Abstract
We present results from a novel comparative approach to the study of the mechanisms of psychiatric disease. Previous work has examined neural activity patterns in the hippocampus of a freely behaving mouse model associated with schizophrenia, the calcineurin knockout mouse. Here, we examined a genetically distinct mouse model that exhibits a similar set of behavioral phenotypes associated with schizophrenia, a transgenic model expressing a putative dominant-negative DISC1 (DN-DISC1). Strikingly, the principal finding of the earlier work is replicated in the DN-DISC1 mice, that is, a selective increase in the numbers of sharp-wave ripple (SWR) events in the hippocampal local field potential (LFP), while at the same time other LFP patterns such as theta and gamma are unaffected. SWRs are thought to arise from hippocampal circuits and reflect the coordinated activity of the principal excitatory cells of the hippocampus in specific patterns that represent reactivated memories of previous experiences and imagined future experiences that predict behavior. These findings suggest that multiple genetic alterations could converge on distinct patterns of aberrant neurophysiological function to give rise to common behavioral phenotypes in psychiatric disease.
Key Words: Schizophrenia, Calcineurin knockout mice, DISC1 transgenic mice, Sharp-wave ripples, Hippocampus
Introduction
Psychiatric diseases such as schizophrenia have devastating effects on millions of people worldwide; however, an understanding of the diseases at a mechanistic level remains an elusive goal. For example, in the case of schizophrenia, no single gene is uniquely associated with the disorder, although many genes confer risk [1]. In this paper, we consider the properties of mice that are genetically engineered to exhibit behavioral phenotypes reminiscent of the symptoms of psychiatric disease [2]. We explore the hypothesis that convergent behavioral phenotypes in genetically different mouse models may be associated with convergent abnormalities in neural activity. Previous work has examined mice with a forebrain-specific knockout of the phosphatase calcineurin [3] exhibiting a range of schizophrenia-like behavioral phenotypes [4] and reported a dramatic pattern of overexcitability in neural circuits [5]. Using local field potential (LFP) and single-unit recordings from the hippocampus during free exploration, it was found that neural activity was normal during exploration but exhibited a 6-fold increase whenever mice paused. This overactivity was associated specifically with sharp-wave ripple (SWR) events in the hippocampal LFP, and an SWR-associated temporal pattern of unit activity known as ‘replay’ was abolished.
To address the hypothesis that convergent activity patterns underlie characteristic phenotypes relevant to schizophrenia, we have now examined a second mouse model that has been associated with schizophrenia. DISC1 was originally identified from a large Scottish family as a disrupted transcript by the hereditary balanced (1;11)(q42.1;q14.3) translocation that co-segregates mainly with schizophrenia and major depression [6,7]. Many biological studies have suggested that DISC1 plays a key role in brain development by modulating neurogenesis, outgrowth, dendritic arborization, and synapse formation [7,8,9]. In order to test the influence of DISC1 perturbation on circuitry and behavioral changes, many hereditary DISC1 genetic models have been generated [10,11]. C'-truncated DISC1 is postulated to act in a dominant-negative fashion (DN-DISC1) [12,13]. Transgenic mice expressing human DN-DISC1 under the αCaMKII promoter in the forebrain exhibit a range of anatomical and behavioral characteristics related to schizophrenia [10,11,14].
Here, we applied LFP recording methods to DN-DISC1 mice. We were thus able to compare electrophysiological phenotypes in the DN-DISC1 model with those of the genetically distinct calcineurin knockout model and correlate this comparison with the behavioral phenotypes in the two models. Using this novel comparative approach, we present evidence for a convergence at the level of neural activity between disparate potential mechanisms of psychiatric disease.
Methods
Subjects
All procedures were approved by the Johns Hopkins University Animal Care and Use Committee and followed US National Institutes of Health animal use guidelines. All neural recordings took place between 8 a.m. and 2 p.m., and animals were housed on a standard, noninverted, 12-hour light cycle. We used a transgenic mouse model expressing a C'-truncated human DISC1 under the αCaMK promoter (line 37), which was previously published [14]. The mouse line was maintained by heterozygous × C57BL/6N mating, and littermate adult (3-5 months) male heterozygous (n = 6) and control (n = 6) mice were compared in the experiments.
Implantation
We designed a lightweight 8-tetrode (2-gram) drive, which we implanted unilaterally into the left hippocampus. The drive was designed using Solidworks and produced using an epoxy resin (Accura 60). It had 2 bundles of 4 tetrodes, and each bundle was adjustable via a screw mechanism. Implantation required the placement of a craniotomy at −2.0 and +1.0 mm of bregma, the placement of 3 screws in the skull to provide stabilization of the drive, and 1 additional screw at +1.0 and +1.0 mm of bregma to provide an animal ground. The drive was then lowered and cemented in place using grip dental cement (Dentsply, York, Pa., USA). Animals were given 1 day for recovery from surgery, and tetrodes were lowered into the hippocampus over the following 3 days.
Behavior
After implantation, animals were housed individually and daily moved to the recording location for either adjustment or recording. When wires reached the hippocampus, animals were given 1 additional day to allow the wires and brain to settle. On the following day, animals were placed on a 120-cm track with 8-cm walls. The track was colored black with high-contrast white local cues equally spaced along the walls. Animals were allowed to freely explore the track for 60 min or 20 laps. After either 60 min elapsed or the animal completed 20 laps, whichever occurred first, animals were moved back into their home cage. For each animal, 2 days of track exploration were analyzed.
In vivo Recording
Data were collected using the Digital Lynx 4SX (Neuralynx, Bozeman, Mont., USA), and an overhead video system collected position data continuously at 60 Hz. The animal's position and heading direction were determined via two distinctly colored head-mounted LEDs. Continuous LFP was recorded continuously at 3,200 Hz and digitally filtered between 0.1 and 500 Hz.
Neural Data Analysis
For LFP analysis, 1 representative electrode was selected from each tetrode, and the 3 tetrodes with the strongest ripple activity were selected for further analysis. The z-score of each channel was taken to normalize for differences in recordings between animals. On these 3 tetrodes, the z-score of the LFP was band-pass filtered between 100 and 300 Hz, and the absolute value of the Hilbert transform of this filtered signal was then smoothed (Gaussian kernel, SD = 10 ms). This processed signal was then averaged across all tetrodes, and ripple events were identified as local peaks with an amplitude >5 SD above the mean during periods that the mouse's velocity was <1 cm/s. The start and end boundaries of each ripple event were defined as the point when the signal crossed mean + 2 SD. Finally, to prevent inappropriate splitting of ripple events, those that occurred within 100 ms of the end of the previous event were combined into a single event. The same ripple finding algorithm was also used for high gamma (frequency range 65-140). For theta power analysis, the raw LFP trace was band-pass filtered between 4 and 12 Hz during bouts of activity when the mouse's velocity exceeded 4 cm/s. The absolute value of the Hilbert transform for the filtered signal was z-scored. The power spectral density was then calculated using Welch's power spectral density estimate of this data. Low gamma (frequency range 30-90) was examined with the same algorithm used in the theta analysis as it is predominantly expressed during bouts of activity.
Statistical Analysis
For statistical analysis of ripple and high-gamma events, we used a nested ANOVA design to take into account each event, but not bias the data based on the differences in ripple numbers between animals (SAS, JMP 11).
Theta, gamma, and ripple numbers were statistically compared using a 2-way ANOVA followed by Bonferroni post hoc testing to account for changes over sessions as well as genotypes (GraphPad, Prism 6).
Results
To study ripple events in DN-DISC1 animals, we implanted microdrives targeting the CA1 region of the hippocampus in DN-DISC1 heterozygous animals and littermate controls. Following the previously described recording protocol used for calcineurin mice [5], recordings were made during exploration and stopping behavior in a novel linear track environment (see Methods section). To assess behavioral activity differences between the DN-DISC1 and control mice, we measured both running speed and total time spent in a behaviorally quiet state. No significant differences in behavior were observed as measured by running speed (12.14 ± 1.72 vs. 10.66 ± 2.07 cm/s, control vs. DN-DISC1, p = 0.602) or by total time active (38.42 ± 2.00 vs. 41.0 ± 2.60 min, control vs. DN-DISC1, p = 0.42).
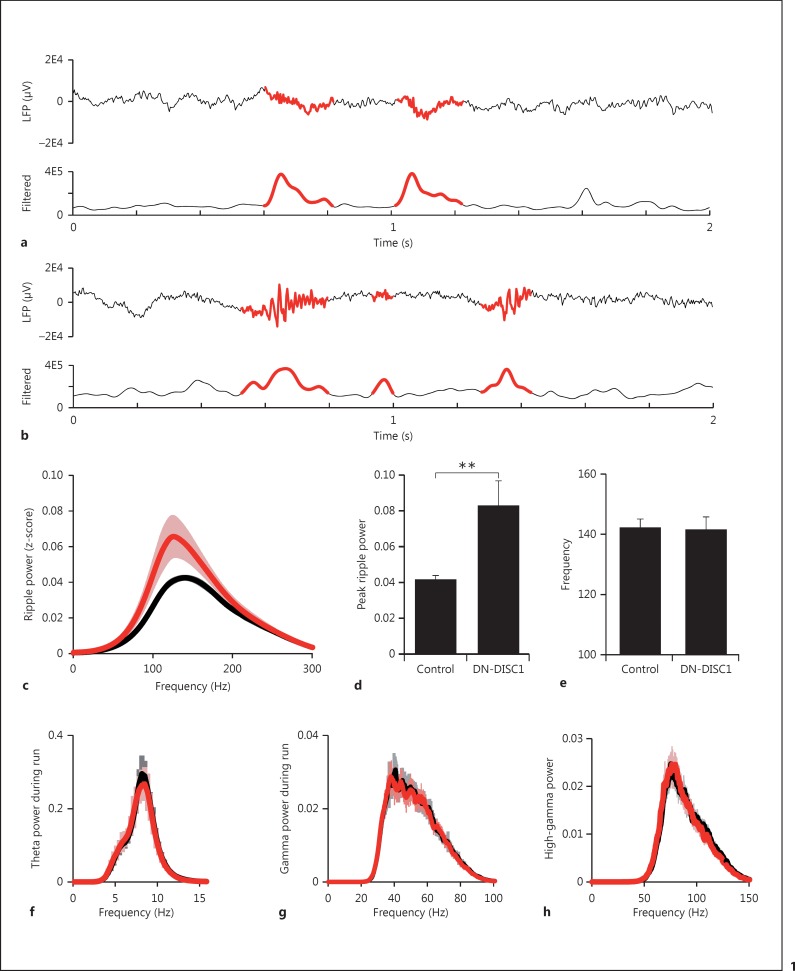
We identified ripple events from LFP filtered between 100 and 300 Hz using previously described methods [15,16] and determined the times during which the power of z-scored EEG within this frequency range exceeded 5 SD above the mean during periods of behavioral inactivity. Using this methodology, we determined that both transgenic and control animals exhibited distinct SWR events (fig. 1a, b). To test the hypothesis that changes in ripple event power underlie phenotypic differences associated with mouse models of schizophrenia, we examined the power of ripple events. We found that DN-DISC1 animals exhibited an increase in peak power (fig. 1c, d; 0.043 ± 0.0017 vs. 0.083 ± 0.14, control vs. DN-DISC1, p < 0.0038) but no changes in peak frequency (fig. 1e; 142.4 ± 2.6 vs. 141.7 ± 4.0 Hz, control vs. DN-DISC1, p = 0.7268).
Fig. 1.
DN-DISC1 animals show specific alterations in ripple activity. For both control (a) and DN-DISC1 (b) animals, the average of 3 LFP traces is shown in the upper panels with ripple events highlighted in red. In the lower panels, the amplitude of the filtered trace is shown with ripple events highlighted in red. c Comparison of spectral power of z-scored EEG filtered in the ripple frequency band (100-300 Hz) for control (black) and DN-DISC1 (red) animals during quiet wake ripple events. The mean total power of ripple events is more than 2-fold increased in DN-DISC1 animals (d); however, no change is observed in the peak ripple frequency of DN-DISC1 animals (e). f Spectral power of z-scored EEG filtered in the theta frequency band (4-12 Hz) during run shows overall similarity between control (black) and DN-DISC1 animals (red) of the run-associated oscillatory activity. g Spectral power of gamma (25-80) during run. h Spectral power in high gamma (65-140) during quiet wake. No changes were found between control and DN-DISC1 animals in either peak frequency or mean total power for theta, gamma, or high-gamma frequency ranges. ** p < 0.01.
To test the specificity of these changes in ripple event power, we examined power in the theta and gamma ranges, which are associated with stimulus-driven activity states such as exploratory behavior. We observed no change in theta power during periods when the animal was behaviorally active (fig. 1f; 0.47 ± 0.04 vs. 0.46 ± 0.5, control vs. DN-DISC1, p = 0.41) nor a significant change in theta frequency (fig. 1f; 7.07 ± 0.22 vs. 7.01 ± 0.22 Hz, control vs. DN-DISC1, p = 0.433). Similarly, we found no change in the gamma range during behaviorally active periods (fig. 1g; frequency: 43.5 ± 2.9 vs. 40.2 ± 1.68 Hz, p = 0.30; power: 0.05 ± 0.019 vs. 0.053 ± 0.018, control vs. DN-DISC1, p = 0.68).
To explore the possibility that all oscillations associated with quiet wake activity are altered in DN-DISC1 animals, we isolated high gamma associated with quiet wake behavior. In contrast to the significant increase identified in DN-DISC1 ripple power, no changes were found in high gamma (fig. 1h; frequency: 77.3 ± 1.69 vs. 76.21 ± 1.01 Hz, p = 0.58; power: 0.045 ± 0.006 vs. 0.047 ± 0.0084, control vs. DN-DISC1, p = 0.63). These results suggest that the alterations in hippocampal oscillations associated with the transgenic expression of truncated DN-DISC1 are specific to high-frequency ripple events and not associated with all behaviorally quiet neural activity.
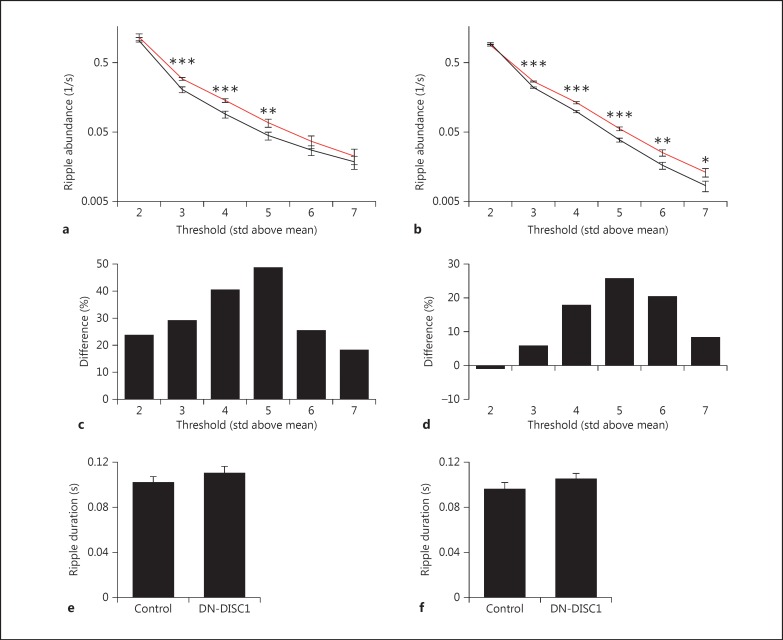
The specific change in ripple oscillations in the hippocampus led us to hypothesize that the increase in power may be accompanied by further changes in ripple events, such as the frequency of occurrence and the average duration. To determine the frequency of occurrence, ripple events were detected across a range of thresholds from 2 through 7 SD above the mean ripple-filtered power, and the total number was normalized to the total time spent behaviorally quiet. On both the track and in the home cage, as the ripple threshold was raised, the number of events decreased; however, across multiple thresholds, DN-DISC1 animals exhibited a significantly increased frequency of event occurrence (fig. 2a, b). When the events were binned to analyze changes at each threshold range, the greatest difference was observed between 5 and 6 SD above the mean ripple power (fig. 2c, d). An increased frequency of occurrence as well as an increased ripple power during ripple events suggest that DN-DISC1 animals have a hyperexcitable neural circuit regulating hippocampal ripple events. The average duration was examined on the track and in the home cage; however, no significant differences were found (fig. 2e, f; track: 0.102 ± 0.0048 vs. 0.110 ± 0.0057 s, p = 0.286; home cage: 0.096 ± 0.0056 vs. 0.1056 ± 0.0043 s, control vs. DN-DISC1, p = 0.1922).
Fig. 2.
DN-DISC1 animals have increased ripple activity. The number of ripple events per second is significantly greater in DN-DISC1 animals across multiple thresholds (2-7 SD) on the track (a) and in the home cage (b). * p < 0.05; ** p < 0.01; *** p < 0.001. Binned data show that the most dramatic increase in events occurs between 4 and 6 SD above the mean and tapers at thresholds above and below both on the track (c) and in the home cage (d). No change in ripple duration was detected between control and DN-DISC1 animals on the track (e) and in the home cage (f).
Discussion
A variety of genetic and environmental factors can contribute to the development of schizophrenia. In the present study, we hypothesized that different etiologies may result in common pathophysiology or circuitry changes underlying the characteristic symptoms. To test this idea, we have used a novel comparative approach in which genetically distinct models of disease are compared to identify shared neural activity phenotypes (table 1). Recent work in a mouse model with behavioral phenotypes relevant to severe psychiatric illness, the forebrain-specific calcineurin knockout mouse, has shown specific changes in high-frequency ripple events within the hippocampus [5]. Specifically, when animals were at rest, high-frequency events associated with internally generated sequences were disrupted, while hippocampal oscillations associated with stimulus-driven activity states, such as theta, were not altered. Previous studies have shown severe impairments in long-term depression in these mice, likely creating an imbalance in synaptic plasticity that could play a significant role in the resulting phenotype [3]. We hypothesized that other genetic models with phenotypes relevant to schizophrenia might also show increased ripple activity. To test this hypothesis, we examined hippocampal EEG in another model of schizophrenia, the DN-DISC1 transgenic mouse line [14,17,18]. This mouse line has a behavioral profile that mirrors that seen in the forebrain-specific calcineurin knockout model; however, the mechanisms thought to underlie the phenotypes are unrelated. Strikingly, we found that at the level of hippocampal population activity, both animal models exhibit a similar pattern of selective overactivity during rest periods, in particular during SWRs. These behavioral and neurophysiological similarities are summarized in table 1. Intriguingly, hyperexcitable hippocampal circuits may underlie the similarities between these two mouse lines. Speculatively, hyperexcitability could result from impaired hippocampal long-term depression in the calcineurin knockout model, but in the DN-DISC1 mouse model it could result from the reduced function of parvalbumin-containing inhibitory interneurons. In each case, the resulting increase in the excitability of pyramidal cells might have caused both an increased abundance of ripple events and an increased participation of these cells in ripple events. The former result has now been established directly for both mouse models, and the latter result has been shown directly for calcineurin knockout mice and is strongly suggested for DN-DISC1 mice by the increased power of ripples that we observed. Given the putative role of SWRs in working memory and flexible cognition [19,20,21,22], this suggests a novel circuit mechanism for psychiatric disease. To our knowledge, this work represents the first demonstration that two distinct animal models show a convergence of altered neural activity relevant to a psychiatric disorder.
Table 1.
Comparison between mouse models of psychiatric disease
| Feature | Calcineurin knockout | DN-DISC1 |
|---|---|---|
| Promoter | αCaMKII | αCaMKII |
| Background strain | C57BL/6 [3] | C57BL/6N [14] |
| Gross brain morphology | No change [3] | No change [14] |
| Noncognitive behaviors | ||
| Locomotion | ↑ [3] | ↑ [14] |
| Anxiety | ↓ [3] | No change [14] |
| FST immobility | ↓ [3] | ↑ [14] |
| Amphetamine response | No change [4] | ↑ [17] |
| Cognitive behaviors | ||
| Prepulse inhibition | ↓ [4] | ↓ [14] |
| Reference memory | No change [3] | No change [14] |
| Social interaction | ↓ [4] | ↓ [18] |
| Working memory | ↓ [3] | Unknown |
| Neurophysiology | ||
| Ripple frequency | No change [5] | No change |
| Ripple occurrence | ↑ [5] | ↑ |
| Ripple power | ↑ [5] | ↑ |
| Theta frequency | No change [5] | No change |
| Theta power | No change [5] | No change |
| Gamma frequency | No change [5] | No change |
| Gamma power | No change [5] | No change |
Behavioral comparison of forebrain-specific calcineurin knockout and DN-DISC1 animals shows several key similarities including impaired prepulse inhibition and decreased social interaction. Neurophysiological phenotypes associated with cognition are also similarly affected with specific changes in ripple parameters but not theta or gamma.
Figures in brackets are the corresponding references.
Recent fMRI studies in schizophrenia patients have found altered neural activity in the default mode network (DMN), which consists of several brain regions involved in quiet wake behaviors such as daydreaming [23,24,25]. These studies revealed a higher correlation in activity among the DMN areas, an increase in network activity associated with positive symptoms (hallucinations, delusions, and thought confusions) during quiet wake periods, and accentuated DMN activity during passive task epochs, and suggest that the DMN may be overactive and that the heterogeneous etiology of schizophrenia may converge upon similar neurophysiological alterations. Multiunit and EEG recordings in the rodent hippocampus have revealed activity thought to relate to high-order cognitive processes and activity within the DMN [26]. Specifically, reactivation of the neural ensembles involved in spatial navigation occurs during quiet wake immediately following active exploration in an activity pattern known as replay [16,19,27,28,29]. These replay activity patterns also occur during decision-making, where they play a necessary role [21], and predict the animal's future behavior [22,30]. The replay of neural ensemble activity is necessary for normal memory consolidation [20,31,32] and has been shown to be more than a simple recall of a recent experience [29]; for example, it can correspond to the depiction of imagined trajectories that the animal has not taken before but is planning to take [22]. EEG from region CA1 within the hippocampus shows that high-frequency ripple events co-occur with replay and are thought to correspond to the depolarization of large numbers of pyramidal cells [33,34,35]. Both hippocampal replay and hippocampal ripples provide an accessible means of studying circuit activity in the hippocampus as they may relate to changes in DMN activity.
The confluence of human imaging studies and neural recordings in two distinct mouse models relevant to schizophrenia suggests that the DMN and, in particular, the hippocampus are overactive during quiet wake behavior in schizophrenia. Furthermore, the fact that two distinct mouse models exhibit similar neurophysiological phenotypes suggests that heterogeneous etiology can lead to a convergence of the neural phenotype that results in a common behavioral phenotype. This work raises the possibility of many distinct mutations that may lead to symptoms of psychiatric disease and perhaps also many pathways in which these mutations might be involved that would lead to common neurophysiological changes responsible for the ultimate phenotypic differences. One approach to treating these convergent disorders may be therapeutic intervention at the level of the neurophysiological phenotype.
Disclosure Statement
The authors declare no competing financial interests.
Acknowledgements
This work was supported by the National Institute for Mental Health R21 MH086702 (to D.J.F.), a NARSAD Young Investigator grant (to D.J.F.), F32 MH102023 (to C.A.), P50 MH094268 (to A.S.), and RO1 MH069853 (to A.S).
References
- 1.McCarroll SA, Hyman SE. Progress in the genetics of polygenic brain disorders: significant new challenges for neurobiology. Neuron. 2013;80:578–587. doi: 10.1016/j.neuron.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Tuathaigh CM, Waddington JL. Mutant mouse models: phenotypic relationships to domains of psychopathology and pathobiology in schizophrenia. Schizophr Bull. 2010;36:243–245. doi: 10.1093/schbul/sbq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- 4.Miyakawa T, Leiter LM, Gerber DJ, Gainetdinov RR, Sotnikova TD, Zeng H, Caron MG, Tonegawa S. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci USA. 2003;100:8987–8992. doi: 10.1073/pnas.1432926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh J, Foster DJ, Davoudi H, Wilson MA, Tonegawa S. Impaired hippocampal ripple-associated replay in a mouse model of schizophrenia. Neuron. 2013;80:484–493. doi: 10.1016/j.neuron.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders - cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porteous DJ, Millar JK, Brandon NJ, Sawa A. DISC1 at 10: connecting psychiatric genetics and neuroscience. Trends Mol Med. 2011;17:699–706. doi: 10.1016/j.molmed.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 2011;12:707–722. doi: 10.1038/nrn3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayan S, Nakajima K, Sawa A. DISC1: a key lead in studying cortical development and associated brain disorders. Neuroscientist. 2013;19:451–464. doi: 10.1177/1073858412470168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaaro-Peled H. Gene models of schizophrenia: DISC1 mouse models. Prog Brain Res. 2009;179:75–86. doi: 10.1016/S0079-6123(09)17909-8. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone M, Thomson PA, Hall J, McIntosh AM, Lawrie SM, Porteous DJ. DISC1 in schizophrenia: genetic mouse models and human genomic imaging. Schizophr Bull. 2011;37:14–20. doi: 10.1093/schbul/sbq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, Sawamura N, Park U, Kudo C, Okawa M, Ross CA, Hatten ME, Nakajima K, Sawa A. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 13.Sawa A, Snyder SH. Genetics. Two genes link two distinct psychoses. Science. 2005;310:1128–1129. doi: 10.1126/science.1121114. [DOI] [PubMed] [Google Scholar]
- 14.Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andrade M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci USA. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skaggs WE, McNaughton BL, Permenter M, Archibeque M, Vogt J, Amaral DG, Barnes CA. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. J Neurophysiol. 2007;98:898–910. doi: 10.1152/jn.00401.2007. [DOI] [PubMed] [Google Scholar]
- 16.Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63:497–507. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson AW, Jaaro-Peled H, Shahani N, Sedlak TW, Zoubovsky S, Burruss D, Emiliani F, Sawa A, Gallagher M. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc Natl Acad Sci USA. 2013;110:12462–12467. doi: 10.1073/pnas.1307925110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaaro-Peled H, Niwa M, Foss CA, Murai R, de Los Reyes S, Kamiya A, Mateo Y, O'Donnell P, Cascella NG, Nabeshima T, Guilarte TR, Pomper MG, Sawa A. Subcortical dopaminergic deficits in a DISC1 mutant model: a study in direct reference to human molecular brain imaging. Hum Mol Genet. 2013;22:1574–1580. doi: 10.1093/hmg/ddt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 20.Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meda SA, Ruano G, Windemuth A, O'Neil K, Berwise C, Dunn SM, Boccaccio LE, Narayanan B, Kocherla M, Sprooten E, Keshavan MS, Tamminga CA, Sweeney JA, Clementz BA, Calhoun VD, Pearlson GD. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci USA. 2014;111:E2066–E2075. doi: 10.1073/pnas.1313093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant ‘default mode’ functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 26.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singer AC, Carr MF, Karlsson MP, Frank LM. Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron. 2013;77:1163–1173. doi: 10.1016/j.neuron.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 32.Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buzsaki G. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 34.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 35.Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J Neurosci. 1999;19:RC20. doi: 10.1523/JNEUROSCI.19-16-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]