Abstract
Purpose
To estimate the diagnostic accuracy and lead time gained by retinal nerve fiber layer (RNFL) thickness measurements from optical coherence tomography (OCT) for detecting glaucoma before the development of visual field defects.
Design
Observational cohort study.
Participants
The study group included 75 eyes of 75 patients suspected of glaucoma followed as part of the Diagnostic Innovations in Glaucoma (DIGS) study. These eyes had normal standard automated perimetry (SAP) visual fields at baseline and developed repeatable (3 consecutive) abnormal tests during a median follow-up of 6.3 years. A control group of 75 eyes of 75 healthy subjects matched by age and number of OCT tests during follow-up was included.
Methods
RNFL thickness measurements were obtained at the time of development of the earliest SAP defect (Time 0) and also at times −1, −2, −3, etc., corresponding to one year, two years, three years, etc., before the development of field loss. OCT measurements at corresponding time intervals were analyzed for controls. Time-dependent receiver operating characteristic (ROC) curves were used to evaluate diagnostic accuracy of OCT.
Main Outcome Measures
ROC curve areas and sensitivities at fixed specificities at different times before development of field loss.
Results
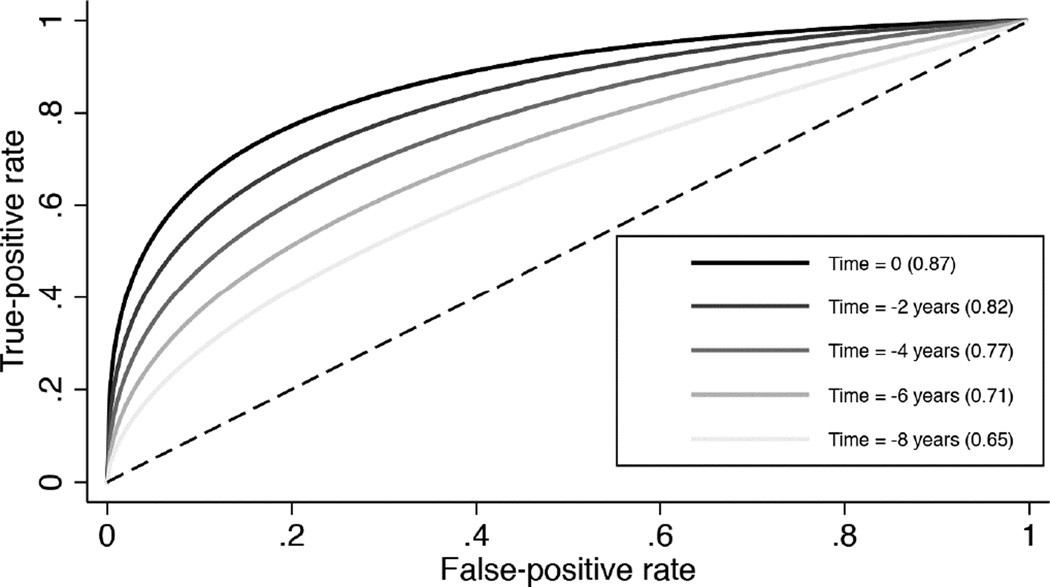
At the date of conversion to the earliest visual field defect (Time 0), mean ± SD average RNFL thickness was 75.0 ± 9.8µm in glaucomatous eyes and 90.6 ± 8.0µm for controls (P<0.001). Significant differences were seen up to 8 years before development of visual field defects (86.3 ± 8.2µm versus 91.4 ± 7.6µm, respectively; P=0.021). ROC curve areas decreased with increasing times before detection of field defects. At times 0, −4 and −8 years, ROC curve areas were 0.87, 0.77 and 0.65, respectively. At 95% specificity, up to 35% of eyes had abnormal average RNFL thickness 4 years before development of visual field loss, and 19% of eyes had abnormal results 8 years before field loss.
Conclusion
Assessment of RNFL thickness with OCT was able to detect glaucomatous damage before the appearance of visual field defects on SAP. In many subjects, significantly large lead times were seen when applying OCT as an ancillary diagnostic tool.
INTRODUCTION
Glaucoma is an optic neuropathy characterized by progressive neuroretinal rim thinning and excavation of the optic nerve head.1 The loss of neural tissue may result in functional deficits, which are usually assessed by standard automated perimetry (SAP). Although the disease may remain asymptomatic until late stages, the diagnosis of moderate and severe cases is usually straightforward and can be confirmed based on the presence of typical visual field defects on SAP, such as arcuate defects, nasal step or paracentral losses, associated with corresponding signs of glaucomatous optic nerve damage.
Glaucoma may occur in many patients before visual field defects are detectable on SAP, but the diagnosis can be challenging in this circumstance.2, 3 Due to the wide variability in the appearance of the optic nerve head, fundoscopic examination at a single visit is generally insufficient to confirm the diagnosis.3 Several imaging technologies have been used as ancillary diagnostic tests in this situation. The use of imaging devices may assist the clinician in identifying glaucomatous damage by providing objective quantification of the integrity of neural structures that may be affected by the disease, such as the neuroretinal rim and retinal nerve fiber layer (RNFL).4–15 One of these technologies, optical coherence tomography (OCT), has been widely used for this purpose.4–12
Several previous studies have evaluated the diagnostic accuracy of OCT in glaucoma.4–11, 16 However, most of these studies have evaluated patients with clearly defined visual field defects, frequently showing moderate or even severe damage. Even in studies that have proposed to evaluate diagnostic accuracy in patients with early glaucoma, all cases had well defined visual field defects that would be clearly diagnostic by themselves. When detecting the presence of disease, it should be obvious that an ancillary diagnostic test would not be necessary in cases where the diagnosis can be clearly confirmed by the presence of repeatable visual field defects. In this situation, one is most often interested in applying OCT to detect damage in cases with questionable field losses or where visual field defects cannot yet be demonstrated.
A longitudinal study involving a cohort of glaucoma suspects provides an optimum design to evaluate the diagnostic performance of OCT at the earliest stages of the disease. By following suspects until they develop the first evidence of repeatable SAP defects, one can assess whether OCT is able to detect structural damage at the point of earliest confirmed functional loss. In addition, one can analyze the historical data and evaluate how far back in the past OCT started to show the earliest signs of damage before the appearance of visual field defects. Such design would provide information about the lead-time that is gained by applying OCT as an ancillary test before patients develop confirmed visual field loss.
In the present study, we evaluated imaging results obtained with OCT in a cohort of glaucoma suspects followed over time who developed the earliest repeatable visual field defects. We evaluated OCT data that was available for several years before the development of visual field defects in order to estimate the lead time that could be gained by applying OCT for diagnosing the disease before the development of field defects.
METHODS
This was an observational study. Participants from this study were included in a prospective longitudinal study designed to evaluate optic nerve structure and visual function in glaucoma (the Diagnostic Innovations in Glaucoma Study [DIGS]) conducted at the Hamilton Glaucoma Center at the Department of Ophthalmology, University of California-San Diego (UCSD). The institutional review board approved the study methodology, which adhered to the tenets of the Declaration of Helsinki and to the Health Insurance Portability and Accountability Act.
At each visit during follow-up, subjects underwent a comprehensive ophthalmologic examination including review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement, gonioscopy, dilated fundoscopic examination, stereoscopic optic disc photography, and automated perimetry using Swedish Interactive Threshold Algorithm (SITA Standard 24-2). Only subjects with open angles on gonioscopy were included. Subjects were excluded if they presented with a best-corrected visual acuity less than 20/40, spherical refraction outside ± 5.0 diopters and/or cylinder correction outside 3.0 diopters, or any other ocular or systemic disease that could affect the optic nerve or the visual field.
Participants
The study group (cases) consisted of 75 eyes of 75 patients suspected of having glaucoma who were followed as part of the DIGS cohort and developed repeatable abnormal visual fields during follow-up, i.e., converted to glaucoma. Initial diagnosis as glaucoma suspect was based on the presence of suspicious appearance of the optic disc or elevated (>21mmHg) intraocular pressure, but normal standard automated perimetry testing at baseline. Normal visual fields were defined based on mean deviation (MD) and pattern standard deviation (PSD) within 95% confidence limits and a Glaucoma Hemifield Test (GHT) within normal limits. These eyes had a median follow-up of 6.3 years (1st quartile: 4.1 years, 3rd quartile: 8.9 years) until the development of repeatable abnormal SAP defects. Repeatable abnormal SAP was defined based on the presence of a sequence of 3 consecutive abnormal SAPs with PSD with P<5% or Glaucoma Hemifield Test outside normal limits. Imaging assessment of the RNFL with optical coherence tomography was performed at the time (within ± 3 months) of the first visual field of the sequence of 3 repeatable abnormal fields. In addition, OCT data was also available and analyzed for the period of follow-up before development of the earliest visual field defect (details on data analysis are described below).
A control group matched by age and number of OCT tests during follow-up was included in the study consisting of 75 eyes from 75 healthy participants. These subjects were recruited from the general population and were required to have a normal ophthalmologic examination and IOP below 22mmHg in both eyes. In addition, they had normal SAP visual field tests during follow-up. Healthy eyes were chosen as control group because we were interested in evaluating the amount of neural loss associated with early visual field defects or before these defects, compared to normal expected age-matched results. Although a group of glaucoma suspects who did not develop visual field loss could be initially thought as a control group, these eyes could have sustained structural damage before functional losses and, therefore, would not constitute a suitable control group for the purposes of this study. Our design best replicates the common clinical practice situation of comparing the results of a test acquired in a patient suspected of glaucoma with a normative age-matched database.
Visual Field Testing
All patients underwent SAP testing using SITA-standard 24-2 strategy during follow-up. All visual fields were evaluated by the UCSD Visual Field Assessment Center (VisFACT).17 Visual fields with more than 33% fixation losses, or more than 15% false-positive errors were excluded. Visual fields exhibiting a learning effect (i.e., initial tests showing consistent improvement on visual field indexes) were also excluded. Visual fields were further reviewed for the following artifacts: lid and rim artifacts, fatigue effects, inappropriate fixation, evidence that the visual field results were due to a disease other than glaucoma (such as homonymous hemianopia), and inattention. The VisFACT requested repeats of unreliable visual field test results, and these were obtained whenever possible.
Optical Coherence Tomography Testing
In order to maximize the amount of OCT data available during follow-up, we analyzed tests from both time-domain OCT (Stratus OCT, Carl-Zeiss Meditec, Dublin, CA), as well as spectral-domain OCT (Cirrus HDOCT, Carl Zeiss Meditec Inc., Dublin, CA). The principles of operation of these instruments have been described in detail elsewhere. RNFL thickness measurements were acquired at a peripapillary 3.46-mm diameter circular scan (10870µm length) placed around the optic disc. To be included, all images were reviewed for non-centered scans and had to have signal strength >6, absence of major movement artifacts, and good centering on the optic disc. The parameter investigated in this study was the global average thickness, corresponding to the average of RNFL thickness measurements obtained in the 360-degree peripapillary circle. This parameter was chosen as it has been reported as having one of the best, if not the best, diagnostic accuracy in previous studies with OCT.4, 6, 12, 18, 19 Also, this parameter has shown the best reproducibility in previous studies with longitudinal OCT data.20–22
As the result of change in OCT technology over time, Stratus OCT images were acquired from 2002 to 2009 and images with Cirrus HDOCT were acquired from 2009 until 2014. As measurements from these two instruments are not directly interchangeable, a conversion factor was obtained from a subgroup of 63 eyes of 63 subjects who had testing with both Stratus OCT and Cirrus HDOCT on the same day during the transition period. The following conversion formula was used by applying a Passing-Bablock regression23:
Cirrus HD average thickness = 8.121 + 0.837* Stratus OCT average thickness
Data Analysis
For the purposes of this study, time zero was defined as the date of conversion to the earliest visual field defect for cases. The conversion date was considered the date of the first of the 3 repeatable abnormal visual fields. OCT exams that were obtained within 3 months of the conversion date and also before the date of conversion were analyzed for cases. We report OCT measurements obtained at time −1, −2, −3, etc., corresponding to one year, two years, three years, etc., before the development of visual field defects. The OCT measurements at corresponding time intervals were also analyzed for the controls, where time zero was then determined to be the last available OCT during follow-up.
One would expect that as time t becomes more negative, i.e., further before the date of conversion, the ability of OCT to detect the presence of glaucomatous damage would decrease. In order to evaluate the effect of time before visual field loss on diagnostic accuracy of OCT, we used time-dependent receiver operating characteristic (ROC) curves obtained from an ROC regression model. Application of ROC regression to the ophthalmic literature has been described in detail previously by Medeiros et al18, 24–26. In brief, this model allows the investigation of the effect of covariates on the ROC curve by modeling these curves using a generalized linear regression model (ROC-GLM). In the current application, time was included as a disease-related covariate, allowing one to obtain estimates of diagnostic accuracy at specific points in time.27, 28 Parameters were estimated using probit regression. To obtain confidence intervals for regression parameters, a bootstrap (with case-control sampling) procedure was used (n=1000 resamples).29 The area under the ROC curve was used to summarize the diagnostic accuracy. The ROC curve area ranges from 0.5 to 1.0, with 1.0 indicating perfect discrimination between cases and controls and 0.5 indicating chance discrimination.
All statistical analyses were performed with commercially available software (STATA, version 13; Stata Corp LP, College Station, TX). The alpha level (type I error) was set at 0.05.
RESULTS
Table 1 shows demographic and clinical characteristics of included subjects at the date of development of the earliest visual field defect for glaucoma patients and at the closest corresponding date for controls. Mean age was 68.3 ± 11.2 years and 65.4 ± 9.0 years for glaucomatous and control subjects, respectively (P=0.082). The median number of OCT tests was 5 (1st quartile: 3, 3rd quartile: 7) for both groups (P = 0.879). At the date of visual field conversion, average MD and PSD of glaucomatous eyes were −1.97 ± 2.1 dB and 2.7 ± 1.1 dB, respectively.
Table 1.
Demographic and clinical variables for glaucoma cases and controls.
| Glaucoma (n=75) | Controls (n=75) | P | |
|---|---|---|---|
| Age, years | 68.3 ± 11.2 | 65.4 ± 9.0 | 0.082 |
| Sex, %F | 61% | 61% | 1.000 |
| Race, | |||
| Caucasian | 48 (64%) | 53 (71%) | 0.506 |
| Black | 26 (35%) | 20 (27%) | |
| Asian | 1 (1%) | 2 (2%) | |
| SAP MD at baseline (dB) | −0.84 ± 1.31 | −0.47 ± 1.17 | 0.068 |
| SAP PSD at baseline (dB) | 1.64 ± 0.32 | 1.54 ± 0.24 | 0.092 |
| SAP MD at time 0* (dB) | −1.97 ± 2.07 | −0.02 ± 1.37 | <0.001 |
| SAP PSD at time 0* (dB) | 2.65 ± 1.15 | 1.65 ± 0.35 | <0.001 |
| Number of OCT tests during follow-up, median (1st quartile, 3rd quartile) | 5 (3, 7) | 5 (3, 7) | 0.879 |
| Total follow-up time, years | 6.4 ± 3.3 | 6.6 ± 3.6 | 0.739 |
Time 0 corresponds to the date of conversion to the earliest detectable visual field defect in glaucoma cases and last follow-up date for controls.
Table 2 shows average RNFL thickness measurements at the date of conversion for glaucomatous eyes and at the corresponding time for controls. At conversion, mean ± SD average RNFL thickness was 75.0 ± 9.8µm in glaucomatous eyes and 90.6 ± 8.0µm for controls (P<0.001). Mean values are also shown for different periods before the date of conversion. For example, at t=−4 years, corresponding to 4 years before conversion, mean average RNFL thickness was 81.3 ± 9.9µm and 91.0 ± 8.0µm for glaucoma and controls, respectively (P<0.001). Significant differences between glaucomatous and healthy eyes were still seen even at 8 years before the date of visual field conversion (t=− 8 years) with mean RNFL thicknesses of 86.3 ± 8.2µm and 91.4 ± 7.6µm, respectively (P=0.021).
Table 2.
Mean (± SD) retinal nerve fiber layer thickness measurements (µm) obtained by optical coherence tomography at different times during follow-up.
| Time | N* | Glaucoma | Controls | P |
|---|---|---|---|---|
| 0 | 150 | 75.0 ± 9.8 | 90.6 ± 8.0 | <0.001 |
| −2 years | 136 | 79.7 ± 9.0 | 90.4 ± 7.8 | <0.001 |
| −4 years | 108 | 81.3 ± 9.9 | 91.0 ± 8.0 | <0.001 |
| −6 years | 90 | 83.6 ± 8.0 | 91.2 ± 7.6 | 0.002 |
| −8 years | 52 | 86.3 ± 8.2 | 91.4 ± 7.6 | 0.021 |
Time 0 corresponds to the date of conversion to the earliest detectable visual field defect in glaucoma cases and last follow-up date for controls. Negative time values correspond to years before time 0.
N corresponds to the total sample size (matched cases and controls) available at each time point.
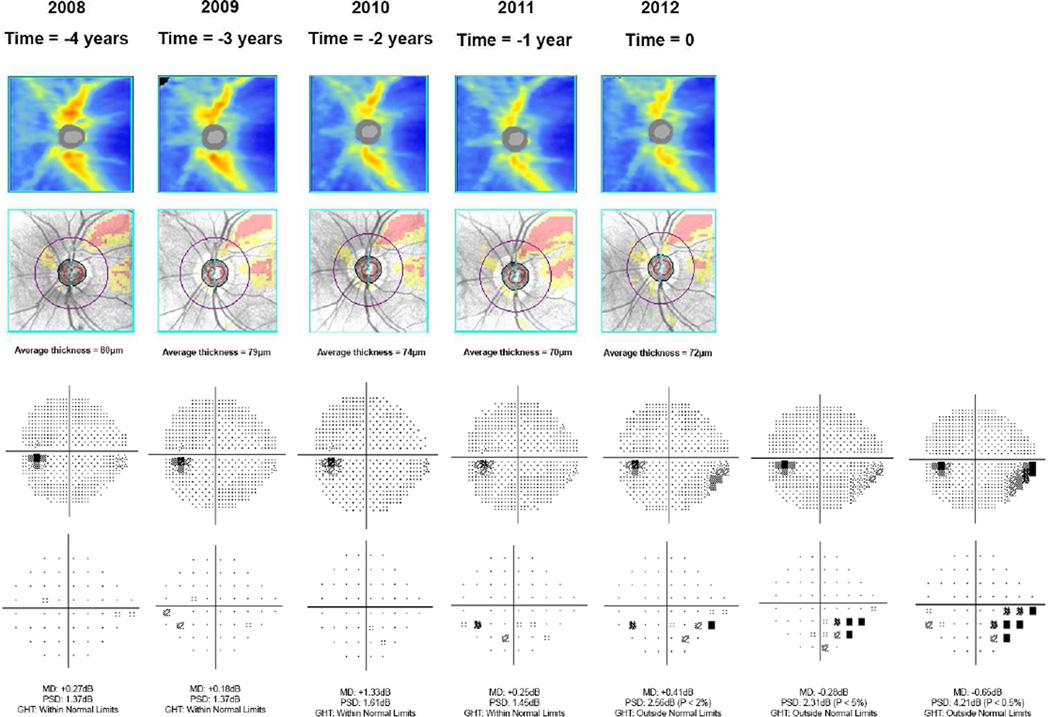
Table 3 shows ROC curve areas for discriminating between glaucomatous and control eyes. Figure 1 shows the corresponding ROC curves. At the date of conversion to the earliest visual field defect, the ROC curve area was 0.87 (95% CI: 0.82 – 0.92). As expected, ROC curve areas decreased with increasingly longer times before detection of the earliest field defect. For example, at 4 years before field losses were detected, the ROC curve area was 0.77 (95% CI: 0.70 – 0.84). Although a significant decrease in ROC curve areas was seen, a statistically significant ROC curve area (i.e., discrimination better than chance) was seen up to 8 years before development of visual field defect (ROC curve area = 0.65; 95% CI: 0.52 – 0.78). Sensitivities for fixed specificities at 95% and 80% are also shown on Table 3. Figure 2 illustrates a case from the study.
Table 3.
Areas under the receiver operating characteristic curves with sensitivities at fixed specificities for discriminating glaucomatous from control eyes at different times during follow-up.*
| Time | ROC curve area | Sensitivity at specificity = 95% |
Sensitivity at specificity = 80% |
|---|---|---|---|
| 0 | 0.87 (0.82 – 0.92) | 53% (40% – 67%) | 77% (68% – 87%) |
| −2 years | 0.82 (0.77 – 0.88) | 44% (31% – 56%) | 69% (60% – 79%) |
| −4 years | 0.77 (0.70 – 0.84) | 35% (21% – 48%) | 61% (49% – 72%) |
| −6 years | 0.71 (0.62 – 0.81) | 26% (12% – 40%) | 51% (36% – 66%) |
| −8 years | 0.65 (0.52 – 0.78) | 19% (5% – 33%) | 42% (23% – 60%) |
Time 0 corresponds to the date of conversion to the earliest detectable visual field defect in glaucoma cases and last follow-up date for controls. Negative time values correspond to years before time 0.
Figure 1.
Receiver operating characteristic curves for discriminating glaucomatous from control eyes. Time 0 corresponds to the date of conversion to the earliest detectable visual field defect in glaucoma cases and last follow-up date for controls. Negative time values correspond to years before time 0.
Figure 2.
Example of a glaucomatous eye included in the study. The figure shows the color-coded retinal nerve fiber layer (RNFL) thickness map and deviation map from spectral domain optical coherence tomography (SDOCT), as well as grayscale and pattern deviation plots from standard automated perimetry. Time 0 corresponds to the date of conversion to the earliest detectable visual field defect, which occurred in 2012 for this eye. The subsequent visual fields confirmed the defect. SDOCT results for this eye were available up to time=−4 years, i.e., 4 years before development of visual field defect.
DISCUSSION
In the present study, we demonstrated that significant loss of the RNFL was detected by OCT several years before the development of visual field loss. By following a cohort of glaucoma suspects over time, we were able to quantify neural losses at different times before development of the earliest signs of visual field damage. Our approach allowed us to evaluate the lead-time that could be gained by applying OCT in diagnosing glaucoma before a visual field defect is detectable by SAP. These results may be important in the assessment of OCT as an ancillary diagnostic tool in glaucoma.
In order to be able to estimate RNFL thickness abnormalities associated with the earliest detectable visual field losses on SAP, we longitudinally followed a cohort of glaucoma suspects over time until they showed evidence of repeatable visual field defects. The criteria used to define visual field losses were those applied by the Ocular Hypertension Treatment Study (OHTS)30, 31 and widely used in clinical practice, requiring confirmation of abnormalities in 3 consecutive visual fields. This greatly decreases the chance that the abnormalities seen on perimetry may represent just variability rather than true defects. At the time of development of the earliest visual field defect, mean average RNFL thickness was 75µm in glaucomatous eyes, compared to 90µm from the age-matched healthy group (P<0.001). Interestingly, this value is almost identical to the threshold for RNFL thickness loss (75.3µm) where visual field defects become detectable as estimated in a previous cross-sectional study.32 Importantly, OCT showed good ability to discriminate glaucomatous from healthy eyes at this point, with a ROC curve area of 0.87. At specificities of 80% and 95%, the sensitivities were 77% and 53%. A sensitivity of 53% for specificity at 95% implies a positive likelihood ratio of 0.53/(1–0.95) = 10.6, which is considered quite large and able to substantially change the probability of disease.12, 33
When used as an ancillary tool to diagnose disease, it is important to evaluate the benefit that OCT assessment can provide in addition to standard diagnostic tools, such as fundoscopy and visual field testing. By including only cases with confirmed visual field defects, most previous studies on diagnostic accuracy of OCT failed to evaluate this issue. For example, in the study of diagnostic accuracy published by Leung and colleagues6, the average MD of the visual field of glaucoma cases was −8.66dB, indicating that most glaucoma patients had at least moderate visual field damage. For comparison, the average MD of our patients at the time of the earliest field defect was −1.97dB. It should be obvious that if a repeatable glaucomatous visual field defect is present, the defect itself is already diagnostic, so there is no need to apply another potentially costly tool for this purpose in clinical practice. Therefore, the design of our study attempted to evaluate the ability of OCT in detecting neural loss before repeatable visual field defects would become clearly apparent. We demonstrated that significant differences between glaucomatous and controls were seen for several years preceding the earliest field defect. However, as expected, the diagnostic ability decreased with increase in the time before field losses.
Our results may have important implications in considering whether the use of OCT as an ancillary test for diagnosis is justifiable. If the lead-time that could be gained by applying OCT is too short, its use in this circumstance may not be justifiable, as one could as easily monitor subjects until repeatable visual field defects would become recognizable, without being penalized by late detection of damage. In our study, we demonstrated that a lead time of up to 8 years could be obtained in some patients by using OCT. However, it is important to note that the proportion of patients showing abnormal results with OCT before development of field loss decreased progressively with increase in the time before the appearance of a field defect. For example, at 95% specificity cut-off, up to 44% of subjects had abnormal average RNFL thickness at 2 years before development of a field defect (Table 3). This number reduced to 35% at 4 years and to 19% at 8 years. Although 19% may be seen as an apparently small proportion, it is important to consider that 8 years is a relatively large amount of time and such lead-time could have significant management implications to a significant group of patients. In fact, our results suggest that OCT could detect damage in approximately 1/3 of glaucoma patients up to 5 years before appearance of the earliest field defects. It is important to emphasize, however, that the benefit of treatment at this stage in preventing future functional disability from glaucoma has not yet been demonstrated. Regardless of this fact, detection of early damage may still have important implications in management decisions, such as establishing frequency of follow-up and patient counseling.
Only 53% of glaucoma patients could be declared to have an abnormal OCT at the time of earliest development of field defects using a 95% specificity cut-off. It is likely that this number could be higher if multiple OCT parameters investigating damage by hemifields or by localized regions had been used. However, use of multiple parameters may also increase false-positives. Subjects in our study were followed carefully over time until development of field defects. It is possible that in clinical practice the frequency of visual field testing would be lower, resulting in longer delays in detecting a confirmed visual field defect. Therefore, the benefit provided by OCT on the percentage of subjects detected before field damage and on lead times could be higher in clinical practice. Despite this fact, our results should not be seen as indicating that OCT should replace visual field as a diagnostic tool for glaucoma, but rather that it can provide useful information as an ancillary diagnostic tool. It is likely that many patients who still present with OCT within normal limits at the time of development of earliest field losses may have had progressive RNFL thickness change over time. This highlights the importance of longitudinal monitoring of glaucoma suspects over time. A recent study by Miki et al34 showed that eyes of suspects who converted to glaucoma had a rate of RNFL thickness change of −2.02 µm/year compared to −0.82 µm/year (P<0.001) for eyes that did not convert to glaucoma. Detection of longitudinal change may improve the ability of OCT in both diagnosing the disease and detecting progression. However, the purpose of our study was not to investigate longitudinal changes over time, but to assess the validity of OCT as an ancillary diagnostic tool when used in a cross-sectional assessment, as commonly performed in clinical practice. Approaches that combine structure and function have also been shown to improve diagnosis both in cross-sectional35, 36 as well as longitudinal investigations37–40 and it would be interesting to assess whether they can increase the lead time for diagnosis before a field defect is apparent.
It is important to emphasize that the analysis of diagnostic accuracy provided in our study should be evaluated in the appropriate context of use of OCT. Our study targeted subjects who would be considered glaucoma suspects and referred for further evaluation at a tertiary center. Our purpose was not to evaluate the accuracy or benefit that could be provided by using OCT as a diagnostic tool in population-based screening. The concept of lead time would also be different in a screening situation. From a public health perspective, early diagnosis would actually mean diagnosis at a stage earlier than would have presented symptomatically. Given that symptomatic presentation of glaucoma is likely to occur only very late in the course of the disease, detecting cases with even moderate damage could still substantially increase the lead time until appearance of symptoms, facilitating appropriate treatment to prevent functional impairment.
Our study had limitations. In order to maximize the amount of OCT data available, we included scans obtained with the older version of the technology, time-domain OCT and a conversion factor to SDOCT was applied. This was necessary due to the relatively recent introduction of SDOCT. Although it could be argued that this approach might introduce bias, the cross-sectional diagnostic accuracy of TDOCT has been reported to be comparable to SDOCT in previous studies.4, 6, 19, 41 In addition, cases and controls were matched by number of OCT tests during follow-up and we did not attempt to estimate longitudinal changes over time, which could be more problematic when mixing tests from different versions of OCT. It should also be noted that our ability to estimate the lead time was limited by the duration of follow- up of the study. It is possible that longer lead times could have been obtained for some subjects with longer follow-up times. Another limitation of our study is that some of the suspects had different treatments, which were introduced at different periods during follow-up at the discretion of the attending ophthalmologist. The treatments may have lengthened the lead time until development of visual field loss. However, our estimates of diagnostic accuracy would still be valid and the lead times would still represent those expected for patients following standard clinical care.
In conclusion, assessment of RNFL thickness with OCT was able to detect glaucomatous damage before the appearance of visual field defects on SAP. In many subjects, significantly large lead times would be gained when applying OCT as an ancillary diagnostic tool. Future research should evaluate the implications of OCT-assisted decision-making in preventing functional impairment and disability in glaucoma.
Acknowledgments
Supported in part by National Institutes of Health/National Eye Institute grants EY021818 (F.A.M.), core grant P30EY022589, EY11008 (L.M.Z.), EY14267 (L.M.Z.), EY019869 (L.M.Z.),; an unrestricted grant from Research to Prevent Blindness (New York, NY); grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck and Santen; Natural Science Foundation of Heilongjiang Province for Returned Scholars, China. No.LC2012C21 (C.Z.); Innovation research special fund of the Science and Technology of Harbin of Heilong Jiang Province, China. No.2011RFLYS029 (C.Z.). Research fund of the First Affiliated Hospital of Harbin Medical University, China No.2007021(C.Z.). Scientific and technical research fund of Education Bureau of Heilongjiang Province, China. No.12511311 (C.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author(s) have made the following disclosure(s):
T.K – none; C.Z. – none; L.M.Z – F: Carl Zeiss Meditec Inc., Heidelberg Engineering GmbH, Optovue Inc., Topcon Medical Systems Inc. Nidek Inc, Quark. P; Carl Zeiss Meditec Inc; R.N.W. – F: Aerie, Carl Zeiss Meditec, Genentech, Heidelberg Engineering GmbH, Nidek, Novartis, Optovue, Topcon; C: Alcon, Allergan, Amatek, Aquesys, Bausch&Lomb, Carl Zeiss Meditec, Topcon, Valeant; F.A.M. – F: Alcon Laboratories, Bausch & Lomb, Carl Zeiss Meditec, Heidelberg Engineering, Merck, Allergan, Sensimed, Topcon, Reichert, National Eye Institute, R: Alcon Laboratories, Allergan, Carl Zeiss Meditec, Reichert, C: Allergan, Carl-Zeiss Meditec, Novartis.
REFERENCES
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 3.Medeiros FA, Alencar LM, Zangwill LM, et al. Prediction of functional loss in glaucoma from progressive optic disc damage. Arch Ophthalmol. 2009;127:1250–1256. doi: 10.1001/archophthalmol.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehi M, Grewal DS, Sheets CW, Greenfield DS. Diagnostic ability of Fourier-domain vs time-domain optical coherence tomography for glaucoma detection. Am J Ophthalmol. 2009;148:597–605. doi: 10.1016/j.ajo.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SB, Sung KR, Kang SY, et al. Comparison of glaucoma diagnostic Capabilities of Cirrus HD and Stratus optical coherence tomography. Arch Ophthalmol. 2009;127:1603–1609. doi: 10.1001/archophthalmol.2009.296. [DOI] [PubMed] [Google Scholar]
- 6.Leung CK, Cheung CY, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009;116:1257–1263. 63 e1–63 e2. doi: 10.1016/j.ophtha.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Kim JS, Ishikawa H, Gabriele ML, et al. Retinal nerve fiber layer thickness measurement comparability between time domain optical coherence tomography (OCT) and spectral domain OCT. Invest Ophthalmol Vis Sci. 2010;51:896–902. doi: 10.1167/iovs.09-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao HL, Zangwill LM, Weinreb RN, et al. Comparison of different spectral domain optical coherence tomography scanning areas for glaucoma diagnosis. Ophthalmology. 2010;117:1692–1699. 9 e1. doi: 10.1016/j.ophtha.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Leite MT, Rao HL, Zangwill LM, et al. Comparison of the diagnostic accuracies of the Spectralis, Cirrus, and RTVue optical coherence tomography devices in glaucoma. Ophthalmology. 2011;118:1334–1339. doi: 10.1016/j.ophtha.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung CK, Lam S, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: analysis of the retinal nerve fiber layer map for glaucoma detection. Ophthalmology. 2010;117:1684–1691. doi: 10.1016/j.ophtha.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Li S, Fu J, et al. Comparative study of retinal nerve fibre layer measurement by RTVue OCT and GDx VCC. Br J Ophthalmol. 2011;95:509–513. doi: 10.1136/bjo.2009.163493. [DOI] [PubMed] [Google Scholar]
- 12.Medeiros FA, Zangwill LM, Bowd C, Weinreb RN. Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and stratus OCT optical coherence tomograph for the detection of glaucoma. Arch Ophthalmol. 2004;122:827–837. doi: 10.1001/archopht.122.6.827. [DOI] [PubMed] [Google Scholar]
- 13.Alencar LM, Zangwill LM, Weinreb RN, et al. Agreement for detecting glaucoma progression with the GDx guided progression analysis, automated perimetry, and optic disc photography. Ophthalmology. 2010;117:462–470. doi: 10.1016/j.ophtha.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann EM, Bowd C, Medeiros FA, et al. Agreement among 3 optical imaging methods for the assessment of optic disc topography. Ophthalmology. 2005;112:2149–2156. doi: 10.1016/j.ophtha.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Vizzeri G, Balasubramanian M, Bowd C, et al. Spectral domain-optical coherence tomography to detect localized retinal nerve fiber layer defects in glaucomatous eyes. Opt Express. 2009;17:4004–4018. doi: 10.1364/oe.17.004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sull AC, Vuong LN, Price LL, et al. Comparison of spectral/Fourier domain optical coherence tomography instruments for assessment of normal macular thickness. Retina. 2010;30:235–245. doi: 10.1097/IAE.0b013e3181bd2c3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Racette L, Liebmann JM, Girkin CA, et al. African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol. 2010;128:551–559. doi: 10.1001/archophthalmol.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leite MT, Zangwill LM, Weinreb RN, et al. Effect of disease severity on the performance of Cirrus spectral-domain OCT for glaucoma diagnosis. Invest Ophthalmol Vis Sci. 2010;51:4104–4109. doi: 10.1167/iovs.09-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takagishi M, Hirooka K, Baba T, et al. Comparison of retinal nerve fiber layer thickness measurements using time domain and spectral domain optical coherence tomography, and visual field sensitivity. J Glaucoma. 2011;20:383–387. doi: 10.1097/IJG.0b013e3181efb371. [DOI] [PubMed] [Google Scholar]
- 20.Medeiros FA, Zangwill LM, Alencar LM, et al. Detection of glaucoma progression with stratus OCT retinal nerve fiber layer, optic nerve head, and macular thickness measurements. Invest Ophthalmol Vis Sci. 2009;50:5741–5748. doi: 10.1167/iovs.09-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung CK, Chiu V, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a comparison between spectral-domain and time-domain optical coherence tomography. Ophthalmology. 2011;118:1558–1562. doi: 10.1016/j.ophtha.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123:464–470. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payne RB. Method comparison: evaluation of least squares, Deming and Passing/Bablok regression procedures using computer simulation. Ann Clin Biochem. 1997;34(Pt 3):319–320. doi: 10.1177/000456329703400317. [DOI] [PubMed] [Google Scholar]
- 24.Medeiros FA, Sample PA, Zangwill LM, et al. A statistical approach to the evaluation of covariate effects on the receiver operating characteristic curves of diagnostic tests in glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2520–2527. doi: 10.1167/iovs.05-1441. [DOI] [PubMed] [Google Scholar]
- 25.Girkin CA, Liebmann J, Fingeret M, et al. The effects of race, optic disc area, age, and disease severity on the diagnostic performance of spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:6148–6153. doi: 10.1167/iovs.10-6698. [DOI] [PubMed] [Google Scholar]
- 26.Rao HL, Leite MT, Weinreb RN, et al. Effect of disease severity and optic disc size on diagnostic accuracy of RTVue spectral domain optical coherence tomograph in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:1290–1296. doi: 10.1167/iovs.10-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonzo TA, Pepe MS. Distribution-free ROC analysis using binary regression techniques. Biostatistics. 2002;3:421–432. doi: 10.1093/biostatistics/3.3.421. [DOI] [PubMed] [Google Scholar]
- 28.Pepe MS. An interpretation for the ROC curve and inference using GLM procedures. Biometrics. 2000;56:352–359. doi: 10.1111/j.0006-341x.2000.00352.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X-H, Obuchowski NA, McClish DK. Analysis of Correlated ROC Data. In: Zhou X-H, Obuchowski NA, McClish DK, editors. Statistical Methods in Diagnostic Medicine. New York: John Wiley & Sons, Inc.; 2002. [Google Scholar]
- 30.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829-30. [DOI] [PubMed] [Google Scholar]
- 31.Keltner JL, Johnson CA, Quigg JM, et al. Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Ocular Hypertension Treatment Study Group. Arch Ophthalmol. 2000;118:1187–1194. doi: 10.1001/archopht.118.9.1187. [DOI] [PubMed] [Google Scholar]
- 32.Wollstein G, Kagemann L, Bilonick RA, et al. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol. 2012;96:47–52. doi: 10.1136/bjo.2010.196907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lisboa R, Mansouri K, Zangwill LM, et al. Likelihood ratios for glaucoma diagnosis using spectral-domain optical coherence tomography. Am J Ophthalmol. 2013;156:918–926. e2. doi: 10.1016/j.ajo.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miki A, Medeiros FA, Weinreb RN, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121:1350–1358. doi: 10.1016/j.ophtha.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medeiros FA, Lisboa R, Weinreb RN, et al. A combined index of structure and function for staging glaucomatous damage. Arch Ophthalmol. 2012;130:1107–1116. doi: 10.1001/archophthalmol.2012.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medeiros FA, Lisboa R, Weinreb RN, et al. Retinal ganglion cell count estimates associated with early development of visual field defects in glaucoma. Ophthalmology. 2013;120:736–744. doi: 10.1016/j.ophtha.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medeiros FA, Leite MT, Zangwill LM, Weinreb RN. Combining structural and functional measurements to improve detection of glaucoma progression using Bayesian hierarchical models. Invest Ophthalmol Vis Sci. 2011;52:5794–5803. doi: 10.1167/iovs.10-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medeiros FA, Zangwill LM, Anderson DR, et al. Estimating the rate of retinal ganglion cell loss in glaucoma. Am J Ophthalmol. 2012;154:814–824. e1. doi: 10.1016/j.ajo.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medeiros FA, Zangwill LM, Girkin CA, et al. Combining structural and functional measurements to improve estimates of rates of glaucomatous progression. Am J Ophthalmol. 2012;153:1197–1205. e1. doi: 10.1016/j.ajo.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell RA, Malik R, Chauhan BC, et al. Improved estimates of visual field progression using bayesian linear regression to integrate structural information in patients with ocular hypertension. Invest Ophthalmol Vis Sci. 2012;53:2760–2769. doi: 10.1167/iovs.11-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim KE, Kim SH, Jeoung JW, et al. Comparison of ability of time-domain and spectral-domain optical coherence tomography to detect diffuse retinal nerve fiber layer atrophy. Jpn J Ophthalmol. 2013;57:529–539. doi: 10.1007/s10384-013-0270-8. [DOI] [PubMed] [Google Scholar]