Abstract
Objective
We sought to investigate the progression of human papillomaviruses (HPV) infection in HIV-positive women after cryotherapy.
Methods
We examined changes in detection of high-risk HPV (hrHPV) cervical infections among HIV-infected women over a 12-week period following cryotherapy using stored specimens from a cohort study conducted between June 2009 and March 2011 in Lusaka, Zambia. Samples from visits at baseline and weeks 4, 8, and 12 were tested using the Roche Linear Array assay.
Results
A total of 89 women were included in the analysis. The median age was 32 years (interquartile range [IQR]: 28–36 years). The median CD4+ cell count was 350 cells/μL (IQR: 214–470 cells/μL) and 66% of women were receiving antiretroviral therapy. At baseline, the prevalence of hrHPV was 91% (95% confidence interval [CI]: 83–95%). HPV45 was the most common HPV type, present in (30%) women, followed by HPV16 (27%), HPV18 (27%), HPV51 (20%), and HPV58 (22%). Among women with valid results both at baseline and 12 weeks, 17/67 (25%) cleared their initial hrHPV infection within 12 weeks of treatment, though 65% (11/17) had new hrHPV types detected.
Conclusions
Cryotherapy led to clearance of 25% of hrHPV infections within 12 weeks of treatment. However, hrHPV infection remained persistent in most women and new hrHPV types were detected often, explaining the high rate of persistence and recurrence of cervical disease in this population. Continued efforts to scale-up HPV vaccination and cervical screening should remain a priority in high HIV burden settings such as Zambia.
Keywords: human papillomavirus, cervix, cervical cancer, cryotherapy, HIV, Zambia
Introduction
Cervical cancer is among the most common cancers and causes of cancer-related death in women worldwide. Zambia has one of the highest cervical cancer incidence and mortality rates in the world, with an estimated 2,330 new diagnoses and 1,380 deaths in the adult population in 2012 [1].
Human papillomaviruses (HPVs) are grouped into oncogenic (high-risk) and non-oncogenic (low-risk) groups based on their association with precancer and invasive cervical cancer. Persistent infection of the cervix with any of the 13 high-risk (hrHPV) types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) [2] is a necessary precursor to cervical cancer [3].
The prevalence and persistence of cervical HPV infection in HIV-negative women has been reported to vary widely [4, 5]. Among these immunocompetent women, the majority of HPV infections regress spontaneously [6] and do not commonly result in the development of invasive cervical cancer. By contrast, HIV-positive women have a greater prevalence of persistent HPV infection and infection with multiple hrHPV types, contributing to an increased incidence of precancerous lesions and faster disease progression to invasive cervical cancer [7-9]. Therefore, in many countries, it is recommended that HIV-positive women be screened for cervical cancer annually, compared with screening every 3–5 years for HIV-negative women [10].
Many studies have reported the effect of cryotherapy (a treatment for precancer) on HPV prevalence and clearance among HIV-negative women, with several demonstrating a decreased prevalence and frequency of multiple HPV infections following cryotherapy [11, 12]. However, few studies have investigated the impact of cryotherapy on the clearance of HPV infections in women infected with HIV. A randomized controlled study in Thailand of 60 women with precancer reported that cryotherapy was not associated with increased clearance of prevalent HPV infections within a 12 month follow-up period compared with no therapy [13], but this study included only five HIV-positive women.
As “single visit approach” cervical cancer prevention initiatives using visual inspection with acetic acid (VIA) and cryotherapy are scaled up across the African continent, [14, 15] it is important to understand the possible impact of cryotherapy on the clearance of hrHPV infection in populations with a high HIV prevalence. In this study, we examined cervical hrHPV clearance among HIV-positive women over a 12-week period following cryotherapy for VIA positive lesions.
Methods
Design
We used data and stored specimens from a prospective cohort study entitled “Factors associated with post-cryotherapy wound healing among HIV-infected women in a low-resource sub-Saharan African setting”. The parent study was conducted between June 2009 and March 2011 in Lusaka, Zambia, and enrolled 101 HIV-positive women with acetowhite cervical lesions detected by VIA. The primary objective of the parent study was to compare the accuracy of three tests (naked eye assessment, digital cervicography, and cervicovaginal lavage for assessment of biomarkers of inflammation and cervicovaginal HIV shedding) to assess the adequacy of post-cryotherapy wound healing. Cervicovaginal lavage (CVL) samples and blood were collected at the time of cryotherapy (baseline) and each subsequent study visit (scheduled 4, 8 and 12 weeks after treatment). In the current study, we examined HPV DNA in cervical specimens from women who were enrolled in the parent study.
Study participants
To be eligible for the parent study, women were ≥18 years, HIV-1-positive, and had an acetowhite cervical lesion detected by VIA and/or digital cervicography that was eligible for cryotherapy (i.e., an acetowhite lesion involving less than 75% of the transformation zone, with no cervical canal involvement and no evidence of invasive cancer). Women were excluded from participation if they had a history of hysterectomy with removal of the cervix, evidence of acute or chronic infection of the cervix, or a cervical lesion deemed too extensive for treatment with cryotherapy. Pregnant women and those less than 6 months post-partum were also excluded.
To be eligible for inclusion in the current analysis, participants had to have a CVL sample with a valid HPV DNA result at baseline (n = 89). (We deemed HPV test results invalid when the β-globin control was negative after three repeats.) For inclusion in the analysis of hrHPV clearance, incidence, and persistence, women had to have valid HPV test results for both the baseline and 12 week visits (n = 68).
Study procedures
The baseline study visit included interviews about the participants’ medical, sexual, and reproductive histories, demographic characteristics, and pelvic examinations. CVL samples were collected at baseline and at each study visit by directing 10mL of sterile phosphate-buffered saline solution (contained in a sterile plastic syringe) at the cervix. The fluid was then aspirated from the vaginal vault, placed into sterile 2mL sample collection tubes, and transported to the Centre for Infectious Disease Research in Zambia (CIDRZ) Central Laboratory for storage at −80°C until processing. Blood was collected for HIV viral load testing and a CD4+ cell count. Women with CD4+ cell counts <200cells/μL were eligible for combination antiretroviral treatment (cART) based on the Zambian national HIV treatment guidelines at the time of the study. Cryotherapy was performed using a freeze-thaw-freeze method (3 minute freeze, 5 minute thaw, 3 minute freeze) with nitrous oxide.
HPV DNA processing
Cervicovaginal lavage specimens were tested for hrHPV DNA using the Roche Linear Array HPV test (LA; Roche Molecular, Pleasanton, CA, USA). This test uses biotinylated PGMY primers to amplify a 450-bp fragment within the polymorphic L1 region of the HPV genome and allows for the identification of 37 anogenital HPV types [6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73 (MM9), 81, 82 (MM4), 83 (MM7), 84 (MM8), IS39, and CP6108]. The HPV52 detected by the LA test could represent concurrently present HPV33, HPV35 and HPV58, because the probe for HPV52 is a mixed probe that cross-reacts with these 3 types [16]. The LA HPV test also amplifies a 265-bp region of the human β-globin gene that serves as an internal control for DNA extraction and amplification. Cervicovaginal lavage specimens, as well as HPV positive and negative controls, were processed in parallel using the AmpliLute liquid media kit (Roche Molecular Systems) on a QIAvac 24 plus vacuum system, according to the manufacturer’s instructions. (The results for the LA positive and negative controls were as expected for every run.) Extracted DNA was eluted into 120μL of buffer AVE, and purified DNA was immediately used for PCR amplification.
HPV DNA Amplification (PCR), Hybridization, and HPV typing
To 50μL of eluted DNA, 50μL of LA PCR mastermix was added, and amplification was performed on an Applied Biosystems Gold-plated 96-well GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA) using the following thermal profile: 2 minutes at 50°C; 9 minutes at 95°C; 40 cycles of 30 seconds at 95°C, 1 minute at 55°C, and 1 minute at 72°C with a ramp rate set at 50%; 5 minutes at 72°C; and a final hold at 72°C for not more than 4 hours.
Following amplification, PCR amplicons were immediately denatured by the addition of 100μL of LA denaturation reagent, and hybridization for HPV typing analysis was performed immediately. Hybridization and HPV typing were performed according to the manufacturer’s instructions, and the strips were manually interpreted within 24 hours using the LA HPV reference guide.
Statistical Analysis
The primary outcome was clearance of type-specific hrHPV infection at 12 weeks after cryotherapy. HPV clearance was defined as not detecting the same hrHPV type(s) at 12 weeks among women who were positive for at least one hrHPV at baseline.
Secondary outcomes were type-specific persistent and new hrHPV infections at 12 weeks post cryotherapy. Persistence was defined as detecting a type-specific hrHPV type at 12 weeks after detecting the same hrHPV type at baseline. New infections are termed “incident,” defined as detection of a new hrHPV type at 12 weeks after not detecting that specific hrHPV type at baseline. In addition to these analyses, the prevalence of low-risk (lr) HPV types (6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39 and CP6108) and other hrHPV types (31, 33, 35, 39, 52, 56, 59, and 68) was calculated, as was the multiplicity of HPV infections. Proportions with 95% confidence intervals were calculated. Data were analyzed with SAS™ version 9.2 (SAS Institute Inc., 2010, Cary, NC, USA).
Ethical approval
The protocol was approved by the local institutional review committee (University of Zambia Biomedical Research Ethics Committee) and the Institutional Review Boards of the University of Alabama at Birmingham and the University of North Carolina at Chapel Hill.
Results
Study Population
Of the 101 HIV-positive women with acetowhite cervical lesions detected by VIA enrolled in the cohort study, 89 women had a valid result for HPV DNA analysis at baseline (Figure 1), while 10 did not have a CVL sample and two had an invalid result due to undetectable β-globin after three repeats. The number of women with a valid HPV result at 4, 8 and 12 weeks was 63, 56, and 68, respectively (Figure 1).
Figure 1.
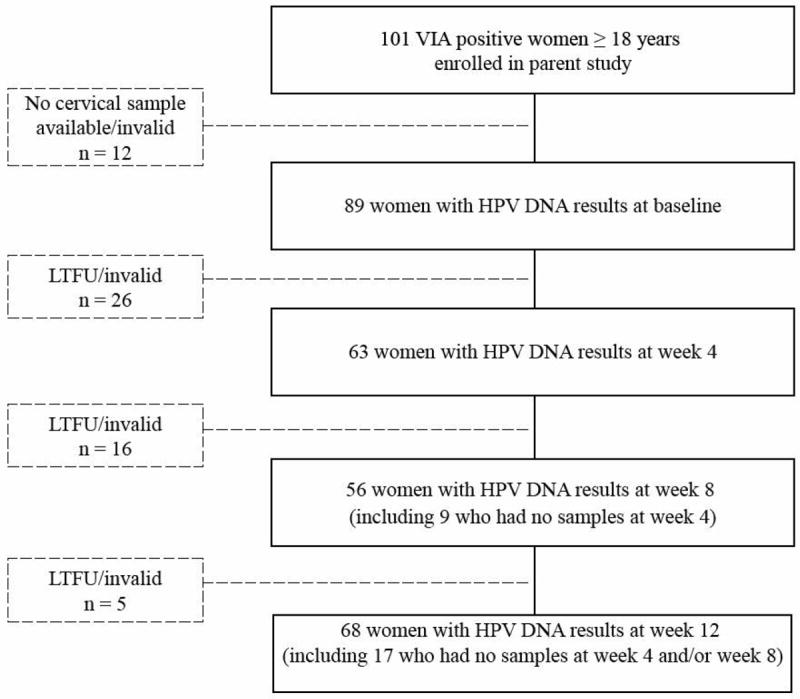
Cohort flow diagram
LTFU=Lost to follow-up
Patient Characteristics
Demographic characteristics included a median age of 32 years [interquartile range (IQR): 28–36 years], with 63% married and 23% having completed high school (Table 1). A total of 54% of women reported being on cART prior to the study; their median CD4 count was 350 cells/μL (IQR: 214–470 cells/μL) and 22% had a CD4+ cell count <200 cells/μL at baseline. The median baseline HIV-1 viral load among women on cART was 1,332 copies/mL (IQR: 95–42,776 copies/mL), and among cART-naïve women it was 28,987 copies/mL (IQR: 16,032–108,914 copies/mL).
Table 1.
Characteristics of the study population
| Variable | N (%) |
|---|---|
| Age (years), n=89 | |
| Median, IQR | 32 [28, 36] |
| 18–30 yrs | 34 (38%) |
| 31–40 yrs | 44 (49%) |
| >40 yrs | 11 (12%) |
| Age at sexual debut (years), n=89 | |
| Median, IQR | 18 [17-20] |
| Number of pregnancies, n=89 | |
| Median, IQR | 3 [2-4] |
| Marital status, n=86 | |
| Not married | 32 (37%) |
| Married | 54 (63%) |
| Condom use with regular partner, n=83 | |
| Always | 9 (11%) |
| Never | 34 (41%) |
| Sometimes | 40 (48%) |
| Number of lifetime partners, n=89 | |
| 1–2 | 26 (29%) |
| 3–5 | 55 (62%) |
| >5 | 8 (9%) |
| Education, n=87 | |
| High school not completed | 67 (77%) |
| High school completed | 20 (23%) |
| Employment, n=86 | |
| Not employed outside the home | 29 (34%) |
| Employed in the formal sector | 24 (28%) |
| Employed in the informal sector | 26 (30%) |
| Other | 7 (8%) |
| CD4+ cell count at baseline, n=87 | |
| Median, IQR | 350 [214–470] |
| <200 cells/μL | 19 (22%) |
| 200–350 cells/μL | 24 (28%) |
| >350 cells/μL | 44 (51%) |
| Variable | N (%) |
| HIV-1 viral load at baseline, n=31 | |
| Median, IQR | 19,250 [1,023–64,898] |
| Below limit of detection (< 20 copies/mL) | 11 (36%) |
| 20–5,000 copies/mL | 8 (26%) |
| >5,000 copies/mL | 12 (39%) |
| HIV-1 viral load at 12 weeks, n=42 | |
| Median, IQR | 12,389 [3,402–72,082] |
| Below limit of detection (< 20 copies/mL) | 19 (45%) |
| 20–5,000 copies/mL | 6 (14%) |
| >5,000 copies/mL | 17 (41%) |
| cART*, n=85 | |
| cART-naive | 29 (34%) |
| cART started during study | 10 (12%) |
| cART started prior to study | 46 (54%) |
cART: combination antiretroviral therapy
HPV Infections at Baseline and Follow-Up Visits
Among 89 women included in this analysis, only two women were HPV negative at baseline. The prevalence of any HPV infection was 98% (95% confidence interval [CI]: 92–99%). The prevalence of hrHPV infection was 91% (95% CI: 83–95%), while the prevalence of lrHPV was 86% (95% CI: 78–92%). HPV45 was the most common HPV type, present in 30% (27/89) of women. Other common types included HPV16 (27%; 24/89), HPV18 (27%; 24/89), HPV51 (20%; 18/89), and HPV58 (23%; 20/89) (Figure 2).
Figure 2.
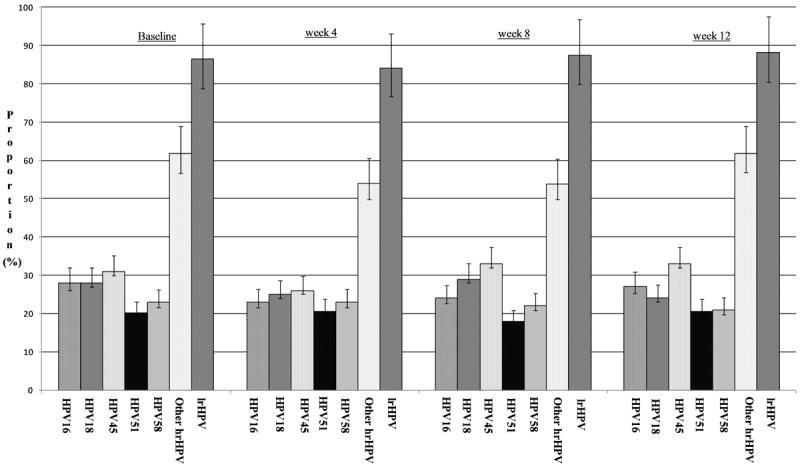
Proportions and 95% confidence intervals of all valid tests with individual HPV genotypes and genotype groupings by visit
At 4 weeks, the overall prevalence of HPV infection was 95% (95% CI: 87–98%) and the prevalence of hrHPV was 89% (95% CI: 79–95%). At 8 weeks, the overall prevalence of HPV infection was 91% (95% CI: 81–96%) and the prevalence of hrHPV was 88% (95% CI: 76–94%). At both of these visits, the most common HPV types were similar to the baseline results (Figure 2).
At the 12-week visit, the overall prevalence of HPV infection was 97% (95% CI: 90–99%) and the prevalence of hrHPV infection was 88% (95% CI: 79–94%), both very similar to the other time points. The prevalence of the most common types also remained similar to previous study visits: HPV16 (27%; 18/68), HPV18 (24%; 16/68), HPV45 (32%; 22/68), HPV51 (21%; 14/68), and HPV58 (21%; 14/68) (Figure 2).
Clearance, Incidence, and Persistence of hrHPV Infections
A total of 68 women had valid results both at baseline and 12 weeks and were included in the analysis to examine hrHPV clearance, incidence, and persistence. One woman had no hrHPV infection detected at baseline or at 12 weeks. Among the remaining 67 with at least one hrHPV type detected at baseline, six women had no hrHPV types detected at 12 weeks, 11 women had new hrHPV types detected (incident detection), 28 had the same hrHPV types detected at baseline that was detected at the 12-week visit (type-specific persistent detection), and 23 participants had both type-specific incident and persistent hrHPV detection. Women with only incident detection had cleared their initial infection(s) but new hrHPV types were detected by the 12 week visit. Combining these 11 women with those who cleared their initial hrHPV types (n=6) yields a 25% (17/67) type-specific clearance of hrHPV at 12 weeks after cryotherapy. Among these 17 women, 65% (11/17) had new hrHPV types detected.
The median CD4 count among women with no hrHPV types detected at 12 weeks was 499 cells/μL (IQR: 483–541 cells/μL), while women with prevalent infections had a median CD4 of 302 cells/μL (IQR: 172–447 cells/μL) and women with incident infections had a median CD4 of 307 cells/μL (IQR: 232–395 cells/μL). Among those women included in the type-specific clearance of hrHPV at 12 weeks (n=17), we did not have information for cART for one (6%) woman, one (6%) woman started cART during the study, seven (41%) women started before study enrollment and eight (47%) were cART naïve.
Multiplicity of HPV Infections
Multiple HPV infections were detected in 76/89 (85%) women at baseline, of which 12 were positive for two HPV types and 64 were positive for three or more HPV types. The most frequently detected hrHPV types in the 76 women with multiple HPV infections were HPV16, 18, 45, 51, and 58. A similar distribution of HPV types was seen at the week 12 follow-up visit (Table 2).
Table 2.
Number of single and multiple infections at baseline and 12 weeks after cryotherapy treatment.
| Baseline visit, n (%) (total N = 89) | 12 week visit, n (%) (total N = 68) | |||
|---|---|---|---|---|
| hrHPV | Single infection | Multiple infections | Single infection | Multiple infections |
| 16 | 1 (1%) | 23 (26%) | 1 (2%) | 17 (25%) |
| 18 | 1 (1%) | 23 (26%) | 0 | 16 (24%) |
| 31 | 0 | 4 (5%) | 0 | 6 (9%) |
| 33 | 0 | 10 (11%) | 0 | 9 (13%) |
| 35 | 2 (2%) | 14 (16%) | 0 | 9 (13%) |
| 39 | 0 | 6 (7%) | 0 | 3 (5%) |
| 45 | 2 (2%) | 25 (28%) | 1 (2%) | 21 (31%) |
| 51 | 0 | 18 (20%) | 0 | 14 (21%) |
| 52 | 1 (1%) | 10 (11%) | 1 (2%) | 8 (12%) |
| 56 | 0 | 8 (9%) | 0 | 6 (9%) |
| 58 | 1 (1%) | 19 (21%) | 0 | 14 (21%) |
| 59 | 0 | 10 (11%) | 0 | 7 (10%) |
| 68 | 0 | 12 (14%) | 0 | 9 (13%) |
| lrHPV | ||||
| 6 | 0 | 11 (12%) | 0 | 9 (13%) |
| 11 | 0 | 3 (3%) | 0 | 3 (5%) |
Discussion
The aim of this study was to determine the effect of cryotherapy on cervical HPV infection in HIV-positive women with acetowhite cervical lesions. We found that only 25% of women with hrHPV infections at baseline cleared their infections by 12 weeks after cryotherapy, indicating that the majority of women had persistent HPV infections. In addition, 11/17 (65%) of the women who cleared their baseline infections had a new hrHPV type detected within 12 weeks of treatment. Lastly, cryotherapy did not decrease the high frequency of multiple HPV infections.
Our study demonstrates a very high (85%) prevalence of multiple HPV infections in HIV-positive women with acetowhite cervical lesions. Previously, studies have reported prevalences of 36–80% [17-19]. Our results are consistent with those of Sahasrabuddhe et al., who reported that among HIV-positive women seeking HIV/AIDS treatment and care at the University Teaching Hospital in Zambia who also attended cervical cancer screening, 87% of women were infected with multiple HPV types [20].
The effect of cryotherapy on HPV infections in HIV-positive women has not been definitively established. The rate of HPV clearance that we report (25%) is lower than Elfgren et al. and Aerssens et al., who report 48% and 65% clearance of HPV infections at the week 12 visit and at 6-months follow-up period, respectively [11, 12]. Our rate of clearance is also much lower than the 90% reported by Chumworathayi et al., who studied cryotherapy in predominantly HIV-negative women [13].
Schneider et al. demonstrated the volatility of HPV 16 infection in immunocompetent women over a one-year span [19]. Our data indicate that while the majority (75%) of women have persistent hrHPV infections at 12 weeks, acquisition of new hrHPV infections are also relatively common. We think it more likely that newly detected infections are incident infections, given the sensitivity of PCR, though we cannot rule out false negative type-specific baseline assessments.
Strengths of our study include the prospective nature of the cohort and the collection of cervical samples before cryotherapy. Limitations include the lack of histological diagnosis results to confirm the presence of cervical disease and a short follow-up period of 12 weeks. The majority of longitudinal studies had a mean follow-up time between 6 months and 16 months [11, 12]. It is possible that our relatively short follow-up period led to lower clearance of HPV than that previously reported [11-13]. Lastly, we acknowledge that some incident infections may have been persistent, but not detected at the baseline assessment, either because they fell below the limit of detection (i.e., false negatives) and/or that newly detected infections were reactivated infections that were latent at the baseline visit.
The high burden of HPV disease and multiplicity of hrHPV infections in HIV-positive women in Zambia underscore a need for primary prevention. Bivalent and quadrivalent vaccines are currently available in many countries around the world [21, 22], and a nonavalent vaccine (including HPV6, 11, 16, 18, 31, 33, 45, 52, and 58) was approved by the U.S. Food and Drug Administration in December 2014 [23]. The effectiveness of these vaccines in preventing the acquisition of vaccine-related HPV types and cervical disease is well documented [24-27]. Additionally, the immune response elicited by the quadrivalent HPV vaccine also appears to be robust among HIV-positive women [28], and vaccine recommendations are identical in both groups. Modeling studies estimate that, with adequate coverage, vaccination with HPV16/18 vaccine could prevent 36–45% of invasive cervical cancers in countries like Zambia [29]. The new nonavalent vaccine will likely protect many more. With expanded access programs now accessible to many low-income countries, this cervical cancer prevention strategy should be a high priority.
Secondary prevention is also a critical component of cervical cancer prevention and control; this is the approach that will have the strongest public health impact over the short term. Cervical screening programs across sub-Saharan Africa need to be expanded and strengthened to ensure that all women have access to this life-saving service. It is estimated that fewer than 20% of Zambian women have ever had a pelvic examination and far fewer have ever been screened with VIA or a Pap smear [30]. Improving the coverage of cervical screening and treatment for precancer and invasive cervical cancer, particularly among HIV-positive Zambian women, will certainly reduce the incidence of this cancer, as it has in high-income nations.
Conclusion
Approximately one-fourth of women with hrHPV clear their infections within 12 weeks of cryotherapy treatment, yet the majority of these women are found to have new hrHPV infections within a relatively short period of time. Our study confirms that there is a high prevalence of HPV infection and that infection with multiple hrHPV types is common among HIV-positive Zambian women who screen positive by VIA. Given the high burden of HPV and cervical disease in this population, primary prevention through vaccination and secondary prevention through cervical screening should be scaled-up more rapidly in counties with generalized HIV epidemics.
Acknowledgments
Disclosure of source(s) of financial support
Original data collection was supported by grants D43TW001035 and R24TW007988 from the National Institutes of Health (NIH). This research was supported by grant 1D43CA153784-01 from the NIH/National Cancer Institute (NCI). Additional investigator support was provided through Fogarty International Center Award R25TW009340 to the UNC Hopkins Morehouse Tulane Fogarty Global Health Fellows Program.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
List of abbreviations and acronyms
- CIDRZ
Centre for Infectious Disease Research in Zambia
- CVL
Cervicovaginal Lavage
- cART
Combination antiretroviral treatment
- CI
Confidence Interval
- DNA
Deoxyribonucleic Acid
- hrHPV
High-risk Human papillomaviruses
- HIV
Human Immunodeficiency Virus
- HPV
Human papillomaviruses
- IQR
Interquartile Range
- lrHPV
Low-risk Human papillomaviruses
- NCI
National Cancer Institute
- NIH
National Institutes of Health
- LA
Roche Linear Array HPV test
- VIA
Visual Inspection with Acetic acid
Footnotes
Disclosure Statement
The authors have no conflict of interest to declare.
IRB Status: IRB Approved by Expedited Review
References
- 1. [January 22, 2015];Estimated Cancer Incidence, Mortality and Prevalence Wordwide in 2012. 2012 Available at: http://globocan.iarc.fr.
- 2.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright TC, Bosch FX, Franco EL, et al. Chapter 30: HPV vaccines and screening in the prevention of cervical cancer; conclusions from a 2006 workshop of international experts. Vaccine. 2006;24(suppl 3):251–261. doi: 10.1016/j.vaccine.2006.06.064. [DOI] [PubMed] [Google Scholar]
- 6.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 7.De Vuyst H, Lillo F, Broutet N, Smith JS. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev. 2008;17:545–554. doi: 10.1097/CEJ.0b013e3282f75ea1. [DOI] [PubMed] [Google Scholar]
- 8.Didelot-Rousseau MN, Nagot N, Costes-Martineau V, et al. Human papillomavirus genotype distribution and cervical squamous intraepithelial lesions among high-risk women with and without HIV-1 infection in Burkina Faso. Br J Cancer. 2006;95:355–362. doi: 10.1038/sj.bjc.6603252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denny LA, Franceschi S, de Sanjose S, Heard I, Moscicki AB, Palefsky J. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine. 2012;30(suppl 5):F168–174. doi: 10.1016/j.vaccine.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 10.WHO. [November 16, 2014];WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. 2013 Available at: http://apps.who.int/iris/bitstream/10665/94830/1/9789241548694_eng.pdf. [PubMed]
- 11.Aerssens A, Claeys P, Garcia A, et al. Natural history and clearance of HPV after treatment of precancerous cervical lesions. Histopathology. 2008;52:381–386. doi: 10.1111/j.1365-2559.2007.02956.x. [DOI] [PubMed] [Google Scholar]
- 12.Elfgren K, Jacobs M, Walboomers JM, Meijer CJ, Dillner J. Rate of human papillomavirus clearance after treatment of cervical intraepithelial neoplasia. Obstetrics and Gynecology. 2002;100:965–971. doi: 10.1016/s0029-7844(02)02280-9. [DOI] [PubMed] [Google Scholar]
- 13.Chumworathayi B, Thinkhamrop J, Blumenthal PD, Thinkhamrop B, Pientong C, Ekalaksananan T. Cryotherapy for HPV clearance in women with biopsy-confirmed cervical low-grade squamous intraepithelial lesions. Int J Gynaecol Obstet. 2010;108:119–122. doi: 10.1016/j.ijgo.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Moten A, Schafer D, Farmer P, Kim J, Ferrari M. Redefining global health priorities: Improving cancer care in developing settings. J Glob Health. 2014;4:010304. doi: 10.7189/jogh.04.010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fokom-Domgue J, Vassilakos P, Petignat P. Is screen-and-treat approach suited for screening and management of precancerous cervical lesions in Sub-Saharan Africa? Prev Med. 2014;65:138–140. doi: 10.1016/j.ypmed.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Jaquet A, Horo A, Charbonneau V, et al. Cervical human papillomavirus and HIV infection in women of child-bearing age in Abidjan, Cote d’Ivoire, 2010. Br J Cancer. 2012;107:556–563. doi: 10.1038/bjc.2012.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palefsky JM, Minkoff H, Kalish LA, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst. 1999;91:226–236. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- 18.Hanisch RA, Sow PS, Toure M, et al. Influence of HIV-1 and/or HIV-2 infection and CD4 count on cervical HPV DNA detection in women from Senegal, West Africa. J Clin Virol. 2013;58:696–702. doi: 10.1016/j.jcv.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider A, Kirchhoff T, Meinhardt G, Gissmann L. Repeated evaluation of human papillomavirus 16 status in cervical swabs of young women with a history of normal Papanicolaou smears. Obstetrics and Gynecology. 1992;79:683–688. [PubMed] [Google Scholar]
- 20.Sahasrabuddhe VV, Mwanahamuntu MH, Vermund SH, et al. Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. Br J Cancer. 2007;96:1480–1483. doi: 10.1038/sj.bjc.6603737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5:705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 22.Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006;24(suppl 3):106–113. doi: 10.1016/j.vaccine.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 23.Kirby T. FDA approves new upgraded Gardasil 9. Lancet Oncol. 2015;16:e56. doi: 10.1016/S1470-2045(14)71191-X. [DOI] [PubMed] [Google Scholar]
- 24.Kojic EM, Kang M, Cespedes MS, et al. Immunogenicity and Safety of the Quadrivalent Human Papillomavirus Vaccine in HIV-1-Infected Women. Clin Infect Dis. 2014;59:127–35. doi: 10.1093/cid/ciu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis. 2007;196:1438–1446. doi: 10.1086/522864. [DOI] [PubMed] [Google Scholar]
- 26.Clark LR, Myers ER, Huh W, et al. Clinical trial experience with prophylactic human papillomavirus 6/11/16/18 vaccine in young black women. J Adolesc Health. 2013;52:322–329. doi: 10.1016/j.jadohealth.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 28.Kahn JA, Xu J, Kapogiannis BG, et al. Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women. Clin Infect Dis. 2013;57:735–744. doi: 10.1093/cid/cit319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campos NG, Kim JJ, Castle PE, et al. Health and economic impact of HPV 16/18 vaccination and cervical cancer screening in Eastern Africa. Int J Cancer. 2012;130:2672–2684. doi: 10.1002/ijc.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5(6):e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]


