Abstract
Purpose
To develop a novel framework for free-breathing MRI called XD-GRASP, which sorts dynamic data into extra motion-state dimensions using the self-navigation properties of radial imaging and reconstructs the multidimensional dataset using compressed sensing.
Methods
Radial k-space data are continuously acquired using the golden-angle sampling scheme and sorted into multiple motion-states based on respiratory and/or cardiac motion signals derived directly from the data. The resulting under-sampled multidimensional dataset is reconstructed using a compressed sensing approach that exploits sparsity along the new dynamic dimensions. The performance of XD-GRASP is demonstrated for free-breathing three-dimensional (3D) abdominal imaging, two-dimensional (2D) cardiac cine imaging and 3D dynamic contrast-enhanced (DCE) MRI of the liver, comparing against reconstructions without motion sorting in both healthy volunteers and patients.
Results
XD-GRASP separates respiratory motion from cardiac motion in cardiac imaging, and respiratory motion from contrast enhancement in liver DCE-MRI, which improves image quality and reduces motion-blurring artifacts.
Conclusion
XD-GRASP represents a new use of sparsity for motion compensation and a novel way to handle motions in the context of a continuous acquisition paradigm. Instead of removing or correcting motion, extra motion-state dimensions are reconstructed, which improves image quality and also offers new physiological information of potential clinical value.
Keywords: compressed sensing, radial sampling, golden-angle, free-breathing, motion compensation
Introduction
Respiratory motion remains a major challenge in MRI, particularly for abdominal and cardiovascular imaging. Due to the limited encoding speed in conventional MRI, k-space lines may be acquired in different respiratory motion states during free-breathing, resulting in ghosting artifacts and image blurring (1,2). The simplest approach to avoid respiratory motion effects is to suspend respiration during data acquisition (3), an approach that is currently widely used in routine clinical MRI exams. However, breathhold capabilities are subject-dependent and can be significantly limited in some patients. In addition, typical breathhold durations (10–15 s) also limit achievable spatial resolution and volumetric coverage. An alternative approach is to use either navigator signals (4) or respiratory bellows (5) to monitor respiratory motion and acquire data only at a specific respiratory state (e.g., end-expiration). However, such gated data acquisition significantly reduces imaging efficiency and further prolongs the total examination times. Real-time MRI can be also used for free-breathing cardiac cine imaging (6–9), but the acquisitions usually comprise only a single slice with limited spatial and temporal resolution. Non-Cartesian k-space sampling schemes, such as radial or spiral, are substantially less sensitive to respiratory motion and enable free-breathing imaging at the expense of increased scan times (10–12). For example, radial imaging eliminates k-space gaps due to motion-related phase shifts, by repeated sampling of the k-space center. However, substantial motion is still a challenge for non-Cartesian imaging and can result in blurring and aliasing artifacts, e.g., streaks for radial trajectories (13,14). Non-Cartesian acquisitions also offer the potential benefit of retrospective self-gating, owing to the continuous passage of the radial lines through the center of k-space, and thus can eliminate the need to use navigator signals or external devices (15,16). For example, Liu et al have proposed an image reconstruction approach for free-breathing cardiac cine MRI (16), in which the cardiac and respiratory motion signals retrospectively obtained from the data are used for self-gating and view-sharing reconstruction with reduced motional blurring. However, these approaches are still time-inefficient, because typically only data within a predefined motion state (e.g., close to expiration) are used for the image reconstruction.
Compressed sensing with temporal sparsifying transforms has enabled high accelerations in dynamic MRI studies (17–19), and several approaches have been proposed to integrate an image registration framework into the reconstruction problem, for correcting respiratory motion in free-breathing imaging. For example, rigid-body motion registration techniques have been applied to compressed sensing cardiac perfusion imaging (20) and more complex deformable registration techniques that account for nonrigid body motion were used in compressed sensing reconstruction of cardiac cine (21), cardiac perfusion (22), and abdominal DCE-MRI examinations (23). A more advanced method, which learns the motion fields from the data itself to guide image reconstruction, was recently introduced, which, in addition to performing motion compensation, can also provide access to specific motion information (24).
With the goal of combining the motion-robustness of radial imaging and the acceleration capabilities of compressed sensing, the iterative Golden-angle RAdial Sparse Parallel (iGRASP) technique has recently been proposed for highly accelerated motion-robust DCE-MRI (25). Successful applications of iGRASP for free-breathing imaging have been demonstrated in various organs affected by respiratory motion, such as liver (26), prostate (27), and small bowel (28). However, our clinical evaluation suggests that iGRASP still suffers from some degree of respiratory motion blurring, especially in sick or elderly patients, who tend to be less cooperative in following a shallow breathing pattern during data acquisition. The resulting motion-blurring effects reduce vessel-tissue contrast and may prevent the detection of small lesions.
In this work, we propose a novel image reconstruction framework, called eXtra-Dimensional GRASP (XD-GRASP), which combines iGRASP with the self-navigation property of radial imaging and uses motion detection schemes adapted from previous work (16,29). Instead of removing or correcting the motion in question, XD-GRASP reconstructs extra motion dimensions, where continuously acquired k-space data are sorted into multiple sets of undersampled datasets with distinct motion states, using motion signals extracted directly from the data (30,31). This approach may also be generalized to account for multiple sources of motion or dynamic signal change simultaneously, such as cardiac motion and contrast enhancement in addition to respiratory motion, by sorting the data into multiple additional motion-state dimensions. A compressed sensing algorithm is used to reconstruct the motion-sorted datasets by exploiting sparsity along the corresponding motion-state dimensions. From a clinical perspective, the extra dimensions may also provide new physiological information, because images of different kinds of motion states may be disentangled during reconstruction. The performance of XD-GRASP is demonstrated by comparing against reconstructions without motion sorting in representative free-breathing imaging applications, including: (i) 3D abdominal imaging with respiratory motion only; (ii) 2D cardiac cine imaging with cardiac and respiratory motion, and (iii) 3D liver DCE-MRI with respiratory motion and time-dependent contrast-enhancement.
Methods
A Simple Example of XD-GRASP
Successful implementation of XD-GRASP has two principal requirements: (a) reliable physiological (e.g., respiratory and/or cardiac) motion signals and (b) preservation of approximately uniform k-space coverage in each motion state after data sorting. Golden-angle radial sampling, which uses ∼111.25° angular increment between consecutive spokes (32), is used for data acquisition, because the repeated sampling of the k-space center enables extraction of motion-state signals (16,29), and it allows the possibility of arbitrary data sorting with approximately uniform k-space coverage in each motion state while maintaining sufficient incoherence in the sampling pattern along the new motion-state dimension for robust compressed sensing reconstruction.
Figure 1 illustrates the basic concept of XD-GRASP, in which the continuously acquired radial k-space data are sorted into a specific number of respiratory states spanning from expiration (top) to inspiration (bottom) using a respiratory motion signal derived from the acquired data (Fig. 1a). The sorting procedure is performed so that the number of spokes grouped in each motion state is the same (as shown in Supporting Figure S1a, which is available online). Approximately uniform coverage of k-space with distinct sampling patterns in each motion state is achieved by using the golden-angle acquisition scheme (Fig. 1b). Data sorting removes blurring and clearly resolves respiratory motion (indicated by the purple dashed line), at the expense of generating undersampling artifacts (Fig. 1c). A compressed sensing reconstruction that exploits sparsity along the new respiratory-state dimension can be used to remove undersampling artifacts (Fig. 1d).
Fig. 1.

Schematic illustration of the XD-GRASP method. a: Continuously acquired radial k-space data are sorted into respiratory states from expiration (top) to inspiration (bottom), using a respiratory motion signal extracted directly from the data. Different colors indicate different motion states. The number of spokes grouped in each motion state is the same. b: Approximately uniform coverage of k-space, with distinct sampling patterns in each respiratory motion state, is achieved using the golden-angle acquisition scheme. c: Data sorting removes blurring and clearly resolves respiratory motion, at the expense of introducing undersampling artifacts. The purple dashed line shows the distinct respiratory motion states after data sorting. d: Sparsity is exploited along the extra dimension to remove aliasing artifacts due to undersampling.
Motion Estimation and Data Sorting
Estimation of motion signals and data sorting were performed in a slightly different way for each target application (e.g., cardiac versus abdominal imaging) and k-space trajectory (2D golden-angle radial versus 3D stack-of-stars golden-angle radial).
Motion Estimation and Data Sorting in 2D Cardiac Cine MRI
For cardiac cine imaging, the center of k-space (DC component) in each spoke (Fig. 2a), which reflects the change in average signal level due to changes of the volume of lung and heart in the excited slab, was used to extract information about physiological motion over time (33). Information from multiple coils was used to obtain separate signals representing respiratory or cardiac motion, as shown in Supporting Figure S2b. Conceptually, the motion signal from the coil nearest to the heart provides predominantly cardiac motion information, and the motion signal from the coil nearest to the diaphragm provides predominantly respiratory motion information. Because these motions are known to have different frequency contents, the motion signal in the coil-element with the highest peak in the frequency range of 0.1–0.5 Hz was automatically selected to represent respiratory motion; and the motion signal in the coil-element with the highest peak in the frequency range of 0.5–2.5 Hz was automatically selected to represent cardiac motion. A filtering procedure can be performed on the detected motion signals for denoising (16). Figure 2b shows an example of detected motion signals and Figure 2c shows the corresponding frequency information. End-systolic motion-states were identified as the valleys in the cardiac motion signal and thus any abnormal cardiac cycles, in case of arrhythmias, can be identified according to the difference between cycle lengths for rejection or a separate reconstruction. Given the selected motion signals, the continuously acquired 2D cardiac dataset can be sorted into an expanded dataset containing two dynamic dimensions, representing predominantly cardiac and respiratory motions, respectively. Specifically, the continuously acquired golden-angle radial dataset were first sorted into a dynamic cardiac series by grouping several consecutive spokes (e.g., 15 spokes) as one cardiac phase (Fig. 2d). All the cardiac cycles, identified using the cardiac motion signal, were then sorted into an expanded dataset to generate an extra respiratory state dimension tR (Fig. 2e), so that sparsity along both cardiac and respiratory dimensions can be exploited in the compressed sensing reconstruction.
Fig. 2.

XD-GRASP motion estimation and data sorting for cardiac cine imaging. a: 2D golden-angle radial trajectory. Motion signals are estimated from the central k-space position of each radial line (gray dot). b,c: Estimation of cardiac and respiratory motion signals using information from multiple coils. The signals with the highest peaks in the frequency range of 0.1–0.5 Hz and 0.5–2.5 Hz are automatically selected for respiratory and cardiac motion signals, respectively. d: Conventional iGRASP sorting of cardiac phases, given by grouping consecutive spokes in each frame. e: XD-GRASP sorting, in which all the cardiac cycles are concatenated into an expanded dataset with one cardiac dimension (tC) and an extra respiratory dimension (tR), so that sparsity along tC and tR can be exploited in the multidimensional compressed sensing reconstruction.
Motion Estimation and Data Sorting in 3D Abdominal MRI
The 3D stack-of-stars sampling scheme (Fig. 3a), in which golden angle radial sampling is used in the kx−ky plane and Cartesian sampling is used along the kz dimension, acquires all spokes along kz for a given rotation angle and then repeats the procedure for the next rotation angle, i.e., an inner loop is defined along kz and an outer loop along the rotation angle. A straightforward approach for motion detection would be to use the DC component of central spokes along the kz dimension (34) and perform the same procedure as was just described for 2D imaging. However, prior study has shown that motion detection is more robust using the projections along the slice dimension for 3D stack-of-stars imaging (35).
Fig. 3.
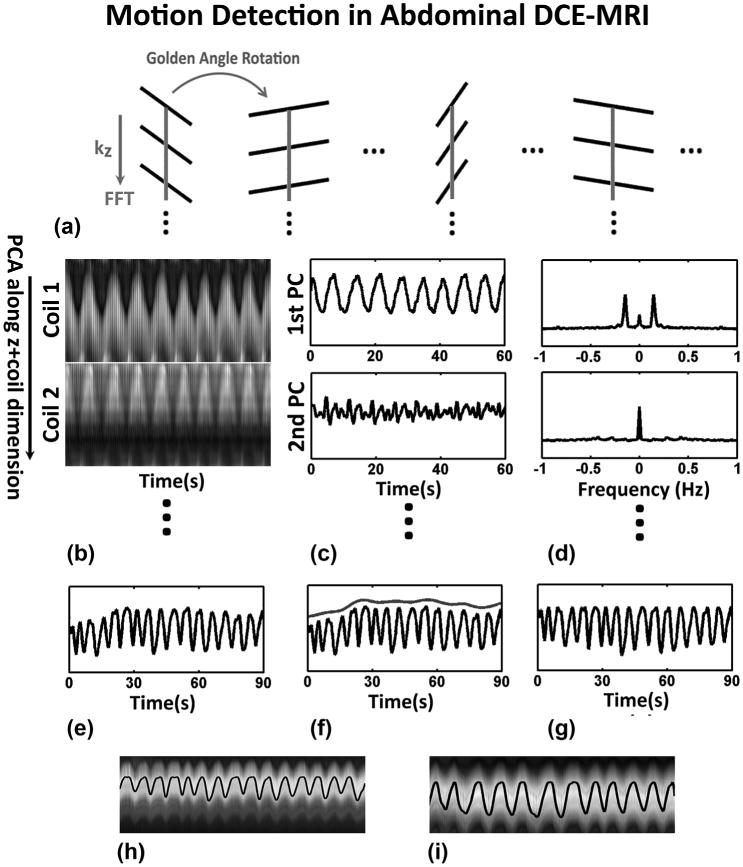
XD-GRASP motion estimation and data sorting for DCE-MRI imaging. a: 3D stack-of-stars radial trajectory with golden-angle rotation, where all spokes along kz for a given rotation angle are acquired before rotating the sampling direction to the next angle. b: A 1D Fourier transform along the series of k-space central points of each slice is performed to obtain a projection profile of the entire volume for each angle and all the projection profiles from all coils are concatenated into a large 2D matrix, followed by principal component analysis (PCA) along the z+coil dimension. c,d: The principal component with the highest peak in the frequency range of 0.1–0.5 Hz is selected to represent respiratory motion. e–g: Contrast-enhancement effect is approximately removed by estimating and subtracting the envelope of the composite signal. h,i: Processed respiratory motion signals are shown superimposed on the z-projection profiles for normal breathing (left) and heavy breathing (right), demonstrating reliable motion estimation.
In this work, an adapted version of the projection approach was used for respiratory motion detection in 3D abdominal imaging. Specifically, a projection profile of the entire volume was computed for each acquisition angle by taking a 1D partition-direction Fourier transform of the series of kx = ky = 0 central points (gray lines in Figure 3a). Respiratory motion detection was performed by first concatenating the projection profiles from all coils into a large 2D matrix, followed by principal component analysis (PCA) along the concatenated z+coil dimension (Fig. 3b). As proposed by Pang et al (29), PCA can be interpreted as a procedure to determine the most common signal variation mode among all coils, and the principal component with the highest peak in the frequency range of 0.1–0.5 Hz was selected to represent respiratory motion (Fig. 3c,d).
For DCE-MRI, contrast-enhancement has to be separated from respiratory motion. In this work, the envelope of the detected motion signal was estimated using a spline data fitting procedure and then subtracted to generate the respiratory motion signal (Fig. 3e-g). Figure 3h&i show two representative examples of respiratory motion in both normal breathing (left) and deep breathing (right) detected using the proposed approach, where motion signals were superimposed on the slice projection profiles. Given the respiratory motion signal, the continuously acquired golden-angle radial dataset was first divided into successive contrast-enhancement phases (dynamic dimension tcontrast) and each phase was then further sorted into multiple respiratory states (dynamic dimension tR), in which the number of spokes is the same in each motion state, as shown in Supporting Figure S1b.
Image Reconstruction
XD-GRASP reconstruction extends the iGRASP pipeline (25) by enforcing a different sparsity constraint along each dynamic dimension. Specifically for 2D free-breathing cardiac imaging, reconstruction was performed by solving the following optimization problem:
| [1] |
Here F is the nonuniform fast Fourier transform (NUFFT) (36) operator defined for the radial sampling pattern, represents the n-elements coil sensitivity maps with dimensionality of x-y-coil, where x and y represent two spatial dimensions. d is the 2D dynamic image-series with one cardiac motion dimension and one respiratory-state dimension (x-y-tC-tR), and are the corresponding multicoil radial k-space data sorted according to the new dimensions (x-y-tC-tR-coil). S1 is the sparsifying transform applied in the cardiac motion dimension with regularization parameter λ1 and S2 is the sparsifying transform applied along the extra respiratory-state dimension with regularization parameter λ2. R is a reordering operator along the tR dimension that sorts all the respiratory phases at a given cardiac position from expiratory state to inspiratory state. This sorting procedure will ensure a smooth transition between adjacent motion states, which improves the performance of total variation minimization along the dynamic dimensions, as proposed by Adluru and Dibella (37).
For 3D liver imaging, reconstruction was performed by solving the following optimization problem:
| [2] |
Here F is the same as before, represents the n-elements coil sensitivity maps with dimensionality of x-y-z-coil, where z is the partition dimension. d is the 3D dynamic image-series with one contrast-enhancement dimension and one respiratory-state dimension (x-y-z- tContrast-tR), and are the corresponding multicoil radial k-space data sorted according to the new dimensions (x-y-z-tContrast-tR-coil). S1 is the sparsifying transform applied in the contrast-enhancement dimension with regularization parameter λ1 and S2 is the sparsifying transform applied along the extra respiratory-state dimension with regularization parameter λ2. For abdominal imaging without contrast injection, λ1 is just set as zero. Because each contrast enhancement phase is already sorted from end-expiratory state to end-inspiratory state, which promotes a smooth transition between respiratory motion states, the reordering operator R applied in Eq. [1] is not needed for Eq. [2].
In this work, temporal finite differences (a.k.a. total variation minimization) along the dynamic dimensions was selected for both S1 and S2 based on our prior experience (9,25), but with different weights λ1 and λ2 tailored to reflect the different degrees of sparsity along each dynamic dimension. For example, stronger regularization was applied along the sparser dynamic dimension and vice versa. (More details on the selection of regularization parameters are provided in subsequent sections.)
Imaging Studies
The performance of XD-GRASP was tested in free-breathing 3D abdominal imaging, 2D cardiac cine imaging and 3D liver DCE-MRI, on both healthy volunteers and patients. Human imaging was approved by the IRB and was HIPAA compliant. Written informed consent was obtained from all subjects before imaging studies.
Free-Breathing 3D Abdominal Imaging: Respiratory Motion Only
3D abdominal imaging (without contrast injection) was performed on one healthy volunteer (female, age = 32) on a whole-body 3.0 Tesla (T) scanner (Tim Trio, Siemens AG, Erlangen, Germany) equipped with the standard 12-element body matrix coil array. A 3D stack-of-stars golden-angle radial FLASH pulse sequence with frequency-selective fat suppression was used and three scans were performed in transverse, coronal, and sagittal orientations to test the robustness to motion in different imaging planes. Relevant imaging parameters included: repetition time/echo time (TR/TE) = 3.52/1.41 ms, field of view (FOV) = 300 × 300 × 140 mm3, number of readout points in each spoke = 192, number of partitions = 28 and spatial resolution =1.5 × 1.5 × 5 mm3. A total of 510 spokes were acquired for each partition, with a total scan time of ∼57 s.
Six respiratory motion-states (84 spokes in each state) were generated by sorting the continuously acquired data as described in Figure 1 and also Supporting Figure S1a. XD-GRASP was performed with one sparsifying transform along the respiratory-state dimension (S2 in Eq. [2]). The results were compared with NUFFT reconstruction of the whole dataset without motion sorting.
Free-Breathing 2D Cardiac Cine Imaging: Cardiac and Respiratory Motions
The 2D cardiac cine data were acquired in one healthy volunteer (female, age = 32), one patient with normal sinus rhythm (male, age = 46), one patient with premature ventricular contractions (PVCs) (female, age = 33) and another patient with second-degree atrioventricular block (female, age = 49). Imaging was performed during normal free-breathing on a whole-body 1.5T scanner (Avanto, Siemens AG, Erlangen, Germany) without any external triggering or gating, using a 2D radial bSSFP pulse sequence with golden-angle acquisition scheme. Three short-axis slices (SAX) in apical, middle, and basal ventricular positions, and one slice in a four-chamber plane (4CH) were acquired in the volunteer scans. Relevant imaging parameters included: TR/TE = 2.8/1.4 ms, FOV = 320 × 320 mm2, number of readout points in each spoke = 160, spatial resolution = 2 × 2 mm2, slice thickness = 8 mm, and the total acquisition time for each slice was ∼20 s. For comparison purposes, cardiac cine images with similar imaging orientations and parameters were also acquired using the routine clinical approach with breathhold, Cartesian k-space sampling and retrospective ECG gating. In the patient scans, one middle ventricular SAX slice was acquired in each subject with the following imaging parameters: TR/TE = 2.8/1.4 ms, FOV = 256 × 256 mm2, number of readout points in each spoke = 128, spatial resolution = 2 × 2 mm2, slice thickness = 8 mm, and the total acquisition time for each slice was ∼15–20 s. For comparison purposes, cardiac cine images with similar imaging orientations but with relatively higher spatial resolution (∼1.6 × 1.6 mm2) were also acquired using the routine clinical approach with breathhold, Cartesian k-space sampling and retrospective ECG gating.
Cardiac cycles with arrhythmias were first detected and separated using the cardiac motion signals in the patient datasets. Every 15 consecutive spokes were grouped to generate one dynamic phase, achieving a temporal resolution of ∼45 ms, as showing in Figure 2d. XD-GRASP reconstruction was performed on an expanded time-series of undersampled datasets (Fig. 2e) with ∼18–26 cardiac phases and ∼10–16 respiratory phases, depending on the heart rate of the subjects. The cardiac cycles with arrhythmias in the patient with PVCs were rejected because there were only two cardiac cycles with arrhythmias in the entire acquisition. Thus the gain of performing XD-GRASP in arrhythmia cycles is small because of the limited number of respiratory phases and limited sparsity along the respiratory dimension. In the patient with second-degree atrioventricular block, there were more arrhythmia cardiac cycles and thus a separate XD-GRASP reconstruction was performed on the cardiac cycles with arrhythmias. For comparison, iGRASP reconstruction (without respiratory sorting) was also performed (on the same datasets) on a time-series of undersampled cardiac phases, where each cardiac phase was formed by grouping 15 consecutive spokes in the volunteer datasets. A 1.5-fold zero-filling was performed in all the results for visualization purposes, and a 5th-order temporal medial filter was performed along the cardiac dimension after image reconstructions to further reduce the residual streaking artifacts (8).
Free-Breathing 3D Liver DCE-MRI: Contrast Enhancement and Respiratory Motion
3D liver DCE-MRI was performed on four volunteers (males, age = 32.5 ± 1.3) as well as one patient (male, age = 69) with a suspected liver lesion on a whole-body 3.0T scanner (Verio, Siemens AG, Erlangen, Germany) equipped with the standard 12-element body matrix coil. Three volunteers and the patient were asked to breathe normally and one volunteer was asked to breath deeply during the scans. The 3D stack-of-stars pulse sequence was used to acquire data in transversal orientation and intravenous injection of 10 mL of gadopentetate dimeglumine (Gd-DTPA) (Magnevist; Bayer Healthcare, Leverkusen) was initialized simultaneously with the onset of data acquisition, followed by a 20-mL saline flush, both injected at a rate of 2 mL/s. Relevant imaging parameters for the volunteer scan included: TR/TE ≈ 3.52/1.41 ms, FOV = 360 × 360 × 240 mm3, number of readout points in each spoke = 256, spatial resolution = 1.4 × 1.4 × 3 mm3, number of partitions = 80, with 60% slice resolution reduction and 6/8 partial Fourier applied along the slice dimension. A total of 600 spokes were continuously acquired in each partition, for a total scan time of ∼95 s. Imaging parameters for the patient scans were similar, except that the number of readout points in each spoke was 320, resulting in a spatial resolution of 1.1 × 1.1 × 3 mm3.
For comparison purposes, iGRASP reconstruction (without respiratory sorting) was first performed on a time-series of undersampled contrast-enhancement phases, where each phase was formed by grouping 84 consecutive spokes (temporal resolution of ∼13 s). XD-GRASP reconstruction was then performed on a multidimensional undersampled dataset, in which the 84 spokes in each contrast-enhancement phase were further sorted into 4 respiratory states spanning from end-expiration to end-inspiration. The sorting procedure was performed such that the number of spokes in each motion state was the same, as shown in Supporting Figure S1b.
Image Reconstruction Implementation
A tailored version of nonlinear conjugate gradient optimization, originally proposed in (38), was used to solve the optimization problem in both Eqs. [1] and [2]. Coil sensitivity maps were computed from a fully sampled reference given by NUFFT reconstruction of the whole dataset, using the adaptive array combination method (39). Regularization parameters λ1 and λ2 were empirically selected by two experienced cardiac and body radiologists. Specifically, the best value of λ1 was selected first (λ2 was set as zero) by testing different values and comparing image quality as well as temporal fidelity, as previously performed in our prior works (9,25). In the next step, different values of λ2 were then compared in combination with the λ1 value selected in the first step and the radiologist selected the best value of λ2. According to our prior experience with compressed sensing MRI (9,19,25,40,41), regularization parameters selected in this manner can be used reliably in the reconstruction of similar datasets. Image reconstruction was performed in MATLAB (Mathworks, Natick, MA), using a workstation with a 16-core Intel Xeon CPU and 96 GB RAM. XD-GRASP reconstruction time was ∼5 min/slice for 3D abdominal imaging, ∼40–60 min for 2D cardiac cine imaging, and ∼15 min/slice for 3D liver DCE-MRI.
Results
Free-Breathing 3D Abdominal Imaging
Figure 4 compares the conventional NUFFT reconstruction of the full dataset without respiratory sorting (corresponding to the motion average) to XD-GRASP reconstruction with six respiratory motion states. XD-GRASP improves the depiction of vessels and removes the blurring effects at the edges of the liver (white arrows).
Fig. 4.

Conventional NUFFT reconstruction without respiratory sorting (motion average) and XD-GRASP reconstruction with six respiratory states for datasets acquired in transverse, coronal and sagittal orientations. XD-GRASP significantly reduces motion-blurring, as indicated by the white arrows.
Comparison of Different Regularization Parameters for the Extra Respiratory Dimension
Figure 5 shows XD-GRASP reconstruction results for four representative respiratory sparsity regularization parameters (λ2) in cardiac imaging and liver DCE-MRI. Usage of a sparsity constraint along the extra respiratory-state dimension improved the removal of undersampling artifacts, when compared with the nonregularized reconstruction (λ2=0). Very low values of λ2 resulted in residual aliasing artifacts, while very high values of λ2 introduced blurring. A λ2 on the order of 0.01 in cardiac cine imaging and 0.015 in liver DCE-MRI provided a good tradeoff between residual aliasing artifacts and temporal fidelity. The results comparing different values of λ2 were not shown because similar comparisons have been demonstrated previously in (9,25) and many other compressed sensing publications.
Fig. 5.

XD-GRASP reconstruction results for four representative respiratory sparsity regularization parameters (λ2) in cardiac imaging and liver DCE-MRI. Usage of a sparsity constraint along the extra respiratory-state dimension improved the removal of under-sampling artifacts, when compared with the nonregularized case (λ2=0). Very low values of λ2 resulted in residual aliasing artifacts, while very high values introduced blurring. A λ2 of 0.01 in cardiac cine imaging and 0.015 in liver DCE-MRI provided a good tradeoff between residual aliasing artifacts and temporal fidelity.
Free-Breathing 2D Cardiac Cine Imaging
Figure 6 compares XD-GRASP with the standard breath-hold approach using retrospective ECG-gating at end-diastolic and end-systolic cardiac phases in the volunteer scans. Free-breathing XD-GRASP achieved similar image quality to the conventional breathhold approach but also enabled the evaluation of the effects of respiratory motion at each cardiac phase, which can be potentially valuable for examination of conditions such as constrictive pericardial heart disease (42). Figure 7a and Supporting Video S1 clearly show respiratory-related motion of the interventricular septum, especially near end-diastolic cardiac phases, which indicates left–right ventricular interaction during respiration.
Fig. 6.

Comparison of XD-GRASP against the standard breathhold approach used in routine clinical studies (i.e., with retrospective ECG-gating) at end-diastolic and end-systolic cardiac phases in the volunteer scans. XD-GRASP provided similar performance to the routine clinical breathhold method.
Fig. 7.

a: XD-GRASP provides access to respiratory motion information for each cardiac phase, where respiratory-related motion of the interventricular septum, especially at diastolic cardiac phases (top row) can be seen, indicating left–right ventricular interaction during respiration. Gray arrows indicate different respiratory motion states. b: Comparison of XD-GRASP reconstruction exploiting sparsity along two dynamic dimensions (right-hand column) with iGRASP reconstruction exploiting sparsity along a single dynamic dimension only (left-hand column), using the same data set acquired during free-breathing.
Figure 7b and Supporting Video S2 show the comparison of XD-GRASP reconstruction exploiting sparsity along two dynamic dimensions (right-hand column) with iGRASP reconstruction exploiting sparsity along a single dynamic dimension only (left-hand column), using the same data set acquired during free-breathing. XD-GRASP reconstruction achieved superior image quality, particularly in the removal of aliasing artifacts due to the separation of cardiac and respiratory motion into different dimensions, which enables exploitation of extra sparsity along the respiratory dimension.
Figure 8a compares XD-GRASP and the standard breath-hold approach with retrospective ECG-gating for the patients. Although the conventional breathhold approach produced good image quality in the patient with normal sinus rhythm, it produced poor image quality for patients with arrhythmia, due to the failure to properly synchronize cardiac cycles with different length in the reconstruction. XD-GRASP, on the other hand, achieved consistent image quality by enabling separation of cardiac cycles with arrhythmia. In addition, the data from cardiac cycles with arrhythmia can be used for a separate XD-GRASP reconstruction to provide additional physiological information, as shown in the case of 2nd degree AV block (Fig. 8b). Supporting Videos S3 and S4 show the difference between normal and arrhythmic cardiac cycles, where arrhythmic cycles are prolonged due to the missed heart contraction in 2nd degree AV block. Figure 8c shows the corresponding cardiac motion signals for three patients, where the cardiac cycle length is varying in the patients with arrhythmia, indicated by gray arrows. The availability of these signals offers the possibility to identify and separately reconstruct images of the cardiac cycles affected by arrhythmia.
Fig. 8.

a: Comparison of XD-GRASP and the standard breathhold approach with retrospective ECG-gating for the patients. Conventional breathhold scans achieved good image quality in a patient with normal sinus rhythm, but it produced poor image quality for patients with arrhythmias. XD-GRASP achieved consistent image quality by separating the cardiac cycles with arrhythmia. b: In the patient with 2nd degree AV block, the arrhythmic cardiac cycles were further sorted for a separate XD-GRASP reconstruction to provide additional physiological information. c: Corresponding cardiac motion signals for three patients with varying length of the cardiac cycle indicated by gray arrows.
Free-Breathing 3D Liver DCE-MRI
Figure 9 shows the aortic and portal venous contrast-enhancement phases in four representative partitions, for both iGRASP and XD-GRASP reconstruction of the first two volunteer datasets. The reduction of respiratory motion blurring in XD-GRASP improved the delineation of vessels and vessel-tissue contrast compared with iGRASP.
Fig. 9.
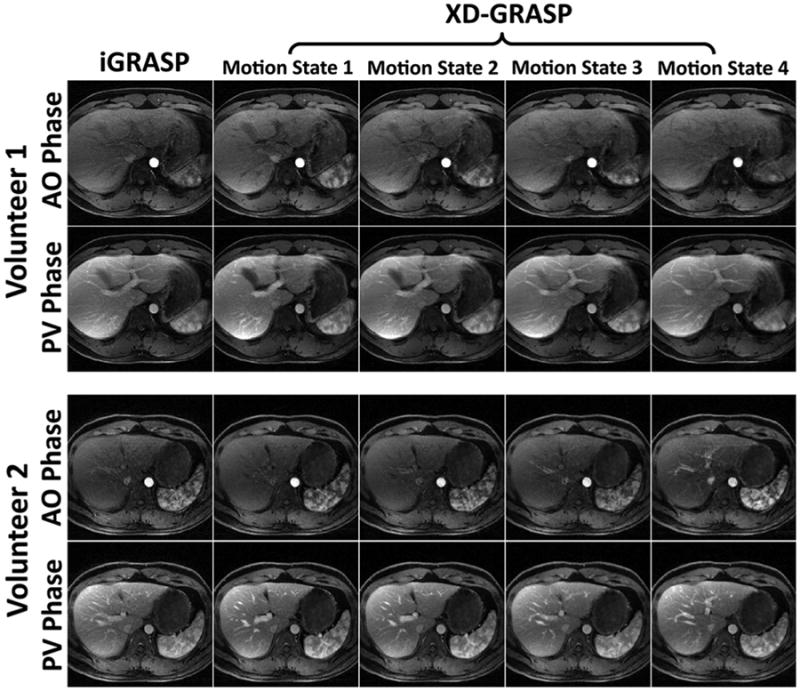
Comparison of iGRASP with XD-GRASP in both aortic (AO) and portal-venous (PV) enhancement phases in two representative partitions each from two volunteer datasets. XD-GRASP improved delineation of the liver and vessels with enhanced vessel-tissue contrast.
The first four rows of Figure 10 show the portal vein enhancement phase for two representative partitions each from volunteers 3 (normal breathing) and 4 (deep breathing). iGRASP suffered from significant intra-frame respiratory motion blurring, especially in the dataset acquired during deep breathing. XD-GRASP improved the delineation of vessels and borders in the liver, improved vessel-tissue contrast and enhanced the depiction of the kidney and bowel. The bottom row of Figure 10 shows the same comparison for one representative partition from the patient dataset. The white arrow indicates a suspected liver lesion that can be seen in iGRASP but is better delineated in XD-GRASP.
Fig. 10.
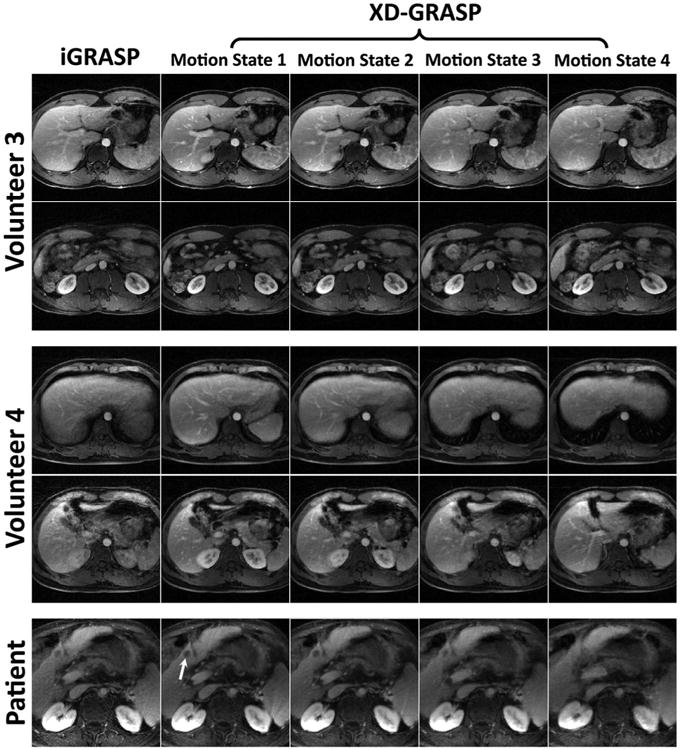
Comparison of iGRASP with XD-GRASP in a total of five representative partitions from two volunteers and one patient. Volunteer 4 was asked to breathe deeply. XD-GRASP achieved superior overall image quality, with reduced motion-blurring. The white arrow indicates a suspected liver lesion, which was better delineated in XD-GRASP.
Discussion
XD-GRASP provides a novel way to handle respiratory and other types of motion in free-breathing MRI. Instead of removing motion, e.g., using self-gating, extra motion-state dimensions are reconstructed and a compressed sensing approach is used to exploit the correlations in these dynamic dimensions. XD-GRASP does not require the use of specific motion models, and therefore it is immune to interpolation errors and offers notable advantages as compared with previously proposed registration-based compressed sensing reconstruction approaches (43,44), which co-register images in different respiratory states to correct motion. In addition, XD-GRASP also enables access to motion information that was not available before, and thus it could potentially be used to study interesting clinical problems, such as evaluation of the interaction of the left and right ventricles during respiration, e.g., for the diagnosis of conditions such as constrictive pericardial heart disease (42), and evaluation of the respiration-dependent flow patterns in “Fontan physiology” (45). Furthermore, there have been recent concerns about dyspnea caused by certain hepatobiliary contrast agents (46), and the use of XD-GRASP for evaluation of the impact of contrast injection on respiratory motion in abdominal DCE-MRI is currently underway. Moreover, the ability to reconstruct images in both inspiratory and expiratory phases from the same acquisition may also be helpful, for example, in discriminating persistent stenosis of the celiac artery from physiologic celiac artery narrowing during expiration.
The XD-GRASP framework is not limited to golden-angle radial sampling, and it can be extended to other trajectories, as long as reliable physiological motion information can be obtained (e.g., using external ECG or respiration monitor devices) and arbitrary data sorting can be performed with approximately uniform k-space coverage in each motion state. For example, novel trajectories based on 3D Cartesian sampling with butterfly navigators have been recently introduced for continuous data acquisition, following a golden-angle spiral pattern in the ky−kz plane (47). These trajectories could be well-suited for XD-GRASP reconstruction (48).
In addition to cardiac cine imaging, other applications, such as coronary MR angiography, can also benefit from simultaneous cardiac and respiratory motion sorting. In these applications, data acquisition is usually performed in a quiescent cardiac phase (e.g., mid-diastole) when cardiac motion is minimal, and a navigator echo is used to monitor the movement of the diaphragm to minimize respiratory motion effects. XD-GRASP can be used to reconstruct datasets acquired continuously covering the entire cardiac cycles and cardiac phases with the best delineation of a particular artery can be retrospectively selected for visualization.
Although XD-GRASP shares similarities with previous work (16) in the motion-detection mechanism, major differences exist in image reconstruction. The method proposed in Liu et al (16) used the respiratory motion signal for retrospective self-gating and only 50% of the data were used to reconstruct one cardiac cycle with reduced motional blurring. The view sharing reconstruction used in Liu et al (16) also leads to decreased temporal resolution. In contrast, XD-GRASP achieves 100% imaging efficiency without discarding any of the acquired data and provides better temporal resolution without using view sharing. In addition, the extra respiratory dimension provides new physiological information that was not presented in Liu et al (16).
The temporal resolution demonstrated in this work for abdominal DCE-MRI (11–12 s) may not be adequate for perfusion analysis, which usually requires 2–3 s temporal resolution. Higher temporal resolutions for DCE-MRI are restricted due to the fact that the contrast enhancement is a nonperiodic process, which limits the number of spokes that can be combined for each respiratory state. One way to achieve higher effective temporal resolutions would be to use a soft-gating approach, which weights k-space data according to the respiratory motion signal, as proposed in Zhang et al (49).
For abdominal DCE-MRI, the number of respiratory states was selected such that they can adequately resolve respiratory motion without introducing residual aliasing artifacts (As the number of motion states increases, the number of radial spokes available for each state decreases, resulting in increased undersampling). A small number of respiratory states (e.g., two) facilitates the removal of aliasing artifacts at the expense of limited depiction of respiratory motion. A large number of respiratory states (e.g., 8 or 10), on the other hand, potentially enhances the visualization of respiratory motion and reduces blurring at the expense of introducing residual aliasing artifacts. As shown in Supporting Figure S3, we found empirically that four respiratory motion states represents a good balance between removal of aliasing artifacts and motion-related blurring. Using more respiratory states (e.g., six) led to lower image quality due to increased undersampling ratio and intrinsic limits in the performance of compressed sensing reconstruction.
The reconstruction of the additional motion-state dimensions increases the computational burden, particularly because one forward and one backward NUFFT operation must be performed separately for each motion state in each iteration. This issue can be addressed using parallel computing, following the parallelization concept of our clinical implementation of iGRASP (50).
Conclusions
XD-GRASP demonstrates a new use of sparsity for motion compensation and offers a new way to handle respiratory or other types of motion in free-breathing MRI. Instead of removing or correcting motion, extra motion-state dimensions are reconstructed using a compressed sensing approach that exploits sparsity along the new dimensions. XD-GRASP reduces motion-induced blurring and allows separation of respiratory motion from cardiac motion in cardiac cine MRI and from contrast enhancement in DCE-MRI. Moreover, the reconstruction of additional motion dimensions offers additional complementary information, which can be of potential value for specific clinical applications.
Supplementary Material
Supp. Fig. S1. a: Data sorting procedure for XD-GRASP in abdominal MRI without contrast ejection. Respiratory motion was first sorted from end-expiration to end-inspiration and the corresponding set of spokes were evenly distributed into multiple respiratory states so that the number of spokes is the same in each motion state. b: For DCE-MRI, the respiratory motion sorting procedure described in (a) is performed in each contrast-enhancement phase separately.
Supp. Fig. S2. Selection of cardiac and respiratory motion signals from multiple coils. a: 2D golden-angle radial trajectory for free-breathing 2D cardiac cine MRI and (b) estimation of cardiac and respiratory motion signals using information from multiple coils. The motion signal in the coil-element with the highest peak in the frequency range of 0.1–0.5 Hz was automatically selected to represent respiratory motion; and the motion signal in the coil-element with the highest peak in the frequency range of 0.5–2.5 Hz was automatically selected to represent cardiac motion. A filtering procedure can be performed on the detected motion signals for denoising.
Supp. Fig. S3. Comparison of XD-GRASP reconstructions with different number of respiratory motion states in abdominal DCE-MRI (end-expiratory motion state only). 4 and 6 respiratory states achieved better resolved respiratory motion than 2 states and 1 state. 6 respiratory states resulted in slightly lower performance than 4 respiratory states. White arrows indicate motional blurring for a choice of 1 motion state, and residual blurring for a choice of 2 motion states.
Supp. Video S1. Myocardial wall movement at different cardiac phases during normal breathing. The clear respiratory-related movement of the interventricular septum at the end-diastolic phase suggests that left–ventricle and right–ventricle interaction can be evaluated using XD-GRASP. This can be of potential use for diagnosis of constrictive pericardial heart disease.
Supp. Video S2. Comparison of XD-GRASP reconstruction with cardiac-respiratory motion separation against iGRASP reconstruction with a single cardiac motion dimension only. XD-GRASP reconstruction presents improved image quality, particularly with the reduction of aliasing artifacts due to the separation of cardiac and respiratory motion into different dimensions, which enables exploitation of extra sparsity along the respiratory dimension and thus improves suppression of aliasing artifacts. The regularization parameter along the cardiac dimension in XD-GRASP reconstruction was selected to be the same as the regularization parameter in iGRASP reconstruction for fair comparison.
Supp. Videos S3 and S4. Comparison of reconstruction of normal (Supporting Video S3) and arrhythmic (Supporting Video S4) cardiac cycles in a patient with 2nd degree atrioventricular block. The arrhythmic cycle is prolonged due to the missed heart contraction in 2nd degree atrioventricular block.
Acknowledgments
The authors thank Dr. Jing Liu from UCSF for helpful discussion about motion estimations and Mr. Jian Xu from Siemens Healthcare USA for radial bSSFP sequence support. This work was supported in part by the NIH and was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R), a NIBIB Biomedical Technology Resource Center.
Grant sponsor: NIH; Grant numbers: R01 EB002568; P41 EB017183.
Footnotes
Supporting Information: Additional Supporting Information may be found in the online version of this article.
References
- 1.Schultz CL, Alfidi RJ, Nelson AD, Kopiwoda SY, Clampitt ME. The effect of motion on two-dimensional Fourier transformation magnetic resonance images. Radiology. 1984;152:117–121. doi: 10.1148/radiology.152.1.6729101. [DOI] [PubMed] [Google Scholar]
- 2.Axel L, Summers RM, Kressel HY, Charles C. Respiratory effects in two-dimensional Fourier transform MR imaging. Radiology. 1986;160:795–801. doi: 10.1148/radiology.160.3.3737920. [DOI] [PubMed] [Google Scholar]
- 3.Paling MR, Brookeman JR. Respiration artifacts in MR imaging: reduction by breath holding. J Comput Assist Tomogr. 1986;10:1080–1082. doi: 10.1097/00004728-198611000-00046. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Rossman PJ, Grimm RC, Riederer SJ, Ehman RL. Navigator-echo-based real-time respiratory gating and triggering for reduction of respiration effects in three-dimensional coronary MR angiography. Radiology. 1996;198:55–60. doi: 10.1148/radiology.198.1.8539406. [DOI] [PubMed] [Google Scholar]
- 5.Ehman RL, McNamara MT, Pallack M, Hricak H, Higgins CB. Magnetic resonance imaging with respiratory gating: techniques and advantages. AJR Am J Roentgenol. 1984;143:1175–1182. doi: 10.2214/ajr.143.6.1175. [DOI] [PubMed] [Google Scholar]
- 6.Brinegar C, Wu YJ, Foley LM, Hitchens TK, Ye Q, Ho C, Liang ZP. Real-time cardiac MRI without triggering, gating, or breath holding. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference; 2008; pp. 3381–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao B, Haldar JP, Liang ZP. PSF model-based reconstruction with sparsity constraint: algorithm and application to real-time cardiac MRI. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference; 2010; pp. 3390–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uecker M, Zhang S, Voit D, Karaus A, Merboldt KD, Frahm J. Real-time MRI at a resolution of 20 ms. NMR Biomed. 2010;23:986–994. doi: 10.1002/nbm.1585. [DOI] [PubMed] [Google Scholar]
- 9.Feng L, Srichai MB, Lim RP, Harrison A, King W, Adluru G, Dibella EV, Sodickson DK, Otazo R, Kim D. Highly accelerated real-time cardiac cine MRI using k-t SPARSE-SENSE. Magn Reson Med. 2013;70:64–74. doi: 10.1002/mrm.24440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI. Magn Reson Med. 1992;28:275–289. doi: 10.1002/mrm.1910280209. [DOI] [PubMed] [Google Scholar]
- 11.Chandarana H, Block TK, Rosenkrantz AB, Lim RP, Kim D, Mossa DJ, Babb JS, Kiefer B, Lee VS. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Invest Radiol. 2011;46:648–653. doi: 10.1097/RLI.0b013e31821eea45. [DOI] [PubMed] [Google Scholar]
- 12.Liao JR, Pauly JM, Brosnan TJ, Pelc NJ. Reduction of motion artifacts in cine MRI using variable-density spiral trajectories. Magn Reson Med. 1997;37:569–575. doi: 10.1002/mrm.1910370416. [DOI] [PubMed] [Google Scholar]
- 13.Lauzon ML, Rutt BK. Effects of polar sampling in k-space. Magn Reson Med. 1996;36:940–949. doi: 10.1002/mrm.1910360617. [DOI] [PubMed] [Google Scholar]
- 14.Lauzon ML, Rutt BK. Polar sampling in k-space: reconstruction effects. Magn Reson Med. 1998;40:769–782. doi: 10.1002/mrm.1910400519. [DOI] [PubMed] [Google Scholar]
- 15.Larson AC, Kellman P, Arai A, Hirsch GA, McVeigh E, Li D, Simonetti OP. Preliminary investigation of respiratory self-gating for free-breathing segmented cine MRI. Magn Reson Med. 2005;53:159–168. doi: 10.1002/mrm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Spincemaille P, Codella NC, Nguyen TD, Prince MR, Wang Y. Respiratory and cardiac self-gated free-breathing cardiac CINE imaging with multiecho 3D hybrid radial SSFP acquisition. Magn Reson Med. 2010;63:1230–1237. doi: 10.1002/mrm.22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lustig M, Santos J, Donoho D, Pauly JM. k-t SPARSE: high frame rate dynamic MRI exploiting spatio-temporal sparsity. Proceedings of the 14th Annual Meeting of ISMRM; Seattle, Washington, USA. 2006; Abstract 2420. [Google Scholar]
- 18.Gamper U, Boesiger P, Kozerke S. Compressed sensing in dynamic MRI. Magn Reson Med. 2008;59:365–373. doi: 10.1002/mrm.21477. [DOI] [PubMed] [Google Scholar]
- 19.Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med. 2010;64:767–776. doi: 10.1002/mrm.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging with respiratory motion correction for highly-accelerated first-pass cardiac perfusion MRI. Proceedings of the 19th Annual Meeting of ISMRM; Montreal, Canada. 2011; Abstract 66. [Google Scholar]
- 21.Usman M, Atkinson D, Odille F, Kolbitsch C, Vaillant G, Schaeffter T, Batchelor PG, Prieto C. Motion corrected compressed sensing for free-breathing dynamic cardiac MRI. Magn Reson Med. 2013;70:504–516. doi: 10.1002/mrm.24463. [DOI] [PubMed] [Google Scholar]
- 22.Likhite D, Ganesh A, McGann CJ, DiBella EV. Use of deformable registration for quantification of cardiac perfusion in patients with arrhythmia. Proceedings of the 21st Annual Meeting of ISMRM; Salt Lake City, Utah, USA. 2013; Abstract 318. [Google Scholar]
- 23.Chen Y, Lee GR, Wright KL, Griswold MA, Seiberlich N, Gulani V. 3D High spatiotemporal resolution quantitative liver perfusion imaging using a stack-of-spirals acquisition and through-time non-cartesian GRAPPA acceleration. Proceedings of the 21st Annual Meeting of ISMRM; Salt Lake City, Utah, USA. 2013; Abstract 601. [Google Scholar]
- 24.Otazo R, Koesters T, Candès E, Sodickson DK. Motion-guided low-rank plus sparse (L+S) reconstruction for free-breathing dynamic MRI. Proceedings of the 22nd Annual Meeting of ISMRM; Milan, Italy. 2014; Abstract 742. [Google Scholar]
- 25.Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014;72:707–717. doi: 10.1002/mrm.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandarana H, Feng L, Block KT, Rosenkrantz AB, Lim P, Babb J, Sodickson DK, Otazo R. Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling. Invest Radiol. 2013;48:10–16. doi: 10.1097/RLI.0b013e318271869c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenkrantz AB, Geppert C, Grimm R, et al. Dynamic contrast-enhanced MRI of the prostate with high spatiotemporal resolution using compressed sensing, parallel imaging, and continuous golden-angle radial sampling: preliminary experience. J Magn Reson Imaging. 2014 doi: 10.1002/jmri.24661. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ream J, Doshi AM, Block KT, Kim S, Otazo R, Feng L, Chandarana H. High spatiotemporal dynamic contrast-enhanced MRI of the small bowel in active Crohn's terminal ileitis using compressed sensing, parallel imaging, and golden-angle radial sampling. Proceedings of the 22nd Annual Meeting of ISMRM; Milan, Italy. 2014; Abstract 2122. [Google Scholar]
- 29.Pang J, Sharif B, Fan Z, Bi X, Arsanjani R, Berman DS, Li D. ECG and navigator-free four-dimensional whole-heart coronary MRA for simultaneous visualization of cardiac anatomy and function. Magn Reson Med. 2014;72:1208–1217. doi: 10.1002/mrm.25450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng L, Liu J, Block K, Xu J, Axel L, Sodickson DK, Otazo R. Compressed sensing reconstruction with an additional respiratory-phase dimension for free-breathing imaging. Proceedings of the 21st Annual Meeting of ISMRM; Salt Lake City, Utah, USA. 2013; Abstract 606. [Google Scholar]
- 31.Feng L, Axel L, Latson L, Xu J, Sodickson DK, R O. Compressed sensing with synchronized cardio-respiratory sparsity for free-breathing cine MRI: initial comparative study on patients with arrhythmias. J Cardiovasc Magn Reson. 2014;16:17. [Google Scholar]
- 32.Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007;26:68–76. doi: 10.1109/TMI.2006.885337. [DOI] [PubMed] [Google Scholar]
- 33.Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP. Self-gated cardiac cine MRI. Magn Reson Med. 2004;51:93–102. doi: 10.1002/mrm.10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimm R, Block KT, Hutter J, Forman C, Hintze C, Kiefer B, Hornegger J. Self-gating reconstructions of motion & perfusion for free-breathing T1-weighted DCE-MRI of the thorax using 3D stack-of-stars GRE imaging. Proceedings of the 20th Annual Meeting of ISMRM; Melbourne, Australia. 2012; Abstract 598. [Google Scholar]
- 35.Spincemaille P, Liu J, Nguyen T, Prince MR, Wang Y. Z intensity-weighted position self-respiratory gating method for free-breathing 3D cardiac CINE imaging. Magn Reson Imaging. 2011;29:861–868. doi: 10.1016/j.mri.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fessler JA, Sutton BP. Nonuniform Fast Fourier transforms using Min-Max interpolation. IEEE Trans Signal Process. 2003;51:560–574. [Google Scholar]
- 37.Adluru G, Dibella EV. Reordering for improved constrained reconstruction from undersampled k-space data. Int J Biomed Imaging. 2008;2008:341684. doi: 10.1155/2008/341684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lustig M, Donoho D, Pauly JM. Sparse MRI: the application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58:1182–1195. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 39.Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000;43:682–690. doi: 10.1002/(sici)1522-2594(200005)43:5<682::aid-mrm10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 40.Feng L, Otazo R, Jung H, Jensen JH, Ye JC, Sodickson DK, Kim D. Accelerated cardiac T2 mapping using breath-hold multiecho fast spin-echo pulse sequence with k-t FOCUSS. Magn Reson Med. 2011;65:1661–1669. doi: 10.1002/mrm.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim D, Dyvorne HA, Otazo R, Feng L, Sodickson DK, Lee VS. Accelerated phase-contrast cine MRI using k-t SPARSE-SENSE. Magn Reson Med. 2012;67:1054–1064. doi: 10.1002/mrm.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McConnell MV, Wu HH. Respiratory-mode display of echocardiographic images highlights effects of pericardial disease. JACC Cardiovasc Imaging. 2013;6:917–919. doi: 10.1016/j.jcmg.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen MS, Sorensen TS, Arai AE, Kellman P. Retrospective reconstruction of high temporal resolution cine images from real-time MRI using iterative motion correction. Magn Reson Med. 2012;68:741–750. doi: 10.1002/mrm.23284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Usman M, Atkinson D, Odille F, Kolbitsch C, Vaillant G, Schaeffter T, Batchelor P, Prieto C. Motion corrected compressed sensing for free-breathing dynamic cardiac MRI. Magn Reson Med. 2013;70:504–516. doi: 10.1002/mrm.24463. [DOI] [PubMed] [Google Scholar]
- 45.Vukicevic M, Chiulli JA, Conover T, Pennati G, Hsia TY, Figliola RS, Network M. Mock circulatory system of the Fontan circulation to study respiration effects on venous flow behavior. ASAIO J. 2013;59:253–260. doi: 10.1097/MAT.0b013e318288a2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davenport MS, Viglianti BL, Al-Hawary MM, Caoili EM, Kaza RK, Liu PS, Maturen KE, Chenevert TL, Hussain HK. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013;266:452–461. doi: 10.1148/radiol.12120826. [DOI] [PubMed] [Google Scholar]
- 47.Cheng JY, Zhang T, Alley MT, Lustig M, Vasanawala SS, Pauly JM. Variable-density radial view-ordering and sampling for time-optimized 3D Cartesian imaging. Proceedings of the ISMRM Workshop on Data Sampling and Image Reconstruction; Sedona, Arizona, USA. 2013. [Google Scholar]
- 48.Cheng JY, Zhang T, Pauly JM, Vasanawala SS, Lustig M. Free breathing dynamic contrast enhanced 3D MRI with resolved respiratory motion. Proceedings of the 22nd Annual Meeting of ISMRM; Milan, Italy. 2014; Abstract 330. [Google Scholar]
- 49.Zhang T, Cheng JY, Alley MT, Uecker M, Lustig M, Pauly JM, Vasanawala SS. Fast 3D Free-breathing abdominal dynamic contrast enhanced MRI with high spatiotemporal resolution. Proceedings of the 22nd Annual Meeting of ISMRM; Milan, Italy. 2014; Abstract 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Block KT, Grimm R, Feng L, Otazo R, Chandarana H, Bruno M, C G, Sodickson DK. Bringing compressed sensing to clinical reality: prototypic setup for evaluation in routine applications. Proceedings of the 21st Annual Meeting of ISMRM; Salt Lake City, Utah, USA. 2013; Abstract 3809. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. Fig. S1. a: Data sorting procedure for XD-GRASP in abdominal MRI without contrast ejection. Respiratory motion was first sorted from end-expiration to end-inspiration and the corresponding set of spokes were evenly distributed into multiple respiratory states so that the number of spokes is the same in each motion state. b: For DCE-MRI, the respiratory motion sorting procedure described in (a) is performed in each contrast-enhancement phase separately.
Supp. Fig. S2. Selection of cardiac and respiratory motion signals from multiple coils. a: 2D golden-angle radial trajectory for free-breathing 2D cardiac cine MRI and (b) estimation of cardiac and respiratory motion signals using information from multiple coils. The motion signal in the coil-element with the highest peak in the frequency range of 0.1–0.5 Hz was automatically selected to represent respiratory motion; and the motion signal in the coil-element with the highest peak in the frequency range of 0.5–2.5 Hz was automatically selected to represent cardiac motion. A filtering procedure can be performed on the detected motion signals for denoising.
Supp. Fig. S3. Comparison of XD-GRASP reconstructions with different number of respiratory motion states in abdominal DCE-MRI (end-expiratory motion state only). 4 and 6 respiratory states achieved better resolved respiratory motion than 2 states and 1 state. 6 respiratory states resulted in slightly lower performance than 4 respiratory states. White arrows indicate motional blurring for a choice of 1 motion state, and residual blurring for a choice of 2 motion states.
Supp. Video S1. Myocardial wall movement at different cardiac phases during normal breathing. The clear respiratory-related movement of the interventricular septum at the end-diastolic phase suggests that left–ventricle and right–ventricle interaction can be evaluated using XD-GRASP. This can be of potential use for diagnosis of constrictive pericardial heart disease.
Supp. Video S2. Comparison of XD-GRASP reconstruction with cardiac-respiratory motion separation against iGRASP reconstruction with a single cardiac motion dimension only. XD-GRASP reconstruction presents improved image quality, particularly with the reduction of aliasing artifacts due to the separation of cardiac and respiratory motion into different dimensions, which enables exploitation of extra sparsity along the respiratory dimension and thus improves suppression of aliasing artifacts. The regularization parameter along the cardiac dimension in XD-GRASP reconstruction was selected to be the same as the regularization parameter in iGRASP reconstruction for fair comparison.
Supp. Videos S3 and S4. Comparison of reconstruction of normal (Supporting Video S3) and arrhythmic (Supporting Video S4) cardiac cycles in a patient with 2nd degree atrioventricular block. The arrhythmic cycle is prolonged due to the missed heart contraction in 2nd degree atrioventricular block.


