Abstract
The lack of novel antimicrobial drugs in development for tuberculosis treatment has provided an impetus for the discovery of adjunctive host-directed therapies (HDTs). Several promising HDT candidates are being evaluated, but major advancement of tuberculosis HDTs will require understanding of the master or “core” cell signaling pathways that control intersecting immunologic and metabolic regulatory mechanisms, collectively described as “immunometabolism.” Core regulatory pathways conserved in all eukaryotic cells include poly (ADP-ribose) polymerases (PARPs), sirtuins, AMP-activated protein kinase (AMPK), and mechanistic target of rapamycin (mTOR) signaling. Critical interactions of these signaling pathways with each other and their roles as master regulators of immunometabolic functions will be addressed, as well as how Mycobacterium tuberculosis is already known to influence various other cell signaling pathways interacting with them. Knowledge of these essential mechanisms of cell function regulation has led to breakthrough targeted treatment advances for many diseases, most prominently in oncology. Leveraging these exciting advances in precision medicine for the development of innovative next-generation HDTs may lead to entirely new paradigms for treatment and prevention of tuberculosis and other infectious diseases.
Keywords: tuberculosis, host-directed therapy, precision medicine, immunometabolism, signaling pathways
Despite identification of many potential bacterial targets, the current development pipeline for antimicrobial drugs against Mycobacterium tuberculosis (Mtb) is meager. Very few drugs of new classes are likely to enter clinical evaluation within the foreseeable future. In response, tuberculosis therapeutic research now includes efforts to identify adjunctive host-directed therapies (HDTs), with a focus on drugs already approved or in clinical development for other diseases [1]. Two important factors must help to guide this new research. First, responses to infections are governed by essential core regulatory mechanisms that have been conserved in all eukaryotic cells throughout the course of evolution, including all immune cells. Second, immune cells of any lineage must be able to function well as a cell in general before they can be effective in host defense. When the core regulatory mechanisms of cellular metabolism and other functions are pathologically disrupted, all cells, including immune cells, experience stress and their functions are compromised. In immune cells, the core regulatory mechanisms for metabolic and immune functions broadly intersect. The overlap and interactions between metabolic and immune regulation has been termed “immunometabolism” [2].
Although cytokines and interferons are essential for host immunity, none alone can prevent or eradicate Mtb infection. Immune responses must be viewed in the context of key genetic/epigenetic programmed regulatory pathways that are being progressively uncovered by basic molecular biology research. These discoveries are being applied for the development of an amazing spectrum of new targeted therapeutics for many diseases, most notably in oncologic, autoimmune, and metabolic disorders. Although the connections between core immunometabolism regulation and Mtb have only begun to be established, many studies have documented modification of some cell signaling pathways by Mtb to facilitate its survival. Lack of knowledge of the interactions between Mtb infection and the central cellular regulation pathways operating in immune cells constitutes a major scientific gap. This review will provide a broad, but not nearly exhaustive, overview of core immunometabolism regulation, the known and probable connections with Mtb pathogenesis, and the many opportunities to leverage new interventions being developed for precision medicine treatment of diseases now known to result from dysfunction of these fundamental core control processes.
PREVIOUSLY STUDIED TUBERCULOSIS IMMUNE MECHANISMS WITH NEW HDT RESEARCH OPPORTUNITIES
MAPK Signaling and the RAS/RAF/MEK/ERK Cascade
Mitogen-activated protein kinases (MAPKs) regulate several cellular processes, including stress responses, apoptosis, autophagy, metabolism, inflammation, and immune cell development with nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) as a significant downstream activation target. Innate immune cells recognize pathogen-associated molecular pattern (PAMPs), through pattern recognition receptors (PRRs). These receptors signal through a variety of pathways with dual functions in regulation of inflammation/immunity and metabolism. PAMP-PRR interaction–initiated signaling pathways include MAPKs. The 14 human MAPKs include extracellular signal-regulated kinase (ERK), c-Jun N terminal kinase (JNK), and p38, serine/threonine kinases that regulate transcription factor activity and have regulatory cross-talk with several other pathways. MAPK signaling is a progressive cascade that begins with activation of MAP3K kinases (by upstream kinases, small GTPases, or PRR-related adaptors) activating MAP2K kinases that then activate MAPKs, which have many downstream substrates [3].
Mtb adversely affects immune cell regulatory and effector functions by interaction with many of these interconnected signaling nodes and pathways. Several well-characterized Mtb ligands modulate immune cells through PAMPs to facilitate survival in phagocytes [4]. The PRRs of innate immune cells recognize Mtb PAMPs, including lipoarabinomannan and lipoproteins, which modulate MAPK signaling for enhancing Mtb survival in several ways. For example, prolonged ERK signaling through Toll-like receptor (TLR) activation by an Mtb lipoprotein induces interleukin (IL)-10 production while suppressing IL-12 secretion and T-helper (Th) 1 cell activation [5]. Inhibitors for several members of the MAPK family and related cascades are now US Food and Drug Administration (FDA)-approved or in clinical evaluation for therapy of malignant, inflammatory, and hyperimmune diseases.
See Table 1 for a list of some potential candidate tuberculosis HDT drug classes and specific agents with various molecular targets.
Table 1.
Candidate Tuberculosis Host-Directed Therapeutic Agents
| Drug Class/Target | Drug Examples | Probable Therapeutic Mechanism |
|---|---|---|
| MAPK cascade inhibitors [4] RAF-B MEK ERK JNK |
Vemurafeniba, dabrafenib Trametiniba [6] SCH772984 CC-930 [7], sitagliptina |
Varies: anti-inflammatoryb/metabolic dysfunction – OR – interfering with tuberculosis pathogenic effect on signaling |
| Small GTPase inhibitors [8] Ras (RAF-MEK-ERK) Rho/ROCK [9] |
Tipifarnib [10], salirasib [11], fasudilc [12], statinsa [13], metformina [14] |
Same |
| Wnt inhibitors [15] | OMP-54F28 [16], tankyrase inhibitors [17], clofaziminea [18] | Same, but more complex |
| Protein kinase inhibitors Tyrosine kinase inhibitors [19, 20] c-abl, c-kit JAK/STAT VEGF EGFR Ser-thr kinase inhibitors SIK inhibitors |
Imatiniba [21, 22] and others Tofactiniba [23], ruxolitiniba Pazopaniba [24] Gefitiniba [25] Dasatiniba, bosutiniba [26] (approved as TKIs) |
Increase autophagy and myeloid cell mobilization Anti-inflammatory Normalize vasculature in granulomas to improve drug penetration Increase autophagy, anti-inflammatory Anti-inflammatory and decrease M2 polarization |
| AMPK activators [27] |
Metformina [28], AICAR [29], AZD-769662 Berberinea [30], resveratrola [31], acetylsalicylic acida |
Anti-inflammatory, increase autophagy, and improve DC, TH1 CD4 cell, and CD8 memory cell development |
| AMPA channel receptor blockers | Topiramatea [32], perampanela [33] | Anti-inflammatory |
| PARP inhibitors [34, 35] | NAD intermediates (NAMa, NRa, NMNa), tetracyclinesa, olapariba, many in development | Anti-inflammatory, increase autophagy, improve effector T-cell function, and inhibit Tregs |
| Sirtuins Activators [36] Inhibitors [37] |
Resveratrola [31], NAD intermediates, statinsa [38], metformina, berberinea [30], and many STACs in development Sirtinol, cambinol, tenovin, others |
Anti-inflammatory and increase autophagy Increase Th1/Treg ratio |
| PI3K-AKT-mTOR pathway inhibitors [39,40] Direct mTOR inhibitors [41, 42] |
Idelalisiba, afuresertib [43], perifosine [44], MK-2206 [45], GSK-609693, [46], triciribine [47] Sirolimusa, everolimusa, ridaforolimus |
Increase autophagy, decrease M2 polarization, and improve DC, Th1 CD4 cell, and CD8 memory cell development Same |
| PTEN activator | Resveratrola [48] | Increase autophagy and decrease M2 polarization |
| p53 activator | Nutlin 3A [49] | Increase autophagy and decrease M2 polarization |
| Autophagy inducers [50] | Imatiniba/other TKIs, metformina, statinsa, verapamila, selective serotonin reuptake inhibitorsa, carbamazepinea, sirolimusa | Increase autophagy: improve pathogen killing, clearance of proinflammatory organism components, and processing of antigenic material for T-cell presentation |
| Oxidative stress reduction agents [51] | Silymarina [52], Tanshinone [53] | Anti-inflammatory and improve macrophage functions, including autophagy |
| ERS/UPR reduction agents Inflammasome inhibitors [54] |
Phenylbutyratea [55], ursolic acida [56] Fasudilc [57], tauroursodeoxycholic acida [58] β-hydroxybutyratea [59], MCC950 [60], sitagliptina |
Anti-inflammatory and improve macrophage functions, including autophagy |
| LOX-1 and other scavenger receptor suppressors Angiotensin II receptor inhibitors [61] |
Ellagic acida [62], coenzyme Q10a [63] Docosahexaenoic acida [64], sitagliptina, statinsa [65], Tanshinone derivatives [66] Telmisartana [67], others |
Decrease M2 polarization/foam cell development, improve macrophage functions |
| Cathelicidin inducers [68] | Vitamin Da, phenylbuturatea, nicotinamidea, resveratrola, pterostilbenea | Induction of antimicrobial peptides, improve lipid metabolism, and decrease M2 polarization |
| Dipeptide dipeptiase-4 inhibitors | Sitagliptina [69], others | Anti-inflammatory/decrease inflammasomes, improve lipid metabolism and macrophage function, decrease M2 polarization, and preserve CXCL10 on effector T cells |
| Mevalonate metabolism inhibitors | Amino-bisphophonatesa, eg, zolandronate [70] | Enhance γδ T-cell activity and bridging between and innate and adaptive immunity |
| Highly pleiotropic agents | Metformina, statinsa, phenylbutyrate, Fasudilc, berberinea, sitagliptina | |
| Combinations | Fasudilc and statinsa (ROCK inhibition) [71] Vitamin Da and phenylbutyratea [72] (cathelicidin induction) Tipifarnib and statinsa [73] (RAS-ERK pathway inhibition) |
Bold text indicates that agent has been evaluated for potential tuberculosis host-directed therapeutic activity in a published study.
Abbreviations: AICAR, 5-Aminoimidazole-4-carboxamide ribonucleotide; AKT, serine/threonine protein kinase; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; AMPK, adenosine monophosphate-activated protein kinase; DC, dendritic cell; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; ERS, endoplasmic reticulum stress; JAK, Janus tyrosine kinase; JNK, c-Jun N-terminal kinase; LOX-1, lectin-like oxidized low-density lipoprotein receptor 1; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase; mTOR, mechanistic target of rapamycin; NAM, nicotinamide; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside; PARP, poly(ADP-ribose) polymerase; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatidylinositol-3, 4, 5,-trisphosphate 3-phosphatase; RAF, rapidly accelerated fibrosarcoma; RAS, Rat sarcoma protein; ROCK, Rho-associated coiled-coil containing kinase; SIK, salt-inducible kinase; STACs, sirtuin activating compounds; STAT, signal transducers and activators of transcription; TKI, tyrosine kinase inhibitor; UPR, unfolded protein response; VEGF, vascular endothelial growth factor.
a US Food and Drug Administration approved or available over the counter.
b Decreasing inflammatory reaction may allow improved drug and immune cell access to lesions; decrease tissue damage; possibly allow “wake and whack” strategies to improve antimicrobial response by allowing activation of nonreplicating bacilli with low metabolism levels.
c Approved in other countries with stringent regulatory authorities.
Small Molecule GTPase Superfamily
Small GTPases include the highly complex Ras, Rho, Rab, Ran, and ARF (ADP ribosylation factor) superfamilies. The Ras superfamily in particular has extensive crosstalk with MAPKs to regulate immunometabolic functions [74]. Mtb infection inhibits some GTPases and activates others. Mtb nucleoside diphosphate kinase binds to and inactivates the small GTPase Rac1 (a Rho kinase) in the macrophage, causing a defect of both NOX2 assembly and antimicrobial reactive oxygen species (ROS) production [75]. Some Rho GTPases activate Rho-associated coiled-coil–containing kinase (ROCK) as a downstream effector to regulate several different metabolic and immune functions [76]. Overactive Rho/ROCK signaling occurs in pathologic inflammatory conditions, including postischemic damage and diabetic complications. The ROCK-specific inhibitor, fasudil, is approved in some countries for treatment of vasospasm and decreasing stroke damage [9]. Alone, or in combination with rosuvastatin, fasudil can decrease inflammation by reducing downstream NF-κB signaling and tissue damage, for example, in animal models of cerebral ischemia [71].
During mycobacterial infection, Rheb (Ras family kinase) inhibits autophagy, and host cells attempt to utilize microRNA-155 to reverse this inhibition and limit bacterial growth [77]. Mevalonate pathway metabolism can upregulate the activity of Ras small GTPases through farnesylation. Some Ras members are involved with upregulation of inflammation caused by various etiologies, including in a rheumatoid arthritis model based on heat-killed Mtb. In this model, the Ras inhibitor farnesylthiosalicylic acid had significant anti-inflammatory activity [78]. Tipifarnib, another drug that disrupts Ras farnesylation, suppresses Ras/ERK signaling and NF-kB induction with synergistic effects in combination with simvastatin [73]. Selective targeting of GTPases has been suggested for HDT against Mtb, but this approach has not yet been studied.
Wnt Signaling Pathway
Signaling by Wnt (19 known ligands) and their 10 different G-coupled Frizzled (Fz) receptors has pivotal roles in immune responses to many pathogens [79] and is often induced through TLR/NF-κB signaling. Downstream Wnt signaling is either canonical (through β-catenin) or noncanonical (through Ca2+, JNK, or Rho GTPase signaling). Wnt signaling regulates several aspects of immunity, including lymphocyte development, dendritic cell (DC) differentiation, and cytokine production and has extensive interactions with other major signaling families, including PRR-initiated pathways [80]. Pro- or anti-inflammatory effects may result, depending on interaction of particular combinations of the many possible Wnt and Fz ligand–receptor pairings.
The highly complex role of Wnt signaling in tuberculosis has begun to be explored [81–83]. However, further research is needed to understand the specific effects of different Wnt signaling components at different stages of Mtb infection. Therapeutic use of Wnt pathway inhibitors has been slow because of toxicity issues, but new drug classes may prove to have therapeutic benefit [15]. Interestingly, clofazimine inhibits canonical Wnt signaling in vitro, has several immunomodulatory effects observed in clinical use, and is in evaluation for treatment of Wnt-dependent cancers [18].
PROMISING INNOVATIVE TUBERCULOSIS HDT AGENTS IN PRECLINICAL STUDIES
Protein Kinase Signaling Inhibition: Imatinib
Tyrosine kinases (TKs), both receptor and nonreceptor, are involved in signaling pathways controlling most cellular processes. In many cancers, TKs are dysregulated and targeted by new-generation anticancer drugs [19]. Imatinib targets Abl kinase and effectively treats chronic myelogenous leukemia. In a murine macrophage cell line, imatinib reduced Mtb intracellular survival and bacterial loads with no direct effect on Mtb [21]. Mechanisms of action are not proven, but likely involve enhancing autophagy and phagosomal acidification [84, 85]. Imatinib also boosts the number of mobilized myeloid progenitor cells in a murine tuberculosis model [22], possibly by inhibition of c-kit or other TKs, resulting in “emergency hematopoiesis.” Imatinib is currently being evaluated as a treatment adjuvant in a rhesus macaque tuberculosis model.
Other TKs possibly involved with tuberculosis pathogenesis include Janus tyrosine kinase (JAK)/signal transducers and activators of transcription (STAT) [23], vascular endothelial growth factor receptor (VEGFR) [24, 86], and epidermal growth factor receptor (EGFR) [25]. Inhibitors continue to be developed for these and many other classes of kinases, and several are now available for clinical use [19, 20]. Other kinase families are involved in immune/inflammatory and/or angiogenesis regulation. For example, inhibition of salt-inducible kinases enhances immune functions of macrophages and DCs in vitro [87].
AMP-Activated Protein Kinase Activators
Adenosine monophosphate (AMP)–activated protein kinase (AMPK) senses low cellular adenosine triphosphate (ATP) levels and initiates signaling to increase ATP by decreasing anabolism and inducing catabolism [88]. The AMPK/peroxisome proliferator–activated receptor γ coactivator-1alpha (PGC-1α) pathway is a key mechanism of antimicrobial defense by activating autophagy and can also reduce inflammation [29]. AMPK intersects with several signaling pathways, including blocking ERK activation and inhibiting the Ras family GTPase Rheb and its downstream signaling partner mechanistic target of rapamycin (mTOR) complex 1 (mTORC1), an autophagy inhibitor [88]. Metformin (MET) has been a useful type 2 diabetes mellitus (T2DM) treatment for 50 years and is in extensive clinical evaluation for cancer and cardiovascular disease [89]. MET both directly and indirectly increases AMPK activity. MET also has AMPK-independent effects that enhance functions of many immune cell types, including macrophages [90].
AMPK regulates effector T-cell differentiation during responses to infections by control of a glucose-sensitive metabolic immune checkpoint to maintain cell energy levels and viability through modulating metabolism. T cells lacking AMPK displayed reduced mitochondrial bioenergetics and cellular ATP production in response to pathogenic challenge in vivo. AMPK is essential for Th1 and Th17 cell development and effective primary T-cell responses to viral and bacterial infections in vivo [91]. Also, AMPK monitors energy stress related to glucose levels and facilitates CD8 T-cell memory development by controlling the transition of metabolically active (primarily utilizing glycolysis) effector CD8 T cells to metabolically (primarily lipid oxidation) quiescent memory T cells during the contraction phase of the immune response [92].
In Mtb-infected mouse models, increasing AMPK activity by MET and other agents improves autophagy and mitochondrial function and decreases Mtb growth [28, 29]. Singhal et al demonstrated that this effect may be mediated by increased macrophage production of ROS upon mitochondrial recruitment to phagosomes to kill intracellular bacteria [28]. MET also suppressed inflammation by an AMPK-dependent mechanism with decreased pulmonary damage. Several new AMPK pathway signaling activators are in development.
Statins: Pleiotropic HDT Effects
Statins inhibit 3-hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) reductase to modify cholesterol levels and have well-known anti-inflammatory and oxidative stress reduction effects independent of lipid alterations [93]. Statins are highly pleiotropic, causing a wide spectrum of effects on immune cells, including suppressing Rho/ROCK and other Ras GTPase pathways to decrease vascular inflammation [13, 94], improve dysregulated macrophage lipid metabolism, downregulate matrix metalloproteinases (MMPs), and enhance autophagy [95]. Simvastatin added to a standard tuberculosis regimen in a murine model significantly enhanced bacillary killing in lung tissue [96]. Choices of an optimal statin and its dosing regimen for clinical evaluation remain to be established.
LEVERAGING INNOVATIVE ADVANCES IN PRECISION MEDICINE FOR TUBERCULOSIS HDT BASED ON TARGETING CORE REGULATORY PATHWAYS
The “NAD World” Concept
Nicotinamide adenine dinucleotide (NAD) and related metabolites have essential roles in regulation of a vast range of critical cell functions [97, 98]. The ratio between the oxidized (NAD+) and reduced (NADH) forms is a critical regulator of energy physiology for all eukaryotic cells. Additionally, NAD+ is a substrate for enzymes that utilize the molecule to either catalyze covalent modifications of target proteins and/or convert NAD+ into active signaling metabolites. These enzymatic processes, more than redox mechanisms, can quickly deplete cellular NAD+ levels. Knowledge of NAD biology has played a critical role in the recent advances in understanding the pathogenesis of and in developing innovative targeted therapeutics for many diseases.
Three core cell regulatory enzyme families utilize NAD+. Membrane-bound CD38 (ADP-ribose cyclase/NAD glycohydrolase) hydrolyzes NAD+ into cyclic ADP-ribose (cADPR). cADPR triggers calcium mobilization (via transient receptor potential cation channel, subfamily M, member 2 [TRPM2] and other channels), leading to immune cell activation and proliferation and is involved with many immune and inflammatory processes [99]. Inhibitors of CD38 enzymatic activity are in preclinical evaluation [100]. The other two enzymes, poly-(ADP-ribosyl) polymerases (PARPs) and sirtuins, will be a focus of this review due to their connections with many essential regulatory signaling pathways and available therapeutic interventions.
ADP-Ribosylation: Poly-(ADP-Ribose) Polymerases
All eukaryotic cells have PARPs or equivalent enzymes that form polymers of ADP-ribose (PAR) from NAD+ that are attached to a substrate protein [98, 101]. The mammalian PARP family includes 17 members (Table 2); the most thoroughly studied are PARP1 and PARP2 [102]. Despite the nomenclature, only 6 members synthesize PAR. PAR chains are typically composed of up to 200 linked ADP-ribose units with extensive branching coupled onto target proteins. Five other members add only a single ADP-ribose onto a targeted protein [103]. Chromosome structure/chromatin modification, epigenetic gene expression regulation, RNA processing, telomere maintenance, cell differentiation, aging, and cell cycle control are among the many key functions of PARP family members.
Table 2.
Location and Function of the Poly-(ADP-Ribosyl) Polymerase and Sirtuin Families
| Name | Enzymatic Activity | Cellular Location | Biologic Function Examples |
|---|---|---|---|
| PARP1 | Poly(ADP-ribosyl)transferase | Nuclear | DNA repair, inflammation, metabolic regulation antiviral, cell differentiation and death |
| PARP2 | Poly(ADP-ribosyl)transferase | Nuclear/cytoplasmic | DNA repair, inflammation, metabolic regulation |
| PARP3 | Mono(ADP-ribosyl)transferase | Nuclear/cytoplasmic | Cell cycle regulation, DNA repair |
| PARP4 | Poly(ADP-ribosyl)transferase | Nuclear/cytoplasmic | Cellular defense to toxins, tumorigenesis |
| PARP5a tankyrase 1 | Poly(ADP-ribosyl) transferase | Cytoplasmic/stress granules | Antiviral, inflammation, metabolic regulation, telomere maintenance |
| PARP5b tankyrase 2 | Poly(ADP-ribosyl)transferase | Cytoplasmic | Inflammation, metabolic regulation, Telomere maintenance |
| PARP6 | Unknown | Cytoplasmic | Cell proliferation, DNA repair |
| PARP7 | Unknown | Unknown | Antiviral, cytosolic RNA processing |
| PARP8 | Unknown | Unknown | Unknown |
| PARP9 | Inactive | Nuclear/cytoplasmic | Cell migration |
| PARP10 | Mono(ADP-ribosyl)transferase | Cytoplasmic | Antiviral, cell proliferation, cytosolic RNA processing |
| PARP11 | Unknown | Unknown | Unknown |
| PARP12 | Mono(ADP-ribosyl)transferase | Cytoplasmic/golgi/stress granules | Antiviral, cytosolic RNA processing |
| PARP13 | Inactive | Cytoplasmic/stress granules | Antiviral, cytosolic RNA processing |
| PARP14 | Mono(ADP-ribosyl)transferase | Nuclear/cytoplasmic/stress Granules | Nuclear RNA processing, inflammation, metabolic regulation |
| PARP15 | Unknown | Stress granules | Cytosolic RNA processing |
| PARP16 | Mono(ADP-ribosyl)transferase | Cytoplasmic | Unfolded protein response |
| SIRT1 | Deacetylase | Nuclear/cytoplasmic | Metabolic regulation, anti-inflammatory, Stress response, cell senescence |
| SIRT2 | Deacetylase | Cytoplasmic | Cell cycle regulation |
| SIRT3 | Deacetylase | Mitochondrial | Mitochondrial metabolism and respiration |
| SIRT4 | Mono(ADP-ribosyl)transferase /lipoamidase | Mitochondrial | Metabolic regulation |
| SIRT5 | Deacetylase | Mitochondrial | Metabolic regulation |
| SIRT6 | Mono(ADP-ribosyl)transferase /deacetylase | Nuclear | Metabolic regulation, DNA repair/PARP1 activation |
| SIRT7 | Deacetylase | Nucleolar | Cellular homeostasis regulation, stress response, epigenomic maintenance |
Abbreviations: PARP, poly(ADP-ribose) polymerase; SIRT, sirtuin.
LPS induces PARP1 activity through MAPK signaling, causing an increase in inflammatory mediators, including tumor necrosis factor alpha (TNF-α), IL-1β, IL-6, interferon gamma (IFN-γ), inducible nitric oxide synthase, MMPs, and adhesion and chemotaxis molecules [104], largely driven through NF-κB signaling. PARP1 and ERK stimulate each other in a positive feedback cycle during responses to stress inducers [105]. Increased PARP1 activity occurs in a variety of pathologies including infections, diabetes, cancer, and neurodegenerative conditions and increases inflammation and suppresses autophagy [106–110]. PARP activity can also become dysregulated in stressful conditions caused by buildup of free radicals/oxidative stress and misfolded proteins, triggering endoplasmic reticulum stress. PARP activation increases expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor triggering ion channels in many cell types [111], and in a macrophage cell line, AMPA receptor activity increases TNF-α levels and generation of ROS [112].
PARPs are known to be involved in regulation of host defenses and in pathogenic mechanisms of several infectious organisms. Some pathogens activate PARP1 to modulate host cell signaling during infection to their advantage. For example, Helicobacter pylori induces intracellular PAR production during infection of gastric epithelial cells, playing a role in H. pylori–mediated chronic inflammation and disease [108]. Trypanosoma cruzi induces the ROS-PARP1-RelA pathway for upregulation of cytokine expression in cardiomyocytes, resulting in sustained inflammation [113]. Mycoplasma fermentans inhibits host DNA topoisomerase I by activation of PARP1 via the induction of MAPK signaling [114]. In contrast, Chlamydia trachomatis causes degradation of PARP1, most likely to downregulate inflammation [115]. Several PARPs have key roles in antiviral defenses by inhibiting transcription and translation [116, 117].
A connection between Mtb and PARPs has yet to be established, but Mtb is known to modulate MAPK activity and other pathways regulated by or regulating PARPs. One potential link is the recent report that Mtb produces tuberculosis necrotizing toxin, a NAD+ glycohydrolase, depletes host cellular NAD+ levels, and causes macrophage cell death [118]. Production of a NAD+ glycohydrolase by Streptococcus pyogenes causes death of infected cells primarily by NAD/ATP depletion. However, an early burst of PARP1 activity causes PARylation leading to release of the damage-associated molecular pattern molecule, high mobility group box 1 protein (HMGB1) from the nucleus that contributes to cell death. This release of HMGB1 appears to be dependent on PARP1 activity [119]. HMGB1 is released during experimental tuberculosis [120]. One direct connection of these pathways with mycobacterial infection is a recent finding that Wnt/β-catenin signaling can inhibit Bacillus Calmette-Guerin (BCG)-induced macrophage necrosis by increasing the production of glutathione to scavenge ROS in part through repression of PARP1/AIF signaling [121]. Also, PARP1 regulates functions of many types of immune cells, including DCs, macrophages, and T and B lymphocytes and influences Th1 and Th2 differentiation [122]. PARPs downregulate Treg cells and function [123].
PARP Inhibitors
PARP inhibitors (including NAD+ intermediates, nicotinamide [NAM], nicotinamide riboside [NR], and nicotinamide mononucleotide [NMN]) can decrease lipopolysaccharide (LPS)–induced inflammation and are being studied for treatment of many inflammatory diseases [34, 35]. Targeted PARP inhibition with newer drugs has been successful in cancer treatment, and the FDA has approved olaparib for BRCA-mutated advanced-stage ovarian cancers [124]. The PARP inhibitor 3-aminobenzamide attenuated progression of heat-killed Mtb adjuvant-induced arthritis in a mouse model [125]. Pyrazinamide's (PZA) structure is similar to NAM, and PZA may possibly be a PARP inhibitor.
Sirtuins
The other posttranslational protein modification requiring NAD+ is deacetylation by sirtuins (SIRTs). SIRTs have similarities with histone deacetylases (HDACs), but SIRTs also have many nonhistone substrates, are not inhibited by drugs targeting HDACs 1, 2, and 4, and their activity levels are highly NAD+ dependent [98, 126]. SIRTs are also activated by cyclic adenosine monophosphate (cAMP)/PKA-AMPK signaling independent of control by NAD+ levels to deacetylate some substrates [127]. Seven human SIRTs have been identified, distinguished by their subcellular locations and deacylation targets [128]. Some SIRTs have additional nondeacetylase activities (Table 2). SIRT1 is the most studied and has a major role in regulating transcription. Histone deacetylation by SIRT1 leads to increased compaction of chromatin and decreased gene transcription. Also, SIRT1 represses transcription on a continuing basis by recruitment of nuclear enzymes involved in histone methylation and DNA methylation, suggesting a broad role for SIRT1 in epigenetic gene regulation [129].
SIRT1 also deacetylates a broad range of transcription factors to regulate target gene expression both positively and negatively. The promoters for several transcription factors of genes involved in metabolism, inflammation, and oxidative stress, including NF-kB, FOXO1, p53, COX-2, and hypoxia-inducible factor (HIF) 1α, are SIRT1 substrates, making SIRT1 signaling a vital linkage between energy availability and innate immunity [128, 130]. In general, activation of SIRT1 results in reduction of cell stress, inflammation, apoptosis, and rate of senescence [131]. Disruption of the antagonistic SIRT1 interactions with NF-kB causes increasing severity of inflammatory and metabolic disorders, including increased risk of diabetes, atherosclerosis, and aging-related diseases [132, 133]. SIRT1 activation upregulates expression of phosphatidylinositol-3, 4, 5,-trisphosphate 3-phosphatase (PTEN), an antagonist of phosphatidylinositol 3-kinase (PI3K)–serine/threonine protein kinase (AKT)–mTOR signaling to enhance autophagy [48].
SIRT1 regulates immune cell differentiation by multiple mechanisms, including shifting metabolic activity from glycolysis to fatty acid oxidation in monocytes progressing from the hyperinflammatory stage to the hypoinflammatory stage of sepsis [134, 135]. SIRT1 switches the cell energy source by enhancing downstream peroxisome proliferator-activated receptor gamma (PPAR-γ) activity through deacetylating PGC-1α. SIRT1 is a key downregulator of the IL-12/IL-23 balance in human DCs [136] and also maintains T-cell immune tolerance [137].
The roles of the SIRT family in Mtb infection remain unexplored. However, SIRTs are involved with host defenses against pathogens, including as evolutionarily conserved broad-spectrum antiviral host factors [138]. In contrast, SIRT activity may be modified by pathogens to achieve a survival advantage. Human immunodeficiency virus (HIV) Tat inhibits SIRT1 deacetylase activity resulting in hyperactivation of NF-kB, causing chronic activation of infected cells [139]. Herpes simplex virus type 1 modulates the AMPK/SIRT1 axis differentially during the course of infection [140]. During Listeria monocytogenes infection, SIRT2 plays a critical role in an epigenetic mechanism to reprogram host responses to enhance infection by deacetylating a specific histone locus in the presence of bacterial factor InlB [141]. Leishmania infantum hijacks the SIRT1-AMPK axis to switch macrophage mitochondrial metabolism from glycolytic metabolism to oxidative phosphorylation crucial for parasite survival in vitro and in vivo [142].
Sirtuin Modulators
To take advantage of SIRT's protective effects against inflammation, oxidative stress, and degenerative diseases observed in a wide range of animal models of diseases, many pharmacologic activators have been developed [36]. Resveratrol is a natural polyphenol being studied for treatment of several disorders involving dysregulated metabolism and inflammation/immunity, degenerative diseases, and malignancies. Resveratrol has many mechanisms of action including SIRT1 activation, and the relative importance of each mechanism is unclear [31]. Resveratrol induces cAMP signaling by suppressing cAMP phosphodiesterase to modulate inflammation and further activate SIRT1 [143]. To target SIRT directly, many SIRT activating compounds (STACs) are in development [36]. One, SRT1720, decreased the severity of pulmonary, renal, metabolic, and cardiovascular diseases in animal models [144]. Simvastatin attenuates TNF-α–induced apoptosis via the upregulation of SIRT1 [38]. Overall, therapeutically increased SIRT activity has been associated with improved health and a longer lifespan in several experimental models.
SIRT1 inhibition can improve T-cell–mediated antimicrobial function by enhancing HIF1α activity in DCs to decrease transforming growth factor beta (TGF-β) expression and increase DC-derived IL-12 and Th1/T-regulatory cell balance [130] and increase inflammatory microbial responses. SIRT1 inhibition could potentially be useful when enhanced immunity is essential (eg, very early during infection) in persons with suboptimal defensive responses, and possibly for improving vaccine effectiveness. Several SIRT inhibitors are in clinical trials. Conversely, drug stimulation of SIRT1 activity might be useful to reduce tissue damage during the later stage of infections or with a hyperinflammatory response.
PARP-SIRT-AMPK Interactions
SIRT1 and PARP1 have extensive regulatory crosstalk [98]. Both require NAD+ to function, but PARP1 binds with a higher affinity and can deplete NAD+ when highly active, suppressing SIRT1 activity. Also, intermediates and enzymes of the NAD+ salvage pathway contribute to regulating PARP and SIRT activity by inhibiting PARP1 while boosting SIRT1 [145, 146]. To counteract PARP1 suppression, SIRT1 interacts with and deacetylates PARP1 to inhibit PARP1 activity and maintain cellular NAD+ levels and its own activity. In contrast, SIRT6 increases PARP1 activity by mono-ADP-ribosylation to promote DNA repair under stress [147]. Notably, PZA directly inhibits SIRT6 activity [148].
AMPK can cross-regulate SIRT and PARP activity. PARP activation depletes NAD+ and ATP levels. In response, AMPK is activated and induces autophagy, preventing PARP-induced necrotic cell death. AMPK also phosphorylates PARP1, causing PARP1 disassociation from intron binding sites of several genes [149]. SIRT1 and AMPK are cross-activating signaling partners [150]. SIRT1 is required for AMPK activation through deacetylation of liver kinase B1 (LKB1). Activated AMPK increases NAD+ levels, enhancing SIRT activity.
PI3K-AKT-mTOR Pathway
In human cell-based screening systems, inhibition of the kinase AKT (within the PI3K-AKT-mTORC1 pathway) significantly decreased growth of Mtb [151]. This key pathway also mediates polarization of monocytes to M2 macrophages [152, 153], and selective inhibition of AKT/mTOR signaling promotes autophagy [154]. mTORC1 and AMPK have an antagonistic relationship with both able to regulate the other and having opposite functions in several cellular processes [155]. TLR4 signaling can induce HIF1α expression by activating MMP9 to cleave AMPK leading to mTORC1 activation [156]. Since Mtb is known to interact with TLR4 it may utilize this pathway to suppress AMPK activity in infected macrophages. PI3K-AKT-mTOR pathway activity also can interfere with DC, CD4, and CD8 T-cell maturation and development.
Several approaches are available to manipulate this pathway, including sirolimus inhibition of mTORC1. Sirolimus enhances Mtb killing in macrophages by increasing autophagy and possibly by other mechanisms [157]. Inhibition of mTORC1 has been used to improve vaccine effectiveness for many types of antigens in animal models [158], including one for BCG, with enhanced Th1 responses [159]. Inhibition of mTORC1 has improved generation of antigen-specific memory CD8+ T cells with vaccinations or viral infections during both the expansion and contraction phases of response in animals. These cells had higher proliferation, improved function, and increased longevity. Many newer mTOR inhibitors are in development. Combined PI3K/mTOR inhibitors are now in clinical evaluation and may be more effective than sirolimus [160].
PTEN and p53 are cooperating tumor suppressor proteins that have key roles in macrophage polarization [161, 162] and enhancing autophagy [163]. PTEN is a phosphatase that antagonizes AKT/mTOR signaling, has regulatory roles in innate immune cell activation [164], inhibits BCG infection of several cell lines [165], and is induced by resveratrol [48]. AMPK stimulates PTEN to negatively regulate inflammation [166]. p53 expression is downregulated in BCG-infected cell lines [167]. p53 expression activators are now in clinical evaluation for cancer treatment [49].
See Figures 1 and 2 for interactions and outcomes of activation of these core signaling pathways.
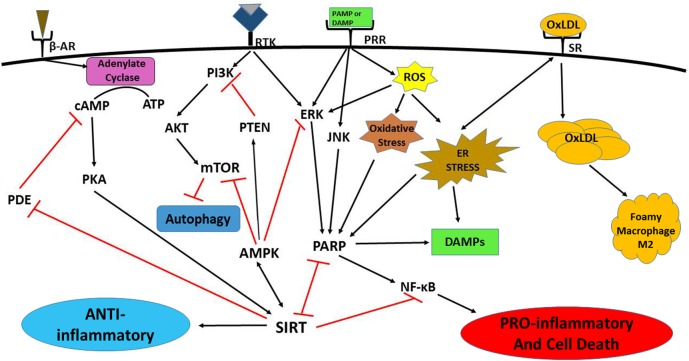
Figure 1.
Signaling pathways of immunometabolism and its dysregulation. Growth receptors (including receptor tyrosine kinases) and pattern recognition receptor (PRRs) induce mechanistic target of rapamycin (mTOR) and poly(ADP-ribose) polymerase (PARP) activation through phosphatidylinositol 3-kinase (PI3K)/serine-threonine protein kinase (AKT) and c-Jun N-terminal kinase (JNK)/extracellular signal-regulated kinase (ERK) signaling, respectively, to stimulate inflammation, largely by upregulation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB). Phosphatidylinositol-3, 4, 5,-trisphosphate 3-phosphatase (PTEN) directly inhibits PI3K activation. Sirtuin (SIRT) and AMP-activated protein kinase (AMPK) cross-activate each other. SIRT dampens inflammation by blocking PARP directly. SIRT signaling also downregulates NF-kB activation. AMPK inhibits PARP through suppression of ERK signaling. AMPK stimulates PTEN, blocks mTOR and induces autophagy. Increased reactive oxygen species (ROS)/oxidative cellular stress induces ERK signaling and PARP activation, and endoplasmic reticulum stress (ERS) increases PARP activation. Both types of stress lead to inflammation, cell damage, and death, and damage-associated molecular pattern (DAMP) molecule release. DAMPs further increase these signaling patterns, resulting in a vicious cycle of progressive inflammation and cell death. The stress responses also lead to increased uptake of oxidized low-density lipoprotein (ox-LDL) in macrophages via scavenger receptors. Increased ox-LDL causes lipid droplet formation that may lead to foam cell development. Foamy macrophages are most often M2 polarized, producing a hypoinflammatory response and increasing susceptibility to Mycobacterium tuberculosis infection. β-Adrenergic and some G-protein–coupled receptors can activate adenylate cyclase that in turn increases protein kinase A (PKA) and SIRT activities. SIRT activation can also inhibit cyclic adenosine monophosphate (cAMP) phosphodiesterase (PDE).
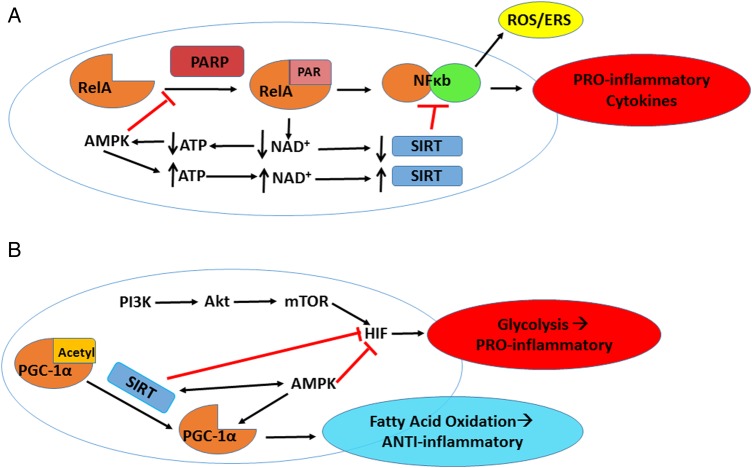
Figure 2.
Poly(ADP-ribose) polymerase (PARP) and sirtuin (SIRT) effects on immunometabolism. A, PARP “PARylates” many substrates including RelA for activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in an oxidized nicotinamide adenine dinucleotide (NAD+)–dependent manner, leading to elevated reactive oxygen species (ROS)–oxidative stress/endoplasmic reticulum stress (ERS) and proinflammatory cytokine production. PARP hyperactivity also causes chronic inflammation by depleting NAD+ that subsequently decreases SIRT activity and lowers adenosine triphosphate (ATP) concentrations. SIRT can counteract NF-κB activation through deacetylation of the RelA/p65 subunit of NF-kB. Depleted ATP activates AMP-activated protein kinase (AMPK), which in turn increases ATP and NAD+ levels, leading to increased SIRT activity and suppressed PARP activity that may bring the cell back to equilibrium. B, SIRT regulates immune cell metabolic activity largely through regulation of hypoxia-inducible factor (HIF1α) and peroxisome proliferator–activated receptor γ coactivator (PGC-1α). Increased phosphatidylinositol 3-kinase (PI3K)/serine-threonine protein kinase (AKT)/mechanistic target of rapamycin (mTOR) signaling activates HIF to convert cell energy production mechanism to aerobic glycolysis during immune activation enhancing T-helper 1 cell differentiation. SIRT, along with AMPK, converts T-cell energy metabolism to fatty acid oxidation by blocking HIF activity and activating PGC-1α to enhance differentiation toward memory T cells and T-regulatory cells.
Autophagy
Xenophagy is the form of autophagy that disposes of foreign materials, enhances efficiency of pathogen killing, clears proinflammatory organism components, and processes antigenic material for T-cell presentation. The relevance of xenophagy in host immunity to Mtb is well established [168]. However, the metabolic sensors and signaling pathways that induce autophagy and the optimal pharmacological intervention targets for infections are not fully characterized. Many of the signaling pathways discussed in this review modulate autophagy, including AMPK and mTOR as focal points [169]. For example, glycogen synthase kinase 3-β (GSK3-β), in tandem with AMPK, inhibit mTORC1. Canonical Wnt signaling blocks GSK3-β activity leading to increased mTORC1 signaling and decreased autophagy [155].
SIRT1 may influence autophagy by deacetylation of key components of its induction network, including autophagy-related proteins 5, 7, and 8. SIRT1 also induces expression of autophagy components through activation of FoxO family transcription factors [170]. Enhancement of autophagy to increase clearance of Mtb and decrease excess inflammation and cellular damage is an exciting new area for HDT research.
CELLULAR STRESS RESPONSES
Cell stress may be caused by harmful oxidants/oxidized molecules and misfolded proteins. Cells attempt to reverse these stresses by initiating inflammatory responses that further disrupt normal cell functions, and if unsuccessful, will cause apoptosis. Many pathologies, including diabetes, cancer, and infectious diseases, cause such stress responses that are primarily initiated by signaling pathways described in this review [171, 172].
Oxidative Stress and Mtb Infection
Reactive oxygen species are byproducts of energy metabolism and have an important role in signaling pathways and as antimicrobial effectors [173]. However, high levels of ROS lead to DNA, lipid, and protein damage that must be limited. Cells control activity of ROS-generating enzymes and normally produce sufficient antioxidants to limit damage [174]. Molecular damage caused by excess ROS results in cellular oxidative stress. Lipid peroxidation products are elevated and antioxidant levels are decreased in myeloid cells obtained from tuberculosis patients, resulting in oxidative stress [175, 176]. However, the full role of oxidative stress in Mtb pathogenesis and the effects of new agents to reverse oxidative stress have not been well studied.
Endoplasmic Reticulum Stress/Unfolded Protein Response and Mtb Infection
Newly synthesized proteins are transported to the endoplasmic reticulum (ER) for posttranslational modification, proper folding, and secretion to their ultimate location. Accumulation of misfolded proteins in the ER disrupts cell function and induces the unfolded protein response (UPR) [177]. UPR utilizes ER-localized transmembrane proteins to restrict protein synthesis and influx into the ER, while activating transcription of chaperone proteins to facilitate unfolded protein removal. If ER stress still progresses, C/EBP-homologous protein (CHOP) is activated to induce apoptosis. Mtb-induced ER stress (ERS) in infected granuloma macrophages correlates with increased apoptosis, and contributes to Mtb survival [178–182]. Testing of therapeutic agents known to reduce ERS for use as tuberculosis HDTs should be greatly expanded.
Cell stress induces many proinflammatory effectors, including NF-κB. Targeting NF-κB directly is limited by intrinsic pathway complexity, cross-talk with other pathways, and poor drug specificity. Efforts to improve NF-κB targeting are ongoing and may involve multimodal therapies [183]. Most NF-κB signaling inhibitors in development are IκB kinase ε (IKKε) inhibitors [184]. Inflammasomes are stress-induced protein complexes that form around intracellular Nod-like receptors and upregulate IL-1β and IL-18 production, leading to further inflammation [54]. Inflammasomes can be inhibited by drug therapy.
OTHER INNATE IMMUNE CELL TYPES AND MEVALONATE METABOLISM
The recent review by Bhatt et al clearly presents the pressing need to study key signaling pathways, master regulators, and cellular stresses/reactions in other immune cells besides myeloid to determine how they are affected by tuberculosis and investigate potential interventions [185]. For example, γδ T cells have been hypothesized to play important roles in host defense against Mtb infection as early infection detection sentinels bridging innate and adaptive immunity. Human Vg9Vd2 T cells are activated by prenyl pyrophosphates produced by mevalonate metabolism [186]. Drugs targeting the mevalonate pathway by inhibiting farnesyl pyrophosphate synthase cause upstream accumulation of the cognate antigen, isopentenyl pyrophosphate, and can rapidly activate Vg9Vd2 T cells [70]. Whether drugs enhancing γδ T-cell function by this mechanism may be effective for tuberculosis HDT is an unanswered question.
LIPID METABOLISM AND MTB INFECTION
Release of free radicals from stressed or damaged cells causes formation and release of oxidized low-density lipoproteins (ox-LDLs) and other oxidized lipids. Macrophages express several scavenger receptors, including CD36, lectin-type ox-LDL receptor 1 (LOX-1), and SR-1A, that transport ox-LDL into cells [187]. Scavenger receptor expression is regulated by Wnt, PPAR-γ, and SIRT signaling [188, 189] and induced by ERS [190]. Increased ox-LDL within macrophages causes accumulation of lipid droplets and development of foam cells. Foam cells often become polarized into M2 macrophages with suppressed IL-12 production and increased IL-10 expression, and are permissive for growth of mycobacteria. Also, a recent study in a cancer model demonstrated that ERS-induced accumulation of abnormal lipid bodies within DCs inhibits T-cell activity by interfering with antigen presentation and immunostimulatory activity, suggesting that targeting ER stress response may be a unique approach to enhance anticancer immunity [191]. Angiotensin II type 1 receptor activity also upregulates the expression of LOX-1 in several cell types [192] and initiates TLR4 signaling, resulting in oxidative stress [193]. CD36 and LOX-1 expression are elevated in Mtb animal models, whereas in CD36-deficient mice, tuberculosis infection is attenuated [194, 195]. Several agents are available to evaluate for reversal of these abnormalities.
VITAMIN D, PHENYLBUTYRATE, AND OTHER ANTIMICROBIAL PEPTIDE INDUCERS
Vitamin D plays an essential role in modulation of lipid metabolism abnormalities and related inflammation and has a prominent role in cellular immune function [196]. Activated vitamin D enhances expression of antimicrobial peptides [197, 198] including cathelicidin [199]. Clinical trials of adjunctive vitamin D in tuberculosis treatment have not shown consistent benefit [200]. However, effects of vitamin D in combination with other HDT drugs have only begun to be explored. Sodium phenylbutyrate (NaPB) is an FDA-approved agent for treatment of urea cycle disorders that is also an HDAC inhibitor increasing cathelicidin expression. The combination of vitamin D and NaPB results in additive enhancement of cathelicidin levels in cell lines [72]. A pilot clinical trial of this combination as adjunctive therapy for tuberculosis treatment demonstrated safety but no difference in 8-week culture conversion [201]. Determination of the most effective dose for NPB may need further study. NaPB also relieves ERS as a protein chaperone. Addition of resveratrol, pterostilbene, or nicotinamide to vitamin D causes synergistic induction of cathelicidin and other antimicrobial peptides in cell lines [68].
TUBERCULOSIS AND DIABETES MELLITUS: UNFORTUNATE PATHOGENIC SYNERGY
T2DM is both a metabolic and inflammatory disease with complications caused by many inputs, including high levels of PAMP-initiated signaling related to the formation of advanced glycation end products (AGEs) that are ligands for many PRRs, including receptors of AGE (RAGE) [202]. Other inputs include oxidative stress, ERS/UPR/inflammasome activity, dysregulated lipid metabolism, oxidized lipid accumulation/foam cell formation, M2 polarization, increased PARP activation, and decreased SIRT activity. Poorly controlled T2DM causes macrophages to produce more inflammatory cytokines and chemokines as part of the ongoing positive feedback loop of chronic “metaflammation” [203].
T2DM increases the likelihood of active tuberculosis disease, slower treatment response, and death [204, 205]. Macrophages from patients with T2DM have reduced phagocytosis and antimicrobial peptide production, and highly increased ROS and proinflammatory cytokine secretion. These altered innate immune responses also delay development of adaptive immunity responses with IFN-γ–producing T cells arriving at infection sites almost a week later in the presence of T2DM than without T2DM. After arrival, these T cells produce increased levels of Th1 and Th17 cytokines and are likely to cause increased pulmonary tissue damage. Several parallels exist between the pathogenic mechanisms of T2DM and Mtb, leading to ineffectiveness of macrophage and DC functions (eg, impaired chemotaxis, phagocytosis, autophagy, antigen processing), cell necrosis/apoptosis, and tissue damage. T2DM and tuberculosis have common and additive mechanisms that disrupt cell regulatory signaling that are very likely to play prominent roles in the worsened course of tuberculosis infection with T2DM.
Given these common features, interventions designed to prevent or decrease diabetic metabolic dysfunctions, pathogenic mechanisms, and resulting complications may also be useful for Mtb HDT with and without concurrent T2DM. These include metformin, other AMPK and SIRT1 activators, imatinib, and PARP and dipeptidyl peptidase-4 inhibitors (DPP-4I). DPP-4Is are in wide clinical use and potently inhibit inflammation in mononuclear cells obtained from patients with diabetes by downregulating NF-kB expression through TLR2/4 and JNK signaling [69], and decreasing inflammasome formation and foam cell development [206, 207]. DPP-4Is can enhance effector T-cell trafficking to improve antitumor immunity in mice by preserving the active form of the chemokine CXCL-10. Sitaglipitin inhibits posttranslational processing (cleavage) of CXCL-10 by DDP-4 that also produces an antagonistic derivative. This derivative binds to its receptor, CXCR3, but does not induce chemotaxsis [208].
CONCLUSIONS AND THE WAY FORWARD
A revolution based on advances in innovative targeted molecular interventions and precision medicine is rapidly progressing in other medical research areas with rich pipelines of potential interventions that must be leveraged if progress is to be accelerated for tuberculosis HDT research. In the face of increasing antimicrobial drug resistance, the movement for developing tuberculosis HDT is growing rapidly and overlaps with innovative new approaches to develop more effective tuberculosis vaccines. New approaches can be based on better understanding of the master/core controllers of all cells (including immune cells), how to strengthen host defense mechanisms, and how to reverse the disruptive effects, as is being done for other diseases. However, the roles of the master regulator pathways of cellular metabolism and immunity and their linkage (immunometabolism) in Mtb infection have barely been explored. Only two references were identified addressing the roles of PARPs or SIRTs in tuberculosis pathogenesis by a recent literature review [121, 209], and only a few have addressed the roles of AMPK and mTOR. Improving knowledge of the effects of cellular dysfunction in immune cells (eg, ERS and oxidative stress) caused by infections is also critically important for advancing tuberculosis HDT research.
Complexity of the myriads of cell signaling, metabolic, and immune effector interactions is a huge challenge, and some targeted interventions to modulate fundamental cell regulatory mechanisms may have unexpected adverse effects. Research must proceed to determine which HDT targets are most promising and how to best use the potential interventions to be effective and safe for improving tuberculosis therapy, as is being accomplished in oncology. Ongoing development of pathway inhibitory and activating agents that are highly specific for individual molecules—for example, active for only 1 of the PARPs or SIRTs (instead of acting on several of them)—will be important advances. Oncology trials indicate that many of the new agents that modulate key pathways are quite tolerable. Only short-term (8–12 weeks or less) use of these agents may be needed as adjuvants for tuberculosis treatment. Of course, even with short-term drug use, long-term safety is unknown and will remain a concern to be carefully addressed in all future HDT clinical research. Safety endpoints must include possible worsening of tissue damage/pulmonary function, tuberculosis treatment response, and HIV replication status.
Hundreds of drugs are approved or in various stages of development for modulation of regulatory signaling pathways, and more are being approved for clinical use at a rapid rate. Selected drugs from the relevant classes that are now approved or in clinical trials can be screened for activity to restrict Mtb growth in appropriate cell lines. To help address issues regarding feasibility of application in nations most impacted by tuberculosis and of patient drug tolerance and adherence, none of the agents listed in Table 1 are biologic, parenteral, or highly toxic traditional chemotherapeutic agents. Even if only a few of these new-generation HDT agent classes can be repurposed for clinical use against Mtb, the number of drugs in the tuberculosis therapeutic pipeline would be greatly expanded. Also, repurposing allows avoidance of the vast majority of drug development costs. As with antiretroviral therapy and some other essential/life-saving medicines, the initially high costs of these agents for use in developing nations will significantly decrease over time. Prices of these drugs drop most rapidly after generic versions can be manufactured in or imported by countries within the regions impacted most by tuberculosis. Such lower-priced generics now undergo review by the FDA for “tentative approval” to allow US government funding to be used for their purchase and distribution to low-income countries.
To quote the Director of the US National Institutes of Health, Dr Francis Collins, precision medicine interventions are “… drugs and antibodies designed to counter the influence of specific molecular drivers. Many targeted therapies have been (and are being) developed, and several have been shown to confer benefits, some of them spectacular” [210]. Many pathogens clearly produce specific molecular drivers to subvert the same core cellular regulatory mechanisms that are modulated by malignancies. Because precision medicine agents are aimed at fundamental immune cell regulatory mechanisms, they are likely to also be effective for treatment of HIV and many other pathogens. A wide spectrum of new knowledge, research tools, and candidate therapies await adaption for tuberculosis HDT development and should advance powerful and innovative approaches to fundamentally transform antimicrobial therapy.
Notes
Disclaimer. This paper was written by one of the authors as a National Institute of Allergy and Infectious Diseases (NIAID) employee, but the views expressed in this paper do not necessarily represent those of NIAID.
Financial support. This project was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), US Department of Health and Human Services (contract number HHSN272200800014C).
Supplement sponsorship. This article appears as part of the supplement “Advances in Tuberculosis Research: A Blueprint for Opportunities.” This article was sponsored by the Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflict of interest. Both authors: No reported conflicts.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol 2015; 15:255–63. [DOI] [PubMed] [Google Scholar]
- 2.Mathis D, Shoelson E. Immunometabolism: an emerging frontier. Nat Rev Immunol 2011; 11:81–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 2013; 13:679–92. [DOI] [PubMed] [Google Scholar]
- 4.Stamm CE, Collins AC, Shiloh MU. Sensing of Mycobacterium tuberculosis and consequences to both host and bacillus. Immunol Rev 2015; 264:204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson ET, Shukla S, Sweet DR, et al. TLR2-dependent ERK signaling in Mycobacterium tuberculosis-infected macrophages drives anti-inflammatory responses and inhibits Th1 polarization of responding T cells. Infect Immun 2015; 83:2242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372:30–9. [DOI] [PubMed] [Google Scholar]
- 7.Plantevin Krenitsky V, Nadolny L, Delgado M, et al. Discovery of CC-930, an orally active anti-fibrotic JNK inhibitor. Bioorg Med Chem Lett 2012; 22:1433–8. [DOI] [PubMed] [Google Scholar]
- 8.Samatar AA, Poulikakos PI. Targeting RAS-ERK signaling in cancer: promises and challenges. Nat Rev Drug Discov 2014; 13:928–42. [DOI] [PubMed] [Google Scholar]
- 9.Pan P, Shen M, Yu H, Li Y, Li D, Hou T. Advances in the development of Rho-associated protein kinase (ROCK) inhibitors. Drug Discov Today 2013; 18:1323–33. [DOI] [PubMed] [Google Scholar]
- 10.Stieglitz E, Ward AF, Gerbing RB, et al. Phase II/III trial of a pre-transplant farnesyl transferase inhibitor in juvenile myelomonocytic leukemia: a report from the children's oncology group. Pediatr Blood Cancer 2015; 62:629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badar T, Cortes JE, Ravandi F, et al. Phase I study of S-trans, trans-farnesylthiosalicylic acid (salirasib), a novel oral RAS inhibitor in patients with refractory hematologic malignancies. Clin Lymphoma Myeloma Leuk 2015; 15:433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J, Wei L. Rho kinases in cardiovascular physiology and pathophysiology: the effect of fasudil. J Cardiovasc Pharmacol 2013; 62:341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawada N, Liao JK. Rho/Rho-associated coiled-coil forming kinase pathway as therapeutic targets for statins in atherosclerosis. Antioxid Redox Signal 2014; 20:1251–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Huang C, Ceng C, Zhan H, Zheng D, Han W. Metformin enhances nitric oxide production and diminishes Rho kinase activity in rats with hyperlipidemia. Lipids Health Dis 2014; 13:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song L, Li Y, He B, Gong Y. Development of small molecules targeting the Wnt signaling pathway in cancer stem cells for the treatment of colorectal cancer. Clin Colorectal Cancer 2015; 14:133–45. [DOI] [PubMed] [Google Scholar]
- 16.Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther 2015; 146:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulak O, Chen H, Holohan B, et al. Disruption of Wnt/β-catenin signaling and telomeric shortening are inextricable consequences of tankyrase inhibition in human cells. Mol Cell Biol 2015; 35:2425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koval AV, Vlasov P, Shichkova P, et al. Anti-leprosy drug clofazimine inhibits growth of triple-negative breast cancer cells via inhibition of canonical Wnt signaling. Biochem Pharmacol 2014; 87:571–8. [DOI] [PubMed] [Google Scholar]
- 19.Rask-Anderson M, Zhang UJ, Fabbro D, Schiöth HB. Advances in kinase targeting: current clinical use and clinical trials. Trends Pharmacol Sci 2014; 35:604–20. [DOI] [PubMed] [Google Scholar]
- 20.Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci 2015; 36:422–39. [DOI] [PubMed] [Google Scholar]
- 21.Napier RJ, Rafi W, Cheruvu M, et al. Imatinib-sensitive tyrosine kinase regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe 2011; 10:475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napier RJ, Norris BA, Swimm A, et al. Low doses of imatinib induce myelopoiesis and enhance host anti-microbial immunity. PLoS Pathog 2015; 11:e1004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiga M, Ahidjo BA, Maiga MC, et al. Efficacy of adjunctive tofacitinib therapy in mouse models of tuberculosis. EBioMedicine 2015; doi:10.1016/j.ebiom.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oehlers SH, Cronan MR, Scott NR, et al. Interception of host angiogenic signaling limits mycobacterial growth. Nature 2014; 517:512–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanley SA, Barczak AK, Silvis MR, et al. Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth. PLoS Pathog 2014; 10:e1003946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozanne J, Prescott AR, Clark K. The clinically approved drugs dasatinib and bosutinib induce anti-inflammatory macrophages by inhibiting the salt-inducible kinases. Biochem J 2015; 465:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 2014; 7:241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singhal A, Jie L, Kumar P, et al. Metformin as adjunct antituberculosis therapy. Sci Trans Med 2014; 6:263ra159. [DOI] [PubMed] [Google Scholar]
- 29.Yang CS, Kim JJ, Lee HM, et al. The AMPK-PPARGC1A pathway is required for antimicrobial host defense through activation of autophagy. Autophagy 2014; 10:785–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang W, Chen L, Hatch GM. Berberine as a therapy for type 2 diabetes and its complications: from mechanism of action to clinical studies. Biochem Cell Biol 2014; 1:1–8. [DOI] [PubMed] [Google Scholar]
- 31.Inoue H, Nakata R. Resveratrol targets in inflammation. Endocr Metab Immune Disord Drug Targets 2015; 15:1–10. [DOI] [PubMed] [Google Scholar]
- 32.Chung SS. A review of the efficacy and safety of extended-release topiramate in the adjunctive treatment for refractory partial-onset seizures. Ther Adv Neurol Disord 2015; 8:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko D, Yang H, Williams B, Xing D, Laurenza A. Perampanel in the treatment of partial seizures: time to onset and duration of most common adverse events from pooled phase III and extension studies. Epilepsy Behav 2015; 48:45–52. [DOI] [PubMed] [Google Scholar]
- 34.Sonnenblick A, de Azambuja E, Azim HA, Jr, Piccart M. An update on PARP inhibitors-moving to the adjuvant setting. Nat Rev Clin Oncol 2015; 12:27–41. [DOI] [PubMed] [Google Scholar]
- 35.Banasik M, Stedeford T, Strosznajder RP. Natural inhibitors of poly(ADP-ribose) polymerase-1. Mol Neurobiol 2012; 46:55–63. [DOI] [PubMed] [Google Scholar]
- 36.Sinclair DA, Guarente L. Small-molecule allosteric activators of sirtuins. Annu Rev Pharmacol Toxicol 2014; 54:363–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu J, Jing H, Lin H. Sirtuin inhibitors as anticancer agents. Future Med Chem 2014; 6:945–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du G, Song Y, Zhang T, et al. Simvastatin attenuates TNF-α-induced apoptosis in endothelial progenitor cells via the upregulation of SIRT1. Int J Mol Med 2014; 34:177–82. [DOI] [PubMed] [Google Scholar]
- 39.Bertacchini J, Heidari N, Mediani L, et al. Targeting PI3K/AKT/mTOR network for treatment of leukemia. Cell Mol Life Sci 2015; 72:2337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiarini F, Evangelisti C, McCubrey JA, Martelli AM. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci 2015; 36:124–35. [DOI] [PubMed] [Google Scholar]
- 41.Huang Z, Wu Y, Zhou X, et al. Clinical efficacy of mTOR inhibitors in solid tumors: a systematic review. Future Oncol 2015; 11:1687–99. [DOI] [PubMed] [Google Scholar]
- 42.Vicier C, Dieci MV, Arnedos M, Delaloge S, Viens P, Andre F. Clinical development of mTOR inhibitors in breast cancer. Breast Cancer Res 2014; 16:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spencer A, Yoon SS, Harrison SJ, et al. The novel AKT inhibitor afuresertib shows favorable safety, pharmacokinetics, and clinical activity in multiple myeloma. Blood 2014; 124:2190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman DR, Lanasa MC, Davis PH, et al. Perifosine treatment in chronic lymphocytic leukemia: results of a phase II clinical trial and in vitro studies. Leuk Lymphoma 2014; 55:1067–75. [DOI] [PubMed] [Google Scholar]
- 45.Ramanathan RK, McDonough SH, Kennecke HF, et al. Phase 2 study of MK-2206, an allosteric inhibitor of AKT, as second-line therapy for advanced gastric and gastroesophageal junction cancer: a SWOG cooperative group trial (S1005). Cancer 2015; 121:2193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cani A, Simioni C, Martelli AM, et al. Triple Akt inhibition as a new therapeutic strategy in T-cell acute lymphoblastic leukemia. Oncotarget 2015; 6:6597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampath D, Malik A, Plunkett W, et al. Phase I clinical, pharmacokinetic, and pharmacodynamics study of the Akt-inhibitor triciribine phosphate monohydrate in patients with advanced hematologic malignancies. Leuk Res 2013; 37:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen G, Tang J, Ni Z, et al. Antiasthmatic effects of resveratrol in ovalbumin-induced asthma model mice involved in the upregulation of PTEN. Biol Pharm Bull 2015; 38:507–13. [DOI] [PubMed] [Google Scholar]
- 49.Secchiero P, Bosco R, Celeghini C, Zauli G. Recent advances in the therapeutic perspectives of Nutlin-3. Curr Pharm Des 2011; 17:569–77. [DOI] [PubMed] [Google Scholar]
- 50.Levine B, Packer M, Codogno P. Development of autophagy inducers in clinical medicine. J Clin Invest 2015; 125:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramprasath T, Vasudevan V, Sasikumar S, Puhari SS, Saso L, Selvam GS. Regression of oxidative stress by targeting eNOS and Nrf/ARE signaling: a guided drug target for cardiovascular diseases. Curr Top Med Chem 2015; 15:857–71. [DOI] [PubMed] [Google Scholar]
- 52.Ebrahimpour Koujan S, Gargari BP, Mobasseri M, Valizadeh H, Asghari-Jafarabadi M. Effects of Silybum marianum (L.) Gaertn. (silymarin) extracts supplementation on antioxidant status and hs-CRP in patients with type 2 diabetes mellitus: a randomized, triple-blind, placebo-controlled clinical trial. Phytomedicine 2015; 22:290–6. [DOI] [PubMed] [Google Scholar]
- 53.Hu H, Zhai C, Qian G, et al. Protective effects of tanshinone IIA on myocardial ischemia reperfusion injury by reducing oxidative stress, HMGB1 expression and inflammatory reaction. Pharm Biol 2015; 13:1–7. [DOI] [PubMed] [Google Scholar]
- 54.Levy M, Thaiss CA, Elinav E. Taming the inflammasome. Nat Med 2015; 21:213–5. [DOI] [PubMed] [Google Scholar]
- 55.Lenin R, Maria MS, Agrawal M, Balasubramanyam J, Mohan V, Balasubramanyam M. Amelioration of glucolipotoxicity-induced endoplasmic reticulum stress by a “chemical chaperone” in human THP-1 monocytes. Exp Diabetes Res 2012; doi:10.1155/2012/356487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li JS, Wang WJ, Sun Y, Zhang YH, Zheng L. Ursolic acid inhibits the development of nonalcoholic fatty liver disease by attenuating endoplasmic reticulum stress. Food Funct 2015; 6:1643–51. [DOI] [PubMed] [Google Scholar]
- 57.Kawanami D, Matoba K, Okada R, et al. Fasudil inhibits ER stress-induced VCAM-1 expression by modulating unfolded protein response in endothelial cells. Biochem Biophys Res Commun 2013; 435:171–5. [DOI] [PubMed] [Google Scholar]
- 58.Gani AR, Uppala JK, Ramaiah KV. Tauroursodeoxycholic acid prevents stress induced aggregation of proteins in vitro and promotes PERK activation in HepG2 cells. Arch Biochem Biophys 2015; 568:8–15. [DOI] [PubMed] [Google Scholar]
- 59.Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015; 21:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015; 21:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Passos-Silva DG, Brandan E, Santos RA. Angiotensins as therapeutic targets beyond heart disease. Trends Pharmacol Sci 2015; 36:310–20. [DOI] [PubMed] [Google Scholar]
- 62.Lee WJ, Ou HC, Hsu WC, et al. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J Vasc Surg 2010; 52:1290–300. [DOI] [PubMed] [Google Scholar]
- 63.Tsai KL, Chen LH, Chiou SH, et al. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1 mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Mol Nutr Food Res 2011; 55(suppl 2):S227–40. [DOI] [PubMed] [Google Scholar]
- 64.Yamagata K, Tusruta C, Ohtuski A, Tagami M. Docosahexaenoic acid decreases TNF-α-induced lectin-like oxidized low-density lipoprotein receptor-1 expression in THP-1 cells. Prostaglandins Lekot Essent Fatty Acids 2014; 90:125–32. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, Cheng L, Wang Q, et al. Atorvastatin protects cardiomyocytes from oxidative stress by inhibiting LOX-1 expression and cardiomyocyte apoptosis. Acta Biochim Biophys Sin 2015; 47:174–82. [DOI] [PubMed] [Google Scholar]
- 66.Li XB, Cheng X, Zhang DL, et al. Syntheses of tanshinone anhydrides and their suppression on oxidized LDL uptake in macrophages and foam cell formation. Pharmazie 2014; 69:163–7. [PubMed] [Google Scholar]
- 67.Guerra GC, Araújo AA, Lira GA, et al. Telmisartan decreases inflammation by modulating TNF-α, IL-10, and RANK/RANKL in a rat model of ulcerative colitis. Pharmacol Rep 2015; 67:520–6. [DOI] [PubMed] [Google Scholar]
- 68.Guo C, Sinnott B, Niu B, Lowry MB, Fantacone ML, Gombart AF. Synergistic induction of human cathelicidin antimicrobial peptide gene expression by vitamin D and stilbenoids. Mol Nutr Food Res 2014; 58:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Makdissi A, Ghanim H, Vora M, et al. Sitagliptin exerts an anti-inflammatory action. J Clin Endocrinol Metab 2012; 97:3333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nussbaumer O, Gruenbacher G, Gander H, Komuczki J, Rahm A, Thurnher M. Essential requirement of zoledronate-induced cytokine and γδ T cell proliferative responses. J Immunol 2013; 191:1346–55. [DOI] [PubMed] [Google Scholar]
- 71.Naraoka M, Munakata A, Matsuda N, Shimamura N, Ohkuma H. Suppression of the Rho/Rho-kinase pathway and prevention of cerebral vasospasm by combination treatment with statin and fasudil after subarachnoid hemorrhage in rabbit. Transl Strok Res 2013; 4:368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mily A, Rekha RS, Kamal SM, et al. Oral intake of phenylbutyrate with or without vitamin D3 upregulates the cathelicidin LL-37 in human macrophages: a dose finding study for treatment of tuberculosis. BMC Pulm Med 2013; 13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahmed TA, Hayslip J, Leggas M. Simvastatin interacts synergistically with tipifarnib to induce apoptosis in leukemia cells through the disruption of RAS membrane localization and ERK pathway inhibition. Leuk Res 2014; 38:1350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wennerberg K, Rossman KL, Der CJ. The RAS superfamily at a glance. J Cell Sci 2005; 118:843–6. [DOI] [PubMed] [Google Scholar]
- 75.Sun J, Singh V, Lau A, et al. Mycobacterium tuberculosis nucleoside diphosphate kinase inactivates small GTPases leading to evasion of innate immunity. PLoS Pathog 2013; 9:e1003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases 2014; 5:e29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang J, Yang K, Zhou L, et al. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog 2013; 9:e1003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aizman E, Blacher E, Ben-Moshe O, Kogan T, Kloog Y, Mor A. Therapeutic effect of farnesylthiosalicylic acid on adjuvant-induced arthritis through suppressed release of inflammatory cytokines. Clin Exp Immunol 2014; 175:458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silva-García O, Valdez-Alarcón JJ, Baizabal-Aguirre VM. The Wnt/β-catenin signaling pathway controls the inflammatory response in infections caused by pathogenic bacteria. Mediators Inflamm 2014; doi:10.1155/2014/310183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y, Pizzute T, Pei M. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res 2014; 358:633–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blumenthal A, Ehlers S, Lauber J, et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 2006; 108:965–73. [DOI] [PubMed] [Google Scholar]
- 82.Schaale K, Brandenburg J, Kispert A, Leitges M, Ehlers S, Reiling N. Wnt6 is expressed in granulomatous lesions of Mycobacterium tuberculosis-infected mice and is involved in macrophage differentiation and proliferation. J Immunol 2013; 191:5182–95. [DOI] [PubMed] [Google Scholar]
- 83.Wu X, Deng G, Hao X, et al. A caspase-dependent pathway is involved in Wnt/β-catenin signaling promoted apoptosis in bacillus Calmette-Guerin infected RAW264.7 macrophages. Int J Mol Sci 2014; 15:5045–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elzinga BM, Nyhan MJ, Crowley LC, O'Donovan TR, Cahill MR, McKenna SL. Induction of autophagy by imatinib sequesters Bcr-Abl in autophagosomes and down-regulates Bcr-Abl protein. Am J Hematol 2013; 88:455–62. [DOI] [PubMed] [Google Scholar]
- 85.Bruns H, Stegelmann F, Fabri M, et al. Abelson tyrosine kinase controls phagosomal acidification required for killing of Mycobacterium tuberculosis in human macrophages. J Immunol 2012; 189:4069–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Datta M, Via LE, Kamoun WS, et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc Natl Acad Sci U S A 2015; 112:1827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sundberg TB, Choi HG, Song JH, et al. Small-molecule screening identifies inhibition of salt-inducible kinases as a therapeutic strategy to enhance immunoregulatory functions of dendritic cells. Proc Natl Acad Sci U S A 2014; 111:12468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hardie DG. AMPK-sensing energy while talking to other signaling pathways. Cell Metab 2014; 20:939–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab 2014; 20:953–66. [DOI] [PubMed] [Google Scholar]
- 90.Hur KY, Lee MS. New mechanisms of metformin action: focusing on mitochondria and the gut. J Diabetes Investig 2015; doi:10.1111/jdi.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blagih J, Coulombe F, Vincent EE, et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 2015; 42:41–54. [DOI] [PubMed] [Google Scholar]
- 92.Rolf J, Zarrouk M, Finlay DK, Foretz M, Viollet B, Cantrell DA. AMPKα1: A glucose sensor that controls CD8 T-cell memory. Eur J Immunol 2013; 43:889–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 2005; 45:89–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsubaki M, Takeda T, Sakamoto K, et al. Bisphosphonates and statins inhibit expression and secretion of MIP-1α via suppression of Ras/MEK/ERK/AML-1A and Ras/PI3K/Akt/AML-1A pathways. Am J Cancer Res 2015; 5:168–79. [PMC free article] [PubMed] [Google Scholar]
- 95.Wei YM, Li X, Xu M, et al. Enhancement of autophagy by simvastatin through inhibition of Rac1-mTOR signaling pathway in coronary arterial myocytes. Cell Physiol Biochem 2013; 31:925–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Skerry C, Pinn ML, Bruiners N, Pine R, Gennaro ML, Karakousis PC. Simvastatin increases the in vivo activity of the first-line tuberculosis regimen. J Antimicrob Chemother 2014; 69:2453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Imai S. The NAD World: a new systematic regulatory network for metabolism and aging-Sirt1, systemic NAD biosynthesis and their importance. Cell Biochem Biphys 2009; 53:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cantó C, Sauve AA, Bai P. Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes. Mol Aspects Med 2013; 34:1168–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wei W, Graeff R, Ye J. Roles and mechanisms of the CD38/cyclic adenosine diphosphate ribose/Ca2+ signaling pathway. World J Biol Chem 2014; 26:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haffner CD, Becherer JD, Boros EE. Discovery, synthesis, and biological evaluation of thiazoloquin(az)olin(on)es as potent CD38 inhibitors. J Med Chem 2015; 58:3548–71. [DOI] [PubMed] [Google Scholar]
- 101.Schreiber V, Dantzer F, Amé JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 2006; 7:517–28. [DOI] [PubMed] [Google Scholar]
- 102.Leung A, Todorova T, Ando Y, Chang P. Poly(ADP-ribose) regulates post-transcriptional gene regulation in the cytoplasm. RNA Biol 2012; 9:542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bai P. Biology of poly(ADP-ribose) polymerases: the factotums of cell maintenance. Mol Cell 2015; 58:947–58. [DOI] [PubMed] [Google Scholar]
- 104.Ba X, Garg NJ. Signaling mechanism of poly(ADP-ribose) polymerase-1 (PARP-1) in Inflammatory diseases. Am J Pathol 2011; 178:946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cohen-Armon M. PARP-1 activtion in the ERK signaling pathway. Trends Pharmacol Sci 2007; 28:556–60. [DOI] [PubMed] [Google Scholar]
- 106.Racz B, Hanto K, Tapodi A, et al. Regulation of MKP-1 expression and MAPK activation by PARP-1 in oxidative stress: a new mechanism for the cytoplasmic effect of PARP-1 activation. Free Radic Biol Med 2010; 49:1978–88. [DOI] [PubMed] [Google Scholar]
- 107.Zheng L, Szabó C, Kern TS. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-κB. Diabetes 2004; 53:2960–7. [DOI] [PubMed] [Google Scholar]
- 108.Nossa CW, Blanke SR. Helicobacter pylori activation of PARP-1: usurping a versatile regulator of host cellular health. Gut Microbes 2010; 1:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weaver AN, Yang ES. Beyond DNA repair: additional functions of PARP-1 in cancer. Front Oncol 2013; 3:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martire S, Mosca L, d'Erme M. PARP-1 involvement in neurodegeneration: a focus on Alzheimer's and Parkinson's diseases. Mech Ageing Dev 2015; 146:53–64. [DOI] [PubMed] [Google Scholar]
- 111.Gerace E, Masi A, Resta F. PARP-1 activation causes neuronal death in the hippocampal CA1 region by increasing the expression of Ca2+-permeable AMPA receptors. Neurobiol Dis 2014; 70:43–52. [DOI] [PubMed] [Google Scholar]
- 112.Cheng XL, Ding F, Li H, et al. Activation of AMPA receptor promotes TNF-α release via the ROS-cSrc-NFκB signaling cascade in RAW264.7. Biochem Biophys Res Commun 2015; 461:275–80. [DOI] [PubMed] [Google Scholar]
- 113.Ba X, Gupta S, Davidson M, Garg NJ. Trypanosoma cruzi induces the reactive oxygen species-PARP-1-RelA pathway for up-regulation of cytokine expression in cardiomyocytes. J Biol Chem 2010; 285:11596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Afriat R, Horowitz S, Priel E. Mycoplasma fermentans inhibits the activity of cellular DNA topoisomerase I by activation of PARP1 and alters the efficacy of its anti-cancer inhibitor. PLoS One 2013; 8:72377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu H, Schwarzer K, Förster M, et al. Role of high-mobility group box 1 protein and poly(ADP-ribose) polymerase 1 degradation in Chlamydia trachomatis-induced cytopathicity. Infect Immun 2010; 78:3288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Atasheva S, Frolova EI, Frolov I. Interferon-stimulated poly (ADP-ribose) polymerases are potent inhibitors of cellular translation and virus replication. J Virol 2014; 88:2116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Daugherty MD, Young JM, Kerns JA, Malik HS. Rapid evolution of PARP genes suggests a broad role for ADP-ribosylation in host-virus conflicts. PLoS Genet 2014; 10:e1004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun J, Siroy A, Lokareddy RK, et al. The tuberculosis necrotizing toxin kills macrophages by hydrolyzing NAD. Nat Struct Mol Biol 2015; doi:10.1038/nsmb.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chandrasekaran S, Caparon MG. The Streptococcus pyogenes NAD+ glycohydrolase modulates epithelial cell PARylation and HMGB1 release. Cell Microbiol 2015; doi:10.1111/CMI.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hernandez-Pando R, Barrios-Payan J, Mata-Epinosa D, et al. The role of High Mobility Group Box 1 protein (HMGB1) in the immunopathology of experimental pulmonary tuberculosis. PLoS One 2015; 10:e0133200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu X, Deng G, Li M, et al. Wnt/β-Catenin signaling reduces bacillus Calmette-Guerin-induced macrophage necrosis through a ROS–mediated PARP/AIF-dependent pathway. BMC Immunol 2015; 16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sambucci M, Laudisi F, Novelli F, Bennici E, Rosado MM, Pioli C. Effects of PARP-1 deficiency on Th1 and Th2 cell differentiation. Sci World J 2013; doi:10.1155/2013/375024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang P, Maruyama T, Konkel JE, et al. PARP-1 controls immunosuppressive function of regulatory T cells by destabilizing Foxp3. PLoS One 2013; 8:e71590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.US Food and Drug Administration FDA approves Lynparza to treat advanced ovarian cancer. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427554.htm Accessed 13 August 2015.
- 125.Ahmad SF, Attia SM, Zoheir KM, Ashour AE, Bakheet SA. Attenuation of the progression of adjuvant-induced arthritis by 3-aminobenzamide treatment. Int Immunopharmacol 2014; 19:52–9. [DOI] [PubMed] [Google Scholar]
- 126.Martínez-Redondo P, Vaquero A. The diversity of histone versus nonhistone sirtuin substrates. Genes Cancer 2013; 4:148–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gerhart-Hines Z, Dominy JE, Jr, Blättler SM, et al. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD+. Mol Cell 2011; 44:851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol Mech Dis 2010; 5:253–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bosch-Presegué L, Vaquero A. Sirtuin-dependent epigenetic regulation in the maintenance of genome integrity. FEBS J 2014; 282:1745–67. [DOI] [PubMed] [Google Scholar]
- 130.Liu G, Bi Y, Xue L, et al. Dendritic cell SIRT1-HIF1α axis programs the differentiation of CD4+ T cells through IL-12 and TGF-β1. Proc Natl Acad Sci U S A 2015; 112:E957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hwang JW, Yao H, Caito S, Sundar IK, Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med 2013; 61:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Turkmen K, Karagoz L, Kucuk A. Sirtuins as novel players in the pathogenesis of diabetes mellitus. World J Diabetes 2014; 5:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guarente L. Sirtuins, aging, and medicine. New Engl J Med 2011; 364:2235–44. [DOI] [PubMed] [Google Scholar]
- 134.Vachharajani VT, Liu T, Brown CM, et al. SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. J Leukoc Biol 2014; 96:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu TF, Vachharajani V, Millet P, Bharadwaj MS, Molina AJ, McCall CE. Sequential actions of SIRT1-RELB-SIRT3 coordinate nuclear mitochondrial communication during immunometabolic adaptation to acute inflammation and sepsis. J Biol Chem 2015; 290:396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alvarez Y, Rodríguez M, Municio C, et al. Sirtuin 1 is a key regulator of the interleukin-12 p70-interleukin-23 balance in human dendritic cells. J Biol Chem 2012; 287:35689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gao B, Kong Q, Kemp K, Zhao YS, Fand D. Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2 mediated reversal of T-cell tolerance. Proc Natl Acad Sci U S A 2012; 109:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Koyuncu E, Budayeva HG, Miteva YV, et al. Sirtuins are evolutionarily conserved viral restriction factors. mBio 2014; 5:e02249–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kwon HS, Brent MM, Getachew R, et al. Human immunodeficiency virus type 1 Tat protein inhibits the SIRT1 deacetylase and induces T cell hyperactivation. Cell Host Microbe 2008; 3:158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martin C, Leyton L, Arancibia Y, et al. Modulation of the AMPK/Sirt1 axis during neuronal infection by herpes simplex virus type 1. J Alzheimers Dis 2014; 42:301–12. [DOI] [PubMed] [Google Scholar]
- 141.Eskandarian HA, Impens F, Nahori MA, et al. A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science 2013; 341:1238858. [DOI] [PubMed] [Google Scholar]
- 142.Moreira D, Rodrigues V, Abengozar M, et al. Leishmania infantum modulates host macrophage mitochondrial metabolism by hijacking the SIRT1-AMPK axis. PLoS Pathog 2015; 11:e1004684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park SJ, Ahmad F, Philp A, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phospodiesterases. Cell 2012; 148:421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mitchel SJ, Martin-Montalvo A, Mercken EM, et al. The SIRT1 ACTIVATOR srt1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep 2014; 6:836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mouchiroud L, Houtkooper RH, Auwerx J. NAD+ metabolism: a therapeutic target for age-related metabolic disease. Crit Rev Biochem Mol Biol 2013; 48:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Imai S, Yoshino J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes Metab 2013; 15(suppl 3):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mao Z, Hine C, Tian X, et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science 2011; 332:1443–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bolivar B, Welch JT. Understanding PZA's host directed activity [abstract J3 1017]. In: Program and Abstracts of the Host Response in Tuberculosis Symposium (Santa Fe) Silverthorne: Keystone Symposia on Molecular and Cellular Biology, 2015:76. [Google Scholar]
- 149.Gongol B, Marin T, Peng IC, et al. AMPKα2 exerts its anti-inflammatory effects through PARP-1 and Bcl-6. Proc Natl Acad Sci U S A 2013; 110:3161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ruderman NB, Xu XJ, Nelson L, et al. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 2010; 298:E751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kuijl C, Savage ND, Marsman M, et al. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature 2007; 450:725–30. [DOI] [PubMed] [Google Scholar]
- 152.Barrett JP, Minogue AM, Falvey A, Lynch MA. Involvement of IGF-1 and Akt in M1/M2 activation state in bone marrow-derived macrophages. Exp Cell Res 2015; 335:258–68. [DOI] [PubMed] [Google Scholar]
- 153.Rocher C, Singla DK. SMAD-PI3K-Akt-mTOR pathway mediates BMP-7 polarization of monocytes into M2 macrophages. PLoS One 2013; 8:e84009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhai C, Cheng J, Mujahid H, et al. Selective inhibition of PI3K/Akt/mTOR signaling pathway regulates autophagy of macrophage and vulnerability of atherosclerotic plaque. PLoS One 2014; 9:390563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hindupur SK, Gonzalez A, Hall MN. The opposing actions of target of rapamycin and AMP-activated protein kinase in cell growth control. Cold Spring Harb Perspect Biol 2015; 7:a019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Z, Amorosa LF, Coyle SM, et al. Proteolytic cleavage of AMPKalpha and intracellular MMP9 expression are both required for TLR4-mediated mTORC1 activation and HIF-1alpha expression in leukocytes. J Immunol 2015; 195:10.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zullo AJ, Jurcic Smith KL, Lee S. Mammalian target of rapamycin inhibition and mycobacterial survival are uncoupled in murine macrophages. BMC Biochem 2014; 15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kamphorst AO, Araki K, Ahmed R. Beyond adjuvants: Immunomodulation strategies to enhance T cell immunity. Vaccine 2015; 33(suppl 2):B21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jagannath C, Bakhru P. Rapamycin-induced enhancement of vaccine efficacy in mice. Methods Mol Biol 2012; 821:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Üstün S, Lassnig C, Preitschopf A, et al. Effects of the mTOR inhibitor everolimus and the PI3K/mTOR inhibitor NVP-BEZ235 in murine acute lung injury models. Transpl Immunol 2015; 33:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li N, Qin J, Lan L, et al. PTEN inhibits macrophage polarization from M1 to M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer Biol Ther 2015; 16:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li L, Ng DS, Mah WC, et al. A unique role for p53 in the regulation of M2 macrophage polarization. Cell Death Differ 2015; 22:1081–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sui X, Han W, Pan H. p53-induced autophagy and senescence. Oncotarget 2015; 6:11723–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 2012; 13:283–96. [DOI] [PubMed] [Google Scholar]
- 165.Huang G, Redelman-Sidi G, Rosen N, Glickman MS, Jiang X. Inhibition of mycobacterial infection by the tumor suppressor PTEN. J Biol Chem 2012; 287:23196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim SA, Choi HC. Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells. Biochem Biophys Res Commun 2012; 425:866–72. [DOI] [PubMed] [Google Scholar]
- 167.Holla S, Ghorpade DS, Singh V, Bansal K, Balaji KN. Mycobacterium bovis BCG promotes tumor cell survival from tumor necrosis factor-α-induced apoptosis. Mol Cancer 2014; 13:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bradfute SB, Castillo EF, Arko-Mensah J, et al. Autophagy as an immune effector against tuberculosis. Curr Opin Microbiol 2013; 16:355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell 2014; 159:1263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ng F, Tang BL. Sirtuins’ modulation of autophagy. J Cell Physiol 2013; 228:2262–70. [DOI] [PubMed] [Google Scholar]
- 171.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 2015; 6:456–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Victorino VJ, Pizzatti L, Michelletti P, Panis C. Oxidative stress, redox signaling and cancer chemoresistance: putting together the pieces of the puzzle. Curr Med Chem 2014; 21:3211–26. [DOI] [PubMed] [Google Scholar]
- 173.Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol 2012; doi:10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 29:44–8. [DOI] [PubMed] [Google Scholar]
- 175.Kwiatkowska S, Piasecka G, Zieba M, Piotrowski W, Nowak D. Increased serum concentrations of conjugated diens and malondialdehyde in patients with pulmonary tuberculosis. Respir Med 1999; 93:272–6. [DOI] [PubMed] [Google Scholar]
- 176.Lamsal M, Gautam N, Bhatta N, Toora BD, Bhattacharya SK, Baral N. Evaluation of lipid peroxidation product, nitrite and antioxidant levels in newly diagnosed and two months follow-up patients with pulmonary tuberculosis. Southeast Asian J Trop Med Public Health 2007; 38:695–703. [PubMed] [Google Scholar]
- 177.Bettigole SE, Glimcher LH. Endoplasmic reticulum stress in immunity. Annu Rev Immunol 2015; 33:5.1–5.31. [DOI] [PubMed] [Google Scholar]
- 178.Seimon TA, Kim MJ, Blumenthal A, et al. Induction of ER stress in macrophages of tuberculosis granulomas. PLoS One 2010; 5:e12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lim YJ, Choi JA, Choi HH, et al. Endoplasmic reticulum stress pathway-mediated apoptosis in macrophages contributes to the survival of Mycobacterium tuberculosis. PLoS One 2011; 6:e28531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Choi JA, Lim YJ, Cho SN, et al. Mycobacterial HBHA induces endoplasmic reticulum stress-mediated apoptosis through the generation of reactive oxygen species and cytosolic Ca2+ in murine macrophage RAW 264.7 cells. Cell Death Dis 2013; 4:e957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Choi HH, Shin DM, Kang G, et al. Endoplasmic reticulum stress response is involved in Mycobacterium tuberculosis protein ESAT-6-mediated apoptosis. FEBS Lett 2010; 584:2445–54. [DOI] [PubMed] [Google Scholar]
- 182.Lim YJ, Choi JA, Lee JH, Choi CH, Kim HJ, Song CH. Mycobacterium tuberculosis 38-kDa antigen induces endoplasmic reticulum stress-mediated apoptosis via toll-like receptor 2/4. Apoptosis 2015; 20:358–70. [DOI] [PubMed] [Google Scholar]
- 183.Erstad DJ, Cusack JC., Jr Targeting the NF-κB pathway in cancer therapy. Surg Oncol Clin N Am 2013; 22:705–46. [DOI] [PubMed] [Google Scholar]
- 184.Verhelst K, Verstrepen L, Carpentier I, Beyaert R. IκB kinase ε (IKKε): a therapeutic target in inflammation and cancer. Biochem Pharmacol 2013; 85:873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bhatt K, Verma S, Ellner JJ, Salgame P. Quest for correlates of protection against tuberculosis. Clin Vaccine Immunol 2015; 22:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thurnher M, Gruenbacher G. T lymphocyte regulation by mevalonate metabolism. Sci Signal 2015; 8:re4. [DOI] [PubMed] [Google Scholar]
- 187.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol 2013; 13:621–34. [DOI] [PubMed] [Google Scholar]
- 188.Mahajan S, Dkhar HK, Chandra V, et al. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARγ and TR4 for survival. J Immunol 2012; 188:5593–603. [DOI] [PubMed] [Google Scholar]
- 189.Wang S, Sun Z, Zhang X, et al. Wnt1 positively regulates CD36 expression via TCF4 and PPARγ in macrophages. Cell Physiol Biochem 2015; 35:1289–302. [DOI] [PubMed] [Google Scholar]
- 190.Yao S, Miao C, Tian H, et al. Endoplasmic reticulum stress promotes macrophage-derived foam cell formation by up-regulating cluster of differentiation 36 (CD36) expression. J Biol Chem 2014; 289:4032–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 2015; 161:1527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang X, Phillips MI, Mehta JL. LOX-1 and angiotensin receptors, and their interplay. Cardiovasc Drugs Ther 2011; 25:401–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nakashima T, Umemoto S, Yoshimura K, et al. TLR4 is a critical regulator of angiotensin II-induced vascular remodeling: the roles of extracellular SOD and NADPH oxidase. Hypertens Res 2015; doi:10.1038/hr.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Palanismay GS, Kirk NM, Ackart DF, et al. Uptake and accumulation of oxidized low-density lipoprotein during Mycobacterium tuberculosis infection in guinea pigs. PLoS One 2012; 7:e34148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hawkes M, Li X, Crockett M, et al. CD36 deficiency attenuates experimental mycobacterial infection. BMC Infect Dis 2010; 10:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Christakos S, Seth T, Hirsch J, Porta A, Moulas A, Dhawan P. Vitamin D biology revealed through the study of knockout and transgenic mouse models. Annu Rev Nutr 2013; 33:71–85. [DOI] [PubMed] [Google Scholar]
- 197.Riek AE, Oh J, Sprague JE, et al. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J Biol Chem 2012; 287:38482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Larriba MJ, González-Sancho JM, Bonilla F, Muñoz A. Interaction of vitamin D with membrane-based signaling pathways. Front Physiol 2014; 5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009; 6:231–43. [DOI] [PubMed] [Google Scholar]
- 200.Daley P, Jagannathan V, John KR, et al. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2015; 15:528–34. [DOI] [PubMed] [Google Scholar]
- 201.Raqib R, Mily A, Kamal SM, et al. Clinical trial of oral phenylbutyrate and vitamin D adjunctive therapy in pulmonary tuberculosis in Bangladesh [abstract OAP-257–30]. In: Program and Abstracts Of the 45th World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease, Barcelona, 28 October–1 November 2014 Paris: The Union, 2014:S233–4. [Google Scholar]
- 202.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011; 11:98–107. [DOI] [PubMed] [Google Scholar]
- 203.Gregor MF, Hotamisligi GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011; 29:415–45. [DOI] [PubMed] [Google Scholar]
- 204.Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol 2014; 44:617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Restrepo BI, Schlesinger LS. Host-pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis 2013; 93:S10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dai Y, Dai D, Wang X, Ding Z, Mehta JL. DPP-4 inhibitors repress NLRP3 inflammasome and interleukin-1beta via GLP-1 receptor in macrophages through protein kinase C pathway. Cardiovasc Drugs Ther 2014; 28:425–32. [DOI] [PubMed] [Google Scholar]
- 207.Dai W, Wang X, Ding Z, Dai D, Mehta JL. DPP-4 inhibitors repress foam cell formation by inhibiting scavenger receptors through protein kinase C pathway. Acta Diabetol 2014; 51:471–8. [DOI] [PubMed] [Google Scholar]
- 208.Barreira da Silva R, Laird ME, Yatim N, Fiette L, Ingersoll MA, Albert ML. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat Immunol 2015; 16:850–8. [DOI] [PubMed] [Google Scholar]
- 209.Cardoso F, Castro F, Moreira-Teixeria L, et al. Myeloid sirtuin 2 expression does not impact long-term Mycobacterium tuberculosis control. PLoS One 2015; 10:e0131904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015; 372:793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]