Abstract
BACKGROUND
Working memory (Work-Mem), the capacity to hold and manipulate information, activates the anterior cingulate cortex (ACC), especially its caudal subregion. Impaired Work-Mem and structural and functional abnormalities of the ACC are reported in schizophrenia. This study aims to elucidate the pathogenesis of Work-Mem dysfunction in schizophrenia by comparing metabolite concentrations across ACC subregions.
MATERIALS AND METHODS
This retrospective study of 18 schizophrenia cases and 10 matched controls used proton magnetic resonance spectroscopic imaging (1H-MRSI, TR/TE=1800/35 ms, 0.5 cm3 spatial resolution) to test whether the Wechsler Adult Intelligence Scale, third edition Work-Mem Index is associated with the differences in the rostral to caudal ACC ratios of N-acetylaspartate (NAA) and creatine (Cr).
RESULTS
Higher caudal:rostral ACC Cr (but not NAA) concentrations were associated with decreased Work-Mem index in cases (r=−0.6, p=0.02), with a similar trend in controls (r=−0.56, p=0.10), although caudal:rostral ACC Cr correlated with NAA in cases and controls (r=0.67 and 0.62, p<0.05 for both). NAA and Cr ratios did not correlate with myo-inositol, excluding gliosis as the underlying process. Subjects’ sex and age had no effects on these relationships.
CONCLUSIONS
The findings suggest that rostral ACC energy hypo-metabolism, possibly arising from neurodevelopmental processes, is associated with working memory impairment in schizophrenia. Changes in the rostral (not the expected caudal) subregion underscore the interconnections between the ACC subregions and may offer laboratory markers for treatment trials, etiology studies and perhaps even enhanced identification of prodromal “at risk” subjects.
INTRODUCTION
Cognitive impairment, a core feature of schizophrenia, profoundly impacts patient outcomes but remains largely resistant to current therapies (Reichenberg and Harvey 2007). It often spans multiple domains, including deficits in working memory (Work-Mem), executive functions and processing speed (Reichenberg 2010), arising subtly in childhood and preceding development of adult disease (Cannon, Bearden, Hollister, Rosso, Sanchez, and Hadley 2000; David, Malmberg, Brandt, Allebeck, and Lewis 1997; Davidson, Reichenberg, Rabinowitz, Weiser, Kaplan, and Mark 1999; Jones, Rodgers, Murray, and Marmot 1994). Interestingly, early Work-Mem dysfunction may be predictive of conversion to psychosis in prodromal individuals (Pukrop, Ruhrmann, Schultze-Lutter, Bechdolf, Brockhaus-Dumke, and Klosterkotter 2007; Seidman, Thermenos, Poldrack, Peace, Koch, Faraone, and Tsuang 2006). Furthermore, in early-onset schizophrenia patients (<18 years old), fMRI shows reduced blood oxygenation level-dependent signals in the Work-Mem network, comprising the dorsolateral prefrontal cortex (DLPFC), frontal operculum and anterior cingulate cortex (ACC) (Kyriakopoulos, Dima, Roiser, Corrigall, Barker, and Frangou 2012).
Disruption of normal remodeling of the Work-Mem network in adolescence may cause the impairment seen in future adult cases of schizophrenia; however, the exact leading mechanisms are unknown (Kyriakopoulos et al. 2012). Abnormalities of the ACC, a key component of the Work-Mem network, are strongly associated with schizophrenia (Bush, Luu, and Posner 2000; Fornito, Yucel, Dean, Wood, and Pantelis 2009). The ACC contains two distinct subregions: a rostral segment linked to emotional processing and volition and a caudal region associated with cognitive operations, e.g., error detection and conflict monitoring (Botvinick, Braver, Barch, Carter, and Cohen 2001; Bush, Luu, and Posner 2000; Paus 2001; Ridderinkhof, Ullsperger, Crone, and Nieuwenhuis 2004; Rushworth, Buckley, Behrens, Walton, and Bannerman 2007; Walton, Croxson, Behrens, Kennerley, and Rushworth 2007). Structural and functional MRI studies show both ACC segments to be smaller (Baiano, David, Versace, Churchill, Balestrieri, and Brambilla 2007; Preuss, Zetzsche, Pogarell, Mulert, Frodl, Muller, Schmidt, Born, Reiser, Moller, Hegerl, and Meisenzahl 2010) and functionally aberrant in patients (Habel, Chechko, Pauly, Koch, Backes, Seiferth, Shah, Stocker, Schneider, and Kellermann 2010; Taylor, Chen, Tso, Liberzon, and Welsh 2011; Wilmsmeier, Ohrmann, Suslow, Siegmund, Koelkebeck, Rothermundt, Kugel, Arolt, Bauer, and Pedersen 2010).
Histopathology findings in the ACC of patients demonstrate decreased cortical thickness and alterations in synaptic architecture (Bouras, Kovari, Hof, Riederer, and Giannakopoulos 2001; Broadbelt, Byne, and Jones 2002; Kreczmanski, Schmidt-Kastner, Heinsen, Steinbusch, Hof, and Schmitz 2005; Todtenkopf, Vincent, and Benes 2005). Furthermore, a meta-analysis of four separate cell counting studies consistently shows lower densities of nonpyramidal neurons in layer II of the ACC in their brains, despite differences in methodologies and subject demographics (Todtenkopf, Vincent, and Benes 2005). Collectively, these findings suggest abnormal synaptic connectivity and decreased neuronal density in the ACC may contribute to Work-Mem impairment in schizophrenia.
PET studies have shown reduced metabolic activity in the ACC of patients with schizophrenia performing cognitive tasks, suggesting that dysfunction in energy metabolism may contribute to cognitive impairment, including Work-Mem (Carter, Mintun, Nichols, and Cohen 1997; Fujimoto, Takeuch, Matsumoto, Kamimura, Hamada, Nakamura, and Kato 2007; Haznedar, Buchsbaum, Hazlett, Shihabuddin, New, and Siever 2004). However, a PET study of first-episode, medication-naïve schizophrenia patients found no significant ACC metabolic differences from matched healthy controls, suggesting that ACC hypo-metabolism in older patients may be due to chronic illness or medication (Yucel, Brewer, Harrison, Fornito, O’Keefe, Olver, Scott, Egan, Velakoulis, McGorry, and Pantelis 2007). In light of these findings, it is unclear if hypo-metabolism represents a primary insult in the pathogenesis of cognitive impairment or a secondary effect.
Proton magnetic resonance spectroscopic imaging (1H-MRSI) is a non-invasive MR modality that can monitor neuronal viability, energy metabolism, cell membrane turnover and glial proliferation via their surrogate markers: N-acetyl-aspartate (NAA), creatine (Cr), choline (Cho) and myo-inositol (mI), respectively (Andres, Ducray, Schlattner, Wallimann, and Widmer 2008; Bertholdo, Watcharakorn, and Castillo 2013; Mountford, Stanwell, Lin, Ramadan, and Ross 2010; Soares and Law 2009). 1H-MRSI studies have consistently identified neurometabolic abnormalities in patients with schizophrenia, including decreased NAA in brain regions implicated in its pathogenesis (Deicken, Zhou, Schuff, and Weiner 1997; Kraguljac, Reid, White, Jones, den Hollander, Lowman, and Lahti 2012; Reid, Stoeckel, White, Avsar, Bolding, Akella, Knowlton, den Hollander, and Lahti 2010; Schwerk, Alves, Pouwels, and van Amelsvoort 2014). Indeed, our recent study found differences in caudal to rostral ACC NAA and Cr concentrations in patients, but not in controls (Hardy, Tal, Babb, Perry, Messinger, Antonius, Malaspina, and Gonen 2011).
Our goal in this study, therefore, is to test whether this PET-detected hypo-metabolism represents a primary insult in the pathogenesis of cognitive impairment, or a secondary effect in previously published data of 18 cases of schizophrenia compared to 10 matched controls. Specifically, whether 1H-MRSI-detected lower caudal to rostral ACC NAA and Cr ratios, reflecting decreased neuronal density and metabolic activity in the cognition-oriented caudal region, will predict impaired Work-Mem Index, as measured by the Wechsler Adult Intelligence Scale third edition (WAIS-III). We chose this index from other possible clinical metrics due to its ubiquity as a core feature of cognitive dysfunction, potential utility predicting prodromal conversion to schizophrenia and its virtual non-responsiveness to current pharmacotherapies.
MATERIALS AND METHODS
Participants
Data from this study were previously published in Hardy et al. (2011). This retrospective study examined 18 cases with DSM-IV schizophrenia or schizoaffective disorder, recruited from inpatient and outpatient research and clinical units at Bellevue Hospital; and 10 healthy matched controls, recruited from medical center postings. It should be noted that only a subset of the original subjects was included in this study because some subjects dropped out before comprehensive WAIS-III testing could be performed within 1 month post-scan. Their demographics are compiled in Table 1. All cases were on stable medication regimens for at least one month and clinically stable. All procedures were performed by trained mental health professionals whose training entailed initial calibrations for validity followed by regular tests of inter-rater reliability. All participants gave local Institutional Review Board approved written informed consent.
Table 1.
Demographics, caudal:rostral metabolite concentrations ratios, and Working Memory Indices for All Subjects (controls: 1 – 10, cases: 11 – 29) sorted by age (in years).
| # | Age/sex | DD | Medication | NAA | Cr | mI | WMI |
|---|---|---|---|---|---|---|---|
| 1 | 23/M | — | 1.02 | 1.12 | 1.54 | 111 | |
| 2 | 28/M | — | 0.97 | 0.99 | 0.99 | 95 | |
| 3 | 29/F | — | 0.85 | 0.84 | 1.24 | 119 | |
| 4 | 31/M | — | 1.01 | 1.44 | 0.97 | 84 | |
| 5 | 34/F | — | 0.99 | 0.97 | 1.04 | 119 | |
| 6 | 36/M | — | 0.99 | 1.35 | 1.16 | 113 | |
| 7 | 43/M | — | 0.94 | 1.04 | 0.96 | 94 | |
| 8 | 46/M | — | 0.93 | 0.87 | 0.92 | 136 | |
| 9 | 47/M | — | 1.06 | 1.13 | 1.04 | 102 | |
| 10 | 55/F | — | 0.88 | 1.04 | 0.88 | 95 | |
| 11 | 22/M | 48 | Clozapine | 1.03 | 1.10 | 1.14 | 108 |
| 12 | 23/M | 3 | Risperidone | 1.00 | 1.03 | 1.02 | 113 |
| 13 | 25/M | 60 | Clozapine, valproic acid | 0.91 | 0.98 | 0.95 | 99 |
| 14 | 26/M | 8 | Ziprasidone | 1.07 | 1.43 | 0.77 | — |
| 15 | 29/F | 8 | Aripiprazole, bupropion, fluphenazine | 1.19 | 1.26 | 1.24 | 82 |
| 16 | 38/F | 276 | Clonazepam, fluphenazine | 1.12 | 1.36 | 0.94 | 63 |
| 17 | 41/M | 264 | Fluphenazine | 1.65 | 1.18 | 1.09 | 80 |
| 18 | 42/F | 23 | Ziprasidone, bupropion, eszopiclone | 1.25 | 1.32 | 1.38 | 65 |
| 19 | 43/F | 240 | Aripiprazole, escitalopram, fluphenazine | 1.00 | 1.06 | 0.99 | 78 |
| 20 | 44/M | 26 | Clozapine, valproic acid | 1.10 | 1.17 | 1.79 | — |
| 21 | 44/M | 312 | Clozapine, valproic acid | 1.41 | 1.36 | 0.68 | 69 |
| 22 | 44/F | 324 | Quetiapine | 1.42 | 1.29 | 1.02 | 90 |
| 23 | 48/M | 276 | Quetiapine | 0.86 | 0.72 | 0.88 | 95 |
| 24 | 51/F | 420 | Ziprasidone, lithium, gabapentin | 1.16 | 1.10 | 1.08 | 73 |
| 25 | 51/F | 35 | None | 0.79 | 0.75 | 0.53 | 104 |
| 26 | 52/M | 32 | Citalopram | 1.19 | 1.07 | 1.06 | 97 |
| 27 | 53/M | 31 | Clozapine, gabapentin, venlafaxine | 1.29 | 1.28 | 1.32 | 104 |
| 28 | 55/F | 432 | Aripiprazole, quetiapine, trazadone | 1.18 | 1.14 | 1.26 | 88 |
Note: F=female, M=male, DD=Schizophrenia disease duration (in months, patients only), NAA, Cr, mI = caudal:rostral ratio of this metabolite, WMI=WAIS-III Working Memory index.
Psychiatric Assessments
The Diagnostic Interview for Genetic Studies was administered and case charts reviewed to determine current and lifetime diagnoses (Nurnberger, Blehar, Kaufmann, York-Cooler, Simpson, Harkavy-Friedman, Severe, Malaspina, and Reich 1994). Inclusion criteria required controls to be free of current Axis I diagnoses and have no family history of psychosis. The inter-rater reliability, kappa, was 0.95 for DSM-IV diagnosis and 0.80 for individual symptoms. Current (state) symptoms were assessed with Positive and Negative Syndrome Scale (PANSS) yielding five symptom factors: positive, negative, dysthymia, activation and autistic preoccupation (Kay, Fiszbein, and Opler 1987; White, Harvey, Opler, and Lindenmayer 1997). Enduring (trait) negative symptoms were assessed with the Schedule for the Deficit Syndrome (Kirkpatrick, Buchanan, McKenney, Alphs, and Carpenter 1989). Cognition was assessed using the WAIS-III, providing the Working Memory, Verbal Comprehension, Perceptual Organization and Processing Speed indices, which comprised fourteen subtests, yielding Verbal, Performance and Full Scale IQ scores for all subjects. The Work-Mem Index is a composite metric of both arithmetic and digit span scores.
MR Data Acquisition
All experiments were done in a 3 T whole-body MR imager (Trio, Siemens AG, Erlangen Germany), as described previously (Hardy et al. 2011). Specifically, for anatomic reference and 1H-MRSI volume-of-interest (VOI) guidance, we acquired 3D T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) MR images at repetition time/echo (TR/TE)/inversion times of 1360/2.6/800 ms; 256×256×160 mm3 field-of-view, 1 mm spatial resolution, in each participant. They were reformatted into axial, sagittal, and coronal image stacks at isotropic 1 mm resolution.
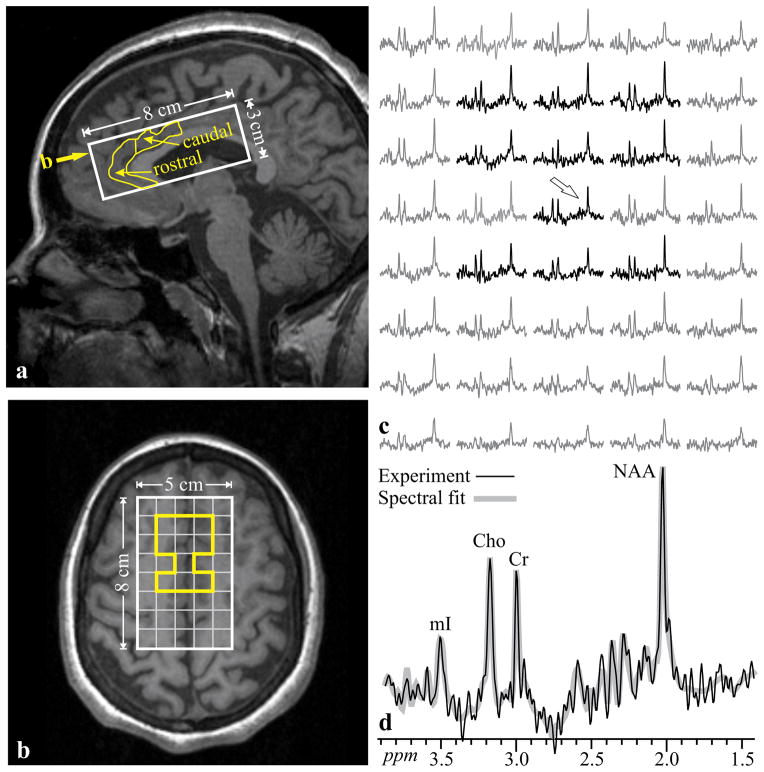
For 1H-MRSI we first shimmed the whole-head to 27±3 Hz using our automated chemical-shift-imaging (CSI) based procedure in 3–5 minutes (Hu, Javaid, Arias-Mendoza, Liu, McNamara, and Brown 1995). We then image-guided an 8 cm anterior-posterior (AP) ×5 cm left-right (LR) ×3 cm inferior-superior (IS) parallelepiped VOI over the ACC, as shown in Fig. 1a and 1b. This VOI was selectively excited with point-resolved spectroscopy (PRESS: TR/TE=1800/35 ms) and partitioned into 16×16×6 voxels (LR×AP×IS), a nominal 0.5 cm3 each. Of these, 180 voxels were inside the VOI (Hardy et al. 2011). Note that the in-plane size of the VOI was selected to ensure that the ACC will not fall in any of the “edge voxels,” that suffer chemical shift displacement (CSD) errors due to the selective PRESS 180° pulses in the LR×AP planes, as shown in Fig. 1b. Furthermore, the slice-selective 90° of the PRESS was under very strong, 9 mT/m gradients, reducing the CSD in the IS direction to less than (a negligible) 4% of the slice thickness between NAA and mI (1.56 ppm apart) and to 2% between Cr and mI (0.56 ppm apart).
Fig. 1.
(a) Sagittal and T1-weighted image from a 29 year-old woman with schizophrenia (#14 in Table 1) superimposed with the 5×8×3 cm3 (LR×AP×IS) VOI (solid white line) and manually delineated ACC (yellow line) with its rostral and caudal parts. Yellow arrow denotes the level of b. (b) Axial slice from the level denoted by yellow arrow on a, showing the the 5×8 cm (LR×AP) VOI and 1×1 cm MRSI grid (white frame, yellow-highlighted voxels contain ACC). Note that ACC voxels are away from the VOI edges and do not incur chemical-shift-displacement errors. (c) Real part of the spectra from the 5×8 VOI voxel matrix in b, each representing 0.5 cm3, on common frequency (1.7 – 4.0 ppm) and intensity scales. Spectra within the ACC (yellow region on b) are black, outside - gray. (d) Spectrum indicated by arrow on c, expanded to show details, superimposed with its spectral-fit. Note the signal-to-noise ratio, excellent spectral resolution (5.3±1.2 Hz linewidth) and spectral fitting fidelity, from these 0.5 cm3 voxels in ~30 minute 1H-MRSI acquisition
1H-MRSI post-processing was described by Hardy et al. (Hardy et al. 2011). Owing to their irregular shape, the caudal and rostral ACC were manually outlined on the axial MRI of each subject, as shown in Fig. 1a, b. Our software then added the phased and aligned, CSF-partial-volume corrected, spectra from all voxels that fell completely or partially within the outlined region, as shown in Fig. 1b and 1c. The metabolites’ relative concentrations were estimated with the SITools-FITT parametric spectral modeling software, using aspartate, glutamate, glutamine, Cho, Cr, mI, NAA [comprising NAA and NAA-glutamate – (NAAG, which resonates only a few Hz downfield and therefore, difficult to resolve) model functions at 7:1 NAA:NAAG ratio] and taurine as model functions, as shown in Fig. 1d (Soher, Young, Govindaraju, and Maudsley 1998). The relative values in voxels where these values’ Crammer-Rao lower bounds were lower than 20%, were converted into absolute concentrations in the caudal and rostral ACC, using phantom replacement (Hardy et al. 2011).
Statistical Analyses
Data were entered and verified using the SIR Database Management Software (SIR 2002, SIR Pty Ltd). IBM SPSS (Statistic 21) was used for analyses. Descriptive statistics (means and standard deviations) and distributions of all measures were examined, whether continuous or categorical to identify key features, e.g., non-normal distribution, outliers, skewness, that might impact inferential methods. The caudal and rostral concentrations of each metabolite were used to calculate respective caudal:rostral ratios. The association between the caudal:rostral ratios and the WAIS-III indices were examined in cases and controls separately using Pearson correlations. In order to generate hypotheses, statistical corrections were not applied to the correlation coefficients. All tests were two-tailed and the alpha for significance was set at p<0.05.
RESULTS
Metabolic and Work-Mem metrics
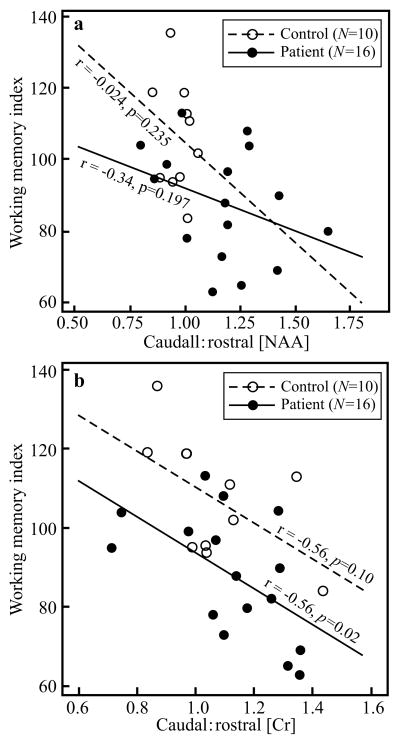
Caudal:rostral ACC metabolite concentrations ratios and Work-Mem Index for each subject are compiled in Table 1. The mean concentrations in each region and each subject cohort, for all the metabolites that had consistent Cramer-Rao lower bounds <20% in every ACC voxel, are given in Table 2. Higher caudal:rostral ACC NAA concentrations showed an inverse correlation with the Work-Mem Index that was not statistically significant for either cases (r=−0.34, p=0.197) or controls (r=−0.024, p=0.235), as shown in Fig. 2b. Lower Work-Mem Index showed a strong inverse correlation with higher caudal:rostral Cr concentrations only in cases (r=−0.56, p=0.02), with a similar magnitude in controls, although only at the trend level, most likely reflecting sample size (r=−0.56, p=0.10), as shown in Fig. 2b.
Table 2.
Means ± standard deviation of the absolute NAA, Cr and Cho concentrations (millimolar) in the caudal and rostral acc of controls and patients. Note that there are no significant differences between the groups for each region but significant differences between regions only in the patients. The pi-s designate: p1 differences between the caudal and rostral concentration of that metabolite; and p3 same as p1 except for the patients, both from mixed model ANOVA to compare regions in terms of metabolite levels within each subject group. p2 is for the difference between patients and controls in that metabolite in that region, from t test to compare the groups in terms of the mean metabolite levels.
| Metabolite | Region | p1 | Control [mM] | p2 | Patient [mM] | p3 |
|---|---|---|---|---|---|---|
| NAA | Caudal | 6.5±1.1 | 0.312 | 7.1±1.63 | ||
| Rostral | 0.643 | 6.6±0.8 | 0.513 | 6.2±1.3 | 0.006 | |
| Cr | Caudal | 5.8±1.1 | 0.361 | 6.3±1.6 | ||
| Rostral | 0.163 | 5.5±0.8 | 0.303 | 5.7±1.4 | 0.012 | |
| Cho | Caudal | 1.6±0.3 | 0.261 | 1.8±0.5 | ||
| Rostral | 0.272 | 1.7±0.3 | 0.554 | 1.7±0.5 | 0.951 | |
| mI | Caudal | 2.6±0.7 | 0.50 | 2.5±0.5 | ||
| Rostral | 0.5101 | 2.5±0.7 | 0.64 | 2.3±0.6 | 0.027 |
Boldface p values indicate statistical significance: pi ≤ 0.05. Note the significantly higher concentration of NAA, Cr and mI in the caudal than the rostral ACC.
Fig. 2.
Top, a: Scatter plot of the association between caudal:rostral ACC NAA concentration and working memory index in cases and controls. Note that although both show an inverse relationship, in neither cohort is it statistically significant.
Bottom, b: Scatter plot of caudal:rostral ACC Cr concentrations versus working memory index in cases and controls. Note the strong significant inverse correlation in the cases but just a “trend” in the controls. These correlations are at similar levels and differences in significance level, therefore, is probably due to different number of subjects
Metabolic metrics
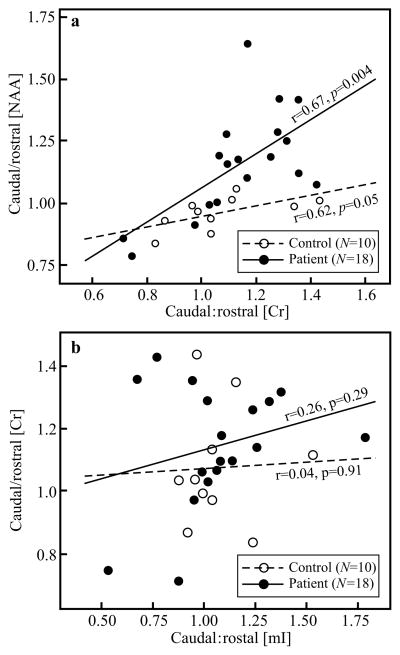
To ascertain whether the caudal:rostral Cr concentration ratios reflect an increase or decrease in the numerator or denominator, we examined the correlation between the Cr and NAA ratios, which was robust both in the cases (r=0.67, p=0.004) and controls (r=0.62, p=0.05), as shown in Fig. 3a. To also ascertain whether the caudal:rostral ACC Cr increase could represent gliosis, we tested its relationship with the mI concentration ratios [both metabolites are higher in glial cells than in neurons (Mountford et al. 2010)] and found only weak insignificant correlations in both cases (r=0.26, p=0.29) and controls (r=0.04, p=0.91), as shown in Fig. 3b.
Fig. 3.
Top, a: Scatter plot of the association between caudal:rostral ACC NAA and creatine concentrations in the cases and the controls). Note that the correlations are both strong and significant in both cohorts, indicating NAA decline in the rostral ACC, i.e., since the latter is synthesized in the mitochondria, a Cr decline in that region as well. Bottom, b: Scatter plot of the association between caudal:rostral ACC Cr and mI concentrations in cases and controls. Note the lack of correlation, suggesting that Cr changes likely represent energy metabolism dysfunction and not gliosis (increased mI).
Age and gender effects
There were no significant correlations between the caudal:rostral NAA, Cr, mI, or Work-Mem Index and subjects’ age for either cases or controls (p>0.1 for both). There were also no gender differences with regard to any of the metrics (p>0.1 for all). Finally, there were no notable structural post-MRI findings in either cases or controls.
DISCUSSION
We used retrospective 1H-MRSI data to test whether previously found differences in NAA and Cr concentrations between the caudal and rostral ACC subregions, an integral component of the Work-Mem network, are associated Work-Mem performance in schizophrenia patients (Hardy et al. 2011). Surprisingly, caudal:rostral ACC concentration of NAA, a metric of neuronal integrity, did not correlate with Work-Mem Index. In contrast, the relative caudal:rostral elevations of ACC Cr did correlate with lower Work-Mem in cases, but not controls.
Since it is uncertain whether the numerator, denominator (or both) are responsible for changes in the magnitude of the Cr and NAA ratios, we also investigated their relationships. With the exception of Canavan’s disease (Baslow and Resnik 1997), NAA levels are reported to only decrease in CNS disorders (Benarroch 2008). An increase in its caudal:rostral ratio, therefore, must indicate a decline in the denominator, not a numerator increase. The strong positive correlation between caudal:rostral ACC Cr and NAA concentrations (Fig. 3a), and the fact that the latter is synthesized in the mitochondria (Baslow and Resnik 1997; Madhavarao, Chinopoulos, Chandrasekaran, and Namboodiri 2003), suggests that the change in the former is likewise consistent with a denominator decline, i.e., the rostral ACC region. This rostral hypo-metabolism, as opposed to a numerator increase, i.e., caudal hyper-metabolism, is in line with previous PET studies (Carter, Mintun, Nichols, and Cohen 1997; Fujimoto et al. 2007; Haznedar et al. 2004). Note that although the caudal:rostral Cr concentrations correlated both with the NAA’s and with the (patients’) Work-Mem Index, the latter did not correlate with the NAA concentration ratio. This, however, is not a contradiction, since correlations are not necessarily transitive (Langford, Schwertman, and Owens 2001).
The Cr peak in 1H-MRS represents overlapping Cr and phosphocreatine (PCr) resonances, therefore, Cr⇔PCr imbalances representing energy metabolism disorders are not assessable from this line alone. Therefore, to further ascertain that the change in the Cr ratio reflects a rostral decline rather than a caudal increase unrelated to cellular metabolic function, we also examined the relationship with the caudal:rostral ACC mI concentration (see Fig. 3b). Since: (i) mI is a glial marker; and (ii) glial cells also contain higher Cr concentrations than neurons, a positive correlation would suggest gliosis, as opposed to impaired energy metabolism, since under normal conditions, brain NAA level fluctuations are expected to link neuronal to mitochondrial activity (Moffett, Ross, Arun, Madhavarao, and Namboodiri 2007), and its synthesis can be disrupted when ATP production is decreased by inhibitors of the mitochondrial respiratory chain (Bates, Strangward, Keelan, Davey, Munro, and Clark 1996). Consequently, we assume that an NAA decline is linked to ATP production impairment, that is reflected by observable PCr+Cr decline. The lack of correlation with mI but strong correlation with NAA may suggest, therefore that Cr caudal:rostral changes are not reflective of gliosis, thereby but possibly rostral ACC energy hypo-metabolism (Carter, Mintun, Nichols, and Cohen 1997; Fujimoto et al. 2007; Haznedar et al. 2004).
Although schizophrenia may be associated with decreased information input to both ACC subregions, our findings are consistent with relative rostral deficits, which may play a larger role in cognitive operations than previously thought. This notion is supported by PET studies linking decreased rostral ACC activation to deficits in selective attention and error detection (Carter, Mintun, Nichols, and Cohen 1997; Fujimoto et al. 2007; Haznedar et al. 2004). Moreover, as our findings did not support an inflammatory process, neurodevelopmental etiologies may be salient. The rostral ACC defect could derive from fetal or early life stress, shown for schizophrenia (Malaspina, Corcoran, Kleinhaus, Perrin, Fennig, Nahon, Friedlander, and Harlap 2008), since children with fetal glucocorticoid exposure show a thinner cortex, primarily in the rostral anterior cingulate (Davis, Sandman, Buss, Wing, and Head 2013). There also may be genetic variation in various growth factor morphogens, some of which differentially impact regional brain development and could differentially disadvantage the rostral ACC (Borello and Pierani 2010; Dityatev, Seidenbecher, and Schachner 2010; Terwisscha van Scheltinga, Bakker, Kahn, and Kas 2013). For example, a genetic variation in the morphogen Fgf8, which is predominantly expressed at the rostral midline of the anterior neural ridge, may cause imbalanced alterations in rostral-caudal patterning during neurodevelopment (Borello and Pierani 2010). Thus, various genetic and environmental factors may converge on this region to increase the vulnerability for working memory dysfunction, a cardinal finding in individuals with severe mental illness.
Early reduction in Work-Mem has been shown to precede overt development of schizophrenia (Cannon et al. 2000; David et al. 1997; Davidson et al. 1999; Jones, Rodgers, Murray, and Marmot 1994). Therefore, our findings of no age or disease duration effects suggest that developmental processes, either prenatally or during brain remodeling in adolescence, may be key drivers of its impairment, rather than a late effect or confounder. Future studies in prodromal and medication naïve cases will be needed to confirm this biomarker for schizophrenia Work-Mem impairment.
Disease heterogeneity may also make it difficult to identify correlations between metabolic differences and cognitive performance in a relatively small sample size, a potential limitation of this study. This may also effect these relationships in the controls that are just “trends” in our study (see Fig. 2b). Furthermore, it should be noted that rostral and caudal ACC subregions are more functionally interconnected than once thought, with one study demonstrating that they share inputs with a common, bilateral and symmetrical network involving associative, limbic, and sensorimotor regions, including the DLPFC, a structure independently linked to working memory (Habas 2010). The relatively limited sample size (18 subjects) is another potentially significant limitation, since it is possible that a larger sample would have yielded correlations undetected by our study. Additional limitations include possible effects of psychotropic medications on ACC metabolites, operator bias resulting from manual tracing of the ACC subregions, and subsequent partial volume effects with surrounding white matter introduced by the voxels’ size (Tal, Kirov, Grossman, and Gonen 2012), although tracings were performed blind to subjects’ illness status and Work-Mem scores. Finally, it should be noted that our conjecture of energy dysfunction in the ACC is only an inference from the Cr+PCr concentration decline correlating with the NAA’s (which in turn is linked to ATP production in the mitochondria), and is not, therefore, a direct measurement.
In conclusion, validation by further 1H-MRSI studies could lead to an effort-independent imaging measure of Work-Mem performance, improved risk stratification in prodromal individuals and even novel (energy-utilization improvement) therapies for deficits in Work-Mem and other cognitive domains poorly responsive to today’s limited armamentarium.
Acknowledgments
This work was supported by NIH Grants NS050520, EB01015, RC1MH088843 and MH01699.
Footnotes
CONFLICT OF INTEREST
The authors have no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Res Bull. 2008;76:329–43. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Baiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P. Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophr Res. 2007;93:1–12. doi: 10.1016/j.schres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Baslow MH, Resnik TR. Canavan disease. Analysis of the nature of the metabolic lesions responsible for development of the observed clinical symptoms. J Mol Neurosci. 1997;9:109–25. doi: 10.1007/BF02736855. [DOI] [PubMed] [Google Scholar]
- Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–400. [PubMed] [Google Scholar]
- Benarroch EE. N-acetylaspartate and N-acetylaspartylglutamate: neurobiology and clinical significance. Neurology. 2008;70:1353–7. doi: 10.1212/01.wnl.0000311267.63292.6c. [DOI] [PubMed] [Google Scholar]
- Bertholdo D, Watcharakorn A, Castillo M. Brain proton magnetic resonance spectroscopy: introduction and overview. Neuroimaging Clin N Am. 2013;23:359–80. doi: 10.1016/j.nic.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Borello U, Pierani A. Patterning the cerebral cortex: traveling with morphogens. Curr Opin Genet Dev. 2010;20:408–15. doi: 10.1016/j.gde.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bouras C, Kovari E, Hof PR, Riederer BM, Giannakopoulos P. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol. 2001;102:373–9. doi: 10.1007/s004010100392. [DOI] [PubMed] [Google Scholar]
- Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26:379–93. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154:1670–5. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- David AS, Malmberg A, Brandt L, Allebeck P, Lewis G. IQ and risk for schizophrenia: a population-based cohort study. Psychol Med. 1997;27:1311–23. doi: 10.1017/s0033291797005680. [DOI] [PubMed] [Google Scholar]
- Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry. 1999;156:1328–35. doi: 10.1176/ajp.156.9.1328. [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA, Buss C, Wing DA, Head K. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol Psychiatry. 2013;74:647–55. doi: 10.1016/j.biopsych.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deicken RF, Zhou L, Schuff N, Weiner MW. Proton magnetic resonance spectroscopy of the anterior cingulate region in schizophrenia. Schizophr Res. 1997;27:65–71. doi: 10.1016/S0920-9964(97)00082-0. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Seidenbecher CI, Schachner M. Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci. 2010;33:503–12. doi: 10.1016/j.tins.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: bridging the gap between neuroimaging and neuropathology. Schizophr Bull. 2009;35:973–93. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T, Takeuch K, Matsumoto T, Kamimura K, Hamada R, Nakamura K, Kato N. Abnormal glucose metabolism in the anterior cingulate cortex in patients with schizophrenia. Psychiatry Res. 2007;154:49–58. doi: 10.1016/j.pscychresns.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Habel U, Chechko N, Pauly K, Koch K, Backes V, Seiferth N, Shah NJ, Stocker T, Schneider F, Kellermann T. Neural correlates of emotion recognition in schizophrenia. Schizophr Res. 2010;122:113–23. doi: 10.1016/j.schres.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Hardy CJ, Tal A, Babb JS, Perry NN, Messinger JW, Antonius D, Malaspina D, Gonen O. Multivoxel proton MR spectroscopy used to distinguish anterior cingulate metabolic abnormalities in patients with schizophrenia. Radiology. 2011;261:542–50. doi: 10.1148/radiol.11110675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, Shihabuddin L, New A, Siever LJ. Cingulate gyrus volume and metabolism in the schizophrenia spectrum. Schizophr Res. 2004;71:249–62. doi: 10.1016/j.schres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Hu J, Javaid T, Arias-Mendoza F, Liu Z, McNamara R, Brown TR. A fast, reliable, automatic shimming procedure using 1H chemical-shift-imaging spectroscopy. J Magn Reson B. 1995;108:213–9. doi: 10.1006/jmrb.1995.1126. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT., Jr The Schedule for the Deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30:119–23. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, Lahti AC. Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res. 2012;203:111–25. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreczmanski P, Schmidt-Kastner R, Heinsen H, Steinbusch HW, Hof PR, Schmitz C. Stereological studies of capillary length density in the frontal cortex of schizophrenics. Acta Neuropathol. 2005;109:510–8. doi: 10.1007/s00401-005-1003-y. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Dima D, Roiser JP, Corrigall R, Barker GJ, Frangou S. Abnormal functional activation and connectivity in the working memory network in early-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2012;51:911–20 e2. doi: 10.1016/j.jaac.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Langford E, Schwertman N, Owens M. Is the property of being positively correlated transitive? The American Statistician. 2001;55:322–325. [Google Scholar]
- Madhavarao CN, Chinopoulos C, Chandrasekaran K, Namboodiri MA. Characterization of the N-acetylaspartate biosynthetic enzyme from rat brain. J Neurochem. 2003;86:824–35. doi: 10.1046/j.1471-4159.2003.01905.x. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, Friedlander Y, Harlap S. Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC Psychiatry. 2008;8:71. doi: 10.1186/1471-244X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountford CE, Stanwell P, Lin A, Ramadan S, Ross B. Neurospectroscopy: the past, present and future. Chem Rev. 2010;110:3060–86. doi: 10.1021/cr900250y. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–59. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–4. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–24. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Zetzsche T, Pogarell O, Mulert C, Frodl T, Muller D, Schmidt G, Born C, Reiser M, Moller HJ, Hegerl U, Meisenzahl EM. Anterior cingulum volumetry, auditory P300 in schizophrenia with negative symptoms. Psychiatry Res. 2010;183:133–9. doi: 10.1016/j.pscychresns.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Pukrop R, Ruhrmann S, Schultze-Lutter F, Bechdolf A, Brockhaus-Dumke A, Klosterkotter J. Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophr Res. 2007;92:116–25. doi: 10.1016/j.schres.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Reichenberg A. The assessment of neuropsychological functioning in schizophrenia. Dialogues Clin Neurosci. 2010;12:383–92. doi: 10.31887/DCNS.2010.12.3/areichenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–58. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- Reid MA, Stoeckel LE, White DM, Avsar KB, Bolding MS, Akella NS, Knowlton RC, den Hollander JA, Lahti AC. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2010;68:625–33. doi: 10.1016/j.biopsych.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Curr Opin Neurobiol. 2007;17:220–7. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Schwerk A, Alves FD, Pouwels PJ, van Amelsvoort T. Metabolic alterations associated with schizophrenia: a critical evaluation of proton magnetic resonance spectroscopy studies. J Neurochem. 2014;128:1–87. doi: 10.1111/jnc.12398. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Thermenos HW, Poldrack RA, Peace NK, Koch JK, Faraone SV, Tsuang MT. Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophr Res. 2006;85:58–72. doi: 10.1016/j.schres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clinical radiology. 2009;64:12–21. doi: 10.1016/j.crad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40:822–31. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- Tal A, Kirov, Grossman RI, Gonen O. The role of gray and white matter segmentation in quantitative proton MR spectroscopic imaging. NMR Biomed. 2012;25:1392–400. doi: 10.1002/nbm.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Chen AC, Tso IF, Liberzon I, Welsh RC. Social appraisal in chronic psychosis: role of medial frontal and occipital networks. J Psychiatr Res. 2011;45:526–38. doi: 10.1016/j.jpsychires.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwisscha van Scheltinga AF, Bakker SC, Kahn RS, Kas MJ. Fibroblast growth factors in neurodevelopment and psychopathology. Neuroscientist. 2013;19:479–94. doi: 10.1177/1073858412472399. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Vincent SL, Benes FM. A cross-study meta-analysis and three-dimensional comparison of cell counting in the anterior cingulate cortex of schizophrenic and bipolar brain. Schizophr Res. 2005;73:79–89. doi: 10.1016/j.schres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Walton ME, Croxson PL, Behrens TE, Kennerley SW, Rushworth MF. Adaptive decision making and value in the anterior cingulate cortex. Neuroimage. 2007;36(Suppl 2):T142–54. doi: 10.1016/j.neuroimage.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L, Harvey PD, Opler L, Lindenmayer JP. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia. A multisite, multimodel evaluation of the factorial structure of the Positive and Negative Syndrome Scale. The PANSS Study Group. Psychopathology. 1997;30:263–74. doi: 10.1159/000285058. [DOI] [PubMed] [Google Scholar]
- Wilmsmeier A, Ohrmann P, Suslow T, Siegmund A, Koelkebeck K, Rothermundt M, Kugel H, Arolt V, Bauer J, Pedersen A. Neural correlates of set-shifting: decomposing executive functions in schizophrenia. J Psychiatry Neurosci. 2010;35:321–9. doi: 10.1503/jpn.090181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Brewer WJ, Harrison BJ, Fornito A, O’Keefe GJ, Olver J, Scott AM, Egan GF, Velakoulis D, McGorry PD, Pantelis C. Anterior cingulate activation in antipsychotic-naive first-episode schizophrenia. Acta Psychiatr Scand. 2007;115:155–8. doi: 10.1111/j.1600-0447.2006.00902.x. [DOI] [PubMed] [Google Scholar]