Abstract
Background. Highly challenging exercises have been suggested to induce neuroplasticity in individuals with Parkinson’s disease (PD); however, its effect on clinical outcomes remains largely unknown. Objective. To evaluate the short-term effects of the HiBalance program, a highly challenging balance-training regimen that incorporates both dual-tasking and PD-specific balance components, compared with usual care in elderly with mild to moderate PD. Methods. Participants with PD (n = 100) were randomized, either to the 10-week HiBalance program (n = 51) or to the control group (n = 49). Participants were evaluated before and after the intervention. The main outcomes were balance performance (Mini-BESTest), gait velocity (during normal and dual-task gait), and concerns about falling (Falls Efficacy Scale–International). Performance of a cognitive task while walking, physical activity level (average steps per day), and activities of daily living were secondary outcomes. Results. A total of 91 participants completed the study. After the intervention, the between group comparison showed significantly improved balance and gait performance in the training group. Moreover, although no significant between group difference was observed regarding gait performance during dual-tasking; the participants in the training group improved their performance of the cognitive task while walking, as compared with the control group. Regarding physical activity levels and activities of daily living, in comparison to the control group, favorable results were found for the training group. No group differences were found for concerns about falling. Conclusions. The HiBalance program significantly benefited balance and gait abilities when compared with usual care and showed promising transfer effects to everyday living. Long-term follow-up assessments will further explore these effects.
Keywords: dual task, exercise, gait, physical activity, postural control
Introduction
Parkinson’s disease (PD) leads to the deterioration of gait and balance abilities,1 a negative trend of falls and injuries,2 fear of falling,3 a decline in physical activity,4,5 and decreased quality of life.6 Pharmacological treatment is the first-choice therapy for PD; however, despite optimal medical management, individuals with PD still experience balance impairments.7 Therefore, to prevent balance-related problems and to facilitate the maintenance of a physically active lifestyle, effective nonpharmaco-logical strategies such as novel exercise regimes need to be established for PD.
The pathophysiology of balance impairments in PD incorporates multiple subsystems (sensory, motor, and cognition).1,8 Sensory problems compromise equilibrium, particularly owing to impaired proprioception and problems integrating different sensory modalities into a frame of reference for the body.8 Motor features (eg, bradykinesia, impaired coordination, and inflexible motor commands) further constrain the efficiency of postural adjustments to anticipate upcoming perturbations or to allow recovery from instability.1 Also essential for balance control is the ability to perform a motor task while simultaneously engaging in a cognitively demanding task, that is, dual-tasking (DT). In individuals with PD, DT leads to degraded balance and gait performance, resulting in vulnerability to falls during many daily activities.9-11 Although recent studies have indicated improved gait performance after DT training in individuals with PD,12-14 hitherto no randomized controlled trial has confirmed this finding.
To be successful, all types of training, including balance exercises, need to be performed at or near the limits of one’s capacity.15 For individuals with PD, exercise is an essential part of treatment,16,17 and recent experimental studies have shown that training, particularly when highly challenging, induces neuroplasticity in animals18,19 and in humans with PD.20,21 However, balance training compromising highly challenging exercises has been sparsely tested in clinical trials involving persons with PD.17 Furthermore, training that involves challenging motor and cognitive demanding exercises may induce synergistic effects for brain plasticity,18 which could lead to enhanced transfer of training effects to real-life situations.22
Based on a translational approach that integrates knowledge from basic science, motor learning, and clinical practice, we have designed a program that emphasizes highly challenging aspects of balance control, the HiBalance program.23 To address PD-specific balance impairments, this program was developed by linking PD symptoms to core areas of balance control and was subsequently translated into principles of training.23,24 The present study aimed to investigate the short-term effects of a 10-week balance program compared with usual care in the elderly with mild to moderate PD. We hypothesized that balance training would lead to specific improvements on balance and gait performance (single-task and DT, respectively), and that these effects also would transfer to everyday living (ie, concerns about falling, physical activity levels and activities of daily living).
Methods
Design
This study, approved by the Regional Ethical Board in Stockholm, was carried out as a randomized controlled study for elderly individuals with PD (trial registration: NCT01417598; for study protocol, see Conradsson et al23). Data were collected from the spring of 2012 to the spring of 2013.
Study Population
Community-dwelling individuals with a clinical diagnosis of idiopathic PD (Queens Square Brain Bank criteria)25 were recruited via advertisements in local newspapers, from Karolinska University Hospital and outpatient neurological clinics in Stockholm County. Based on a clinical assessment, we included individuals with impaired balance, such as instability during postural transfers and gait impairments. This approach aimed to apply clinical reasoning by recruiting individuals who would be assigned to balance training in clinical practice. In addition, inclusion criteria included a Hoehn and Yahr score of 2 or 3,26 age ≥60 years, the ability to independently ambulate indoors without a walking aid, and ≥3weeks of stable anti-Parkinson’s medication. Exclusion criteria were a Mini-Mental State Examination score27 of <24 and other medical conditions that would substantially influence balance performance or participation in the intervention. The assessment for eligibility covered 3 steps: First, participants reported their interest to one of the study coordinators. This was followed by a telephone interview to screen whether the volunteer met the inclusion criteria. Finally, eligible participants were called for baseline testing that evaluated their cognitive function and balance performance. All participants signed an informed consent form before entering the study.
Participants who met the criteria for inclusion were divided into 2 geographic cohorts, and after baseline testing the participants in each cohort were randomized in blocks of 4 to either the training group or the control group. The random sequence for group allocation was performed by one of the study coordinators using Web-based software. To ensure that testers and participants were unaware of the group allocation, opaque envelopes (sealed and numbered) were used. Masking of the test leaders was not possible after baseline assessments, given that some also served as trainers for the balance training. During the follow-up assessments, participants were never assessed by a test leader who had been involved in their training.
The sample-size calculation, detailed in the study protocol,23 was based on a pilot study24 and similar intervention studies in PD.12,28 The power calculation was performed separately for the 3 main outcome measures: (a) balance performance assessed with the Mini Balance Evaluation Systems Test (Mini-BESTest), (b) gait velocity measured during single-task and DT conditions, and (c) concerns about falling evaluated with the Falls Efficacy Scale–International (FES-I). In order to achieve 80% power with a 2-sided α level of 5%, the number of subjects required per group and the hypothesized effect size, respectively, was 24 (effect size = 0.83) for Mini-BESTest, 27 (effect size = 0.83) for gait velocity and 32 (effect size = 0.71) for FES-I. Altogether, by taking an anticipated dropout rate of 15% into account, a sample size of 40 in each group was warranted (total n = 80). However, because of long-term follow-up (not included in the present study), the group size was increased to 50 subjects (total n = 100).
Testing Procedure
Participants were assessed by experienced physiotherapists at baseline (pretest) and at the 10-week follow-up (posttest). All participants followed their normal scheme for PD medication and were tested during the on-phase at the same time of the day at the pre- and posttest. Data collection comprised 2 steps: first, physical tests of gait and balance performance and self-reported questionnaires in a movement laboratory; and subsequently, objectively measured physical activity levels in free-living conditions. To avoid bias, the order of the balance and gait tests was randomized and preceded by a standardized learning session. Demographic data (age, gender, body weight, and length), fall history, and the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS) were recorded at pretest. In addition, at both test occasions, participants’ daily levodopa equivalency dose was recorded.29
Balance and Gait Outcomes
The Mini-BESTest is a 14-item clinical test that covers 4 components of balance control (anticipatory postural adjustments, postural responses, sensory orientation, and stability in gait).30 Each item is scored from 0 (unable or requiring help) to 2 (normal), and the maximum score is 28 points.30
Gait characteristics were assessed with a 9-meter electronic walkway system, GAITRite (CIR Systems, Inc, Havertown, PA, USA) during normal walking and while performing an additional cognitive task (DT; reciting every second letter of the Swedish alphabet). The participants were instructed to walk at a normal pace during both conditions, placing equal focus on the walking and the cognitive task. Each gait condition was performed 6 times, and an average value was used for analysis. In addition to gait velocity, step length and cadence were also analyzed for both gait conditions. To gain more insight into DT performance, we evaluated the performance of the cognitive task during walking (DT) and as a single task while seated. For the performance of the cognitive task, percentage of errors (ie, the number of errors/total number of letters recited) was used for analysis.
Balance performance was also assessed with the Modified Figure-of-Eight test (MFE).31 The participants were instructed to walk 2 cycles on a figure-of-eight course (marked on the floor with a 40 mm wide tape, each loop having an internal diameter of 1.63 m) as fast as possible while trying to step on the tape. The test was performed twice where the average time and number of oversteps (ie, steps not touching the tape) were used for analysis.31
Activity Outcomes
Physical activity level (steps per day) was assessed during free-living conditions, using an accelerometer worn around the waist (Actigraph GT3X+, Pensacola, FL, USA). Participants were instructed to wear the accelerometer throughout the day and to record on a log sheet the exact times the device was worn. Data were downloaded and processed with ActiLife 6 software. Participants with 4 or more valid days were included in the analysis since this has shown to be reliable in adult and elderly populations.32,33 In order for a day to be considered valid for analysis, we required data of at least 9 hours (>90 consecutive minutes of zeros was considered nonwear time).32
Activities of daily living were assessed with the second part of the UPDRS, a 13-item questionnaire focusing on the effects of PD on various daily activities (eg, speech, handwriting, dressing, and mobility). The UPDRS-ADL is reported on a 4-point scale and is summarized into a total score (range 0-52), where a higher score indicates more limitations.34
Concerns About Falling Outcome
Concerns about falling was assessed with FES-I, a 16-item questionnaire measuring concern about falling during different activities. Each item, graded on a 4-point scale, is calculated to a sum score (range 16-64), where higher scores indicate greater concerns about falling.35
The HiBalance Program
The HiBalance training program has proved feasible in clinical practice,24 and its content and progression have been detailed in a previous publication.23 The balance training was performed in groups of 4 to 7 participants, 3 times per week, 60 minutes per session, for 10 weeks at a university hospital. Because of the highly challenging exercises, each session was supervised by 2 physiotherapists. Importantly, no fixed scheme of predetermined exercises was used in the intervention. Instead, a framework based on motor-learning principles (specificity, progressive overload, and variation) was used as a foundation for the application and adaptation of exercises to the participants’ individual abilities. Consequently, this approach not only resembles clinical practice but also requires continuous evaluation, modification, and planning of the training. Therefore, all the trainers involved in this study were physiotherapists (n = 10) educated in the framework of this training concept during two 4-hour sessions of both theory and practice. In addition, to ensure alignment to the HiBalance program, the trainers were supported in the practical aspects of the training when needed.
To target cognitive impairments, DT exercises were gradually integrated into the program by adding concurrent cognitive (eg, counting, remembering items) and/or motor tasks (eg, carrying and/or manipulating objects) to the balance exercises. These DT exercises were not the same tasks as those used during pre- and posttest. Moreover, 4 balance components specific to PD impairments were emphasized: (a) sensory integration (walking tasks on varying surfaces with or without visual constraints); (b) anticipatory postural adjustments (voluntary arm/leg/trunk movements, postural transitions, and multidirectional stepping, emphasizing movement velocity and amplitude); (c) motor agility (interlimb coordination under varying gait conditions and quick shifts of movement characteristic during predictable and unpredictable conditions); and (d) stability limits (controlled leaning tasks performed while standing with varying bases of support, stimulating weight shifts in multiple directions).
Highly challenging training conditions were defined as exercises where the participants were forced, intermittently, to use reactive postural adjustments to control their balance during single-tasking. Similarly, the level of difficulty for DT exercises was aimed at a level where there was consistent interference of the participants’ motor performance. Moreover, to promote progressive overload and exercise variation, the 10-week period was divided into 3 blocks (A, B, C). In block A (weeks 1-2), participants were introduced to the single-task exercises of each balance component separately (no DT-exercises were practiced), emphasizing quality of movement and the objectives of the exercises. In block B (weeks 3-5), basic DT exercises were introduced (approximately 40% of each session), and the level of difficulty for each balance component was increased. The level of difficulty of all the exercises was further enhanced in block C (weeks 6-10) by increasing the variation through exercises that combined several balance components, as well as the level of difficulty and time spent on DT exercises (approximately 60% of each session).
The participants in the control group were encouraged to maintain their normal physical activities and were not restricted from participation in ongoing rehabilitation programs. All participants were advised to keep up their normal level of exercise throughout the intervention period.
Data Analysis
Statistical analysis was conducted using STATISTICA software (Statsoft, version 12, Tulsa, OK, USA). The Student t test, Mann-Whitney test, and the χ2 test were used to assess the homogeneity of the groups at baseline. To test for equality of variance and data normality, Levene’s test was used, combined with a visual inspection of the normally distributed and residual curve. On fulfillment of these criteria, a 2-factor repeated-measures analysis of variance was performed to test for interaction effects between groups (training group vs control group) and with time (pretest and posttest). In the case of significant interaction effects, Tukey’s honestly significant difference (HSD) post hoc analyses were performed to assess differences between pre- and posttest. For outcomes with skewed data distributions, log-transformations were conducted, and if normally distributed afterward, an analysis of variance was used. For outcomes without normal distribution even after log-transformation (ie, performance of the cognitive DT and MFE), the Mann-Whitney U test was used to determine between-group differences (ie, calculated as the difference between pre- and posttest performances) and if significant, the Wilcoxon signed rank test was used to determine within-group differences between pre- and posttest in each group separately. Effect size between the 2 independent groups was computed using Cohen’s d calculation.36 We used both an intention-to-treat (last value carried forward data imputation) and a per-protocol approach. However, since these analyses revealed similar results, and given the small dropout rate, only the results for the per-protocol analysis are reported. Significance level was set at P ≤ .05.
Results
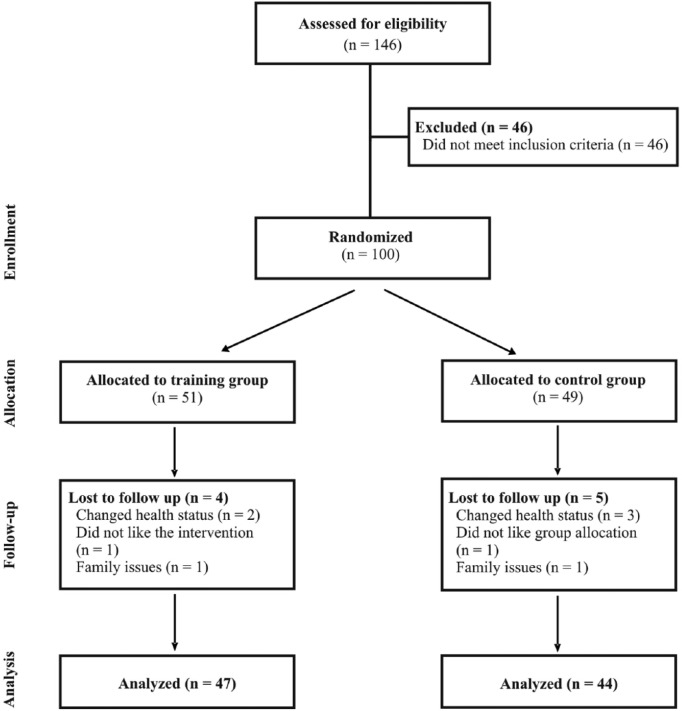
There were no significant differences between groups regarding demographic data, PD severity, daily levodopa equivalent dose, fall history (Table 1) or for any outcome measure at pretest (P > .05). The dropout rate and reasons were similar between groups (see Figure 1). Regarding the daily levodopa equivalency dose throughout the intervention, 70% in both groups had an unchanged dosage, and no significant group differences were found for the proportion that increased (training group 17%, control group 25%; P = .498) or decreased their dosage (training group 13%, control group 5%; P = .311).
Table 1.
Participant Characteristics.a
| Training Group (n = 47) | Control Group (n = 44) | P | |
|---|---|---|---|
| Age (years) | 72.9 (6.0) | 73.6 (5.3) | .488 |
| Gender, male/female | 28 (60) / 19 (40) | 23 (51) / 22 (49) | .200 |
| Body weight (kg) | 75.8 (14.1) | 76.4 (13.9) | .955 |
| Body height (cm) | 171.8 (9.2) | 171.2 (9.0) | .922 |
| H&Y stage, 2/3 | 20 (43) / 27 (57) | 19 (43) / 25 (57) | 1.000 |
| UPDRS motor | 36 (10) | 37 (11) | .711 |
| PD duration (years) | 6.0 (5.1) | 5.6 (5.0) | .693 |
| Levodopa equivalent dosageb | 581 (295) | 645 (404) | .821 |
| Recurrent faller, yes/noc | 25(53) / 22 (47) | 24 (55) / 20 (45) | .777 |
Abbreviations: H&Y, Hoehn and Yahr; UPDRS, Unified Parkinson’s Disease Rating Scale; PD, Parkinson’s disease.
Continuous data presented as mean (standard deviation) and nominal data as proportions (percentages).
Daily levodopa dose equivalency calculated in accordance to Tomlinson et al.29
Participants who had experienced ≥2 falls during the previous 12 months were classified as recurrent fallers.
Figure 1.
CONSORT flow diagram illustrating recruitment, randomization, and tracking of the participants over the course of the study.
Effect of Training on Balance and Gait
We found highly significant interaction effects for the Mini-BESTest, F(1, 89) = 15.49, P = .001, gait velocity, F(1, 88) = 7.19, P = .009, and step length during normal walking, F(1, 88) = 7.90, P = .006 (Table 2, Figure 2), representing a significant improvement over time in the training group by 3 points, 0.1 m/s, and 0.04 m, respectively (P < .001), while no statistical difference occurred in the control group. Regarding DT gait performance, there were no interaction effects for any of the gait parameters (velocity, step length, and cadence). However, we found a significant between group difference at posttest for the performance of cognitive DT while walking (P = .006), demonstrating an 8% improvement in the training group (P = .006), contrasting with the unchanged performance in the control group (P = .291). There were no differences between groups for the performance of the cognitive task while seated or MFE.
Table 2.
Treatment Effects for the Training Group and Control Group at Baseline and 10-Week Follow-up.
| Training Group |
Control Group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SE) |
Mean (95% CI) |
n | Mean (SE) |
Mean (95% CI) |
Pb |
|||||
| n | Pretest | Posttest | Difference | Pretest | Posttest | Difference | Effect Sizea | Interaction Effect | ||
| Mini-BESTest (0-28) | 47 | 19.2 | 22.2 | 3.0 | 44 | 18.4 | 19.3 | 0.9 | 0.82 | <.001 |
| (0.5) | (0.5) | (2.3 to 3.7) | (0.5) | (0.5) | (0.0 to 1.7) | |||||
| Norm velocity (m/s)c | 46 | 1.19 | 1.28 | 0.10 | 44 | 1.16 | 1.17 | 0.00 | 0.58 | .009 |
| (0.03) | (0.03) | (0.04 to 0.14) | (0.03) | (0.03) | (–0.03 to 0.05) | |||||
| Norm step length (m)c | 46 | 0.63 | 0.67 | 0.04 | 44 | 0.62 | 0.62 | 0.00 | 0.72 | .006 |
| (1.27) | (1.32) | (0.02 to 0.06) | (1.30) | (1.35) | (–0.01 to 0.02) | |||||
| Norm cadence (steps/min)c | 46 | 113 | 115 | 3 | 44 | 113 | 113 | 0 | 0.43 | .077 |
| (1) | (1) | (1 to 5) | (1) | (1) | (–2 to 2) | |||||
| DT velocity (m/s) | 45 | 0.98 | 1.07 | 0.09 | 42 | 0.90 | 0.96 | 0.06 | 0.15 | .547 |
| (0.04) | (0.04) | (0.03 to 0.15) | (0.04) | (0.04) | (0.00 to 0.13) | |||||
| DT step length (m) | 45 | 0.59 | 0.63 | 0.04 | 42 | 0.56 | 0.58 | 0.03 | 0.14 | .340 |
| (1.46) | (1.51) | (0.02 to 0.06) | (1.51) | (1.56) | (0.00 to 0.05) | |||||
| DT cadence (steps/min) | 45 | 100 | 103 | 3 | 42 | 97 | 99 | 3 | 0.00 | .946 |
| (3) | (3) | (–2 to 7) | (3) | (3) | (–1 to 6) | |||||
| Average steps per day | 37 | 4842 | 5123 | 282 | 32 | 4695 | 4147 | −548 | 0.52 | .033 |
| (528) | (545) | (–206 to 768) | (568) | (587) | (–1164 to 68) | |||||
| FES-Id (16-64) | 47 | 30.1 | 27.3 | −2.8 | 44 | 28.8 | 26.5 | −2.3 | 0.07 | .636 |
| (1.4) | (1.2) | (–5.1 to −0.5) | (1.4) | (1.2) | (–4.6 to −0.1) | |||||
| UPDRS-ADL (0-52) | 47 | 14.0 | 12.3 | −1.7 | 44 | 12.8 | 13.2 | 0.4 | 0.69 | .001 |
| (0.7) | (0.7) | (–2.6 to −0.8) | (0.7) | (0.7) | (–0.5 to 1.3) | |||||
| Training Group |
Control Group |
|||||||||
| Median (IQR) |
Median (IQR) |
n |
Median (IQR) |
Median (IQR) |
P |
|||||
| n | Pretest | Posttest | Difference | Pretest | Posttest | Difference | Effect Size | Between Group | ||
| Cognitive DT (% error) | 45 | 24 | 16 | −9 | 42 | 19 | 24 | 2 | 0.48 | .006 |
| (13) | (20) | (18) | (30) | (23) | (15) | |||||
| Cognitive ST (% error) | 45 | 11 | 10 | 0 | 42 | 16 | 12 | 0 | 0.19 | .634 |
| (21) | (20) | (19) | (28) | (31) | (17) | |||||
| MFE-time (seconds) | 44 | 25 | 24 | −3 | 39 | 27 | 26 | −2 | 0.00 | .506 |
| (13) | (13) | (6) | (16) | (9) | (5) | |||||
| MFE-steps | 44 | 2 | 2 | −3 | 39 | 2 | 2 | −2 | 0.25 | .393 |
| (4) | (3) | (6) | (4) | (3) | (7) | |||||
Abbreviations: SE, standard error; CI, confidence interval; DT, dual task; ST, single task; UPDRS-ADL, Unified Parkinson’s Disease Rating Scale–activities of daily living; MFE, modified figure of eight; FES-I, Falls Efficacy Scale–International; IQR, interquartile range.
Cohen’s effect size, calculated on between-group differences.
P values in boldface indicate statistical significance (ie, P ≤ .05).
Normal walking conditions.
Analysis performed on log-transformed data.
Figure 2.
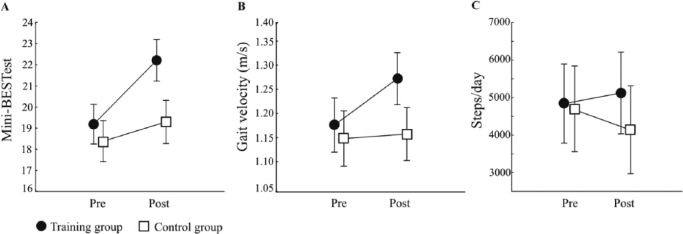
(A) Mini-BESTest score, (B) gait velocity (m/s), and (C) steps per day in the training group and control group at pre- and postintervention. Data are plotted as mean and standard error.
Effect of Training on Daily Activity
For physical activity levels, a significant interaction effect was found for the average number of steps per day, F(1, 67) = 4.72, P = .033 (Table 2 and Figure 2). However, post-hoc analyses did not reveal any significant differences between the pre- and posttest occasions. Thus, the statistical interaction effect occurred because of a nonsignificant increase in activity level in the training group (average increase of 282 steps/day; 6%) and decrease in activity level in the control group (average decrease of 548 steps per day; 12%). Regarding activities of daily living, a significant interaction effect was found, F(1, 89) = 10.91, P = .001, revealing improvements in the training group by 1.7 points on the UPDRS-ADL at posttest (P = .002), whereas the control group remained unchanged (P = .810).
Effect of Training on Concerns About Falling
We found no interaction effect for concerns about falling (Table 2).
Compliance and Adverse Events
The average attendance rate for the training group was 90%. In all, participants took part in 1380 training sessions, resulting in a total of 13 adverse events (all were falls during training) and an incidence rate of 0.9%. None of these events caused injury or pain that interfered with the participants’ ability to proceed with the balance training or other activities.
Discussion
In this study, we investigated the short-term effects of a 10-week program with an emphasis on motor and cognitively challenging balance exercises in elderly individuals with PD. The findings of this trial revealed that the training group that received a specific intervention targeting balance and gait performance improved significantly better in terms of balance control and gait performance when compared with the control group that received usual care.
Consistent with 2 meta-analyses reporting the beneficial short-term effects of exercise on balance performance in individuals with PD,16,17 we found a significant effect and a large effect size (0.82) for the Mini-BESTest in the training group. Our effect size on balance performance was similar to previous studies that evaluated challenging balance exercises in PD.37-39 However, the training stimuli in the balance training, that is the level of challenge on postural demands in relation to individual capacities, are inconsistently reported between studies. Therefore, from a methodology perspective, to enable comparison, there is a need for psychometrically validated tools to monitor balance training and its progression.40
In contrast to our results of the Mini-BESTest, we found no training effects for the single-item balance test MFE. The Mini-BESTest is a multi-item test that aims to cover the complexity of balance performance,30 hence our results indicate that in PD, it may be important to use a combination of tests in order to cover different aspects of balance impairments.41 This 3-point improvement on the Mini-BESTest exceeds the previously found standard error of measurement (1.5 points) in elderly individuals with mild to moderate PD.42 However, comparable trials using the Mini-BESTest are sparse and data on minimal clinically important difference (MCID) in PD is lacking. Nevertheless, our results are promising, especially since enhanced balance performance was also linked to improved gait velocity (0.10 m/s) in the training group. Gait velocity is a vital indicator of health in older adults,43,44 and our effect exceeded the pooled effect for exercise in PD (0.04-0.05 m/s), which was recently reported in 2 meta-analyses.16,17 In fact, few randomized controlled trials in PD38,45,46 have shown training effects exceeding an MCID of 0.10 m/s for gait velocity.43,44 In addition, short step length, a typical feature in individuals with PD, has rarely been improved by training interventions.16 In our study, the increased step length (0.04 m) in the training group provides further confirmation that this intervention efficiently targets gait impairments.
To date, this is the first randomized controlled trial showing that DT training can improve certain aspects of DT performance in individuals with PD. Although we found no between group differences regarding DT gait performance, the training group improved the performance of the cognitive task while walking compared with the control group. Since the performance of the cognitive task was unchanged when performed as a single task (while seated), it provides evidence of a specific improvement regarding DT performance in the training group. These DT improvements may derive from increased automatization of single tasks, or it might be a consequence of improved efficiency in integrating both tasks.14,47 Furthermore, although no interaction effect was found for gait velocity during DT, the effect in the training group (0.09 m/s) was close to the MCID (0.10 m/s). It is noteworthy that this effect is similar to the results from previous studies regarding DT training in PD.12-14 However, none of these studies included a control group—hence, potential training effects cannot be differentiated from learning effects owing to repeated assessments. In summary, even though the field of DT training for individuals with PD is nascent, our findings indicate that DT performance might be improved in this population. However, the underlying mechanisms of DT impairments and the potential for rehabilitation need further investigation.
Despite growing evidence regarding the advantages of exercise for individuals with PD, it is unclear whether specific improvements in balance and gait can translate into “real-world” settings.16,17 In our study, the notion of short-term transfer effects is supported by physical activity levels and activities of daily living. In particular, although not significant, the training group showed an increasing trend in physical activity (6%), while the control group, in line with the progressiveness of PD,48 showed a declining trend (12%). Even though the absolute difference between groups is rather small, we believe that these findings are important, especially since benefits in terms of general health and disease modification could be expected to result from maintaining physical activity throughout the course of PD.49 Only 2 previous studies, the ParkFit trial50 and the RESCUE trial,51 have used objective measures (eg, accelerometry) to investigate the impact of rehabilitation programs on daily physical activity in individuals with PD. In line with their results, our study showed that enhanced physical activity occurred at the same time as improvement in physical functioning. Regarding activities of everyday living, the improvement of 1.7 units on the UPDRS-ADL must be cautiously interpreted since it only approached the MCID values between 2 and 3 units for the UPDRS-ADL.52
In accordance with the current body of research in PD,16,53 we found no short-term effects of balance exercise on concerns about falling. Although this intervention was greatly appreciated by the participants, it is uncertain how the experience of threatening postural situations in highly challenging exercises may influence patients’ concerns about falling and their awareness of personal boundaries. These aspects require another approach; therefore a qualitative study of the participants’ experience of this intervention is in progress.
The main strengths of this study are the large sample size, the low dropout rate, and the comprehensive assessment of outcomes related to balance impairments (clinical tests, objective measures of gait, physical activity levels, and self-perceived disability). However, the study also had several limitations. First, a majority of the participants were recruited by advertisement, a method that can lead to a convenience sample of individuals interested in training and improving balance abilities. Second, the results can only be generalized to elderly, specifically community-dwelling individuals with mild- to moderate-stage PD without known cognitive impairments. Third, some participants were unable to maintain a constant dosage of anti-Parkinson medication throughout the trial. However, reported changes of medication were equivalent between groups and not related to pre/post difference in dyskinesia (a possible cause for medication change and balance improvements). Moreover, analyses on the subgroups that changed medication dosage revealed no difference in balance performance. Finally, the assessors were not masked to group allocation at the posttest assessment, thus bias related to the physical testing cannot be ruled out. On the other hand, we believe that the clinical approach of this study strengthens the ecological validity of this research.
In conclusion, the results of this randomized controlled trial showed that highly challenging exercises, when compared with usual care, improved balance and gait performance in elderly individuals with mild to moderate PD. This study also showed positive transfer effects to activities performed in real-world settings, indicating that appropriate training programs could promote physical activity and daily activities. Further explorations of these effects in a long-term follow-up are underway.
Acknowledgments
The authors would like to thank all the participants, physiotherapists, and research assistants who contributed to this work.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the Swedish Research Council, the Swedish Parkinson Foundation, the Karolinska Institutet, the Loo and Hans Ostermans Foundation, the Gun and Bertil Stohnes Foundation, the Swedish NEURO Foundation, the Norrbacka Eugenia Foundation, and the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet.
References
- 1. Kim SD, Allen NE, Canning CG, Fung VS. Postural instability in patients with Parkinson’s disease. Epidemiology, pathophysiology and management. CNS Drugs. 2013;27:97-112. [DOI] [PubMed] [Google Scholar]
- 2. Pickering RM, Grimbergen YA, Rigney U, et al. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov Disord. 2007;22:1892-1900. [DOI] [PubMed] [Google Scholar]
- 3. Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. On the nature of fear of falling in Parkinson’s disease. Behav Neurol. 2011;24:219-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dontje ML, de Greef MH, Speelman AD, et al. Quantifying daily physical activity and determinants in sedentary patients with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:878-882. [DOI] [PubMed] [Google Scholar]
- 5. Lord S, Godfrey A, Galna B, Mhiripiri D, Burn D, Rochester L. Ambulatory activity in incident Parkinson’s: more than meets the eye? J Neurol. 2013;260:2964-2972. [DOI] [PubMed] [Google Scholar]
- 6. Schrag A, Jahanshahi M, Quinn N. How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov Disord. 2000;15:1112-1118. [DOI] [PubMed] [Google Scholar]
- 7. Bohnen NI, Cham R. Postural control, gait, and dopamine functions in parkinsonian movement disorders. Clin Geriatr Med. 2006;22:797-812, vi. [DOI] [PubMed] [Google Scholar]
- 8. Konczak J, Corcos DM, Horak F, et al. Proprioception and motor control in Parkinson’s disease. J Mot Behav. 2009;41:543-552. [DOI] [PubMed] [Google Scholar]
- 9. Bloem BR, Grimbergen YA, van Dijk JG, Munneke M. The “posture second” strategy: a review of wrong priorities in Parkinson’s disease. J Neurol Sci. 2006;248:196-204. [DOI] [PubMed] [Google Scholar]
- 10. Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dual-task walking deficits in people with Parkinson’s disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis. 2012;2012:918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. LaPointe LL, Stierwalt JA, Maitland CG. Talking while walking: cognitive loading and injurious falls in Parkinson’s disease. Int J Speech Lang Pathol. 2010;12:455-459. [DOI] [PubMed] [Google Scholar]
- 12. Yogev-Seligmann G, Giladi N, Brozgol M, Hausdorff JM. A training program to improve gait while dual tasking in patients with Parkinson’s disease: a pilot study. Arch Phys Med Rehabil. 2012;93:176-181. [DOI] [PubMed] [Google Scholar]
- 13. Canning CG, Ada L, Woodhouse E. Multiple-task walking training in people with mild to moderate Parkinson’s disease: a pilot study. Clin Rehabil. 2008;22:226-233. [DOI] [PubMed] [Google Scholar]
- 14. Brauer SG, Morris ME. Can people with Parkinson’s disease improve dual tasking when walking? Gait Posture. 2010;31:229-233. [DOI] [PubMed] [Google Scholar]
- 15. Thompson W, Gordon N, Pescatello L. ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Wolters Kluwer; 2010. [Google Scholar]
- 16. Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev. 2013;9:CD002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson’s disease: a meta-analysis of the effect of exercise and motor training. Mov Disord. 2011;26:1605-1615. [DOI] [PubMed] [Google Scholar]
- 18. Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013;12:716-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77:288-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sehm B, Taubert M, Conde V, et al. Structural brain plasticity in Parkinson’s disease induced by balance training. Neurobiol Aging. 2014;35:232-239. [DOI] [PubMed] [Google Scholar]
- 21. Fisher BE, Li Q, Nacca A, et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport. 2013;24:509-514. [DOI] [PubMed] [Google Scholar]
- 22. Kantak SS, Winstein CJ. Learning-performance distinction and memory processes for motor skills: a focused review and perspective. Behav Brain Res. 2012;228:219-231. [DOI] [PubMed] [Google Scholar]
- 23. Conradsson D, Löfgren N, Ståhle A, Hagströmer M, Franzén E. A novel conceptual framework for balance training in Parkinson’s disease-study protocol for a randomised controlled trial. BMC Neurol. 2012;12:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conradsson D, Löfgren N, Ståhle A, Franzén E. Is highly challenging and progressive balance training feasible in older adults with Parkinson’s disease? Arch Phys Med Rehabil. 2014;95:1000-1003. [DOI] [PubMed] [Google Scholar]
- 25. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427-442. [DOI] [PubMed] [Google Scholar]
- 27. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198. [DOI] [PubMed] [Google Scholar]
- 28. Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26:132-143. [DOI] [PubMed] [Google Scholar]
- 29. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649-2653. [DOI] [PubMed] [Google Scholar]
- 30. Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42:323-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jarnlo G, Nordell E. Reliability of the modified figure of eight—a balance performance test for elderly women. Physiother Theory Pract. 2003;19:35-43. [Google Scholar]
- 32. Matthews CE, Hagströmer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44:68-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fahn S, Elton RL. Unified Parkinson’s Disease Rating Scale. In: Fahn S MD, Calne D, eds. Recent Developments in Parkinson Diseases. London, England: Macmillan; 1987:153-163. [Google Scholar]
- 35. Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale–International (FES-I). Age Ageing. 2005;34:614-619. [DOI] [PubMed] [Google Scholar]
- 36. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 37. Smania N, Corato E, Tinazzi M, et al. Effect of balance training on postural instability in patients with idiopathic Parkinson’s disease. Neurorehabil Neural Repair. 2010;24:826-834. [DOI] [PubMed] [Google Scholar]
- 38. Protas EJ, Mitchell K, Williams A, Qureshy H, Caroline K, Lai EC. Gait and step training to reduce falls in Parkinson’s disease. NeuroRehabilitation. 2005;20:183-190. [PubMed] [Google Scholar]
- 39. Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch Phys Med Rehabil. 2003;84:1109-1117. [DOI] [PubMed] [Google Scholar]
- 40. Farlie MK, Robins L, Keating JL, Molloy E, Haines TP. Intensity of challenge to the balance system is not reported in the prescription of balance exercises in randomised trials: a systematic review. J Physiother. 2013;59:227-235. [DOI] [PubMed] [Google Scholar]
- 41. Jacobs JV, Horak FB, Tran VK, Nutt JG. Multiple balance tests improve the assessment of postural stability in subjects with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:322-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Löfgren N, Lenholm E, Conradsson D, Ståhle A, Franzén E. The Mini-BESTest—a clinically reliable tool for balance evaluations in mild to moderate Parkinson’s disease? BMC Neurol. 2014;14:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. 2009;32:46-49. [PubMed] [Google Scholar]
- 44. Chui K, Hood E, Klima D. Meaningful change in walking speed. Top Geriatr Rehabil. 2012;28:97-103. [Google Scholar]
- 45. Ellis T, de Goede CJ, Feldman RG, Wolters EC, Kwakkel G, Wagenaar RC. Efficacy of a physical therapy program in patients with Parkinson’s disease: a randomized controlled trial. Arch Phys Med Rehabil. 2005;86:626-632. [DOI] [PubMed] [Google Scholar]
- 46. Thaut MH, McIntosh GC, Rice RR, Miller RA, Rathbun J, Brault JM. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov Disord. 1996;11:193-200. [DOI] [PubMed] [Google Scholar]
- 47. Ruthruff E, Van Selst M, Johnston JC, Remington R. How does practice reduce dual-task interference: integration, automatization, or just stage-shortening? Psychol Res. 2006;70:125-142. [DOI] [PubMed] [Google Scholar]
- 48. Cavanaugh JT, Ellis TD, Earhart GM, Ford MP, Foreman KB, Dibble LE. Capturing ambulatory activity decline in Parkinson’s disease. J Neurol Phys Ther. 2012;36:51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Speelman AD, van de Warrenburg BP, van Nimwegen M, Petzinger GM, Munneke M, Bloem BR. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol. 2011;7:528-534. [DOI] [PubMed] [Google Scholar]
- 50. van Nimwegen M, Speelman AD, Overeem S, et al. Promotion of physical activity and fitness in sedentary patients with Parkinson’s disease: randomised controlled trial. BMJ. 2013;346:f576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lim I, van Wegen E, Jones D, et al. Does cueing training improve physical activity in patients with Parkinson’s disease? Neurorehabil Neural Repair. 2010;24:469-477. [DOI] [PubMed] [Google Scholar]
- 52. Schrag A, Sampaio C, Counsell N, Poewe W. Minimal clinically important change on the Unified Parkinson’s Disease Rating Scale. Mov Disord. 2006;21:1200-1207. [DOI] [PubMed] [Google Scholar]
- 53. Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson’s disease: a systematic review across the disability spectrum. J Neurol Phys Ther. 2009;33:14-26. [DOI] [PubMed] [Google Scholar]