Abstract
Background
The prevalence of marijuana (MJ) use among youth and its legalization for medical or recreational use has intensified public health endeavors of understanding MJ effects on brain structure and function. Studies indicate that MJ use is related to impaired cognitive performance, and altered functional brain activation and chemistry in adolescents and adults, but MJ effects on brain morphology in emerging adults are less understood.
Methods
15 MJ users (age 21.8±3.6, 2 females) and 15 non-using (NU) participants (age 22.3±3.5, 2 females) were included, demographically matched on age, education and alcohol use. High-resolution structural MR images were acquired at 3 Tesla. Cortical thickness (CT) and volumetric analyses were performed using Freesurfer. A priori regions of interest (ROI) included orbitofrontal and cingulate cortices, amygdala, hippocampus and thalamus.
Results
Whole brain CT analysis did not find significant group differences in a priori ROIs but revealed MJ users had significantly less CT (i.e., thinness) in right fusiform gyrus (rFG) compared to NU (p<0.05). Thalamic volume was significantly smaller in MJ users compared to NU (right, p=0.05; left, p=0.01) and associated with greater non-planning (p<0.01) and overall impulsivity (p=0.04). There were no other group volume differences.
Conclusions
RFG cortical thinness and smaller thalamic volume in emerging adults is associated with MJ abuse. Furthermore, smaller thalamic volume associated with greater impulsivity contributes to growing evidence that the thalamus is neurobiologically perturbed by MJ use. Collectively, altered thalamic and rFG structural integrity may interfere with their known roles in regulating visuoperceptual and object information processing.
Keywords: cortical thickness, volume, marijuana, young adults, thalamus, fusiform gyrus
1. INTRODUCTION
In 2013, 180.6 million or 3.9 percent of the world population aged 15–64 used marijuana (United Nations Office on Drug and Crime, 2013). In the US, the rate of past month marijuana use among adolescents aged 12–17 years increased from 6.7 to 7.9 percent between 2007 and 2011 (SAMHSA, 2013). It is expected that the prevalence of marijuana use will continue to escalate at a rapid rate in the US across all age groups, given the recent legalization of medical and recreational use, and a decrease in public perception of harm associated with MJ use (Palamar et al., 2014; Schuermeyer et al., 2014). Accordingly, 54.6% of adolescents aged 12 to 17 perceived smoking marijuana once or twice a week as a “great risk” in 2007, which has decreased almost 10%, to 44.8% in 2011 (SAMHSA, 2013). Therefore, a better understanding of the long-term effects of marijuana on the brain, particularly the developing brain, as young adolescent users transition into emerging adulthood (ages 18–24) is an increasingly important public health endeavor. To this end, previous studies show that when marijuana use is initiated before age 17, the negative impact of chronic marijuana use on cognitive function and brain morphology can last several years and may even be permanent (Gruber et al., 2011; Jacobus et al., 2009, 2014; Meier et al., 2012; Schweinsburg et al., 2008b; Wilson et al., 2000). As such, investigating the effects of marijuana exposure initiated during adolescence and continuing during emerging adulthood on vulnerable prefrontal and subcortical regions will offer unique insight into structural consequences of short-term persistent marijuana use in emerging adults.
The major psychoactive component in marijuana (MJ) is delta-9-tetrahydrocannabinol (THC). The main cannabinoid receptor in the brain is the CB1 receptor, which is a G-coupled protein that is widely distributed throughout the central nervous system (CNS), with greatest densities in the associational areas of frontal and limbic lobes, cerebellar cortex, thalamus, pallidum, amygdala, hippocampus and substantia nigra pars reticulata (Glass et al., 1997). Marijuana use can broadly affect cognitive processes, and prior research demonstrates MJ-related alterations in executive functioning, attention, memory, learning, decision-making, and processing speed (Becker et al., 2014; Lisdahl et al., 2014; Meier et al., 2012; Swift et al., 2008). Moreover, MJ use has been associated with mental health issues, including co-morbid mood symptoms, especially depression and anxiety (Weinstein et al., 2013), lower age of onset of psychosis, mania, increased risk of suicide attempts and a more severe course of illness (Kvitland et al., 2014). The effects of MJ on mood and other psychiatric symptoms has been linked to the ability of the endocannabinoid system to modulate the activity of other neurotransmitter systems, energy metabolism and immune functions (Leweke and Koethe, 2008).
Findings from neuroimaging studies of MJ users, often focused on either adolescent users or adult users, document significant associations between marijuana use and alterations in neurobiology, including brain structure, function and neurochemistry (for review, see Batalla et al., 2013; Martin-Santos et al., 2010; Sneider et al., 2014). Findings of alterations in brain structure reported using magnetic resonance imaging (MRI) are somewhat heterogenous, however, and the significance of the changes identified using this technology remains controversial because of conflicting findings among existing studies. For instance, while some studies report alterations in whole brain and regional volumes, and in cortical thickness and subcortical volumes (Ashtari et al., 2011; Cousijn et al., 2012; Matochik et al., 2005; Yucel et al., 2008), other investigations fail to report significant differences in brain structure between adult MJ users and comparison subjects (Block et al., 2000; Jager et al., 2007; Tzilos et al., 2005). Importantly, there is mounting evidence that MJ use, particularly exposure to THC, may be more deleterious during adolescence, a time when cognitive development and brain maturation are rapidly ongoing (Lisdahl et al., 2013). For instance, adolescent MJ users exhibit altered frontal region and insula cortical thickness, suggestive of aberrant gray matter (GM) development or maturation that could persist beyond adolescence (Lopez-Larson et al., 2011), and also exhibit alterations in prefrontal cortex, amygdala and cerebellum, some of which are sex-specific (McQueeny et al., 2011; Medina et al., 2009, 2010). It also has been reported that smaller orbitofrontal cortex volumes observed at age 12 years predict initiation of MJ use by age 16 years, whereas volumes of other regions such as amygdala, hippocampus, and anterior cingulate cortex were not predictive of later MJ use (Cheetham et al., 2012). Furthermore, in a longitudinal study of adolescent MJ users, greater lifetime exposure to MJ predicted greater cortical thickness in the left and right superior frontal gyri, left pars opercularis, right pars triangularis, right supramarginal, and left inferior parietal cortex after adjusting for baseline cortical thickness, suggesting that heavy MJ use during adolescence alters the trajectory of cortical GM development (Epstein and Kumra, 2015).
Collectively, previous investigations have focused on identifying regions that exhibit structural alterations related to the effects of MJ use measured during adolescence or during adulthood, but there are few investigations specifically examining structural alterations in emerging adults who initiated MJ use in adolescence. Thus, the present study aimed to characterize potential neurobiological consequences of MJ use on cortical thickness and subcortical volumetric differences in emerging adult MJ users compared with age-matched non-using subjects. A priori regions of interest (ROIs) included orbito-frontal, dorsolateral prefrontal, and anterior cingulate cortices (OFC, DLPFC, and ACC respectively), and superior and middle frontal gyri, as well as subcortical amygdala, thalamus, and hippocampus regions.
Frontal ROIs were chosen based on previous reports of MJ-related functional alterations in each region during cognitive task performance. Specifically, altered OFC and DLPFC activity is associated with impaired decision-making and poor adaptation to negative consequences (Bolla et al., 2005), and ACC hypoactivity is associated with error awareness and performance monitoring (Hester et al., 2009) and memory retrieval during spatial navigation (Sneider et al., 2013). Increased activity has been observed in superior and middle frontal gyri in MJ users during inhibitory processing, suggesting inefficient functional responses and potential overcompensation by neighboring tissue to adequately perform the task (Tapert et al., 2007). Given the sub-optimal functional response of these a priori regions in MJ users during cognitive task performance, it is possible that surface-based cortical thickness analyses will reveal corresponding structural changes, with lower cortical thickness related to MJ use in these regions.
Hippocampus, amygdala and thalamus, regions demonstrating high CB1 receptor distribution (Herkenham et al., 1991), also were selected for volumetric analysis based on evidence that MJ users exhibit functional and neurochemical alterations in these regions related to marijuana use (Ashtari et al., 2011; Bolla et al., 2005; Cousijn et al., 2012; Demirakca et al., 2011; Gilman et al., 2014; Glass et al., 1997; Hester et al., 2009; Mashhoon et al., 2013; Matochik et al., 2005; Schacht et al., 2012; Sneider et al., 2013; Sneider et al., 2014; Yucel et al., 2008). Clinical measures of mood and impulsivity were examined relative to cortical thickness and brain volume to further probe potential links with neurobiological consequences of marijuana use.
2. MATERIAL AND METHODS
2.1. Participants, demographics and procedure
Fifteen marijuana users (MJ; 2 females, age 21.8 ± 3.6) and fifteen non-using controls (NU; 2 females, age 22.3 ± 3.5) were included in this study. Participants were selected from a larger pool of subjects that underwent structural MRI scanning at McLean Hospital as part of two larger functional magnetic resonance (MR) imaging and MR spectroscopy studies (Silveri et al., 2011; Sneider et al., 2013). All participants were matched on age, education and alcohol use, and underwent a Structured Clinical Interview for DSM-IV Non-Patient Edition (SCID-I/NP), which is widely used to reliably determine Axis I disorders in general research populations (First et al., 2002). All subjects were screened for other substance use prior to study enrollment. Subjects were excluded if they had: (1) A history of having consumed more than 10 alcoholic drinks per week for a period of two months or more at any time in their lives or more than 3 drinks in a 24 hour period more than once a week; (2) A history of having used any other psychoactive substance more than 6 times in the last six months and more than 10 times in their lifetime; (3) A positive drug urine screen on the day of scanning, except for THC positive test in MJ users. Other exclusion criteria included history of head injury, loss of consciousness, seizure disorder, any Axis I diagnosis except for the MJ abuse, and any contraindication for MR scanning.
Marijuana users were included if they met the minimum inclusion criteria: smoked marijuana a minimum of 1450 times as indicated by self report (at least 500 times in the past two years), used marijuana at least 5 times in the last seven days prior to the study visit, tested positive for urinary cannabinoids on the day of scanning and met DSM-IV criteria for marijuana abuse or dependence (Silveri et al., 2011; Sneider et al., 2013). NU participants reported fewer than 5 lifetime episodes of marijuana use. Nicotine use was assessed in all subjects. None of the control subjects were nicotine smokers. The nicotine use was minimal in the marijuana users, with only three out of fifteen marijuana users reporting nicotine use. Their use was reported as one pack per day, one pack per month and occasional use respectively.
McLean Hospital Institutional Review Board approved all study procedures. All participants gave informed consent prior to beginning the study and received monetary compensation for participating. All participants completed questionnaires about alcohol use and received a urine drug screen prior to the scanning session.
2.2. Clinical variables
Participants were assessed on a battery of clinical instruments prior to imaging. The Profile of Mood States (POMS; McNair et al., 1971) was used to identify both transient and enduring mood states and feelings across six mood states that include tension–anxiety, depression–dejection, anger–hostility, vigor–activity, fatigue–inertia, and confusion–bewilderment. The Barratt Impulsiveness Scale (BIS-11, Patton et al., 1995) was used to measure of impulsivity, including a total score for trait impulsivity, and subscale scores for cognitive (rapid shifts in attention/impatience with complexity), motor (impetuous action), and non-planning (lack of future orientation) impulsivity. The Positive Affect Negative Affect Schedule (Watson et al., 1988) was used to distinguish positive and negative affect.
2.3. Structural MR imaging
High-resolution anatomical images were obtained on a 3.0 Tesla Siemens Trio (Siemens Medical Solutions USA Inc., Malvern, PA, USA) whole body MRI scanner at the McLean Hospital Imaging Center. A standard quadrature head coil was utilized to acquire magnetization-prepared, rapid acquisition with gradient echoes (MPRAGE) T1-weighted images for 3D reconstruction. The parameters utilized for imaging were as follows: 128 sagittal slices; 1.0 × 1.0 × 1.3mm3 spatial resolution, 256×256 matrix, echo time (TE) = 2.7 ms; repetition time (TR) = 2100 ms; inversion time (TI) = 1100 ms; flip angle = 12°. Imaging parameters were chosen to optimize signal contrast between white matter (WM) and gray matter (GM) as well as between GM and cerebro-spinal fluid (CSF), to facilitate subsequent cortical surface segmentation and reconstruction processes.
2.4. Cortical thickness processing
The Freesurfer morphometric analysis suite (v5.3.0; freesurfer.net) was utilized for all cortical surface reconstruction, cortical thickness, and volumetric estimate processing. The technical details of these methods have been described previously (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2002; Segonne et al., 2004), and have been utilized in previous cortical thickness studies by our group (Mashhoon et al., 2014). Briefly, the Freesurfer standard pipeline automates procedures that incorporate removal of non-brain tissue and skull (Segonne et al., 2004), linear Talairach atlas registration, voxel intensity classification and normalization, and cortical surface segmentation (Fischl et al., 2002). Freesurfer processing produced cortical models with tesselated GM and WM boundaries that were then registered to a spherical atlas that applies individual cortical folding patterns to closely align cortical geometry across all participants and enable accurate morphological matching of cortical vertices and regions along the reconstructed surface (Dale et al., 1999). During an automated process, each hemisphere was then parcellated into 74 distinct cortical regions (Desikan et al., 2006; Fischl et al., 2004). Automated algorithms calculate cortical thickness by mapping spatial intensity gradients across different tissue types and measuring the closest distance between the GM/WM boundary to the GM/CSF boundary at each vertex on the tessellated cortical surface (Fischl and Dale, 2000). Accordingly, the surface maps do not rely on absolute signal intensity and are capable of distinguishing submillimeter microarchitectural differences between groups (Fischl and Dale, 2000). Furthermore, automated cortical thickness procedures have been tested and validated against histological analysis (Rosas et al., 2002) and manual measurements (Salat et al., 2004). To ensure quality control, each data set was manually checked and necessary edits were made to ensure accurate Talairach transform, skull stripping, pial surface boundary placement, tissue intensity normalization, white matter segmentation and to check for topological defects.
2.5. Volumetric measurements and analysis
Following all automated processing, parcellation, and completed quality control procedures, Freesurfer was again utilized to extract absolute segmented volumes of subcortical regions that included the amygdala, thalamus, hippocampus, pallidum, caudate, putamen, and cerebellum, in addition to estimated intracranial volume (ICV), and total gray matter and total white matter volumes (Fischl et al., 2002). In order to control for individual variability in brain volume (Giedd et al., 1996), subcortical volumes were analyzed as ratios to ICV. All subcortical and total estimated volumes were imported from Freesurfer to SPSS for analysis.
2.6. Statistical analyses
Whole-brain surface maps were statistically assessed using a two-group unpaired t-test, controlling for age and sex, implemented in Freesurfer to investigate differences in cortical thickness between MJ and NU. Resulting p-value maps were thresholded at p=0.01 and were smoothed using a Gaussian kernel with a full-width half-maximum level of 10, to identify contiguous clusters of significant cortical thickness differences between groups in each hemisphere. In order to account and correct for multiple comparisons corrections, a Monte Carlo simulation cluster analysis, which is part of the Freesurfer processing stream, was performed with 10,000 iterations to identify significant differences with a cluster-corrected threshold of p = 0.05.
SPSS 19.0 (SPSS, Chicago, IL, USA) was also used for statistical analyses. A p<0.05 was used as the statistical significance threshold. Demographic, substance use and clinical measures were analyzed using ANCOVAs to compare age- and sex-matched MJ and NU groups. Analyses of subcortical volume differences were performed using repeated measures ANCOVAs with hemisphere (right vs. left) as the within-subjects factor and group (MJ vs. NU) as the between-subjects factor. Effect size f (ES) was calculated for significant main effects and interactions using G*power (Version 3.1.9.2; Faul, Erdfelder, Lang, and Buchner, Dusseldorf, Germany). Correlations of significant ROI cortical thickness measures and subcortical volume measures with clinical measures and self-reported MJ use were assessed using Pearson s r correlation coefficients. Bootstrap confidence intervals (BSCI) at 90% were calculated for significant correlations.
3. RESULTS
3.1. Demographics and substance use
There were no significant differences between MJ and NU groups in age, gender distribution, education or amount of alcohol consumed weekly (Table 1). All MJ use variables are also reported in Table 1.
Table 1.
Demographic and Substance Use Measures
| Measures | NU (n=15) | MJ (n=15) | p |
|---|---|---|---|
| % Male | 87% | 87% | ns |
| Age | 22.3 ± 3.5 | 21.8 ± 3.6 | ns |
| Education | 15.2 ± 2.1 | 14.0 ± 1.6 | ns |
| Number of alcohol drinks/week | 2.8 ± 3.8 | 4.9 ± 3.9 | ns |
| Age at MJ use onset | - | 16.1 ± 1.9 | |
| Duration of MJ use (years) | - | 5.2 ± 2.5 | |
| Lifetime MJ smokes | - | 3808 ± 1812 | |
| THC-COOH (ng/ml): Creatinine (mg/dL) | - | 249.5 ± 99.7 | |
| BIS motor | 19.7 ± 5.2 | 23.4 ± 4.8 | .05 |
| BIS non-planning | 23.0 ± 4.8 | 28.1 ± 4.4 | .01 |
| BIS cognitive | 15.3 ± 3.8 | 17.5 ± 4.3 | ns |
| BIS total | 58.0 ± 10.4 | 69.0 ± 11.2 | 0.01 |
| POMS Vigor | 19.6 ± 6.0 | 17.3 ± 5.4 | ns |
| POMS Anger | 3.3 ± 3.7 | 4.3 ± 6.5 | ns |
| POMS Confusion | 5.9 ± 2.9 | 7.5 ± 3.4 | ns |
| POMS Tension | 6.1 ± 4.0 | 5.4 ± 3.2 | ns |
| POMS Fatigue | 5.1 ± 5.7 | 4.3 ± 4.5 | ns |
| POMS Depression | 3.2 ± 4.5 | 6.2 ± 10.6 | ns |
| PANAS Positive | 34.2 ± 7.7 | 32.1 ± 9.4 | ns |
| PANAS Negative | 11.4 ± 2.4 | 12.6 ± 3.5 | ns |
| PANAS Total | 45.6 ± 8.2 | 44.7 ± 9.3 | ns |
Values represent mean ± standard deviation.
3.2. Clinical data
There were no significant differences between MJ and NU groups on total or subscale scores on the POMS or PANAS (Table 1). MJ users did differ significantly from the NU group on multiple BIS measures (Table 1). MJ exhibited higher scores on the BIS motor (F(1,29) = 4.21, p=0.05, ES=.39) and non-planning (F(1,29) = 9.38, p=0.005, ES=.59) impulsivity subscales, and higher BIS total impulsivity scores (F(1,29) = 7.79, p=0.009, ES=.53) compared to NU. Groups did not differ on the BIS cognitive/attention impulsivity subscale.
3.3. Structural MRI data
3.3.1. Cortical thickness
Whole brain voxelwise analyses revealed a number of clusters in each hemisphere that exhibited differences in cortical thickness between MJ and NC at an uncorrected threshold of p<0.01, including a priori OFC (p<0.008) and middle frontal gyrus (p<0.005) regions. There were no differences at this level of significance observed, however, in a priori regions in the superior frontal gyrus, ACC, and DLPFC regions.
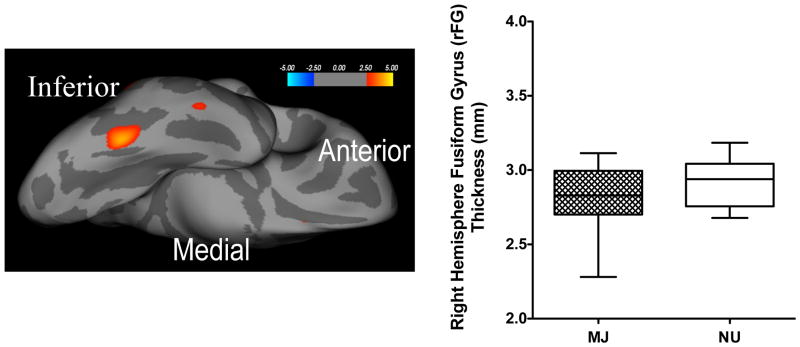
The cluster-wise correction for multiple comparisons analysis revealed a single cluster in the right hemisphere fusiform gyrus (rFG) as the only surviving cluster to show significant group differences at a corrected significance of p<0.05) (Figure 1).
Figure 1.
Significant differences in right hemisphere fusiform gyrus (rFG) cortical thickness between marijuana (MJ) users and non-users (NU) are shown. Left. Representation of statistically significant cluster of fusiform gyrus cortical thinness in right hemisphere (inflated medial view) in MJ users compared to (NU). Right. Group differences in rFG cortical thickness. All values are the means ± SD.
3.3.2. Volumetric analysis
There were no differences in ICV (p=0.54) between MJ users and NU participants, thus subcortical volumes were analyzed as ratios to ICV. Repeated measures ANCOVA showed a main effect of group on thalamic volume, (F(1,28)=6.91, p=0.014, ES=.50) and post-hoc analysis revealed that thalamic volume in both hemispheres was significantly smaller in MJ users compared to NU participants (right, F(1,29)= 4.1, p=0.053, ES=.38; left, F(1,29)=8.07, p=.008, ES=.54). Given that thalamic volume was significantly smaller in MJ users across both hemispheres, the data were collapsed into total thalamic volume (F(1,29)=6.91, p=0.014, ES=.50) (Figure 2). Other a priori subcortical region volume measures, including the amygdala and hippocampus, were not significantly different between groups.
Figure 2.
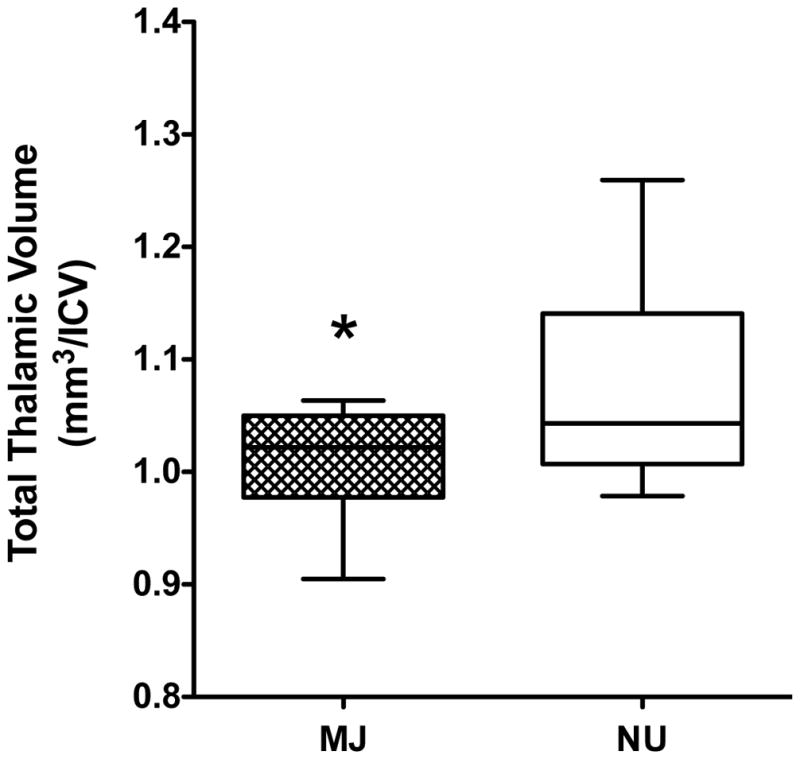
Significant group differences in total thalamic volume between marijuana (MJ) users and non-users (NU) are shown. Volume is shown as a ratio to intracranial volume (ICV). All values are the means ± SD. *p≤0.01
3.4. Correlations: Cortical Thickness, Volume, Clinical Measures and Drug Use
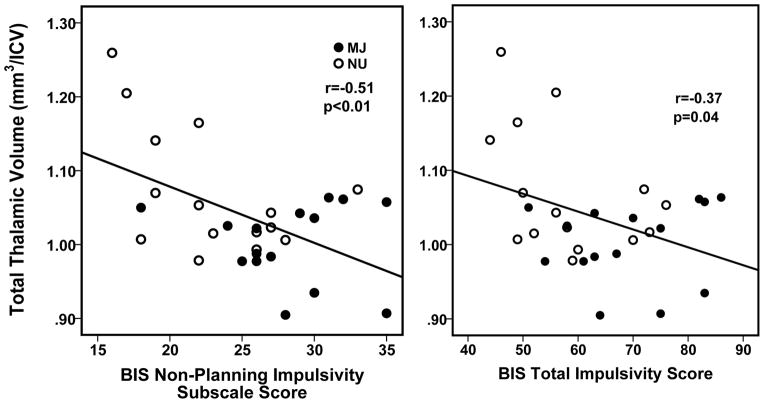
Right FG thickness was not correlated with any MJ use variables, clinical measures, or with reported current alcohol use in either MJ or NU groups. Total thalamic volume was negatively correlated with the BIS non-planning subscale (r = −0.510, p<0.004, BSCI: r = −0.706 to −0.241) and BIS total impulsivity score (r = −0.371, p=0.043, BSCI: r = −0.608 to −0.073) (Figure 3) but was not correlated with any other MJ use variables (such as age of onset or frequency of use), any clinical measures, or with current reported alcohol use in either MJ or NU groups. There were no significant correlations between current alcohol use (number of alcohol drinks consumed per week) and cortical thickness or thalamic volume.
Figure 3.
Scatterplots representing individual marijuana (MJ) users and non-users (NU) data and showing significant relationships between smaller total thalamic volume and Barratt Impulsiveness Scale (BIS) non-planning subscale and total impulsivity scores. Volume is shown as a ratio to intracranial volume (ICV).
4. DISCUSSION
Emerging adult MJ users exhibited cortical thickness and volumetric differences relative to healthy emerging adult NU. Findings revealed less rFG cortical thickness (i.e., rFG cortical thinness), and smaller thalamic volumes in MJ users. Unlike cortical thinning, which is a continuous measure collected with longitudinal data, cortical thinness is a descriptive term that has previously been operationally defined (Mashhoon et al., 2014) as indicating less or lower cortical thickness measured between populations at a cross-sectional time point. Other regional a priori cortical thickness analyses in orbitofrontal, cingulate and cerebellar cortices, and superior and middle frontal gyri did not result in any significant group differences, although there were some trends towards cortical thickness differences in OFC and middle frontal gyri between groups. Additional volumetric analyses also did not result in significant differences in other subcortical regions, including hippocampus or amygdala, between MJ and NU groups. Smaller total thalamic volume was significantly associated with higher BIS non-planning and overall impulsivity scores in MJ and NU participants. MJ users also had higher BIS motor scores, compared to NU, but these measures were not associated with smaller thalamic volume or rFG cortical thinness. Other clinical measures reflecting mood state did not correlate significantly with cortical or subcortical differences.
The current finding of rFG cortical thinness and smaller thalamic volume contributes toward a growing literature elucidating persistent measurable differences in brain alterations associated with heavy MJ use in MJ users relative to non-using individuals. Such findings are observed across the age span, to include adolescents, emerging adults and adults. Indeed, the period of emerging adulthood occurs during the final stage of neuromaturation (Bennett and Baird, 2006), indicating an extended vulnerability of the brain to psychoactive substances that lasts into the early 20s. White matter myelination, synaptogenesis, gray matter synaptic pruning, dendritic growth and proliferation, and axonal growth are all neurodevelopmental processes that guide target-specific synaptic connectivity and neuronal communication patterns (Berghuis et al., 2007; Rakic, 2006; Wang et al., 2003; Wonders and Anderson, 2006). While primarily initiated during embryonic and early postnatal development, these neuromaturational transformations continue throughout adolescence and into early emerging adulthood (Armstrong et al., 1995; Spear, 2013), which prolongs their structural vulnerability to MJ use and repeated cannabinoid CB1 receptor activation.
CB1 receptor activation has been previously associated with morphological and functional changes in glia and neurons and, consequently, neuronal signaling (for review, Cachope, 2012; Stella, 2010). As an inhibitory G-protein-coupled receptor, CB1 receptors bind to and suppress excitatory actions of lipids, neurotransmitters, and molecules such as adenylyl cyclase, which is an important enzymatic component of the cyclic adenosine monophosphate (cAMP) signaling pathway (Haj-Dahmane and Shen, 2010; Kano et al., 2009; Schlicker and Kathmann, 2001). The cAMP pathway plays a prominent role in regulating different types of associative learning, long-term memory, and memory consolidation (Baldwin et al., 2002; Ma et al., 2009) in addition to modulating synaptic morphology and thus the strength of connectivity between neurons (Kandel, 2012). CB1 receptor inhibition of adenylyl cyclase, and in turn cAMP signaling, has been previously shown to interfere with the formation and recruitment of new functional synapses, suggesting that cannabinoids can directly alter and inhibit the strength of synaptic connectivity between neurons as well as prevent new synapse formation (Kim and Thayer, 2001). This neuromodulatory process would be particularly disruptive and maladaptive during neurodevelopment and maturation of the CNS in adolescence, and likely extending into emerging adulthood.
The FG is moderately distributed with cannabinoid CB1 receptors (Wong et al., 2010), which may underlie the reported structural vulnerability of this region to chronic marijuana use (James et al., 2011; Matochik et al., 2005). Furthermore, the FG displays elevated sensitivity to acute MJ exposure, as healthy volunteers who received an acute MJ challenge prior to performing a visual stimulation functional magnetic resonance imaging (fMRI) task exhibited increased activation in the left FG (Winton-Brown et al., 2011). MRI studies implementing face and object visual processing tasks have frequently reported finding greater face-sensitive (relative to object-sensitive) activation in the FG, though notably, the rFG commonly exhibits dominant face perception activation bias (Hasson et al., 2002; Rossion et al., 2000; Yovel et al., 2008). The role of the rFG in regulating face perception and recognition is a highly specialized product of neurodevelopmental processes throughout adolescence and into adulthood that involves experience-dependent dynamic synaptic pruning and reorganization of neural connections (Cohen Kadosh and Johnson, 2007; Golarai et al., 2007). Typically, pruning eliminates asymmetrical excitatory synapses, indicating that glutamatergic transmission is commonly affected throughout neuromaturation in adolescence and early adulthood (Brenhouse and Andersen, 2011). Previous preclinical studies have shown that THC exposure during the adolescent developmental window may disrupt normative patterns of excitatory synapse elimination, thereby interfering with maturation of regional glutamatergic systems and functional network connections (Rubino et al., 2014). Thus, the cortical thinness observed in the rFG may be a consequence of abnormal pruning patterns related to persistent long-term MJ use throughout adolescence and into emerging adulthood.
Similar to the FG, the thalamus is also moderately distributed with CB1 receptors (Glass et al., 1997; Wong et al., 2010) and has also exhibited altered gray matter tissue density (Matochik et al., 2005) and neurometabolite levels (Silveri et al., 2011; Mashhoon et al., 2013) in MJ users. Chronic consumption of MJ, such as the patterns established by MJ users in the present study, and consequent regular binding of THC to CB1 receptors may directly alter the volume of the thalamus through a combination of axonal, neuronal and myelin loss (Evangelou et al., 2000; Hof et al., 2003). Oligodendrocytes are myelin-producing neuroglia widely distributed throughout the central nervous system (CNS) that express CB1 receptors (Molina-Holgado et al., 2002). Repeated marijuana use and overstimulation of oligodendrocyte CB1 receptors may result in regional oligodendrocyte downregulation possibly associated with demyelination and myelin loss (Dalton and Zavitsanou, 2010; Zalesky et al., 2012). Similar to the potential pathophysiology of cortical thinness in the FG, neuronal loss in the thalamus also may result from MJ-related alterations in normative regional synaptic pruning patterns. Furthermore, the thalamus may sustain neuronal loss, and consequently loss in volume, from retrograde neuronal degeneration as a consequence of MJ-related pathology in frontal cortex that can damage axon terminations of thalamocortical relay neurons (Gilbert et al., 2001; Zikopoulos and Barbas, 2007). Changes to the structural morphology of the thalamus could have drastic consequences for the critical function of this substrate in relaying multisensory and adaptive cognitive information processing to the cortex as well as maintaining thalamocortical network neurotransmission (Marzinzik et al., 2008).
The present finding of thalamic volume differences in MJ users is consistent with and contributes to growing evidence that suggests the thalamus is neurobiologically perturbed by chronic MJ use. Consequences of chronic MJ exposure are evident in measurable alterations to the microarchitectural integrity of the thalamus. Greater gray matter tissue density has been reported in the right thalamus in male MJ users, relative to non-MJ-users, (Matochik et al., 2005) that may be related to abnormal synaptic pruning. Previous work also has revealed neurochemical differences in the left thalamus, a hypothesized component of left-hemisphere lateralized cognitive inhibition circuitry, in male MJ users compared to non-using individuals (Mashhoon et al., 2013). Lower levels of myo-Inositol (mI), a glial marker involved in neuronal metabolism, neural signaling, and regulating cellular energy use (Ho et al., 1995; Maragakis and Rothstein, 2006) were reported in the left thalamus of MJ users that were associated with greater cognitive impulsivity (Mashhoon et al., 2013).
The current study also demonstrated associations between smaller total thalamic volume and greater impulsivity, specifically non-planning and overall impulsivity. The BIS non-planning impulsivity subscale is associated with lack of forethought and poor future planning. In MJ smokers, this likely reflects lack of behaving with forethought of negative consequences and frequently impulsive decision-making. The thalamus is a critical hub in neural circuitry regulating cognitive inhibition; MJ-related neurochemical (Mashhoon et al., 2013) and volumetric alterations to thalamic structural stability and integrity could directly interfere with cortico-thalamic signaling that functionally supports cognitive inhibition and manages impulsive decision-making by integrating and relaying information across reciprocal connections to frontal cortex (Haber, 2003). Furthermore, chronic MJ use could negatively inhibit the efficient transmission of information through thalamo-cortical projection networks via persistent CB1 receptor-mediated actions on synaptic activity, such as disrupted cAMP signaling, within the thalamus.
One influential thalamo-cortical projection network is the pathway that regulates attention by transmission of visual information from input sources in the visual cortex through thalamic projections to the frontal cortex that is significant for behavioral contexts (Rees, 2009; Saalmann and Kastner, 2009). Moreover, the thalamus is also involved in visual object localization and organization (Ward and Arend, 2007). Importantly, the rFG also plays a principal role in visual information processing. Though often functionally associated with face-selective perception and recognition processing, the FG is also involved in elemental stages of visual object information retrieval and categorization (James et al., 2011; Wheatley et al., 2005). Studies utilizing fMRI have shown that the rFG is part of the ventral visual pathway (Mahon et al., 2007a; Mahon et al., 2007b; van der Linden et al., 2011) and a critical lateralized component of visuoperceptual processing of basic-level object identification (Bruffaerts et al., 2013), as well as of object size and location (Grill-Spector et al., 1999). These visuoperceptual representations are subsequently often stored in visual working memory. Thus, findings of rFG cortical thinness and smaller thalamic volume in emerging adult MJ users may be connected through their key roles in the visual information processing pathway.
The current study did not probe visual working memory directly, however a growing body of evidence suggests that MJ users demonstrate difficulty and less cognitive efficiency in performing visual working memory tasks (e.g. (Bolla et al., 2002; Kanayama et al., 2004; King et al., 2011; Padula et al., 2007; Schweinsburg et al., 2008a, 2008b; Smith et al., 2010). It is possible that chronic MJ use measurably alters cortical pathways that transmit basic-level visuoperceptual information that is applied toward a behavioral response. The rFG cortical thinness and smaller thalamic volume measured in MJ users in the present study may reflect neurobiological alterations related to persistent MJ use in regions specifically involved in visuoperceptual and object information processing. Impaired visuoperceptual processing, particularly in visuomotor or visuospatial domains, can be of significant concern in practical realms such as navigational ability and applying cognitive-motor skills to drive a vehicle (Weinstein et al., 2008).
A limitation of the study was that differences in morphology within discrete thalamic nuclei cannot accurately be measured and thus our interpretations of the smaller thalamic volume findings in MJ users are limited. Furthermore, the current cross-sectional structural and volumetric group differences cannot aid in determining if the alterations were pre-existing in participants prior to initiation of MJ use or if they are a consequence of persistent use. Though other structural neuroimaging studies have shown MJ-related differences in hippocampal and amygdala volume relative to non-using counterparts (e.g., Ashtari et al., 2011; Cousijn et al., 2012; Demirakca et al., 2011; Schacht et al., 2012; Yucel et al., 2008), our study did not find any structural differences in these regions between MJ and NU groups. Other investigations have also previously reported a lack of hippocampal volume alterations in male and female young adult short-term MJ users (Block et al., 2000) and heavy long-term MJ users (Tzilos et al., 2005) and suggest that the direct influence of MJ use on hippocampal volume may be impacted by other variables; these include potential underreporting and/or inaccurate reporting of other lifetime drug and alcohol use in both MJ and NU groups (Tzilos et al., 2005) and possible hippocampal volume abnormalities that are not detectable by current assessment techniques (Block et al., 2000). An additional study limitation was the small overall sample size of n=15 MJ and n=15 NU groups, which could have precluded finding relevant differences in other brain regions due a limited sample. Importantly, groups were age- and sex-matched, alcohol use (drinks per week) did not differ between groups, and nicotine use was minimal in study participants. With regard to nicotine use, a significantly smaller thalamic volume (p=.039) and evidence for reduced cortical thickness in the fusiform (uncorrected) were maintained when the three MJ subjects with positive nicotine use histories were removed from analyses. As even minimal nicotine use could impact study findings, future studies should more thoroughly account for smoking status. Nonetheless, significant differences observed between groups on impulsivity and brain measures produced medium to large effect sizes. Sex differences in measures of ICV (Block et al., 2000) and amygdala volume (McQueeny et al., 2011) in MJ users have been previously reported. It is possible that MJ use during neurodevelopment may have interfered with sex-specific maturational processes in the amygdala, and thus subtle alterations to amygdala morphometry between males and females may obscure the influence of MJ use (McQueeny et al., 2011). We cannot presently parse apart sex differences in regional structure or volume, given that there were only two females in each group. Future investigations of MJ-related morphological alterations should account for sex-related differences in neural composition. Finally, this study relied on self-report for establishing patterns of past and current marijuana use, as well as for measures of mood states and impulsivity. Self-report can often be negatively influenced or compromised by individual willingness to respond accurately, estimation ability, and social context and perceived admissibility (Del Boca and Darkes, 2003). Participants were assured that responses to all drug use questions, in addition to all other responses on mood and impulsivity measures, would be kept confidential, with sufficient time permitted to recall and report MJ use. To this end, it is plausible that this self-selected group of MJ users was willing and able to effectively produce reliable detailed reports.
In conclusion, the present investigation revealed rFG cortical thinness and smaller thalamic volumes in MJ using emerging adults, relative to NU healthy age-matched control participants, that may be related to neurobiological consequences of heavy MJ use. Furthermore, the present finding of smaller thalamic volume associated with greater impulsivity contributes to growing evidence that the thalamus is uniquely neurobiologically perturbed by persistent MJ use. Collectively, alterations to the structural integrity of the thalamus and the rFG, a lateralized component of visuospatial identity processing, may interfere with established roles in regulating visuoperceptual and object information processing. As such, MJ users may experience greater difficulty in completing visual working memory tasks that rely on efficient visuoperceptual and visuospatial processing.
Research Highlights.
Used Freesurfer to measure cortical thickness and volume in emerging adult marijuana (MJ) users
Less cortical thickness in right hemisphere fusiform gyrus in MJ users v. nonusers
Smaller thalamic volume in MJ users, v. non-users, related to greater impulsivity
RFG and thalamus may be connected through visual information processing pathway
Altered rFG and thalamus integrity may impair visuoperceptual processing in MJ users
Acknowledgments
Role of funding sources
This investigation was supported in part by National Institute on Drug Abuse grants K01034028 (YM) and R03DA022482 (JTS), and National Institute on Alcohol Abuse and Alcoholism grant K01AA014651 (MMS).
Footnotes
Contributors
Drs. Sava, Sneider and Silveri conceptualized the study. Drs. Mashhoon and Sava conducted the data processing and analysis. Drs. Mashhoon, Sava and Silveri drafted the manuscript. Drs. Sneider and Nickerson made contributions and edited the manuscript. Dr. Mashhoon consolidated edits from coauthors. All authors approved the final manuscript.
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, Gee J, Sevy S, Kumra S. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res. 2011;45:1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K, Holahan MR, Kelley AE. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol Learn Mem. 2002;77:44–62. doi: 10.1006/nlme.2000.4002. [DOI] [PubMed] [Google Scholar]
- Batalla A, Bhattacharyya S, Yucel M, Fusar-Poli P, Crippa JA, Nogue S, Torrens M, Pujol J, Farre M, Martin-Santos R. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MP, Collins PF, Luciana M. Neurocognition in college-aged daily marijuana users. J Clin Exp Neuropsychol. 2014;36:379–398. doi: 10.1080/13803395.2014.893996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CM, Baird AA. Anatomical changes in the emerging adult brain: a voxel-based morphometry study. Hum Brain Mapp. 2006;27:766–777. doi: 10.1002/hbm.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11:491–496. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruffaerts R, Dupont P, Peeters R, De Deyne S, Storms G, Vandenberghe R. Similarity of fMRI activity patterns in left perirhinal cortex reflects semantic similarity between words. J Neurosci. 2013;33:18597–18607. doi: 10.1523/JNEUROSCI.1548-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R. Functional diversity on synaptic plasticity mediated by endocannabinoids. Philos Trans R Soc Lond B Biol Sci. 2012;367:3242–3253. doi: 10.1098/rstb.2011.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yucel M, Lubman DI. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol Psychiatry. 2012;71:684–692. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh K, Johnson MH. Developing a cortex specialized for face perception. Trends Cogn Sci. 2007;11:367–369. doi: 10.1016/j.tics.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dalton VS, Zavitsanou K. Cannabinoid effects on CB1 receptor density in the adolescent brain: an autoradiographic study using the synthetic cannabinoid HU210. Synapse. 2010;64:845–854. doi: 10.1002/syn.20801. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98(Suppl 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- Demirakca T, Sartorius A, Ende G, Meyer N, Welzel H, Skopp G, Mann K, Hermann D. Diminished gray matter in the hippocampus of cannabis users: possible protective effects of cannabidiol. Drug Alcohol Depend. 2011;114:242–245. doi: 10.1016/j.drugalcdep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Epstein KA, Kumra S. Altered cortical maturation in adolescent cannabis users with and without schizophrenia. Schizophren Res. 2015 doi: 10.1016/j.schres.2014.11.029. in press. [DOI] [PubMed] [Google Scholar]
- Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM. Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol. 2000;47:391–395. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York Psychiatric Institute; New York: 2002. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry. 2001;158:618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N, van der Kouwe A, Blood AJ, Breiter HC. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. 2014;34:5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JD, Grill-Spector K. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat Neurosci. 2007;10:512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Dahlgren MK, Yurgelun-Todd D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Exp Clin Psychopharmacol. 2011;19:231–242. doi: 10.1037/a0023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. Regulation of plasticity of glutamate synapses by endocannabinoids and the cyclic-AMP/protein kinase A pathway in midbrain dopamine neurons. J Physiol. 2010;588:2589–2604. doi: 10.1113/jphysiol.2010.190066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Levy I, Behrmann M, Hendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:479–490. doi: 10.1016/s0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y, Wigglesworth K, Eppig JJ, Schultz RM. Preimplantation development of mouse embryos in KSOM: augmentation by amino acids and analysis of gene expression. Mol Reprod Dev. 1995;41:232–238. doi: 10.1002/mrd.1080410214. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, Jr, Byne W, Buitron C, Perl DP, Davis KL. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 2009;92:559–565. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, Tapert SF. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. J Stud Alcohol Drugs. 2014;75:729–743. doi: 10.15288/jsad.2014.75.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Van Hell HH, De Win MM, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol. 2007;17:289–297. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- James A, Hough M, James S, Winmill L, Burge L, Nijhawan S, Matthews PM, Zarei M. Greater white and grey matter changes associated with early cannabis use in adolescent-onset schizophrenia (AOS) Schizophren Res. 2011;128:91–97. doi: 10.1016/j.schres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004;176:239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kim D, Thayer SA. Cannabinoids inhibit the formation of new synapses between hippocampal neurons in culture. J Neurosci. 2001;21:RC146. doi: 10.1523/JNEUROSCI.21-10-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GR, Ernst T, Deng W, Stenger A, Gonzales RM, Nakama H, Chang L. Altered brain activation during visuomotor integration in chronic active cannabis users: relationship to cortisol levels. J Neurosci. 2011;31:17923–17931. doi: 10.1523/JNEUROSCI.4148-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitland LR, Melle I, Aminoff SR, Lagerberg TV, Andreassen OA, Ringen PA. Cannabis use in first-treatment bipolar I disorder: relations to clinical characteristics. Early Interv Psychiatry. 2014 doi: 10.1111/eip.12138. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Koethe D. Cannabis and psychiatric disorders: it is not only addiction. Addict Biol. 2008;13:264–275. doi: 10.1111/j.1369-1600.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Wright NE, Kirchner-Medina C, Maple KE, Shollenbarger S. The effects of regular cannabis use on neurocognition in adolescents and young adults. Curr Addict Rep. 2014;1:144–156. doi: 10.1007/s40429-014-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Abel T, Hernandez PJ. Exchange protein activated by cAMP enhances long-term memory formation independent of protein kinase A. Learn Mem. 2009;16:367–370. doi: 10.1101/lm.1231009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Costa A, Peterson R, Vargas KA, Caramazza A. Lexical selection is not by competition: a reinterpretation of semantic interference and facilitation effects in the picture-word interference paradigm. J Exp Psychol Learn Mem Cogn. 2007a;33:503–535. doi: 10.1037/0278-7393.33.3.503. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Milleville SC, Negri GA, Rumiati RI, Caramazza A, Martin A. Action-related properties shape object representations in the ventral stream. Neuron. 2007b;55:507–520. doi: 10.1016/j.neuron.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Martin-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, Fusar-Poli P, Borgwardt S, Seal M, Busatto GF, McGuire P. Neuroimaging in cannabis use: a systematic review of the literature. Psychol Med. 2010;40:383–398. doi: 10.1017/S0033291709990729. [DOI] [PubMed] [Google Scholar]
- Marzinzik F, Wahl M, Schneider GH, Kupsch A, Curio G, Klostermann F. The human thalamus is crucially involved in executive control operations. J Cogn Neurosci. 2008;20:1903–1914. doi: 10.1162/jocn.2008.20124. [DOI] [PubMed] [Google Scholar]
- Mashhoon Y, Jensen JE, Sneider JT, Yurgelun-Todd DA, Silveri MM. Lower left thalamic myo-inositol levels associated with greater cognitive impulsivity in marijuana-dependent young men: preliminary spectroscopic evidence at 4T. J Addict Res Ther Suppl. 2013:4. doi: 10.4172/2155-6105.S4-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Czerkawski C, Crowley DJ, Cohen-Gilbert JE, Sneider JT, Silveri MM. Binge alcohol consumption in emerging adults: anterior cingulate cortical “thinness” is associated with alcohol use patterns. Alcohol Clin Exp Res. 2014;38:1955–1964. doi: 10.1111/acer.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppelman LF. EDITS Manual For The Profile Of Mood States. Educational and Industrial Service; San Diego: 1981. [Google Scholar]
- McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res. 2011;224:128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol. 2009;14:457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res. 2010;182:152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci US A. 2012;109:E2657–2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Almazan G, Molina-Holgado F, Borrell J, Guaza C. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychol Addict Behav. 2007;21:478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Ompad DC, Petkova E. Correlates of intentions to use cannabis among US high school seniors in the case of cannabis legalization. Int J Drug Policy. 2014;25:424–435. doi: 10.1016/j.drugpo.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Rakic P. A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb Cortex. 2006;16(Suppl 1):i3–17. doi: 10.1093/cercor/bhk036. [DOI] [PubMed] [Google Scholar]
- Rees G. Visual attention: the thalamus at the centre? Curr Biol. 2009;19:R213–214. doi: 10.1016/j.cub.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Rossion B, Dricot L, Devolder A, Bodart JM, Crommelinck M, De Gelder B, Zoontjes R. Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. J Cogn Neurosci. 2000;12:793–802. doi: 10.1162/089892900562606. [DOI] [PubMed] [Google Scholar]
- Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M, Sagheddu C, Ligresti A, Tonini R, Di Marzo V, Parolaro D. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis. 2014;73C:60–69. doi: 10.1016/j.nbd.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S. Gain control in the visual thalamus during perception and cognition. Curr Opin Neurobiol. 2009;19:408–414. doi: 10.1016/j.conb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- SAMHSA; Substance Abuse and Mental Health Services Administration, C.f.B.H.S.a.Q, editor. The NSDUH Report: Trends in Adolescent Substance Use and Perception of Risk from Substance Use. Rockville, MD: 2013. [PubMed] [Google Scholar]
- Schacht JP, Hutchison KE, Filbey FM. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology. 2012;37:2368–2376. doi: 10.1038/npp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Schuermeyer J, Salomonsen-Sautel S, Price RK, Balan S, Thurstone C, Min SJ, Sakai JT. Temporal trends in marijuana attitudes, availability and use in Colorado compared to non-medical marijuana states: 2003–11. Drug Alcohol Depend. 2014;140:145–155. doi: 10.1016/j.drugalcdep.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008a;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008b;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Jensen JE, Rosso IM, Sneider JT, Yurgelun-Todd DA. Preliminary evidence for white matter metabolite differences in marijuana-dependent young men using 2D J-resolved magnetic resonance spectroscopic imaging at 4 Tesla. Psychiatry Res. 2011;191:201–211. doi: 10.1016/j.pscychresns.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Longo CA, Fried PA, Hogan MJ, Cameron I. Effects of marijuana on visuospatial working memory: an fMRI study in young adults. Psychopharmacology (Berl) 2010;210:429–438. doi: 10.1007/s00213-010-1841-8. [DOI] [PubMed] [Google Scholar]
- Sneider JT, Gruber SA, Rogowska J, Silveri MM, Yurgelun-Todd DA. A preliminary study of functional brain activation among marijuana users during performance of a virtual water maze task. J Addict. 2013;2013:461029. doi: 10.1155/2013/461029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneider JT, Mashhoon Y, Silveri MM. A review of magnetic resonance spectroscopy studies in marijuana using adolescents and adults. J Addict Res Ther Suppl. 2014:4. doi: 10.4172/2155-6105.S4-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurodevelopment. J Adolesc Health. 2013;52:S7–13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift W, Coffey C, Carlin JB, Degenhardt L, Patton GC. Adolescent cannabis users at 24 years: trajectories to regular weekly use and dependence in young adulthood. Addiction. 2008;103:1361–1370. doi: 10.1111/j.1360-0443.2008.02246.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzilos GK, Cintron CB, Wood JB, Simpson NS, Young AD, Pope HG, Jr, Yurgelun-Todd DA. Lack of hippocampal volume change in long-term heavy cannabis users. Am J Addict. 2005;14:64–72. doi: 10.1080/10550490590899862. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drug and Crime. World Drug Report. 2013. [Google Scholar]
- van der Linden M, van Turennout M, Fernandez G. Category training induces cross-modal object representations in the adult human brain. J Cogn Neurosci. 2011;23:1315–1331. doi: 10.1162/jocn.2010.21522. [DOI] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Keller E, Hurd YL. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience. 2003;118:681–694. doi: 10.1016/s0306-4522(03)00020-4. [DOI] [PubMed] [Google Scholar]
- Ward R, Arend I. An object-based frame of reference within the human pulvinar. Brain. 2007;130:2462–2469. doi: 10.1093/brain/awm176. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Person Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Brickner O, Lerman H, Greemland M, Bloch M, Lester H, Chisin R, Sarne Y, Mechoulam R, Bar-Hamburger R, Freedman N, Even-Sapir E. A study investigating the acute dose-response effects of 13 mg and 17 mg Delta 9- tetrahydrocannabinol on cognitive-motor skills, subjective and autonomic measures in regular users of marijuana. J Psychopharmacol. 2008;22:441–451. doi: 10.1177/0269881108088194. [DOI] [PubMed] [Google Scholar]
- Weinstein AM, Miller H, Bluvstein I, Rapoport E, Schreiber S, Bar-Hamburger R, Bloch M. Treatment of cannabis dependence using escitalopram in combination with cognitive-behavior therapy: a double-blind placebo-controlled study. Am J Drug Alcohol Abuse. 2013;40:16–22. doi: 10.3109/00952990.2013.819362. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Weisberg J, Beauchamp MS, Martin A. Automatic priming of semantically related words reduces activity in the fusiform gyrus. J Cogn Neurosci. 2005;17:1871–1885. doi: 10.1162/089892905775008689. [DOI] [PubMed] [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- Winton-Brown TT, Allen P, Bhattacharyya S, Borgwardt SJ, Fusar-Poli P, Crippa JA, Seal ML, Martin-Santos R, Ffytche D, Zuardi AW, Atakan Z, McGuire PK. Modulation of auditory and visual processing by delta-9-tetrahydrocannabinol and cannabidiol: an FMRI study. Neuropsychopharmacology. 2011;36:1340–1348. doi: 10.1038/npp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevara M, Ye W, Dannals RF, Ravert HT, Nandi A, Rahmim A, Ming JE, Grachev I, Roy C, Cascella N. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage. 2010;52:1505–1513. doi: 10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovel G, Tambini A, Brandman T. The asymmetry of the fusiform face area is a stable individual characteristic that underlies the left-visual-field superiority for faces. Neuropsychologia. 2008;46:3061–3068. doi: 10.1016/j.neuropsychologia.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Solowij N, Yucel M, Lubman DI, Takagi M, Harding IH, Lorenzetti V, Wang R, Searle K, Pantelis C, Seal M. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012;135:2245–2255. doi: 10.1093/brain/aws136. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Parallel driving and modulatory pathways link the prefrontal cortex and thalamus. PLoS One. 2007;2:e848. doi: 10.1371/journal.pone.0000848. [DOI] [PMC free article] [PubMed] [Google Scholar]