Abstract
Mechanotransduction, the conversion of physical forces into biochemical signals, is an essential component of numerous physiological processes including not only conscious senses of touch and hearing, but also unconscious senses such as blood pressure regulation. Mechanically activated (MA) ion channels have been proposed as sensors of physical force, but the identity of these channels and an understanding of how mechanical force is transduced has remained elusive. A number of recent studies on previously known ion channels along with the identification of novel MA ion channels have greatly transformed our understanding of touch and hearing in both vertebrates and invertebrates. Here, we present an updated review of eukaryotic ion channel families that have been implicated in mechanotransduction processes and evaluate the qualifications of the candidate genes according to specified criteria. We then discuss the proposed gating models for MA ion channels and highlight recent structural studies of mechanosensitive potassium channels.
Introduction
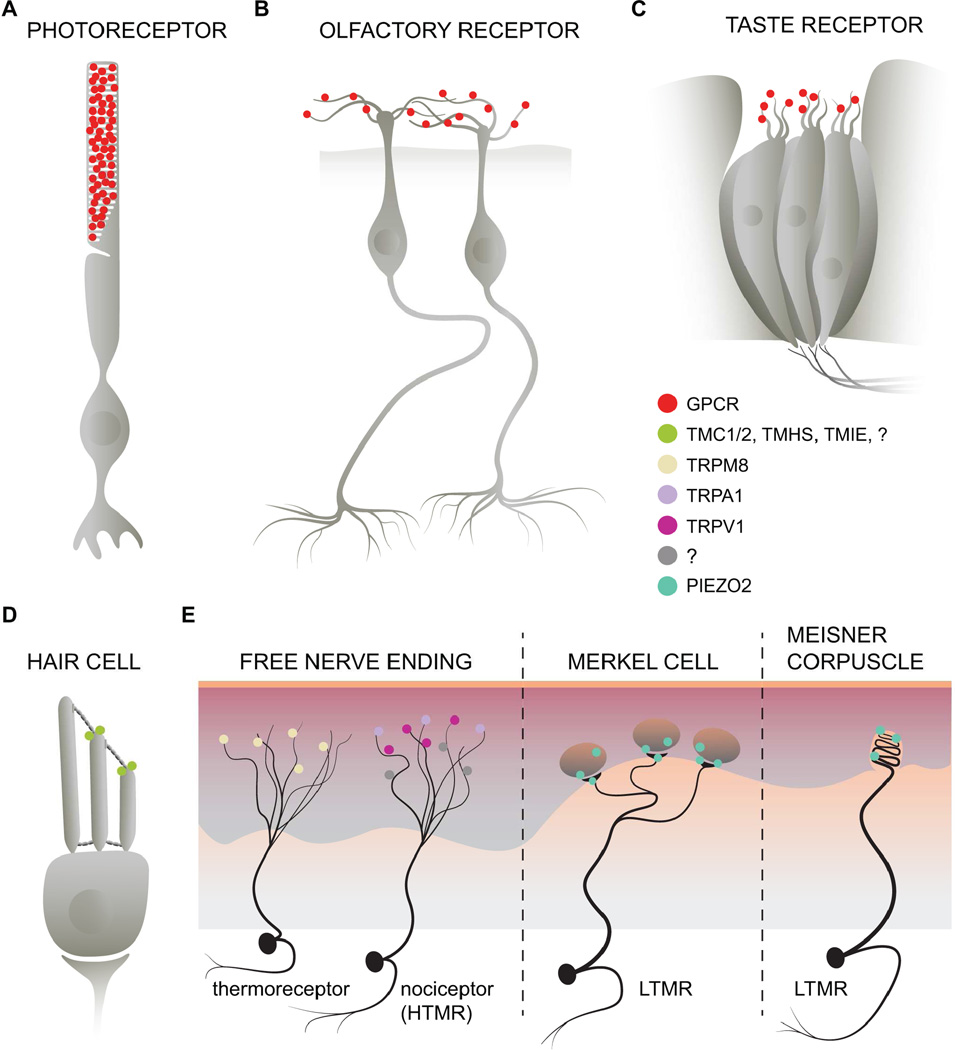
Our sensory system allows us to perceive the external world. Each of the five senses contains a unique receptor cell in which integral membrane proteins, such as G Protein-Coupled Receptors (GPCRs) or ion channels, convert external stimuli into electrical signals that are relayed to our brain. For example, rod cells in the eye express high levels of rhodopsin, a GPCR conjugated to a chromophore and activated by photons of light (Figure 1A). Olfactory epithelial cells contain a diverse array of GPCRs that are activated by volatile small molecules in the air and allow us to sense smells (Figure 1B). Similarly, GPCRs expressed in taste receptor cells, are activated by chemicals in the food that we eat (Figure 1C). Unlike these three senses, our ability to feel touch and hear sounds comes from the activation of ion channels that respond to mechanical forces such as vibration, indentation, gravity and sound waves (Figures 1D and 1E). Epithelial hair cells contain a stereocilia bundle that are inter-connected by tip links. The mechanical force imparted by sound waves bends the stereocilia and pulls on the tip links. The strain induced by tip link pulling is thought to open a mechanically activated ion channel complex (Figure 1D). The peripheral endings of somatosensory neurons consist of either free nerve terminals thought to detect noxious stimuli or specialized terminals that detect innocuous physical stimuli (Figure 1E).
Figure 1. Sensory Receptors.
(A) The high levels of rhodopsin expressed in rod cells allowed for biochemical isolation and characterization before the advent of many molecular biology cloning techniques. (B and C) Olfactory and Taste receptor cells are also activated by the binding of small molecule ligands to GPCRs. (D and E) Somatosensory neurons detect a wide array of mechanical and thermal inputs. Un-myelinated DRG neurons with free nerve endings embedded in the skin express ion channels such as TRPM8 for cool, innocuous temperature sensing. Some DRG neurons, classified as high threshold mechanoreceptors (HTMR), express TRPV1 and TRPA1 and detect noxious temperature or mechanical forces. At present, the sensor for noxious mechanical force is unknown (illustrated as a “?” in the figure legend). Light touch is mediated by activation of low threshold mechanoreceptors (LTMR) such as the Merkel cell-neurite complex or the Meissner corpuscle. Piezo2 has been shown to play a major role for light touch sensation in mice (Ranade et al., 2014b; Woo et al., 2014a).
Fundamental insight into the function of the sensory system has been gained by the identification and characterization of the receptor genes for each system. Partly because it is expressed in high enough levels to facilitate biochemical analysis, rhodopsin was the first sensory receptor gene to be identified and sequenced (Hargrave et al., 1983; Nathans and Hogness, 1983). Advances in molecular cloning techniques and new strategies to identify genes based on sequence similarities to known GPCRs led to the landmark discovery of a multi-gene GPCR family of olfactory receptors, as well as the identification of receptors for taste (Buck and Axel, 1991; Hoon et al., 1999). Electrophysiology recordings of auditory receptor cells were the first experiments to suggest the existence of ion channels that could be directly activated by mechanical force (Corey and Hudspeth, 1979). Stretch activated cation channels were then recorded in other non-sensory tissues, such as skeletal muscle cells (Guharay and Sachs, 1984).
Unlike the structural similarities of GPCRs, ion channels vary greatly in sequence and function. Indeed, the only common structural feature of ion channels is that they all contain a minimum of two transmembrane domains. Whereas rhodopsin is highly enriched in rod cells, mechanosensitive ion channels are usually expressed at low levels in endogenous cells and may be associated in complexes with auxiliary proteins (Arnadottir and Chalfie, 2010). These limitations have delayed the identification of mechanically activated ion channels compared to other receptors. Indeed, many of the candidate mechanosensitive channels in vertebrates and invertebrates were ultimately identified by genetic or genomic screens, and not via biochemical or homology approaches.
It has previously been suggested that certain qualifications must be met in order for a candidate ion channel to be considered a transducer of mechanical force (Arnadottir and Chalfie, 2010; Christensen and Corey, 2007). The candidate gene must be expressed in a mechanosensitive cell and loss of that gene should abolish mechanosensitivity without affecting the development of that cell. Ideally, expression of the candidate gene should be sufficient to confer mechanosensitivity to a naïve cell and deletion of the gene in a model organism should lead to deficits in mechanotransduction processes in vivo. Further characterization includes validation that the candidate gene encodes for the pore forming subunit, either by engineering point mutations that alter ion selectivity and conductance or by structural analysis. If auxiliary subunits are not involved in gating, the candidate ion channel should retain mechanosensitivity when purified and reconstituted into artificial lipid bilayers.
The identification of receptors remains a critical first step towards an understanding of mechanotransduction processes in vivo. Indeed, the characterization of temperature activated Transient Receptor Potential ion channels has greatly advanced our understanding of thermosensation in both vertebrates and invertebrates (Caterina et al., 1997; Peier et al., 2002). Only a few candidate mechanosensitive ion channels have been discovered that meet all of the prerequisite qualifications. However, over the past few years, a number of advances have been made through the identification of new ion channels, such as the Piezo family, as well as the elucidation of high-resolution crystal structures of mechanosensitive potassium channels.
In this review, we summarize research into eukaryotic ion channel families that contain candidate genes implicated as mechanically activated ion channels. While we focus primarily on the sensory systems of touch and hearing, it is important to note that mechanotransduction processes extend to numerous physiological systems including cardiovascular, pulmonary, vestibular, baroreceptor reflex etc. (Teng et al., 2015). Mechanosensitive ion channels are expressed in nearly all cell types, including commonly studied heterologous expression systems like the Neuro2A cell line from which Piezo1 was identified (Coste et al., 2010). Although we describe eukaryotic channels in greater detail, we also present an analysis of studies from bacterial MscL and MscS ion channels in the context of two general models for how physical forces can gate mechanically activated ion channels. Recent studies of K2P potassium channels have shown that the principles established for gating prokaryotic mechanosensitive ion channels are more similar to eukaryotic channels than previously appreciated.
Assays of Mechanotransduction
The development of assays to probe the function of mechanically activated ion channels has played an important role in the identification and characterization of these elusive ion channels. Mechanically activated ion channels can respond to a diverse range of physical forces such as vibration, stretch, or sound waves (Delmas et al., 2011). Numerous in vitro and ex vivo assays have been developed to apply various forms of mechanical force either to isolated cells or to intact tissue preparations. Ion channel activation is usually recorded by directly measuring ion flow across the lipid bilayer using patch clamp electrophysiology of isolated cells or by recording action potential firing activity from nerve fibers in intact tissue preparations (Reeh, 1986). Along with electrophysiology techniques, calcium sensitive fluorescent small molecules such as Fura-2 or genetically encoded calcium indicator (GECI) proteins have also been used to measure the activity of mechanically activated cation channels (Tian et al., 2009; Tsien, 1989).
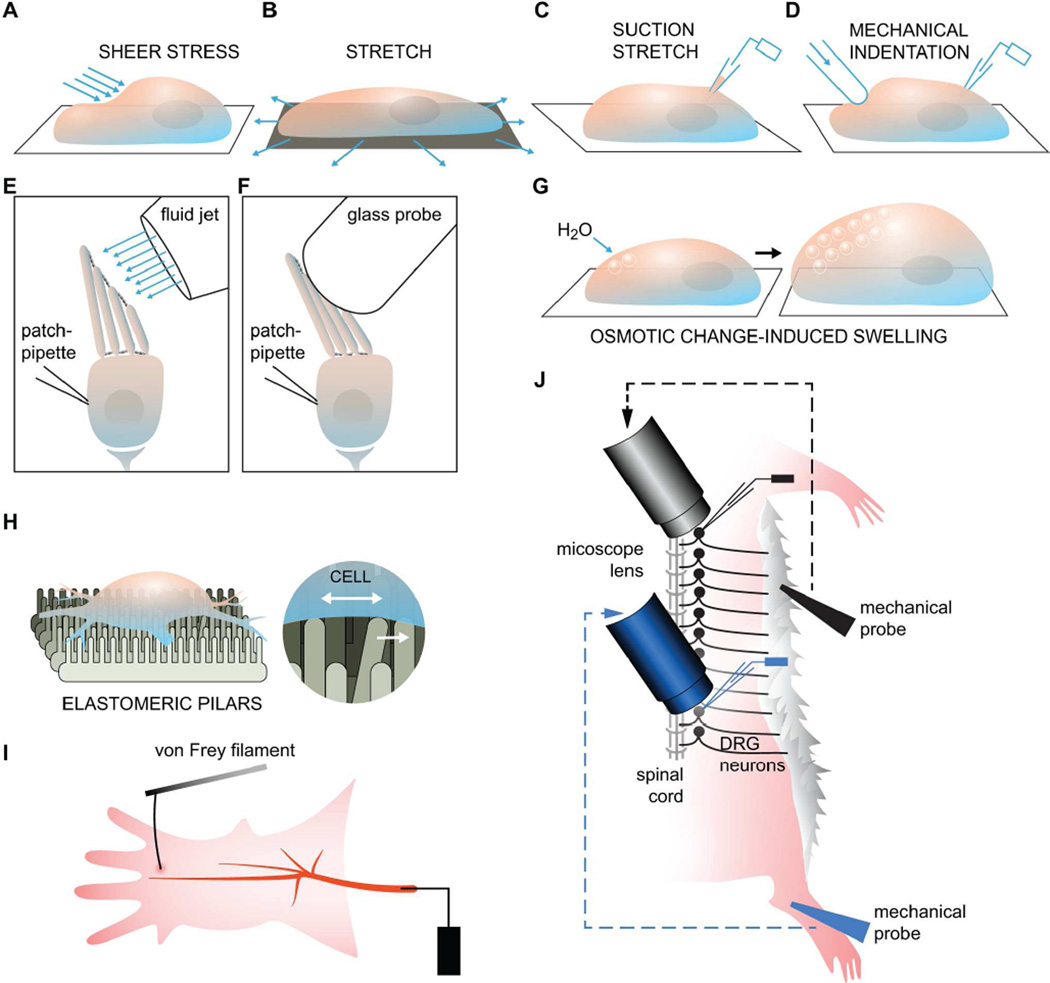
The application of mechanical force to isolated cells is achieved primarily by fluid shear stress, membrane stretch or membrane indentation with a glass pipette (Figures 2A–2D). Membrane stretch can also be applied to purified, lipid-solubilized ion channels reconstituted into proteoliposomes (Brohawn et al., 2014b). Each of these potentially distinct mechanical stimuli is thought to be relevant for specific cell types. For example, endothelial cells experience shear stress; muscles cells, stretch; somatosensory neurons, membrane indentation. Both fluid shear stress and membrane indentation have also been used to apply mechanical force to the stereocilia bundle of isolated epithelial hair cells (Figures 2E and 2F) (Fettiplace and Kim, 2014). Whereas mechanical indentation is used primarily to deflect the stereocilia bundle in one direction, fluid shear stress can deflect the stereocilia bundle in both forward and reverse directions (Kim et al., 2013). Interestingly, changes to osmotic potentials can induce cell swelling and can also exert mechanical strain onto the cell membrane (Figure 2G). However, cellular swelling due to changes in osmotic potentials is slower and less uniform compared to other mechanical forces such as stretch or membrane indentation, and likely involves numerous compensatory cytoskeletal changes (Sachs, 2010). Swelling can also cause activation of ion channels via indirect mechanisms including reduction of ionic strength. Therefore, cell swelling alone cannot be used as evidence of direct mechanosensitivity.
Figure 2. Mechanotransduction Assays.
(A) To generate shear stress, a perfusion tube with a small opening at the tip is placed near the cell and bath solution is then injected onto the cell membrane (McCarter et al., 1999; Olesen et al., 1988). (B and C) Membrane stretch can either be applied generally to the entire basal side of a cell or a liposome that has been seeded onto a flexible material, or, more locally, to a small patch of membrane using a glass pipette to generate a protrusion or “bleb” (Bhattacharya et al., 2008; Maingret et al., 1999a; Sukharev and Sachs, 2012). (D) Physical indentation, or poking of the cell membrane or a lipid bilayer by a glass pipette controlled by a piezoelectric device, can also be used to apply force onto a local region of the membrane (McCarter et al., 1999). (E and F) Isolated epithelial hair cells can be stimulated with either a glass probe or fluid shear stress. Both methods serve to bend the stereocilia but only the shear stress method can be used for applying force in both positive and negative directions. (G) Changes to the osmotic potential can cause an influx of water and lead to cell swelling. (H) New assays such as the elastomeric pillars have been developed to more precisely deliver mechanical strain and can be precise enough to apply force to specific parts of a cell, such as the nerve endings. (I) The classic setup for the saphenous nerve preparation used to record action potential firing upon stimulation with a von Frey filament. (J) A variant form of the skin-nerve preparation that can be used to monitor the activity of DRG neurons along the entire spinal cord.
New methods have been developed to apply mechanical force to specific locations of a cell, including the nerve terminals, or neurites, where mechanically activated ion channels are thought to be functioning (Poole et al., 2015). Poole et al. have developed an elastomeric pillar array where each pilus is controlled by a piezoelectric device and can be deflected to apply local mechanical indentation (Figure 2H). Using this system, the authors were able to show distinct populations of mechanically activated currents in Dorsal Root Ganglia (DRG) neurons by substrate deflection at both the soma and the neurite (Poole et al., 2014).
Along with studies of isolated cells, ex vivo preparations have also been used to study the function of mechanically activated somatosensory neurons. The primary advantage of this setup is the ability to keep intact the native organization of mechanoreceptors that innervate the skin. The skin-nerve preparation technique is used to record action potentials from the saphenous nerve upon mechanical indentation of the associated skin layer (Figure 2I) (Zimmermann et al., 2009). A variation of this technique is to record directly from DRG neuron cell bodies instead of the saphenous nerve (Figure 2J) (Koerber and Woodbury, 2002; Li et al., 2011).
Although distinct forms of mechanical force such as shear stress and membrane stretch have been described to be relevant to specific cell types, it is important to note that mechanically activated ion channels can often be activated by multiple types of physical forces. While it has been shown that certain cell types are tuned to respond preferentially to one form of mechanical force, the commonality to all of the mechanical forces described above is the perturbation of the membrane lipid bilayer (Arnadottir and Chalfie, 2010; Hoffman et al., 2011; Kung, 2005). Later in this review, we discuss the two leading proposed models for transmission of physical forces to mechanically activated ion channels.
Candidate Gene Families
DEG/ENaC/ASIC Ion Channels
Sodium ion channels expressed in somatosensory neurons of C. elegans were among the first identified eukaryotic mechanically activated ion channels (Figure 3A) (Arnadottir and Chalfie, 2010; Chalfie, 2009). The Degenerin (DEG) genes were so named because mutations that caused constitutively active sodium currents led to the degeneration of mechanosensitive neurons and rendered worms incapable of escaping from a physical poking stimulus (Schafer, 2015). Among the DEG genes, MEC-4 and MEC-10 were shown to be pore-forming subunits of an ion channel complex required for the activity of the gentle touch receptor neurons ALM and PLM (O'Hagan et al., 2005). Loss of MEC-4 or MEC-10 ablates the mechanosensitivity of PLM neurons in vivo and missense mutations to Mec-10 alters the ion conductivity of these cells upon mechanical stimulation (O'Hagan et al., 2005). However, neither MEC-4 nor MEC-10 is sufficient to confer mechanically activated currents in heterologous expression systems. When a constitutively active (degenerin) mutant form of MEC-4 or MEC-10 was expressed in Xenopus oocytes, an amiloride-sensitive sodium current was recorded, and the current amplitude was dramatically increased when these proteins were co-expressed with MEC-2, a stomatin-related integral membrane protein, and MEC-6, a paraoxonase-related integral membrane protein (Chelur et al., 2002; Goodman et al., 2002). Importantly, biochemical evidence for the existence of a multi-subunit complex has not yet been established.
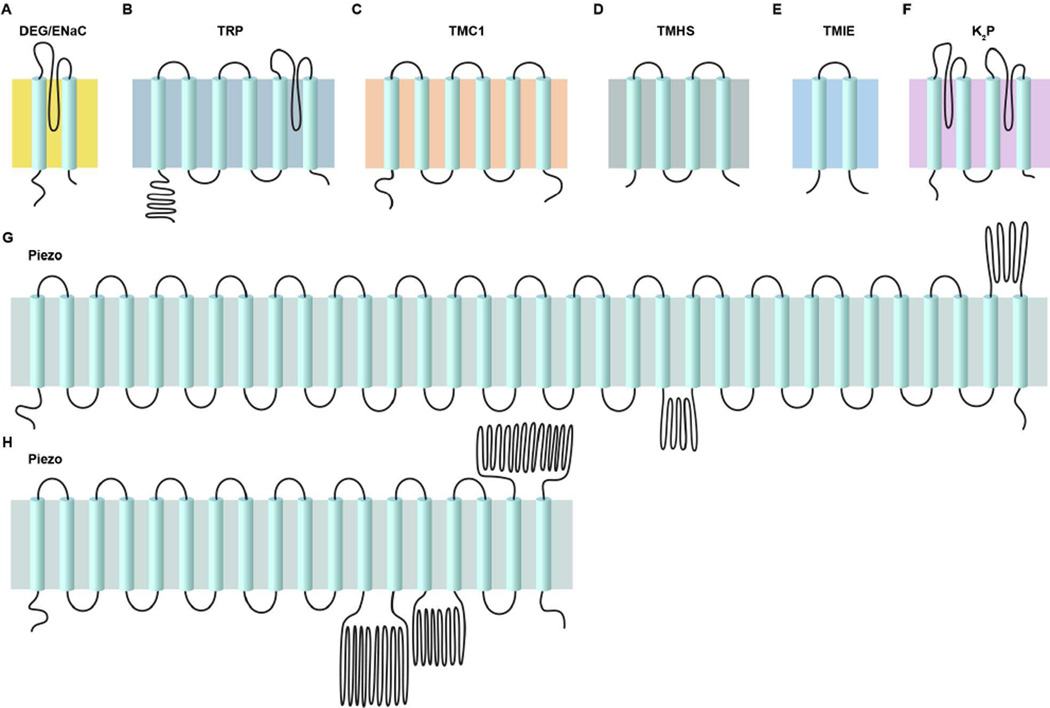
Figure 3. Eukaryotic Ion Channel Subunits.
The topology of ion channel subunits implicated in eukaryotic mechanotransduction illustrates the vast diversity compared to other sensory receptors. (A) DEG/ENaC proteins have 2 TM domains and associate as trimeric complexes. (B) The general structure of many TRP channels is a tetrameric complex where each subunit contains 4 TM domains and a pore loop domain between TM5 and TM6. An extraordinary amount of structural diversity exists between TRP channel members, particularly within the structure of the N and C terminal soluble domains of the proteins. (C – E) At present, four proteins (TMC1/2, TMIE and TMHS) have been shown to be necessary for mechanotransducer currents in hair cells. The exact composition of the various subunits to the channel complex and whether this composition varies along the tonotopic axis is unknown. (F) K2P channels are unique among other ion channel complexes in that each subunit contains 2 poor loop helices and the formation of a functional pore requires an association of dimers. (G and H) Biochemical and computational evidence suggest that Piezo ion channels contain at least 18 TM domains and potentially up to 38 TM domains.
Two other DEG genes, DEGT-1 and DEG-1, have been implicated as mechanotransducers in C. elegans. DEGT-1, along with MEC-10, are necessary for mechanically induced calcium transients in the nociceptive PVD neuron (Chatzigeorgiou et al., 2010). DEGT-1 and MEC-10 co-localize in dendritic puncta of PVD neurons and loss of MEC-10 in PVD neurons abolishes the escape response of worms to a physical poking stimulus. Whereas the PVD neurons sense noxious mechanical forces applied to the body of the worm, the ASH neuron is required for sensing nociceptive signals at the nose. In ASH neurons, DEG-1 is required for the majority (∼80%), but not entirety, of the mechanically induced currents (Geffeney et al., 2011). Mutations to the putative pore domain of DEG-1 alter the ion selectivity, arguing that DEG-1 is the pore forming subunit of the mechanically activated channel in these cells.
While DEG genes have been well characterized in C. elegans, a role for the evolutionarily conserved orthologs in mechanotransduction in Drosophila or mammals is less clear. Two DEG/ENaC (Epithelial Sodium Channels) genes, Pickpocket and Balboa, are necessary for mechanical activation of class IV dendritic arborization neurons in Drosophila larvae (Mauthner et al., 2014; Zhong et al., 2010). However, similar to MEC-4 and MEC-10, neither gene is sufficient to generate mechanically induced currents in heterologous expression systems (Mauthner et al., 2014). In mammals, the evolutionarily related Acid Sensing Ion Channel genes (ASIC1–3) are expressed in DRG neurons but do not contribute to mechanosensitivity of these cells (Drew et al., 2004; Page et al., 2004). ASIC genes are also expressed in nodose ganglia neurons that project to the aortic arch and the carotid sinus arteries and express mechanoreceptors that detect changes in blood pressure in a process termed the baroreceptor reflex (Lu et al., 2009). ASIC2−/− mice displayed elevated basal arterial pressure and heart rates, and showed signs of Dysautonomia, a broad term referring here to an impaired ability to regulate arterial function upon acute changes. Isolated nodose ganglia neurons from ASIC2−/− mice showed slightly decreased mechanically induced depolarization compared to wild type controls, suggesting a role for ASIC2 in modulating the mechanosensitivity of these cells.
Interestingly, the stomatin-like gene, STOML3 is evolutionarily related to MEC-2 and modulates the activity of mechanosensitive DRG neurons (Wetzel et al., 2007). Loss of STOML3 increased the number of mechanically insensitive DRG nerve fibers and led to deficits in the ability of mice to sense a sandpaper-like textured surface. STOML3 can modulate the sensitivity of both Piezo1 and Piezo2 in vitro by decreasing the mechanical threshold needed for activation of these channels (Poole et al., 2014).
TRP Ion Channels
Transient Receptor Potential (TRP) ion channels are an evolutionarily conserved family of genes that are essential to a wide range of sensory functions (Dhaka et al., 2006; Venkatachalam and Montell, 2007). TRP channels can be generally classified into seven categories based on sequence homology: TRPA, TRPC, TRPM, TRPML, TRPN, TRPP and TRPV (Christensen and Corey, 2007). TRP channels are non-selective cation channels that associate as tetramers where each monomer contains 6 transmembrane (TM) domains and a pore loop domain between TM5 and TM6 (Figure 3B) (Cao et al., 2013; Liao et al., 2013). TRP channels are polymodal in that they are activated by numerous stimuli including voltage, temperature, and small molecules and a number of TRP channel members have been implicated as candidate mechanotransducers in flies, worms and mammals (Arnadottir and Chalfie, 2010; Delmas and Coste, 2013).
Research in invertebrate model organisms has elegantly demonstrated the function of TRP channels, and in particular TRPN orthologs, in touch and hearing. In Drosophila larvae, the TRPN ortholog, NOMPC, is expressed in Class III dendritic arborization neurons and is necessary for the response of larvae to light touch stimuli (Walker et al., 2000; Yan et al., 2013). NOMPC is sufficient to confer mechanically activated currents when mis-expressed in class IV neurons and when heterologously expressed in the Drosophila S2 cell line. Furthermore, mutations in the putative pore domain alter the ion selectivity (Yan et al., 2013). These qualities establish NOMPC as a mechanically activated ion channel required for Drosophila touch sensation. NOMPC also plays a critical role in auditory mechanotransduction in Drosophila Johnston's organ, although there are conflicting reports about whether NOMPC or two TRPV proteins, Nanchung and Inactive, are required for the receptor currents (Effertz et al., 2012; Lehnert et al., 2013).
In C. elegans, a TRPN ortholog, TRP-4, is expressed in the cilium of the Cephalic neuron (CEP) that is responsible for basal slowing responses when activated by light touch stimuli (Kang et al., 2010; Li et al., 2006). Deletion of TRP-4 abolishes mechanically activated currents in CEP neurons and leads to deficits in the behavioral slowing response. Overexpression of TRP-4 confers mechanically activated currents and rescues the behavioral response to touch, arguing that TRP-4 is both necessary and sufficient for this response. Similar to the experiments in Drosophila NOMPC, mutations to the pore domain alters the ion selectivity of the mechanically activated current in CEP neurons (Kang et al., 2010). These data establish TRPN orthologs in both flies and worms as bona fide sensors of mechanical force. Similar to TRPN, the TRPV genes, nanchung and inactive, as well as the TRPA ortholog, painless, are necessary for hearing and touch responses in flies, respectively, while the C. elegans TRPA-1 ortholog is involved in somatosensation (Gong et al., 2004; Kim et al., 2003; Kindt et al., 2007; Tracey et al., 2003).
In mammals, however, a TRPN ortholog does not exist and the importance of vertebrate TRP channels in mechanotransduction remains less clear than for the invertebrate orthologs. For example, TRPV4 expressed in HEK293 cells did not respond to membrane stretch but others have reported that TRPV4 expressed in Xenopus oocytes or endogenous TRPV4 in urothelial cells can be activated by stretch (Loukin et al., 2010; Mochizuki et al., 2009; Strotmann et al., 2000). TRPV4 can also be activated indirectly by osmotic force induced cell swelling and fluid shear stress, but TRPV4−/− mice have only slight deficits in response thresholds to a tail pinch stimulus (Liedtke et al., 2000; Suzuki et al., 2003; Vriens et al., 2004). These results available to date suggest that TRPV4 plays a minor role in mechanotransduction processes in vivo. TRPA1 has been definitively shown not to be required for hearing in mammals (Bautista et al., 2006; Kwan et al., 2006). However, some groups have reported a potential role for TRPA1 in mediating mechanically activated currents in a subset of DRG neurons (Kwan et al., 2009; Vilceanu and Stucky, 2010). Chemical inhibition of TRPA1 evoked subtle behavioral deficits, suggesting that TRPA1 may play a modulatory role in noxious mechanosensitive DRG neurons (Lennertz et al., 2012; Petrus et al., 2007).
Beyond the sensory systems, TRP channels have also been implicated in cilia mechanotransduction. Primary cilia have been considered as shear stress sensors in renal epithelial cells as well as in endothelial cells (AbouAlaiwi et al., 2009; Nauli et al., 2003). Fluid shear stress applied to cultured Madin-Darby Canine Kidney (MDCK) cells was shown to bend the primary cilium and correlated with an increase in cytoplasmic calcium concentration (Praetorius and Spring, 2001; Schwartz et al., 1997). Two genes, PKD1 and PKD2 (or TRPP2), were localized to the cilia of mouse kidney epithelial cells and calcium imaging revealed that cells isolated from PKD1−/− mice were no longer responsive to shear stress (Nauli et al., 2003; Pazour et al., 2002; Yoder et al., 2002). These results established a model that fluid shear stress activates the PKD1/PKD2 complex, leading to calcium signaling through the IP3 pathways as well as release of calcium from ER stores through the ryanodine receptor (Nauli et al., 2003). Deficits to the function of these pathways would then presumably lead to the cyst formations found in diseases such as autosomal dominant polycystic kidney disease (ADPKD).
Recent studies have begun to highlight complications associated with this model. Importantly, the primary cilium is relatively small in proportion to the entire cell (less than 1 um in width and a ∼10 um in length) and neither PKD1 nor PKD2 have been shown to be mechanically activated in heterologous expression systems (DeCaen et al., 2013; Delling et al., 2013; Peyronnet et al., 2012). Application of fluid shear stress to MDCK cells leads to ATP release and calcium influx into the cytoplasm via activation of P2XR and P2YR (Rodat-Despoix et al., 2013). In this study, the authors showed that calcium influx upon shear stress occurred regardless of the presence of cilia, arguing for an indirect role of cilia in mechanotransduction processes.
Advances have been made by multiple groups using Genetically Encoded Calcium Indicators (GECI) to specifically label the primary cilium and record calcium transients in the cilia distinct from the calcium influx into the cytoplasm. Using a cilia targeting G-GECO1.0 construct expressed in ciliated mouse inner medullary collecting duct (mIMCD3) cells, Su et al. could visualize shear stress mediated calcium influx in the primary cilium, however these responses started only at 15 seconds after the onset of flow (Su et al., 2013). This slow latency for response complicates the interpretation of whether cilia are direct sensors of shear stress.
Delling and DeCaen et al. expanded on the use of GECI methods to label cilia and used patch clamp electrophysiology to directly record a non-selective cation channel current, termed Icilia (DeCaen et al., 2013; Delling et al., 2013). In a human retina pigmented epithelial cell line (hRPE), direct measurements of cilia using whole cell patch clamp electrophysiology or single channel recordings from detached cilia revealed calcium currents that were also present in primary RPE cells, kidney IMCD cells and embryonic fibroblasts (MEFs). While many putative ciliary ion channels were expressed in hRPE cells, siRNA knockdown of only PKD1L1 and PKD2L1 ablated the Icilia current. Biochemical analysis showed that PKD1L1 and PKD2L1 physically interact and, while PKD2L1 can form a homomeric channel, the endogenous Icilia current is likely mediated by a heteromeric channel of PKD1L1 and PKD2L1. Interestingly, the authors showed that this heteromeric channel is only weakly activated by membrane stretch and temperature and propose that this weak activation is negligible to the cytoplasmic calcium concentration as a whole. Delling et al. further found that ciliary calcium concentrations are much higher relative to the cytoplasmic calcium concentration. PKD1L1 had been shown to have a ciliary phenotype in left/right symmetry, and the authors now showed that loss of PKD2L1 led to subtle malrotation defects in the intestines through alteration in sonic hedgehog (shh) signaling.
Overall, the data from direct recording of cilia indicate that the main function of the cilia is likely as a calcium-rich organelle critical for signaling pathways. While Delling and DeCaen showed that PKD1 and PKD2 are not required for Icilia currents in hRPE cells, the function of these genes in kidney epithelial cells under shear stress (and the implication to the mechanism of ADPKD cyst formation) is still not known. Although PKD1 and PKD2 are not mechanically activated in heterologous expression systems, their main role could be in modification or tuning of other mechanosensitive ion channels such as TREK/TRAAK and Piezos. In smooth muscle cells, the heteromeric PKD1/PKD2 complex can regulate the activity of a stretch activated cation channel through an interaction with the actin cytoskeleton (Sharif-Naeini et al., 2009). In renal epithelial cells, PKD1 and PKD2 interact with the stretch activated potassium channels TREK-1 and TRAAK to protect against mechanical strain induced apoptosis (Peyronnet et al., 2012). In these cells, PKD1/PKD2 can also modulate the stretch induced activation of Piezo1 (Peyronnet et al., 2013). This “mechanoprotection” role argues that the importance of PKD1 and PKD2 activation upon mechanical stimulation may be as modulators of other mechanically activated ion channels.
TMC/TMHS/TMIE (transduction channel in hair cells)
The sense of hearing is mediated by an extraordinarily intricate process in which hair cells convert mechanical forces from sound waves into electrical signals that are relayed to the brain (Gillespie and Muller, 2009; Hudspeth, 2014). All vertebrate hair cells contain stereocilia bundles on their apical surface that are arranged in rows of increasing height and are connected to each other by small strands called tip links, composed of Cadherin 23 (Cdh23) and Protocadherin 15 (Pcdh15) (Kazmierczak et al., 2007; Siemens et al., 2004). Hair cells are positioned in the cochlea along a tonotopic gradient where the frequency of the sound waves detected varies from the apex to the base (Beurg et al., 2006). Mechanical forces produced by sound waves deflect the hair cell stereocilia bundles and the physical strain induced by tip link movement is thought to gate a mechanically activated (or mechanotransducer, MT) ion channel located at the lower end of each tip link (Beurg et al., 2009). Tip links are thought to be essential for the gating of the ion channel and a number of genes have been described that are required for the proper connection of the tip link to the stereocilia at both the upper and lower ends (Assad et al., 1991; Fettiplace and Kim, 2014). Although a number of candidate genes have been suggested, the identity of the mammalian mechanotransducer channel complex remains incomplete. At present, four genes have been shown to be necessary components of the hair cell mechanotransducer complex: Transmembrane Channel Like proteins 1 and 2 (TMC1 and TMC2), Tetraspan Membrane Protein of Hair Cell Stereocilia (TMHS), and Transmembrane Inner Ear Expressed Gene (TMIE).
Mutations in the TMC1 gene were identified through genetic sequencing analysis of human patients with hearing disorders and mouse models for deafness that mapped to mutations in the TMC1 locus (Deol, 1958; Kurima et al., 2002; Vreugde et al., 2002). TMC genes are evolutionarily conserved and biochemical analysis of mouse TMC1 revealed a topology with 6 putative TM domains (Figure 3C) (Kawashima et al., 2015; Labay et al., 2010). Although this topology is generally similar to voltage gated potassium and TRP ion channels, a pore domain has not yet been identified. Expression of TMC1 and TMC2 RNA transcripts in the utricle and cochlea correlated with the onset of mechanotransduction in newborn mice (Kawashima et al., 2011). Interestingly, TMC2 but not TMC1 expression in the cochlea decreased after P5. Using null allele mouse lines, Kawashima et al. reported that TMC1 deficient mice were deaf whereas TMC2 -deficient mice did not have hearing defects. Mice deficient in either TMC1 or TMC2 alone did not display vestibular defects, however, mice deficient in both TMC1 and TMC2 were deaf and had characteristic vestibular defects such as circling, head-bobbing and ataxic gate. Electrophysiology recordings of the outer hair cells upon physical deflection of the sterocilia bundles showed that mechanically activated currents were not present in TMC1/2 DKO mice. These deficits occurred without gross alterations to hair bundle morphology in newborn pups and, in organotypic explants, mechanically activated currents in TMC1/2 DKO mice could be rescued by viral transfection of either TMC1 or TMC2 (Kawashima et al., 2011).
Further analysis showed that mechanotransducer currents were also abolished in the inner hair cells of TMC1/2 DKO mice (Pan et al., 2013). Pan et al. recorded single channel currents from inner hair cells of the Beethoven mouse model and found alterations in calcium ion permeation (Kurima et al., 2002; Vreugde et al., 2002). The Beethoven mouse model contains a Methionine to Lysine (M412K) point mutation in the TMC1 gene that the authors hypothesize lies within the pore domain and leads to the decreased calcium permeation. However, the pore domain of either TMC1 or TMC2 has not been experimentally identified and the only evidence for ion conductivity from any TMC proteins comes from the C. elegans ortholog, TMC-1 (Chatzigeorgiou et al., 2013). Heterologous expression of worm TMC-1 reportedly led to a sodium selective current upon stimulation of cells with 150 mM NaCl. These results have not yet been reproduced by other groups and mechanically induced currents have not been reported. Pan et al. also recorded a range of mechanotransducer channel properties in cells that express TMC1, TMC2 or a combination of both genes, and proposed that TMC oligomers could vary in composition along the tonotopic axis (Pan et al., 2013). One problem with this argument is that TMC-2 is not expressed in cochlear hair cells of adult mice, and thus a multimeric channel with different stoichiometries of TMC1 and TMC2 might only exist during a limited developmental timeframe. Because the mechanotransducer ion channel complex has been shown to reside in the lower end of the tip link where PCDH15 is localized, and because tip links are necessary for gating the mechanically activated channel complex, a physical association with PCDH15 should presumably be a requirement for any candidate gene. In line with this requirement, a recent report showed that mouse TMC1 can physically associate with PCDH15 (Beurg et al., 2014b; Maeda et al., 2014).
These data together argue that TMC1/TMC2 are expressed in mechanosensitive cells, are necessary components of the hair cell mechanotransducer complex and that TMC1 may potentially be the pore-forming subunit. However, TMC genes, like the MEC complexes in C. elegans, have not been shown to confer mechanically activated channel activity in naïve cells. This could be because heterologous expression of TMC genes lead to retention of the protein in the ER (Labay et al., 2010). Another complication for the role of TMC1/2 as the mechanotransducer channel arose from reports of an anomalous mechanically activated current from hair cells of TMC1/2 DKO mice. Using a fluid-jet shear stress mechanical stimulus capable of both pushing and pulling the stereocilia bundles, Kim et al. reported a mechanotransducer current that was present upon deflection of the stereocilia in the reverse direction (Kim et al., 2013). The conduction properties of the reversed polarity current was similar to the wild type mechanotransducer current and was blocked by FM1–43, dihydrostreptomycin, and extracellular Ca2+ (Beurg et al., 2014a; Kim et al., 2013). Interestingly, ablation of tip links also presented this similar reversed polarity current, and the authors argued that this activity is likely mediated by a mechanotransducer channel that does not associate with tip links. The reverse polarity MT currents in TMC1/2 double mutant mice were not affected by tip link breakage, suggesting a gating mechanism distinct from the wild type MT channel complex (Fettiplace and Kim, 2014; Kim et al., 2013). At the moment, the physiological relevance of this aberrant current is unclear and, notably, the reverse polarity current can only be recorded in atypical conditions, such as in mouse lines containing mutations in PCDH15 and CDH23 that affect tip link architecture (Alagramam et al., 2011). In light of these data, Fettiplace and colleagues suggest that TMC genes act as the vestibule in a complex with the pore-forming subunit of an unidentified mechanotransducer channel.
The hypothesis that the mechanotransducer channel exists as a complex with multiple genes as oligomers is in line with the findings that two membrane bound proteins, TMHS and TMIE, are both required subunits of the mechanotransducer complex (Figures 3D and 3E) (Xiong et al., 2012; Zhao et al., 2014). Loss of TMHS in mice led to a nearly complete (∼90%) reduction in the activity of the mechanotransducer channel. Although gross morphological alterations to stereocilia structure were not observed, the number of tip links was greatly reduced in TMHS−/− mice. TMHS is expressed at the ends of the stereocilia, physically associates with PCDH15 and regulates the surface expression of PCDH15. Importantly, single channel recordings revealed a decrease in maximum amplitude of channel conductance, and changes to channel adaption properties including an increased latency for channel activation and channel open time (tau). Given that tip links are critical to gating of the mechanotransducer channel complex and that loss of TMHS alters the MT channel pore properties, TMHS likely serves as an auxiliary subunit that can couple the MT channel complex to the tip links (Xiong et al., 2012). A recent report further showed that loss of TMHS also lead to a concomitant decrease in the expression of TMC1 (Beurg et al., 2014b). TMHS can physically associate with TMC1 but not TMC2, and the authors showed that the residual MT currents observed in TMHS deficient hair cells likely stems from TMC2 activity. Interestingly, both macroscopic and single channel recordings revealed that loss of TMHS attenuated the tonotopic gradient in conductance and channel amplitude, respectively (Beurg et al., 2014b).
Along with TMHS, TMIE can form a multimeric complex with TMHS and PCDH15 (Zhao et al., 2014). TMIE contains 2 TM domains and has been previously implicated in human deafness (Mitchem et al., 2002; Naz et al., 2002). TMIE is expressed at the tips of the stereocilia, near the lower end of the tip link, and loss of TMIE abolishes the mechanotransducer current. In cochlear explant experiments, transfection of TMIE could rescue the MT currents in TMIE deficient hair cells. However, heterologous expression of TMIE was not sufficient for mechanically activated channel activity. An elegant set of experiments using variant isoforms of the cytosolic domains of PCDH15 showed that TMIE is required for coupling the MT channel complex to PCDH15 (Zhao et al., 2014). These data suggest that alternate splicing of PCDH15 and the various interactions with TMIE and TMHS could be yet another mechanism for generating diversity to the MT channel complex.
Given all of the recent data, it seems clear that TMC1, TMHS and TMIE are all necessary components of the mechanotransducer complex and that TMC2 can functionally substitute for TMC1. However, it is not clear if there are yet unidentified subunits of this complex, and the relationship of each protein to the pore-forming subunit is not fully defined. While ion selectivity measurements of hair cells from the Beethoven mouse suggest that TMC1 may be the pore-forming subunit, precedents exist for mutations in proteins within an ion channel complex to alter ion selectivity without necessarily lining the pore. For example, a point mutation in the hydrophobic domain of MinK, a single pass transmembrane protein, led to altered ion selectivity and open channel block of a slowly activating potassium current in Xenopus oocytes (Goldstein and Miller, 1991; Takumi et al., 1988). These results initially led the authors to conclude that MinK itself was an ion channel. Later studies, however, showed that MinK was not the pore-lining subunit and instead associated with the pore of KvLQT1 (Kv7.1) to form a fully functional channel (Barhanin et al., 1996; Sanguinetti et al., 1996). More recently, ion selectivity of the calcium release-activated calcium channel (CRAC1), was shown to be modulated by varying concentrations of the associated protein STIM1, even though STIM1 is not a pore-forming subunit, but in fact is present in the ER (McNally et al., 2012). These results highlight the ability of accessory proteins to modulate ion selectivity and suggest that more experiments are needed to validate TMC1 as the pore-forming subunit of the hair cell mechanotransducer complex.
Finally, the identity of the channel responsible for the reverse polarity current and the physiological roles of this current are also unknown. In fact, it is not fully clear if the channel responsible for the reversed polarity currents is entirely separate from or is the same as the pore-forming subunit of the native mechanotransducer current. The existence of multiple genes that are necessary for the MT current fits with the model of a channel complex whose subunit composition varies in a manner that could explain the tonotopic gradient.
Mechanosensitive Potassium Channels
The existence of mechanosensitive potassium channels adds a layer of complexity to the basic model of neuronal activation by mechanical force: whereas the opening of mechanosensitive cation channels leads to membrane depolarization and triggers action potentials or neurotransmitter release, mechanosensitive potassium channels would hyperpolarize the membrane potential and thus decrease the likelihood of an action potential. While this may seem counter-intuitive, mechanosensitive potassium channels can counteract, or balance, the activity of mechanosensitive cation channels. Although the physiological roles of potassium channels in mechanotransduction are not yet fully understood, biophysical and structural studies are starting to provide novel information on how mammalian mechanically activated ion channels are gated.
Potassium channels are generally divided into three classes: 6/7 transmembrane (TM) domain voltage gated (Kv) and calcium activated (Kca) potassium channels, 2 TM domain inwardly rectifying channels (Kir) and 4 TM domain “two pore domain” channels (K2P) (Berkefeld et al., 2010; Noel et al., 2011; Stocker, 2004). Kv, Kca, and Kir channels associate as tetramers where each monomer contains one pore domain (PD) region (hence each subunit contributes one quarter of the ion conducting pore in a tetrameric assembly), while the K2P channels are dimers where both monomers contain two pore domains regions (and each subunit contributes half of the ion conducting pore in a dimeric assembly) (Figure 3F) (Enyedi and Czirjak, 2010; Pardo and Stuhmer, 2014).
K2P channels were one of the first identified mammalian mechanically activated ion channels and recent studies have provided evidence that members of this family directly sense mechanical forces imparted through the lipid membrane (Brohawn et al., 2014b). Originally discovered in yeast (Ketchum et al., 1995), sequence homology screens of the pore domain region led to the identification of the first mammalian K2P channel, TWIK-1 (Tandem of P domains in a Weak Inward rectifying K+ channel) (Lesage et al., 1996). Thus far, 15 mammalian K2P channels have been identified (Honore, 2007), and three channels in particular have been shown to be mechanically activated: TREK-1 (TWIK-1 related K+ channel) (Fink et al., 1996), TREK-2 (Bang et al., 2000; Lesage et al., 2000) and TRAAK (TWIK-related arachidonic acid-stimulated K+ channel) (Fink et al., 1998).
TREK-1 is a polymodal ion channel whose activity is regulated by a wide range of stimuli including mechanical force, temperature, polyunsaturated fatty acids (PUFA) such as arachidonic acid, inhalation anesthetics and pH (Maingret et al., 2000; Maingret et al., 1999b; Patel et al., 1999; Patel et al., 1998). Expression of TREK-1 in Xenopus oocytes and in COS-7 cells led to membrane stretch, osmotic swelling and laminar shear stress induced channel activity (Patel et al., 1998). Stretch-induced channel activity was still present in excised patch recordings, arguing that TREK-1 mechanosensitivity is likely not occurring indirectly through cytoskeleton-associated proteins. TREK-1 is expressed throughout the central nervous system and in DRG neurons, where it is co-expressed with the nociceptive ion channel TRPV1. Mechanically activated potassium currents were recorded from cultured DRG neurons and this activity was lost in TREK-1−/− animals (Alloui et al., 2006). Interestingly, TREK-1−/− mice were more sensitive than wild type controls to von Frey stimuli and displayed more nocifensive behaviors upon injection of osmotic stimuli. TREK-1−/− mice were also more susceptible to chemically induced seizures and were insensitive to some inhalant anesthetics such as halothane (Heurteaux et al., 2004). These behavioral results are consistent with the model that TREK-1 decreases the activity of central and peripheral neurons and can coordinately act alongside ion channels that activate these neurons (Brohawn et al., 2014b). These data also show that TREK-1 expression in mechanosensitive cells is physiologically relevant, and that TREK-1 is both necessary and sufficient for detecting mechanical forces. However, since K2P channels are polymodal in nature, it is not clear if mechanical forces or other stimuli that modulate these channels are the cause of these phenotypes.
TREK-2 and TRAAK are the only other K2P ion channels that can be activated by mechanical forces (Brohawn et al., 2014b; Honore, 2007). Similar to TREK-1, both rat and human TREK-2 can be activated by membrane stretch in heterologous expression systems (Bang et al., 2000; Lesage et al., 2000). TRAAK channels, which bear lower sequence homology to TREKs but contain a similar tandem 2 pore domain topology, are activated by mechanical forces in excised patch recordings (Maingret et al., 1999a). Interestingly, TRAAK channels are also activated by amphipathic compounds that selectively insert into one side of the lipid bilayer and create a convex curvature of the membrane (Maingret et al., 1999a). Consistent with this finding, TRAAK channels are activated by negative pressure in inside-out patches and by positive pressure in outside-out patches. These data again highlight the ability of membrane tension to gate TREK/TRAAK channels and suggest that asymmetric tension induced by curvature is an important activating stimulus. Mice deficient in both TREK-2 and TRAAK also are more sensitive to von Frey filaments and paw injection of hypotonic solution (Noel et al., 2009; Pereira et al., 2014).
Recent biochemical and structural studies have begun to address the question of how mammalian mechanosensitive ion channels can sense mechanical forces in the membrane and potentially integrate all of the various activating modalities. Two groups were able to express, purify and reconstitute TREK-1 and TRAAK in proteoliposomes and show that mechanosensitivity is maintained in an isolated membrane environment (Berrier et al., 2013; Brohawn et al., 2014b). Brohawn et al. recorded potassium currents from excised patches of human TRAAK and zebrafish TREK-1 reconstituted in proteoliposomes, and showed that both channels were activated by membrane stretch. Channel properties were largely similar in excised patches compared to whole cell recordings, and both TREK-1 and TRAAK were activated by either negative or positive pressure. These studies show that TRAAK and TREK-1 are directly sensitive to and intrinsically gated by membrane tension.
Along with biochemical studies, the high-resolution crystal structures of TWIK-1, TRAAK and TREK-2 have provided confirmation of the model that the individual pore domain regions coordinate potassium ions by converging in a manner similar to other four-fold symmetrical tetrameric ion channels (Brohawn et al., 2012; Dong et al., 2015; Miller and Long, 2012). All three structures show that K2P channels contain large extracellular loop helices between TM1 and P1 that can form a physical barrier or “helical cap,” forcing potassium ions to exit through lateral openings and providing a possible explanation for why numerous classical extracellular potassium channel blockers do not affect K2P channels (Honore, 2007). Interestingly, all three structures show a wide opening or “fenestration” below the selectivity filter in the region that corresponds to the inner leaflet of the lipid bilayer. It has been proposed that this opening could provide an access point for membrane bound lipids such as PUFAs to modulate channel activity.
Brohawn et al. reported the x-ray crystal structures of TRAAK in conductive and non-conductive conformations (Brohawn et al., 2014a). In a non-conductive conformation, a lipid acyl chain density was observed that is proposed to cause an occluded pore. A 5A° wide lateral opening in the membrane inner leaflet is suggested to be an access window for the lipid acyl chain to enter channel’s cavity. In the conductive conformation, the lipid acyl chain was absent and the presence of Tl+ ion led the authors to predict an unhindered and open permeation pathway. The conductive state conformation also showed rotation of TM4 about a central hinge that could presumably block the intra-membrane opening, preventing lipid access to the cavity and permitting ion entry. In a separate study, Lolicato et al. concluded that the TM4 conformational change of TRAAK hinges on the conserved Glycine (G124) that resides on the pore helix (P1) that contacts the top of TM4 (Lolicato et al., 2014). The authors report crystal structures of two mutants, G124I and W262S, that render TRAAK channels in an activated state as compared to wild type. Comparison and superposition of mutants vs. wild type show that straightening of the TM4 helix repositions the TM4 C-terminal end by 23–27 degrees. This large change in TM4 position is also associated with disruption of inter-helical packing between TM2-TM3 transmembrane helices, and an opening of a wide pathway to the inner leaflet of the bilayer. The salient feature of the TRAAK structure by Brohawn et al. is that membrane tension leads to channel activation. This idea is supported by the presence and absence of the ions and lipid-like moiety in the open and closed conformation, respectively. A caveat to the TRAAK structure by Lolicato et al. is that the authors used activating mutations that induce the open conformation. Despite the differences in the models of channel gating, the structural data from both studies identified conformation changes in TM4 of TRAAK channels as a critical component required to favor an open state (Brohawn et al., 2014a; Lolicato et al., 2014).
The x-ray crystal structure of TREK-2 in the absence and presence of a chemical antagonist offers additional clues to the mechanism of K2P channel gating (Dong et al., 2015). Dong et al. were able to resolve the structure of TREK-2 in both a conductive and non-conductive state, based on the conformational changes of TM2-TM4. Similar to the TWIK-1 and TRAAK structures, in the “down” state, or closed state, an intra-membrane fenestration beneath the selectivity filter is accessible for lipids and membrane bound chemical modulators. In the “up” state, the intra-membrane fenestration is inaccessible due to conformational changes in TM2-TM4. As further evidence that the “down” state is non-conductive, the authors show that the state dependent inhibitors, fluoxetine and norfluoxetine, bind TREK-2 only in the “down” state, where the intra-membrane fenestration is accessible. These data are consistent with biochemical results that norfluoxetine inhibition is decreased when TREK-2 is activated with arachidonic acid or by membrane stretch, and that TREK-2 activation by membrane stretch is inhibited by pre-incubation with norfluoxetine.
Although K2P channels have received the most attention for their mechanotransduction properties, the modulation of voltage gated potassium channels by mechanical force has also been recorded (Anishkin et al., 2014; Morris, 2011). Schmidt et al. showed that the Kv1.2 Paddle chimera, Shaker Kv1.1 and Kv2.1 exhibit low threshold mechanosensitivity and over time change their gating properties upon formation of a gigaseal patch for electrophysiology recordings (Schmidt et al., 2012). Two different patch configurations -whole cell vs. on cell- were tested to account for the differences in the lateral membrane tension. In all three potassium channels, on cell patch configuration (high tension membrane) caused the leftward shift of voltage activation curves, irrespective of the cell types tested. These data suggest that membrane tension indirectly affects the activity of voltage gated potassium channels, and could counteract the activity of cation channels in mechanosensitive cells. In line with this hypothesis, Hao et al. reported the presence of a novel voltage dependent mechanically activated potassium current present in mouse DRG neurons (Hao et al., 2013). Using a panel of toxins and recordings from neurons of mice deficient in TREK-1, TREK-2 and TRAAK, the authors identified Kv1.1 subunits as being necessary for this current. A dominant negative Kv1.1 mouse line dramatically reduced the endogenous response and Kv1.1 oligomers were sufficient to produce MA currents in heterologous expression systems. The authors showed that mechanical force leads to voltage dependent channel activation and, in DRG neurons, Kv1.1 oligomers negatively regulate the mechanical activation of nociceptive high threshold C fibers (C-HTMRs). Consequently, mice that contain a dominant negative form of Kv1.1 display decreased response thresholds to von Frey stimuli. These results show that voltage gated potassium channels can act as negative regulators or brakes against the function of excitatory ion channels and, like the K2P channels, tune the overall response.
Recent findings have solidified the role of K2P channels TREK1, TREK2 and TRAAK as bona fide mechanically activated ion channels that are physiologically important for tuning the activity of mechanosensitive DRG neurons. One key question that remains is how mechanical forces are integrated in cells where mechanosensitive potassium channels are co-expressed with mechanosensitive cation channels. Interestingly, Brohawn et al. showed that expression of TRAAK in the neuroblastoma cell line, N2A, led to decreased depolarization of the membrane potential upon mechanical indentation (Brohawn et al., 2014b). This reduction in the depolarization produced by TRAAK channels is likely due to the simultaneous activation of endogenous Piezo1 in these cells, suggesting a similar mechanism for mechanotransduction in vivo.
Piezo Ion Channels
The activation of mammalian touch receptor neurons and other mechanosensitive cells, such as endothelial or smooth muscle cells, is thought to occur primarily because of mechanically activated cation channels (Corey and Hudspeth, 1979; Guharay and Sachs, 1984). Because the role of DEG/ENaC and TRP channels in mammalian mechanotransduction remains unclear, the identification of novel ion channel families, such as the Piezo ion channel family, has been critical to furthering our understanding of mammalian mechanotransduction (Coste et al., 2010). Piezos are evolutionarily conserved in animals and plants, but bear no sequence homology to other known ion channel families (Coste et al., 2010). In mammals, Piezos are broadly expressed in a wide range of mechanosensitive cells and are enriched in the lungs, kidneys and DRG neurons. Although the precise topology of Piezo ion channels has not yet been determined, computational models and biochemical studies suggest that Piezo ion channels contain at least 18 and, potentially 38 transmembrane domains, suggesting that Piezos might be the largest identified ion channel complex to date (Figures 3G and 3H) (Coste, 2015; Coste et al., 2012).
Piezo1, the founding member of this family, was initially identified as the mechanically activated ion channel necessary for membrane indentation and membrane stretch induced currents in a neuroblastoma cell line, N2A. Piezo1 associates as a putative tetramer and is a non-selective cation channel that is blocked by chemical antagonists such as gadolinium (Gd), Ruthenium Red (RR) and GsMTx4, a peptide toxin isolated from tarantula venom known to block stretch activated channels (Bae et al., 2011; Coste et al., 2010; Coste et al., 2012). Piezo2, a related mammalian homolog, was also shown to be necessary for a subset of mechanically activated currents in isolated DRG neurons (Coste et al., 2010). Importantly, heterologous expression of mouse Piezo1 and Piezo2, as well as Drosophila Piezo, was sufficient to confer robust mechanically activated currents in naïve cells (Coste et al., 2010; Coste et al., 2012).
Over the past few years, numerous studies have defined the importance of Piezo ion channels to mechanotransduction processes in vivo. The Drosophila Piezo ortholog is expressed in Class IV dendritic arborization neurons that respond to high intensity or noxious touch stimuli (Kim et al., 2012; Yan et al., 2013). Loss of dPiezo abolished membrane stretch induced currents in isolated sensory neurons and attenuated the ability of larvae to escape from noxious levels of touch stimulation. The deletion of both Piezo and the DEG/ENaC gene, Pickpocket, in Class IV neurons abolished the behavioral response of larvae to noxious touch stimuli. These results have led to an in-depth understanding of peripheral touch sensation in Drosophila larvae: Piezo and Pickpocket function in separate but parallel circuits that constitute mechanical responses to noxious stimuli in Class IV dendritic arborization neurons, whereas light touch sensation is mediated by the TRP channel, NOMPC, in Class III neurons (Kim et al., 2012; Yan et al., 2013).
The function of Piezo ion channels in mammalian mechanotransduction has also been elucidated by multiple groups. Whereas Piezo deficient flies are viable, constitutive deletion of both Piezo1 and Piezo2 in mice led to developmental lethality (Li et al., 2014; Ranade et al., 2014a; Woo et al., 2014b). Piezo1 is required for the development of the mouse vasculature through a mechanism involving impaired mechanotransduction of hemodynamic shear stress by endothelial cells (Li et al., 2014; Ranade et al., 2014a). Furthermore, Piezo1 is required for mechanical force induced cation influx in red blood cells and conditional deletion of Piezo1 in red blood cells of adult mice leads to increased fragility and dehydration (Cahalan et al., 2015). Piezo2 is expressed in a subset of DRG neurons that innervate the skin and hair follicles to form low threshold mechanoreceptors such as lanceolate endings, circumferential endings and Meissner’s corpuscles (Ranade et al., 2014b). Piezo2 is also expressed in Merkel cells, specialized touch receptors embedded in the skin that detect light touch, and is required for the mechanosensitivity of these cells (Ikeda et al., 2014; Maksimovic et al., 2014; Woo et al., 2014b). Deletion of Piezo2 in sensory neurons and in Merkel cells led to a dramatic reduction in a subset of isolated DRG neurons that normally display rapidly adapting mechanically activated currents. In skin-nerve preparation measurements, the activity of nearly all types of low threshold mechanoreceptors was markedly reduced or ablated. These deficits in the function of sensory neurons in vitro and ex vivo correlated with profound behavioral deficits in light touch sensation without any adverse effects to noxious touch (Ranade et al., 2014b). These results establish Piezo2 as the primary sensor of light touch in mammals and predict that other ion channels likely constitute noxious touch sensation. Interestingly, using a novel differentiation protocol to convert human embryonic stem cells (ES) into low threshold mechanoreceptor DRG neurons, Schrenk-Seimens et al. showed that Piezo2 is required for mechanically activated currents in those cells (Schrenk-Siemens et al., 2015). These results in mice, flies and other model organisms including zebrafish and waterfowl, establish the critical function of Piezo2 in touch sensation (Faucherre et al., 2013; Schneider et al., 2014).
A mechanistic understanding of how Piezo ion channels sense mechanical forces is still unknown. Piezo1 has been shown to form an oligomeric complex that retains activity when purified and reconstituted into asymmetric droplet lipid bilayers as well as in asolectin proteoliposomes (Coste et al., 2012). The conductance of Piezo1 in cells and lipid bilayers closely matches when similar ionic conditions are compared. For example, conductance in asymmetric bilayers recorded in 200 mM KCl and in cells recorded in 150 mM NaCl (without divalent ions) is equal within an error of ∼58 pS for both conditions. However, ionic conditions can affect conductance values in both systems. For example, the presence of calcium ions reduces single channel conductance to ∼30 pS in cells (Coste et al., 2015; Coste et al., 2012); while recording in standard asymmetric droplet bilayer conditions of 500mM KCl increases the conductance to ∼120 pS. Overall, the data that purified Piezo1 oligomers act as ion channels in bilayers cemented the idea that Piezo1 is a bona-fide ion channel.
It is interesting to note that Piezo1 channel activity in bilayers was observed in the absence of applied mechanical forces (such as pressure or bilayer indentation), (Coste et al., 2012). It is not yet clear if other accessory proteins are required to regulate the Piezo channel or if the asymmetric lipid composition of the bilayer could affect channel gating similar to results from prokaryotic and eukaryotic K2P mechanosensitive channels (Nilius and Honore, 2012). Indeed, this question remains to be resolved before Piezo1 can be called to be independently mechanosensitive. Regardless, mechanical activation of Piezo1 is shown to be modulated by other proteins such as PKD2 (PC2) and STOML3, however the mechanism of this interaction is still unclear (Peyronnet et al., 2013; Poole et al., 2014).
Until recently, no small molecule agonists of Piezo ion channels had been reported and it was not known if Piezos could be activated by stimuli other than mechanical force. To address this issue, Syeda et al. conducted a high throughput chemical library screen for agonists of Piezo1 and identified Yoda1 as an activator of both mouse and human Piezo1 but not Piezo2 (Syeda et al., 2015). In cell-attached patch clamp recordings of Piezo1 transiently expressed in HEK293 cells, Yoda1 led to slower channel inactivation kinetics and a shift in the current-pressure relationship, indicating sensitization to membrane stretch induced activation of Piezo1. Yoda1 also activated Piezo1 channels that were purified and reconstituted in artificial lipid bilayers, ruling out the possibility that other proteins are required for Yoda1 mediated effects on Piezo1 mechanosensitivity. Whether Yoda1 affects Piezo1 directly or exerts its effects through changes in the membrane lipid tension is not yet known and Yoda1 could be a valuable tool to gain new insights into gating of Piezo channels.
Structure/function analysis of Piezo channels is limited, and critical features of the channels, including the pore domain, has yet to be defined. Coste et al. used an epitope tagging strategy and confirmed that 9 of the putative extracellular loops predicted from bioinformatics analyses are indeed accessible from outside of the cell to antibody recognition (Coste et al., 2015). The authors then generated chimeric proteins consisting of distinct regions of both Drosophila Piezo and mouse Piezo1 and identified two transmembrane domains in the C-terminus of mouse Piezo1 that is required for unitary conductance, ion selectivity and Ruthenium Red sensitivity. Within this region, mutations to a specific glutamate reside, E2133, affected unitary conductance and channel block by Ruthenium Red. These results suggest that the channel pore lies within C-terminal domain and that E2133 is located physically near the pore (Coste et al., 2015).
Further biochemical and structural analyses are needed to define the exact topology of Piezo ion channels, including identification of the pore forming residues, and to elucidate the mechanism of activation to both mechanical and chemical stimuli. A recent study has also highlighted the ability of membrane bound lipids, such as PIP2, to modulate Piezo1 mechanosensitivity (Borbiro et al., 2015). Regardless, it is clear that Piezo ion channels are both necessary and sufficient to transduce mechanical forces and are physiologically relevant in numerous mechanotransduction pathways in vivo. Recent studies linking diseases in humans to mutations in Piezo1 and Piezo2 provide further evidence for the importance of these genes in vivo (Albuisson et al., 2013; Coste et al., 2013).
Models for Gating Mechanisms of Mechanosensitive Ion Channels
Mechanical activation of ion channels is thought to be mediated by forces transmitted either directly through the lipid bilayer or by auxiliary proteins in addition to the lipid bilayer (Kung, 2005). Here we discuss two models that have been hypothesized for the gating of ion channels by mechanical forces: “tethered” models and a membrane delimited “force from lipid” model (Anishkin et al., 2014; Arnadottir and Chalfie, 2010). The primary difference between these two models is that the tethered models involve transmission of force to the channel complex by auxiliary structures such as the extracellular matrix and/or the intracellular cytoskeleton, whereas the “force from lipid” models do not require additional components beyond the lipid bilayer. Initially, the tethered model was favored over the more simple “force from lipid” model for eukaryotic ion channels because of the extensive cytoskeletal and extracellular matrix networks adjacent to the membrane (Chalfie, 2009; Kung, 2005). However, recent studies of mammalian mechanosensitive potassium channels have shown that these channels, similar to MscS and MscL channels in prokaryotes, do not require auxiliary proteins to transduce mechanical forces.
A “dual-tethered” gating model was first suggested to describe biophysical features of mechanical transduction in auditory and vestibular hair cells (Chalfie, 2009). The transduction channel was thought to be tethered to extracellular tip links that connect to the adjacent stereocilia and to actin filaments of the cytoskeleton (Pickles et al., 1984). Positive deflection of the stereocilia would stretch the channel open between the two tethering points. This model also implied that transduction is unidirectional and movement of the stereocilia only in the direction that stretches the tethered connections opens the channels (Hudspeth and Corey, 1977). Apart from the hair cells of the cochlea and the vestibular system, tip link-like tethered structures are also present in skin cells (Hu et al., 2010; Li and Ginty, 2014). Hu et al. showed that low threshold mechanoreceptors (LTMR) and some nociceptive high threshold mechanoreceptors (HTMR) require an extracellular tether protein to function. A protein filament of ∼100 nm size is synthesized by sensory neurons and links mechanosensitive channels to the extracellular matrix, as previously described for tip links. Treatment of sensory neurons with non-specific and site-specific endopeptidases to terminate the protein tether abolished mechanosensitive currents. Another example of the tethered model for somatosensory function involves epithelial hair follicle longitudinal lanceolate complexes. These mechanically sensitive structures transform hair follicle deflections into electrical impulses. Li and Ginty suggest that “epithelial cell–lanceolate complex tethers” serve a function analogous to the tip links. The authors combined genetic labeling of low threshold mechanoreceptors, electron microscopic analyses, and genetic manipulations to propose that the deflection of hair follicles would place strain on the tethers to activate mechanically gated ion channels, thus leading to depolarization.
The “force from lipids” model developed from the identification and functional reconstitution of the prokaryotic mechanosensitive ion channel, MscL (Sukharev et al., 1994). These biochemical studies were followed by a high-resolution crystal structure of MscL at 3.5Å resolution in a closed state, as well as the electron paramagnetic resonance (EPR) analysis to trap the open and intermediate states of the channel (Chang et al., 1998; Perozo et al., 2002a; Perozo et al., 2002b). MscL, a pentameric channel with each monomer containing 2 TM domains, has served as an ideal system to study mechanotransduction at molecular level. In a closed state, all five TM1 helices of MscL cross near the intracellular side of the membrane to create the hydrophobic seal. The TM2 helices then interact with the lipid bilayer and TM1 helices. In a modeled open state, based on the EPR data, the TM1 helices have moved away from the axis of symmetry, thereby breaking the hydrophobic seal. Moreover, the crystal structure of a related prokaryotic mechanosensitive ion channel, MscS, at 3.9 Å resolution also revealed a tension sensor contained within the protein itself (Bass et al., 2002). Unlike MscL, MscS associates as a homo-heptamer and each subunit contains 3 TM domains. TM3 helices line the channel pore and TM1 and TM2 helices constitute the sensors for membrane tension and voltage (Bass et al., 2002). The characterization of bacterial MscL and MscS has been discussed at length (Hamill and Martinac, 2001; Perozo, 2006). Here we will focus on the gating mechanism since the membrane-gated model is extensively studied and relatively well understood for bacterial channels.
The common consensus among all the investigations is that the lipid bilayer tension alone is sufficient to gate prokaryotic mechanosensitive channels because purified MscL and MscS retain their mechanosensitivity when reconstituted into liposomes (Hase et al., 1995; Martinac, 2001; Perozo and Rees, 2003; Sukharev et al., 1994). What makes these channels respond to membrane tension is less clear and topic of great interest. Spencer et al. proposed a concerted mechanism, in which tension within the membrane leads to a simultaneous motion of all helices in the TM1 and TM2 leading to the pore opening (Spencer et al., 1999). On the other hand, Sukharev et al. postulated that the outer TM2 helices act as a tension sensor and can expand significantly before the internal part of the channel opens (Sukharev et al., 2001). The channel opens when the tension-sensing rim transfers enough force to pull the internal TM1 bundle into the open state.
It has been long postulated and later established that mechanosensitive channels respond to mechanical forces along the plane of the cell membrane for example membrane tension but not to hydrostatic pressure perpendicular to it (Sokabe and Sachs, 1990; Sokabe et al., 1991). The conclusion was drawn by varying the pressure in the patch clamp recordings and simultaneously calculating the radius of the patch to quantify tension by Laplace’s Law. The key properties that provide the lipid bilayer with mechanical force are: hydrophobic mismatch, intrinsic curvature and the physical state or fluidity of the lipids. Perozo et al. evaluated two potential causes of MscL gating by the bilayer mechanism: (1) protein–lipid-bilayer hydrophobic mismatch and (2) membrane curvature (Perozo et al., 2002b). The gating of MscL is found to be dependent on changes in lipid composition upon addition of lysophosphatidylcholine (LPC) into one leaflet. Addition of LPC to one monolayer of the bilayer creates local stresses leading to reorganization of the transbilayer pressure profile, hence opening the channel. The authors concluded that cone shaped lipids (with high lateral tension) favors the fully open state of MscL (Perozo and Rees, 2003) and that the hydrophobic mismatch is probably a component but not the main trigger for mechanical activation of MscL. These findings were also supported by other reports that emphasized lateral tension rather than the membrane curvature as being an important biophysical parameter for MscL gating (Moe and Blount, 2005).
Recent biochemical and structural studies have convincingly shown that the “force from lipid” model is also applicable to mammalian mechanically activated ion channels. As discussed previously in this review, the mechanosensitivity of TREK-1 and TRAAK channels was shown to be mediated directly through the lipid bilayer in the absence of other cellular components (Brohawn et al., 2014b). However, the molecular mechanism of K2P channel gating and the influence of the lipid bilayer to channel mechanosensitivity remain unclear. To address the structural aspects of the gating mechanism, three groups have published the high-resolution crystal structures of TRAAK and TREK-2 (Brohawn et al., 2014a; Dong et al., 2015; Lolicato et al., 2014). The TRAAK structure by Lolicato et al., suggested that the conformational changes induced by activating-mutants opens up a wide-pathway towards the inner leaflet of the bilayer. The TRAAK structure by Brohawn et al., and TREK-2 structure by Dong et al., proposes that conformational changes within the TM domains allow channel gating upon changes to membrane tension. These finding extends the “force-from-lipid” model for K2P mechanosensors as is established for bacterial channels.
The emerging data predicts that force detection of K2P channels must be happening at the channel-lipid interface and suggests a common mechanism for mechanotransduction between bacterial and mammalian channels (Anishkin et al., 2014). Incredibly, the structural data suggest that K2P channels may employ a similar mechanism where membrane tension leads to conformational changes of the transmembrane domain region beneath the selectivity filter. The lateral opening beneath the selectivity filter as observed in TRAAK and a fenestration observed in TREK-2 could provide means for hydrophobic modulators and lipids to affect channel function. The polymodal activation of TRAAK and TREK2 by AA, PUFA and local anesthetics is reminiscent of MscS and MscL gating by lipids (LPC) and local anesthetics. These studies also highlight the importance of high-resolution structural data that support biochemical and previous functional analyses of K2p, and bacterial MSc in the literature. These studies also highlight the importance of high-resolution structural data that now support biochemical and previous functional analyses of K2p in the literature. Based on converging similarities between prokaryotic and eukaryotic channels on the “force from lipid” model, it is likely that the tethered model might be a reiteration of basic force from lipid model. This idea, put forth by Kung et al. over a decade ago, suggests that tethered links pull the channel within the membrane while deforming and tensing up the bilayer around channel (Kung, 2005). In depth studies are required to confirm the convergence of both the models but the challenge lies in reconstituting multimeric components of proteins to study tethered model. Nevertheless, understanding the molecular mechanism of mechanotransduction not only requires a quantitative narrative of the physical forces but also a thorough investigation of protein–lipid interactions.
Conclusions/Future Directions
The ability of cells to sense mechanical force is a fundamental process conserved throughout evolution. Diverse mechanotransduction processes allow us to sense tactile inputs, hear sounds, coordinate our bodies in space and regulate blood pressure in response to changes in heart rate. Over the past few years, numerous advances have been made in understanding mechanotransduction processes in mammals but fundamental questions still remain. Biochemical and structural studies have established that the K2P channels TREK-1, TREK-2 and TRAAK are exquisitely sensitive to mechanical forces in a manner similar to the bacterial MscL and MscS channels. How K2P channels are gated by mechanical forces and other stimuli is only beginning to be understood, and will lead to a greater understanding of the physiological functions of these channels. The publication of multiple high-resolution crystal structures also offer the possibility for drug design and the development of specific antagonists. While TMC1/2, TMHS and TMIE are all required for hearing in mammals, an in-depth understanding of the hair cell mechanotransducer channel complex has yet to be described. Further investigation is needed to address the intriguing possibility that distinct components to the mechanotransducer channel complex could explain the tonotopic gradient from the apex to the base of the cochlea.
Piezo ion channels have been shown to be the receptor of touch sensation in a range of model organisms. Furthermore, Piezo ion channels are expressed in a wide range of mechanosensitive cell types and recent studies have shown that Piezo1 is required for mechanotransduction in endothelial cells as well as in red blood cells. The surprising finding that Piezo ion channels are not only required for detecting touch stimuli in somatosensory neurons but also for shear stress responses in endothelial cells suggests that these seemingly diverse stimuli may not be fundamentally different. How these various forces impact the lipid membrane will certainly be a major avenue for future research. As molecular identification of sensory receptors has led to a fundamental understanding of other senses, insight into a general role for Piezo ion channels in mechanotransduction processes in various physiological systems should further our understanding of how mechanical forces regulate a range of cellular functions, and contribute to a deeper understanding of mechanotransduction. Interestingly, while cell volume regulation is not classically defined as a mechanotransduction process, the recent identification of SWELL1 could lead to new insights into similarities between volume regulation and mechanotransduction (Qiu et al., 2014; Voss et al., 2014). Finally, in mice, loss of Piezo2 did not affect noxious force sensation. This suggests that other ion channels that have yet to be identified are likely required for this process.
Mechanically activated ion channels, unlike the GPCRs of smell, taste and vision, are strikingly diverse receptors in structure and function. Of the gene families discussed in this review, the TRP ion channels and the DEG/ENaC channels are critically involved in invertebrate mechanotransduction whereas mammals make more use of the Piezo ion channels. Why this occurs is unknown and there is still a need to identify new ion channels that are responsible for mechanotransduction in both vertebrates and invertebrates.
Acknowledgements
We would like to acknowledge Julia Kuhl for illustration of figures. We also thank Professor Uli Mueller and members of the Patapoutian lab for critical evaluation of our manuscript and helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circulation research. 2009;104:860–869. doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagramam KN, Goodyear RJ, Geng R, Furness DN, van Aken AF, Marcotti W, Kros CJ, Richardson GP. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PloS one. 2011;6:e19183. doi: 10.1371/journal.pone.0019183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Feneant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nature communications. 2013;4:1884. doi: 10.1038/ncomms2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloui A, Zimmermann K, Mamet J, Duprat F, Noel J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, et al. TREK-1, a K+ channel involved in polymodal pain perception. The EMBO journal. 2006;25:2368–2376. doi: 10.1038/sj.emboj.7601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anishkin A, Loukin SH, Teng J, Kung C. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7898–7905. doi: 10.1073/pnas.1313364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annual review of biophysics. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- Assad JA, Shepherd GM, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–994. doi: 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50:6295–6300. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. The Journal of biological chemistry. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Bass RB, Strop P, Barclay M, Rees DC. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298:1582–1587. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Berkefeld H, Fakler B, Schulte U. Ca2+-activated K+ channels: from protein complexes to function. Physiological reviews. 2010;90:1437–1459. doi: 10.1152/physrev.00049.2009. [DOI] [PubMed] [Google Scholar]
- Berrier C, Pozza A, de Lacroix de Lavalette A, Chardonnet S, Mesneau A, Jaxel C, le Maire M, Ghazi A. The purified mechanosensitive channel TREK-1 is directly sensitive to membrane tension. The Journal of biological chemistry. 2013;288:27307–27314. doi: 10.1074/jbc.M113.478321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Evans MG, Hackney CM, Fettiplace R. A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:10992–11000. doi: 10.1523/JNEUROSCI.2188-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Fettiplace R, Nam JH, Ricci AJ. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nature neuroscience. 2009;12:553–558. doi: 10.1038/nn.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Kim KX, Fettiplace R. Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. The Journal of general physiology. 2014a;144:55–69. doi: 10.1085/jgp.201411173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Xiong W, Zhao B, Muller U, Fettiplace R. Subunit determination of the conductance of hair-cell mechanotransducer channels. Proceedings of the National Academy of Sciences of the United States of America. 2014b doi: 10.1073/pnas.1420906112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya MR, Bautista DM, Wu K, Haeberle H, Lumpkin EA, Julius D. Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20015–20020. doi: 10.1073/pnas.0810801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbiro I, Badheka D, Rohacs T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Science signaling. 2015;8:ra15. doi: 10.1126/scisignal.2005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, Campbell EB, MacKinnon R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature. 2014a;516:126–130. doi: 10.1038/nature14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, del Marmol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335:436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, Su Z, MacKinnon R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proceedings of the National Academy of Sciences of the United States of America. 2014b;111:3614–3619. doi: 10.1073/pnas.1320768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Cahalan SM, Lukacs V, Ranade SS, Chien S, Bandell M, Patapoutian A. Piezo1 links mechanical forces to red blood cell volume. Elife. 2015;4 doi: 10.7554/eLife.07370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chalfie M. Neurosensory mechanotransduction. Nature reviews Molecular cell biology. 2009;10:44–52. doi: 10.1038/nrm2595. [DOI] [PubMed] [Google Scholar]
- Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- Chatzigeorgiou M, Bang S, Hwang SW, Schafer WR. tmc-1 encodes a sodium-sensitive channel required for salt chemosensation in C. elegans. Nature. 2013;494:95–99. doi: 10.1038/nature11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou M, Yoo S, Watson JD, Lee WH, Spencer WC, Kindt KS, Hwang SW, Miller DM, Treinin M, 3rd, Driscoll M, et al. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nature neuroscience. 2010;13:861–868. doi: 10.1038/nn.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelur DS, Ernstrom GG, Goodman MB, Yao CA, Chen L, R OH, Chalfie M. The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel. Nature. 2002;420:669–673. doi: 10.1038/nature01205. [DOI] [PubMed] [Google Scholar]
- Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nature reviews Neuroscience. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- Corey DP, Hudspeth AJ. Response latency of vertebrate hair cells. Biophysical journal. 1979;26:499–506. doi: 10.1016/S0006-3495(79)85267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Houge G, Murray MF, Stitziel N, Bandell M, Giovanni MA, Philippakis A, Hoischen A, Riemer G, Steen U, et al. Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4667–4672. doi: 10.1073/pnas.1221400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Murthy SE, Mathur J, Schmidt M, Mechioukhi Y, Delmas P, Patapoutian A. Piezo1 ion channel pore properties are dictated by C-terminal region. Nature communications. 2015;6:7223. doi: 10.1038/ncomms8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Murthy SE, Mathur J, Schmidt M, Mechioukhi Y, Delmas P, Patapoutian A. Piezo1 Ion Channel Pore Properties are Dictated by C-terminal region. Nature communications. 2015 doi: 10.1038/ncomms8223. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaen PG, Delling M, Vien TN, Clapham DE. Direct recording and molecular identification of the calcium channel of primary cilia. Nature. 2013;504:315–318. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature. 2013;504:311–314. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Coste B. Mechano-gated ion channels in sensory systems. Cell. 2013;155:278–284. doi: 10.1016/j.cell.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nature reviews Neuroscience. 2011;12:139–153. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- Deol MSaKW. A new gene for deafness in the mouse. The Journal of heredity. 1958;12:463–466. [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annual review of neuroscience. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Dong YY, Pike AC, Mackenzie A, McClenaghan C, Aryal P, Dong L, Quigley A, Grieben M, Goubin S, Mukhopadhyay S, et al. K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science. 2015;347:1256–1259. doi: 10.1126/science.1261512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. The Journal of physiology. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effertz T, Nadrowski B, Piepenbrock D, Albert JT, Gopfert MC. Direct gating and mechanical integrity of Drosophila auditory transducers require TRPN1. Nature neuroscience. 2012;15:1198–1200. doi: 10.1038/nn.3175. [DOI] [PubMed] [Google Scholar]
- Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiological reviews. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- Faucherre A, Nargeot J, Mangoni ME, Jopling C. piezo2b regulates vertebrate light touch response. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:17089–17094. doi: 10.1523/JNEUROSCI.0522-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R, Kim KX. The physiology of mechanoelectrical transduction channels in hearing. Physiological reviews. 2014;94:951–986. doi: 10.1152/physrev.00038.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. The EMBO journal. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. The EMBO journal. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffeney SL, Cueva JG, Glauser DA, Doll JC, Lee TH, Montoya M, Karania S, Garakani AM, Pruitt BL, Goodman MB. DEG/ENaC but not TRP channels are the major mechanoelectrical transduction channels in a C. elegans nociceptor. Neuron. 2011;71:845–857. doi: 10.1016/j.neuron.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Muller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nature reviews Genetics. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Miller C. Site-specific mutations in a minimal voltage-dependent K+ channel alter ion selectivity and open-channel block. Neuron. 1991;7:403–408. doi: 10.1016/0896-6273(91)90292-8. [DOI] [PubMed] [Google Scholar]
- Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, et al. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB, Ernstrom GG, Chelur DS, O'Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. The Journal of physiology. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiological reviews. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- Hao J, Padilla F, Dandonneau M, Lavebratt C, Lesage F, Noel J, Delmas P. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron. 2013;77:899–914. doi: 10.1016/j.neuron.2012.12.035. [DOI] [PubMed] [Google Scholar]
- Hargrave PA, McDowell JH, Curtis DR, Wang JK, Juszczak E, Fong SL, Rao JK, Argos P. The structure of bovine rhodopsin. Biophysics of structure and mechanism. 1983;9:235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- Hase CC, Le Dain AC, Martinac B. Purification and functional reconstitution of the recombinant large mechanosensitive ion channel (MscL) of Escherichia coli. The Journal of biological chemistry. 1995;270:18329–18334. doi: 10.1074/jbc.270.31.18329. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. The EMBO journal. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK1. Nature reviews Neuroscience. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Hu J, Chiang LY, Koch M, Lewin GR. Evidence for a protein tether involved in somatic touch. The EMBO journal. 2010;29:855–867. doi: 10.1038/emboj.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. Integrating the active process of hair cells with cochlear function. Nature reviews Neuroscience. 2014;15:600–614. doi: 10.1038/nrn3786. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ, Corey DP. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive Abeta-afferent impulses. Cell. 2014;157:664–675. doi: 10.1016/j.cell.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Geleoc GS, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, et al. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. The Journal of clinical investigation. 2011;121:4796–4809. doi: 10.1172/JCI60405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Kurima K, Pan B, Griffith AJ, Holt JR. Transmembrane channel-like (TMC) genes are required for auditory and vestibular mechanosensation. Pflugers Archiv : European journal of physiology. 2015;467:85–94. doi: 10.1007/s00424-014-1582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- Ketchum KA, Joiner WJ, Sellers AJ, Kaczmarek LK, Goldstein SA. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995;376:690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, Lee HW, Son W, Yim J, Park CS, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- Kim KX, Beurg M, Hackney CM, Furness DN, Mahendrasingam S, Fettiplace R. The role of transmembrane channel-like proteins in the operation of hair cell mechanotransducer channels. The Journal of general physiology. 2013;142:493–505. doi: 10.1085/jgp.201311068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, Patapoutian A, Schafer WR. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nature neuroscience. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Woodbury CJ. Comprehensive phenotyping of sensory neurons using an ex vivo somatosensory system. Physiology & behavior. 2002;77:589–594. doi: 10.1016/s0031-9384(02)00904-6. [DOI] [PubMed] [Google Scholar]
- Kotsis F, Boehlke C, Kuehn EW. The ciliary flow sensor and polycystic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28:518–526. doi: 10.1093/ndt/gfs524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436:647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, Naz S, Arnaud D, Drury S, Mo J, Makishima T, et al. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nature genetics. 2002;30:277–284. doi: 10.1038/ng842. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labay V, Weichert RM, Makishima T, Griffith AJ. Topology of transmembrane channel-like gene 1 protein. Biochemistry. 2010;49:8592–8598. doi: 10.1021/bi1004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnert BP, Baker AE, Gaudry Q, Chiang AS, Wilson RI. Distinct roles of TRP channels in auditory transduction and amplification in Drosophila. Neuron. 2013;77:115–128. doi: 10.1016/j.neuron.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennertz RC, Kossyreva EA, Smith AK, Stucky CL. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PloS one. 2012;7:e43597. doi: 10.1371/journal.pone.0043597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. The EMBO journal. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. The Journal of biological chemistry. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ginty DD. The structure and organization of lanceolate mechanosensory complexes at mouse hair follicles. Elife. 2014;3:e01901. doi: 10.7554/eLife.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Feng Z, Sternberg PW, Xu XZ. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolicato M, Riegelhaupt PM, Arrigoni C, Clark KA, Minor DL., Jr Transmembrane helix straightening and buckling underlies activation of mechanosensitive and thermosensitive K(2P) channels. Neuron. 2014;84:1198–1212. doi: 10.1016/j.neuron.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukin S, Zhou X, Su Z, Saimi Y, Kung C. Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. The Journal of biological chemistry. 2010;285:27176–27181. doi: 10.1074/jbc.M110.143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, et al. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009;64:885–897. doi: 10.1016/j.neuron.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda R, Kindt KS, Mo W, Morgan CP, Erickson T, Zhao H, Clemens-Grisham R, Barr-Gillespie PG, Nicolson T. Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:12907–12912. doi: 10.1073/pnas.1402152111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Fosset M, Lesage F, Lazdunski M, Honore E. TRAAK is a mammalian neuronal mechano-gated K+ channel. The Journal of biological chemistry. 1999a;274:1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honore E. TREK-1 is a heat-activated background K(+) channel. The EMBO journal. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano-or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. The Journal of biological chemistry. 1999b;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509:617–621. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B. Mechanosensitive channels in prokaryotes. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2001;11:61–76. doi: 10.1159/000047793. [DOI] [PubMed] [Google Scholar]
- Mauthner SE, Hwang RY, Lewis AH, Xiao Q, Tsubouchi A, Wang Y, Honjo K, Skene JH, Grandl J, Tracey WD., Jr Balboa binds to pickpocket in vivo and is required for mechanical nociception in Drosophila larvae. Current biology : CB. 2014;24:2920–2925. doi: 10.1016/j.cub.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter GC, Reichling DB, Levine JD. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neuroscience letters. 1999;273:179–182. doi: 10.1016/s0304-3940(99)00665-5. [DOI] [PubMed] [Google Scholar]
- McNally BA, Somasundaram A, Yamashita M, Prakriya M. Gated regulation of CRAC channel ion selectivity by STIM1. Nature. 2012;482:241–245. doi: 10.1038/nature10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AN, Long SB. Crystal structure of the human two-pore domain potassium channel K2P1. Science. 2012;335:432–436. doi: 10.1126/science.1213274. [DOI] [PubMed] [Google Scholar]
- Mitchem KL, Hibbard E, Beyer LA, Bosom K, Dootz GA, Dolan DF, Johnson KR, Raphael Y, Kohrman DC. Mutation of the novel gene Tmie results in sensory cell defects in the inner ear of spinner, a mouse model of human hearing loss DFNB6. Human molecular genetics. 2002;11:1887–1898. doi: 10.1093/hmg/11.16.1887. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. The Journal of biological chemistry. 2009;284:21257–21264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe P, Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry. 2005;44:12239–12244. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- Morris CE. Voltage-gated channel mechanosensitivity: fact or friction? Frontiers in physiology. 2011;2:25. doi: 10.3389/fphys.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J, Hogness DS. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983;34:807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature genetics. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Naz S, Giguere CM, Kohrman DC, Mitchem KL, Riazuddin S, Morell RJ, Ramesh A, Srisailpathy S, Deshmukh D, Riazuddin S, et al. Mutations in a novel gene, TMIE, are associated with hearing loss linked to the DFNB6 locus. American journal of human genetics. 2002;71:632–636. doi: 10.1086/342193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Honore E. Sensing pressure with ion channels. Trends in neurosciences. 2012;35:477–486. doi: 10.1016/j.tins.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Noel J, Sandoz G, Lesage F. Molecular regulations governing TREK and TRAAK channel functions. Channels. 2011;5:402–409. doi: 10.4161/chan.5.5.16469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. The EMBO journal. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nature neuroscience. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Martinez-Salgado C, Wemmie JA, Brennan TJ, Symonds E, Omari T, Lewin GR, Welsh MJ, et al. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127:1739–1747. doi: 10.1053/j.gastro.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Pan B, Geleoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron. 2013;79:504–515. doi: 10.1016/j.neuron.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo LA, Stuhmer W. The roles of K(+) channels in cancer. Nature reviews Cancer. 2014;14:39–48. doi: 10.1038/nrc3635. [DOI] [PubMed] [Google Scholar]
- Patel A. The Primary cilium calcium channels and their role in flow sensing. Pflugers Archiv : European journal of physiology. 2015;467:157–165. doi: 10.1007/s00424-014-1516-0. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nature neuroscience. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. The EMBO journal. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Current biology : CB. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Pereira V, Busserolles J, Christin M, Devilliers M, Poupon L, Legha W, Alloui A, Aissouni Y, Bourinet E, Lesage F, et al. Role of the TREK2 potassium channel in cold and warm thermosensation and in pain perception. Pain. 2014;155:2534–2544. doi: 10.1016/j.pain.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Perozo E. Gating prokaryotic mechanosensitive channels. Nature reviews Molecular cell biology. 2006;7:109–119. doi: 10.1038/nrm1833. [DOI] [PubMed] [Google Scholar]
- Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 2002a;418:942–948. doi: 10.1038/nature00992. [DOI] [PubMed] [Google Scholar]
- Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nature structural biology. 2002b;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- Perozo E, Rees DC. Structure and mechanism in prokaryotic mechanosensitive channels. Current opinion in structural biology. 2003;13:432–442. doi: 10.1016/s0959-440x(03)00106-4. [DOI] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Molecular pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyronnet R, Martins JR, Duprat F, Demolombe S, Arhatte M, Jodar M, Tauc M, Duranton C, Paulais M, Teulon J, et al. Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO reports. 2013;14:1143–1148. doi: 10.1038/embor.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyronnet R, Sharif-Naeini R, Folgering JH, Arhatte M, Jodar M, El Boustany C, Gallian C, Tauc M, Duranton C, Rubera I, et al. Mechanoprotection by polycystins against apoptosis is mediated through the opening of stretch-activated K(2P) channels. Cell reports. 2012;1:241–250. doi: 10.1016/j.celrep.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hearing research. 1984;15:103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Poole K, Herget R, Lapatsina L, Ngo HD, Lewin GR. Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nature communications. 2014;5:3520. doi: 10.1038/ncomms4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K, Moroni M, Lewin GR. Sensory mechanotransduction at membrane-matrix interfaces. Pflugers Archiv : European journal of physiology. 2015;467:121–132. doi: 10.1007/s00424-014-1563-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. The Journal of membrane biology. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157:447–458. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proceedings of the National Academy of Sciences of the United States of America. 2014a;111:10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014b;516:121–125. doi: 10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neuroscience letters. 1986;66:141–146. doi: 10.1016/0304-3940(86)90180-1. [DOI] [PubMed] [Google Scholar]
- Retailleau K, Duprat F. Polycystins and partners: proposed role in mechanosensitivity. The Journal of physiology. 2014;592:2453–2471. doi: 10.1113/jphysiol.2014.271346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodat-Despoix L, Hao J, Dandonneau M, Delmas P. Shear stress-induced Ca(2)(+) mobilization in MDCK cells is ATP dependent, no matter the primary cilium. Cell calcium. 2013;53:327–337. doi: 10.1016/j.ceca.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Sachs F. Stretch-activated ion channels: what are they? Physiology. 2010;25:50–56. doi: 10.1152/physiol.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. Journal of cell science. 2010;123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer WR. Mechanosensory molecules and circuits in C. elegans. Pflugers Archiv : European journal of physiology. 2015;467:39–48. doi: 10.1007/s00424-014-1574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, del Marmol J, MacKinnon R. Mechanistic basis for low threshold mechanosensitivity in voltage-dependent K+ channels. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10352–10357. doi: 10.1073/pnas.1204700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ER, Mastrotto M, Laursen WJ, Schulz VP, Goodman JB, Funk OH, Gallagher PG, Gracheva EO, Bagriantsev SN. Neuronal mechanism for acute mechanosensitivity in tactile-foraging waterfowl. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14941–14946. doi: 10.1073/pnas.1413656111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenk-Siemens K, Wende H, Prato V, Song K, Rostock C, Loewer A, Utikal J, Lewin GR, Lechner SG, Siemens J. PIEZO2 is required for mechanotransduction in human stem cell-derived touch receptors. Nature neuroscience. 2015;18:10–16. doi: 10.1038/nn.3894. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. The American journal of physiology. 1997;272:F132–F138. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, et al. Polycystin-1 and −2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, Gillespie PG, Muller U. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature. 2004;428:950–955. doi: 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- Sokabe M, Sachs F. The structure and dynamics of patch-clamped membranes: a study using differential interference contrast light microscopy. The Journal of cell biology. 1990;111:599–606. doi: 10.1083/jcb.111.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe M, Sachs F, Jing ZQ. Quantitative video microscopy of patch clamped membranes stress, strain, capacitance, and stretch channel activation. Biophysical journal. 1991;59:722–728. doi: 10.1016/S0006-3495(91)82285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RH, Chang G, Rees DC. 'Feeling the pressure': structural insights into a gated mechanosensitive channel. Current opinion in structural biology. 1999;9:448–454. doi: 10.1016/S0959-440X(99)80063-3. [DOI] [PubMed] [Google Scholar]
- Stocker M. Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nature reviews Neuroscience. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nature cell biology. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, Inoue T. Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nature methods. 2013;10:1105–1107. doi: 10.1038/nmeth.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S, Durell SR, Guy HR. Structural models of the MscL gating mechanism. Biophysical journal. 2001;81:917–936. doi: 10.1016/S0006-3495(01)75751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S, Sachs F. Molecular force transduction by ion channels: diversity and unifying principles. Journal of cell science. 2012;125:3075–3083. doi: 10.1242/jcs.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. The Journal of biological chemistry. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, Matzen J, Lao J, Tully DC, Engels IH, et al. Chemical activation of the mechanotransduction channel Piezo1. Elife. 2015:4. doi: 10.7554/eLife.07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988;242:1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Teng J, Loukin S, Anishkin A, Kung C. The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflugers Archiv : European journal of physiology. 2015;467:27–37. doi: 10.1007/s00424-014-1530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Tsien RY. Fluorescent probes of cell signaling. Annual review of neuroscience. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annual review of biochemistry. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilceanu D, Stucky CL. TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PloS one. 2010;5:e12177. doi: 10.1371/journal.pone.0012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344:634–638. doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- Vreugde S, Erven A, Kros CJ, Marcotti W, Fuchs H, Kurima K, Wilcox ER, Friedman TB, Griffith AJ, Balling R, et al. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nature genetics. 2002;30:257–258. doi: 10.1038/ng848. [DOI] [PubMed] [Google Scholar]
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, et al. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2007;445:206–209. doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- Woo SH, Lumpkin EA, Patapoutian A. Merkel cells and neurons keep in touch. Trends in cell biology. 2014a doi: 10.1016/j.tcb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014b;509:622–626. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Grillet N, Elledge HM, Wagner TF, Zhao B, Johnson KR, Kazmierczak P, Muller U. TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell. 2012;151:1283–1295. doi: 10.1016/j.cell.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493:221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. Journal of the American Society of Nephrology : JASN. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- Zhao B, Wu Z, Grillet N, Yan L, Xiong W, Harkins-Perry S, Muller U. TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron. 2014;84:954–967. doi: 10.1016/j.neuron.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Current biology : CB. 2010;20:429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Hein A, Hager U, Kaczmarek JS, Turnquist BP, Clapham DE, Reeh PW. Phenotyping sensory nerve endings in vitro in the mouse. Nature protocols. 2009;4:174–196. doi: 10.1038/nprot.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]