Abstract
Introduction
Previous research shows immune response to vaccination differs by sex but this has not been explored for IMVAMUNE®, a replication-deficient smallpox vaccine developed in response to the potential for bioterrorism using smallpox.
Methods
We conducted a participant-level meta-analysis (N=275, 136 men, 139 women) of 3 randomized trials of IMVAMUNE conducted at 13 centers in the US through a federally-funded extramural research program. Studies were eligible for inclusion if they tested the standard dose (1×108 TCID50/mL on Days 0 and 28) of liquid formulation IMVAMUNE, were completed at the time of our search, and enrolled healthy vaccinia-naïve participants. Models of the peak log2 ELISA and PRNT titers post-second vaccination were constructed for each study with sex as a covariate. Results from these models were combined into random effects meta-analyses of the sex difference in response to IMVAMUNE. We then compared this approach with fixed effects models using the combined participant level data.
Results
In each study the mean peak log2 ELISA titer was higher in men than women but no single study demonstrated a statistically significant difference. Combination of the adjusted study-specific estimates into the random effects model showed a higher mean peak log2-titer in men compared with women (absolute difference [men-women]: 0.32, 95% CI: 0.02-.60). Fixed effects models controlling for study showed a similar result (log2 ELISA titer, men-women: 0.34, 95% CI: 0.04–0.63). This equates to a geometric mean peak titer that is approximately 27% higher in men than women (95% CI:3%–55%). Peak log2 PRNT titers were also higher (although not significantly) in men (men-women: 0.14, 95% CI: −0.30–0.58).
Conclusion
Our results show statistically significant differences in response to IMVAMUNE comparing healthy, vaccinia-naïve men with women and suggest that sex should be considered in further development and deployment of IMVAMUNE and other MVA-based vaccines.
Keywords: Vaccines, Smallpox, Vaccinia virus, MVA vaccine, Imvamune, Meta-analysis
INTRODUCTION
The potential to prevent infectious diseases (ID) through vaccination was recognized in the late 18th century, although it wasn’t until the 19th and 20th centuries that vaccination significantly impacted public health.[1–3] Advances in knowledge of pathogens and Omics, studies of pathogen-host interactions, and mechanisms of immunity have improved the efficiency and success of vaccine development.[1, 4, 5] However, the fundamental assumption underlying vaccine deployment has remained largely unchanged, i.e., that a single vaccine for a given pathogen can be used in a large population.[6] This assumption is incongruous with contemporary recognition that the immune response is heterogeneous and that a single vaccine may have varying utility in population subgroups.[7]
Heterogeneous post-vaccination immune responses in men and women have been reported across a range of vaccines and in populations with different characteristics.[8] The effect of sex on immune response to vaccination may depend on several factors, including the vaccine antigen itself, with men responding better to some antigens than women and vice versa.[8] Recent studies of military personnel and civilian healthcare workers vaccinated against smallpox using Dryvax vaccine showed that females maintain stronger long-term humoral immunity than males,[9] but that sex differences in cellular immune response are less consistent, with the female (or male) dominance depending on individual cytokines.[10] The possibility that population subgroups respond differently to smallpox vaccination is of concern given the development of novel smallpox vaccines intended for emergency use against a bioterrorist attack with weaponized smallpox.[11]
Vaccines against smallpox are based on the poxvirus vaccinia, which induces immunity against variola virus, the causative agent of smallpox.[12] Unfortunately, live-virus vaccines such as Dryvax, which were successfully used to eradicate smallpox, are associated with rare but potentially fatal adverse events, e.g., disseminated vaccinia and myopericarditis,[13] and the newly developed vaccinia-virus smallpox vaccine ACAM2000®, which is now licensed in the US for limited use in people at risk, is associated with similar safety concerns.[14] IMVAMUNE® is a highly attenuated smallpox vaccine developed as a safer alternative to existing live virus vaccines. IMVAMUNE is based on the Modified Vaccinia Ankara (MVA) virus, which is a replication-deficient vaccinia virus, first experimented with in the 1970s as a priming agent intended to reduce adverse reactions of subsequent vaccination with live vaccinia virus vaccines.[15] The possibility of bioterrorist attack using smallpox generated renewed interested in MVA as a smallpox vaccine and this led to development of IMVAMUNE, which is now licensed by the European Medicines Agency and Health Canada for prevention of smallpox and continues to be tested in clinical trials in the United States.[15–17]
Sex differences in response to IMVAMUNE have not been explored, and although men and women have been included in randomized trials of IMVAMUNE, individual trials were not powered to detect differences in immune response between sexes. Therefore, we conducted a participant-level meta-analysis of completed randomized trials of IMVAMUNE to evaluate sex differences in humoral immune response to this novel smallpox vaccine. Our objective was to inform the design of future studies of IMVAMUNE and other MVA-based vaccines, and to explore the importance of sex in human immunity in general.
METHODS
Our approach to conducting and reporting this analysis followed established standards for meta-analysis of clinical trials.[18, 19]
Identification of Studies
Since 2002, the Division of Microbiology and Infectious Diseases (DMID) at the National Institute of Allergy and Infectious Diseases (NIAID) has sponsored clinical trials of IMVAMUNE through its extramural research program. During February of 2014, in collaboration with DMID/NIAID staff, we identified all DMID-sponsored clinical trials of IMVAMUNE for which participant-level data were available at the DMID/NIAD data coordinating center (The EMMES Corporation, Rockville, MD). We then selected studies for our meta-analysis from this portfolio.
Eligibility Criteria
Studies eligible for inclusion in our meta-analysis of sex differences in humoral immune response to IMVAMUNE were: randomized clinical trials (these studies offer high-quality evidence that IMVAMUNE elicits a humoral immune response), completed at the time of our search (required for extraction of results), included healthy participants only (to exclude effects of established pathological processes on immune function), enrolled participants who were naïve to smallpox vaccine (to exclude the effect of immunological experience on immune response to IMVAMUNE), and tested the liquid formulation of IMVAMUNE in the standard dose, 1×108 TCID50/ml via subcutaneous needle injection on Days 0 and 28 (chosen because this formulation, dose, and administration timing elicits the strongest humoral immune response).[20]
Data Extraction
Participant-level data were obtained for each of the included studies. We extracted data for all participants from each study who received two doses (on Days 0 and 28) of liquid formulation IMVAMUNE at 1×108 TCID50/mL. Data were not extracted for participants receiving placebo or other IMVAMUNE regimens. Included studies measured humoral immune response at various time points after each vaccination. We focused our analysis on measurements taken after the second vaccination as this is the time period when IMVAMUNE is shown to elicit the strongest humoral immune response.[20] Antibody titers, measured by enzyme linked immunosorbent assay (ELISA) and plaque reduction neutralizing titer (PRNT), were extracted from each included study for several time points post-second vaccination (Table 1). The primary endpoint for our meta-analysis was the highest log2-transformed titer achieved for each individual, which we interpreted as an estimate of the peak titer. We chose the mean difference in the log2-transformed titer, comparing men to women, as the summary measure for our meta-analysis as this allowed us to assess both the presence and magnitude of the sex difference in response to IMVAMUNE. This method also allowed us to interpret the anti-logarithm of the sex difference as a relative measure of geometric mean titer in men vs. women.
Table 1.
Characteristics of Studies Included in Meta-Analysis of Standard Dose IMVAMUNE (1×108 TCID50, liquid formulation, subcutaneous needle injection on Days 0 and 28)
| Study | Year Completed | Population | Clinical Centers | Total Enrolled | Number With Antibody Measurements | Number Receiving Standard Dose IMVAMUNE | Antibody Measurement Timepoints |
|---|---|---|---|---|---|---|---|
| NCT 0043702120 | 2009 | Healthy vaccinia-naive adults, Age 18–35, born after 1971 | Case Western Reserve University, Cleveland, OH; Duke University, Durham, NC; Saint Louis University, Saint Louis, MO; University of Iowa, Iowa City, IA; University of Maryland, College Park, MD; University of Rochester, Rochester, NY; University of Texas - Galveston, Galveston, TX | 226 | 189 | 66 | Day of Second Vaccination; Day 04; Day 08; Day 14; Day 28; Day 180; Day 365 |
| NCT 0087976222 | 2011 | Healthy vaccinia-naive adults, Age 18–37, born after 1971 | Saint Louis University, Saint Louis, MO; University of Iowa, Iowa City, IA | 91 | 91 | 45 | Day of Second Vaccination; Day 14; Day 28; Day 180 |
| NCT 00914732 | 2011 | Healthy vaccinia-naive adults, Age 18–38, born after 1971 | Baylor University, Waco, TX; Emory University, Atlanta, GA; Group Health Cooperative, Seattle, WA; Johns Hopkins University, Baltimore, MD; Saint Louis University, Saint Louis, MO; University of Iowa, Iowa City, IA; University of Maryland, College Park, MD; University of Washington, Seattle, WA; Vanderbilt University, Nashville, TN | 525 | 512 | 164 | Day of Second Vaccination; Day 14; Day 28; Day 180 |
Statistical Analysis
We began by plotting the mean of the log2-titer over time by sex, separately for each study. This allowed us to qualitatively evaluate the presence of sex differences in response to IMVAMUNE prior to conducting any formal analyses. Our meta-analysis of the peak log2-titer followed a two-stage approach, in which we estimated the sex difference in response to IMVAMUNE in each study separately (the first stage) and then combined the study-specific results into a random effects model (the second stage) using the method described by Der Simonian and Laird.[21] For each study, we applied the generalized linear modeling framework, with identity link and normal errors, to estimate the absolute difference in peak log2-titer between men and women. We selected four models for each study: an unadjusted and adjusted model of the peak log2-titer as measured by ELISA and PRNT. Selection of adjusted models was performed by exploring the importance of the following factors, one at a time, in a model containing sex: age (continuous and categorical), study center, log2-titer immediately prior to second vaccination, and race (White vs. non-White). Any factors that were significant (alpha=0.10) after controlling for sex, or that substantially improved model fit over a model containing only sex (as indicated by the Akaike or the Bayseian information criteria) were entered simultaneously into a model containing sex. Then, backward elimination was applied until all predictors in the model (other than sex) were significant at alpha=0.05. All models used females as the referent group. Statistical significance was evaluated using the likelihood ratio chi-square test, and model assumptions were verified graphically. The coefficient and standard error for sex were extracted from each of the final models for incorporation into random effects models. Finally, we evaluated the impact of inter-study heterogeneity on the summary estimate of the sex difference in log2-titer by fitting joint fixed effects models (i.e., treating all of the participant-level data as if it were collected as part of a single study) and informally comparing results with the random effects models. Joint fixed effects models were built using a similar approach as described above and included a model covariate indicating the study that the data were drawn from. Analyses were done using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
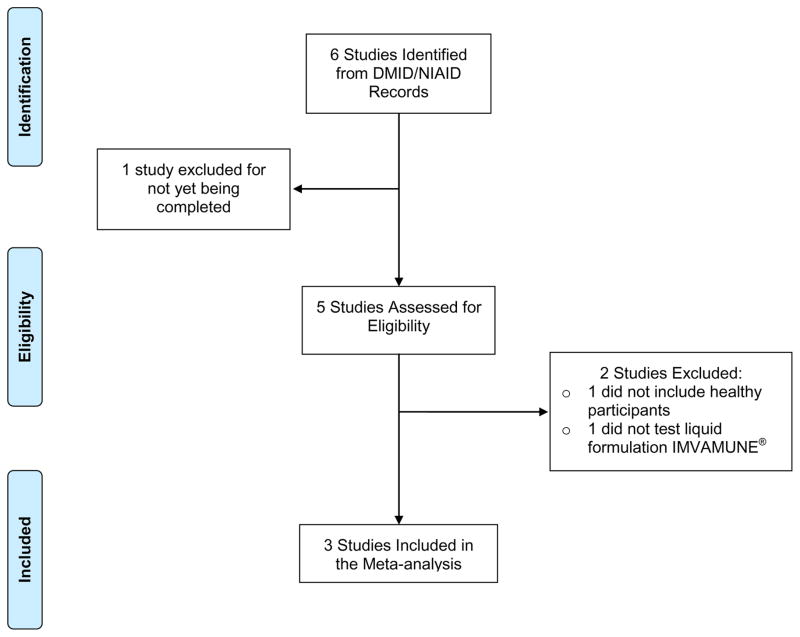
Our search for randomized trials of IMVAMUNE identified 6 studies (Figure 1). One of these was not yet completed and was excluded. Among the remaining 5 completed studies, 1 was excluded because it did not enroll healthy participants, and 1 was excluded because liquid formulation IMVAMUNE was not tested. Therefore, 3 studies were included in our analysis. Results of two studies were published (ClinicalTrials.gov NCT #00437021[20] and NCT #00879762[22]) and a manuscript for the third study (NCT #00914732) was in peer review at the time of this writing.
Figure 1.
PRISMA Flow Diagram Illustrating Selection of Randomized Trials of IMVAMUNE for Meta-Analysis
PRISMA=Preferred Reporting Items for Systematic Reviews and Meta-Analyses.19 DMID=Division of Microbiology and Infectious Diseases. NIAID=National Institute of Allergy and Infectious Diseases.
Characteristics of studies included in our analysis are shown in Table 1. All studies enrolled participants of a similar age from 13 sites in the Eastern, Mid-Western, and North-Western United States, with substantial overlap in clinic sites across studies. Antibody titers were available for 84%, 100%, and 98% of participants in the three studies. In two studies, approximately 1/3 of participants received standard dose IMVAMUNE while in the third study nearly half of participants were randomized to standard dose IMVAMUNE. One study measured humoral immune response at six time points, and two studies measured it at 3 time points, post-second vaccination. In total, we extracted data for 275 participants (136 men [median age 26 years, range: 18–37], and 139 women [median age 26 years, range: 18–38) who received standard dose IMVAMUNE. ELISA and PRNT assays for each study were conducted using the same method at a single laboratory as described previously.[23]
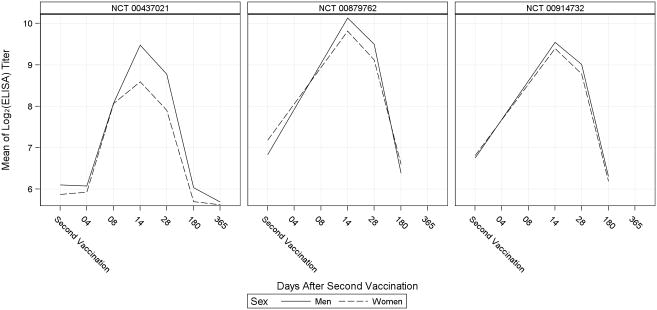
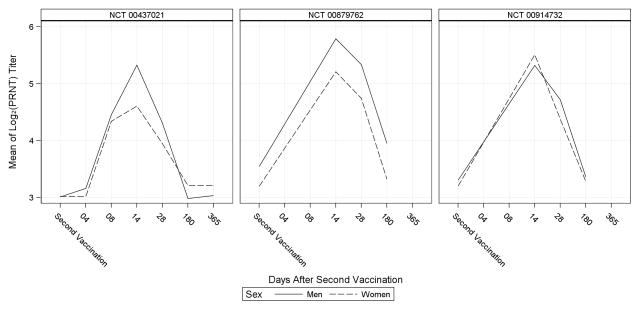
Humoral immune response over time for men and women, as measured by ELISA and PRNT, is shown for each included study in Figures 2 and 3. The pattern of immune response measured by ELISA showed moderate differences between men and women in each study; particularly at Day 14 post-second vaccination when the mean log2-titer was consistently higher in men than women. The PRNT assay also revealed higher titers in men than women at Day 14 post-second vaccination, with the exception of one study in which PRNT results differed from the ELISA results at Day 14 post-second vaccination. Notably, the starkest differences between men and women measured by either assay were observed in studies with the smallest sample sizes.
Figure 2.
Mean of the log2 ELISA Titer over Time in Men and Women
ELISA=enzyme linked immunosorbent assay.
Figure 3.
Mean of the log2 PRNT Titer over Time in Men and Women
PRNT= plaque reduction neutralizing titer.
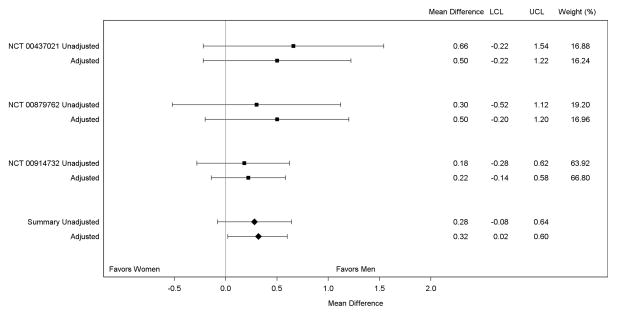
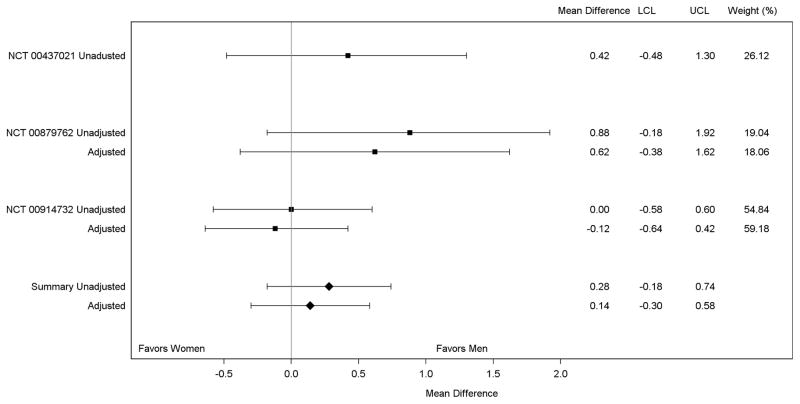
Results of our random effects meta-analysis of the peak log2 ELISA titer are shown in Figure 4. In each of the included studies, the peak log2-titer was higher in men compared with women, although no single study showed a statistically significant difference between men and women before or after adjustment. However, combination of the adjusted study-specific estimates into the random effects model showed a statistically significantly higher mean peak log2-titer in men compared with women (absolute difference [men-women]: 0.32, 95% CI: 0.02–0.60). Joint fixed effects regression analysis showed a nearly identical result to the random effects model, implying little impact of inter-study heterogeneity on the summary difference in immune response between men and women (absolute difference [men-women]: 0.34, 95% CI: 0.04–0.63). The predicted mean peak log2-titer was 5.25 (95% CI: 4.45–6.06) in men and 4.92 (95% CI: 4.11–5.73) in women (not tabulated). The random effects meta-analysis of the peak log2 PRNT titer (Figure 5) also suggested higher titers in men compared with women, although the difference was not statistically significant (adjusted absolute difference [men-women]: 0.14, 95% CI: −0.30–0.58).
Figure 4.
Meta-Analysis of the Mean of the Peak log2 ELISA Titer in Men and Women
ELISA=enzyme linked immunosorbent assay.
Figure 5.
Meta-Analysis of the Mean of the Peak log2 PRNT Titer in Men and Women
PRNT= plaque reduction neutralizing titer. There was no adjusted model chosen for NCT 00437021. The summary adjusted estimate includes the unadjusted estimate from NCT 00437021.
DISCUSSION
Using a participant-level meta-analysis of completed randomized trials, we observed a statistically significant sex difference in humoral immune response to IMVAMUNE smallpox vaccine in healthy volunteers not previously exposed to vaccinia. Results from both our random and fixed effects analyses suggested an absolute difference in peak log2-ELISA titer between men and women of approximately 0.3, which equates to a peak geometric mean titer that is nearly 25% higher in men than women. The magnitude of this difference is unlikely to modify any protection against smallpox afforded by IMVAMUNE in the presence of optimal administration of the vaccine.[24, 25] However, our results provide further evidence of population subgroup differences in response to vaccination that may be relevant in further development and deployment of IMVAMUNE or other MVA-based vaccines, and that may inform design of future vaccine clinical trials.
We are unaware of any reports of sex differences in response to IMVAMUNE for direct comparison with our results. However, sex differences have been observed in humoral immunity after vaccination with Dryvax. A recent observational study enrolling 1,076 healthy military personnel and civilian healthcare workers age 18–40 who received a single dose of Dryvax observed that women had higher neutralizing antibody titers compared with men.[9] This finding differs from our results, which show males with higher titers than females for total (ELISA) and neutralizing antibodies (PRNT). However, in our meta-analysis we analyzed the peak titer for each participant during the short term immediately following vaccination. Identifying the highest titer for each participant during a defined follow-up period provides a robust assessment of individual differences in immune response following vaccination. It is unlikely that the aforementioned study identified the peak titer as the majority of participants were measured more than 1 month after vaccination.[9] However, we must acknowledge that antibody titers in persons vaccinated with live vaccinia virus smallpox vaccines decline only slightly over time, and that mean titers measured at distant time points after vaccination may be more relevant when considering long-term protection from infection.[26] Our plots of mean antibody titers after vaccination with IMVAMUNE do not suggest any difference in decline of mean antibody titer in men and women immediately following vaccination. However, we are unable to make statements about long-term declines as our meta-analysis included studies that followed participants for only 6 months (2 studies) or 12 months (1 study). The previous study did not report sex differences in antibody titer stratified by follow-up time,[9] and other studies of long-term humoral immunity after vaccination with live vaccinia virus smallpox vaccines do not report antibody titers by sex,[27–33] so we are unable to directly compare our results with published data.
Sex differences in response to other (not smallpox) vaccines are common. A 2008 review of published literature uncovered 97 studies reporting sex differences in antibody response to vaccination with 14 different vaccines against a wide variety of viral and bacterial infections.[8] Sex differences were observed after vaccination against diseases with a range of pathological mechanisms (e.g., mucosal replication, toxin production, and viraemia) but the female/male dominance varied with the particular type of vaccine.[8] For example, females had greater response to hepatitis A and B vaccines whereas men had greater antibody response to diphtheria and measles vaccines.[8] Our search for clinical trial results published after the aforementioned literature review did not reveal any additional studies reporting sex differences in response to vaccination in healthy individuals. However, one study comparing Cervarix and Gardasil vaccines in HIV-positive volunteers observed higher antibody response in women receiving Cervarix compared with men, while no sex difference was observed in response to Gardasil.[34]
Several plausible mechanisms for sex differences in response to vaccination are discussed in a recent review by Klein et al,[35] including immunological, hormonal, genetic and microbiota differences between males and females. However, none of these mechanisms alone are adequate to explain the range of different responses to vaccination that have been observed. For example, hormonally-mediated differences in immune response cannot explain the greater antibody response to influenza vaccine observed in post-menopausal elderly women compared with elderly men.[8] Furthermore, biological differences between sexes that affect immunity may act differently in the presence of different antigens.[36] The mechanism underlying the sex difference in antibody response observed in our study of an MVA-based vaccine is currently unknown. However, preliminary data from animal studies suggests that immune responses in volunteers of reproductive age, such as the volunteers in the studies we analyzed, may be at least partially determined through interaction of estrogen and Th2 cytokines in production of antibodies.[37] Additional research is necessary to explore such hypotheses.
Our findings are accompanied by several unique strengths. First, our results are derived from randomized clinical trials characterized by close, protocol-specified follow-up with excellent adherence to the scheduled evaluation of pre-specified study endpoints. Furthermore, we studied a homogenous population with respect to immunological experience with vaccinia virus, age, and health status. The intervention we studied was also homogenous with respect to factors known to be associated with immune response to IMVAMUNE: dose, route, formulation, and timing of administration. Furthermore, our use of participant-level data allowed us to explore demographic and study-specific factors as confounders in our meta-analysis.
Despite these methodological strengths, we must acknowledge important caveats to our findings. Of particular note, we did not have information regarding polymorphisms in genes that are relevant to humoral immune function.[7] However, assignment to IMVAMUNE in each of the included studies was randomized. Therefore, if genotype confounded our results then randomization would have systematically assigned men with high-responding genotypes to IMVAMUNE and this is unlikely to have been the case. Nonetheless, we cannot rule out the possibility of residual confounding by unmeasured factors that may be associated with both sex and humoral immune response to IMVAMUNE. We must also point out that the magnitude of difference we observed in titers comparing men and women may not be of consequence in conferring protection against smallpox. However, previous research suggests sex differences may influence protection from infection afforded by other vaccines and therefore sex should be considered in vaccine development.[8, 36, 38, 39] In addition, we point out that while our results are applicable to healthy vaccinia-naïve persons exposed to IMVAMUNE in the standard dose and formulation, and adhering to the standard regimen, this is in practice is unlikely to occur and IMVAMUNE may in the future be used in populations with different immunological characteristics.[40] It is not clear how or if sex will be important in determining immune response under these circumstances. Finally, our analysis included only studies from a single sponsor’s portfolio. While this provided certain advantages through access to participant level data, it is unclear whether our results would differ materially with the inclusion of data from other sponsors who have studied IMVAMUNE.
In summary, we report for the first time evidence of a sex difference in response to vaccination with IMVAMUNE, a novel smallpox vaccine, using a participant-level meta-analysis of completed randomized trials. Our results are consistent with a body of literature suggesting sex is a potentially important determinant of human immunity, and our work provides an impetus for considering sex in the development of future vaccines.
We studied sex differences in response to IMVAMUNE® using meta-analysis
We analyzed study-specific estimates and patient-level data from randomized trials
Men had consistently higher ELISA titers than women over time
The geometric mean peak titer in men was 27% higher than women
Sex should be considered in future testing of IMVAMUNE and other MVA-based vaccines
Acknowledgments
The authors acknowledge the efforts of the investigators who conducted the studies included in this meta-analysis. The expertise and diligence of these investigators yielded high-quality data from well-conducted randomized trials that made the present work possible. The authors extend their gratitude to: Patricia L. Winokur, MD (University of Iowa, Iowa City, IA), Robert B. Belshe, MD (Saint Louis University, Saint Louis, MO), Hana M. El Sahli, MD (Baylor College of Medicine, Houston, TX), Sri Edupuganti, MD (Emory University, Atlanta, GA), Lisa A. Jackson, MD, MPH (Group Health Research Institute, Seattle, WA), Jack Stapleton, MD (University of Iowa, Iowa City, IA), Samer S. El-Kamary, MB, ChB, MPH (University of Maryland, Baltimore, MD), Ana Wald, MD, MPH (University of Washington, Seattle, WA), Kathryn M. Edwards, MD (Vanderbilt University, Nashville, TN), Robert A. Salata, MD (Case Western Reserve University, Cleveland, OH), Christine B. Turley, MD (University of Texas Medical Branch, Galveston, TX), Emmanuel B. Walter Jr., MD (Duke University, Durham, NC), and Christine Mhorag Hay, MD (University of Rochester, Rochester, NY).
This work was supported by National Institutes of Health HHSN272201500002C and HHSN27220130021I.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jesse D. Troy, Email: jesse.troy@duke.edu.
Heather R. Hill, Email: hhill@emmes.com.
Marian G. Ewell, Email: mewell@emmes.com.
Sharon E. Frey, Email: freyse@slu.edu.
References
- 1.Nabel GJ. Designing tomorrow’s vaccines. N Engl J Med. 2013;368:551–60. doi: 10.1056/NEJMra1204186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Panhuis WG, Grefenstette J, Jung SY, Chok NS, Cross A, Eng H, et al. Contagious diseases in the United States from 1888 to the present. N Engl J Med. 2013;369:2152–8. doi: 10.1056/NEJMms1215400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauci AS, Morens DM. The perpetual challenge of infectious diseases. N Engl J Med. 2012;366:454–61. doi: 10.1056/NEJMra1108296. [DOI] [PubMed] [Google Scholar]
- 4.Rinaudo CD, Telford JL, Rappuoli R, Seib KL. Vaccinology in the genome era. The Journal of clinical investigation. 2009;119:2515–25. doi: 10.1172/JCI38330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana JM, Alexander E, Salvatore M. Translational research in infectious disease: current paradigms and challenges ahead. Translational research : the journal of laboratory and clinical medicine. 2012;159:430–53. doi: 10.1016/j.trsl.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poland GA, Ovsyannikova IG, Jacobson RM. Personalized vaccines: the emerging field of vaccinomics. Expert opinion on biological therapy. 2008;8:1659–67. doi: 10.1517/14712598.8.11.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mentzer AJ, O’Connor D, Pollard AJ, Hill AV. Searching for the human genetic factors standing in the way of universally effective vaccines. Philosophical transactions of the Royal Society of London Series B. Biological sciences. 2015;370 doi: 10.1098/rstb.2014.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26:3551–5. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy RB, Ovsyannikova IG, Pankratz VS, Vierkant RA, Jacobson RM, Ryan MA, et al. Gender effects on humoral immune responses to smallpox vaccine. Vaccine. 2009;27:3319–23. doi: 10.1016/j.vaccine.2009.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haralambieva IH, Ovsyannikova IG, Kennedy RB, Larrabee BR, Shane Pankratz V, Poland GA. Race and sex-based differences in cytokine immune responses to smallpox vaccine in healthy individuals. Human immunology. 2013;74:1263–6. doi: 10.1016/j.humimm.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell PK. Vaccines in civilian defense against bioterrorism. Emerging infectious diseases. 1999;5:531–3. doi: 10.3201/eid0504.990413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shchelkunov SN. Emergence and reemergence of smallpox: the need for development of a new generation smallpox vaccine. Vaccine. 2011;29(Suppl 4):D49–53. doi: 10.1016/j.vaccine.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Poland GA, Grabenstein JD, Neff JM. The US smallpox vaccination program: a review of a large modern era smallpox vaccination implementation program. Vaccine. 2005;23:2078–81. doi: 10.1016/j.vaccine.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Petersen BW, Damon IK, Pertowski CA, Meaney-Delman D, Guarnizo JT, Beigi RH, et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2015;64:1–26. [PubMed] [Google Scholar]
- 15.Kennedy JS, Greenberg RN. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert review of vaccines. 2009;8:13–24. doi: 10.1586/14760584.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imvanex: Modified vaccinia Ankara virus. European Medicines Agency; London, UK: [Accessed: May 19, 2015]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002596/human_med_001666.jsp&mid=WC0b01ac058001d124. [Google Scholar]
- 17.Products for Human Use. Submission #144762. Register for Innovative Drugs. [Accessed: May 19, 2015];Health Canada. http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/regist/reg_innov_dr-eng.php.
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151:264–9. w64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 20.Frey SE, Winokur PL, Salata RA, El-Kamary SS, Turley CB, Walter EB, Jr, et al. Safety and immunogenicity of IMVAMUNE(R) smallpox vaccine using different strategies for a post event scenario. Vaccine. 2013;31:3025–33. doi: 10.1016/j.vaccine.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Frey SE, Winokur PL, Hill H, Goll JB, Chaplin P, Belshe RB. Phase II randomized, double-blinded comparison of a single high dose (5×10(8) TCID50) of modified vaccinia Ankara compared to a standard dose (1×10(8) TCID50) in healthy vaccinia-naive individuals. Vaccine. 2014;32:2732–9. doi: 10.1016/j.vaccine.2014.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Krempelhuber A, Vollmar J, Pokorny R, Rapp P, Wulff N, Petzold B, et al. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine. 2010;28:1209–16. doi: 10.1016/j.vaccine.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack TM, Noble J, Jr, Thomas DB. A prospective study of serum antibody and protection against smallpox. The American journal of tropical medicine and hygiene. 1972;21:214–8. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar JK, Mitra AC, Mukherjee MK. The minimum protective level of antibodies in smallpox. Bulletin of the World Health Organization. 1975;52:307–11. [PMC free article] [PubMed] [Google Scholar]
- 26.Taub DD, Ershler WB, Janowski M, Artz A, Key ML, McKelvey J, et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. The American journal of medicine. 2008;121:1058–64. doi: 10.1016/j.amjmed.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Yeo SG, Park KH, Bang JW, Kim HB, Kim NJ, et al. The persistence of humoral and cellular immunities more than three decades after smallpox vaccination. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2007;13:91–3. doi: 10.1111/j.1469-0691.2006.01576.x. [DOI] [PubMed] [Google Scholar]
- 28.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 29.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 30.el-Ad B, Roth Y, Winder A, Tochner Z, Lublin-Tennenbaum T, Katz E, et al. The persistence of neutralizing antibodies after revaccination against smallpox. J Infect Dis. 1990;161:446–8. doi: 10.1093/infdis/161.3.446. [DOI] [PubMed] [Google Scholar]
- 31.Gallwitz S, Schutzbank T, Heberling RL, Kalter SS, Galpin JE. Smallpox: residual antibody after vaccination. Journal of clinical microbiology. 2003;41:4068–70. doi: 10.1128/JCM.41.9.4068-4070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatakeyama S, Moriya K, Saijo M, Morisawa Y, Kurane I, Koike K, et al. Persisting humoral antiviral immunity within the Japanese population after the discontinuation in 1976 of routine smallpox vaccinations. Clinical and diagnostic laboratory immunology. 2005;12:520–4. doi: 10.1128/CDLI.12.4.520-524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putz MM, Alberini I, Midgley CM, Manini I, Montomoli E, Smith GL. Prevalence of antibodies to Vaccinia virus after smallpox vaccination in Italy. The Journal of general virology. 2005;86:2955–60. doi: 10.1099/vir.0.81265-0. [DOI] [PubMed] [Google Scholar]
- 34.Toft L, Storgaard M, Muller M, Sehr P, Bonde J, Tolstrup M, et al. Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind clinical trial. J Infect Dis. 2014;209:1165–73. doi: 10.1093/infdis/jit657. [DOI] [PubMed] [Google Scholar]
- 35.Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2015;109:9–15. doi: 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. The Lancet infectious diseases. 2010;10:338–49. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu FX, Abel K, Ma Z, Rourke T, Lu D, Torten J, et al. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clinical and experimental immunology. 2002;128:10–20. doi: 10.1046/j.1365-2249.2002.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein SL, Poland GA. Personalized vaccinology: one size and dose might not fit both sexes. Vaccine. 2013;31:2599–600. doi: 10.1016/j.vaccine.2013.02.070. [DOI] [PubMed] [Google Scholar]
- 39.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. Journal of autoimmunity. 2012;38:J282–91. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Walsh SR, Wilck MB, Dominguez DJ, Zablowsky E, Bajimaya S, Gagne LS, et al. Safety and immunogenicity of modified vaccinia Ankara in hematopoietic stem cell transplant recipients: a randomized, controlled trial. J Infect Dis. 2013;207:1888–97. doi: 10.1093/infdis/jit105. [DOI] [PMC free article] [PubMed] [Google Scholar]