Abstract
The protective role of B cells and humoral immune responses in tuberculosis infection has been regarded as inferior to cellular immunity directed to the intracellular pathogen Mycobacterium tuberculosis. However, B-cell–mediated immune responses in tuberculosis have recently been revisited in the context of B-cell physiology and antigen presentation. We discuss in this review the diverse functions of B cells in tuberculosis, with a focus on their biological and clinical relevance to progression of active disease. We also present the peptide microarray platform as a promising strategy to discover unknown antigenic targets of M. tuberculosis that could contribute to the better understanding of epitope focus of the humoral immune system against M. tuberculosis.
Keywords: tuberculosis, B cells, antibodies, cytokines, host-directed therapy
For this review, we performed a literature search on PubMed, PubMedCentral, and Google using the following terms: tuberculosis, B cells, humoral immunity, antibodies, tumor-specific B-cell responses, cytokines, and immunotherapy. Results were filtered based on relevance to the respective subsections presented in this review—immunological background of B cells in tuberculosis, intratumor B-cell responses, and significance of antibodies in clinical tuberculosis. We have also incorporated some data generated in-house to illustrate the applicability of our peptide microarray platform for gauging specific antituberculosis antibody responses.
THE ROLE OF B CELLS AND ANTIBODY RESPONSES IN TUBERCULOSIS
Tuberculosis is a communicable disease caused by Mycobacterium tuberculosis (Mtb), which is mainly an intracellular pathogen that kills almost 2 million people annually, leaving at least one-third of the world's population latently infected [1]. The more devastating form of pulmonary tuberculosis disease in adults develops with unspecific and nonproductive inflammation in the lungs, leading to tissue destruction and eventually to organ failure and death [2]. Up to now, protective immune responses in tuberculosis remain poorly understood [3]. While infiltration and activation of CD4+ Th1 cells and CD8+ cytolytic lymphocytes is required for control of human tuberculosis [4], the role of B cells and antituberculosis humoral immune responses remains controversial [5].
Adaptive anti-Mtb immune responses are initiated by effective antigen presentation in secondary lymphoid organs in the upper thoracic region. Upon uptake of live Mtb bacilli or shed antigens, professional antigen-presenting cells (pAPCs), such as dendritic cells and macrophages, traffic from the site of infection in the lungs to the mediastinal lymph nodes. Here, antigen-loaded pAPCs activate CD4+ and CD8+ T cells, followed by an influx of Mtb-specific T cells into infectious foci [6]. Activation of other immune cells at the site of infection includes neutrophils, monocytes/macrophages, and also B cells. This organized orchestration of immune cells leads to the formation of dynamic lymphoid structures (ie, granulomas), which, in most individuals, are able to control further dissemination of Mtb. Complete eradication of Mtb bacilli is rare; instead, latent tuberculosis is established in the human host [7]. Mtb can reside for years within macrophages and monocytes in individuals with latent tuberculosis [6], including CD271+ bone marrow mesenchymal stromal cells [8]. The specific immune responses or factors responsible for progression of active tuberculosis are not well characterized. However, the enrichment of highly specific immune effector cells with potent anti-Mtb activity most probably plays a pivotal role to stop progress of tuberculosis infection to clinical disease.
Both naive and memory B cells have been shown to be present in tuberculosis granulomas and lesions in the human lung, which resemble germinal center–like secondary lymphoid structures [9]. The function of B cells in the Mtb-infected lung may involve presentation of Mtb antigens to T cells and the production of cytokines and Mtb-specific antibodies [10]. Inflammatory effector B-cell subsets, including the newly discovered innate B cells [11], can promote development of Th1 responses via production of interleukin (IL) 12, interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) [12]. A Th1-like “milieu” may play a significant role in the development of clinically relevant antimycobacterial immune responses and early control of tuberculosis infection. Conversely, the presence of anti-inflammatory B cells (with regulatory functions and the ability to secrete anti-inflammatory cytokines such as IL-4, IL-33, and transforming growth factor beta [TGF-β]) may subvert the inflammatory response orchestrated by Th1 and Th17 cells to reduce tissue damage [10, 13]: A “Th2-like” milieu may help maintain equilibrium between productive and destructive cellular immune responses. Mtb-specific antibodies may have clinically relevant effects in adaptive immune responses, in addition to cell-mediated immune response in tuberculosis [5]. Here, various studies in mouse models of tuberculosis over the past decade reveal a potential role for specific antibodies in the host defense against Mtb [5]. High-dose administration of intravenous immunoglobulin (IVIG) has shown protective effects in mouse models of tuberculosis by reducing the hyperinflammatory response marked by reduced granulomatous infiltration into the lung, correlating with better control of Mtb bacillary load [14]. Induction of humoral immune responses in animal models of tuberculosis as well as humans with active tuberculosis disease [10], along with evidence of antibody reactivity to various Mtb antigens primarily found in serum samples from tuberculosis patients, suggests that B cells probably play a significant role in determining the clinical outcome of Mtb infection [5]. B-cell epitopes and T-cell epitopes are often closely related because the uptake of the nominal target antigen by the B-cell receptor protects the target epitope from intracellular proteolysis and favors the presentation in the major histocompatibility complex (MHC) class II antigen processing and presentation pathway by MHC class II molecules [15].
B-CELL ACTIVATION AND EFFECTOR MECHANISMS IN TUBERCULOSIS
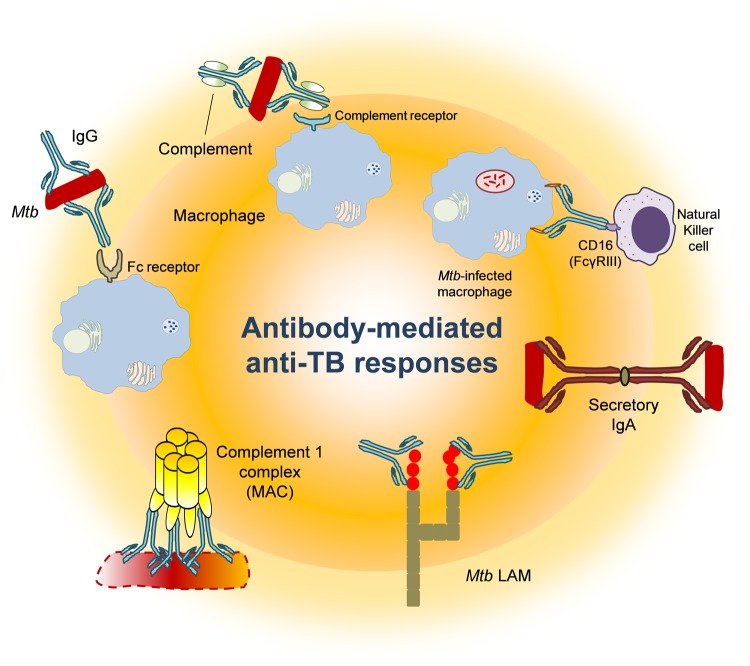
Naive B cells are activated when their surface immunoglobulin-based B-cell receptors bind to antigens presented on MHC class II molecules expressed by antigen-primed CD4+ T cells or pAPCs in addition to maturation signals such as cytokines and CD40–CD40L interactions [16]. Upon activation, some B cells develop into plasma cells, which can produce antibodies and cytokines [12]. Mtb-directed antibodies may mediate effector functions such as opsonization of bacterial cells, neutralization of secreted antigens, and antibody-dependent cellular cytotoxicity (ADCC) [16, 17] (summarized in Figure 1). B cells are effective APCs that can readily respond to either cell-free antigens or entire pathogens that can ultimately be presented to CD4+ T cells [16]. B cells can therefore contribute to early protection and the induction of effective CD4+ T cell responses in tuberculosis.
Figure 1.
Role of antibodies in anti–Mycobacterium tuberculosis (Mtb) infection. Abbreviations: FcγRIII, Fc gamma receptor III; IgA, immunoglobulin A; IgG, immunoglobulin G; LAM, lipoarabinomannan; MAC, membrane-attack complex; TB, tuberculosis.
ANTIBODY RESPONSES IN TUBERCULOSIS: A ROLE IN IMMUNE PROTECTION?
Several preclinical reports support a protective effect of antibodies in tuberculosis. In mice, immunoglobulin A (IgA) appears to provide early protection against intranasal BCG infection, as IgA-deficient animals succumbed to pulmonary mycobacterial disease as opposed to their wild-type counterparts [18]. Immunoglobulin G (IgG)–mediated opsonization of Mtb bacilli leads to enhanced phagocytosis by macrophages via additional binding of complement proteins C3 and C4, and internalization via complement receptors [19]. Both IgG and IgA antibodies can neutralize Mtb-derived antigens, and thus potentially block systemic bacterial dissemination [5]. Although unexplored in tuberculosis, IgG-mediated ADCC could be instrumental in early control of Mtb infection via opsonization of the infected target cell followed by binding of the IgG Fcγ region to CD16 (FcγRIII) expressed on natural killer [16] and effector memory T cells [20]. CD16 engagement triggers the release of perforin and granzymes from cytolytic lymphocytes, resulting in lysis of the infected target cell, as observed in the elimination of transformed cells [16]. Mtb-specific IgG antibodies may promote the depletion of mycobacterial reservoirs in tissue via CD16-mediated ADCC early after infection.
Naturally occurring anti-Mtb immunoglobulin M (IgM) antibodies may potentially exhibit activity for opsonization and neutralization of secreted toxins [17]. Assessment of antibody-mediated antituberculosis responses upon intranasal immunization of mice with human IgA has been shown to protect animals to subsequent Mtb challenge [21], confirming the anti-infective potential of IgA against early Mtb infection. These preclinical data have been substantiated in a clinical setting: Ethiopian individuals with latent tuberculosis were found to have higher serum levels of IgA directed against the secreted Mtb antigens ESAT-6 and Rv2031c compared with patients with active tuberculosis [22]. Passive administration of human IgG has been shown to promote better control of mycobacterial growth and to reduce pathological inflammation in the lung of Mtb-infected mice [5, 14]. This effect apparently requires glycosylation of the Fcγ region, as administration of IVIG without Fc region glycosylation does not protect mice against subsequent Mtb challenge [14]. In this case, antibodies may bind to the Mtb bacilli or to immunodominant Mtb antigens, resulting in elimination of bacteria and bacterial products. IgG antibodies may also gain access to the cytosol of the Mtb-infected cell and promote growth restriction of intracellular bacteria, as previously shown in the context of Salmonella infection [23]. Similarly, antibodies to intracellular nuclear cancer antigens have shown clinical benefit [24], suggesting that the role of antibodies directed against intracellular antigens may be diverse; that is, they may access the cytosol, or, mutually inclusive, they may mediate ADCC and facilitate antigen uptake (from accessible material, ie, after killing of infected macrophages by T cells, or by digested Mtb material from neutrophils [25]).
Nevertheless, B-cell responses and antibodies in tuberculosis have also been associated with progressive clinical disease.
ANTIBODY RESPONSES IN TUBERCULOSIS AS A RESULT OF PROGRESSIVE DISEASE
Although the major focus of this review concerns protective B-cell-mediated and antibody-mediated immune responses in tuberculosis, the B-cell compartment may also be involved in disease progression. As Mtb is an intracellular pathogen, is it likely that antibody-mediated immune responses become most effective in the progressive phase of tuberculosis disease, when extracellular bacteria and antigens are released and spread from destructive tissue lesions in the lung (Figure 2). While Mtb-specific antibodies may be involved in bacterial clearance and subsequent immune control, it is also possible that an enhanced activation of antibody responses in human tuberculosis reflects a consequence of impaired cellular immunity. Thus, elevated antibody responses in the chronic phase of tuberculosis infection could instead indicate exacerbated disease. The observation that elevated levels of Mtb-specific IgG-secreting cells in the peripheral circulation of patients with active tuberculosis are associated with reduced Mtb-specific IFN-γ production and more severe forms of tuberculosis disease [26] would support this point. High levels of total and Mtb-specific serum antibodies have previously been shown in patients with advanced tuberculosis disease [27], including cavitary forms of pulmonary tuberculosis [28, 29]. Likewise, high levels of purified protein derivative (PPD)–specific serum IgG antibodies have been reported in rabbits with chronic pulmonary tuberculosis [30]; similar associations between increased antigen-specific antibodies and tuberculosis disease severity have also been found in Mycobacterium leprae [31] and helminth infections [32]. Along this line, it was recently shown in mice with B cells lacking the ability to produce antibodies that the Mtb load increases in the course of progressive tuberculosis disease due to an excess of IL-10 produced by activated macrophages [33]. IL-10 blockade reduced the bacterial burden in lungs and spleens of these mice to the same level as to wild-type animals, supporting the notion that B cells may modulate cytokine production, macrophage activation, and immunopathology in tuberculosis. Tuberculosis disease may be further exacerbated by cross-reactive antibodies to nontuberculous mycobacteria, which are a common confounder to anti-Mtb humoral immune responses in humans [34]. Thus, Mtb-specific antibody responses in the sera of patients with active tuberculosis may be useful for diagnostic purposes or considered as biomarkers of active and/or progressive disease, rather than a correlate of protection [26, 27].
Figure 2.
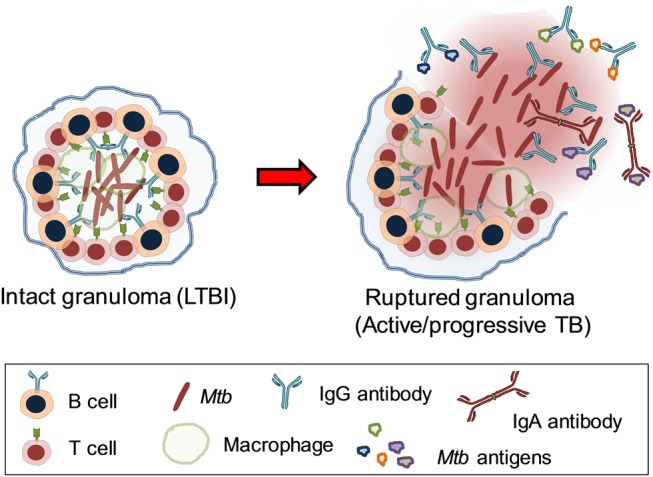
In situ activity of anti–Mycobacterium tuberculosis (Mtb) antibodies in lung granulomas. In addition to secreted Mtb antigens, immunoglobulin G and dimeric immunoglobulin A antibodies recognize Mtb surface structures such as lipoarabinomannan, heparin-binding hemagglutinin, and lipoproteins leading to antigen neutralization. Abbreviations: IgA, immunoglobulin A; IgG, immunoglobulin G; LTBI, latent tuberculosis; TB, tuberculosis.
USE OF Mtb-SPECIFIC B CELLS FOR DIAGNOSIS OF CLINICAL DISEASE
Conventional serology [35] involving assessment of serum antibodies [36] shows variable diagnostic results depending on the sputum (smear) result of patients with active tuberculosis as well as their human immunodeficiency virus (HIV) status [37–39]. Instead, assessment of antibody-secreting cells that are temporarily present in the peripheral circulation of patients with active disease may represent a promising alternate way to diagnose tuberculosis [26, 40, 41]. A simple antibody-based, point-of-care test that could provide rapid results of whether or not the patient has active tuberculosis would be very helpful in the clinical management of tuberculosis disease.
FUNCTIONALITY OF B CELLS IN TISSUE AND NONTISSUE COMPARTMENTS: IMPLICATIONS FOR TUBERCULOSIS
B cells residing in tissue, as well as antibodies secreted therein, may show functional differences from B cells circulating in the periphery. For example, it was recently shown that production of anti-inflammatory IL-10 by pleural fluid B cells can dampen the IFN-γ–secreting property of Th1 cells following in vitro restimulation with irradiated whole Mtb bacilli; these B cells were phenotypically different from those found in the patients' peripheral blood [42]. Furthermore, B-cell–derived IL-10 driven by antigen-specific responses may also be useful in neutralizing chronic, excessive inflammation in the lung at later stages of tuberculosis disease [10].
Regulatory B-Cell Responses in Tuberculosis
A subset of regulatory B cells (Breg cells) that controls inflammation and autoimmunity in both mice [43] and humans [44] was recently described: CD19+CD24hiCD38hi Breg cells isolated from peripheral blood of healthy individuals have the ability to suppress T-cell functions including the differentiation of IFN-γ– and TNF-α–producing Th1 cells, but also IL-17–producing Th17 cells [45, 46]. Breg cells have also been shown to suppress the production of TNF-α by macrophages [44]. Interestingly, a functional link seems to exist between Breg cells and regulatory T cells (Treg cells), as Breg cells could promote pulmonary infiltration of FoxP3+ Treg cells that prevent allergic inflammation in ovalbumin-treated mice [47] and also induce Treg cell expansion in a murine lupus model [48]. Likewise, CD19+CD24hiCD38hi Breg cells have been shown to promote the expansion of FoxP3+ Treg cells with suppressive functions in healthy individuals, while Breg cells from patients with different autoimmune conditions have lost their suppressive ability including the capability to maintain FoxP3+ Treg cells [45, 46]. Immune suppression mediated by Breg cells seems to be primarily dependent on IL-10 [43–46]. Patients with tuberculosis have also been found to have elevated levels of a functionally suppressive CD19+CD1d+CD5+ B-cell subset in peripheral blood [13]. Successful antituberculosis treatment reduced the frequency and function of these Breg cells in peripheral blood of patients with pulmonary tuberculosis [49]. Thus, reduced numbers of Breg cells may fail to limit exaggerated inflammatory responses in patients with autoimmune diseases, whereas excess numbers of Breg cells may prevent antimicrobial effector responses in tuberculosis, in part by allowing local expansion of FoxP3+ Treg cells.
B Cells in Tuberculosis Granulomas
Tuberculosis granulomas in the lung represent the hallmark of human tuberculosis disease [50]. Tuberculosis granulomas are products of lymphoid neogenesis and therefore support in situ antigen processing and presentation, where B cells constitute a major cellular component [10]. Granuloma-associated B cells have been shown to maintain close contact with CXCR5+ T-cell subsets and Mtb-infected macrophages [10]. These B cells are likely to be involved in IL-10– and IL-21–mediated regression of tuberculosis immunopathology [29], as well as antibody production directed against Mtb-cell envelope components and secreted antigens (reviewed in [5]) that may disrupt bacterial dissemination and delay progression of tuberculosis disease (Figure 2). B cells act also as APCs that can engulf entire Mtb bacilli or antigens and present them to T cells locally in the infected tissue [10]. This process reciprocally promotes the differentiation of B cells to activated plasma cells capable of secreting Mtb-specific antibodies. The APC function of B cells has been postulated as a critical component to enhance targeted and protective CD4+ T-cell recall responses to infection, as may be the case in the microenvironment of the tuberculosis granuloma [5, 10]. Therefore, B cells in granulomas are likely to express a vast repertoire of Mtb-antigen specificities and participate in curbing Mtb infection at an early stage. Novel insights into tuberculosis granuloma–associated B-cell populations and their capacity to eliminate Mtb reservoirs and/or control pathological inflammation in the human host promise important clinical significance. Here, solid latent tuberculosis granulomas in surgical resections from patients with lung abnormalities [51] represent an ideal source to study lung-resident effector and memory B-cell subsets in humans. Alternatively, resected tissue from patients with lung cancer who recovered from an episode of clinical pulmonary tuberculosis [52] may contain calcified/healed granulomas harboring memory B cells with therapeutic potential in tuberculosis.
B Cells in Bone Marrow
The maintenance of long-lived memory B cells in the bone marrow (BM) and their regulation by antigen availability is well established [53]. Homing of mycobacterial antigen-experienced, high-affinity memory B cells to the BM following contraction of the primary immune response has been documented [54]. In addition, BM-derived B cells can process and present cognate antigen ex vivo, and readily transform into IgG- and cytokine-producing plasma cells upon antigen rechallenge [54]. Furthermore, allogeneic BM transplant has also been shown to enrich the Haemophilus influenzae type b–specific IgG repertoire in recipients via transfer of memory B cells [55]. There is also evidence for qualitative editing of memory B cells prior to their repopulation of the BM, a process that obliterates several poly- and autoreactive subsets [56]. Thus, one may assume that memory B cells in the BM are highly selective and functional in nature—with a potential impact on productive and/or protective immune responses, also in tuberculosis.
B Cells in Sputum
Microscopic confirmation of Mtb bacilli present in patient sputum is a routine test used in clinical tuberculosis diagnostics [1]. Sputum contains macrophages and keratinocytes as well as lymphocytes (B cells to a lesser extent than T cells) [57]. Sputum-associated B cells have been described to express HLA-DR and CD40, both features of activated B cells [16]. Interestingly, antibodies generated in the airways or lungs have been shown to be present in the sputum of patients with different diseases. For example, high levels of IgA, IgM, and IgG autoantibodies have been reported in the sputum of patients at risk for or diagnosed with early rheumatoid arthritis [58]. Also, IgA antibodies against Pseudomonas aeruginosa found in nasal secretions/sputum of cystic fibrosis patients were shown to be able to distinguish between the various clinical manifestations of lung infection with the pathogen [59]. Presence of anti-Mtb antibodies in sputum are yet to be demonstrated in clinical tuberculosis but present a viable avenue to explore, largely for diagnostic and potentially for therapeutic applications.
THE PEPTIDE MICROARRAY PLATFORM AS A PROMISING TOOL TO DISCOVER NOVEL Mtb ANTIGENS WITH CLINICAL RELEVANCE
Although protein antigens induce antibody production by plasma cells, it is the presence of specific peptides or epitopes within these cognate antigens that evoke this immune response in an organism or individual. Peptide- or epitope-specific responses reflect epitope-antibody complexes targeting the nominal protein, or cross-reacting antibodies that recognize structurally related targets derived from “self” or “nonself” antigens [60]. Peptide microarray technology allows the objective and simultaneous testing of several thousand unique epitopes displayed as stretched linear peptides on glass slides. We have recently used protein microarrays with linear peptides to profile specific antibody responses in autoimmunity [61] as well as to influenza virus [62] and Mtb [63] epitopes. These studies have revealed an unbiased, global view of the human immune response without preselecting target proteins. In the above-mentioned context, a peptide might be important not only because it resides in a particularly biologically relevant protein, but also due to its specific location within the protein. In addition, most of the current analyses focus on the “positive recognition” of targets, yet important information may be missed if they are tagged as “negative events.” This absence of recognition of target structures is not appreciated, as the absence of certain naturally occurring antibodies can nevertheless be biologically relevant [64]. The demonstration of the presence or absence of immunoglobulin reactivity may therefore be clinically relevant in tuberculosis, uncovering potential therapeutic immunoglobulin subclasses.
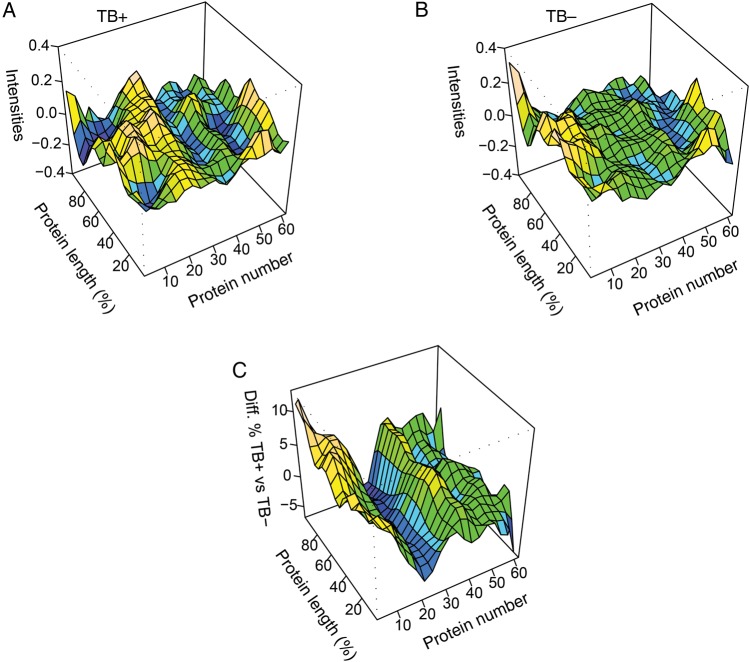
The peptide response can be visualized using a high-content peptide microarray [60], whereas immunoglobulin reactivity may be graphically represented using a 3D regression surface model. The global analysis of immunoglobulin reactivity will allow the appreciation of the presence/absence of certain antibody reactivity profiles, which may be biologically pertinent to antituberculosis humoral responses in different tissue compartments. For example, we have previously used the 3D regression surface model to profile and graphically display the human antibody response against 63 Mtb proteins (listed in Table 1) [63]. This method allows for description of differences between immunoglobulin target recognition in various anatomical compartments and sources (ie, serum, lung, bone marrow, sputum), longitudinally, in a single individual or a group of people who harbor latent tuberculosis or who have active tuberculosis disease (Figure 3A and 3B). Alternatively, this model can also show functional differences in immunoglobulin G recognition patterns in tuberculosis-positive individuals vs tuberculosis-negative individuals (Figure 3C) spanning the entire Mtb proteome. Thus, results from peptide microarray studies can directly contribute to the discovery of previously unknown antigenic Mtb targets with significant clinical relevance.
Table 1.
Ranking of 63 Mycobacterium tuberculosis Proteins Derived From Peptide Microarray Data According to Immunological Significance
| Rank | Accession | Protein Name | Rv No. |
|---|---|---|---|
| Secreted proteins | |||
| 1 | P0A564 | 6 kDa early secretory antigenic target (ESAT-6) | Rv3875 |
| 2 | P0A566 | ESAT-6-like protein esxB (10 kDa culture filtrate antigen CFP-10) | Rv3874 |
| 3 | P0C5B9 | Antigen 85B (30 kDa extracellular protein) (Ag85B) | Rv1886c |
| 4 | P0A5B7 | Alpha-crystallin (Acr) (14 kDa antigen) | Rv2031c |
| 5 | P0A5Q2 | Immunogenic protein MPT63 (antigen MPT63) | Rv1926c |
| 6 | P0A5Q4 | Immunogenic protein MPT64 (antigen MPT64) | Rv1980c |
| 7 | P0A668 | Immunogenic protein MPT70 | Rv2875 |
| 8 | O50430 | Low molecular weight T-cell antigen TB8.4 | Rv1174c |
| 9 | P96213 | ESX-1 secretion–associated protein EspE | Rv3864 |
| 10 | Q7U2C8 | Esat-6 like protein EsxG (conserved protein TB9.8) | Rv0287 |
| 11 | Q933K8 | ESX-1 secretion-associated protein EspB (antigen MTB48) | Rv3881c |
| 12 | P0A570 | ESAT-6-like protein EsxN | Rv1793 |
| 13 | P64091 | ESAT-6-like protein EsxQ | Rv3017c |
| 14 | P64093 | ESAT-6-like protein EsxR | Rv3019c |
| 15 | P0A568 | ESAT-6-like protein EsxH (10 kDa antigen CFP7) (CFP-7) | Rv0288 |
| 16 | P63879 | Probable cutinase Rv1984c/MT2037 (EC 3.1.1.74) | Rv1984c |
| Proteins involved in cell wall maintenance | |||
| 17 | P0A670 | Cell surface lipoprotein MPT83 (lipoprotein p23) | Rv2873 |
| 18 | Q79FV1 | Uncharacterized PPE family protein PPE14 | Rv0915c |
| 19 | L7N675 | PPE family protein (PPE family protein PPE18) | Rv1196 |
| 20 | Q79FE1 | PPE family protein PPE41 | Rv2430c |
| 21 | Q79FC6 | Uncharacterized PPE family protein PPE42 | Rv2608 |
| 22 | Q6MWX9 | PPE family protein PPE55 | Rv3347c |
| 23 | I6Y936 | PE family protein (PE family protein PE7) | Rv0916c |
| 24 | P95130 | PGL/p-HBAD biosynthesis rhamnosyltransferase (EC 2.4.1.-) | Rv2962 |
| 25 | P95134 | PGL/p-HBAD biosynthesis glycosyltransferase/MT3034 (EC 2.4.1.-) | Rv2958c |
| 26 | Q7U1Z4 | Probable cyclopropane-fatty-acyl-phospholipid synthase UfaA1 (cyclopropane fatty acid synthase) | Rv0447c |
| 27 | P0A599 | PGL/p-HBAD biosynthesis glycosyltransferase/MT3031 (EC 2.4.1.-) | Rv2957 |
| 28 | P0A5J0 | Lipoprotein lpqH (19 kDa lipoprotein antigen) | Rv3763 |
| 29 | P30234 | Alanine dehydrogenase (EC 1.4.1.1) (40 kDa antigen) (TB43) | Rv2780 |
| 30 | Q02251 | Mycocerosic acid synthase (EC 2.3.1.111) | Rv2940c |
| 31 | P67157 | UPF0073 membrane protein Rv1085c/MT1117 | Rv1085c |
| 32 | Q79FZ9 | MCE family protein 1A (MCE-family protein Mce1A) | Rv0169 |
| 33 | P67300 | Putative membrane protein insertion efficiency factor | Rv3922c |
| 34 | O33192 | Lipoprotein LprJ (probable lipoprotein LprJ) | Rv1690 |
| 35 | P0A521 | 60 kDa chaperonin 2 (65 kDa antigen) (cell wall protein A) | Rv0440 |
| Proteins involved in central biochemistry of Mtb | |||
| 36 | O05870 | Phosphate-binding protein pstS 2 (PBP 2) (PstS-2) | Rv0932c |
| 37 | P0A5Y2 | Phosphate-binding protein pstS 3 (PBP3) (PstS-3) (antigen Ag88) | Rv0928 |
| 38 | P15712 | Phosphate-binding protein pstS 1 (PBP1) (PstS-1) (antigen Ag78) (protein antigen B) | Rv0934 |
| 39 | O07175 | Probable serine protease PepA (serine proteinase) (MTB32A) | Rv0125 |
| 40 | O05871 | Serine/threonine-protein kinase pknD (EC 2.7.11.1) | Rv0931c |
| 41 | O06186 | Hypoxic response protein 1 (HRP1) | Rv2626c |
| 42 | O53611 | Isocitrate dehydrogenase, NADP-dependent, monomeric type (EC 1.1.1.42) | Rv0066c |
| 43 | P65097 | Isocitrate dehydrogenase [NADP] (EC 1.1.1.42) | Rv3339c |
| 44 | P09621 | 10 kDa chaperonin (10 kDa antigen) (BCG-A heat shock protein) (GroES protein) (protein Cpn10) | Rv3418c |
| 45 | P0A5J4 | Malate synthase G (EC 2.3.3.9) | Rv1837c |
| 46 | P71495 | Acyl-CoA synthase | Rv2941 |
| 47 | O53896 | probable serine protease pepd (serine proteinase) (mtb32b) (EC 3.4.21) | Rv0983 |
| 48 | P63456 | 3-oxoacyl-[acyl-carrier-protein] synthase 2 (EC 2.3.1.41) (β-ketoacyl-ACP synthase 2) (KAS 2) | Rv2246 |
| 49 | P65402 | Probable molybdenum cofactor guanylyltransferase | Rv2453c |
| 50 | P64897 | NAD-specific glutamate dehydrogenase (NAD-GDH) (EC 1.4.1.2) | Rv2476c |
| 51 | O53673 | Heat shock protein (heat-stress-induced ribosome-binding protein A) | Rv0251c |
| 52 | P0A510 | Biotinylated protein TB7.3 | Rv3221c |
| Proteins involved in transcriptional regulation of Mtb | |||
| 53 | O06153 | Universal stress protein Rv1636/MT1672 (USP Rv1636) | Rv1636 |
| 54 | O06189 | Universal stress protein Rv2623/MT2698 (USP Rv2623) | Rv2623 |
| 55 | P95193 | Transcriptional regulatory protein DevR (DosR) | Rv3133c |
| 56 | P0A674 | DNA-directed RNA polymerase subunit β (EC 2.7.7.6) | Rv0668 |
| 57 | P0A680 | DNA-directed RNA polymerase subunit β (EC 2.7.7.6) | Rv0667 |
| 58 | Q7D5S2 | RNA polymerase sigma factor SigF (Sigma factor SigF) | Rv3286c |
| 59 | P0A5V2 | 50S ribosomal protein L7/L12 | Rv0652 |
| Uncharacterized/unknown proteins | |||
| 60 | O06183 | Uncharacterized protein Rv2629/MT2704 | Rv2629 |
| 61 | Q7U2P4 | Conserved protein tb18.5 | Rv0164 |
| 62 | Q7TYY1 | Conserved protein tb16.3 | Rv2185c |
| 63 | O50383 | Putative uncharacterized protein | Rv3354 |
Serum from 34 patients with pulmonary tuberculosis from Armenia, 6 patients from Stockholm, and 35 healthy individuals from the United States were used for differential Mtb epitope recognition analysis using the peptide microarray platform [63]. The peptide microarray technology picked up epitopes within relevant Mtb targets that have been described previously (eg, Ag85, ESAT-6), yet also (intracellular) targets associated with Mtb biochemistry.
Abbreviation: Mtb, Mycobacterium tuberculosis.
Figure 3.
Three-dimensional regression model for visualizing the 63 Mycobacterium tuberculosis (Mtb) antigens listed in Table 1 captured via the peptide microarray platform. Intensity of antibody responses to the peptides constituting a particular protein is aligned against the length of the entire molecule, to indicate possible immunogenic “hotspots” of recognition among patients with tuberculosis (A) and healthy individuals (B). C, Differences in recognition between patients with tuberculosis and healthy individuals are plotted against protein length. Protein number refers to the sequential order of the antigens in Table 1. Abbreviations: Diff., difference; TB, tuberculosis.
APPLICATION OF Mtb-SPECIFIC IMMUNOGLOBULIN G FOR ANTITUBERCULOSIS IVIG
Polyspecific IVIG contains a broad array of multiple IgG antibodies and represents an established treatment to suppress inflammation by blocking the interaction between the Fcγ receptor and proinflammatory ligands [65] while reducing antigen-specific T-cell proliferation without inducing apoptosis [66]. IVIG administration has been clinically used for patients with low antibody production due to pathologies arising from immunodeficiency [67], autoimmunity, immune-mediated and inflammatory diseases, or as adjunctive treatment in clinical manifestations such as neurological disorders [68], bacterial sepsis [69], HIV infection [70], and allogeneic stem cell transplant–associated cytomegalovirus infection [71]. IgG directed against specific Mtb antigens, present in individuals with latent tuberculosis but not in patients with active tuberculosis, could be tailored for intravenous administration, following in vitro confirmation of antituberculosis activity. However, a more precise definition of the nominal Mtb target antigens may be needed to select the best IVIG profile.
CONCLUSIONS
Our current knowledge of the role of B cells in tuberculosis is limited, yet current data suggest an active role in tuberculosis protection, as well as in tuberculosis progression, associated with the nature of antibody specificities and with the cytokine profiles elaborated by B cells. Evaluation of B-cell–mediated antituberculosis immune responses in mice and nonhuman primates suggests a clinically relevant role at the early stages of Mtb infection. Conversely, abundance of antibodies in sera of patients as well as in various animal models (rabbits and mice) suggests that B cells are involved in immunopathology during active disease. In addition, the regulatory function of B cells, concomitant with that of T cells, may actively participate in determining the outcome of Mtb infection in humans. Further insights into tissue-specific anti-Mtb immune reactivity using novel, cutting-edge technology may uncover novel mechanisms by which B cells orchestrate productive, clinically relevant immune responses in tuberculosis and whether the nature of the target antigen, in addition to the milieu interne, determines the cytokine production pattern of antigen-specific B lymphocytes in tuberculosis infection.
Notes
Supplement sponsorship. This article appears as part of the supplement “Advances in Tuberculosis Research: A Blueprint for Opportunities.” This article was sponsored by the Karolinska Institutet.
Potential conflicts of interest. A. Z. receives funding from European Union FW7 Rid-RTI and the National Institute for Health Research Biomedical Research Centre, University College Hospitals, London, UK. S. B. receives funding from the Stockholm County Council; Karolinska Institutet; Magnus Bergwall and Åke Wiberg Foundations; and the Swedish Society for Medical Microbiology. M. M. receives funding from Hjärt-lungfonden (Swedish Heart and Lung Foundation); Vinnova; Vetenskåpsrådet (Swedish Research Council); and the European Developing Countries Clinical Trials Partnership. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global tuberculosis report 2014. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 2.Zumla A, Rao M, Parida SK et al. Inflammation and tuberculosis: host-directed therapies. J Intern Med 2015; 277:373–87. [DOI] [PubMed] [Google Scholar]
- 3.Zumla A, Maeurer M. Rational development of adjunct immune-based therapies for drug-resistant tuberculosis: hypotheses and experimental designs. J Infect Dis 2012; 205(suppl 2):S335–9. [DOI] [PubMed] [Google Scholar]
- 4.Brighenti S, Andersson J. Local immune responses in human tuberculosis: learning from the site of infection. J Infect Dis 2012; 205(suppl 2):S316–24. [DOI] [PubMed] [Google Scholar]
- 5.Achkar JM, Chan J, Casadevall A. Role of B cells and antibodies in acquired immunity against Mycobacterium tuberculosis. Cold Spring Harb Perspect Med 2014; 5:a018432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol 2013; 31:475–527. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers S, Schaible UE. The granuloma in tuberculosis: dynamics of a host-pathogen collusion. Front Immunol 2012; 3:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das B, Kashino SS, Pulu I et al. CD271(+) bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med 2013; 5:170ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozakiewicz L, Phuah J, Flynn J, Chan J. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Adv Exp Med Biol 2013; 783:225–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan J, Mehta S, Bharrhan S et al. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Semin Immunol 2014; 26:588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao Y, Liu X, Han C et al. Identification of IFN-gamma-producing innate B cells. Cell Res 2014; 24:161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol 2008; 20:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Zheng X, Zhang J et al. CD19(+)CD1d(+)CD5(+) B cell frequencies are increased in patients with tuberculosis and suppress Th17 responses. Cell Immunol 2012; 274:89–97. [DOI] [PubMed] [Google Scholar]
- 14.Olivares N, Marquina B, Mata-Espinoza D et al. The protective effect of immunoglobulin in murine tuberculosis is dependent on IgG glycosylation. Pathog Dis 2013; 69:176–83. [DOI] [PubMed] [Google Scholar]
- 15.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature 1985; 314:537–9. [DOI] [PubMed] [Google Scholar]
- 16.Abbas AK, Lichtman AH, Pillai S. Basic immunology: functions and disorders of the immune system. 4th ed Philadelphia: Elsevier Saunders, 2014:320. [Google Scholar]
- 17.Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe 2013; 13:250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez A, Tjarnlund A, Ivanji J et al. Role of IgA in the defense against respiratory infections IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine 2005; 23:2565–72. [DOI] [PubMed] [Google Scholar]
- 19.Manivannan S, Rao NV, Ramanathan VD. Role of complement activation and antibody in the interaction between Mycobacterium tuberculosis and human macrophages. Indian J Exp Biol 2012; 50:542–50. [PubMed] [Google Scholar]
- 20.Clemenceau B, Vivien R, Berthome M et al. Effector memory alphabeta T lymphocytes can express FcgammaRIIIa and mediate antibody-dependent cellular cytotoxicity. J Immunol 2008; 180:5327–34. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez N, Infante JF, Borrero R et al. Histopathological study of the lungs of mice receiving human secretory IgA and challenged with Mycobacterium tuberculosis. Malays J Med Sci 2014; 21:31–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Legesse M, Ameni G, Medhin G et al. IgA response to ESAT-6/CFP-10 and Rv2031 antigens varies in patients with culture-confirmed pulmonary tuberculosis, healthy Mycobacterium tuberculosis-infected and non-infected individuals in a tuberculosis endemic setting, Ethiopia. Scand J Immunol 2013; 78:266–74. [DOI] [PubMed] [Google Scholar]
- 23.McEwan WA, Tam JC, Watkinson RE, Bidgood SR, Mallery DL, James LC. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat Immunol 2013; 14:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabbatini P, Tsuji T, Ferran L et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res 2012; 18:6497–508. [DOI] [PubMed] [Google Scholar]
- 25.Morris D, Nguyen T, Kim J et al. An elucidation of neutrophil functions against Mycobacterium tuberculosis infection. Clin Dev Immunol 2013; 2013:959650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashenafi S, Aderaye G, Zewdie M et al. BCG-specific IgG-secreting peripheral plasmablasts as a potential biomarker of active tuberculosis in HIV negative and HIV positive patients. Thorax 2013; 68:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welch RJ, Lawless KM, Litwin CM. Antituberculosis IgG antibodies as a marker of active Mycobacterium tuberculosis disease. Clin Vaccine Immunol 2012; 19:522–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demkow U, Filewska M, Michalowska-Mitczuk D et al. Heterogeneity of antibody response to mycobacterial antigens in different clinical manifestations of pulmonary tuberculosis. J Physiol Pharmacol 2007; 58(suppl 5 pt 1):117–27. [PubMed] [Google Scholar]
- 29.Rahman S, Rehn A, Rahman J, Andersson J, Svensson M, Brighenti S. Pulmonary tuberculosis patients with a vitamin D deficiency demonstrate low local expression of the antimicrobial peptide LL-37 but enhanced FoxP3 regulatory T cells and IgG-secreting cells. Clin Immunol 2014; 156:85–97. [DOI] [PubMed] [Google Scholar]
- 30.Subbian S, Tsenova L, Yang G et al. Chronic pulmonary cavitary tuberculosis in rabbits: a failed host immune response. Open Biol 2011; 1:110016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussain R, Dockrell HM, Chiang TJ. Dominant recognition of a cross-reactive B-cell epitope in Mycobacterium leprae 10 K antigen by immunoglobulin G1 antibodies across the disease spectrum in leprosy. Immunology 1999; 96:620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Zeng Q, Ellis MK, Xiong T, Balen J, McManus DP. CD4+ T-cell counts, CD4+/CD8+ T-cell count ratios, and antibody levels in migrant fishermen infected with Schistosoma japonicum in the Dongting Lake, China. Am J Trop Med Hyg 2006; 75:910–3. [PubMed] [Google Scholar]
- 33.Torrado E, Fountain JJ, Robinson RT et al. Differential and site specific impact of B cells in the protective immune response to Mycobacterium tuberculosis in the mouse. PLoS One 2013; 8:e61681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis 2006; 10:1192–204. [PubMed] [Google Scholar]
- 35.Steingart KR, Henry M, Laal S et al. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med 2007; 4:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity 1998; 8:363–72. [DOI] [PubMed] [Google Scholar]
- 37.Verma RK, Jain A. Antibodies to mycobacterial antigens for diagnosis of tuberculosis. FEMS Immunol Med Microbiol 2007; 51:453–61. [DOI] [PubMed] [Google Scholar]
- 38.Bothamley GH. Serological diagnosis of tuberculosis. Eur Respir J Suppl 1995; 20:676s–88. [PubMed] [Google Scholar]
- 39.Maekura R, Okuda Y, Nakagawa M et al. Clinical evaluation of anti-tuberculous glycolipid immunoglobulin G antibody assay for rapid serodiagnosis of pulmonary tuberculosis. J Clin Microbiol 2001; 39:3603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raqib R, Kamal SM, Rahman MJ et al. Use of antibodies in lymphocyte secretions for detection of subclinical tuberculosis infection in asymptomatic contacts. Clin Diagn Lab Immunol 2004; 11:1022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raqib R, Mondal D, Karim MA et al. Detection of antibodies secreted from circulating Mycobacterium tuberculosis-specific plasma cells in the diagnosis of pediatric tuberculosis. Clin Vaccine Immunol 2009; 16:521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schierloh P, Landoni V, Balboa L et al. Human pleural B-cells regulate IFN-gamma production by local T-cells and NK cells in a Mycobacterium tuberculosis-induced delayed hypersensitivity reaction. Clin Sci (Lond) 2014; 127:391–403. [DOI] [PubMed] [Google Scholar]
- 43.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008; 28:639–50. [DOI] [PubMed] [Google Scholar]
- 44.Iwata Y, Matsushita T, Horikawa M et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011; 117:530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blair PA, Norena LY, Flores-Borja F et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010; 32:129–40. [DOI] [PubMed] [Google Scholar]
- 46.Flores-Borja F, Bosma A, Ng D et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med 2013; 5:173ra23. [DOI] [PubMed] [Google Scholar]
- 47.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol 2010; 125:1114–24.e8. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe R, Ishiura N, Nakashima H et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol 2010; 184:4801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang M, Zeng G, Yang Q et al. Anti-tuberculosis treatment enhances the production of IL-22 through reducing the frequencies of regulatory B cell. Tuberculosis 2014; 94:238–44. [DOI] [PubMed] [Google Scholar]
- 50.Pagan AJ, Ramakrishnan L. Immunity and immunopathology in the tuberculous granuloma. Cold Spring Harb Perspect Med 2014; doi:10.1101/cshperspect.a018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tully G, Kortsik C, Hohn H et al. Highly focused T cell responses in latent human pulmonary Mycobacterium tuberculosis infection. J Immunol 2005; 174:2174–84. [DOI] [PubMed] [Google Scholar]
- 52.Lee SH, Min JW, Lee CH et al. Impact of parenchymal tuberculosis sequelae on mediastinal lymph node staging in patients with lung cancer. J Korean Med Sci 2011; 26:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ochsenbein AF, Pinschewer DD, Sierro S, Horvath E, Hengartner H, Zinkernagel RM. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc Natl Acad Sci U S A 2000; 97:13263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paramithiotis E, Cooper MD. Memory B lymphocytes migrate to bone marrow in humans. Proc Natl Acad Sci U S A 1997; 94:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lausen BF, Hougs L, Schejbel L, Heilmann C, Barington T. Human memory B cells transferred by allogenic bone marrow transplantation contribute significantly to the antibody repertoire of the recipient. J Immunol 2004; 172:3305–18. [DOI] [PubMed] [Google Scholar]
- 56.Scheid JF, Mouquet H, Kofer J, Yurasov S, Nussenzweig MC, Wardemann H. Differential regulation of self-reactivity discriminates between IgG+ human circulating memory B cells and bone marrow plasma cells. Proc Natl Acad Sci U S A 2011; 108:18044–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lay JC, Peden DB, Alexis NE. Flow cytometry of sputum: assessing inflammation and immune response elements in the bronchial airways. Inhal Toxicol 2011; 23:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willis VC, Demoruelle MK, Derber LA et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum 2013; 65:2545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aanaes K, Johansen HK, Poulsen SS, Pressler T, Buchwald C, Hoiby N. Secretory IgA as a diagnostic tool for Pseudomonas aeruginosa respiratory colonization. J Cyst Fibros 2013; 12:81–7. [DOI] [PubMed] [Google Scholar]
- 60.Valentini D, Gaseitsiwe S, Maeurer M. Humoral ‘reactome’ profiles using peptide microarray chips. Trends Immunol 2010; 31:399–400. [DOI] [PubMed] [Google Scholar]
- 61.Ambati A, Poiret T, Svahn BM et al. Increased beta-haemolytic group A streptococcal M6 serotype and streptodornase B-specific cellular immune responses in Swedish narcolepsy cases. J Intern Med 2015; doi:10.1111/joim.12355. [DOI] [PubMed] [Google Scholar]
- 62.Aditya A, Valentini D, Montomoli E et al. H1N1 viral proteome peptide microarray predicts individuals at risk for H1N1 infection and segregates infection versus pandemrix -vaccination. Immunology 2015; 143:357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaseitsiwe S, Valentini D, Mahdavifar S et al. Pattern recognition in pulmonary tuberculosis defined by high content peptide microarray chip analysis representing 61 proteins from M. tuberculosis. PLoS One 2008; 3:e3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Britschgi M, Olin CE, Johns HT et al. Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer's disease. Proc Natl Acad Sci U S A 2009; 106:12145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: the intravenous IgG paradox. J Exp Med 2007; 204:11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aktas O, Waiczies S, Grieger U, Wendling U, Zschenderlein R, Zipp F. Polyspecific immunoglobulins (IVIg) suppress proliferation of human (auto)antigen-specific T cells without inducing apoptosis. J Neuroimmunol 2001; 114:160–7. [DOI] [PubMed] [Google Scholar]
- 67.Orange JS, Hossny EM, Weiler CR et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol 2006; 117(4 suppl):S525–53. [DOI] [PubMed] [Google Scholar]
- 68.Latov N, Chaudhry V, Koski CL et al. Use of intravenous gamma globulins in neuroimmunologic diseases. J Allergy Clin Immunol 2001; 108(4 suppl):S126–32. [DOI] [PubMed] [Google Scholar]
- 69.Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA 1992; 267:3315–6. [PubMed] [Google Scholar]
- 70.Intravenous immune globulin for the prevention of bacterial infections in children with symptomatic human immunodeficiency virus infection. The National Institute of Child Health and Human Development Intravenous Immunoglobulin Study Group. N Engl J Med 1991; 325:73–80. [DOI] [PubMed] [Google Scholar]
- 71.Winston DJ, Ho WG, Rasmussen LE et al. Use of intravenous immune globulin in patients receiving bone marrow transplants. J Clin Immunol 1982; 2(2 suppl):42S–7. [DOI] [PubMed] [Google Scholar]