Abstract
The matricellular protein CCN1 (CYR61) is known to function in wound healing and is upregulated in colons of patients with Crohn’s disease and ulcerative colitis, yet its specific role in colitis is unknown. Here we have used Ccn1dm/dm knockin mice expressing a CCN1 mutant unable to bind integrins α6β1 and αMβ2 as a model to probe CCN1 function in dextran sodium sulfate (DSS)-induced colitis. Ccn1dm/dm mice exhibited high mortality, impaired mucosal healing, and diminished IL-6 expression during the repair phase of DSS-induced colitis compared to wild type mice, despite having comparable severity of initial inflammation and tissue injury. CCN1 induced IL-6 expression in macrophages through integrin αMβ2 and in fibroblasts through α6β1, and IL-6 promoted intestinal epithelial cell (IEC) proliferation. Administration of purified CCN1 protein fully rescued Ccn1dm/dm mice from DSS-induced mortality, restored IEC proliferation and enhanced mucosal healing, whereas delivery of IL-6 partially rectified these defects. CCN1 therapy accelerated mucosal healing and recovery from DSS-induced colitis even in wild type mice. These findings reveal a critical role for CCN1 in restoring mucosal homeostasis after intestinal injury in part through integrin-mediated induction of IL-6 expression, and suggest a therapeutic potential for activating the CCN1/IL-6 axis for treating inflammatory bowel disease.
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC), two major subtypes of inflammatory bowel disease (IBD), are chronic, relapsing, and remitting inflammatory disorders of the gastrointestinal tract that affect 1.4 million people in the United States1. These medically incurable diseases of poorly defined etiology generally begin in young adulthood and continue throughout life, often requiring lifelong management. Traditional treatment modalities have aimed at dampening inflammation in the GI tract to alleviate symptoms in patients, and this rationale prompted the development of monoclonal antibodies targeting the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) for the treatment of IBD. Although blockade of TNF-α induces clinical remission and has become a critical component in the therapeutic arsenal for IBD, many patients (~40%) do not respond, lose their response during treatment, or develop complications due to side effects2, 3. Patients who are refractory to therapies may eventually require colectomy and are at increased risks of developing colorectal cancer, causing significant morbidity. Thus, there is an urgent need for alternative treatment options, and considerable effort has been focused on the identification of novel therapeutic targets4.
Among the targets under investigation for IBD therapy is interleukin-6 (IL-6), a multifunctional cytokine expressed by diverse cell types during inflammation5, 6. IL-6 is induced upon intestinal injury in the blood and colonic tissue of IBD patients, and is thought to be involved in the pathogenesis of IBD by inducing T-cell activation and suppressing T-cell apoptosis6, 7. Consistent with this notion, monoclonal antibodies (mAbs) against IL-6 receptor (IL-6R) prevent T cell-mediated murine colitis7, 8, and a pilot clinical trial using anti-IL6R mAbs showed symptomatic improvement in patients with CD9. Paradoxically, IL-6-deficient mice suffer increased mortality, morbidity, and mucosal damage in infectious and non-infectious models of colitis, suggesting that IL-6 may play pleiotropic and potentially antithetical roles in IBD10, 11. Therefore, further elucidation of the regulation and function of IL-6 in colitis is likely needed to optimize its therapeutic potential.
CCN1 (CYR61), a secreted matricellular protein of ~40 kDa, is emerging as an important regulator of tissue homeostasis and injury repair12. Acting primarily through direct binding to integrin receptors, CCN1 regulates diverse cellular functions in a cell-type and context-dependent manner12. Whereas CCN1 is essential for angiogenesis and cardiovascular development during embryogenesis13, its expression has been linked to inflammation and tissue repair in adulthood14, 15. However, the specific functions of CCN1 in various inflammation-associated pathologies are only beginning to be appreciated. For example, in cutaneous wound healing and in chronic liver injuries induced by hepatotoxin or cholestasis, CCN1 functions to diminish and restrict tissue fibrosis in the maturation phase of tissue repair by triggering cellular senescence in activated myofibroblasts16, 17. CCN1 can also contribute to inflammatory damage by inducing the expression of pro-inflammatory cytokines in macrophages and enhancing the cytotoxicity of TNF family cytokines18–20. Importantly, CCN1 expression is elevated in biopsies of patients with UC or CD, and in mice with experimental colitis21. However, the precise function of CCN1 in IBD remains unknown.
Here we provide the first evidence that CCN1 plays a critical role in promoting recovery and mucosal healing in colitis, in part through integrin-mediated induction of IL-6 expression during the repair phase. Moreover, administration of CCN1 protein accelerated recovery and mucosal healing in wild type and Ccn1 mutant mice. Our findings reveal CCN1 as a critical regulator of mucosal healing in colitis, uncover the importance of CCN1-induced IL-6 in intestinal epithelial restitution, and suggest a therapeutic potential in activating the CCN1/IL-6 axis for the treatment of IBD.
RESULTS
Ccn1dm/dm mice suffer increased mortality as well as impaired recovery and mucosal healing upon DSS challenge
Immunohistochemical analysis showed that CCN1 protein was mainly associated with the surface epithelial cells in the normal colon, but was detected in the entire mucosal epithelium when mice were challenged with 5% DSS to induce colitis22 (supplementary Figure S1A). This expression pattern was confirmed in CCN1-EGFP mice23 in which Ccn1 expression was visualized by anti-GFP staining, with GFP restricted to terminally differentiated intestinal epithelial cells in the normal colon but expanded to the entire crypt upon DSS exposure (supplementary Figure S1B). Consistently, Ccn1 mRNA was increased in the colon of DSS-challenged mice compared to untreated mice (P <0.01)(supplementary Figure S1C), suggesting that CCN1 is actively regulated during colitis. CCN1 exerts diverse effects in various cell types through distinct integrins, among them αMβ2 in macrophages and α6β1 in fibroblasts12. To elucidate the role of CCN1 in IBD, we have used Ccn1dm/dm knockin mice in which the Ccn1 genomic locus is replaced by an allele encoding a CCN1 double mutant protein (DM-CCN1) disrupted in its overlapping binding sites for integrins αMβ2 and α6β124, 25. This genetic model circumvents the embryonic lethality of Ccn1-null mice13, and avoids potential limitations of cell type-specific deletions as CCN1 may be secreted by multiple cell types in the colonic tissue microenvironment. Ccn1dm/dm mice are viable, fertile, morphologically and behaviorally normal16, 18, and display a normal intestinal histology in the absence of pathogenic insults.
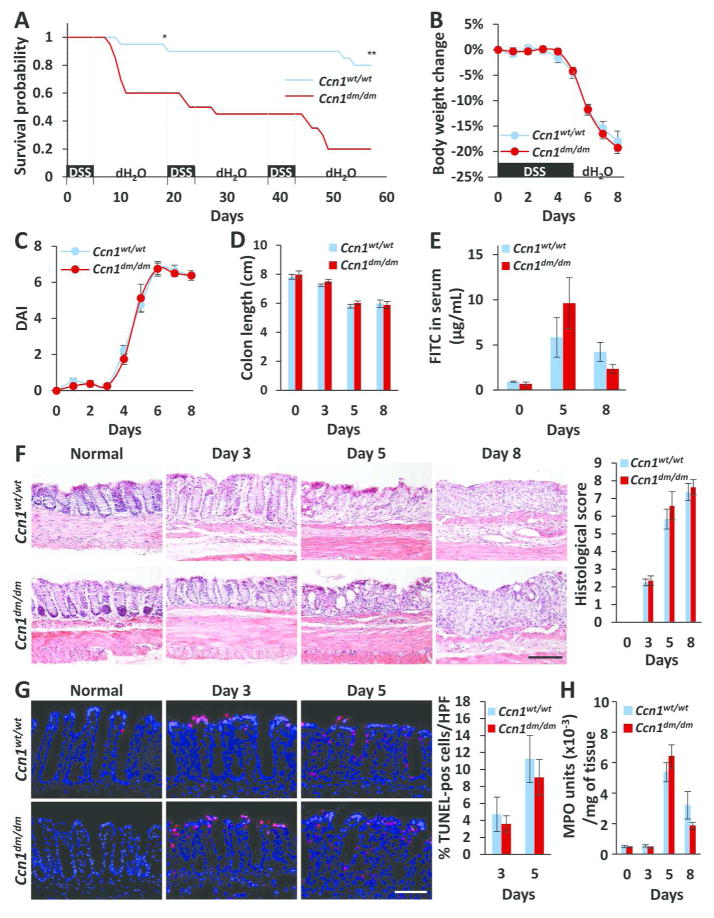
Wild type (WT, Ccn1wt/wt) and Ccn1dm/dm mice were subjected to a single or repeated cycles of DSS challenge, which induced acute or chronic colitis, respectively, in an established protocol for the induction of intestinal inflammation22. When challenged with 5% DSS (w/v), only 60% and 20% of Ccn1dm/dm mice survived through the first and third cycles of DSS feeding, respectively, compared to 95% and 80% of wild type mice (P<0.05 and P<0.001, respectively) (Figure 1A), suggesting that CCN1 serves an important protective role in both acute and chronic DSS-induced colitis. However, increased lethality of Ccn1dm/dm mice was not due to exacerbated disease as judged by body weight loss, DAI, and colonic shortening (Figure 1B–D). The intestinal barrier function after DSS feeding, as evaluated by detection of gavaged FITC-conjugated dextran in the serum, was also similar between the two genotypes (Figure 1E). Histological analyses of the distal colon showed comparable crypt loss and epithelial damage (Figure 1F), with similar numbers of apoptotic cells (Figure 1G). Lastly, myeloperoxidase (MPO) activity in the colonic tissue was comparable in wild type and Ccn1dm/dm mice, indicating similar extent of neutrophil infiltration into the mucosal epithelium and lamina propria (Figure 1H). These results show that CCN1 functions mediated through its α6β1/αMβ2 binding sites are critical for animal survival in DSS-induced colitis, although loss of these functions did not result in exacerbated morbidity or inflammatory damage.
Figure 1. Higher mortality but not morbidity in DSS-challenged Ccn1dm/dm mice.
(A) Wild type and Ccn1dm/dm mice (n=20 per genotype) were given 5% (w/v) of DSS water for 5 days followed by 14 days of recovery with normal drinking water as indicated. Survival was monitored through three cycles of DSS challenge. *P<0.05, **P<0.001 (log-rank test). The disease course of acute colitis was monitored by measuring (B) body weight change, (C) DAI, and (D) colon lengths on indicated days after initiation of DSS exposure. (E) Orally-gavaged FITC particles were detected in the serum to evaluate intestinal barrier integrity (n=3). (F) Mucosal damage was assessed by H&E staining of paraffin-embedded sections of the distal colon; histological scores are shown on right (n=4–6). Scale bar=100 μm. (G) Cell death in the distal colon was measured by TUNEL, and cells were counterstained with 4,6-diamidino-2-phenylindole (DAPI). Quantitation is shown on the right (n=3). Scale bar=50 μm. (H) MPO activity from colonic tissue (n=3–6).
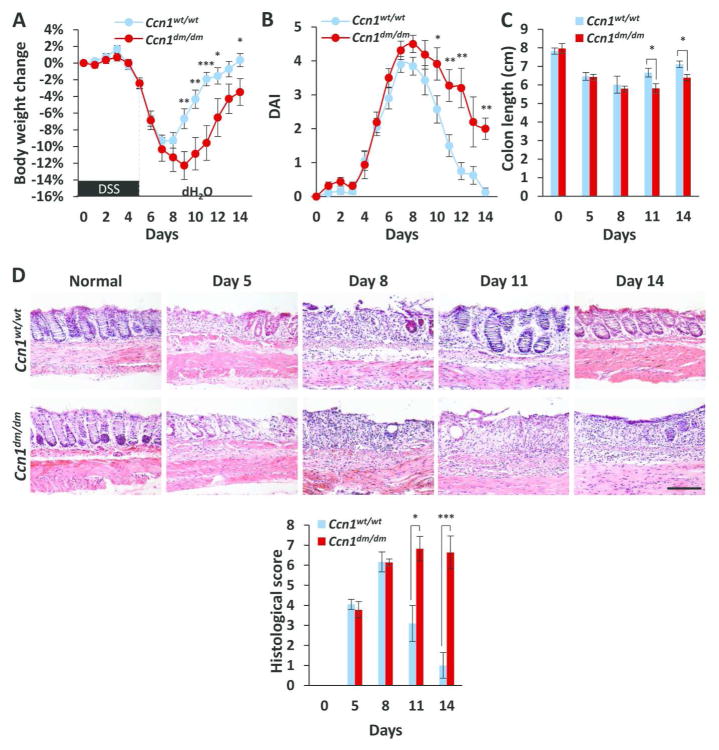
To test the hypothesis that CCN1 may function to promote recovery and mucosal healing in colitis, we lowered the dose of DSS to 3.5% (w/v) to avoid death of Ccn1dm/dm mice, and examined recovery (as judged by DAI) and mucosal healing (as judged by epithelial restitution). When administered 3.5% DSS, Ccn1 mRNA expression was elevated throughout the recovery phase compared to untreated mice (day 8 and 14, P<0.01; day 11, P<0.05)(supplementary Figure S1D). As expected, wild type and Ccn1dm/dm mice showed comparable body weight loss, DAI, colonic shortening, and histological score during the onset of disease from days 0–8 (Figure 2A–D). However, as recovery commenced on day 9 and the DAI began to decline in wild type, Ccn1dm/dm mice experienced a prolonged disease course with elevated DAI (Figure 2B), slow recovery of body weight and colon length (Figure 2A, C), and unrelenting epithelial erosion and ulceration through day 14 (P<0.001)(Figure 2D), indicating that Ccn1dm/dm mice suffer impaired recovery and mucosal healing from colitis.
Figure 2. Impaired recovery and mucosal healing in DSS-challenged Ccn1dm/dm mice.
Wild type and Ccn1dm/dm mice were given DSS water (3.5% w/v) for five days followed by normal drinking water. (A) Changes in body weight and (B) DAI were recorded daily, and (C) colonic length was measured (n=7–8). (D) H&E staining of paraffin-embedded sections of the distal colon from mice 5, 8, 11, and 14 days after initiation of DSS feeding. Histological scores are shown on bottom (n=4–6). *P<0.05, **P<0.01, ***P<0.001. Scale bar=100 μm.
CCN1 protein rescues Ccn1dm/dm mice from lethality and accelerates mucosal healing
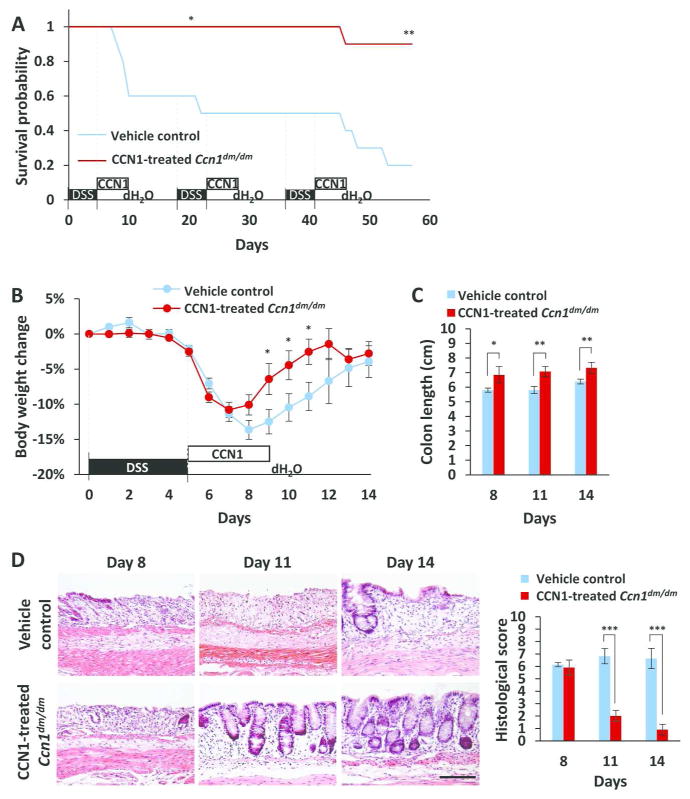
To assess whether increased mortality of Ccn1dm/dm mice is indeed due to compromised CCN1 activity during recovery, we tested the effect of supplying WT-CCN1 protein during the recovery phase. After 5 days of 5% DSS feeding, Ccn1dm/dm mice were injected (i.p.) daily with purified WT-CCN1 for 5 consecutive days. Strikingly, 90% of CCN1-treated Ccn1dm/dm mice survived through three cycles of DSS challenge, compared to only 20% survival in vehicle-treated controls (P<0.01)(Figure 3A) and 80% in wild type mice (Figure 1A). Thus, CCN1 therapy fully restored survival in Ccn1dm/dm mice to wild type level. To evaluate the role of CCN1 during recovery, mice fed 3.5% DSS were similarly injected with WT-CCN1. These mice recovered their body weight (P<0.05) and colon length significantly faster than vehicle controls (day 8, P<0.05; days 11 and 14, P<0.01)(Figure 3B–C), comparable to wild type animals (Figure 2A, C). Histology showed that CCN1-treated Ccn1dm/dm mice completely restored the crypt architecture in the distal colon as early as day 11 after initiation of DSS exposure (P<0.001)(Figure 3D), further indicating that CCN1 promotes tissue repair after DSS-induced colitis.
Figure 3. Treatment with WT-CCN1 promotes survival and mucosal repair.
(A) Ccn1dm/dm mice (n=10) were injected i.p. with 5 μg of WT-CCN1 or vehicle control daily for 5 days after each cycle of 5% DSS feeding and monitored for survival. *P<0.05, **P<0.01 (log-rank test). (B) Mice similarly treated except with 3.5% DSS were monitored for body weight change and (C) colon lengths (n=6–8). (D) Mucosal recovery was evaluated by H&E staining of paraffin-embedded colonic tissue. Histological scores are shown on right. Data are presented as mean ± SEM; n=3–4; *P<0.05, **P<0.01, ***P<0.001. Scale bar=100 μm.
Proliferation of intestinal epithelial cells (IECs) is a critical component in intestinal tissue regeneration. Therefore, we postulated that CCN1 may have a direct role in intestinal tissue repair by promoting proliferation of colonic epithelial cells. To test this possibility, we treated young adult mouse colon (YAMC) IECs with BSA, purified WT-CCN1 or DM-CCN1. Surprisingly, neither WT-CCN1 nor DM-CCN1 had any effect on the proliferation of YAMCs in culture as judged by cell number counts and Ki67 staining (supplementary Figure S2), suggesting that CCN1 may indirectly mediate tissue repair after DSS-induced injury.
CCN1 induces IL-6 in vivo and in macrophages and fibroblasts
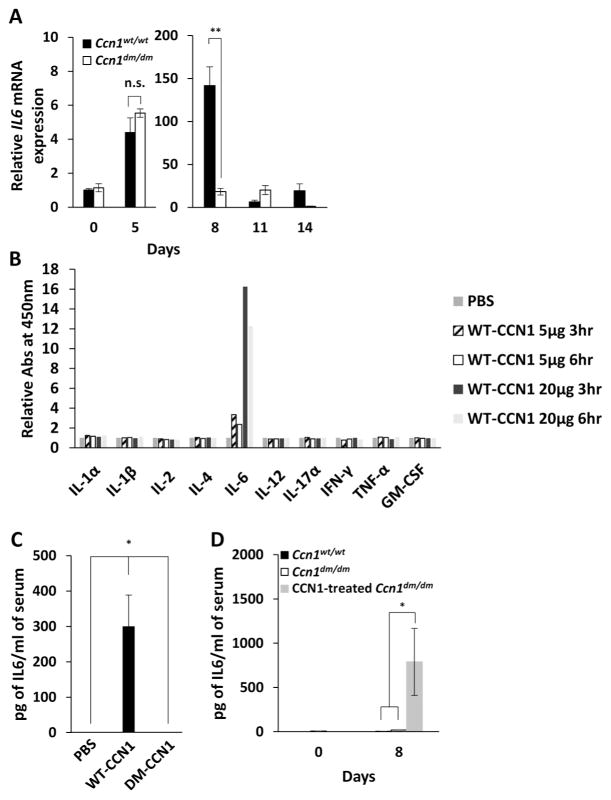
Since cytokines are important in inflammation and repair, we measured cytokine expression in the wild type and Ccn1dm/dm colon after 3.5% DSS challenge. Most differences were small: wild type mice transiently expressed somewhat higher levels of the pro-inflammatory cytokines IFN-γ and IL-17, but TNFα expression was the same in both genotypes (supplementary Figure S3A). Likewise, Ccn1dm/dm mice showed similar or higher levels of the anti-inflammatory cytokines IL-4, TGF-β, and IL-22, whereas IL-10 expression was the same (supplementary Figure S3B). Despite the expression of reparative cytokines such as IL-22, IL-10, and TGF-β26, Ccn1dm/dm mice showed impaired mucosal repair (Fig. 2), suggesting that expression of these cytokines was insufficient to initiate efficient recovery and repair in Ccn1dm/dm mice. By contrast, whereas IL-6 expression was similar in Ccn1dm/dm and WT mice at day 5 when inflammatory damage began to be observed, it was dramatically lower in Ccn1dm/dm mice (>7-fold) by day 8 when damage had peaked and repair began (Figure 4A). Thus, Ccn1dm/dm mice suffered a large deficit in IL-6 expression in the repair phase but not the initiation phase of colitis. IL-6 expression remained induced but at a lower level at later times, and the difference between the two genotypes diminished, suggesting an exquisite control of IL-6 by CCN1 in colitis. To assess the ability of CCN1 to induce the expression of IL-6 and other cytokines in vivo, we injected WT-CCN1 intraperitoneally into wild type mice in the absence of induced colitis. We found that WT-CCN1 upregulated serum IL-6 level in a time and dose-dependent manner as judged by ELISA, whereas DM-CCN1 had no effect (P<0.05)(Figure 4B–C), indicating that CCN1 acts through its α6β1/αMβ2 binding sites to induce IL-6. Moreover, we did not detect an increase in systemic levels of IL-1α, IL-1β, IL-2, IL-4, IL-12, IL-17α, IFN-γ, TNFα, and GM-CSF after injection of WT-CCN1 protein by ELISA, indicating that IL-6 is a specific target of CCN1 regulation (Fig. 4B). In the context of DSS-induced colitis, WT-CCN1 elevated IL-6 in blood from 18 to 787 pg/ml in Ccn1dm/dm mice on day 8 after DSS exposure (P<0.05)(Figure 4D), further confirming that CCN1 can induce IL-6 during the repair phase.
Figure 4. WT-CCN1, but not DM-CCN1, induces IL-6 in vivo.
(A) IL-6 mRNA in the colon of wild type and Ccn1dm/dm mice treated with 3.5% DSS for 5 days was measured by qRT-PCR, and normalized to healthy wild type colon with cyclophilin E as internal reference. Data shown as mean ± SD; n=3–4; n.s., not significant. **P<0.003. (B) IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-12, IL-17α, IFN-γ, TNFα, and GM-CSF protein was measured by ELISA in the serum of wild type mice at indicated times after a single injection i.p. of purified WT-CCN1 protein (5 or 20 μg), or (C) 24 hours after i.p. injection of 10 μg of WT-CCN1, DM-CCN1 protein, or PBS (n=4). (D) IL-6 was measured from the serum of 3.5% DSS-challenged wild type and Ccn1dm/dm mice with and without CCN1 treatment by ELISA. Data are presented as mean ± SEM; n=7; *P<0.05.
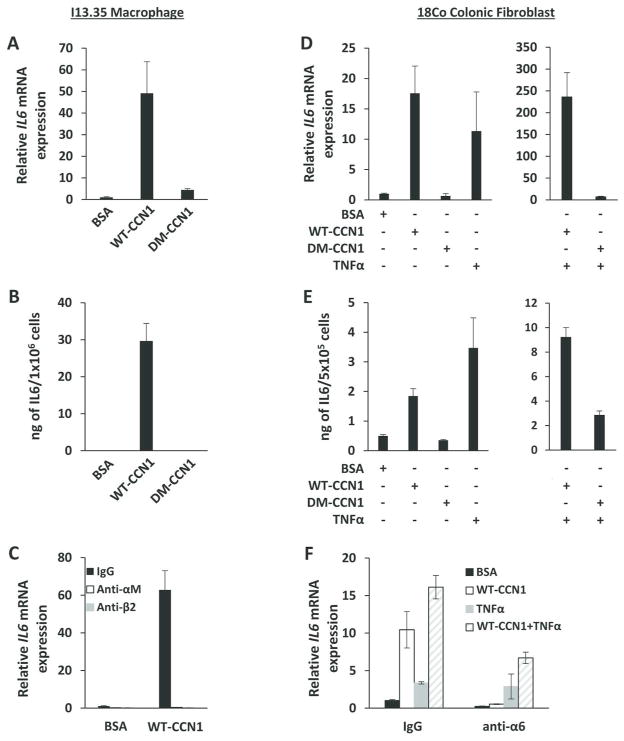
IL-6 is thought to be produced by several cell types in the lamina propria, including macrophages and fibroblasts27. To identify the cellular source of CCN1-induced IL-6, we first tested CCN1 functions in I13.35 macrophages. WT-CCN1 induced IL-6 mRNA and protein levels in these cells, whereas DM-CCN1 did not (Figure 5A–B). Pre-incubation of macrophages with mAbs against integrin αM or β2 abrogated CCN1-induced IL-6 expression, showing the requirement of αMβ2 (Figure 5C). In addition, WT-CCN1, but not DM-CCN1, greatly upregulated IL-6 mRNA and protein levels in 18Co human colonic fibroblasts (Figure 5D–E). Since CCN1 and TNFα are known to be co-expressed in inflammation, we tested whether they act synergistically to regulate IL-6. Remarkably, CCN1 in combination with TNFα induced IL-6 mRNA in 18Co cells by nearly 250-fold, or 15- and 23-fold higher than stimulation by CCN1 or TNFα alone, respectively (Figure 5D). This synergistic effect was also observed by measuring IL-6 protein levels in the conditioned media of 18Co cells (Figure 5E). Consistent with integrin α6β1 being the principal CCN1 receptor in fibroblasts28, pretreatment of 18Co cells with anti-α6 mAb blocked CCN1-induced IL-6 expression (Figure 5F). Although α6 can heterodimerize with β1 and β4 subunits, β4 is predominantly localized to hemidesmosomes and is not express in fibroblasts. Together, these results show that CCN1 upregulates IL-6 mRNA and protein in macrophages through αMβ2 and in fibroblasts through α6β1, consistent with the deficiency in IL-6 expression in Ccn1dm/dm mice (Figure 4B) and inability of DM-CCN1 to induce IL-6 in vivo (Figure 4D).
Figure 5. WT-CCN1, but not DM-CCN1, induces IL-6 in macrophages and fibroblasts.
(A) IL-6 mRNA was measured by qRT-PCR and (B) IL-6 protein from conditioned media was measured by ELISA in serum-starved (overnight) I13.35 macrophages treated with 5 μg/ml of purified WT-CCN1 or DM-CCN1, or BSA for 24 hrs. (C) I13.35 macrophages were incubated with blocking mAbs against integrin αM (50 μg/ml) or β2 (50 μg/ml) 1 hr prior to treatment with WT-CCN1 for 24 hrs. IL-6 mRNA was measured by qRT-PCR. (D) IL-6 mRNA was measured by qRT-PCR and (E) IL-6 protein from conditioned media was measured by ELISA in serum-starved 18Co fibroblasts treated with 5 μg/ml of purified WT-CCN1, DM-CCN1, BSA, or 25 ng/ml of TNFα for 24 hrs. (F) 18Co fibroblasts were incubated with blocking mAb against integrin α6 for 1 hr, then treated with WT-CCN1, TNFα, or BSA for 24 hrs as above. IL-6 mRNA was measured by qRT-PCR. Data shown as mean ± SD of triplicate experiments.
CCN1-induced IL-6 promotes intestinal epithelial healing
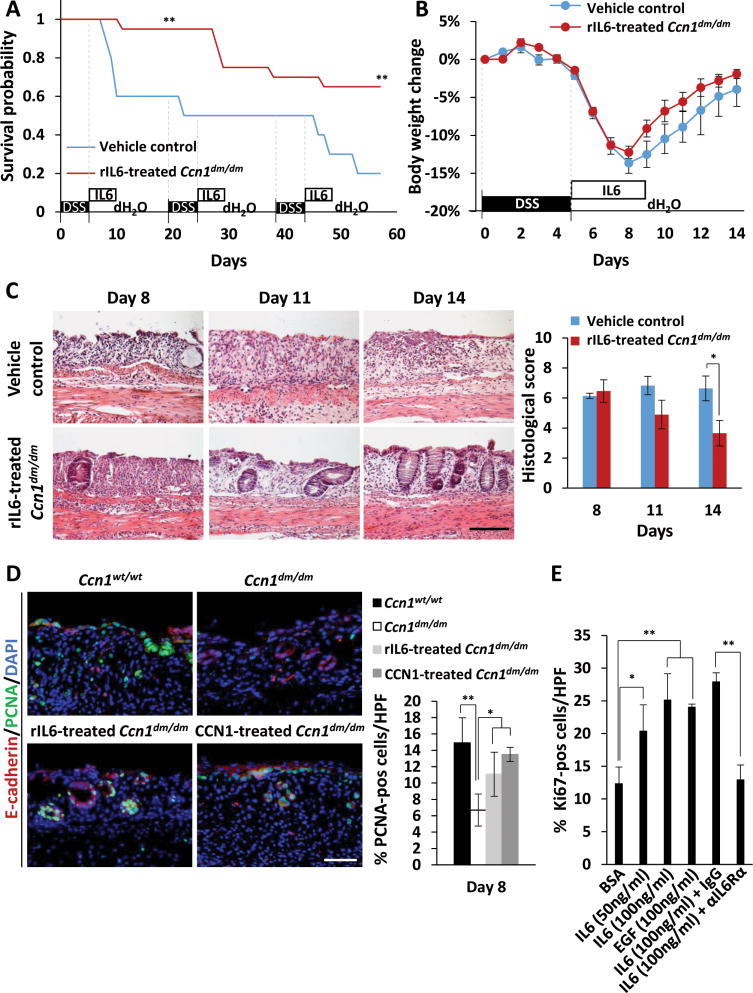
To determine whether deficits in IL-6 expression contributed to impaired healing in Ccn1dm/dm mice, we delivered five daily injections of recombinant IL-6 (rIL-6) to Ccn1dm/dm mice following 5% DSS feeding. Remarkably, rIL-6 treatment increased survival from 60% to 95% in the first cycle of DSS challenge (P<0.01) and from 20% to 65% by the third cycle (P<0.01)(Figure 6A). In Ccn1dm/dm mice fed 3.5% DSS, rIL-6 treatment also enhanced body weight recovery, although the numbers did not reach statistical significance (Figure 6B). Histological evaluation showed that rIL-6 treatment accelerated mucosal restitution in Ccn1dm/dm mice compared to vehicle controls, although improvements in histological scores still lagged behind wild type mice (Figures 2D, 6C). These results indicate that IL-6 treatment partially rescued lethality and accelerated intestinal repair in Ccn1dm/dm mice following DSS-induced colitis.
Figure 6. Delivery of IL-6 enhances survival and mucosal repair of Ccn1dm/dm mice by restoring IEC proliferation.
(A) Ccn1dm/dm mice (n=20) were injected i.p. with rIL-6 (100 ng) or vehicle control daily for 5 consecutive days after each cycle of 5% DSS feeding and were monitored for survival. **P<0.01 (log-rank test). (B) Body weight change was monitored daily in 3.5% DSS-challenged Ccn1dm/dm mice treated with 100 ng of rIL-6 or vehicle control for 5 consecutive days after DSS feeding. (C) Mucosal damage in mice treated in (B) was evaluated by H&E staining of paraffin-embedded sections of the distal colon. Histological scores are shown on the right (n=3–4; *P<0.05). Scale bar=100 μm. (D) Wild type and Ccn1dm/dm mice were challenged with 3.5% DSS, and Ccn1dm/dm mice were further treated with WT-CCN1 (5 μg) or rIL-6 (100 ng) as indicated. PCNA and E-cadherin was detected by immunofluorescence microscopy to assess IEC proliferation. DAPI was used for counterstaining. Quantitation of PCNA staining is shown on right (n=3–4; *P<0.05, **P<0.01). Scale bar=50 μm. (E) YAMC cells were treated with BSA, IL-6 (50 or 100 ng/ml), or EGF (100 ng/ml) for 24 hrs and stained for Ki67 by immunofluorescence and quantitated. Where indicated, YAMC cells were pre-incubated with blocking polyclonal antibodies for IL-6 receptor α (IL-6Rα) or IgG for 1 hr before IL-6 addition. Data are shown as mean ± SD; *P<0.05, **P<0.01. Scale bar=300 μm.
To examine whether CCN1-induced IL-6 stimulated cell proliferation during tissue repair, we counted the number of proliferating cells in tissue sections of wild type, Ccn1dm/dm, rIL-6 treated- and CCN1-treated-Ccn1dm/dm mice stained with antibodies against PCNA, a proliferation marker. There were significantly fewer proliferating cells in Ccn1dm/dm mice compared to wild type mice (P<0.01), and treatment with rIL-6 or WT-CCN1 partially or completely restored cell proliferation, respectively, to wild type levels (Figure 6D). Furthermore, PCNA-positive cells co-localized with E-cadherin, an epithelial cell marker, in wild type and rIL-6 or CCN1-treated-Ccn1dm/dm mice, but not in Ccn1dm/dm mice, indicating that there was a deficit in IECs undergoing proliferation in Ccn1dm/dm mice that was rectified by treatment with rIL-6 or CCN1.
To assess the activity of IL-6 in IEC proliferation directly, YAMC cells were treated with BSA, rIL-6, or EGF as a positive control and stained for Ki67. Treatment of YAMCs with rIL-6 increased Ki67-positive cells in a dose-dependent manner and to the same extent as EGF, a known mitogen for IECs, when both were applied at 100 ng/ml (Figure 6E). Pretreatment of cells with anti-IL-6Rα antibody 1-hr prior to rIL-6 addition blocked the increase in Ki67 positive cells, indicating that rIL-6 acts directly through its specific receptor to enhance IEC proliferation.
CCN1 promotes recovery and mucosal healing in DSS-challenged wild type animals
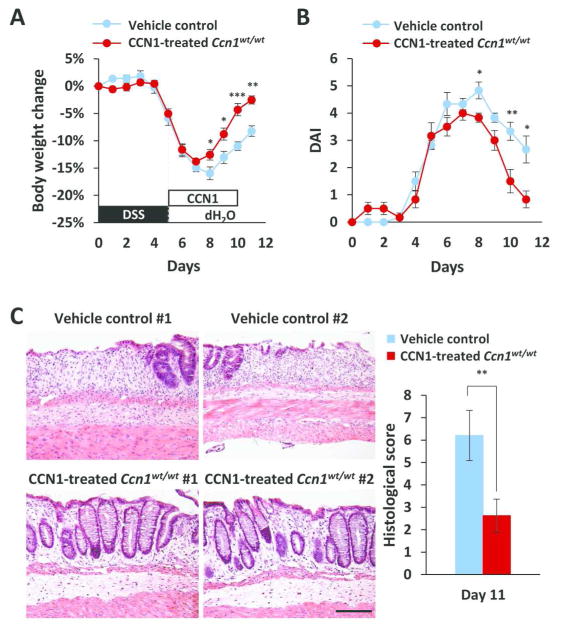
To determine whether the healing effect of CCN1 in colitis is limited to mice with deficiency in CCN1 activity or IL-6 expression, and to test whether CCN1 may have therapeutic value in treating colitis, we delivered five daily injections of purified WT-CCN1 (10 μg i.p.) into wild type animals with colitis induced by 5% DSS. WT-CCN1 protein accelerated body weight recovery and attenuated DAI from days 8–11 in wild type mice with colitis (Figure 7A–B). To investigate whether the improved clinical outcome was a result of enhanced intestinal mucosal repair, we harvested the distal colon on day 11 and evaluated for tissue injury. Histology of the distal colon showed significantly fewer areas of ulceration and a higher number of organized crypts compared to vehicle controls (P<0.01)(Figure 7C). Thus, CCN1 therapy enhances recovery and promotes mucosal healing in colitis even in WT mice with a fully functional CCN1.
Figure 7. CCN1 treatment accelerates recovery and tissue repair in wild type mice.
Wild type mice were challenged with 5% DSS for 5 days, then injected i.p. with vehicle control or 10 μg of purified WT-CCN1 daily for 5 consecutive days post-DSS feeding. (A) Body weight change and (B) DAI were monitored. (C) Histological evaluation by H&E staining of paraffin-embedded sections of the distal colon. Histological scores are on the right. Data are represented as mean ± SEM; n=6; *P<0.05, **P<0.01, ***P<0.001. Scale bar=100 μm.
DISCUSSION
Traditional treatment modalities for IBD have aimed at dampening inflammation in the gastrointestinal tract to alleviate symptoms in patients. Recent studies have shown that IBD patients who achieve and maintain mucosal healing have more favorable long-term outcomes than patients who do not, and thus mucosal healing is emerging as a critical endpoint in clinical trials and practice6, 29. Although anti-TNFα therapy for IBD was initially developed to reduce inflammation, it was found to improve symptoms as well as induce endoscopic remission and sustained mucosal healing30, 31. In this report, we provide the first evidence that CCN1 promotes mucosal healing in murine colitis. Administration of exogenous CCN1 can accelerate mucosal restitution from colitis in both wild type and Ccn1 mutant mice, underscoring a therapeutic potential for CCN1 in IBD.
As a multifunctional matricellular protein, secreted CCN1 is associated with the extracellular matrix and regulates diverse cellular functions through interaction with distinct integrins in various cell types12. Although gene array studies have linked aberrant CCN1 expression with a multitude of inflammation-related diseases, the specific functions of CCN1 in various pathological contexts are only now beginning to emerge14. Interestingly, different aspects of CCN1 activities may be revealed depending on the specific tissue and nature of injury. For example, in skin wound healing and in chronic inflammatory hepatic injuries that induce liver fibrosis, CCN1 functions to dampen fibrosis by acting through integrin α6β1 to induce cellular senescence and expression of the antifibrotic senescence-associated secretory phenotype in myofibroblasts16, 17. In experimental hepatitis induced by concanavalin A, anti-CD95 antibody, or alcohol gavage, CCN1 exacerbates hepatocyte apoptosis by acting in synergy with TNFα and FasL18, 19. Additionally, a role for CCN1 in bone fracture repair has been attributed to its angiogenic activity32.
Ccn1dm/dm mice suffer deficient IL-6 expression in the repair phase of colitis, culminating in increased mortality and impaired mucosal healing. These defects can be substantially rescued by delivery of exogenous IL-6, indicating that CCN1 promotes mucosal healing in part through IL-6 (Figure 6). DSS-induced colitis is linked to damage in the intestinal epithelium, leading to an exuberant inflammatory response due to invasion of the intestinal mucosa by the intraluminal microbiota. Pattern recognition receptors that respond to invading bacteria such as toll-like receptors and the nucleotide-binding oligomerization domain (NOD)-containing protein-like receptors can induce IL-6 through activation of NFκB, and both receptor systems have been implicated in the pathogenesis of IBD1, 33, 34. Furthermore, transcriptional factor interferon regulatory factor 4 has been demonstrated to drive the expression of IL-6 in T-lymphocytes and contribute to the pathogenesis of the colitis35. Thus, it is not surprising that upregulation of IL-6 expression in the initiation phase (~4-fold; day 5) of colitis is independent of CCN1 (Figure 4A). Remarkably, CCN1 exerts exquisite control on IL-6, and a large upregulation of IL-6 expression in the repair phase (~140-fold; day 8) is largely CCN1-dependent. Therefore, Ccn1dm/dm mice provide a unique model in which IL-6 expression in the initiation and repair phases of colitis can be dissociated. CCN1 and TNFα can act synergistically to induce a high level of IL-6 expression (Fig. 5D). It is of interest to note that Ccn1dm/dm mice suffered impaired healing even though they expressed a higher level of IL-22 (Supplementary Fig. S3B), a cytokine known to participate in mucosal repair36, suggesting that CCN1 and IL-6 activities are indispensable for efficient mucosal healing.
A rare though significant adverse effect in patients undergoing anti-IL-6 therapy for rheumatoid arthritis37 and Crohn’s disease (ClinicalTrail.gov identifier NCT01545050)5 is intestinal perforation. This observation is consistent with our finding that downregulation of IL-6 compromises gastrointestinal mucosal healing. However, we have not observed direct evidence of intestinal perforation in Ccn1dm/dm mice, since they suffered similar initial inflammatory damage as WT mice as measured by body weight loss, DAI, colonic length, and intestinal barrier function (days 0–8, Fig. 1B–E). Furthermore, bacterial culture of serum and peritoneal lavage fluid from Ccn1dm/dm mice also did not show evidence of intestinal perforation (data not shown). The overwhelming number of deaths in Ccn1dm/dm mice occurred (days 9–10) when loss of body weight exceeded that in WT mice in the recovery phase (Fig. 2A), suggesting that acute volume depletion might be a significant factor contributing to mortality.
CCN1 regulates diverse cellular functions in a cell type- and integrin-dependent manner12. Here we show that CCN1 induces IL-6 in macrophages through integrin αMβ2 and in fibroblasts through α6β1 (Figure 5). These activities are abrogated in DM-CCN1, consistent with impaired IL-6 expression in Ccn1dm/dm mice. CCN1 may also induce the expression of genes other than IL-6 or provide additional functions that promote mucosal repair, since delivery of CCN1 fully rescued defects in Ccn1dm/dm mice whereas IL-6 was only partially effective (Figures 6–7). However, it is also possible that the IL-6 treatment regimen has not been fully optimized. CCN1 is also known to induce angiogenesis through integrin αVβ3 in endothelial cells38, although the binding site for αVβ3 is unaffected in DM-CCN1 and we did not observe angiogenic defects in the colon of Ccn1dm/dm mice (data not shown).
Several lines of evidence indicate that IL-6 is a pro-inflammatory cytokine required for the establishment of IBD7, 8, and a pilot clinical trial showed symptomatic improvements in CD patients treated with anti-IL-6R antibodies9. Thus, IL-6 has been identified as a therapeutic target for IBD and clinical trials to evaluate the efficacy of anti-IL-6 therapies are in progress4–6. However, accumulating evidence also indicates that IL-6 can serve important protective and homeostatic functions in the intestinal mucosa by promoting IEC proliferation and survival, and regulating intestinal barrier function in mice39–42. Existing results and findings presented herein are consistent with the interpretation that IL-6 plays a dual role in IBD: it is important for the development of colitis as a pro-inflammatory cytokine, but also critical for mucosal repair. Thus, IL-6 deficient mice showed reduced inflammation during colitis induction43, 44, but suffered impaired mucosal healing during recovery10, 45. These findings suggest that although anti-IL-6 therapy may afford benefit by dampening the pro-inflammatory functions of IL-6, it may also impede mucosal healing. Therefore, further optimization of anti-IL-6 treatment regimens and/or selective induction of IL-6 during mucosal healing, potentially through the CCN1/IL-6 axis, may enhance the therapeutic value of targeting IL-6 in IBD.
METHODS
Cell culture, proteins, antibodies, and reagents
Immortalized splenic macrophage cell line I-13.35 (CRL-2471, ATCC) derived from TLR4-defective C3H/HeJ strain and human 18Co colonic fibroblasts (CCL-228, ATCC) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 20% LADMAC conditioned media and Eagle’s minimum essential medium (EMEM), respectively, supplemented with 10% FBS (Hyclone) at 37°C with 10% CO2. The conditionally immortalized young adult mouse colon (YAMC) cell line (a gift of R. Whitehead, Vanderbilt University Medical Center), which carries a heat-labile SV40 large T antigen under the control of an interferon (IFN)-γ-dependent promoter46, was cultured on rat tail collagen (BD Biosciences)-coated plates in RPMI 1640 medium with 5% FBS, 2 mM L-gluatamine, 5 U/mL IFN-γ (Peprotech), and 5 μg/mL of insulin (Gibco) at 32°C (permissive condition). Cells were transferred to RPMI 1640 containing 1% FBS without IFN-γ and insulin at 37°C (nonpermissive condition) 24 hrs prior to experiments. WT-CCN1 and DM-CCN1 proteins were purified as described25, 47. Recombinant mouse IL-6 was from Peprotech (Rocky Hill, NJ) and recombinant TNFα was from Apotech (Epalinges, Switzerland), and epidermal growth factor (EGF) from Sigma-Aldrich. Neutralizing monoclonal antibodies against intergrin αM (clone M1/70.15) was from Abd Serotec (Raleigh, NC), integrin β2 (clone M18/2.a.12.7) from Santa Cruz Biotechnology, integrin α6 (clone GoH3) from Beckman Coulter, and IL-6Rα from R&D.
Mice and DSS-induced colitis
Ccn1dm/dm mice were backcrossed 20X into the C57BL/6 background16–18. Transgenic CCN1(CYR61)-EGFP mice in FVB/N-Swiss webster background harboring a modified bacterial artificial chromosome (BAC) with an EGFP (enhanced green fluorescent protein) inserted upstream of the initiating ATG codon of the first coding exon of the Cyr61 gene was obtained from the Mutant Mouse Regional Resource Centers at University of California, Davis23. Female WT C57BL/6, Ccn1dm/dm, CCN1-EGFP mice (12–16 wks) were given 5% or 3.5% (w/v) DSS (36–50 kDa; MP Biomedicals) in the drinking water for five days followed by 14 days of regular water. This cycle was repeated 3X in survival experiments. Where indicated, mice were injected intraperitoneally (i.p.) with CCN1 or IL-6 in 200 μl of PBS. All animal procedures were approved by the University of Illinois Animal Care Committee.
Disease activity index (DAI) and histological analysis
Mice were examined daily and DAI was assessed based on symptom severities as described48. Distal colons were formalin-fixed and paraffin-embedded; sections were stained with H&E and photomicrographed. Histological score was based on published criteria49 with each parameter multiplied by percentage of tissue involvement for a maximum score of 10.
RNA isolation, qRT-PCR, and ELISA
RNA was extracted using TRIzol (Invitrogen) and RNeasy Mini Kit (Qiagen), and reverse transcribed to cDNA using SuperScript Reverse Transcriptase III following the manufacturer’s protocol (Invitrogen). Quantitative reverse transcription-PCR (qRT-PCR) was performed with iCycler thermal cycler (Bio-Rad) using iQ SYBR Green supermix. Cyclophilin E was used as an internal standard. List of primer sequences used for qRT-PCR is provided in supplementary table 1. ELISA was conducted using IL-6 Quantikine ELISA kit (R&D); serum protein levels were quantified using Inflammatory Cytokine and Chemokine Multi-Analyte ELISArray and IL-6 single analyte ELISA kits (Qiagen) with manufacturer’s protocol.
In vivo permeability assay
Mice were gavaged with fluorescein isothiocyanate (FITC)-dextran (MW 4000; Sigma-Aldrich, St. Louis, MO) at 60 mg/kg of body weight 4 hrs prior to sacrifice. Blood was collected via cardiac puncture, and fluorescence intensity in the serum was measured (excitation, 485 nm; emission, 520 nm) using Fluostar Omega multimode microplate reader. FITC-dextran concentrations were determine from a standard curve generated by a serial dilution of FITC-dextran.
TUNEL and MPO assays
TUNEL assay was performed using ApopTag Red detection kit (Millipore) according to manufacturer’s protocol. Four randomly selected high-power microscopic fields of each sample were examined using Leica DM 4000B microscope mounted with QI Click digital CCD camera. Number of TUNEL-positive cells were scored and normalized by total number of cells. MPO activity was measured as previous described50. Enzymatic activity was measured at 450 nm using 96-well Multiskan microplate reader and determined from a standard curve generated by a serial dilution of purified MPO enzyme (Enzo Life Sciences).
Immunofluorescence microscopy and cell proliferation assay
Paraffin-embedded colon sections were stained with anti-E-cadherin antibody (Santa Cruz Biotech), anti-green fluorescent protein (GFP; Abcam), or mAb against proliferating cell nuclear antigen (PCNA; Abcam), and visualized with anti-rabbit IgG Texas Red and anti-mouse IgG Alexa Fluor 488 (Invitrogen). For cell proliferation, Ki67 was detected using rabbit polyclonal antibodies (Abcam) and anti-rabbit IgG Alexa Fluor 488 (Invitrogen). All assays were done in triplicates, and >300 cells were counted in each sample from random fields.
Statistical analysis
Data are expressed as mean ± SEM. All experiments were performed in triplicates unless stated otherwise. Statistical significance was determined by Student’s t test. Survival curves were calculated using Kaplan-Meier method. Statistical significance for survival between populations was analyzed by log-rank test.
Supplementary Material
Acknowledgments
We thank Robert H. Whitehead for a generous gift of YAMC, Chih-Chiun Chen for helpful suggestions, and Seungwon Shin and Guoqiang Yan for excellent technical assistance. JSC was supported by the UIC Medical Scientist Training Program and NIH grant T32 DK07739. This work was supported by grants from the NIH (R01AR61791 and R01GM78492) to LFL.
Footnotes
Disclosures
The authors have no conflict of interest to disclose.
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013;369:754–762. doi: 10.1056/NEJMct1209614. [DOI] [PubMed] [Google Scholar]
- 3.Leal RF, et al. Identification of inflammatory mediators in patients with Crohn’s disease unresponsive to anti-TNFalpha therapy. Gut. 2015;64:233–242. doi: 10.1136/gutjnl-2013-306518. [DOI] [PubMed] [Google Scholar]
- 4.Scharl M, Vavricka SR, Rogler G. Review: new anti-cytokines for IBD: what is in the pipeline? Curr Drug Targets. 2013;14:1405–1420. doi: 10.2174/13894501113149990159. [DOI] [PubMed] [Google Scholar]
- 5.Allocca M, Jovani M, Fiorino G, Schreiber S, Danese S. Anti-IL-6 treatment for inflammatory bowel diseases: next cytokine, next target. Curr Drug Targets. 2013;14:1508–1521. doi: 10.2174/13894501113146660224. [DOI] [PubMed] [Google Scholar]
- 6.Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6–19. doi: 10.1038/mi.2013.73. [DOI] [PubMed] [Google Scholar]
- 7.Atreya R, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878–4882. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 9.Ito H, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126:989–996. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Tebbutt NC, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 11.Dann SM, et al. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J Immunol. 2008;180:6816–6826. doi: 10.4049/jimmunol.180.10.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci. 2011;68:3149–3163. doi: 10.1007/s00018-011-0778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mo FE, et al. CYR61 (CCN1) Is Essential for Placental Development and Vascular Integrity. Mol Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nature Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emre Y, Imhof BA. Matricellular protein CCN1/CYR61: a new player in inflammation and leukocyte trafficking. Semin Immunopathol. 2014;36:253–259. doi: 10.1007/s00281-014-0420-1. [DOI] [PubMed] [Google Scholar]
- 16.Jun JI, Lau LF. The Matricellular Protein CCN1 Induces Fibroblast Senescence and Restricts Fibrosis in Cutaneous Wound Healing. Nature Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KH, Chen CC, Monzon RI, Lau LF. The Matricellular Protein CCN1 Promotes Regression of Liver Fibrosis through Induction of Cellular Senescence in Hepatic Myofibroblasts. Mol Cell Biol. 2013;33:2078–2090. doi: 10.1128/MCB.00049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CC, et al. Cytotoxicity of TNFα is regulated by Integrin-Mediated Matrix Signaling. EMBO J. 2007;26:1257–1267. doi: 10.1038/sj.emboj.7601596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juric V, Chen CC, Lau LF. Fas-Mediated Apoptosis is Regulated by the Extracellular Matrix Protein CCN1 (CYR61) in vitro and in vivo. Mol Cell Biol. 2009;29:3266–3279. doi: 10.1128/MCB.00064-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai T, Chen CC, Lau LF. The matricellular protein CCN1 activates a pro-inflammatory genetic program in murine macrophages. J Immunol. 2010;184:3223–3232. doi: 10.4049/jimmunol.0902792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koon HW, et al. Substance P-mediated expression of the pro-angiogenic factor CCN1 modulates the course of colitis. Am J Pathol. 2008;173:400–410. doi: 10.2353/ajpath.2008.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 23.Gong S, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 24.Schober JM, Lau LF, Ugarova TP, Lam SC. Identification of a novel integrin αMβ2 binding site in CCN1 (CYR61), a matricellular protein expressed in healing wounds and atherosclerotic lesions. J Biol Chem. 2003;278:25808–25815. doi: 10.1074/jbc.M301534200. [DOI] [PubMed] [Google Scholar]
- 25.Leu SJ, et al. Targeted mutagenesis of the matricellular protein CCN1 (CYR61): selective inactivation of integrin α6β1-heparan sulfate proteoglycan coreceptor-mediated cellular activities. J Biol Chem. 2004;279:44177–44187. doi: 10.1074/jbc.M407850200. [DOI] [PubMed] [Google Scholar]
- 26.Mizoguchi A. Healing of intestinal inflammation by IL-22. Inflamm Bowel Dis. 2012;18:1777–1784. doi: 10.1002/ibd.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 28.Chen N, Chen CC, Lau LF. Adhesion of human skin fibroblasts to Cyr61 is mediated through integrin α6β1 and cell surface heparan sulfate proteoglycans. J Biol Chem. 2000;275:24953–24961. doi: 10.1074/jbc.M003040200. [DOI] [PubMed] [Google Scholar]
- 29.Pineton de CG, Peyrin-Biroulet L, Lemann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 30.Rutgeerts P, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102–1111. doi: 10.1053/j.gastro.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 31.Hebuterne X, et al. Endoscopic improvement of mucosal lesions in patients with moderate to severe ileocolonic Crohn’s disease following treatment with certolizumab pegol. Gut. 2013;62:201–208. doi: 10.1136/gutjnl-2012-302262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Athanasopoulos AN, et al. Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J Biol Chem. 2007;282:26746–26753. doi: 10.1074/jbc.M705200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henckaerts L, et al. Mutations in pattern recognition receptor genes modulate seroreactivity to microbial antigens in patients with inflammatory bowel disease. Gut. 2007;56:1536–1542. doi: 10.1136/gut.2007.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Wang Y, Ouyang X. Beyond toll-like receptors: Porphyromonas gingivalis induces IL-6, IL-8, and VCAM-1 expression through NOD-mediated NF-kappaB and ERK signaling pathways in periodontal fibroblasts. Inflammation. 2014;37:522–533. doi: 10.1007/s10753-013-9766-0. [DOI] [PubMed] [Google Scholar]
- 35.Mudter J, et al. The transcription factor IFN regulatory factor-4 controls experimental colitis in mice via T cell-derived IL-6. J Clin Invest. 2008;118:2415–2426. doi: 10.1172/JCI33227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pickert G, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gout T, Ostor AJ, Nisar MK. Lower gastrointestinal perforation in rheumatoid arthritis patients treated with conventional DMARDs or tocilizumab: a systematic literature review. Clin Rheumatol. 2011;30:1471–1474. doi: 10.1007/s10067-011-1827-x. [DOI] [PubMed] [Google Scholar]
- 38.Chen N, Leu SJ, Todorovic V, Lam SCT, Lau LF. Identification of a novel integrin αvβ3 binding site in CCN1 (CYR61) critical for pro-angiogenic activities in vascular endothelial cells. J Biol Chem. 2004;279:44166–44176. doi: 10.1074/jbc.M406813200. [DOI] [PubMed] [Google Scholar]
- 39.Becker C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 40.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin X, Zimmers TA, Zhang Z, Pierce RH, Koniaris LG. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut. 2010;59:186–196. doi: 10.1136/gut.2008.151175. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Srinivasan S, Theiss AL, Merlin D, Sitaraman SV. Interleukin-6 induces keratin expression in intestinal epithelial cells: potential role of keratin-8 in interleukin-6-induced barrier function alterations. J Biol Chem. 2007;282:8219–8227. doi: 10.1074/jbc.M604068200. [DOI] [PubMed] [Google Scholar]
- 43.Naito Y, et al. Reduced intestinal inflammation induced by dextran sodium sulfate in interleukin-6-deficient mice. Int J Mol Med. 2004;14:191–196. [PubMed] [Google Scholar]
- 44.Gay J, Kokkotou E, O’Brien M, Pothoulakis C, Karalis KP. Interleukin-6 genetic ablation protects from trinitrobenzene sulfonic acid-induced colitis in mice. Putative effect of antiinflammatory cytokines. Neuroimmunomodulation. 2006;13:114–121. doi: 10.1159/000096656. [DOI] [PubMed] [Google Scholar]
- 45.Fattori E, et al. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci U S A. 1993;90:587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kireeva ML, Mo FE, Yang GP, Lau LF. Cyr61, product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol. 1996;16:1326–1334. doi: 10.1128/mcb.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gommeaux J, et al. Colitis and colitis-associated cancer are exacerbated in mice deficient for tumor protein 53-induced nuclear protein 1. Mol Cell Biol. 2007;27:2215–2228. doi: 10.1128/MCB.01454-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vowinkel T, et al. Apolipoprotein A-IV inhibits experimental colitis. J Clin Invest. 2004;114:260–269. doi: 10.1172/JCI21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto S, et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.