Abstract
Arsenic crosses the placenta and may have adverse consequences in utero and later in life. At present, little is known about arsenic concentrations in placenta and their relation to maternal and infant exposures particularly at common levels of exposure.
We measured placenta arsenic in a US cohort potentially exposed via drinking water from private wells, and evaluated the relationships between placenta and maternal and infant biomarker arsenic concentrations.
We measured total arsenic concentrations in placental samples from women enrolled in the New Hampshire Birth Cohort Study (N=766). We compared these data to maternal urinary arsenic (total arsenic and individual species) collected at approximately 24–28 week gestation, along with maternal post-partum toenails and infant toenails using non-parametric multivariate analysis of log10-transformed data. We also examined the association between placental arsenic and household drinking water arsenic.
Placenta arsenic concentrations were related to arsenic concentrations in maternal urine (β 0.55, P value <0.0001), maternal (β 0.30, P value 0.0196) and infant toenails (β 0.40, P value 0.0293) and household drinking water (β 0.09, P value <0.0001). Thus, our data suggest that placenta arsenic concentrations reflect both maternal and infant exposures.
Keywords: Arsenic, placenta, biomarker, low-level
INTRODUCTION
Arsenic readily crosses the placenta, and may adversely affect fetal development.1 Early life exposure to arsenic has also been linked to adverse health effects later in life. These include an increased risk of lower respiratory tract infection in children2, 3 and higher risk of cancer in both animal models and from emerging epidemiological studies of highly exposed populations.2, 4–7 The biological and health effects of low dose arsenic exposure are poorly understood and in some studies contrast with effects seen at higher doses, suggesting a complex dose-response relationship.8 Moreover, populations may experience differences in their underlying risk of disease and lifestyle factors such as dietary habits, which may play a role in susceptibility to arsenic-induced health effects, by influencing arsenic metabolism.9–11
The placenta has critical functions in nutrient and waste transport between mother and fetus and in hormonally regulating the progression of pregnancy, and may be vulnerable to the impacts of arsenic.12 Despite the non-invasive nature of placental tissue collection, and early observations that placental metal levels correlated with those measured in maternal and fetal blood,13, 14 the potential of the placenta as a pregnancy biomarker15 has not been fully realized. Recently placenta has been used to identify early effects of exposure to numerous environmental contaminant metals and metalloids, including lead, mercury and cadmium5, but studies on arsenic are lacking. Even in highly exposed populations, arsenic concentrations are in the ng/g range,16 which present technical challenges for accurate measurement. As a result there are very few epidemiological studies of arsenic in placenta. Therefore, we used sensitive ICP-MS methods to determine whether placenta arsenic concentrations were related to drinking water arsenic and other biomarkers of arsenic exposure in a US birth cohort with a range of concentrations from private well water.
MATERIAL AND METHODS
The study protocols for the New Hampshire Birth Cohort Study (NHBCS) were approved by the Committee for the Protection of Human Subjects at Dartmouth College. All study participants provided written informed consent.
The New Hampshire Birth Cohort Study
To be eligible for the New Hampshire Birth Cohort Study, women were: a) currently pregnant, b) 18 to 45 years old, c) receiving routine prenatal care at one of the study clinics, d) living at residence served by a private water system (e.g., serving <15 households or 25 individuals), e) residing in the same place since their last menstrual period, using the same water supply, and f) not planning to move prior to delivery.
Data and Sample Collection
At the enrolment visit (approximately 24–28 weeks gestation), study participants were asked to complete a prenatal questionnaire about their pregnancy. Participants were also provided with a kit for collecting a sample of their home tap water using a commercially washed, high-density polyethylene bottle that met the Environmental Protection Agency’s water collection standards. Water samples were stored at −20°C or lower until analysis. Tap water samples were analyzed for total arsenic concentration by inductively coupled plasma mass spectrometry (ICP-MS) using an Agilent 7700x (Santa Clara, CA). The detection limit for arsenic in water ranged from 0.009 – 0.074 μg/L and 96% of water samples exceeded the detection limit.
Maternal Urine
At enrollment participants were asked to provide a urine sample. Samples were analyzed at the University of Arizona using high performance liquid chromatography (HPLC) interfaced with ICP-MS to detect arsenate, arsenite, monomethylarsonic acid (MMA) dimethylarsinic acid (DMA) and arsenobetaine. Detection limits for all species in urine ranged from 0.10–0.15 μg/L. The total arsenic concentration used in statistical analysis is the sum of arsenate, arsenite, MMA and DMA, and excludes arsenobetaine, an unmetabolized form of arsenic that is considered non-toxic. Urinary creatinine was determined using Cayman’s Creatinine Assay Kit (Cayman Chemical Company, Ann Arbor, MI).
Placenta
Placental biopsies were uniformly collected from the fetal side, at the base of the cord insertion avoiding vasculature, and measure approximately 1 cm deep and approximately 1–2 cm in diameter. The maternal decidua was removed to avoid inclusion of calcium (Ca) deposits and connective tissue. Biopsies were placed in metal-free tubes and stored at −80°C until analysis.
Prior to analysis samples were transferred to a −20°C freezer, then to 4°C for a maximum of 2 days, and lastly to room temperature. 1 ml of HNO3/HCl at 9:1 ratio (Optima™) was added to samples with of up to 500 mg mass, and 2 ml of was added to samples greater than 500 mg. Samples were digested via microwave (CEM, Microwave Assisted Reaction System), ramping the temperature to 95°C in 15 minutes, and holding at this temperature for 45 minutes. 0.25–0.35 ml H2O2 was added to each tube and the microwave digestion sequence was repeated. Quality control procedures for this study included the use of a laboratory-made reference placental digest and internal standards. For the placental reference digest, de-identified placental tissue was subsampled to aliquots at the midpoint of the sample weight range, subject to the open-vessel digestion, and then pooled. The pooled sample was mixed, analyzed and an aliquot was included with each batch of placental samples analyzed. Arsenic was analyzed by ICP-MS using He as a collision gas on the Agilent 7700x. NIST 1566b Oyster Tissue was digested and analyzed as a reference material; recovery was 93% ± 7% (n=33). Analysis of batches of placental tissues routinely used internal standards, initial calibration verification, initial calibration blanks continuing, calibration verification and analytical duplicates and spikes. The detection limit for arsenic in placenta was 0.0148 ng/g.
Maternal and Infant Nails
At two weeks post partum, participants received an information packet requesting maternal and infant toenail clippings within eight weeks of birth, a timing which was consistent with other studies.17,18 Maternal toenails underwent an additional washing procedure that included manual removal of visible dirt and five washes in an ultrasonic bath using Triton X-100 (LabChem Inc., PA) and acetone followed by deionized water, and allowed to dry. All toenail samples were subject to low-pressure microwave digestion using the method outlined above for placenta digestions and were analyzed via ICP-MS. The detection limit differs on a sample-by-sample basis because of the difference in sample weights used for digestion and analysis. The detection limit of arsenic in maternal toenails ranged from 0.001 – 0.41 μg/g and for infant toenails ranged from 0.005 – 2.5 μg/g. The detection limit differs on a sample-by-sample basis because of the difference in sample weights used for digestion and analysis. The high maximum value was due to the very low mass of some of the infant toenails.
Statistical Analysis
We used Spearman’s correlation coefficients to evaluate relationships between placenta arsenic and maternal and infant biomarkers, and between placenta arsenic and household water arsenic concentrations. We further used linear regression, and to improve model fit and normalize residuals all arsenic biomarker variables (arsenic concentration in maternal urine, maternal toenails, infant toenails and placenta arsenic concentrations) were log10-transformed. All parameters estimates were exponentiated (10β for covariates and 2β for dependent variables) representing the percent change in the covariates, and a doubling in the dependent variables respectively. We examined a variety of potential confounders such as maternal age upon enrollment, pre-pregnancy maternal body mass index (BMI), maternal smoking status, infant sex, birth weight and season of toenail collection. We assessed covariates individually in univariate associations with placental arsenic concentration as well on other exposure measures of interest. We used a one-way analysis of variance test to detect whether the season of tap water collection had any influence on the arsenic concentration (log10-transformed). All statistical analyses were conducted in JMP version 11 (The SAS Institute, Cary, NC). Participants for whom data were missing, for example in the analysis of infant toenail arsenic (N=153), were excluded from our regression models and Spearman’s correlations.
RESULTS
Descriptive characteristics of the cohort
At the time of this study the total number of NHBCS participants was 1033. The number of participants providing infant toenail clippings was 153. The characteristics of this group of participants did not differ from those presented in Table 1. The study population was predominantly white, with an average age of 31.3 and a mean body mass index (BMI) of 25.3 kg/m2 (10th – 90th percentile range of 20.0–32.1), primarily full term pregnancies (>37 weeks) with an equal male/female distribution and an average birthweight of 3455g (±SD 519) (Table 1). Median arsenic concentrations were 78 (SD = 81) ng/g (N=579) for maternal toenail arsenic, 68 (SD = 253) ng/g for infant toenail arsenic concentrations (N=153), 3.62 (SD = 14.7) μg/L for maternal urinary total arsenic (the sum of inorganic and organic species, excluding arsenobetaine) (N=623), and 0.38 (SD = 11.9) μg/L for household drinking water arsenic concentrations (N=716). We used a oneway analysis of variance test to detect any influence of the season of tap water collection on the arsenic concentration. Data for arsenic concentration of household tap water were not normally distributed and were log10-transformed. This analysis showed that season of collection does not significantly influence household tap water arsenic concentration (F ratio 0.7526, P value 0.5211). Season of collection was unrelated to household drinking water arsenic concentration (ANOVA F ratio 0.7526, P value 0.5211). Arsenic in maternal urine was predominantly present in the organic form DMA (dimethylarsinic acid) (mean 80.8% SD=11.9%), with an average of 9.1% MMA (monomethylarsonic acid) (SD = 6.4%). Inorganic arsenic constituted the remaining 10.1% of urinary arsenic, with on average 5.9% arsenite (SD=4.7%) and 4.1% arsenate (SD=7.5%). In the current subgroup of the NHBCS, 9% of participants’ household drinking water exceeded the EPA’s MCL for arsenic of 10 μg/L.
Table 1.
Selected characteristics of the New Hampshire Birth Cohort Study participants according to arsenic concentrations in placenta.
| Characteristics | N (%) | Placenta As (ng/g) Mean (± SD) |
|---|---|---|
|
| ||
| Maternal: | ||
| Age at enrollment (years): | ||
| < 30 years | 308 (40) | 1.08 (1.52) |
| ≥30 years | 456 (60) | 1.15 (1.64) |
| BMI (kg/m2) | ||
| Normal (BMI <25) | 397 (58) | 1.16 (1.63) |
| Overweight (≥25 to < 30) | 170 (25) | 1.06 (1.25) |
| Obese (≥30) | 114 (17) | 0.92 (0.93) |
| Smoking status | ||
| Smoker | 44 (6) | 0.96 (0.85) |
| Non-smoker | 649 (94) | 1.10 (1.42) |
| Parity | ||
| First live birth | 307 (41) | 1.16 (1.42) |
| 1 or more live birth | 447 (59) | 1.12 (1.72) |
| Infant: | ||
| Sex | ||
| Female | 378 (50) | 1.13 (1.55) |
| Male | 385 (50) | 1.15 (1.64) |
| Birth weight (g) | ||
| Low (<2,500) | 28 (4) | 1.04 (1.22) |
| Normal (≥2,500) | 725 (96) | 1.14 (1.62) |
Placenta arsenic concentrations did not differ significantly across the selected maternal and infant characteristics, which included maternal age, pre-pregnancy BMI, smoking status, parity, infant sex, or low birth weight (<2500g). Placenta arsenic concentrations ranged from below detection limits (0.01 ng/g) to 18.35 ng/g (Table 1), with a median of 0.76 ng/g and an interquartile range of 0.83 ng/g.
Correlations between arsenic in placenta, maternal and infant biomarkers and tap water
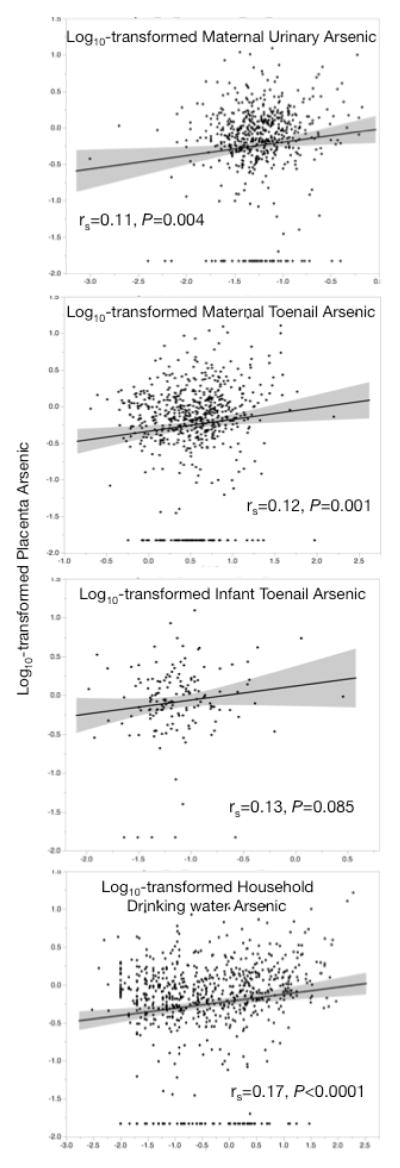
Positive correlations were observed with placental arsenic and maternal postpartum toenail, maternal gestational urine and infant urine (Figure 1). In our regression models, a doubling of arsenic concentration in maternal urine, maternal toenail and infant toenails, was associated with 31.0%, 13.2% and 18.9% increases in placental arsenic concentrations of respectively (Table 2). The regression model between placenta arsenic and maternal urinary arsenic concentrations was adjusted for urinary creatinine concentration, but urinary arsenic levels are expressed as μg/L throughout to remain comparable with prior work 19, 20. Placental arsenic concentrations also were positively associated with measured household water arsenic concentrations (Table 2). For this model, none of the covariates appreciably altered the parameter estimate, and therefore were not adjusted for in the analysis. This model predicted that for a 1 μg/L increase in household water arsenic concentration increased placental arsenic by 2.1%.
Figure 1.
Pairwise correlations between log10-transformed placenta arsenic concentration and log10-transformed maternal and infant biomarkers, and arsenic concentration of household drink water. On each panel, the line of fit is shown with 95% confidence intervals, and results of Spearman’s correlation (rs) and P value are shown.
Table 2.
Change in placenta arsenic concentration in relation to maternal and infant arsenic variables.
| Arsenic Variable | % Increase in placental Arsenic (95% CI) | N | P |
|---|---|---|---|
|
| |||
| Biomarkers | |||
| Maternal urinarya, b (μg/L) | 31.0 (15.4, 48.7) | 431 | <0.0001 |
| Maternal toenail§ (μg/g) | 13.2 (2.0, 25.7) | 579 | 0.0196 |
| Infant toenailc (μg/g) | 18.9 (1.8, 38.8) | 151 | 0.0293 |
| Exposured | |||
| Household drinking water§ (μg/L) | 2.1 (1.3, 3.9) | 716 | <0.0001 |
Excluding arsenobetaine
Adjusted for creatinine concentration (mg/dL)
Adjusted for parity
Based on a doubling of exposure
No factors appreciably altered the estimates (see text).
Discussion
Within the NHBCS, total urinary arsenic and toenail arsenic concentrations have been shown previously to correlate with the arsenic concentration of household drinking water 21, 22, and with the consumption of certain dietary items known to contain elevated concentrations of arsenic 17, 19, 20. The arsenic biomarkers reported in this study, in addition to placenta, mirror previous findings. When participants are grouped in to those with household drinking water either below or above the MCA for arsenic (10 μg/L), there is a significant difference between the arsenic concentrations measured in maternal urine, maternal toenails and placenta (Supplemental Table 1). These differences are less pronounced for infant toenail arsenic concentration, but this may be a result of the smaller number of infant toenails that were available for our analysis.
We detected correlations between the concentration of arsenic in placental tissue and that of both maternal and infant biomarkers. Further, the arsenic burden of the placenta was related to the concentration of arsenic in household drinking water.
To date, only a small number of studies have measured concentrations of arsenic in placenta (summarized in Table 3).16, 23–29 Placental arsenic concentrations reported worldwide between 1976–200030 were on average 6 ng/g (range: 3–12 ng/g) (wet weight). More recently Jin et al24 measured placental arsenic concentrations as part of a study of neural tube defects in a rural area of the Shanxi Province in northern China. Among controls, placenta arsenic concentrations were 11.8 (± SD 7.9) ng/g on average (N=80). In a smaller study of arsenic concentrations in placental tissue from participants living close to a copper smelter in Bulgaria,28 mean arsenic concentrations of 23 (± SD 21) ng/g were detected in placentas of participants living near the smelter (n=30), whereas an average of 7 (± SD 4) ng/g arsenic was measured in placental samples from those outside of the smelter area (N=15). Previous studies have been hindered by instrumental detection limits23, 29, whereas we had the advantage of using ICP-MS for ultra-trace arsenic detection. In light of our low detection limits and our findings of a range of placenta arsenic concentrations that extend below levels previously reported upwards to levels reported in industrially exposed communities, our study presents new information.
Table 3.
Summary of studies reporting arsenic measurements in human placenta.
| First author | Year | Country | N | Other Biomarkers | Placenta As (ng/g) | Exposure/(water μg/L) |
|---|---|---|---|---|---|---|
| Tabacova | 1994 | Bulgaria | 49 | M, C blood | 7, 23D | smelter |
| Concha | 1998 | Argentina | 11 | M, C blood, M urine, breastmilk | 34W | 200 |
| Llanos | 2009 | Chile | 40 | none | 170, 280D | - |
| Kippler | 2010 | Bangladesh | 44 | none | 152D | 1–1,470 |
| Zadorozhnaja | 2010 | Ukraine | 200 | none | 378*D | |
| Amaya | 2013 | Spain | 137 | none | not determined | |
| Leino | 2013 | Finland | 130 | none | 5.68W | |
| Jin | 2013 | China | 130 | none | 6.4–17W |
wet weight measurement
dry weight measurement
maximum
maternal
infant cordblood
Of the prior investigations of placental arsenic, none to our knowledge have directly compared placenta arsenic levels to those measured in other biomarkers. Tabacova et al28 estimated that 34% of the variation in placental arsenic could be explained by the participants’ place of residence relative to a copper smelter area in Bulgaria. Those living closest to the smelter had a fourfold higher placental arsenic concentration than those living in a non-smelter area. Concha et al16 reported placental arsenic levels from a group exposed to drinking water containing ≈200 μg/L arsenic (34 ng/g dry weight) compared to the non-exposed group (7 ng/g dry weight) previously reported in Tabacova et al.28 However, they were unable to compute correlations between placental arsenic and other biomarkers. In addition to correlations with infant and maternal biomarkers, we found drinking water to be a source of arsenic exposure in our study population that is reflected placental arsenic concentrations.
The limitations of our study included the relatively small number of infant toenail samples, which reduced the precision of our analysis. While we only had toenail arsenic concentration for 153 infants, the association with placenta data was statistically significant. Additionally, comparison of the selected characteristics of our study cohort (Table 1) with those for whom infant toenail arsenic data was available showed no significant differences. The collection of infant toenails 8 weeks after birth may also raise concerns that post-natal arsenic exposure is reflected in addition to in utero exposure. However, given the slow rate of infant nail growth (estimated to be between 1–3 mm per month31) it is unlikely that post-natal nail growth would have reached the distal nail edge at the time of sampling.31 Nail growth arises from the proximal end of the nail, under the epidermis; whereas it is the free margin at the distal end of the nail that is sampled.
The collection of a single water sample for arsenic determination from study participants may also be viewed as a limitation in the light of recent studies on temporal variability in well water arsenic concentrations32, but our early study of reproducibility of sequential water samples suggests this is not an issue33.
In conclusion, we found positive associations between placental arsenic concentration and both maternal and infant biomarker concentrations as well as household drinking water arsenic concentration. These data suggest inter-marker reliability between our placental arsenic measurements and arsenic measurements from urine and toenails. Although the magnitude of the correlations were relatively low (<0.2), placenta still may have value as a biomarker of maternal and infant exposures, and be particularly important when considering molecular effects on the placenta as it provides an opportunity to link a biomarker of exposure and biomarker of effect (e.g., changes in gene expression or DNA methylation alterations) in the same functional tissue sample.
Supplementary Material
Acknowledgments
This work was supported in part by the following P20 GM104416 from the National Institute of General Medical Sciences, P01ES022832 and P42 ES007373 from the National Institute of Environmental Health at the NIH and RD83544201 from the Environmental Protection Agency
Footnotes
The authors declare they have no actual or potential competing financial interests.
References
- 1.Ramsey KA, Larcombe AN, Sly PD, Zosky GR. In utero exposure to low dose arsenic via drinking water impairs early life lung mechanics in mice. BMC pharmacology & toxicology. 2013;14:13. doi: 10.1186/2050-6511-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman M, Sohel N, Yunus M, Chowdhury ME, Hore SK, Zaman K, et al. Increased Childhood Mortality and Arsenic in Drinking Water in Matlab, Bangladesh: A Population-Based Cohort Study. PloS one. 2013;8:e55014. doi: 10.1371/journal.pone.0055014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farzan SF, Karagas MR, Chen Y. In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol Appl Pharmacol. 2013;272:384–90. doi: 10.1016/j.taap.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waalkes MP, Liu J, Germolec DR, Trempus CS, Cannon RE, Tokar EJ, et al. Arsenic exposure in utero exacerbates skin cancer response in adulthood with contemporaneous distortion of tumor stem cell dynamics. Cancer Res. 2008;68:8278–85. doi: 10.1158/0008-5472.CAN-08-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esteban-Vasallo MD, Aragones N, Pollan M, Lopez-Abente G, Perez-Gomez B. Mercury, cadmium, and lead levels in human placenta: a systematic review. Environmental Health Perspectives. 2012;120:1369–77. doi: 10.1289/ehp.1204952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waalkes MP, Liu J. Early-life arsenic exposure: methylation capacity and beyond. Environmental Health Perspectives. 2008;116:104. doi: 10.1289/ehp.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liaw J, Marshall G, Yuan Y, Ferreccio C, Steinmaus C, Smith AH. Increased childhood liver cancer mortality and arsenic in drinking water in northern Chile. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:1982–7. doi: 10.1158/1055-9965.EPI-07-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodwell JE, Gosse JA, Nomikos AP, Hamilton JW. Arsenic disruption of steroid receptor gene activation: complex dose-response effects are shared by several steroid receptors. Chemical Research in Toxicology. 2006;19:1619–29. doi: 10.1021/tx060122q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milton AH, Shahidullah SM, Smith W, Hossain KS, Hasan Z, Ahmed KT. Association between chronic arsenic exposure and nutritional status among the women of child bearing age: a case-control study in Bangladesh. International journal of environmental research and public health. 2010;7:2811–21. doi: 10.3390/ijerph7072811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, Factor-Litvak P, et al. Folic acid supplementation lowers blood arsenic. American Journal of Clinical Nutrition. 2007;86:1202–9. doi: 10.1093/ajcn/86.4.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gamble MV, Liu XH, Ahsan H, Pilsner JR, Ilievski V, Slavkovich V, et al. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. American Journal of Clinical Nutrition. 2006;84:1093–101. doi: 10.1093/ajcn/84.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fei DL, Koestler DC, Li Z, Giambelli C, Sanchez-Mejias A, Gosse JA, et al. Association between In Utero arsenic exposure, placental gene expression, and infant birth weight: a US birth cohort study. Environ Health. 2013;12:58. doi: 10.1186/1476-069X-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karp WB, Robertson AF. Correlation of human placental enzymatic activity with trace metal concentration in placentas from three geographical locations. Environ Res. 1977;13:470–7. doi: 10.1016/0013-9351(77)90026-3. [DOI] [PubMed] [Google Scholar]
- 14.Baglan RJ, Brill AB, Schulert A, Wilson D, Larsen K, Dyer N, et al. Utility of placental tissue as an indicator of trace element exposure to adult and fetus. Environ Res. 1974;8:64–70. doi: 10.1016/0013-9351(74)90063-2. [DOI] [PubMed] [Google Scholar]
- 15.Iyengar GV, Rapp A. Human placenta as a ‘dual’ biomarker for monitoring fetal and maternal environment with special reference to potentially toxic trace elements. Part 1. Physiology, function and sampling of placenta for elemental characterization. The Science of the Total Environment. 2001;280:195–206. doi: 10.1016/s0048-9697(01)00825-7. [DOI] [PubMed] [Google Scholar]
- 16.Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicological Sciences. 1998;44:185–90. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- 17.Cottingham KL, Karimi R, JFG, Zens MS, Sayarath V, Folt CL, et al. Diet and toenail arsenic concentrations in a New Hampshire population with arsenic-containing water. The Nutrition Journal. 2013;12:149–59. doi: 10.1186/1475-2891-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis MA, Li Z, Gilbert-Diamond D, Mackenzie TA, Cottingham KL, Jackson BP, et al. Infant toenails as a biomarker of in utero arsenic exposure. Journal of exposure science & environmental epidemiology. 2014 doi: 10.1038/jes.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. Rice consumption and urinary arsenic concentrations in US children. Environmental Health Perpectives. 2012;120:1418–24. doi: 10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, et al. Rice consumption raises a health concern: evidence from U.S. pregnant women. Proceedings of the National Academy of Sciences. 2011 doi: 10.1073/pnas.1109127108. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karagas MR, Le CX, Morris S, Blum J, Lu X, Spate V, et al. Markers of low level arsenic exposure for evaluating human cancer risks in a US population. International journal of occupational medicine and environmental health. 2001;14:171–5. [PubMed] [Google Scholar]
- 22.Karagas MR, Morris JS, Weiss JE, Spate V, Baskett C, Greenberg ER. Toenail samples as an indicator of drinking water arsenic exposure. Cancer Epidemiol Biomarkers Prev. 1996;5:849–52. [PubMed] [Google Scholar]
- 23.Amaya E, Gil F, Freire C, Olmedo P, Fernandez-Rodriguez M, Fernandez MF, et al. Placental concentrations of heavy metals in a mother-child cohort. Environ Res. 2013;120:63–70. doi: 10.1016/j.envres.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Jin L, Zhang L, Li Z, Liu JM, Ye R, Ren A. Placental concentrations of mercury, lead, cadmium, and arsenic and the risk of neural tube defects in a Chinese population. Reproductive Toxicology. 2013;35:25–31. doi: 10.1016/j.reprotox.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Kippler M, Hoque AM, Raqib R, Ohrvik H, Ekstrom EC, Vahter M. Accumulation of cadmium in human placenta interacts with the transport of micronutrients to the fetus. Toxicol Lett. 2010;192:162–8. doi: 10.1016/j.toxlet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Leino O, Kiviranta H, Karjalainen AK, Kronberg-Kippila C, Sinkko H, Larsen EH, et al. Pollutant concentrations in placenta. Food Chem Toxicol. 2013;54:59–69. doi: 10.1016/j.fct.2011.10.058. [DOI] [PubMed] [Google Scholar]
- 27.Llanos MN, Ronco AM. Fetal growth restriction is related to placental levels of cadmium lead and arsenic but not with antioxidant activities. Reproductive Toxicology. 2009;27:88–92. doi: 10.1016/j.reprotox.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 28.Tabacova S, Baird DD, Balabaeva L, Lolova D, Petrov I. Placental arsenic and cadmium in relation to lipid peroxides and glutathione levels in maternal-infant pairs from a copper smelter area. Placenta. 1994;15:873–81. doi: 10.1016/s0143-4004(05)80188-2. [DOI] [PubMed] [Google Scholar]
- 29.Zadorozhnaja TD, Little RE, RKM, Mendel NA, Taylor RJ, Presley BJ, et al. Concentrations of arsenic, cadmium, copper, lead, mercuy and zinc in human placentas from two cities in Ukraine. Journal of toxicology and Environmental Health Part A. 2000;61:255–63. doi: 10.1080/00984100050136571. [DOI] [PubMed] [Google Scholar]
- 30.Iyengar GV, Rapp A. Human placenta as a ‘dual’ biomarker for monitoring fetal and maternal environment with special reference to potentially toxic trace elements. Part 3. Toxic trace elements in placenta and placenta as a biomarker for these elements. Science of the Total Environment. 2001;280:221–38. doi: 10.1016/s0048-9697(01)00827-0. [DOI] [PubMed] [Google Scholar]
- 31.Seaborg B, Bodurtha J. Nail size in normal infants. Establishing standards for healthy term infants. Clinical pediatrics. 1989;28:142–5. doi: 10.1177/000992288902800309. [DOI] [PubMed] [Google Scholar]
- 32.Ayotte JD, Belaval M, Olson SA, Burow KR, Flanagan SM, Hinkle SR, et al. Factors affecting temporal variability of arsenic in groundwater used for drinking water supply in the United States. Sci Total Environ. 2014 doi: 10.1016/j.scitotenv.2014.02.057. [DOI] [PubMed] [Google Scholar]
- 33.Karagas MR, Tosteson TD, Blum J, Klaue B, Weiss JE, Stannard V, et al. Measurement of low levels of arsenic exposure: A comparison of water and toenail concentrations. American Journal of Epidemiology. 2000;152:84–90. doi: 10.1093/aje/152.1.84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.