Abstract
Background
The cholinergic anti-inflammatory pathway (CAP) modulates inflammatory responses through the vagus nerve and the α-7-nicotinic acetylcholine receptor (α7nAChR) on macrophages and immune cells. Sympathetic/parasympathetic imbalance and chronic inflammation are both linked to poor outcome in dialysis patients. The aim of this study was to investigate CAP activity in these patients.
Methods
Twenty dialysis patients, 12 hemodialysis (HD) and 8 peritoneal dialysis (PD) patients (12 male, 8 female; age range 47–83 years) and 8 controls (5 male, 3 female; age range 31–52 years) were analyzed for C-reactive protein (CRP), tumor necrosis factor (TNF), interleukin-1b (IL-1b), IL-6 and IL-10 at baseline. The cytokines were then assessed after whole blood stimulation ex vivo with lipopolysaccharide (LPS) (10 and 100 ng/mL) and again in the presence of 45 and 90 μmol/L GTS-21, a cholinergic α7nAChR agonist.
Results
CRP, TNF, IL-1 and IL-6 were significantly higher, whereas IL-10 was significantly lower at baseline in patients compared with controls. After LPS stimulation, TNF increased significantly more in patients than in controls but decreased to similar levels in both groups after addition of GTS-21. IL-6 attenuation was comparable with TNF and the IL-1b pattern was similar but remained significantly higher in patients. Interestingly, IL-10 increased after GTS-21 in a dose-dependent manner, but only in patients. Results in HD and PD patients did not differ.
Conclusions
The response of immune cells after LPS exposure and cholinergic stimulation suggests a functional CAP in dialysis patients. It may thus be possible to target the α7nAChR control of cytokine release as an anti-inflammatory strategy and thereby improve outcome in these patients.
Keywords: autonomic dysfunction, cholinergic agonist, cytokine attenuation, dialysis, inflammation
Introduction
The autonomic nervous system controls in part the innate immune system through the cholinergic anti-inflammatory pathway (CAP). The current understanding of this pathway is that it operates as a nerve-mediated reflex that, in case of injury, inflammation or infection in the periphery, transmits signals to the central nervous system via the sensory afferent vagus nerve. The motor fibers in the efferent vagus nerve then activate the adrenergic splenic nerve, which releases norepinephrine in the spleen. Acetylcholine-synthesizing T-cells in the spleen located in the proximity of catecholaminergic nerve endings, which are necessary for attenuation of inflammation, have recently been identified. These specialized T-cells respond to the norepinephrine signal and release acetylcholine, which modulates cytokine responses by activating α-7-nicotinic acetylcholine receptors (α7nAChR) on macrophages and other immune cells [1–5].
Renal impairment entails the loss of several bodily functions and is an independent risk factor for cardiovascular events, 10- to 20-fold higher than the general population [6]. It has been shown that dialysis patients have elevated circulating levels of inflammatory markers such as CRP, TNF, IL-6 and HMGB1, the latter identified as a prototypic alarmin [7–9]. Several of these cytokines are associated with poor prognosis in this immunocompromised population [7, 8].
Autonomic dysfunction has been associated with adverse outcome in a number of clinical conditions, including cardiovascular disease (CVD), congestive heart failure and septicemia [10–12]. Chronic inflammatory diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus are associated with autonomic dysfunction and a reduction in vagus nerve activity [13, 14]. This can be assessed with electrocardiogram (ECG) measurements by analyzing the heart rate variability (HRV), which provides a bedside non-invasive method for investigating the autonomic input into the heart or the balance of the sympathetic and parasympathetic (vagus) nervous systems. The HRV method quantifies the amount by which the RR interval or heart rate changes between heart beat cycles and is typically analyzed in relation to time or frequency. HRV can be measured by continuous electrocardiography over a 24-h period, but shorter recordings can also be utilized. In healthy individuals, the rate of beat-to-beat variability is significantly higher than in subjects with autonomic dysfunction. The high-frequency (HF) range (0.15–0.50 Hz) has been linked to cardiac parasympathetic regulation, reflecting vagus nerve tone. The low-frequency (LF) range (0.04–0.15 Hz) reflects mixed parasympathetic and sympathetic contributions, and the LF/HF ratio is considered a measurement of sympathovagal balance [15, 16]. In dialysis patients, autonomic dysfunction is common and has been associated with poor prognosis. It has been proposed that HRV may be used as a surrogate marker for sudden cardiac death in these patients [17, 18]. It has been hypothesized that CAP can be stimulated either by drugs or by vagus nerve stimulation (VNS) and thereby influence the course of disease by reducing inflammation. Preclinical studies in sepsis models and animal models of mice with collagen-induced arthritis and ischaemia-reperfusion injury have demonstrated the efficacy of cholinergic agonists as well as VNS [19–22].
There are no previous studies on cholinergic function in patients with chronic kidney disease (CKD). Our aim in this exploratory study was therefore to investigate possible deficiencies in the CAP in CKD patients with autonomic dysfunction treated with hemodialysis (HD) and peritoneal dialysis (PD).
Materials and Methods
Patients
The study population consisted of 20 subjects: 12 HD patients (7 male, 5 female; age range 26–84 years), 8 PD patients (5 male, 3 female; age range 47–84 years) and 8 healthy controls (5 male, 3 female; age range 31–52 years). The patients were recruited among stable chronic dialysis patients at the Renal Department at Karolinska University Hospital. Patients on HD were treated three times per week on average 4 h per dialysis session, whereas PD patients were treated with a continuous ambulatory PD (CAPD) regimen, except two who used automated PD (APD). Patients with signs of clinical infection were excluded from the study. Healthy controls were recruited among the staff of the Renal Department. A summary of patient characteristics including age, gender, dialysis modality and time on dialysis is found in Table 1.
Table 1.
Clinical and biochemical characteristics of dialysis patients and healthy controls
| Patients (n = 20) | Controls (n = 8) | P-value | |
|---|---|---|---|
| Sex female (%) | 42 | 38 | 0.92 |
| Age (years) | 65 (47–83) | 46 (31–52) | 0.002 |
| Time on dialysis (months) | 27 (2–91) | NA | |
| CRP (mg/L) | 5 (0.3–22.6) | 0.0 (0.0–8) | 0.004 |
| B-Hb (g/L) | 113 (99–136) | 137 (121–154) | 0.0006 |
| B-WBC, 109 | 7.8 (4.4–10.6) | 5.1 (3.8–10.7) | 0.08 |
| B-monocytes | 0.7 (0.3–1.35) | 0.5 (0.4–0.9) | 0.13 |
| B-lymphocytes | 1.5 (0.9–2.2) | 1.9 (1.2–3.1) | 0.16 |
| TNF (pg/mL)a | 33 (16.5–49.7) | 10.1 (7.3–22.2) | 0.001 |
| IL-1b (pg/mL)a | 1.6 (1.3–8.8) | 2.7 (0.2–9.3) | 0.02 |
| IL-6 (pg/mL)a | 7.9 (4.2–15.6) | 0.9 (0.2–8.8) | 0.002 |
| IL-10 (pg/mL)a | 3.9 (2.8–4.8) | 4.6 (2.5–16.4) | 0.002 |
Data are expressed as median and (10–90) percentiles. References CRP, Hb and so on <3 mg/L; B-Hb 117–153 g/L (female), 134–170 g/L (male); B-white blood cell count (WBC) 3.5–8.8 × 109/L; B-monocytes 0.1–1.0 × 109/L; and B-lymphocytes 1.0–4.0 × 109/L. NA, not applicable.
aBaseline values prior to LPS stimulation.
The study protocol was approved by the local ethics committee and informed consent was obtained from each subject.
Methods
All subjects were sampled in the morning. HD patients were sampled on a dialysis day prior to dialysis treatment. Blood samples were analyzed for C-reactive protein (CRP), hemoglobin (Hb), white blood cell count (WBC) with differentials, tumor necrosis factor (TNF), interleukin-1b (IL-1b), IL-6 and IL-10 at baseline. CRP, Hb and WBC were analyzed using routine methods at the Department of Clinical Chemistry at Karolinska University Hospital.
Ex vivo whole blood assay
The whole blood assay was performed by a method previously described [23]. Briefly, whole blood samples were stimulated ex vivo with two concentrations of lipopolysaccharide (LPS) (10 and 100 ng/mL) to induce an inflammatory reaction. TNF, IL-1b, IL-6 and IL-10 were measured at these concentrations and again in the presence of 45 and 90 μmol/L GTS-21, a selective α7nAChR cholinergic agonist.
Whole blood was collected in two heparinized tubes and immediately kept in a 37°C heated container until processed within 60–120 min.
LPS from Escherichia coli 0111:B4 was obtained from Sigma-Aldrich (St Louise, MO, USA; cat. no. L4130). Five milligrams of LPS was dissolved in 1 mL phosphate-buffered saline (PBS), sonicated for 30 min, vortexed well and diluted five times to 1 × 106 ng/mL (=stock solution) and then portioned and frozen at −80°C.
Solutions of GTS-21 and LPS were prepared as follows. The 1 × 106 ng/mL stock solution of LPS was serially diluted with PBS to 10 000 and 1000 ng/mL. GTS-21 (2 mg) was diluted in distilled water to approximately 4.6 and 9.2 mmol/L working solutions, respectively.
One milliliter each of whole blood was pipetted into twelve 2-mL Eppendorf tubes. Ten microliters of PBS was added to tubes 1–4, 10 µL of 4.6 mmol/L GTS-21 working solution (final concentration ∼45 µmol/L) to tubes 5–8 and 10 µL of 9.2 mmol/L GTS-21 working solution (final concentration ∼90 µmol/L) to tubes 9–12. The tubes were incubated at 37°C for 15 min on a rocking platform. Then, 10 µL of PBS was added to tubes 1, 2, 5, 6, 9 and 10; 10 µL of 1000 ng/mL LPS (final concentration ∼10 ng/mL) was added to tubes 3, 7 and 11; and 10 µL of 10 000 ng/mL LPS (final concentration ∼100 ng/mL) was added to tubes 4, 8 and 12. (Thus, tubes 1 and 2 containing whole blood and PBS only served as controls.)
After a 4-h incubation at 37°C on a rocking platform, plasma was collected by centrifugation (2600g, 20 min, 18°C, Eppendorf centrifuge 5804R) and frozen at −80°C pending cytokine analyses.
Cytokine analysis
High-sensitivity TNF, IL-1b, IL-6 and IL-10 were analyzed by immunometric assays on an Immulite 1000 Analyzer (Siemens Healthcare Diagnostics, Los Angeles, CA, USA) according to the instructions of the manufacturer.
Heart rate variability
HRV was measured in a subset of dialysis patients and in all the controls. These measurements were, due to practical reasons, not done at blood sampling but at a separate visit. Twelve patients (seven on HD, five on PD and the eight healthy control subjects) were assessed for HRV, which was done in a quiet examining room in a supine position.
ECG and tetrapolar thoracic bioimpedance were measured over 20 min using a custom-made device, designed by Z-Health Technologies (Borås, Sweden). By this method, the RR interval variability (RRV) is automatically calculated based on the Pan–Tompkins algorithm [24]. Artifacts and ectopic beats were detected and removed visually (<2% of beats were removed). Thoracic bioimpedance measurements were used to calculate respiration rate. The HRV analysis generated data for time and frequency domain parameters according to the European Society of Cardiology Task Force for HRV measurements [15]. The time domain parameter standard deviation of normal-to-normal intervals (SDNN) was recorded as an overall estimate of HRV, and frequency domain was recorded as LF and HF and the LF/HF ratio. Respiration rate and heart rate were also measured.
Statistical methods
All values are expressed as median (10th, 90th percentile) or percentage as appropriate according to the type and distribution of the data. P-values <0.05 were considered statistically significant. Comparisons between groups were performed using non-parametric Wilcoxon's test. Comparisons between groups and various concentrations of LPS were performed using Friedman's non-parametric two-way ANOVA. Fischer's exact test and chi-square test were used for categorical variables. Non-parametric Spearman's rank correlation analysis was used to determine associations between various variables. As P-values are not adjusted for multiple testing, they have to be considered as descriptive. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
There was a significant difference in age between patients and controls but not in gender (Table 1).
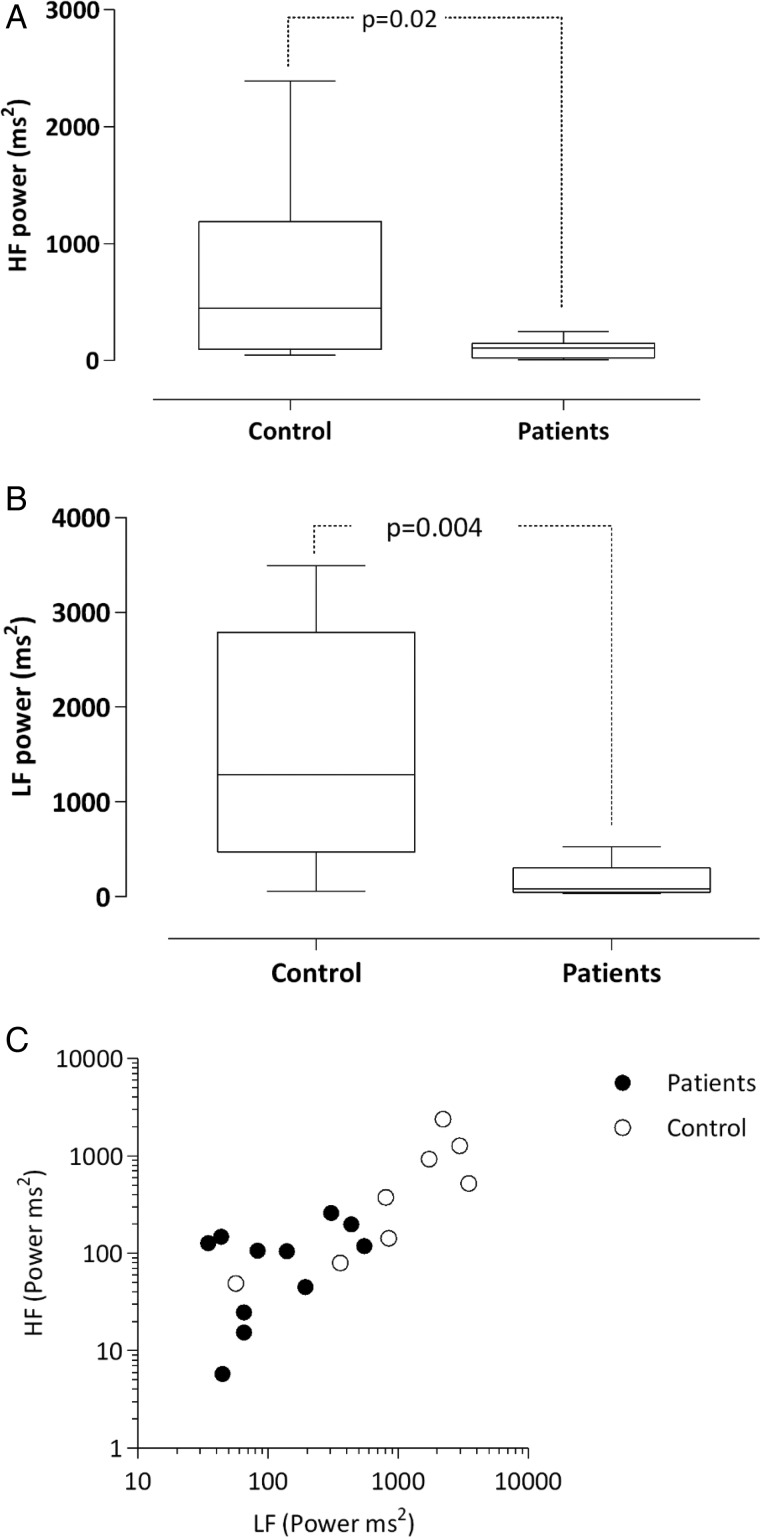
There were no significant differences for HRV in the HD and PD groups (data not shown), and hence the results of all dialysis patients were compared with controls. The result of HRV analysis confirmed significant autonomic dysfunction in patients when compared with controls with regard to the time domain parameter SDNN as well as the frequency domain parameters HF and LF (Table 2 and Figure 1A–C). Respiration rate and heart rate did not differ between groups. However, one HD patient with high heart and respiratory rates was excluded from the analysis as an outlier.
Table 2.
Clinical and HRV parameters of healthy subjects and dialysis patients
| Controls (n = 8) | Patients (n = 11) | P-value | |
|---|---|---|---|
| Age (years) | 46 (31–52) | 66 (31–82) | 0.003 |
| LF (ms2) | 1289 (56–3489) | 83 (36–529) | 0.005 |
| HF (ms2) | 449 (49–2393) | 106 (8–248) | 0.02 |
| LF/HF ratio | 2.2 (0.9–6.7) | 2.2 (0.3–7.1) | 0.56 |
| SDNN (ms) | 39.3 (17.1–70.2) | 16 (9.8–48.8) | 0.047 |
| Heart rate (beats/min) | 62 (48–84) | 70 (65–125) | 0.06 |
| Respiration (breaths/min) | 16 (13–18) | 15 (11–19) | 0.90 |
Data are expressed as median and (10–90) percentiles.
LF, low-frequency; HF, high-frequency; SDNN, standard deviation of normal-to-normal intervals.
Fig. 1.
(A) High-frequency (HF) power in patients and controls. (B) LF power in patients and controls. (C) Relationship of HF and LF powers of HRV for controls and patients HF (y-axis) versus LF (x-axis) with logarithmic scale.
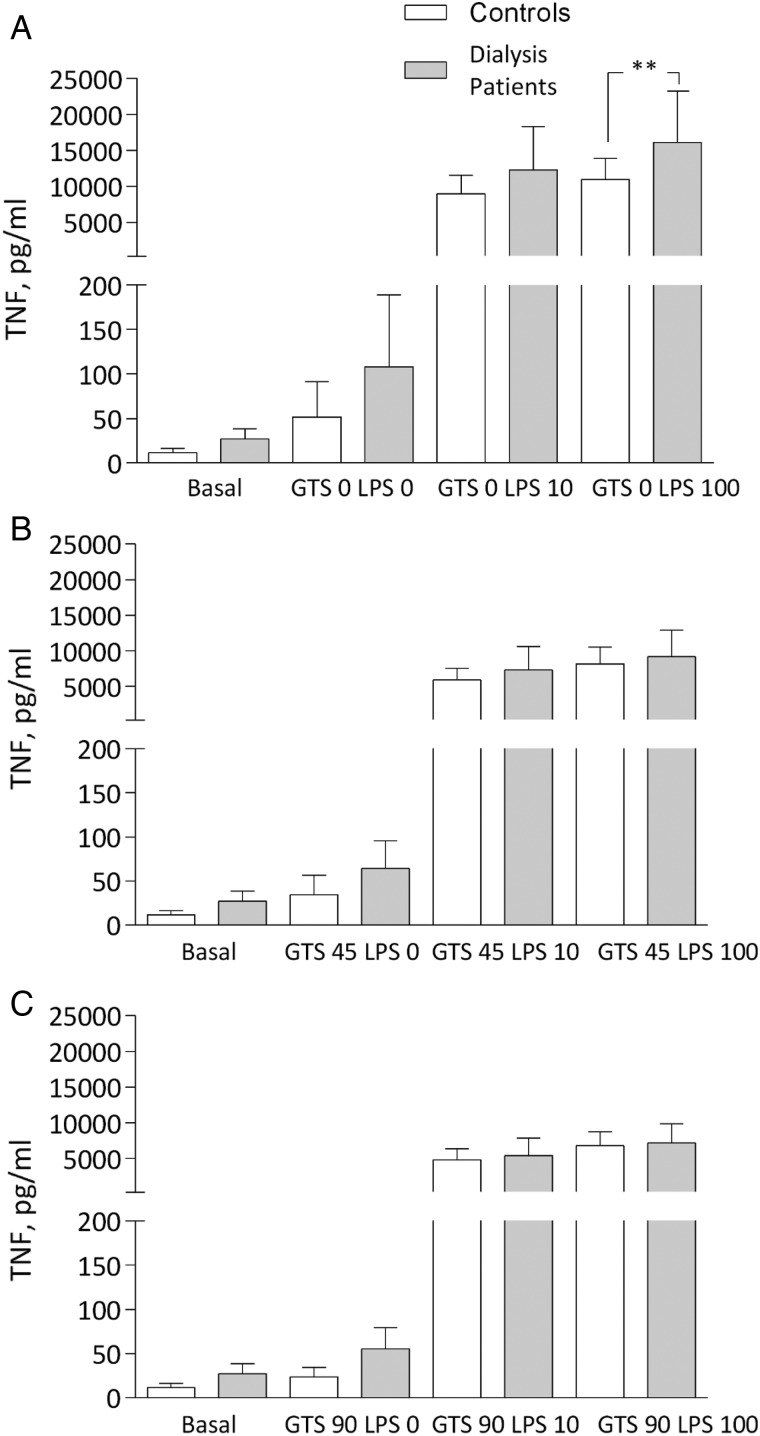
TNF, IL-1b, IL-6, IL-10 and CRP were significantly increased at baseline in patients compared with controls (Table 1). After LPS stimulation ex vivo at the two concentrations (10 and 100 ng/mL), there was an increase of TNF, with a significantly stronger augmentation in patients at maximal stimulation compared with controls. In the presence of GTS-21, there was a robust decrease in TNF levels in patients, which did not differ significantly from controls (Figure 2A–C). There was no difference in TNF levels after LPS exposure or in the presence of GTS-21 between HD and PD patients (data not shown), and the cytokine data are therefore reported as all dialysis patients compared with controls.
Fig. 2.
TNF levels (A) after LPS: 0, 10 and 100 ng/mL; (B) after GTS-21: 45 μmol/L; and (C) 90 μmol/L in dialysis patients and controls. **P < 0.01. All samples except basal incubated for 4 h.
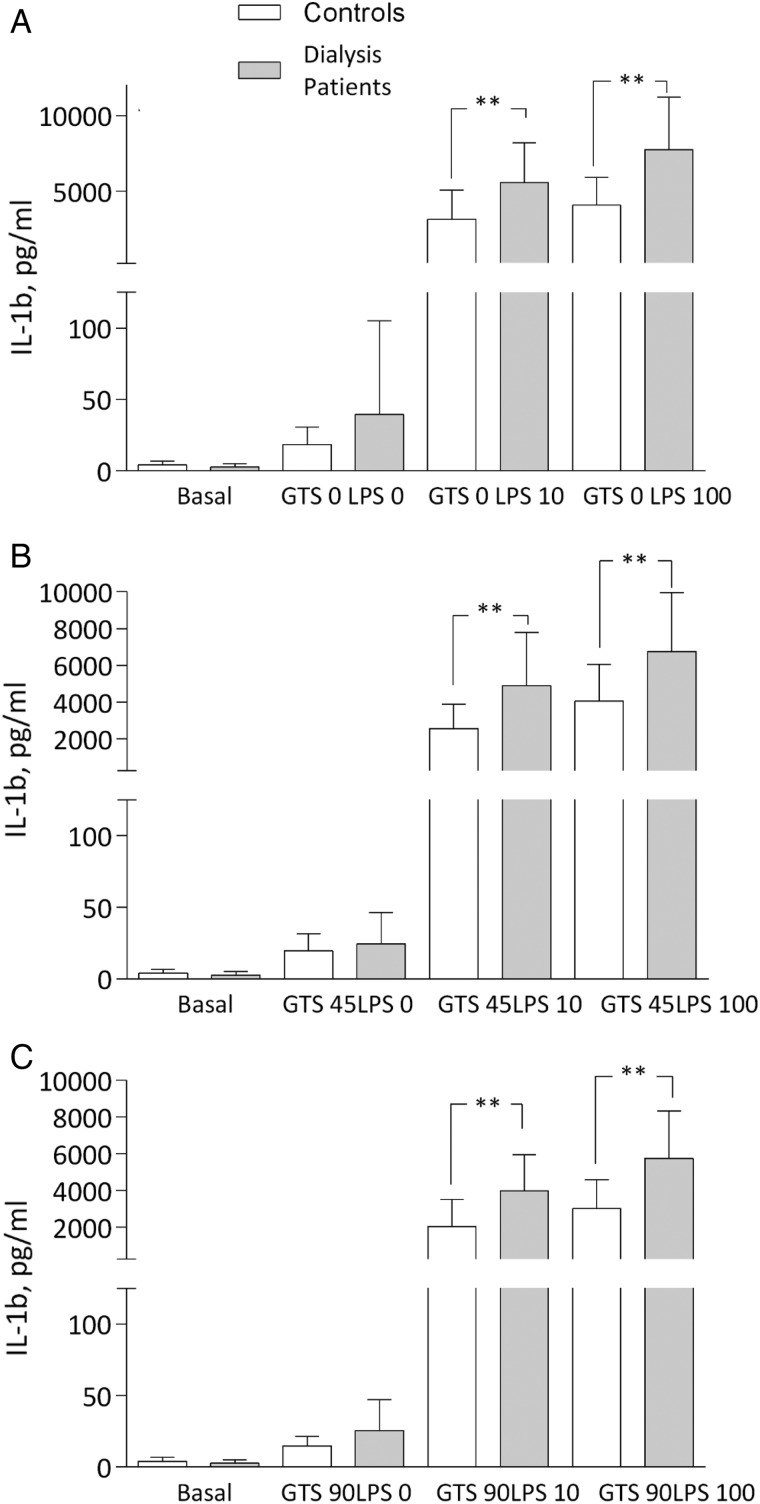
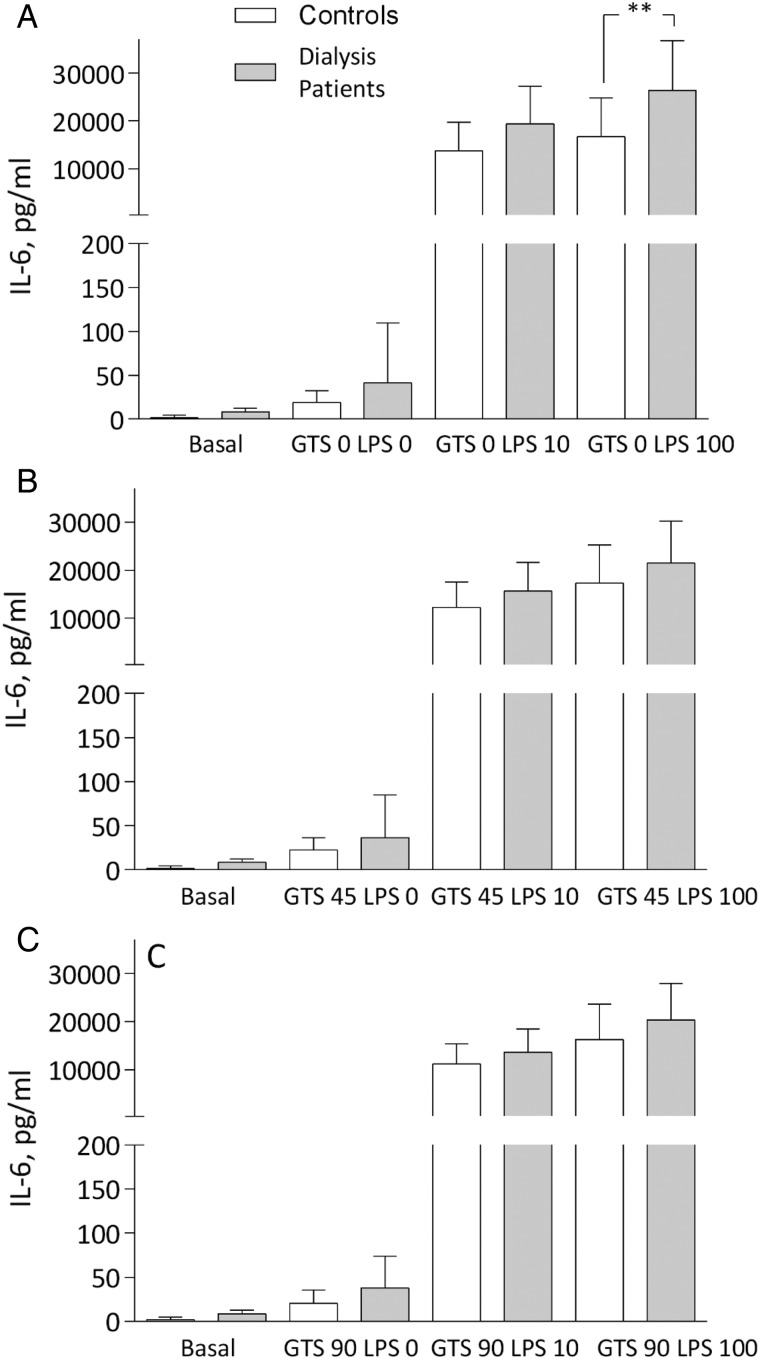
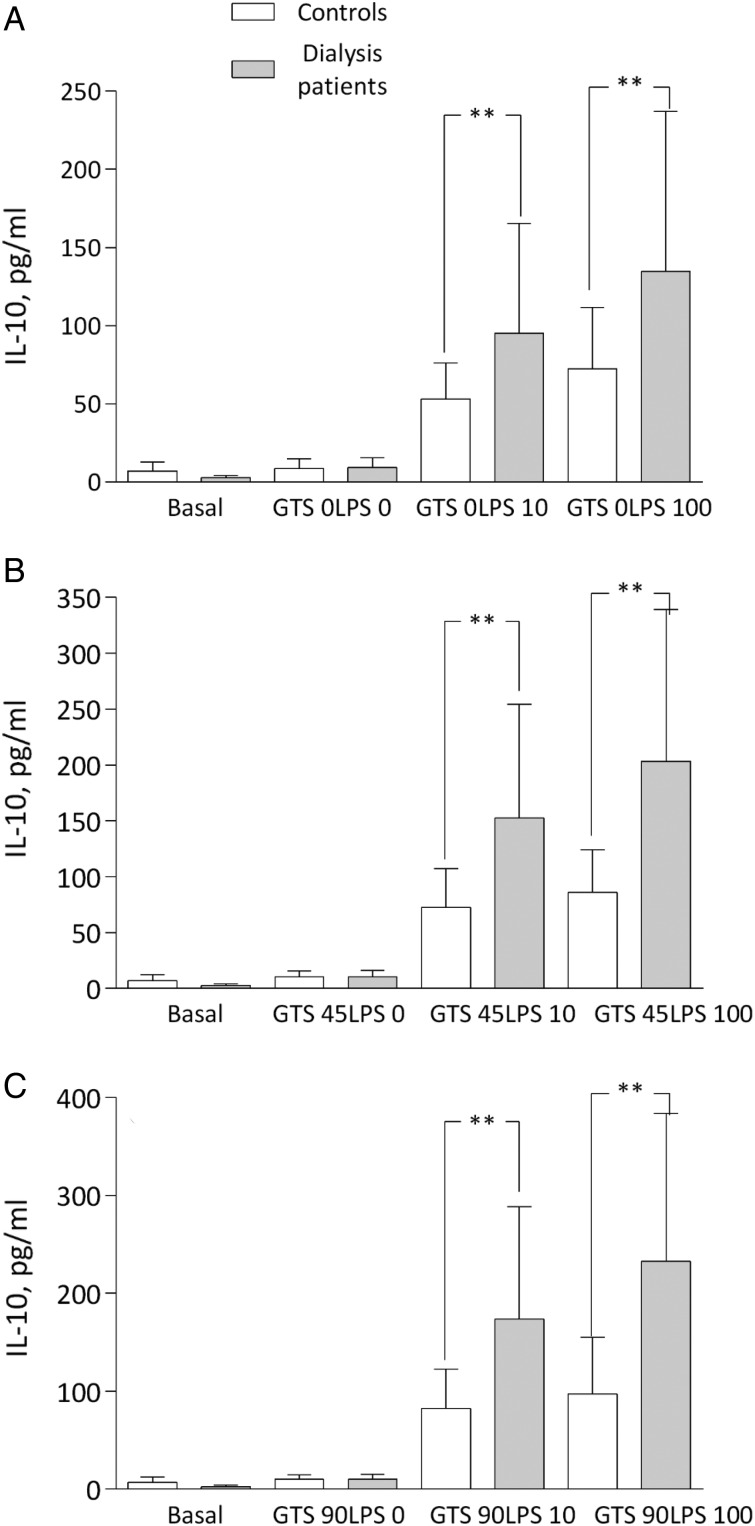
IL-1b was significantly increased in patients at the two LPS concentrations compared with controls. In both groups, IL-1b decreased in the presence of GTS-21, but a statistical difference remained between groups, with higher levels in patients (Figure 3A–C). The IL-6 pattern was similar to TNF at maximal stimulation with LPS, but again with comparable cytokine suppression with GTS-21 in both groups (Figure 4A–C). Finally, IL-10 representing an anti-inflammatory cytokine increased in the presence of GTS-21 in a dose-dependent manner, but only in patients (Figure 5A–C).
Fig. 3.
IL-1b levels (A) after LPS: 0, 10 and 100 ng/mL; (B) after GTS-21: 45 μmol/L; and (C) 90 μmol/L in dialysis patients and controls. **P < 0.01. All samples except basal incubated for 4 h.
Fig. 4.
IL-6 levels (A) after LPS: 0, 10 and 100 ng/mL; (B) after GTS-21: 45 μmol/L; and (C) 90 μmol/L in dialysis patients and controls. **P < 0.01. All samples except basal incubated for 4 h.
Fig. 5.
IL-10 levels (A) after LPS: 0, 10 and 100 ng/mL; (B) after GTS-21: 45 μmol/L; and (C) 90 μmol/L in dialysis patients and controls. **P < 0.01. All samples except basal incubated for 4 h.
Total WBC and differentials did not differ between groups.
Discussion
Increased morbidity and mortality in CKD remains a major challenge [7, 8]. Approximately one-third of deaths among dialysis patients may be attributable to autonomic nervous system dysfunction [25]. Increased sympathetic activity is also noted in patients with chronic renal failure prior to dialysis and has been shown to contribute to the increased risk of CVD and possibly also to renal damage [26, 27]. Although identified as a possible modifiable target, most of the focus in this research field has been on increased sympathetic activity and less on parasympathetic or vagus activity.
HRV is a non-invasive measure of autonomic function. It has repeatedly been shown that decreased HRV can be predictive of survival in the CKD population [17, 18, 25–30]. In this study we found that our cohort did not differ from previously studied dialysis patients.
A state of chronic inflammation affects outcome and is a strong prognostic factor of sudden death in end-stage renal disease patients [7, 31]. Treatment of this persistent condition may therefore be beneficial in reducing morbidity and mortality. Thus far, the effect of various anti-inflammatory interventions on outcome has yet to be proved in this patient population [32].
In the current study we investigated whether immune cells from dialysis patients with autonomic dysfunction were able to react in a functional way ex vivo with cytokine attenuation after endotoxin exposure with LPS and cholinergic suppression. We demonstrated that inflammatory cells from dialysis patients exposed to an environment of autonomic dysfunction with decreased vagus nerve activity in vivo respond to cholinergic agonists ex vivo in a similar manner compared with healthy controls, suggesting a functional CAP. We were surprised that there were no differences between HD and PD patients. Dialysis filters used in HD may trigger inflammation in addition to the underlying inflammatory dysregulation common in most dialysis patients. On the other hand, chronic fluid overload in PD patients contributes to inadequate blood pressure control and left ventricular hypertrophy, which may increase inflammation as shown in a recent study [33]. However, our data suggest that it is the end-stage renal disease and dialysis dependency that is important rather than dialysis modality for cytokine expression in this model.
The major limitations of this study were the relatively small sample size and that patients and controls were sampled only once. However, in a similarly designed study of RA patients who came for one to eight visits, we could not find any significant difference in HRV and whole blood assay results at repeated visits. This suggests that the combined phenotype of autonomic dysfunction and the innate response to endotoxin stimulation with LPS in chronic inflammation is fairly stable. Interestingly, dialysis patients differed from RA patients by having a much stronger inflammatory response to LPS in the whole blood model [23]. This may reflect that the RA patients were treated with anti-inflammatory drugs, which was not the case with the dialysis patients, or possibly that there is an inherent biological difference between RA and CKD. However, the response to the cholinergic agonist was similar, suggesting a functional CAP irrespective of underlying disease. Another issue was that the control population was significantly younger compared with dialysis patients, which could potentially be important since HRV is known to decrease with age [15, 16]. However, in spite of significant differences in HRV, the response to the cholinergic agonist did not differ between groups in the current study. Finally, it may also be argued that the ex vivo situation with GTS-21 does not fully represent the in vivo biology. However, GTS-21 has been proved effective as an immunomodulatory compound by attenuating pro-inflammatory cytokine levels and improving survival in sepsis models [22, 34].
Since the discovery of the CAP, preclinical studies have demonstrated the effect of cholinergic agonists and VNS in sepsis, arthritis and ischaemia-reperfusion injury models [19–21, 34–36]. Hoeger et al. [37] also showed that vagal stimulation in brain dead donor rats could decrease chronic allograft nephropathy in recipients, which suggests that the effect could be sustained in spite of surgery. In a recent pilot study, it was shown that an implantable VNS device in RA patients had a significant effect on inflammation measured as the 28-joint Disease Activity Score, and neurostimulation of the CAP has also been shown to ameliorate disease in the correspondent rat collagen-induced arthritis model [38, 39].
Conclusion
We have shown here that immune cells from dialysis patients, with an underlying autonomic dysfunction and an dysregulated cytokine response, can mount an anti-inflammatory response after cholinergic stimulation. To the best of our knowledge, these are novel findings in this patient population and may be worth exploring in the evolving therapeutic field of neuroimmunomodulation by cholinergic modalities.
Acknowledgements
We thank Annika Nilsson, Ulrika Jensen and Åsa Lindé for collection of samples and Ann-Christin Bragfors-Helin and Monica Eriksson for laboratory analyses.
Funding
This study was supported by grants from the Fund for Renal Research, Karolinska Institutet Funds, the Swedish Society of Medicine, Westman Research Fund and Stockholm County Council (ALF project).
Conflict of interest statement
None declared.
References
- 1.Tracey KJ. The inflammatory reflex. Nature 2002; 420: 853–859 [DOI] [PubMed] [Google Scholar]
- 2.Czura CJ, Tracey KJ. Autonomic neural regulation of immunity. J Intern Med 2005; 257: 156–166 [DOI] [PubMed] [Google Scholar]
- 3.Saeed RW, Varma S, Peng-Nemeroff T, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med 2005; 201: 1113–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosas-Ballina M, Ochani M, Parrish WR, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A 2008; 105: 11008–11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olofsson PS, Katz DA, Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011; 334: 98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32 (5 Suppl 3): S112–S119 [DOI] [PubMed] [Google Scholar]
- 7.Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-α: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int 2005; 67: 1216–1233 [DOI] [PubMed] [Google Scholar]
- 8.Honda H, Qureshi AR, Heimburger O, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 2006; 47: 139–148 [DOI] [PubMed] [Google Scholar]
- 9.Bruchfeld A, Qureshi AR, Lindholm B, et al. High mobility group box protein-1 correlates with renal function in chronic kidney disease (CKD). Mol Med 2008; 14: 109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Rovere MT, Bigger JT, Jr, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998; 351: 478–484 [DOI] [PubMed] [Google Scholar]
- 11.Guzzetti S, La Rovere MT, Pinna GD, et al. Different spectral components of 24 h heart rate variability are related to different modes of death in chronic heart failure. Eur Heart J 2005; 26: 357–362 [DOI] [PubMed] [Google Scholar]
- 12.Chen WL, Chen JH, Huang CC, et al. Heart rate variability measures as predictors of in-hospital mortality in ED patients with sepsis. Am J Emerg Med 2008; 26: 395–401 [DOI] [PubMed] [Google Scholar]
- 13.Louthrenoo W, Ruttanaumpawan P, Aramrattana A, et al. Cardiovascular autonomic nervous system dysfunction in patients with rheumatoid arthritis and systemic lupus erythematosus. QJM 1999; 92: 97–102 [DOI] [PubMed] [Google Scholar]
- 14.Goldstein RS, Bruchfeld A, Yang L, et al. Cholinergic anti-inflammatory pathway activity and high mobility group box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med 2007; 13: 210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heart rate variability: standards of measurement, physiological interpretation and clinical use, Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996; 17: 354–381 [PubMed] [Google Scholar]
- 16.Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol 2005; 10: 88–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oikawa K, Ishihara R, Maeda T, et al. Prognostic value of heart rate variability in patients with renal failure on hemodialysis. Int J Cardiol 2009; 131: 370–377 [DOI] [PubMed] [Google Scholar]
- 18.Ranpuria R, Hall M, Chan CT, et al. Heart rate variability (HRV) in kidney failure: measurement and consequences of reduced HRV. Nephrol Dial Transplant 2008; 23: 444–449 [DOI] [PubMed] [Google Scholar]
- 19.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405: 458–462 [DOI] [PubMed] [Google Scholar]
- 20.van Maanen MA, Stoof SP, Larosa GJ, et al. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann Rheum Dis 2010; 69: 1717–1723 [DOI] [PubMed] [Google Scholar]
- 21.Yeboah MM, Xue X, Duan B, et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int 2008; 74: 62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med 2011; 269: 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruchfeld A, Goldstein RS, Chavan S, et al. Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in RA. J Intern Med 2010; 268: 94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan J, Tompkins W. A real-time QRS detection algorithm. IEEE Trans Biomed Eng 1985; 32: 230–236 [DOI] [PubMed] [Google Scholar]
- 25.Herzog CA, Mangrum JM, Passman R. Sudden cardiac death and dialysis patients. Semin Dial 2008; 21: 300–307 [DOI] [PubMed] [Google Scholar]
- 26.Koomans HA, Blankestijn PJ, Joles JA. Sympathetic hyperactivity in chronic renal failure: a wake-up call. J Am Soc Nephrol 2004; 15: 524–537 [DOI] [PubMed] [Google Scholar]
- 27.Chandra P, Sands RL, Gillespie BW, et al. Predictors of heart rate variability and its prognostic significance in chronic kidney disease. Nephrol Dial Transplant 2012; 27: 700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayano J, Takahashi H, Toriyama T, et al. Prognostic value of heart rate variability during long-term follow-up in chronic haemodialysis patients with end-stage renal disease. Nephrol Dial Transplant 1999; 14: 1480–1488 [DOI] [PubMed] [Google Scholar]
- 29.Fukuta H, Hayano J, Ishihara S, et al. Prognostic value of heart rate variability in patients with end-stage renal disease on chronic haemodialysis. Nephrol Dialysis Transplant 2003; 18: 318–325 [DOI] [PubMed] [Google Scholar]
- 30.Chan CT, Hanly P, Gabor J, et al. Impact of nocturnal haemodialysis on the variability of heart rate and duration of hypoxaemia during sleep. Kidney Int 2004; 65: 661–665 [DOI] [PubMed] [Google Scholar]
- 31.Parekh RS, Plantinga LC, Kao WH, et al. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 2008; 74: 1335–1342 [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto T, Carrero JJ, Stenvinkel P. Inflammation as a risk factor and target for therapy in chronic kidney disease. Curr Opin Nephrol Hypertens 2011; 20: 662–668 [DOI] [PubMed] [Google Scholar]
- 33.Lai S, Molfino A, Russo GE, et al. Cardiac, inflammatory and metabolic parameters: hemodialysis versus peritoneal dialysis. Cardiorenal Med 2015; 5: 20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavlov VA, Ochani M, Yang LH, et al. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med 2007; 35: 1139–1144 [DOI] [PubMed] [Google Scholar]
- 35.Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, et al. The selective α7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med 2009; 15: 195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olofsson PS, Rosas-Ballina M, Levine YA, et al. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev 2012; 248: 188–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoeger S, Fontana J, Jarczyk J, et al. Vagal stimulation in brain dead donor rats decreases chronic allograft nephropathy in recipients. Nephrol Dial Transplant 2014; 29: 544–549 [DOI] [PubMed] [Google Scholar]
- 38.Koopman FA, Schuurman PR, Vervoordeldonk MJ, et al. Vagus nerve stimulation: a new bioelectronics approach to treat rheumatoid arthritis? Best Pract Res Clin Rheumatol 2014; 28: 625–635 [DOI] [PubMed] [Google Scholar]
- 39.Levine YA, Koopman FA, Faltys M, et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS One 2014; 9: e104530. [DOI] [PMC free article] [PubMed] [Google Scholar]