation of reactive oxygen species (ROS, including superoxide, hydrogen peroxide as well as precursor products peroxynitrite or hypochlorous acid) and antioxidant enzymes [1,2], leading to a deviation from the steady state [3]. More recent evidence suggests that adverse redox signaling and oxidative stress are not only side effects of the progression of cardiovascular disease but even potent triggers of their development and pathogenesis [4,5]. According to the “kindling radical” hypothesis (or “bonfire” concept), the formation of ROS may trigger the activation of additional sources of ROS in certain disease conditions or during the aging process [6,7]. According to recent reports, vascular dysfunction in general, but hypertension and coronary artery disease may also be linked to inflammation or low-grade activation of the immune system [8,9]. Uncoupling of endothelial nitric oxide synthase (eNOS) is a hallmark of most cardiovascular disease [10,11] and endothelial dysfunction in coronary and peripheral vessels, measured by acetylcholine-dependent plethysmography or flow-mediated dilation, is an early predictor of cardiovascular events [12,13]. eNOS function is regulated by many different factors such as subcellular localization, calcium levels, binding of different co-factors (e.g., BH4, FAD, FMN, NADPH, zinc), and other proteins (e.g., calmodulin, heat shock proteins). Regulation of eNOS activity by so-called “redox switches” is of great interest for the present review—the oxidative depletion of tetrahydrobiopterin (BH4), oxidative disruption of the dimeric eNOS complex by oxidation of the zinc-sulfur complex, S-glutathionylation of a cysteine in the reductase domain, and adverse phosphorylation at Thr495/Tyr657, as well as ROS-triggered increases in levels of the endogenous eNOS inhibitor asymmetric dimethylarginine (ADMA) (for detailed review see [6,7]). Another focus of research in the cardiovascular field is the “repair” of vascular damage by improvement of the function of endothelial progenitor cells by drugs with antioxidant and other pleiotropic properties [14] or infusion of these cells after a severe insult such as myocardial infarction [15,16]. This topic will only be touched on with some examples but not explored in detail. The major part of this review will discuss antioxidant therapeutic interventions that prevent eNOS uncoupling, thereby normalizing endothelial function in particular and improving cardiovascular disease in general. We will emphasize the importance of low-grade inflammation in the development of endothelial dysfunction and cardiovascular disease and discuss the contribution of specific inflammatory cells and their cytokine profiles to the development and progression of cardiovascular disease.
1.2. Inflammation, Oxidative Stress, and Endothelial Dysfunction
The first description of the role of oxidative stress in the development and progression of cardiovascular disease in an experimental model of hypercholesterolemia was published by Harrison and Ohara [17,18]. According to the abovementioned concept of “kindling radicals” or the “bonfire” hypothesis, the initial formation of superoxide (e.g., from phagocytic NADPH oxidases of infiltrated leukocytes) and subsequent formation of peroxynitrite most likely triggers further damage such as eNOS uncoupling, converting this beneficial nitric oxide synthase into a detrimental superoxide-producing enzyme (Figure 1) [6,8]. Likewise, ROS from infiltrated immune cells can activate or induce expression of vascular cell oxidases such as Nox1, Nox2, or Nox4 (isoform specific catalytic subunits of NADPH oxidases), or mediate the oxidative conversion of the xanthine dehydrogenase to the oxidase form [6,19]. This ROS-induced ROS formation is well known for cross-activation of mitochondrial ROS formation by dysfunctional mitochondria [20]. Mitochondrial ROS formation and release can be stimulated by thiol-oxidation in different mitochondrial structures (e.g., mitochondrial permeability transition pore constituents such as cyclophilin D, p66shc or monoamine oxidases); xanthine dehydrogenase is converted to the oxidase form by oxidation of critical thiol residues; the protective action of eNOS to produce •NO is switched to adverse superoxide formation by oxidative depletion of BH4, adverse phosphorylation by redox-activated kinases, S-glutathionylation ,or oxidative disruption of the zinc-sulfur complex at the dimer binding interface, called the “uncoupling” process. These changes (increased vascular oxidative stress and release of inflammatory signaling molecules) will lead to endothelial cell activation and priming for the adhesion of additional immune cells as well as platelets and switch the vasodilatory, antiaggregatory, and antiatherosclerotic phenotype of the endothelium to a vasoconstrictory, proaggregatory, and proatherosclerotic one.
Figure 1.
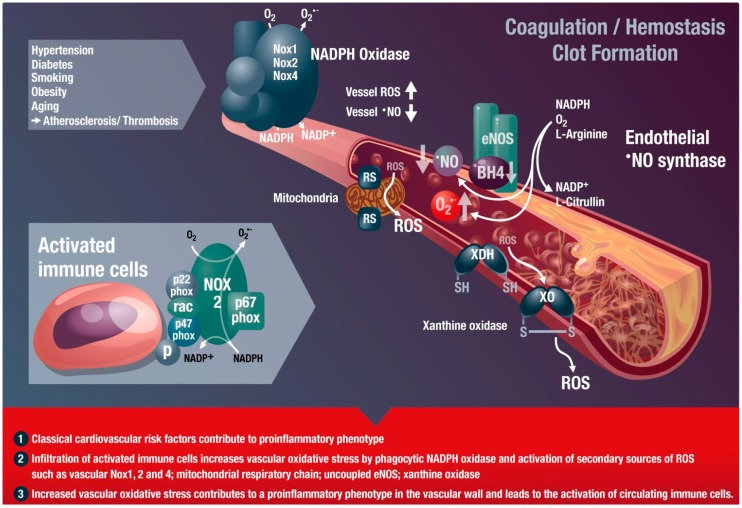
Inflammatory cells, vascular dysfunction, and atherothrombosis. The scheme illustrates the activation of immune cells and recruitment to vascular tissues by classical cardiovascular risk factors, leading to activation of secondary vascular ROS sources such as NADPH oxidase (Nox1, Nox2, and Nox4), xanthine oxidase (conversion of the dehydrogenase (XDH) to the oxidase (XO) form), mitochondria (via mitochondrial redox switches (RS)), and uncoupled eNOS (oxidative depletion of tetrahydrobiopterin (BH4) and other redox switches), all of which contribute to vascular dysfunction. Immune cells such as monocytes need the functional phagocyte-type NADPH oxidase (Nox2) in order to infiltrate vascular tissues. ROS produced by this activated Nox2 from infiltrated immune cells will activate secondary vascular ROS sources in a redox-sensitive fashion (for review see [19,30]). These processes lead to late-stage cardiovascular complications such as atherosclerosis with plaque formation and thrombosis. Modified from [8]. With permission by Bentham Science Publisher. Copyright © 2014, Eureka Science Ltd.
As described above, ROS formation is not only a side effect of cardiovascular diseases but directly contributes to the disease progression in many ways. For example, the induction of endothelial dysfunction by oxidative modification of eNOS or its cofactors as well as redox stimulation of inflammatory cascades fosters the progression of these cardiovascular diseases (Figure 1). Most immune cells express high levels of functional NADPH oxidases and are capable of producing ROS at much higher levels than vascular cells [21,22]. The important role of phagocytic NADPH oxidase in this process was demonstrated by the fact that white blood cells with dysfunctional Nox2 were not able to infiltrate the vascular wall and induce vascular oxidative stress and damage [22,23], e.g., by oxidative conversion of xanthine dehydrogenase to the oxidase form, uncoupling of eNOS, or stimulation of mitochondrial ROS formation and release by specific redox switches [6]. On the other hand, there is clear evidence that ROS formation per se contributes to a pro-inflammatory phenotype, since mitochondrial superoxide/hydrogen peroxide formation has the ability to activate immune cells [24,25,26]. ROS play an important role in inflammation and tissue damage [27]. There is also increasing evidence of a close interaction between vascular oxidative stress and inflammation during the aging process, leading to a vicious cycle in the aging vasculature [28]. By this crosstalk, infiltrated immune cells promote vascular oxidative stress, lead to endothelial cell activation, and prime the endothelium for the adhesion of additional leukocytes and platelets [8], which is of great importance for aging-associated endothelial dysfunction [29]. Vice versa, oxidative stress is a hallmark of all cardiovascular disease and will also lead to endothelial cell activation, priming for adhesion and infiltration of immune cells as well as activation of these infiltrated immune cells. Accordingly, most cardiovascular disease displays a low-grade inflammatory phenotype of the vasculature.
Different immune cells have been reported to contribute to the development of cardiovascular disease, but since they interact with each other, the individual impact of each cell type on the development of cardiovascular disease remains elusive. The contribution of B- and T-cells to the development of hypertension by angiotensin-II infusion was demonstrated by RAG-1−/− mice [22]. Likewise, the onset of angiotensin-II induced hypertension and vascular oxidative stress was also attenuated by removal of myelomonocytic cells [23]. Of note, immune suppressive treatment of patients with rheumatoid arthritis or psoriasis was associated with a reduction of systolic blood pressure [31], underlining the clinical impact of a direct contribution of the immune system to vascular dysfunction.
1.3. Chronic Autoimmune Diseases Associated with Cardiovascular Disease
Chronic autoimmune diseases such as systemic lupus erythematodes, rheumatoid arthritis, and severe psoriasis are associated with an increased risk for cardiovascular events [32,33,34,35]. Importantly, psoriasis was defined as an independent risk factor in addition to the classical cardiovascular risk factors such as smoking, obesity, and diabetes [36]. The European League against Rheumatism even recommends the management of cardiovascular risk in inflammatory arthritis in their guidelines [37]. As an early predictor of cardiovascular events, impaired vascular function was observed under chronic inflammation, which was evident in patients with rheumatoid arthritis by a significant increase in the intima-media thickness [38] or impaired endothelial function measured by flow-mediated dilatation in patients with psoriasis [39]. Therefore, recent clinical trials have demonstrated that increased cardiovascular mortality in patients with chronic inflammatory disease can be managed by targeting specific cytokines or activation of specific immune cells, e.g., in psoriasis the IL-17/IL-23 axis [40,41,42], in systemic lupus erythematodes IL-17A signaling [43], and in rheumatoid arthritis the IL-6, TNF-α, and IL-17A cascades [44,45]. These data provide a feasible link between cardiovascular disease and the chronic autoimmune diseases (also reviewed in [32,34]).
2. Classical Antioxidants and New Strategies to Modulate Oxidative Stress
2.1. Classical Antioxidants
Based on the oxidative stress concept in cardiovascular, neurodegenerative, metabolic, and inflammatory disease [5,7,8], numerous studies were conducted. In vitro and animal studies of these diseases were performed in order to characterize the cytoprotective or therapeutic benefit of antioxidants and to promote phytochemicals, functional foods, and antioxidant (vitamin) supplements. However, antioxidants have failed to show any therapeutic benefit in most large clinical trials that were conducted according to modern standards [46], such as HOPE (Heart Outcome Prevention Evaluation) and HOPE-TOO (Heart Outcome Prevention Evaluation—The Ongoing Outcomes), which demonstrated that vitamin E causes more heart failure and left heart decompensation [47,48,49,50] (for review see [51]). A prospective study with vitamin C in post-menopausal women with diabetes mellitus even demonstrated an increased incidence of cardiovascular events and mortality under antioxidant therapy [52]. The SAINT I trial investigated the therapeutic benefit of the synthetic antioxidant, NXY-059, in acute ischemic stroke but failed to show any neuroprotective effect [53]. According to Bjelakovic and coworkers, meta-analysis of 68 randomized trials with 232,606 participants revealed that the use of lipid-soluble antioxidants without medical indication may even increase mortality in adults [54]. Another meta-analysis of 14 randomized trials with 170,525 individuals by the same author demonstrated a similar trend—lipid-soluble antioxidants increased the mortality of gastrointestinal cancer patients [55]. However, other meta-analyses support the beneficial effects of vitamin C in specific disease conditions or disease-associated impairment of functional parameters, e.g., on the survival of women with breast cancer [56] or on endothelial function in patients with atherosclerosis, diabetes, and heart failure [57].
These large-scale clinical trials on chronic oral antioxidant supplementation are contrasted by multiple small cohort studies with acute (parenteral) administration of antioxidants with highly beneficial effects on the surrogate parameters of disease (e.g., endothelial dysfunction) in chronic smokers or patients with diabetes or coronary artery disease [12,58,59,60] (for review see [51]). The advantage of parenteral administration of water-soluble antioxidants is that high plasma concentrations of the antioxidant are achieved [61], thereby omitting the complications of oral absorption (time-lag, limited capacity) and insufficient compliance. High-dose intravenous infusion of vitamin C also improved endothelial function in patients with Kawasaki disease [62], kidney dysfunction [63], hypertension [64], liver cirrhosis, and portal hypertension [65]. Moreover, parenteral application of vitamin C has also proven to have clinical effects in patients with allergies [66], sudden hearing loss [67], breast cancer, infection, and pancreatitis [68].
A positive example of the beneficial effect of chronic antioxidant therapy is vitamin D. Lack of vitamin D is endemic in the human population and epidemiological data indicate that deficiency of this vitamin is associated with cardiovascular disease [69]. There is some evidence from interventional trials demonstrating that supplementation of vitamin D is beneficial to endothelial function [70,71], blood pressure [72], and cardiac hypertrophy [73,74] in humans. Furthermore, a recent Cochrane analysis revealed that vitamin D supplementation significantly reduces cardiovascular mortality in elderly people [75]. Nevertheless, further large-scale randomized placebo-controlled clinical trial are needed to elucidate the cardiovascular protective effects of vitamin D. In contrast to other vitamins, deficiency of vitamin D is very common, especially in older individuals [76], which might be the explanation for the beneficial effects of vitamin D, especially on cardiovascular disease in the elderly [77].
Based on the disappointing results of most large-scale clinical trials (HOPE, HOPE-TOO) [47,49] (reviewed in [51]), with chronic oral antioxidants supplementation the question arises whether the oxidative stress hypothesis in pathogenesis and disease progression is wrong? We think the answer is no and possible explanations for the lack of clinical efficacy of antioxidants in these studies might be that: (1) vitamins C and E act as pro-oxidants; (2) the coronary artery disease of the patients included in the studies is already irreversible; (3) the coronary artery disease patients are already being treated with drugs displaying antioxidant properties (for example, ACE inhibitors and angiotensin type 1 receptor blockers; see also Section 3); (4) chronic antioxidant therapy inhibits intrinsic ischemic preconditioning, which relies on ROS formation; and (5) oral vitamin treatment does not result In high enough concentrations of the antioxidants at the place of oxidative stress (summarized from [5]). Another important drawback of classical antioxidants may be the slow reaction between O2•− and vitamins C and E (with rate constants of 3.3 × 105 and 4.9 × 103 M−1·s−1, respectively, compared with 1.9 × 1010 M−1·s−1 for the reaction between •NO and O2•−) [78]. Finally, in most of the abovementioned large-scale clinical trials on the use of chronic oral antioxidant supplementation, the compliance of the patients was not controlled (e.g., by measurement of plasma levels of the antioxidants). The important role of controlled antioxidant plasma levels became evident in the EPIC Norfalk study, demonstrating that vitamin C concentrations in the blood inversely correlate with all-cause mortality in healthy volunteers [79]. In addition, there was an inverse correlation between circulating vitamin C concentrations and risk of stroke as reported by a meta-analysis [80]. According to a concept put forward by Lykkesfeldt and colleagues [81], better results of antioxidant therapy might be expected under conditions of antioxidant deficiency (e.g., for vitamin C and E) [82], or often encountered for vitamin D [76]. Some of these reasons would favor a proper prediagnosis of patients for blood levels of antioxidants, strict monitoring of this parameter during antioxidant therapy, and acute infusion of vitamin C in accordance with the observations and advantages of parenteral use discussed above. According to a recent review on the use of antioxidants in translational medicine, future antioxidant strategies will not be based on the classical antioxidant vitamins (apart from some acute situations with subclinical deficiencies as well as parenteral instead of oral therapy) but rather on the activation of endogenous antioxidant enzyme systems, inhibition of critical ROS sources (e.g., NADPH oxidase), the repair of oxidatively damaged structures, or site-directed antioxidant approaches [51].
2.2. New Antioxidant Strategies
Direct and cell organelle-specific targeting of ROS formation is a new promising strategy. A prominent example is scavenging mitochondrial ROS by mitochondria-targeted antioxidants such as mitoquinone (mitoQ) [83,84], a quinone that is coupled to a triphenylphosphonium group to facilitate mitoQ accumulation in mitochondria by up to 10,000-fold. MitoQ showed beneficial effects in an animal model and in human cells from patients with chronic obstructive pulmonary disease [85], improved nitrate-tolerance-associated side effects of nitroglycerin therapy in rats [86], and normalized endothelial function and cardiac hypertrophy in stroke-prone spontaneously hypertensive rats [87]. Moreover, MitoQ showed neuroprotective effects in experimental amyotrophic lateral sclerosis [88], suppressed NLRP3 inflammasome-mediated inflammatory cytokines in a murine colitis model [89], beneficially influenced nephropathy in diabetic mice [90], and prevented cardiac ischemia-reperfusion injury in rats [84]. According to recent data, another mitochondria-targeted antioxidant, mitoTEMPO, prevented adverse effects of angiotensin-II in experimental hypertension [91]. Similar beneficial effects have been reported for the use of mitochondria-targeted SOD mimetics such as Mn(III) 5,10,15,20-tetrakis(N-methylpyridinium-2-yl)porphyrin (MnTM-2-PyP5+) in various disease models [92,93]. Several compounds of the class of mitochondria-targeted antioxidants are currently in late phase clinical trials (for review see [92,93,94,95]; for ongoing clinical trials visit www.clinicaltrials.gov). A limitation of the use of mitochondria-targeted antioxidants might be that viable mitochondria with intact membrane potential are required for their mitochondrial accumulation, which could interfere with their uptake, especially in dysfunctional, ROS-producing mitochondria. A similar idea provides the basis for endothelium-targeted antioxidants. Shuvaev et al. demonstrated that targeting of SOD, but not catalase, to the endothelium reversed angiotensin II-induced endothelial dysfunction [96], nicely demonstrating that O2•− is a more harmful species in the vascular system than H2O2. The antioxidant enzymes SOD and catalase were conjugated with an antibody against PECAM-1 (platelet/endothelial cell adhesion molecule-1) to ensure endothelial binding. Another strategy would be to covalently bind SOD mimetics or antioxidants to heparin [97], which will lead to the binding of these antioxidant compounds to heparin-binding sites on the endothelial cell layer [98,99].
Modulation/regulation of endogenous antioxidant defense systems or ROS sources by miRNAs (e.g., antagomirs), epigenetic drugs (e.g., modulators of histone acetyl transferases or deacetylases), or phytochemicals are other completely new antioxidant strategies [100,101,102,103,104,105,106]. Resveratrol, a phytochemical antioxidant, was previously regarded as a direct ROS scavenger, but more recent data revealed that it mostly acts via indirect antioxidant mechanisms [107], e.g., by modulation of gene expression via miRNAs, epigenetic modifications, and direct effects on proteins of the DNA repair machinery [108,109,110]. Besides resveratrol, there are hundreds of these phytochemical antioxidant compounds, in many cases with proven therapeutic effects and in some cases even with mechanistic explanations for these observed clinical effects. One prominent example is Ginkgo biloba, which has been in clinical use for a long time, especially for dementia therapy but also for its positive effects on cardiovascular disease [111].
Other antioxidant strategies are only briefly mentioned here (we refer to the respective review articles, e.g., [51]) and are based on: (1) the inhibition of disease-relevant ROS sources such as inhibitors of NADPH oxidase (Nox) enzymes [112,113,114,115] or xanthine oxidase [116,117]; (2) upregulation of the endogenous antioxidant defense system such as Nrf2 agonists [118]; and (3) repair of oxidatively damaged protein structures as exemplified by activators of heme-deficient or oxidized soluble guanylyl cyclase [119,120,121]. According to Stocker and colleagues, an antioxidant should ideally be recycled by cellular reducing systems, act catalytically to prevent its consumption, or induce endogenous antioxidant defense systems rather than act as a direct scavenger (for review see [122]).
3. Antioxidants 2.0—Pleiotropic Antioxidant Effects of Established Drugs
It would be beyond the scope of this review to discuss all of the important representatives of this group of drugs, so the following will be limited to some examples of new function for old drugs. The pleiotropic antioxidant effects of these compounds are characterized by different modes of action (as already described above): (1) induction of intrinsic antioxidant systems; (2) inhibition of Nox2-dependent ROS formation; and (3) direct ROS scavenging activity. It remains to be established whether some of these pleiotropic antioxidant effects are just a consequence of the primary pharmacological action of the drugs (e.g., lowering of blood pressure). At least in some of the examples the primary pharmacological action of the drugs can be excluded since data were obtained either in cell culture or even with isolated enzymatic systems.
3.1. Statins, ACE-Inhibitors, and AT1-Receptor Blockers
Angiotensin-converting enzyme inhibitors, type 1 angiotensin II receptor antagonists, statins, and many other cardiovascular drugs display pleiotropic indirect antioxidant properties (e.g., inhibition of Nox enzymes and secondary to this prevention of eNOS uncoupling) [123,124]. In more detail, angiotensin-converting enzyme inhibitors and type 1 angiotensin II receptor antagonists increase the bioavailability of •NO by decreased breakdown of bradykinin and activation of the corresponding B2 receptor [125]. They also prevent the activation of the phagocytic and vascular Nox2 enzyme and thereby decrease cellular superoxide, hydrogen peroxide, and peroxynitrite levels [126]. Inhibition of angiotensin-II signaling decreases oxidative stress since angiotensin-II via its receptor leads to the formation of diacylglycerol, a potent endogenous trigger of NADPH oxidase activity [127]. In addition, these drugs confer potent anti-inflammatory effects by interfering with the adhesion of monocytes to the endothelium [128] and even improving the severity of adjuvant arthritis [129]. In summary, angiotensin-converting enzyme inhibitors and type 1 angiotensin II receptor antagonists promote a vasodilatory, antithrombotic, and antiproliferative milieu and improve the function of endothelial progenitor cells [130]. These protective mechanisms might also explain their benefit for the therapy of patients with heart failure [131]. Statins obviously target exactly the same pathophysiological parameters. In patients with cardiovascular disease, statins reduce vascular inflammation and atherothrombosis, which causes cardiovascular events like myocardial infarction [132,133,134]. Statins reduce NADPH oxidase activity in a Rac1-dependent mechanism [135,136] and improve the bioavailability of •NO through increased levels of the eNOS cofactor BH4, decreased levels of the endogenous eNOS inhibitor asymmetric dimethyl-l-arginine, decreased caveolin-1 activity, improved activating eNOS phosphorylation, and upregulation of eNOS mRNA [137]. The beneficial pleiotropic effects of statins are probably based on the induction of the Nrf2-heme oxygenase-1 system [138,139] but improvement of the function of endothelial progenitor cells could also contribute to their protective profile [140].
3.2. Nebivolol, Hydralazine, and Pentaerythrityl Tetranitrate (PETN)
One of the first known antihypertensive drugs was hydralazine, which is today mainly used for the treatment of pre-eclampsia [141]. However, it experienced a “revival” when the company NitroMed introduced their combination drug BiDil containing hydralazine and isosorbide dinitrate. This combination therapy showed an impressive decrease in mortality in African-Americans with severe heart failure, who responded poorly to ACE inhibitors and other standard medications (the study design was based on data from V-HeFT (Vasodilator Heart Failure Trial) and A-HeFT (African-American Heart Failure Trial) [142,143,144]. According to our previous observations, hydralazine is a highly efficient peroxynitrite scavenger and prevents tyrosine nitration [145,146], which may at least contribute to its beneficial effects on nitroglycerin-induced nitrate tolerance [147] and potentially isosorbide dinitrate-associated side effects. Based on these data, we postulate a direct antioxidant property of hydralazine by scavenging peroxynitrite, a potentially harmful oxidant. This provides the rationale for the beneficial effects of the hydralazine/isosorbide dinitrate combination to prevent side effects of the organic nitrate under chronic therapy (e.g., endothelial dysfunction [148]). Recent data support a reaction between peroxynitrite and dihydralazine sulfate [149]. However, there is also evidence for the indirect antioxidant effects of hydralazine by induction of hypoxia-inducible factor-1α, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases [150]. Among other antihypertensive drugs, hydralazine has been found to possess pleiotropic antioxidant effects in patients beyond the direct blood pressure lowering effects [151]. Also, protective effects of hydralazine on endothelial progenitor cell function were reported [152].
The third generation beta-blocker nebivolol was reported to induce vascular nitric oxide formation via stimulation of eNOS activity in ex vivo studies [153,154,155], providing the rationale for improved •NO bioavailability in patients with essential hypertension [156]. In this clinical study a combination therapy of nebivolol/bendrofluazide, in contrast to atenolol/bendrofluazide treatment, improved •NO bioavailability despite a similar degree of blood pressure lowering. We could detect eNOS-stimulating effects of nebivolol neither in cultured endothelial cells nor in hypertensive mice when comparing wild-type controls with eNOS knockout mice (unpublished data, Karbach et al. and Daiber). We have previously shown that nebivolol, in contrast to metoprolol and atenolol, prevents eNOS uncoupling and induction of phagocytic NADPH oxidase activity in white blood cells, vascular oxidative stress, and endothelial dysfunction in hyperlipidemic Watanabe (WHHL) rabbits [157]. In a subsequent study we characterized nebivolol as a potent Nox2 inhibitor in hypertensive rats as well as isolated cells, which are not shared by first- and second-generation beta-blockers [158]. Nebivolol directly interferes with the assembly of Nox2 and cytosolic subunits p47phox, p67phox, and rac1 in the cytoplasmic membrane, suggesting that Nox2 inhibition leads to reduced superoxide formation, prevents eNOS uncoupling and breakdown of nitric oxide by reaction with superoxide, and finally ameliorates endothelial function. This concept goes hand in hand with human data in which nebivolol normalized oxidative stress in hypertensive patients and led to reduced oxidative degradation of nitric oxide [159]. Finally, nebivolol improved the function of early endothelial progenitor cells in experimental myocardial infarction, which could also contribute to its beneficial clinical profile [160].
After the development of pentaerithrityl tetranitrate (PETN) for the U.S. market, it was abandoned, but then used for many years in the former Eastern German Republic. After the reunion of Germany, PETN became the best-selling nitrate on the German market. PETN is the only organic nitrate in clinical use devoid of induction of nitrate tolerance, endothelial dysfunction, and other nitrate-associated side effects in volunteers [161,162] and patients with coronary artery disease [163,164]. The molecular explanation for the beneficial effects of PETN, not shared by other nitrates, is the induction of heme oxygenase-1 [165,166,167,168] in a Nrf2-dependent fashion [169]. PETN also induced extracellular superoxide dismutase [170], prevented vascular complications in experimental diabetes and hypertension [165,169], prevented the progression of atherosclerosis in a rabbit model [171], and inhibited platelet aggregation in heart failure [172]. In contrast to other organic nitrates, PETN improved the function (migration and incorporation) of endothelial progenitor cells and decreased their NADPH oxidase activity ex vivo and in vivo in humans and rats [173,174,175]. In addition, PETN therapy leads to the regulation of more than 1200 genes and upregulates several cardio-protective transcription factors, whereas nitroglycerin, also a nitrovasodilator, regulated approximately 500 genes different from those regulated by PETN [176]. More recently, PETN was shown to induce heritable epigenetic changes envisaged by H3K27 acetylation, H3K4 trimethylation, and transcriptional activation of eNOS, MnSOD, glutathione peroxidase-1, and heme oxygenase-1, all of which lead to reduced blood pressure in female offspring of PETN-treated hypertensive rats [177]. Of note, these beneficial effects were neither shared by other organic nitrates nor by the •NO donors tested in this study. The ongoing CAESAR trial (Clinical efficacy study of Pentalong for pulmonary hypertension in heart failure; EudraCT Number: 2009-015059-26) will show whether the potent antioxidant and vasculoprotective effects of PETN can be translated to patients with pulmonary hypertension as a result of heart failure.
3.3. Gliptins and Glucagon-Like Peptide-1 (GLP-1) Analogs Display Antioxidant and Anti-Inflammatory Properties
Dipeptidyl peptidase-4 (DPP-4) is an exopeptidase also known as CD26. N-terminal dipeptides are cleaved from alanine- and proline-rich proteins [178]. Besides DPP-4, the family of DPPs consists of several members: DPP-1–DPP-4, DPP-6–DPP-9, quiescent cell proline dipeptidase (QPP), and fibroblast activation protein (FAP) [178,179]. DPP-4 has a wide range of functions, the best characterized of which is the degradation of incretins (glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide GIP) [179]. Furthermore, DPP-4 cleaves non-incretin peptides, possesses non-enzymatic function, and interacts with membrane bound proteins as a chaperone [179]. In various tissues DPP-4 is expressed on the surface of endothelial cells, epithelial cells, and inflammatory cells (monocytes, lymphocytes, dendritic cells, and natural killer (NK) cells) [180,181,182,183].
GLP-1 is an incretin hormone released from L-cells in the intestine after food uptake [184,185]. In the context of glucose homeostasis, circulating GLP-1 binds to its receptor, which is expressed on pancreatic beta-cells, but also on cardiomyocytes, endothelial cells, and inflammatory cells. The GLP-1 receptor belongs to the family of G-protein-coupled receptors. After binding of GLP-1 to its receptor, cAMP levels rise and insulin release is stimulated. On pancreatic alpha-cells, GLP-1 reduces glucagon release (for review see [186]). In summary, GLP-1 is involved in glycemic control, which makes it an attractive target for treatment of diabetes [187,188]. Derived from the prolucagon gene, GLP-1 (7–36-amide) and GLP-1 (7–37) are secreted. Due to rapid degradation of GLP-1 to GLP-1 (9–36-amide) by DPP-4, the half-life of GLP-1 is below 2 min [189,190]. There are two pharmacological strategies for using the GLP-1 effects on glucose metabolism in diabetic patients: (1) inhibition of DPP-4 by gliptins to increase GLP-1 levels and (2) supplementation of modified GLP-1, which resists degradation by DPP-4. At the time of this review five DPP-4 inhibitors are approved by the European Medicines Agency (EMA) (vildagliptin, alogliptin, sitagliptin, linagliptin, and saxagliptin) for treatment of type 2 diabetes mellitus, also reflected by the Global Guideline for Type 2 Diabetes [191]. GLP-1 analogs are represented by liraglutide and exenatide, also reflected by the Global Guideline for Type 2 Diabetes [191].
Besides their potent effects on glycemic control in diabetic patients, research of the last years revealed their effects on several other cell types and tissues. In vivo and in vitro studies demonstrated the beneficial effects of DPP-4 inhibitors on cardiovascular disease [192,193], but also in diseases like psoriasis [194], hepatic steatosis [195], or stroke [196]. Interestingly, pathogenesis of all of these diseases has oxidative stress and most likely inflammation in common. Finally, DPP-4 inhibitors improved the number and “homing” of endothelial progenitor cells in animal [197,198] and human [199] studies, but the number of publications on this association is still low. GLP-1 analogs seem to share these effects on endothelial progenitor cells [200].
Oeseburg et al. published evidence for a protective effect of DPP-4 inhibition on oxidative stress-induced DNA damage and cellular senescence in Zucker diabetic fatty rats [201]. The authors propose elevated GLP-1 to be responsible since the effect could be blocked by exendin fragment 9–39, which is a GLP-1 receptor antagonist. According to this study, induction of the antioxidant enzymes heme oxygenase-1 and NADPH dehydrogenase (quinone) via protein kinase A activation are responsible for the beneficial effects [201]. The mentioned study is an excellent example of the difficulty of differentiating between the direct GLP-1 effects and the GLP-1-independent effects of DPP-4 inhibition. Others detected reduced oxidative stress under DPP-4 inhibitor therapy in animal models of type 1 diabetes [202], cardiac ischemia/reperfusion-injury [203], chronic myocardial infarction [204], abdominal aortic aneurysm [205], Parkinson’s diseases [206], and sepsis [207,208]. Furthermore, limited data are available on the reduction of oxidative stress by DPP-4 inhibition in humans. Shah et al. found reduced 3-nitrotyrosine levels in isolated human pancreatic cells after treatment with linagliptin [209], which agrees with the findings in gliptin-treated type 2 diabetic patients [210]. In humans it remains unclear whether DPP-4 inhibitor-dependent reduction of oxidative stress is independent of glucose-lowering effects. However, there is clear evidence for glucose-independent reduction of oxidative stress by DPP-4 inhibition in different animal models.
DPP4 inhibition has been shown to reduce oxidative stress in various disease models. Reports on diabetes [211,212], atherosclerosis [192,193], sepsis [207,208], and neurological disease [213] can be found in the literature. AMP-activated protein kinase (AMPK) is an important regulator of oxidative stress in the vasculature, more specifically in endothelial cells [214]. Activation of AMPK via GLP-1 receptor signaling has been shown to reduce oxidative stress in cardiomyocytes and reduces activation of NADPH oxidase [215]. On the other hand, suppression of protein kinase C (PKC)/NFκB-dependent Nox activation/upregulation might also be responsible [211,212]. For GLP-1 independent action of DPP-4 inhibition on reduction of oxidative stress, it has been proposed that DPP-4 is an adenosine deaminase (ADA)-binding protein and regulates the subcellular localization and activity of this enzyme, which has known immunomodulatory functions [216,217]. ADA activity also leads to increased inosine levels with subsequent hypoxanthine formation and thereby provides the substrate for the pro-oxidative enzyme xanthine oxidase (XO) [208]. Furthermore, several other protein targets were described for DPP-4 such as caveolin-1, kidney Na+/H+ ion exchanger 3, thromboxane A2 receptor, CXCR4, CXCL12 (SDF-1), fibronectin, and many more [179], most of them being involved in the regulation of inflammation. Immunomodulation by DPP-4 seems to be critical for antioxidant properties of DPP-4 inhibitors.
Since various cell types and tissues are affected by DPP-4 inhibition and also by GLP-1, it is difficult to determine which signaling pathway is predominantly responsible for reduction of oxidative stress in a specific disease model. Most of the studies that investigated the effects of DPP-4 inhibition on oxidative burst performed no experiments with genetic or pharmacological inhibition of the GLP-1 receptor. This limitation prevents a differentiation between DPP-4- and GLP-1-dependent effects. Future studies on cell-specific GLP-1 receptor knock-out animals are needed to draw a clearer picture of the complex interaction of DPP-4 and GLP-1. The following sections will focus on the antioxidant effects of DPP-4 inhibition and GLP-1 analog supplementation in atherosclerosis and sepsis.
3.3.1. Gliptins and GLP-1 in Atherosclerosis
As described above, cardiovascular diseases are closely linked to oxidative stress and inflammation. Endothelial function is a reliable predictor of future cardiovascular events and is directly linked to the burden of oxidative stress in the vessel wall as well as in the blood (e.g., activation state of circulating immune cells) [12]. Recent studies demonstrate that walking distance and critical limb ischemia correlate with the activation state and production of ROS of circulating leukocytes in patients with peripheral artery disease [218,219,220]. Impaired endothelial function leads to inadequate vasodilation and increased disposition for infiltration of inflammatory cells. There is convincing evidence that atherosclerosis can be regarded an inflammatory disease [221].
Matsubara et al. investigated the effects of the DPP-4 inhibitor sitagliptin in an animal model of atherosclerosis [192]. They used ApoE−/− mice on a high-fat diet. Sitagliptin treatment reduced atherosclerotic lesions, improved endothelial function, and reduced infiltration of CD68+ cells into the vascular wall. Vascular inflammation was significantly reduced by sitagliptin treatment, which was proven by reduced mRNA levels of several pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α). Similar reduction of inflammation was found in cultured human monocytes. The authors also showed the anti-inflammatory effects of a GLP-1 analog (envisaged by reduced IL-6), which was additive to the beneficial effects of sitagliptin. This in vitro experiment demonstrated that the anti-inflammatory effects of DPP-4 inhibitor and GLP-1 analog were not connected to each other. Shah et al. made similar observations in LDLr−/− and ApoE−/− mice by using alogliptin treatment [193]. They demonstrated nicely the reduction of chemotaxis and monocyte activation by DPP-4 inhibitor therapy in both models of atherosclerosis. A major limitation of the study is that it does not differentiate between GLP-1- and DPP-4-dependent effects [193]. Besides the beneficial effects of DPP-4 inhibition on atherosclerosis, anti-atherosclerotic effects of GLP-1 supplementation were also demonstrated in animal models. GLP-1 therapy reduced vascular inflammation and increased plaque stability [222]. Others demonstrated improved endothelial function in ApoE−/− mice [223]. Since oxidative stress, derived from inflammatory monocytes, is a major trigger for endothelial dysfunction [23], the beneficial effects of GLP-1 in this context might rely on inhibition of this cell type. This hypothesis is supported by a recent publication reporting on reduced oxidative stress in human monocytes after exendin-4 incubation [224]. Furthermore, the antioxidant capacity (superoxide dismutase activity) in these cells was increased by exendin-4, which could be blocked by the PKA inhibitor H89 [224]. Others attributed the anti-inflammatory effects of GLP-1 to the modulation of NFκB-activity via PKA-dependent signaling pathways [225]. GLP-1 supplementation and DPP-4 inhibition induce protective effects on vascular function in animal models of atherosclerosis. Both reduce vascular inflammation, a main trigger for oxidative stress in the vasculature. Since the GLP-1 receptor and DPP-4 are expressed in endothelial cells, it may be suggested that both can improve endothelial function by direct effects. Indeed, improved function of the endothelial NO synthase in response to GLP-1 analog treatment was demonstrated, followed by reduced activation of endothelial cells via the PI3 kinase/Akt-signaling pathway [226]. Likewise, an activation of the cAMP/PKA-signaling pathway but also the cGMP-signaling pathway was reported for GLP-1 analogs [227,228]. Also, for the DPP-4 inhibitor alogliptin, potent vasodilatory effects were described based on a Src-Akt-eNOS-dependent nitric oxide release [229]. Similar vasodilatory effects were described for linagliptin in an eNOS and soluble guanylyl cyclase-dependent fashion [208]. Furthermore, studies with HUVECs revealed the inhibitory effects of GLP-1 analog treatment on mRNA expression of Nox subunits gp91 an p22phox [230].
Future studies and the use of cell-specific knock-out animals (DPP-4 and GLP-1 receptor) are needed to differentiate between the DPP-4- and GLP-1-dependent effects on vascular oxidative stress and inflammation in models of atherosclerosis.
3.3.2. Gliptins and GLP-1 in Sepsis and Chronic Inflammatory Disease
Sepsis is an inflammatory disease that affects the whole organism. Depending on the immune status of a patient, simple pneumonia can expand to a “systemic inflammatory response syndrome” (SIRS), which is a severe, life-threatening condition. An average mortality of 40% makes it a leading cause of death in the European Union [231]. Because of antibiotic resistance, the use of invasive procedures, and an aging population, sepsis has become more frequent and the absence of efficient causal therapies confers high importance and priority to future research on the septic pathomechanisms but also on more promising therapeutic interventions [232].
Endotoxins (e.g., lipopolysaccharide, LPS) are responsible for the pathogenesis of sepsis in humans and animals. They are part of the outer membrane of gram-negative bacteria and trigger the pathophysiological effects (e.g., circulatory disorders). Hypotension, impaired oxygen utilization, lactic acidosis, and aggravated blood flow in the microcirculation are characteristic of sepsis, by which multiple organ failure is caused [233,234,235]. Previous studies (animal and human) could show a correlation between sepsis and endothelial dysfunction, in which oxidative stress and endothelium-derived mediators (e.g., NO, prostacyclin) are involved [236,237,238]. The oxidative burst is a key feature of neutrophils and monocytes/macrophages, which play a pivotal role in host defense. NADPH oxidase isoform 2 is generating superoxide anion radicals in response to stimuli like LPS (gram-negative bacteria) or zymosan-A (fungi). Chronic granulomatous disease (CGD) underlines the importance of Nox2-derived superoxide anion radicals for host defense. In these patients Nox2 is dysfunctional because of a genetic mutation of the gp91phox gene and they are highly susceptible to infections [239]. Studies on critically ill patients revealed the protective effects of antioxidant therapy with ascorbate and α-tocopherol [240]. It reduced the risk of organ dysfunction and duration of hospitalization in these patients [240], whereas studies on other antioxidants revealed no beneficial effects [241]. The reason for these conflicting results might be that ROS formation is needed for host defense against bacteria.
A global reduction of oxidative stress in sepsis seems not to be the perfect solution to improve survival. The most promising strategy would reduce the overshooting inflammatory response in septic patients but leave the basal host defense intact. Therefore, the question is whether systemic anti-inflammatory therapy of sepsis could counteract the overshooting immune response and might improve survival. The CORTICUS trial investigated whether hydrocortisone application, as a general suppressor of inflammation, might improve survival in septic patients. In this trial no significant improvement of survival in patients suffering from septic shock was found [242]. Furthermore, statins have anti-inflammatory properties and a recent trial tested the use of rosuvastatin in patients suffering from acute respiratory distress syndrome (ARDS). The results were disappointing and statin therapy failed to improve clinical outcome [243].
The immunomodulatory actions of DPP-4 inhibitors and GLP-1 analogs have already been discussed in general and for models of atherosclerosis. Our group investigated the anti-inflammatory and antioxidant effects of DPP-4 inhibition and GLP-1 supplementation in models of endotoxemia. For induction of endotoxemia, the LPS injection model (10 mg/kg) was used, whereas a higher dose of LPS (17 mg/kg) was used to induce partial mortality. In a first study, we were able to show that endothelial dysfunction was severely impaired in endotoxemic rats [208]. This finding was associated with increased oxidative stress in whole blood, vessel walls, and hearts of the animals. Oral treatment with the DPP-4 inhibitor linagliptin strongly improved endothelial function and accordingly reduced oxidative stress levels. Interestingly, the reduced oxidative stress in the vascular wall was accompanied by less infiltration of CD11b+ cells and reduced myeloperoxidase protein expression. Our results revealed two different mechanisms for reduced oxidative stress under endotoxemic conditions in the vascular wall: (1) linagliptin treatment prevents expression of leukocyte adhesion molecules like vascular adhesion molecule-1 (VCAM-1) and thereby reduces endothelial cell activation; and (2) linagliptin directly reduces LPS-induced activation of polymorphonuclear neutrophils (PMN), which was reflected by attenuated adhesion to endothelial cells and oxidative burst. In summary, DPP-4 inhibition reduces the inflammatory state of circulating leukocytes as well as the pro-inflammatory phenotype of the vascular wall and improves the function of endothelial cells, all of which ameliorates vascular function under endotoxemic conditions (Figure 2).
Figure 2.
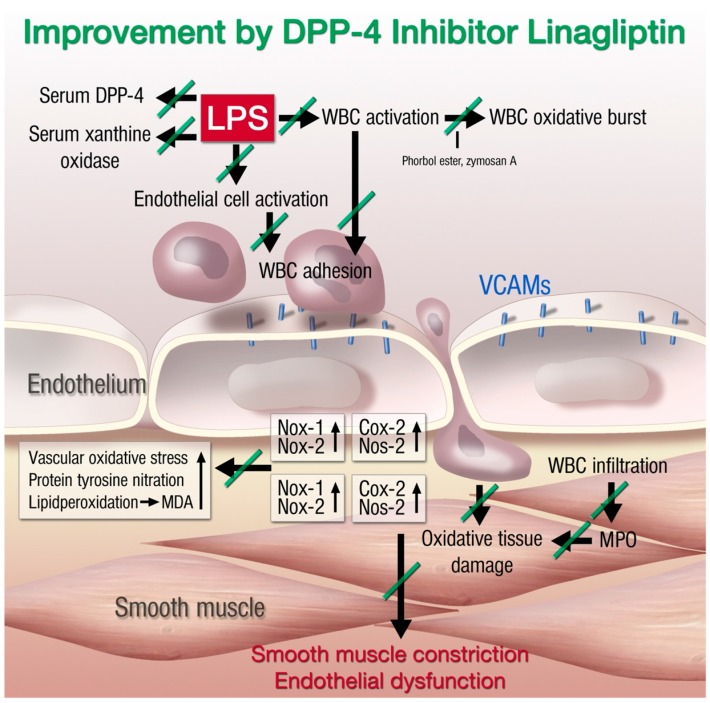
Proposed mechanisms of lipopolysaccharide (LPS)-induced vascular dysfunction and improvement by linagliptin therapy. LPS treatment activates white blood cells (WBC, envisaged by increased oxidative burst), increases serum levels of xanthine oxidase (XO), increases DPP-4 serum activity, and activates vascular cells (detected by expression of endothelial adhesion molecules and inducible cyclooxygenase (Cox-20). This leads to the infiltration of WBC to the vascular wall (detected by aortic FACS analysis for myelomonocytic cells, inducible nitric oxide synthase (Nos-2), Nox2, and myeloperoxidase (MPO) expression) and oxidative damage of the vasculature (Nox1 expression, ROS formation, 3-nitrotyrosine levels, and lipid peroxidation by malondialdehyde (MDA)). Finally, the tissue damage results in smooth muscle constriction and endothelial dysfunction. The green lines on the arrows define the inhibitory effects of linagliptin on septic complications. Adapted from [208]. With permission by Oxford University Press. Copyright © 2012, Oxford University Press.
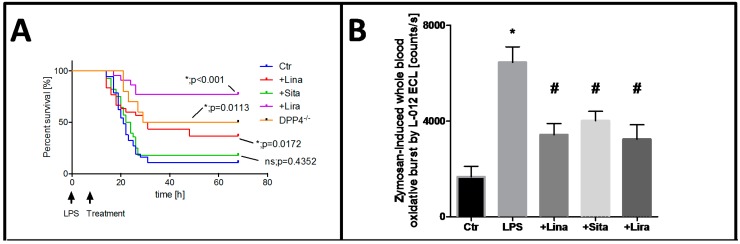
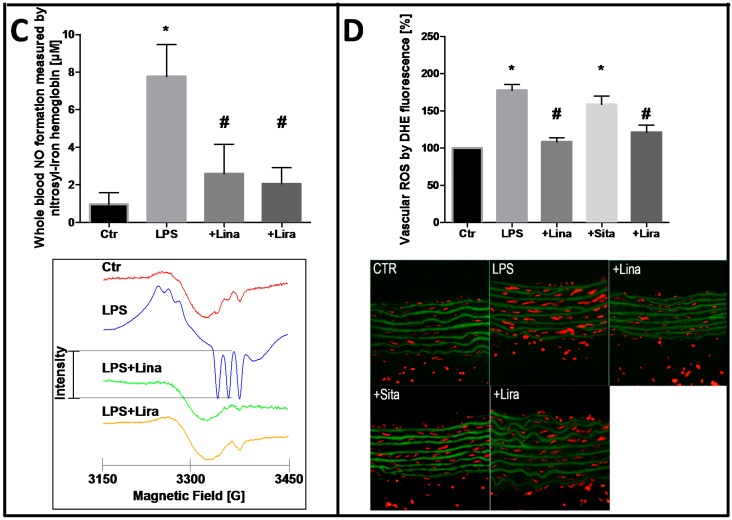
These promising results encouraged us to investigate the impact of DPP-4 inhibition and GLP-1 supplementation on the survival of endotoxemic mice [207]. Pre- as well as post-treatment with linagliptin and the GLP-1 analog liraglutide improved survival of endotoxemic animals significantly (Figure 3A). As a proof of concept, we tested the survival of LPS-treated DPP-4−/− mice and found that these mice are also protected from endotoxic shock-dependent death [207]. Ku et al. found similar results in DPP-4−/− rats and postulated that increased GLP-1 levels are responsible for the improved survival [244]. In line with these murine data, the oxidative burst in whole blood of LPS-treated rats was significantly increased and normalized by DPP-4 inhibition and GLP-1 supplementation (Figure 3B). In accordance with this observation, the nitrosyl-iron hemoglobin (Hb-NO) signal was measured by electron spin resonance (EPR) spectroscopy in whole blood as a direct read-out for increased iNOS activity in LPS-treated animals, which was increased in endotoxemic rats and normalized by linagliptin and liraglutide therapy (Figure 3C). As a marker of vascular oxidative stress, the dihydoethidium fluorescence signal was increased in the vascular wall of LPS-treated rats and normalized by linagliptin and liraglutide treatment (Figure 3D). In summary, these data nicely show the anti-inflammatory and antioxidant potential of DPP-4 inhibitors and GLP-1 analogs, but also reveal substantial differences between the different DPP-4 inhibiting drugs that might be related to their binding affinity and specific location in the active site of DPP-4 [207,208].
Figure 3.
Protective effects of dipeptidyl peptidase-4 inhibition and glucagon-like peptide-1 analog supplementation in an animal model of LPS-induced endotoxemia. (A) Survival of animals was recorded and interpreted by Kaplan–Meier curves; (B) Whole blood oxidative burst upon stimulation with the fungal endotoxin zymosan A was measured by chemiluminescence using the luminol analog L-012; (C) iNOS-derived nitric oxide was determined in whole blood by measurement of nitrosyl-iron hemoglobin by electron paramagnetic resonance spectroscopy; (D) Vascular ROS formation was measured in aortic cryo-sections by dehydrothidium (DHE)-dependent oxidative fluorescence microtopography. Data are mean ± SEM of experiments with 19–36 mice (A) or at least three rats per group (B–D). * p < 0.05 vs. Ctr; # p < 0.05 vs. LPS. Adapted from [207]. With permission by Springer-Verlag Berlin Heidelberg. Copyright © 2015, Springer.
A known adverse side effect of DPP-4 inhibition in humans is an increased risk for infections like nasopharygitis (risk ratio, 1.2 (95% CI, 1.0–1.4)) or urinary tract infection (risk ratio 1.5 (95% CI, 1.0–2.2)). These results from a meta-analysis reflect the abovementioned immunomodulatory effects of DPP-4 inhibition [245]. Furthermore, the GLP-1 analog exendin-4 reduced inflammation in a non-alcoholic steatohepatitis (NASH) animal model by decreasing the infiltration of macrophages (CD68+, F4/80+) [246]. Sitagliptin improved inflammation and fibrosis in methionine/choline-deficient diet-induced steatohepatitis [247]. NASH is a liver inflammatory disease, sharing several features with atherosclerosis [248]. Besides atherosclerosis, sepsis, and NASH, DPP-4 inhibitors and GLP-1 analogs exert immunomodulatory effects in various models of chronic inflammatory diseases such as colitis [249], asthma [250], chronic obstructive lung disease (COPD) [251], and rheumatoid arthritis [252].
4. Immunomodulation as a Therapeutic Strategy
As already outlined above, inflammation represents an independent risk factor for the development of cardiovascular disease. C-reactive protein (CRP) is an acute phase protein and indicates inflammatory processes. According to the PROVE IT-TIMI 22 trial of patients with acute coronary syndrome after initiation of statin therapy, the risk of recurrent myocardial infarction or coronary death was significantly elevated in patients with a high hsCPR (>2 mg/L) compared to patients with low hsCPR levels [253]. The cytokine IL-17 was demonstrated to induce death of human endothelial cells, contributing to plaque destabilization and acute coronary syndrome by disruption of the blood-brain-barrier and activation of NADPH oxidase in brain endothelial cells [254,255]. These adverse effects were suppressed by administration of an IL-17A blocking antibody or by antioxidant therapy [255]. IL-17 induces endothelial cell activation, expression of endothelial adhesion molecules, followed by adhesion and infiltration of neutrophils [256], providing the rational for beneficial effects of therapy with a soluble TNF-α receptor antibody (etanercept) on angiotensin-II induced vascular superoxide production and hypertension [257,258]. The immunosuppressive drug methotrexate (MTX) is used in treatment of cancer and autoimmune disease. Rheumatoid arthritis patients suffer from chronic inflammation and have an increased risk for cardiovascular events. A meta-analysis revealed the antirheumatic drug to be protective against cardiovascular disease in patients with chronic inflammation [259]. Studies on myocardial infarction in dogs showed reduced infarction size by methotrexate treatment [260]. Based on these interesting results, the ongoing TETHYS trial, which investigates the effects of MTX therapy on myocardial infarction with ST-segment elevation, was initiated [261]. An additional clinical trial, which faces the effect of immunosuppression by MTX on the cardiovascular system, is the ongoing CIRT trial (Cardiovascular Inflammation Reduction trial). Patients with myocardial infarction and either type 2 diabetes mellitus or metabolic syndrome will be treated with low-dose MTX or a placebo [262]. Another target currently under investigation for immunomodulation in cardiovascular disease is IL-1β. Animal studies revealed that blockade of IL-1β improves endothelial regrowth, reduces neointima formation, and thereby prevents restenosis following carotid denudation [263]. Furthermore, it ameliorates cardiac remodeling and reduces cardiomyocyte apoptosis after experimental acute myocardial infarction [264]. The ongoing CANTOS trial examines the cardiovascular outcome after blockade of IL-1 β by canakinumab in post myocardial infarction patients [265]. There is convincing evidence for a contribution of inflammation to cardiovascular disease and immunomodulation is a promising new therapeutic approach to treat cardiovascular disease. Nevertheless, it is challenging to find a specific target and tool for modulating the inflammatory cascade. The ongoing clinical trials will provide more answers for these questions.
5. Conclusions
Endothelial dysfunction is an early hallmark of most cardiovascular disease in general [266] and coronary heart disease in particular [13], as well as related future cardiovascular events. Oxidative stress is associated with cardiovascular disease [1,2] but also represents a prognostic marker of future cardiovascular events [12]. Therefore, oxidative stress must be considered a trigger of cardiovascular events; it probably contributes to the progression of cardiovascular disease and represents an attractive target for its therapy [267]. According to more recent data, there is a close correlation between oxidative stress and inflammation in the vasculature [8,28,218,268], making both of them independent triggers/risk factors for the progression of cardiovascular disease and future cardiovascular events [12,36]. Since large clinical trials on chronic oral, systemic, unspecific antioxidant therapy failed to display beneficial effects on cardiovascular events [269], the use of source and cell (organelle)-specific compounds or activators of intrinsic antioxidant systems represents a more promising strategy [122]. Another attractive attempt might be the exploitation of the antioxidant and anti-inflammatory properties of established cardiovascular drugs [5]. Screening for candidates with potent anti-inflammatory effects could represent important additional criteria for the development of cardiovascular drugs in the future. Comparison of drugs with similar primary effects (e.g., blood pressure lowering) but with or without pleiotropic anti-inflammatory and antioxidant effects will allow us to study the importance of these pleiotropic effects. Also, prescreening of patients for markers of inflammation and/or oxidative stress will help us to develop or find the most efficient drug or drug combination for the treatment of the patients in an individual way (in the sense of personalized medicine).
The results obtained with GLP-1 supplementation and DPP-4 inhibition in models of endotoxemia are quite promising, but they remind us of the enthusiasm about statins as a new treatment strategy in sepsis and their failure in large clinical trials [270]. Animal trials and small clinical trials showed convincing evidence for a mortality reduction by statin therapy, which also relied on immunomodulatory effects [271]. Unfortunately, these results could not be reproduced in a large multi-center trial [243] and meta-analysis revealed no improvement of survival [272]. More research is needed to better characterize the antioxidant and anti-inflammatory effects of DPP-4 inhibition and GLP-1 supplementation in the pathogenesis of sepsis. Studies in different models of sepsis (i.e., acute respiratory response syndrome or cecal ligation and puncture) and small clinical trials could shed light on this promising field of sepsis research in the future.
Acknowledgments
We thank Thilo Weckmüller and Margot Neuser for expert graphical assistance. The present work was supported by vascular biology research grants from Boehringer Ingelheim Pharma GmbH & Co. KG, Mainzer Herz Stiftung, and the Center for Translational Vascular Biology (CTVB) (to Andreas Daiber and Thomas Münzel). Sebastian Steven holds a Virchow Fellowship from the Center of Thrombosis and Hemostasis (Mainz, Germany), funded by the Federal Ministry of Education and Research (BMBF 01EO1003). Andreas Daiber and Sebastian Steven were supported by the European Cooperation in Science and Technology (COST Action BM1203/EU-ROS).
Author Contributions
All authors contributed equally to this work. Sebastian Steven and Andreas Daiber drafted the manuscript. Thomas Münzel and Andreas Daiber made critical revisions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Griendling K.K., FitzGerald G.A. Oxidative stress and cardiovascular injury: Part I: Basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 2.Griendling K.K., FitzGerald G.A. Oxidative stress and cardiovascular injury: Part II: Animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 3.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gori T., Munzel T. Oxidative stress and endothelial dysfunction: Therapeutic implications. Ann. Med. 2011;43:259–272. doi: 10.3109/07853890.2010.543920. [DOI] [PubMed] [Google Scholar]
- 5.Chen A.F., Chen D.D., Daiber A., Faraci F.M., Li H., Rembold C.M., Laher I. Free radical biology of the cardiovascular system. Clin. Sci. 2012;123:73–91. doi: 10.1042/CS20110562. [DOI] [PubMed] [Google Scholar]
- 6.Schulz E., Wenzel P., Munzel T., Daiber A. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daiber A., Oelze M., Daub S., Steven S., Schuff A., Kroller-Schon S., Hausding M., Wenzel P., Schulz E., Gori T., et al. Vascular redox signaling, redox switches in endothelial nitric oxide synthase and endothelial dysfunction. In: Laher I., editor. Systems Biology of Free Radicals and Antioxidants. Springer-Verlag; Berlin Heidelberg, Germany: 2014. pp. 1177–1211. [Google Scholar]
- 8.Karbach S., Wenzel P., Waisman A., Munzel T., Daiber A.E. eNOS uncoupling in cardiovascular diseases—The role of oxidative stress and inflammation. Curr. Pharm. Des. 2014;20:3579–3594. doi: 10.2174/13816128113196660748. [DOI] [PubMed] [Google Scholar]
- 9.Harrison D.G., Guzik T.J., Lob H.E., Madhur M.S., Marvar P.J., Thabet S.R., Vinh A., Weyand C.M. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forstermann U., Munzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 11.Munzel T., Daiber A., Ullrich V., Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cgmp-dependent protein kinase. Arterioscler. Thromb. Vasc. Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 12.Heitzer T., Schlinzig T., Krohn K., Meinertz T., Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 13.Schachinger V., Britten M.B., Zeiher A.M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.CIR.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 14.Lin C.P., Lin F.Y., Huang P.H., Chen Y.L., Chen W.C., Chen H.Y., Huang Y.C., Liao W.L., Huang H.C., Liu P.L., et al. Endothelial progenitor cell dysfunction in cardiovascular diseases: Role of reactive oxygen species and inflammation. BioMed Res. Int. 2013;2013:845037. doi: 10.1155/2013/845037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assmus B., Leistner D.M., Schachinger V., Erbs S., Elsasser A., Haberbosch W., Hambrecht R., Sedding D., Yu J., Corti R., et al. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: Migratory capacity of administered cells determines event-free survival. Eur. Heart J. 2014;35:1275–1283. doi: 10.1093/eurheartj/ehu062. [DOI] [PubMed] [Google Scholar]
- 16.Schachinger V., Erbs S., Elsasser A., Haberbosch W., Hambrecht R., Holschermann H., Yu J., Corti R., Mathey D.G., Hamm C.W., et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 17.Ohara Y., Peterson T.E., Harrison D.G. Hypercholesterolemia increases endothelial superoxide anion production. J. Clin. Investig. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison D.G., Ohara Y. Physiologic consequences of increased vascular oxidant stresses in hypercholesterolemia and atherosclerosis: Implications for impaired vasomotion. Am. J. Cardiol. 1995;75:75B–81B. doi: 10.1016/0002-9149(95)80018-N. [DOI] [PubMed] [Google Scholar]
- 19.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 20.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cave A.C., Brewer A.C., Narayanapanicker A., Ray R., Grieve D.J., Walker S., Shah A.M. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 22.Guzik T.J., Hoch N.E., Brown K.A., McCann L.A., Rahman A., Dikalov S., Goronzy J., Weyand C., Harrison D.G. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenzel P., Knorr M., Kossmann S., Stratmann J., Hausding M., Schuhmacher S., Karbach S.H., Schwenk M., Yogev N., Schulz E., et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 24.Bulua A.C., Simon A., Maddipati R., Pelletier M., Park H., Kim K.Y., Sack M.N., Kastner D.L., Siegel R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J. Exp. Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West A.P., Brodsky I.E., Rahner C., Woo D.K., Erdjument-Bromage H., Tempst P., Walsh M.C., Choi Y., Shadel G.S., Ghosh S. Tlr signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 27.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Assar M., Angulo J., Rodriguez-Manas L. Free Radic. Biol. Med. Vol. 65. Angulo; 2013. Oxidative stress and vascular inflammation in aging; pp. 380–401. [DOI] [PubMed] [Google Scholar]
- 29.Mikhed Y., Daiber A., Steven S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int. J. Mol. Sci. 2015;16:15918–15953. doi: 10.3390/ijms160715918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroller-Schon S., Steven S., Kossmann S., Scholz A., Daub S., Oelze M., Xia N., Hausding M., Mikhed Y., Zinssius E., et al. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid. Redox Signal. 2014;20:247–266. doi: 10.1089/ars.2012.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrera J., Ferrebuz A., MacGregor E.G., Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J. Am. Soc. Nephrol. 2006;17:S218–S225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 32.Soltesz P., Kerekes G., Der H., Szucs G., Szanto S., Kiss E., Bodolay E., Zeher M., Timar O., Szodoray P., et al. Comparative assessment of vascular function in autoimmune rheumatic diseases: Considerations of prevention and treatment. Autoimmun. Rev. 2011;10:416–425. doi: 10.1016/j.autrev.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Murdaca G., Colombo B.M., Cagnati P., Gulli R., Spano F., Puppo F. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis. 2012;224:309–317. doi: 10.1016/j.atherosclerosis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Vena G.A., Vestita M., Cassano N. Psoriasis and cardiovascular disease. Dermatol. Ther. 2010;23:144–151. doi: 10.1111/j.1529-8019.2010.01308.x. [DOI] [PubMed] [Google Scholar]
- 35.Hak A.E., Karlson E.W., Feskanich D., Stampfer M.J., Costenbader K.H. Systemic lupus erythematosus and the risk of cardiovascular disease: Results from the nurses’ health study. Arthritis Rheum. 2009;61:1396–1402. doi: 10.1002/art.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta N.N., Azfar R.S., Shin D.B., Neimann A.L., Troxel A.B., Gelfand J.M. Patients with severe psoriasis are at increased risk of cardiovascular mortality: Cohort study using the general practice research database. Eur. Heart J. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peters M.J., Symmons D.P., McCarey D., Dijkmans B.A., Nicola P., Kvien T.K., McInnes I.B., Haentzschel H., Gonzalez-Gay M.A., Provan S., et al. Eular evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann. Rheum. Dis. 2010;69:325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 38.Sodergren A., Karp K., Boman K., Eriksson C., Lundstrom E., Smedby T., Soderlund L., Rantapaa-Dahlqvist S., Wallberg-Jonsson S. Atherosclerosis in early rheumatoid arthritis: Very early endothelial activation and rapid progression of intima media thickness. Arthritis Res. Ther. 2010;12:R158. doi: 10.1186/ar3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balci D.D., Balci A., Karazincir S., Ucar E., Iyigun U., Yalcin F., Seyfeli E., Inandi T., Egilmez E. Increased carotid artery intima-media thickness and impaired endothelial function in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2009;23:1–6. doi: 10.1111/j.1468-3083.2008.02936.x. [DOI] [PubMed] [Google Scholar]
- 40.Di Cesare A., di Meglio P., Nestle F.O. J. Investig. Dermatol. Vol. 129. di Meglio; 2009. The IL-23/Th17 axis in the immunopathogenesis of psoriasis; pp. 1339–1350. [DOI] [PubMed] [Google Scholar]
- 41.Leonardi C., Matheson R., Zachariae C., Cameron G., Li L., Edson-Heredia E., Braun D., Banerjee S. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N. Engl. J. Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 42.Papp K.A., Leonardi C., Menter A., Ortonne J.P., Krueger J.G., Kricorian G., Aras G., Li J., Russell C.B., Thompson E.H., et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N. Engl. J. Med. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 43.Crispin J.C., Tsokos G.C. IL-17 in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010;2010:943254. doi: 10.1155/2010/943254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choy E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2012;51(Suppl. 5):v3–v11. doi: 10.1093/rheumatology/kes113. [DOI] [PubMed] [Google Scholar]
- 45.Pasceri V., Yeh E.T. A tale of two diseases: Atherosclerosis and rheumatoid arthritis. Circulation. 1999;100:2124–2126. doi: 10.1161/01.CIR.100.21.2124. [DOI] [PubMed] [Google Scholar]
- 46.Sesso H.D., Christen W.G., Bubes V., Smith J.P., MacFadyen J., Schvartz M., Manson J.E., Glynn R.J., Buring J.E., Gaziano J.M. Multivitamins in the prevention of cardiovascular disease in men: The physicians’ health study II randomized controlled trial. JAMA. 2012;308:1751–1760. doi: 10.1001/jama.2012.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lonn E., Bosch J., Yusuf S., Sheridan P., Pogue J., Arnold J.M., Ross C., Arnold A., Sleight P., Probstfield J., et al. Effects of long-term vitamin e supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 48.Muntwyler J., Hennekens C.H., Manson J.E., Buring J.E., Gaziano J.M. Vitamin supplement use in a low-risk population of us male physicians and subsequent cardiovascular mortality. Arch. Intern. Med. 2002;162:1472–1476. doi: 10.1001/archinte.162.13.1472. [DOI] [PubMed] [Google Scholar]
- 49.Mann J.F., Lonn E.M., Yi Q., Gerstein H.C., Hoogwerf B.J., Pogue J., Bosch J., Dagenais G.R., Yusuf S. Effects of vitamin e on cardiovascular outcomes in people with mild-to-moderate renal insufficiency: Results of the hope study. Kidney Int. 2004;65:1375–1380. doi: 10.1111/j.1523-1755.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 50.Yusuf S., Dagenais G., Pogue J., Bosch J., Sleight P. Vitamin e supplementation and cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N. Engl. J. Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt H.H., Stocker R., Vollbracht C., Paulsen G., Riley D.P., Daiber A., Cuadrado A. Antioxidants in translational medicine. Antioxid. Redox Signal. 2015 doi: 10.1089/ars.2015.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee D.H., Folsom A.R., Harnack L., Halliwell B., Jacobs D.R., Jr. Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am. J. Clin. Nutr. 2004;80:1194–1200. doi: 10.1093/ajcn/80.5.1194. [DOI] [PubMed] [Google Scholar]
- 53.Shuaib A., Lees K.R., Lyden P., Grotta J., Davalos A., Davis S.M., Diener H.C., Ashwood T., Wasiewski W.W., Emeribe U., et al. Nxy-059 for the treatment of acute ischemic stroke. N. Engl. J. Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 54.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 55.Bjelakovic G., Nikolova D., Simonetti R.G., Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: A systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 56.Harris H.R., Orsini N., Wolk A. Vitamin C and survival among women with breast cancer: A meta-analysis. Eur. J. Cancer. 2014;50:1223–1231. doi: 10.1016/j.ejca.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Ashor A.W., Lara J., Mathers J.C., Siervo M. Effect of vitamin C on endothelial function in health and disease: A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis. 2014;235:9–20. doi: 10.1016/j.atherosclerosis.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Heitzer T., Finckh B., Albers S., Krohn K., Kohlschutter A., Meinertz T. Beneficial effects of α-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: Relation to parameters of oxidative stress. Free Radic. Biol. Med. 2001;31:53–61. doi: 10.1016/S0891-5849(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 59.Heitzer T., Brockhoff C., Mayer B., Warnholtz A., Mollnau H., Henne S., Meinertz T., Munzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers : Evidence for a dysfunctional nitric oxide synthase. Circ. Res. 2000;86:E36–E41. doi: 10.1161/01.RES.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 60.Heitzer T., Just H., Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94:6–9. doi: 10.1161/01.CIR.94.1.6. [DOI] [PubMed] [Google Scholar]
- 61.Levine M., Rumsey S.C., Daruwala R., Park J.B., Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415–1423. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 62.Deng Y.B., Li T.L., Xiang H.J., Chang Q., Li C.L. Impaired endothelial function in the brachial artery after kawasaki disease and the effects of intravenous administration of vitamin C. Pediatr. Infect. Dis. J. 2003;22:34–39. doi: 10.1097/00006454-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 63.Schaufele T.G., Schlaich M.P., Delles C., Klingbeil A.U., Fleischmann E.H., Schmieder R.E. Impaired basal no activity in patients with glomerular disease and the influence of oxidative stress. Kidney Int. 2006;70:1177–1181. doi: 10.1038/sj.ki.5001745. [DOI] [PubMed] [Google Scholar]
- 64.Solzbach U., Hornig B., Jeserich M., Just H. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation. 1997;96:1513–1519. doi: 10.1161/01.CIR.96.5.1513. [DOI] [PubMed] [Google Scholar]
- 65.Hernandez-Guerra M., Garcia-Pagan J.C., Turnes J., Bellot P., Deulofeu R., Abraldes J.G., Bosch J. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology. 2006;43:485–491. doi: 10.1002/hep.21080. [DOI] [PubMed] [Google Scholar]
- 66.Hagel A.F., Layritz C.M., Hagel W.H., Hagel H.J., Hagel E., Dauth W., Kressel J., Regnet T., Rosenberg A., Neurath M.F., et al. Intravenous infusion of ascorbic acid decreases serum histamine concentrations in patients with allergic and non-allergic diseases. Naunyn Schmiedebergs Arch. Pharmacol. 2013;386:789–793. doi: 10.1007/s00210-013-0880-1. [DOI] [PubMed] [Google Scholar]
- 67.Kang H.S., Park J.J., Ahn S.K., Hur D.G., Kim H.Y. Effect of high dose intravenous vitamin C on idiopathic sudden sensorineural hearing loss: A prospective single-blind randomized controlled trial. Eur. Arch. Otorhinolaryngol. 2013;270:2631–2636. doi: 10.1007/s00405-012-2294-y. [DOI] [PubMed] [Google Scholar]
- 68.Du W.D., Yuan Z.R., Sun J., Tang J.X., Cheng A.Q., Shen D.M., Huang C.J., Song X.H., Yu X.F., Zheng S.B. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J. Gastroenterol. 2003;9:2565–2569. doi: 10.3748/wjg.v9.i11.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 70.Harris R.A., Pedersen-White J., Guo D.H., Stallmann-Jorgensen I.S., Keeton D., Huang Y., Shah Y., Zhu H., Dong Y. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight african-american adults. Am. J. Hypertens. 2011;24:557–562. doi: 10.1038/ajh.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sugden J.A., Davies J.I., Witham M.D., Morris A.D., Struthers A.D. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet. Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 72.Pfeifer M., Begerow B., Minne H.W., Nachtigall D., Hansen C. Effects of a short-term vitamin D3 and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J. Clin. Endocrinol. Metab. 2001;86:1633–1637. doi: 10.1210/jc.86.4.1633. [DOI] [PubMed] [Google Scholar]
- 73.Kim H.W., Park C.W., Shin Y.S., Kim Y.S., Shin S.J., Kim Y.S., Choi E.J., Chang Y.S., Bang B.K. Calcitriol regresses cardiac hypertrophy and QT dispersion in secondary hyperparathyroidism on hemodialysis. Nephron. Clin. Pract. 2006;102:c21–29. doi: 10.1159/000088295. [DOI] [PubMed] [Google Scholar]
- 74.Park C.W., Oh Y.S., Shin Y.S., Kim C.M., Kim Y.S., Kim S.Y., Choi E.J., Chang Y.S., Bang B.K. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am. J. Kidney Dis. 1999;33:73–81. doi: 10.1016/S0272-6386(99)70260-X. [DOI] [PubMed] [Google Scholar]
- 75.Bjelakovic G., Gluud L.L., Nikolova D., Whitfield K., Wetterslev J., Simonetti R.G., Bjelakovic M., Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst. Rev. 2014;1:CD007470. doi: 10.1002/14651858.CD007470.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janssen H.C., Samson M.M., Verhaar H.J. Vitamin D deficiency, muscle function, and falls in elderly people. Am. J. Clin. Nutr. 2002;75:611–615. doi: 10.1093/ajcn/75.4.611. [DOI] [PubMed] [Google Scholar]
- 77.Mozos I., Marginean O. Links between vitamin D deficiency and cardiovascular diseases. BioMed Res. Int. 2015;2015:109275. doi: 10.1155/2015/109275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stocker R. The ambivalence of vitamin E in atherogenesis. Trends Biochem. Sci. 1999;24:219–223. doi: 10.1016/S0968-0004(99)01404-8. [DOI] [PubMed] [Google Scholar]
- 79.Khaw K.T., Bingham S., Welch A., Luben R., Wareham N., Oakes S., Day N. Relation between plasma ascorbic acid and mortality in men and women in epic-norfolk prospective study: A prospective population study. European prospective investigation into cancer and nutrition. Lancet. 2001;357:657–663. doi: 10.1016/S0140-6736(00)04128-3. [DOI] [PubMed] [Google Scholar]
- 80.Chen G.C., Lu D.B., Pang Z., Liu Q.F. Vitamin C intake, circulating vitamin C and risk of stroke: A meta-analysis of prospective studies. J. Am. Heart Assoc. 2013;2:e000329. doi: 10.1161/JAHA.113.000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tveden-Nyborg P., Lykkesfeldt J. Does vitamin C deficiency increase lifestyle-associated vascular disease progression? Evidence based on experimental and clinical studies. Antioxid. Redox Signal. 2013;19:2084–2104. doi: 10.1089/ars.2013.5382. [DOI] [PubMed] [Google Scholar]
- 82.Ashor A.W., Siervo M., Lara J., Oggioni C., Mathers J.C. Antioxidant vitamin supplementation reduces arterial stiffness in adults: A systematic review and meta-analysis of randomized controlled trials. J. Nutr. 2014;144:1594–1602. doi: 10.3945/jn.114.195826. [DOI] [PubMed] [Google Scholar]
- 83.Koopman W.J., Verkaart S., Visch H.J., van der Westhuizen F.H., Murphy M.P., van den Heuvel L.W., Smeitink J.A., Willems P.H. Inhibition of complex I of the electron transport chain causes O2−·-mediated mitochondrial outgrowth. Am. J. Physiol. Cell Physiol. 2005;288:C1440–C1450. doi: 10.1152/ajpcell.00607.2004. [DOI] [PubMed] [Google Scholar]
- 84.Adlam V.J., Harrison J.C., Porteous C.M., James A.M., Smith R.A., Murphy M.P., Sammut I.A. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 85.Wiegman C.H., Michaeloudes C., Haji G., Narang P., Clarke C.J., Russell K.E., Bao W., Pavlidis S., Barnes P.J., Kanerva J., et al. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015 doi: 10.1016/j.jaci.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Esplugues J.V., Rocha M., Nunez C., Bosca I., Ibiza S., Herance J.R., Ortega A., Serrador J.M., D’Ocon P., Victor V.M. Complex i dysfunction and tolerance to nitroglycerin: An approach based on mitochondrial-targeted antioxidants. Circ. Res. 2006;99:1067–1075. doi: 10.1161/01.RES.0000250430.62775.99. [DOI] [PubMed] [Google Scholar]
- 87.Graham D., Huynh N.N., Hamilton C.A., Beattie E., Smith R.A., Cocheme H.M., Murphy M.P., Dominiczak A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 88.Miquel E., Cassina A., Martinez-Palma L., Souza J.M., Bolatto C., Rodriguez-Bottero S., Logan A., Smith R.A., Murphy M.P., Barbeito L., et al. Neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2014;70:204–213. doi: 10.1016/j.freeradbiomed.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 89.Dashdorj A., Jyothi K.R., Lim S., Jo A., Nguyen M.N., Ha J., Yoon K.S., Kim H.J., Park J.H., Murphy M.P., et al. Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med. 2013;11:178. doi: 10.1186/1741-7015-11-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chacko B.K., Reily C., Srivastava A., Johnson M.S., Ye Y., Ulasova E., Agarwal A., Zinn K.R., Murphy M.P., Kalyanaraman B., et al. Prevention of diabetic nephropathy in Ins2(+/)−(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem. J. 2010;432:9–19. doi: 10.1042/BJ20100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dikalova A.E., Bikineyeva A.T., Budzyn K., Nazarewicz R.R., McCann L., Lewis W., Harrison D.G., Dikalov S.I. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Batinic-Haberle I., Rajic Z., Tovmasyan A., Reboucas J.S., Ye X., Leong K.W., Dewhirst M.W., Vujaskovic Z., Benov L., Spasojevic I. Diverse functions of cationic Mn(III) N-substituted pyridylporphyrins, recognized as SOD mimics. Free Radic. Biol. Med. 2011;51:1035–1053. doi: 10.1016/j.freeradbiomed.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Batinic-Haberle I., Tovmasyan A., Roberts E.R., Vujaskovic Z., Leong K.W., Spasojevic I. SOD therapeutics: Latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid. Redox Signal. 2014;20:2372–2415. doi: 10.1089/ars.2012.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith R.A., Hartley R.C., Murphy M.P. Mitochondria-targeted small molecule therapeutics and probes. Antioxid. Redox Signal. 2011;15:3021–3038. doi: 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- 95.Murphy M.P. Antioxidants as therapies: Can we improve on nature? Free Radic. Biol. Med. 2014;66:20–23. doi: 10.1016/j.freeradbiomed.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 96.Shuvaev V.V., Christofidou-Solomidou M., Bhora F., Laude K., Cai H., Dikalov S., Arguiri E., Solomides C.C., Albelda S.M., Harrison D.G., et al. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J. Pharmacol. Exp. Ther. 2009;331:404–411. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fernandez C., Hattan C.M., Kerns R.J. Semi-synthetic heparin derivatives: Chemical modifications of heparin beyond chain length, sulfate substitution pattern and N-sulfo/N-acetyl groups. Carbohydr. Res. 2006;341:1253–1265. doi: 10.1016/j.carres.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 98.Kleschyov A.L., Sen V., Golubev V., Munnemann K., Hinderberger D., Lackner K.J., Weber S., Terekhov M., Schreiber L.M., Munzel T. Heparin-polynitroxides: Synthesis and preliminary evaluation as cardiovascular EPR/MR imaging probes and extracellular space-targeted antioxidants. Eur. J. Med. Chem. 2012;58:265–271. doi: 10.1016/j.ejmech.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 99.Kleschyov A.L., Sen V.D. Heparin-polynitroxide derivatives: A platform for new diagnostic and therapeutic agents in cardiovascular disease? Future Med. Chem. 2013;5:385–388. doi: 10.4155/fmc.13.18. [DOI] [PubMed] [Google Scholar]
- 100.Mikhed Y., Gorlach A., Knaus U.G., Daiber A. Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox Biol. 2015;5:275–289. doi: 10.1016/j.redox.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hayes P., Knaus U.G. Balancing reactive oxygen species in the epigenome: NADPH oxidases as target and perpetrator. Antioxid. Redox Signal. 2013;18:1937–1945. doi: 10.1089/ars.2012.4895. [DOI] [PubMed] [Google Scholar]
- 102.Baird A.M., O’Byrne K.J., Gray S.G. Reactive oxygen species and reactive nitrogen species in epigenetic modifications. In: Laher I., editor. Systems Biology of Free Radicals and Antioxidants. Springer-Verlag; Berlin Heidelberg, Germany: 2014. pp. 437–455. [Google Scholar]
- 103.Archer S.L., Marsboom G., Kim G.H., Zhang H.J., Toth P.T., Svensson E.C., Dyck J.R., Gomberg-Maitland M., Thebaud B., Husain A.N., et al. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: A basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cooper M.P., Keaney J.F., Jr. Epigenetic control of angiogenesis via DNA methylation. Circulation. 2011;123:2916–2918. doi: 10.1161/CIRCULATIONAHA.111.033092. [DOI] [PubMed] [Google Scholar]
- 105.Kim G.H., Ryan J.J., Archer S.L. The role of redox signaling in epigenetics and cardiovascular disease. Antioxid. Redox Signal. 2013;18:1920–1936. doi: 10.1089/ars.2012.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang J., Gong L., Tan Y., Hui R., Wang Y. Hypertensive epigenetics: From DNA methylation to micrornas. J. Hum. Hypertens. 2015:2015. doi: 10.1038/jhh.2014.132. [DOI] [PubMed] [Google Scholar]
- 107.Li H., Xia N., Forstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide. 2012;26:102–110. doi: 10.1016/j.niox.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 108.Mukhopadhyay P., Pacher P., Das D.K. Microrna signatures of resveratrol in the ischemic heart. Ann. N. Y. Acad. Sci. 2011;1215:109–116. doi: 10.1111/j.1749-6632.2010.05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Farghali H., Kutinova Canova N., Lekic N. Resveratrol and related compounds as antioxidants with an allosteric mechanism of action in epigenetic drug targets. Physiol. Res. 2013;62:1–13. doi: 10.33549/physiolres.932434. [DOI] [PubMed] [Google Scholar]
- 110.Gatz S.A., Wiesmuller L. Take a break—resveratrol in action on DNA. Carcinogenesis. 2008;29:321–332. doi: 10.1093/carcin/bgm276. [DOI] [PubMed] [Google Scholar]
- 111.Zhou W., Chai H., Lin P.H., Lumsden A.B., Yao Q., Chen C. Clinical use and molecular mechanisms of action of extract of ginkgo biloba leaves in cardiovascular diseases. Cardiovasc. Drug Rev. 2004;22:309–319. doi: 10.1111/j.1527-3466.2004.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 112.Kleikers P.W., Wingler K., Hermans J.J., Diebold I., Altenhofer S., Radermacher K.A., Janssen B., Gorlach A., Schmidt H.H. NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. Int. J. Mol. Med. 2012;90:1391–1406. doi: 10.1007/s00109-012-0963-3. [DOI] [PubMed] [Google Scholar]
- 113.Altenhofer S., Kleikers P.W., Radermacher K.A., Scheurer P., Rob Hermans J.J., Schiffers P., Ho H., Wingler K., Schmidt H.H. The Nox toolbox: Validating the role of NADPH oxidases in physiology and disease. Cell. Mol. Life Sci. 2012;69:2327–2343. doi: 10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Radermacher K.A., Wingler K., Langhauser F., Altenhofer S., Kleikers P., Hermans J.J., Hrabe de Angelis M., Kleinschnitz C., Schmidt H.H. Neuroprotection after stroke by targeting Nox4 as a source of oxidative stress. Antioxid. Redox Signal. 2013;18:1418–1427. doi: 10.1089/ars.2012.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wingler K., Hermans J.J., Schiffers P., Moens A., Paul M., Schmidt H.H. Nox1, 2, 4, 5: Counting out oxidative stress. Br. J. Pharmacol. 2011;164:866–883. doi: 10.1111/j.1476-5381.2011.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee B.E., Toledo A.H., Anaya-Prado R., Roach R.R., Toledo-Pereyra L.H. Allopurinol, xanthine oxidase, and cardiac ischemia. J. Investig. Med. 2009;57:902–909. doi: 10.2310/JIM.0b013e3181bca50c. [DOI] [PubMed] [Google Scholar]
- 117.Higgins P., Dawson J., Lees K.R., McArthur K., Quinn T.J., Walters M.R. Xanthine oxidase inhibition for the treatment of cardiovascular disease: A systematic review and meta-analysis. Cardiovasc. Ther. 2012;30:217–226. doi: 10.1111/j.1755-5922.2011.00277.x. [DOI] [PubMed] [Google Scholar]
- 118.Jazwa A., Cuadrado A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr. Drug Targets. 2010;11:1517–1531. doi: 10.2174/1389450111009011517. [DOI] [PubMed] [Google Scholar]
- 119.Evgenov O.V., Pacher P., Schmidt P.M., Hasko G., Schmidt H.H., Stasch J.P. NO-independent stimulators and activators of soluble guanylate cyclase: Discovery and therapeutic potential. Nat. Rev. Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Follmann M., Griebenow N., Hahn M.G., Hartung I., Mais F.J., Mittendorf J., Schafer M., Schirok H., Stasch J.P., Stoll F., et al. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew. Chem. Int. Ed. Engl. 2013;52:9442–9462. doi: 10.1002/anie.201302588. [DOI] [PubMed] [Google Scholar]
- 121.Schmidt H.H., Schmidt P.M., Stasch J.P. NO- and haem-independent soluble guanylate cyclase activators. Handb. Exp. Pharmacol. 2009;191:309–339. doi: 10.1007/978-3-540-68964-5_14. [DOI] [PubMed] [Google Scholar]
- 122.Lonn M.E., Dennis J.M., Stocker R. Actions of “antioxidants” in the protection against atherosclerosis. Free Radic. Biol. Med. 2012;53:863–884. doi: 10.1016/j.freeradbiomed.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 123.Drexler H., Hornig B. Endothelial dysfunction in human disease. J. Mol. Cell. Cardiol. 1999;31:51–60. doi: 10.1006/jmcc.1998.0843. [DOI] [PubMed] [Google Scholar]
- 124.Warnholtz A., Munzel T. Why do antioxidants fail to provide clinical benefit? Curr. Control. Trials Cardiovasc. Med. 2000;1:38–40. doi: 10.1186/CVM-1-1-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Porsti I., Bara A.T., Busse R., Hecker M. Release of nitric oxide by angiotensin-(1–7) from porcine coronary endothelium: Implications for a novel angiotensin receptor. Br. J. Pharmacol. 1994;111:652–654. doi: 10.1111/j.1476-5381.1994.tb14787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mollnau H., Oelze M., August M., Wendt M., Daiber A., Schulz E., Baldus S., Kleschyov A.L., Materne A., Wenzel P., et al. Mechanisms of increased vascular superoxide production in an experimental model of idiopathic dilated cardiomyopathy. Arterioscler. Thromb. Vasc. Biol. 2005;25:2554–2559. doi: 10.1161/01.ATV.0000190673.41925.9B. [DOI] [PubMed] [Google Scholar]
- 127.Williams H.C., Griendling K.K. NADPH oxidase inhibitors: New antihypertensive agents? J. Cardiovasc. Pharmacol. 2007;50:9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]
- 128.Soehnlein O., Schmeisser A., Cicha I., Reiss C., Ulbrich H., Lindbom L., Daniel W.G., Garlichs C.D. Ace inhibition lowers angiotensin-II-induced monocyte adhesion to HUVEC by reduction of p65 translocation and AT1 expression. J. Vasc. Res. 2005;42:399–407. doi: 10.1159/000087340. [DOI] [PubMed] [Google Scholar]
- 129.Caspritz G., Alpermann H.G., Schleyerbach R. Influence of the new angiotensin converting enzyme inhibitor ramipril on several models of acute inflammation and the adjuvant arthritis in the rat. Arzneimittelforschung. 1986;36:1605–1608. [PubMed] [Google Scholar]
- 130.Durik M., Seva Pessoa B., Roks A.J. The renin-angiotensin system, bone marrow and progenitor cells. Clin. Sci. 2012;123:205–223. doi: 10.1042/CS20110660. [DOI] [PubMed] [Google Scholar]
- 131.Munzel T., Gori T., Keaney J.F., Jr., Maack C., Daiber A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Heart. J. 2015 doi: 10.1093/eurheartj/ehv305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takemoto M., Liao J.K. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler. Thromb. Vasc. Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 133.Ray K.K., Cannon C.P. Pathological changes in acute coronary syndromes: The role of statin therapy in the modulation of inflammation, endothelial function and coagulation. J. Thromb. Thrombolysis. 2004;18:89–101. doi: 10.1007/s11239-004-0205-9. [DOI] [PubMed] [Google Scholar]
- 134.Patel T.N., Shishehbor M.H., Bhatt D.L. A review of high-dose statin therapy: Targeting cholesterol and inflammation in atherosclerosis. Eur. Heart J. 2007;28:664–672. doi: 10.1093/eurheartj/ehl445. [DOI] [PubMed] [Google Scholar]
- 135.Adam O., Laufs U. Rac1-mediated effects of HMG-CoA reductase inhibitors (statins) in cardiovascular disease. Antioxid. Redox Signal. 2014;20:1238–1250. doi: 10.1089/ars.2013.5526. [DOI] [PubMed] [Google Scholar]
- 136.Wenzel P., Daiber A., Oelze M., Brandt M., Closs E., Xu J., Thum T., Bauersachs J., Ertl G., Zou M.H., et al. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis. 2008;198:65–76. doi: 10.1016/j.atherosclerosis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Margaritis M., Channon K.M., Antoniades C. Statins as regulators of redox state in the vascular endothelium: Beyond lipid lowering. Antioxid. Redox Signal. 2014;20:1198–1215. doi: 10.1089/ars.2013.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Habeos I.G., Ziros P.G., Chartoumpekis D., Psyrogiannis A., Kyriazopoulou V., Papavassiliou A.G. Simvastatin activates Keap1/Nrf2 signaling in rat liver. Int. J. Mol. Med. 2008;86:1279–1285. doi: 10.1007/s00109-008-0393-4. [DOI] [PubMed] [Google Scholar]
- 139.Ali F., Zakkar M., Karu K., Lidington E.A., Hamdulay S.S., Boyle J.J., Zloh M., Bauer A., Haskard D.O., Evans P.C., et al. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J. Biol. Chem. 2009;284:18882–18892. doi: 10.1074/jbc.M109.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hibbert B., Simard T., Ramirez F.D., Pourdjabbar A., Raizman J.E., Maze R., Wilson K.R., Hawken S., O’Brien E.R. The effect of statins on circulating endothelial progenitor cells in humans: A systematic review. J. Cardiovasc. Pharmacol. 2013;62:491–496. doi: 10.1097/FJC.0b013e3182a4027f. [DOI] [PubMed] [Google Scholar]
- 141.Magee L.A., Abalos E., von Dadelszen P., Sibai B., Easterling T., Walkinshaw S., Group C.S. How to manage hypertension in pregnancy effectively. Br. J. Clin. Pharmacol. 2011;72:394–401. doi: 10.1111/j.1365-2125.2011.04002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Anand I.S., Tam S.W., Rector T.S., Taylor A.L., Sabolinski M.L., Archambault W.T., Adams K.F., Olukotun A.Y., Worcel M., Cohn J.N. Influence of blood pressure on the effectiveness of a fixed-dose combination of isosorbide dinitrate and hydralazine in the african-american heart failure trial. J. Am. Coll. Cardiol. 2007;49:32–39. doi: 10.1016/j.jacc.2006.04.109. [DOI] [PubMed] [Google Scholar]
- 143.Taylor A.L., Ziesche S., Yancy C.W., Carson P., Ferdinand K., Taylor M., Adams K., Olukotun A.Y., Ofili E., Tam S.W., et al. Early and sustained benefit on event-free survival and heart failure hospitalization from fixed-dose combination of isosorbide dinitrate/hydralazine: Consistency across subgroups in the african-american heart failure trial. Circulation. 2007;115:1747–1753. doi: 10.1161/CIRCULATIONAHA.106.644013. [DOI] [PubMed] [Google Scholar]
- 144.Cohn J.N., Tam S.W., Anand I.S., Taylor A.L., Sabolinski M.L., Worcel M. Isosorbide dinitrate and hydralazine in a fixed-dose combination produces further regression of left ventricular remodeling in a well-treated black population with heart failure: Results from A-HeFT. J. Card. Fail. 2007;13:331–339. doi: 10.1016/j.cardfail.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 145.Daiber A., Oelze M., Coldewey M., Kaiser K., Huth C., Schildknecht S., Bachschmid M., Nazirisadeh Y., Ullrich V., Mulsch A., et al. Hydralazine is a powerful inhibitor of peroxynitrite formation as a possible explanation for its beneficial effects on prognosis in patients with congestive heart failure. Biochem. Biophys. Res. Commun. 2005;338:1865–1874. doi: 10.1016/j.bbrc.2005.10.106. [DOI] [PubMed] [Google Scholar]
- 146.Daiber A., Mulsch A., Hink U., Mollnau H., Warnholtz A., Oelze M., Munzel T. The oxidative stress concept of nitrate tolerance and the antioxidant properties of hydralazine. Am. J. Cardiol. 2005;96:25i–36i. doi: 10.1016/j.amjcard.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 147.Munzel T., Kurz S., Rajagopalan S., Thoenes M., Berrington W.R., Thompson J.A., Freeman B.A., Harrison D.G. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound nadh oxidase. A new action for an old drug. J. Clin. Investig. 1996;98:1465–1470. doi: 10.1172/JCI118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sekiya M., Sato M., Funada J., Ohtani T., Akutsu H., Watanabe K. Effects of the long-term administration of nicorandil on vascular endothelial function and the progression of arteriosclerosis. J. Cardiovasc. Pharmacol. 2005;46:63–67. doi: 10.1097/01.fjc.0000162771.00174.a8. [DOI] [PubMed] [Google Scholar]
- 149.Yang X.F. Chemiluminescence investigation of carbon dioxide-enhanced oxidation of dihydralazine sulfate by peroxynitrite and its application to pharmaceutical analysis. Anal. Chim. Acta. 2008;616:190–195. doi: 10.1016/j.aca.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 150.Knowles H.J., Tian Y.M., Mole D.R., Harris A.L. Novel mechanism of action for hydralazine: Induction of hypoxia-inducible factor-1α, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ. Res. 2004;95:162–169. doi: 10.1161/01.RES.0000134924.89412.70. [DOI] [PubMed] [Google Scholar]
- 151.Aslam S. Cardiovascular disease in dialysis patients: Do some antihypertensive drugs have specific antioxidant effects or is it just blood pressure reduction? Does antioxidant treatment reduce the risk for cardiovascular disease? Curr. Opin. Nephrol. Hypertens. 2008;17:99–105. doi: 10.1097/MNH.0b013e3282f313bd. [DOI] [PubMed] [Google Scholar]
- 152.Hoenig M.R., Bianchi C., Sellke F.W. Hypoxia inducible factor-1 α, endothelial progenitor cells, monocytes, cardiovascular risk, wound healing, cobalt and hydralazine: A unifying hypothesis. Curr. Drug Targets. 2008;9:422–435. doi: 10.2174/138945008784221215. [DOI] [PubMed] [Google Scholar]
- 153.Brehm B.R., Wolf S.C., Bertsch D., Klaussner M., Wesselborg S., Schuler S., Schulze-Osthoff K. Effects of nebivolol on proliferation and apoptosis of human coronary artery smooth muscle and endothelial cells. Cardiovasc. Res. 2001;49:430–439. doi: 10.1016/S0008-6363(00)00253-4. [DOI] [PubMed] [Google Scholar]
- 154.Georgescu A., Pluteanu F., Flonta M.L., Badila E., Dorobantu M., Popov D. The cellular mechanisms involved in the vasodilator effect of nebivolol on the renal artery. Eur. J. Pharmacol. 2005;508:159–166. doi: 10.1016/j.ejphar.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 155.Broeders M.A., Doevendans P.A., Bekkers B.C., Bronsaer R., van Gorsel E., Heemskerk J.W., Egbrink M.G., van Breda E., Reneman R.S., van Der Zee R. Nebivolol: A third-generation β-blocker that augments vascular nitric oxide release: Endothelial β2—adrenergic receptor—Mediated nitric oxide production. Circulation. 2000;102:677–684. doi: 10.1161/01.CIR.102.6.677. [DOI] [PubMed] [Google Scholar]
- 156.Tzemos N., Lim P.O., MacDonald T.M. Nebivolol reverses endothelial dysfunction in essential hypertension: A randomized, double-blind, crossover study. Circulation. 2001;104:511–514. doi: 10.1161/hc3001.094207. [DOI] [PubMed] [Google Scholar]
- 157.Mollnau H., Schulz E., Daiber A., Baldus S., Oelze M., August M., Wendt M., Walter U., Geiger C., Agrawal R., et al. Nebivolol prevents vascular nos III uncoupling in experimental hyperlipidemia and inhibits NADPH oxidase activity in inflammatory cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:615–621. doi: 10.1161/01.ATV.0000065234.70518.26. [DOI] [PubMed] [Google Scholar]
- 158.Oelze M., Daiber A., Brandes R.P., Hortmann M., Wenzel P., Hink U., Schulz E., Mollnau H., von Sandersleben A., Kleschyov A.L., et al. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension. 2006;48:677–684. doi: 10.1161/01.HYP.0000239207.82326.29. [DOI] [PubMed] [Google Scholar]
- 159.Pasini A.F., Garbin U., Nava M.C., Stranieri C., Davoli A., Sawamura T., Cascio V.L., Cominacini L. Nebivolol decreases oxidative stress in essential hypertensive patients and increases nitric oxide by reducing its oxidative inactivation. J. Hypertens. 2005;23:589–596. doi: 10.1097/01.hjh.0000160216.86597.ff. [DOI] [PubMed] [Google Scholar]
- 160.Sorrentino S.A., Doerries C., Manes C., Speer T., Dessy C., Lobysheva I., Mohmand W., Akbar R., Bahlmann F., Besler C., et al. Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional β1-blockade. J. Am. Coll. Cardiol. 2011;57:601–611. doi: 10.1016/j.jacc.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 161.Munzel T., Daiber A., Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ. Res. 2005;97:618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- 162.Gori T., Daiber A. Non-hemodynamic effects of organic nitrates and the distinctive characteristics of pentaerithrityl tetranitrate. Am. J. Cardiovasc. Drugs. 2009;9:7–15. doi: 10.1007/BF03256591. [DOI] [PubMed] [Google Scholar]
- 163.Munzel T., Meinertz T., Tebbe U., Schneider H.T., Stalleicken D., Wargenau M., Gori T., Klingmann I., Investigators C.S. Efficacy of the long-acting nitro vasodilator pentaerithrityl tetranitrate in patients with chronic stable angina pectoris receiving anti-anginal background therapy with β-blockers: A 12-week, randomized, double-blind, placebo-controlled trial. Eur. Heart. J. 2014;35:895–903. doi: 10.1093/eurheartj/eht384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schnorbus B., Schiewe R., Ostad M.A., Medler C., Wachtlin D., Wenzel P., Daiber A., Munzel T., Warnholtz A. Effects of pentaerythritol tetranitrate on endothelial function in coronary artery disease: Results of the penta study. Clin. Res. Cardiol. 2010;99:115–124. doi: 10.1007/s00392-009-0096-z. [DOI] [PubMed] [Google Scholar]
- 165.Schuhmacher S., Wenzel P., Schulz E., Oelze M., Mang C., Kamuf J., Gori T., Jansen T., Knorr M., Karbach S., et al. Pentaerythritol tetranitrate improves angiotensin II-induced vascular dysfunction via induction of heme oxygenase-1. Hypertension. 2010;55:897–904. doi: 10.1161/HYPERTENSIONAHA.109.149542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wenzel P., Oelze M., Coldewey M., Hortmann M., Seeling A., Hink U., Mollnau H., Stalleicken D., Weiner H., Lehmann J., et al. Heme oxygenase-1: A novel key player in the development of tolerance in response to organic nitrates. Arterioscler. Thromb. Vasc. Biol. 2007;27:1729–1735. doi: 10.1161/ATVBAHA.107.143909. [DOI] [PubMed] [Google Scholar]
- 167.Oberle S., Abate A., Grosser N., Hemmerle A., Vreman H.J., Dennery P.A., Schneider H.T., Stalleicken D., Schroder H. Endothelial protection by pentaerithrityl trinitrate: Bilirubin and carbon monoxide as possible mediators. Exp. Biol. Med. (Maywood) 2003;228:529–534. doi: 10.1177/15353702-0322805-21. [DOI] [PubMed] [Google Scholar]
- 168.Oberle S., Abate A., Grosser N., Vreman H.J., Dennery P.A., Schneider H.T., Stalleicken D., Schroder H. Heme oxygenase-1 induction may explain the antioxidant profile of pentaerythrityl trinitrate. Biochem. Biophys. Res. Commun. 2002;290:1539–1544. doi: 10.1006/bbrc.2002.6379. [DOI] [PubMed] [Google Scholar]
- 169.Schuhmacher S., Oelze M., Bollmann F., Kleinert H., Otto C., Heeren T., Steven S., Hausding M., Knorr M., Pautz A., et al. Vascular dysfunction in experimental diabetes is improved by pentaerithrityl tetranitrate but not isosorbide-5-mononitrate therapy. Diabetes. 2011;60:2608–2616. doi: 10.2337/db10-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Oppermann M., Balz V., Adams V., Dao V.T., Bas M., Suvorava T., Kojda G. Pharmacological induction of vascular extracellular superoxide dismutase expression in vivo. J. Cell. Mol. Med. 2009;13:1271–1278. doi: 10.1111/j.1582-4934.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hacker A., Muller S., Meyer W., Kojda G. The nitric oxide donor pentaerythritol tetranitrate can preserve endothelial function in established atherosclerosis. Br. J. Pharmacol. 2001;132:1707–1714. doi: 10.1038/sj.bjp.0704021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Flierl U., Fraccarollo D., Widder J.D., Micka J., Neuser J., Bauersachs J., Schafer A. The nitric oxide donor pentaerythritol tetranitrate reduces platelet activation in congestive heart failure. PLoS ONE. 2015;10:e0123621. doi: 10.1371/journal.pone.0123621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Thum T., Fraccarollo D., Thum S., Schultheiss M., Daiber A., Wenzel P., Munzel T., Ertl G., Bauersachs J. Differential effects of organic nitrates on endothelial progenitor cells are determined by oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2007;27:748–754. doi: 10.1161/01.ATV.0000258787.18982.73. [DOI] [PubMed] [Google Scholar]
- 174.Thum T., Wiebking V., Ertl G., Bauersachs J. Organic nitrates differentially modulate circulating endothelial progenitor cells and endothelial function in patients with symptomatic coronary artery disease. Antioxid. Redox Signal. 2011;15:925–931. doi: 10.1089/ars.2010.3503. [DOI] [PubMed] [Google Scholar]
- 175.DiFabio J.M., Thomas G.R., Zucco L., Kuliszewski M.A., Bennett B.M., Kutryk M.J., Parker J.D. Nitroglycerin attenuates human endothelial progenitor cell differentiation, function, and survival. J. Pharmacol. Exp. Ther. 2006;318:117–123. doi: 10.1124/jpet.106.102129. [DOI] [PubMed] [Google Scholar]
- 176.Pautz A., Rauschkolb P., Schmidt N., Art J., Oelze M., Wenzel P., Forstermann U., Daiber A., Kleinert H. Effects of nitroglycerin or pentaerithrityl tetranitrate treatment on the gene expression in rat hearts: Evidence for cardiotoxic and cardioprotective effects. Physiol. Genom. 2009;38:176–185. doi: 10.1152/physiolgenomics.00035.2009. [DOI] [PubMed] [Google Scholar]
- 177.Wu Z., Siuda D., Xia N., Reifenberg G., Daiber A., Munzel T., Forstermann U., Li H. Maternal treatment of spontaneously hypertensive rats with pentaerythritol tetranitrate reduces blood pressure in female offspring. Hypertension. 2015;65:232–237. doi: 10.1161/HYPERTENSIONAHA.114.04416. [DOI] [PubMed] [Google Scholar]
- 178.Abbott C.A., Yu D.M., Woollatt E., Sutherland G.R., McCaughan G.W., Gorrell M.D. Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8. Eur. J. Biochem. 2000;267:6140–6150. doi: 10.1046/j.1432-1327.2000.01617.x. [DOI] [PubMed] [Google Scholar]
- 179.Zhong J., Rao X., Rajagopalan S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: Potential implications in cardiovascular disease. Atherosclerosis. 2013;226:305–314. doi: 10.1016/j.atherosclerosis.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 180.Buhling F., Junker U., Reinhold D., Neubert K., Jager L., Ansorge S. Functional role of CD26 on human B lymphocytes. Immunol. Lett. 1995;45:47–51. doi: 10.1016/0165-2478(94)00230-O. [DOI] [PubMed] [Google Scholar]
- 181.Buhling F., Kunz D., Reinhold D., Ulmer A.J., Ernst M., Flad H.D., Ansorge S. Expression and functional role of dipeptidyl peptidase IV (CD26) on human natural killer cells. Nat. Immun. 1994;13:270–279. [PubMed] [Google Scholar]
- 182.Gorrell M.D. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin. Sci. 2005;108:277–292. doi: 10.1042/CS20040302. [DOI] [PubMed] [Google Scholar]
- 183.Palmieri F.E., Ward P.E. Dipeptidyl(amino)peptidase iv and post proline cleaving enzyme in cultured endothelial and smooth muscle cells. Adv. Exp. Med. Biol. 1989;247A:305–311. doi: 10.1007/978-1-4615-9543-4_45. [DOI] [PubMed] [Google Scholar]
- 184.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 185.Lund P.K., Goodman R.H., Dee P.C., Habener J.F. Pancreatic preproglucagon cdna contains two glucagon-related coding sequences arranged in tandem. Proc. Natl. Acad. Sci. USA. 1982;79:345–349. doi: 10.1073/pnas.79.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Doyle M.E., Egan J.M. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 2007;113:546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nauck M.A. Incretin-based therapies for type 2 diabetes mellitus: Properties, functions, and clinical implications. Am. J. Med. 2011;124:S3–18. doi: 10.1016/j.amjmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 188.Brubaker P.L., Drucker D.J. Structure-function of the glucagon receptor family of G protein-coupled receptors: The glucagon, GIP, GLP-1, and GLP-2 receptors. Recept. Channels. 2002;8:179–188. doi: 10.1080/10606820213687. [DOI] [PubMed] [Google Scholar]
- 189.Mentlein R., Gallwitz B., Schmidt W.E. Dipeptidyl-peptidase iv hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 190.Kieffer T.J., McIntosh C.H., Pederson R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 191.International Diabetes Federation Guideline Development Group . Diabetes Res. Clin. Pract. Vol. 104. diabetes; 2014. Global guideline for type 2 diabetes; pp. 1–52. [DOI] [PubMed] [Google Scholar]
- 192.Matsubara J., Sugiyama S., Sugamura K., Nakamura T., Fujiwara Y., Akiyama E., Kurokawa H., Nozaki T., Ohba K., Konishi M., et al. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J. Am. Coll. Cardiol. 2012;59:265–276. doi: 10.1016/j.jacc.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 193.Shah Z., Kampfrath T., Deiuliis J.A., Zhong J., Pineda C., Ying Z., Xu X., Lu B., Moffatt-Bruce S., Durairaj R., et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nishioka T., Shinohara M., Tanimoto N., Kumagai C., Hashimoto K. Sitagliptin, a dipeptidyl peptidase-iv inhibitor, improves psoriasis. Dermatology. 2012;224:20–21. doi: 10.1159/000333358. [DOI] [PubMed] [Google Scholar]
- 195.Kern M., Kloting N., Niessen H.G., Thomas L., Stiller D., Mark M., Klein T., Bluher M. Linagliptin improves insulin sensitivity and hepatic steatosis in diet-induced obesity. PLoS ONE. 2012;7:e38744. doi: 10.1371/journal.pone.0038744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Darsalia V., Ortsater H., Olverling A., Darlof E., Wolbert P., Nystrom T., Klein T., Sjoholm A., Patrone C. The DPP-4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: A comparison with glimepiride. Diabetes. 2013;62:1289–1296. doi: 10.2337/db12-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Huang C.Y., Shih C.M., Tsao N.W., Lin Y.W., Huang P.H., Wu S.C., Lee A.W., Kao Y.T., Chang N.C., Nakagami H., et al. Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br. J. Pharmacol. 2012;167:1506–1519. doi: 10.1111/j.1476-5381.2012.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brenner C., Krankel N., Kuhlenthal S., Israel L., Remm F., Fischer C., Herbach N., Speer T., Grabmaier U., Laskowski A., et al. Short-term inhibition of DPP-4 enhances endothelial regeneration after acute arterial injury via enhanced recruitment of circulating progenitor cells. Int. J. Cardiol. 2014;177:266–275. doi: 10.1016/j.ijcard.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 199.Fadini G.P., Boscaro E., Albiero M., Menegazzo L., Frison V., de Kreutzenberg S., Agostini C., Tiengo A., Avogaro A. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: Possible role of stromal-derived factor-1α. Diabetes care. 2010;33:1607–1609. doi: 10.2337/dc10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xiao-Yun X., Zhao-Hui M., Ke C., Hong-Hui H., Yan-Hong X. Glucagon-like peptide-1 improves proliferation and differentiation of endothelial progenitor cells via upregulating vegf generation. Med. Sci. Monit. 2011;17:BR35–BR41. doi: 10.12659/MSM.881383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Oeseburg H., de Boer R.A., Buikema H., van der Harst P., van Gilst W.H., Sillje H.H. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase a. Arterioscler. Thromb. Vasc. biol. 2010;30:1407–1414. doi: 10.1161/ATVBAHA.110.206425. [DOI] [PubMed] [Google Scholar]
- 202.Akarte A.S., Srinivasan B.P., Gandhi S., Sole S. Chronic DPP-IV inhibition with PKF-275-055 attenuates inflammation and improves gene expressions responsible for insulin secretion in streptozotocin induced diabetic rats. Eur. J. Pharm. Sci. 2012;47:456–463. doi: 10.1016/j.ejps.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 203.Chinda K., Palee S., Surinkaew S., Phornphutkul M., Chattipakorn S., Chattipakorn N. Cardioprotective effect of dipeptidyl peptidase-4 inhibitor during ischemia-reperfusion injury. Int. J. Cardiol. 2013;167:451–457. doi: 10.1016/j.ijcard.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 204.Inthachai T., Lekawanvijit S., Kumfu S., Apaijai N., Pongkan W., Chattipakorn S.C., Chattipakorn N. Dipeptidyl peptidase-4 inhibitor improves cardiac function by attenuating adverse cardiac remodelling in rats with chronic myocardial infarction. Exp. Physiol. 2015;100:667–679. doi: 10.1113/EP085108. [DOI] [PubMed] [Google Scholar]
- 205.Bao W., Morimoto K., Hasegawa T., Sasaki N., Yamashita T., Hirata K., Okita Y., Okada K. Orally administered dipeptidyl peptidase-4 inhibitor (alogliptin) prevents abdominal aortic aneurysm formation through an antioxidant effect in rats. J. Vasc. Surg. 2014;59:1098–1108. doi: 10.1016/j.jvs.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 206.Abdelsalam R.M., Safar M.M. Neuroprotective effects of vildagliptin in rat rotenone parkinson’s disease model: Role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J. Neurochem. 2015;133:700–707. doi: 10.1111/jnc.13087. [DOI] [PubMed] [Google Scholar]
- 207.Steven S., Hausding M., Kroller-Schon S., Mader M., Mikhed Y., Stamm P., Zinssius E., Pfeffer A., Welschof P., Agdauletova S., et al. Gliptin and GLP-1 analog treatment improves survival and vascular inflammation/dysfunction in animals with lipopolysaccharide-induced endotoxemia. Basic Res. Cardiol. 2015;110:6. doi: 10.1007/s00395-015-0465-x. [DOI] [PubMed] [Google Scholar]
- 208.Kroller-Schon S., Knorr M., Hausding M., Oelze M., Schuff A., Schell R., Sudowe S., Scholz A., Daub S., Karbach S., et al. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc. Res. 2012;96:140–149. doi: 10.1093/cvr/cvs246. [DOI] [PubMed] [Google Scholar]
- 209.Shah P., Ardestani A., Dharmadhikari G., Laue S., Schumann D.M., Kerr-Conte J., Pattou F., Klein T., Maedler K. The DPP-4 inhibitor linagliptin restores β-cell function and survival in human isolated islets through GLP-1 stabilization. J. Clin. Endocrinol. Metab. 2013;98:E1163–E1172. doi: 10.1210/jc.2013-1029. [DOI] [PubMed] [Google Scholar]
- 210.Rizzo M.R., Barbieri M., Marfella R., Paolisso G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: Role of dipeptidyl peptidase-iv inhibition. Diabetes Care. 2012;35:2076–2082. doi: 10.2337/dc12-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Batchuluun B., Inoguchi T., Sonoda N., Sasaki S., Inoue T., Fujimura Y., Miura D., Takayanagi R. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis. 2014;232:156–164. doi: 10.1016/j.atherosclerosis.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 212.Shiraki A., Oyama J., Komoda H., Asaka M., Komatsu A., Sakuma M., Kodama K., Sakamoto Y., Kotooka N., Hirase T., et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221:375–382. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 213.Salcedo I., Tweedie D., Li Y., Greig N.H. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: An emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br. J. Pharmacol. 2012;166:1586–1599. doi: 10.1111/j.1476-5381.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fisslthaler B., Fleming I. Activation and signaling by the amp-activated protein kinase in endothelial cells. Circ. Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 215.Balteau M., van Steenbergen A., Timmermans A.D., Dessy C., Behets-Wydemans G., Tajeddine N., Castanares-Zapatero D., Gilon P., Vanoverschelde J.L., Horman S., et al. AMPK activation by glucagon-like peptide-1 prevents NADPH oxidase activation induced by hyperglycemia in adult cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H1120–H1133. doi: 10.1152/ajpheart.00210.2014. [DOI] [PubMed] [Google Scholar]
- 216.Martin M., Huguet J., Centelles J.J., Franco R. Expression of ecto-adenosine deaminase and CD26 in human T cells triggered by the TCR-CD3 complex. Possible role of adenosine deaminase as costimulatory molecule. J. Immunol. 1995;155:4630–4643. [PubMed] [Google Scholar]
- 217.Kameoka J., Tanaka T., Nojima Y., Schlossman S.F., Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993;261:466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 218.Dopheide J.F., Scheer M., Doppler C., Obst V., Stein P., Vosseler M., Abegunewardene N., Gori T., Munzel T., Daiber A., et al. Change of walking distance in intermittent claudication: Impact on inflammation, oxidative stress and mononuclear cells: A pilot study. Clin. Res. Cardiol. 2015 doi: 10.1007/s00392-015-0840-5. [DOI] [PubMed] [Google Scholar]
- 219.Dopheide J.F., Obst V., Doppler C., Radmacher M.C., Scheer M., Radsak M.P., Gori T., Warnholtz A., Fottner C., Daiber A., et al. Phenotypic characterisation of pro-inflammatory monocytes and dendritic cells in peripheral arterial disease. Thromb. Haemost. 2012;108:1198–1207. doi: 10.1160/TH12-05-0327. [DOI] [PubMed] [Google Scholar]
- 220.Dopheide J.F., Doppler C., Scheer M., Obst V., Radmacher M.C., Radsak M.P., Gori T., Warnholtz A., Fottner C., Munzel T., et al. Critical limb ischaemia is characterised by an increased production of whole blood reactive oxygen species and expression of TREM-1 on neutrophils. Atherosclerosis. 2013;229:396–403. doi: 10.1016/j.atherosclerosis.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 221.Libby P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Burgmaier M., Liberman A., Mollmann J., Kahles F., Reith S., Lebherz C., Marx N., Lehrke M. Glucagon-like peptide-1 (GLP-1) and its split products GLP-1(9-37) and GLP-1(28-37) stabilize atherosclerotic lesions in apoe−/− mice. Atherosclerosis. 2013;231:427–435. doi: 10.1016/j.atherosclerosis.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 223.Gaspari T., Liu H., Welungoda I., Hu Y., Widdop R.E., Knudsen L.B., Simpson R.W., Dear A.E. A GLP-1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an apoe−/− mouse model. Diabetes Vasc. Dis. Res. 2011;8:117–124. doi: 10.1177/1479164111404257. [DOI] [PubMed] [Google Scholar]
- 224.Buldak L., Labuzek K., Buldak R.J., Machnik G., Boldys A., Okopien B. Exenatide (a GLP-1 agonist) improves the antioxidative potential of in vitro cultured human monocytes/macrophages. Naunyn Schmiedebergs Arch. Pharmacol. 2015 doi: 10.1007/s00210-015-1124-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Arakawa M., Mita T., Azuma K., Ebato C., Goto H., Nomiyama T., Fujitani Y., Hirose T., Kawamori R., Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Erdogdu O., Nathanson D., Sjoholm A., Nystrom T., Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol. Cell. Endocrinol. 2010;325:26–35. doi: 10.1016/j.mce.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 227.Ban K., Noyan-Ashraf M.H., Hoefer J., Bolz S.S., Drucker D.J., Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 228.Green B.D., Hand K.V., Dougan J.E., McDonnell B.M., Cassidy R.S., Grieve D.J. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving katp and camp. Arch. Biochem. Biophys. 2008;478:136–142. doi: 10.1016/j.abb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 229.Shah Z., Pineda C., Kampfrath T., Maiseyeu A., Ying Z., Racoma I., Deiuliis J., Xu X., Sun Q., Moffatt-Bruce S., et al. Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vascul. Pharmacol. 2011;55:2–9. doi: 10.1016/j.vph.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang R., Lu L., Guo Y., Lin F., Chen H., Chen W., Chen M. Effect of glucagon-like peptide-1 on high-glucose-induced oxidative stress and cell apoptosis in human endothelial cells and its underlying mechanism. J. Cardiovasc. Pharmacol. 2015 doi: 10.1097/FJC.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 231.Levy M.M., Artigas A., Phillips G.S., Rhodes A., Beale R., Osborn T., Vincent J.L., Townsend S., Lemeshow S., Dellinger R.P. Outcomes of the surviving sepsis campaign in intensive care units in the USA and europe: A prospective cohort study. Lancet Infect. Dis. 2012;12:919–924. doi: 10.1016/S1473-3099(12)70239-6. [DOI] [PubMed] [Google Scholar]
- 232.Levi M., van der Poll T. Disseminated intravascular coagulation: A review for the internist. Intern. Emerg. Med. 2013;8:23–32. doi: 10.1007/s11739-012-0859-9. [DOI] [PubMed] [Google Scholar]
- 233.Boerma E.C., van der Voort P.H., Spronk P.E., Ince C. Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit. Care Med. 2007;35:1055–1060. doi: 10.1097/01.CCM.0000259527.89927.F9. [DOI] [PubMed] [Google Scholar]
- 234.Bone R.C. Sepsis syndrome. New insights into its pathogenesis and treatment. Infect. Dis. Clin. N. Am. 1991;5:793–805. [PubMed] [Google Scholar]
- 235.Nguyen H.B., Rivers E.P., Knoblich B.P., Jacobsen G., Muzzin A., Ressler J.A., Tomlanovich M.C. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit. Care Med. 2004;32:1637–1642. doi: 10.1097/01.CCM.0000132904.35713.A7. [DOI] [PubMed] [Google Scholar]
- 236.Gkaliagkousi E., Ferro A. Nitric oxide signalling in the regulation of cardiovascular and platelet function. Front. Biosci. 2011;16:1873–1897. doi: 10.2741/3828. [DOI] [PubMed] [Google Scholar]
- 237.Huet O., Dupic L., Harrois A., Duranteau J. Oxidative stress and endothelial dysfunction during sepsis. Front. Biosci. 2011;16:1986–1995. doi: 10.2741/3835. [DOI] [PubMed] [Google Scholar]
- 238.Krotz F., Sohn H.Y., Pohl U. Reactive oxygen species: Players in the platelet game. Arterioscler. Thromb. Vasc. Biol. 2004;24:1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 239.Heyworth P.G., Cross A.R., Curnutte J.T. Chronic granulomatous disease. Curr. Opin. Immunol. 2003;15:578–584. doi: 10.1016/S0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 240.Nathens A.B., Neff M.J., Jurkovich G.J., Klotz P., Farver K., Ruzinski J.T., Radella F., Garcia I., Maier R.V. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann. Surg. 2002;236:814–822. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Domenighetti G., Suter P.M., Schaller M.D., Ritz R., Perret C. Treatment with N-acetylcysteine during acute respiratory distress syndrome: A randomized, double-blind, placebo-controlled clinical study. J. Crit. Care. 1997;12:177–182. doi: 10.1016/S0883-9441(97)90029-0. [DOI] [PubMed] [Google Scholar]
- 242.Sprung C.L., Annane D., Keh D., Moreno R., Singer M., Freivogel K., Weiss Y.G., Benbenishty J., Kalenka A., Forst H., et al. Hydrocortisone therapy for patients with septic shock. N. Engl. J. Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 243.National Heart L., Blood Institute A.C.T.N., Truwit J.D., Bernard G.R., Steingrub J., Matthay M.A., Liu K.D., Albertson T.E., Brower R.G., Shanholtz C., et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N. Engl. J. Med. 2014;370:2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ku H.C., Chen W.P., Su M.J. GLP-1 signaling preserves cardiac function in endotoxemic fischer 344 and DPP4-deficient rats. Naunyn Schmiedebergs Arch. Pharmacol. 2010;382:463–474. doi: 10.1007/s00210-010-0559-9. [DOI] [PubMed] [Google Scholar]
- 245.Amori R.E., Lau J., Pittas A.G. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 246.Wang Y., Parlevliet E.T., Geerling J.J., van der Tuin S.J., Zhang H., Bieghs V., Jawad A.H., Shiri-Sverdlov R., Bot I., de Jager S.C., et al. Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration. Br. J. Pharmacol. 2014;171:723–734. doi: 10.1111/bph.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Jung Y.A., Choi Y.K., Jung G.S., Seo H.Y., Kim H.S., Jang B.K., Kim J.G., Lee I.K., Kim M.K., Park K.G. Sitagliptin attenuates methionine/choline-deficient diet-induced steatohepatitis. Diabetes Res. Clin. Pract. 2014;105:47–57. doi: 10.1016/j.diabres.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 248.Weng S.Y., Schuppan D. AMPK regulates macrophage polarization in adipose tissue inflammation and NASH. J. Hepatol. 2013;58:619–621. doi: 10.1016/j.jhep.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 249.Iwaya H., Fujii N., Hagio M., Hara H., Ishizuka S. Contribution of dipeptidyl peptidase IV to the severity of dextran sulfate sodium-induced colitis in the early phase. Biosci. Biotechnol. Biochem. 2013;77:1461–1466. doi: 10.1271/bbb.130105. [DOI] [PubMed] [Google Scholar]
- 250.Schade J., Schmiedl A., Kehlen A., Veres T.Z., Stephan M., Pabst R., von Horsten S. Airway-specific recruitment of T cells is reduced in a CD26-deficient F344 rat substrain. Clin. Exp. Immunol. 2009;158:133–142. doi: 10.1111/j.1365-2249.2009.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Viby N.E., Isidor M.S., Buggeskov K.B., Poulsen S.S., Hansen J.B., Kissow H. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology. 2013;154:4503–4511. doi: 10.1210/en.2013-1666. [DOI] [PubMed] [Google Scholar]
- 252.Busso N., Wagtmann N., Herling C., Chobaz-Peclat V., Bischof-Delaloye A., So A., Grouzmann E. Circulating CD26 is negatively associated with inflammation in human and experimental arthritis. Am. J. Pathol. 2005;166:433–442. doi: 10.1016/S0002-9440(10)62266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ridker P.M., Cannon C.P., Morrow D., Rifai N., Rose L.M., McCabe C.H., Pfeffer M.A., Braunwald E. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 254.Zhu F., Wang Q., Guo C., Wang X., Cao X., Shi Y., Gao F., Ma C., Zhang L. IL-17 induces apoptosis of vascular endothelial cells: A potential mechanism for human acute coronary syndrome. Clin. Immunol. 2011;141:152–160. doi: 10.1016/j.clim.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 255.Huppert J., Closhen D., Croxford A., White R., Kulig P., Pietrowski E., Bechmann I., Becher B., Luhmann H.J., Waisman A., et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010;24:1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- 256.Roussel L., Houle F., Chan C., Yao Y., Berube J., Olivenstein R., Martin J.G., Huot J., Hamid Q., Ferri L., et al. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J. Immunol. 2010;184:4531–4537. doi: 10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
- 257.Hoch N.E., Guzik T.J., Chen W., Deans T., Maalouf S.A., Gratze P., Weyand C., Harrison D.G. Regulation of t-cell function by endogenously produced angiotensin II. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Geiger H. T-cells in angiotensin-II-induced vascular damage. Nephrol. Dial. Transplant. 2008;23:1107–1108. doi: 10.1093/ndt/gfm867. [DOI] [PubMed] [Google Scholar]
- 259.Micha R., Imamura F., Wyler von Ballmoos M., Solomon D.H., Hernan M.A., Ridker P.M., Mozaffarian D. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am. J. Cardiol. 2011;108:1362–1370. doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Asanuma H., Sanada S., Ogai A., Minamino T., Takashima S., Asakura M., Ogita H., Shinozaki Y., Mori H., Node K., et al. Methotrexate and MX-68, a new derivative of methotrexate, limit infarct size via adenosine-dependent mechanisms in canine hearts. J. Cardiovasc. Pharmacol. 2004;43:574–579. doi: 10.1097/00005344-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 261.Moreira D.M., Lueneberg M.E., da Silva R.L., Fattah T., Mascia Gottschall C.A. Rationale and design of the tethys trial: The effects of methotrexate therapy on myocardial infarction with ST-segment elevation. Cardiology. 2013;126:167–170. doi: 10.1159/000351972. [DOI] [PubMed] [Google Scholar]
- 262.Everett B.M., Pradhan A.D., Solomon D.H., Paynter N., Macfadyen J., Zaharris E., Gupta M., Clearfield M., Libby P., Hasan A.A., et al. Rationale and design of the cardiovascular inflammation reduction trial: A test of the inflammatory hypothesis of atherothrombosis. Am. Heart J. 2013;166:199–207 e115. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Elhage R., Maret A., Pieraggi M.T., Thiers J.C., Arnal J.F., Bayard F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation. 1998;97:242–244. doi: 10.1161/01.CIR.97.3.242. [DOI] [PubMed] [Google Scholar]
- 264.Chi H., Messas E., Levine R.A., Graves D.T., Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein e heterozygote model: Pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 265.Ridker P.M., Howard C.P., Walter V., Everett B., Libby P., Hensen J., Thuren T., Group C.P.I. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: A phase IIB randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 266.Munzel T., Sinning C., Post F., Warnholtz A., Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann. Med. 2008;40:180–196. doi: 10.1080/07853890701854702. [DOI] [PubMed] [Google Scholar]
- 267.Munzel T., Gori T., Bruno R.M., Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart. J. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 268.Zhang L., Zalewski A., Liu Y., Mazurek T., Cowan S., Martin J.L., Hofmann S.M., Vlassara H., Shi Y. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–478. doi: 10.1161/01.CIR.0000080378.96063.23. [DOI] [PubMed] [Google Scholar]
- 269.Steinhubl S.R. Why have antioxidants failed in clinical trials? Am. J. Cardiol. 2008;101:14D–19D. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 270.Warnholtz A., Genth-Zotz S., Munzel T. Should treatment of sepsis include statins? Circulation. 2005;111:1735–1737. doi: 10.1161/01.CIR.0000160382.01347.FF. [DOI] [PubMed] [Google Scholar]
- 271.Merx M.W., Liehn E.A., Janssens U., Lutticken R., Schrader J., Hanrath P., Weber C. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2004;109:2560–2565. doi: 10.1161/01.CIR.0000129774.09737.5B. [DOI] [PubMed] [Google Scholar]
- 272.Deshpande A., Pasupuleti V., Rothberg M.B. Statin therapy and mortality from sepsis: A meta-analysis of randomized trials. Am. J. Med. 2015;128:410–417 e411. doi: 10.1016/j.amjmed.2014.10.057. [DOI] [PubMed] [Google Scholar]