Abstract
Retinoids (vitamin A and its derivatives) are critical for a spectrum of developmental and physiological processes, in which steroid hormones also play indispensable roles. The StAR protein predominantly regulates steroid biosynthesis in steroidogenic tissues. We reported that regulation of retinoid, especially atRA and 9-cis RA, responsive StAR transcription is largely mediated by an LXR-RXR/RAR heterodimeric motif in the mouse StAR promoter. Herein we demonstrate that retinoids are capable of enhancing StAR protein, P-StAR, and steroid production, in granulosa, adrenocortical, glial, and epidermal cells. Whereas transient expression of RARα and RXRα enhanced 9-cis RA-treated StAR gene transcription, silencing of RXRα with siRNA, decreased StAR and steroid levels. An oligonucleotide probe encompassing an LXR-RXR/RAR motif bound to adrenocortical and epidermal keratinocyte NEs in EMSAs. ChIP studies revealed association of RARα and RXRα with the StAR proximal promoter. Further studies demonstrated that StAR mRNA levels decreased in diseased and elderly men and women skin tissues and that atRA could restore steroidogenesis in epidermal keratinocytes of aged individuals. These findings provide novel insights into the relevance of retinoid signaling in the up-regulation of steroid biosynthesis in various target tissues, and indicate that retinoid therapy may have important implications in age-related complications and diseases.
Keywords: Retinoids, RAR, RXR, cAMP/PKA, StAR expression, steroid biosynthesis, steroidogenic cells
1. Introduction
Retinoids, the metabolically active structural derivatives of vitamin A, especially atRA and 9-cis RA, exert a wide range of effects on development, differentiation, reproduction and epidermal homeostasis (Clagett-Dame and Knutson, 2011; Inoue et al., 2012; Jacobs et al., 2006; Vernet et al., 2006). The biological actions of retinoids are principally mediated by the activities of two families of nuclear receptors, the RARs and RXRs, each of which has three subtypes (α, β and γ) with additional isoforms resulting from alternative splicing (Chambon, 2005; Lefebvre et al., 2005). Whereas RARs are activated by both atRA and 9-cis RA, RXRs are induced solely by 9-cis RA. Both RARs and RXRs are members of the steroid/thyroid hormone receptor superfamily of transcription factors that form either hetero- or homo-dimers and bind to a retinoid response element, termed the RARE/RXRE. This element is a direct repeat (DR) of two hexameric half-sites with the consensus sequences 5’-PuG(G/T)TCA-3’, their inverted or everted forms being present in the regulatory region of target genes (Chambon, 2005; Kliewer et al., 1992; Lefebvre et al., 2010; Mangelsdorf et al., 1990). However, RXR-RAR heterodimers are the functional units that transduce the retinoid signal (Chambon, 2005; Lefebvre et al., 2010). RARs and RXRs are expressed in varying amounts in steroidogenic tissues, suggesting they may be independently regulated, respond to discrete ligands, and perform distinct cellular functions (Clagett-Dame and Knutson, 2011; Manna et al., 2014). Of note, mice lacking RARα, RARγ, RXRα, and RXRβ display profound anomalies in gonadal and adrenal functions, including sterility and/or embryonic lethality (Chung et al., 2004; Clagett-Dame and Knutson, 2011; Li and Clagett-Dame, 2009; Mark et al., 2006), underscoring the importance of retinoid signaling in reproduction as well as steroidogenesis.
The intramitochondrial transport of cholesterol is the rate-limiting and regulated step in steroid biosynthesis, a process that is primarily mediated by the StAR protein, a rapidly synthesized mitochondrial phosphoprotein whose expression, activation, and removal is influenced by PKA, PKC, and a host of other signaling pathways that produce both acute and chronic effects on steroidogenesis (Clark et al., 1994; Manna and Stocco, 2005; Miller and Bose, 2011; Stocco et al., 2005). At the level of the mitochondria, cytochrome P450scc (CYP11A1) cleaves the side chain of cholesterol to form the first steroid, pregnenolone, which is further converted by a series of enzymes to various steroid hormones in pertinent tissues [reviewed in Refs. (Manna et al., 2009;Manna and Stocco, 2005; Miller and Bose, 2011; Stocco et al., 2005)]. Noteworthy, phosphorylation of StAR, especially at Ser194, has been demonstrated to be an indispensable event to obtain the maximal cholesterol transferring activity of StAR for steroid biosynthesis (Jo et al., 2005; Stocco et al., 2005; Manna et al., 2006; Manna et al., 2009a; Manna et al., 2013). In a recent study, we have reported that retinoids, by interacting with an LXR-RXR/RAR motif, synergistically activate cAMP/PKA stimulated StAR expression and steroid biosynthesis in MA-10 mouse Leydig cells (Manna et al., 2014). Additionally, the cooperation/interaction between LXR and RXR/RAR in influencing StAR transcription and steroidogenesis has been shown in gonadal and adrenal cells (Manna et al., 2013; Manna et al., 2014). However, the mechanisms of action of retinoids in the regulation of the steroidogenic response remain elusive.
With aging, multifaceted changes in the neuroendocrine system result in a decline in various hormones, including steroids and, thus, many physiological activities. Age-related complications and diseases are also frequently associated with decreases in retinoid metabolism and signaling (Mihaly et al., 2011; Olson and Mello, 2010; Ono and Yamada, 2012). Noteworthy, the systemic administration of retinoids, especially RAs, reverses most reproductive and developmental blocks in vitamin A deficient (VAD) rats/mice, demonstrating that retinoid signaling rescues reproductive defects as well as steroidogenesis in VAD animals (Chung and Wolgemuth, 2004; Clagett-Dame and Knutson, 2011; Mark et al., 2006). The involvement of retinoids in skin physiology/pathophysiology has long been known. Human skin possesses endocrine functions that include the capabilities to synthesize cholesterol and express StAR and also house the functional biochemical apparatus for the synthesis of glucocorticoids and sex steroids de novo or from precursors of systemic origin (Cirillo and Prime, 2011; Slominski and Wortsman, 2000; Slominski et al., 2013a; Slominski et al., 2014; Zouboulis, 2009). A question remains as to whether retinoids can reverse the decline in steroid biosynthesis in target tissues and thereby maintain steroid requiring physiological activities that become diminished during aging. The experimental approaches utilized here provide evidence that retinoids up-regulate the steroidogenic response in several different endocrine tissues and that retinoid signaling is able to enhance/restore the age associated decline in steroid biosynthesis in epidermal keratinocytes.
2. Materials and methods
2.1. Cells, plasmids, transfections, and luciferase assays
Mouse granulosa KK-1 (Manna et al., 2009b; Manna et al., 2013), human astroglial A172 (Davis and Syapin, 2004), adrenocortical H295R (Clark et al., 1995; Tu et al., 2014) and epidermal keratinocyte HaCaT (Boukamp et al., 1988; Slominski et al., 2013a) cells were respectively cultured in DMEM/F12 medium (Invitrogen Life Technologies, Inc., Grand Island, NY) plus 10% FBS, DMEM/F12 with 1% ITS plus, 2.5% NuSerum, DMEM/F12 with 10% FBS and 1% nonessential amino acids, and F-12K medium with 10% HS, containing antibiotics.
The 5-flanking −254/−1 bp region of the mouse StAR promoter was synthesized using a PCR based cloning strategy and inserted into the XhoI and HindIII sites of the pGL3 basic vector (Promega, Madison, WI) that contains firefly luciferase as a reporter gene (Manna et al., 2002; Manna et al., 2013; Manna et al., 2014). The −254/−1 bp StAR segment was used for generating mutations in the LXR-RXR/RAR heterodimeric motif 5’-TGACCCCTGCTTTCCC-3’ (−200/−185 bp region in the mouse StAR promoter, Wt-LXR-RXR/RAR) using the Quikchange site directed mutagenesis kit (Stratagene, La Jolla, CA) (Manna et al., 2014). The sense strand of the oligonucleotide sequence used in mutating the LXR-RXR/RAR site was 5’-CCGTGAattCTGCTTgatCTATATG-3’ (Mut-LXR-RXR/RAR; mutated bases in lowercase boldface letters) and the mutation was verified by EcoRI and Sau3A1. The pRL-SV40 plasmid containing the Renilla luciferase gene driven by SV40 promoter was obtained from Promega. Expression plasmids for RARα and RXRα isoforms have been previously described (Manna et al., 2014). All plasmids were confirmed by either restriction edonuclease digestion or sequencing on a PE Biosystems 310 Genetic Analyzer (Perkin-Elmer, Boston, MA).
For transfection studies, different cell types were cultured in either 6- or 12-well plates to ~70% confluence and were transfected using Lipofectamine 2000 transfection reagent (Invitrogen), under optimized conditions (Manna et al., 2011; Manna et al., 2013; Manna et al., 2014). Transfection efficiency was normalized by co-transfecting 10-20 ng pRL-SV40 vector. The amount of DNA used in transfections was equalized with an empty expression vector.
Luciferase activity in the cell lysates was determined by the Dual-luciferase reporter assay (Promega) system (Manna and Stocco, 2007; Manna et al., 2009a; Manna et al., 2014). Following treatments, cells were washed with 0.01 M PBS and 250 μl of the reporter lysis buffer was added to the cells. Cellular debris was pelleted by centrifugation at 12,000 × g for 10 min at 4 °C, and the supernatant was measured for relative light units (luciferase/Renilla) using a TD 20/20 Luminometer (Turner Designs, Sunnyvale, CA).
2.2. Immunoblotting
Immunoblotting studies were carried out using total cellular protein (Manna et al., 2011; Manna et al., 2013; Manna et al., 2014). Briefly, equal amounts of protein were loaded onto 10-12% SDS-PAGE (Bio-Rad Laboratories, Inc., Hercules, CA). The proteins were electrophoretically transferred onto Immuno-Blot PVDF membranes, which were probed with the specific antibodies (Abs) that recognize StAR (Bose et al., 1999), P-StAR [Ser194; (Manna et al., 2006)], RARα, RXRα (Cell Signaling Technology, Beverly, MA), CYP11A1 (Chemicon International Inc., Temecula, CA), and β-actin (Applied Biosystems/Ambion, Austin, TX). Following overnight incubation with primary Abs, the membranes were washed and incubated with the appropriate horseradish peroxidase-conjugated secondary Abs for 1h at room temperature. The immunodetection of different proteins was determined using a Chemiluminescence Imaging Kit (Perkin-Elmer), and the intensity of bands was quantified using a computer-assisted image analyzer (Quantity One Software, Bio-Rad Laboratories).
2.3. Isolation of epidermal keratinocytes from human skin tissues
De-identified human skin tissues of elderly men and women (64-83 years) were obtained from the Department of Dermatology clinic, Texas Tech University Health Sciences Center (TTUHSC; Institutional Review Board (IRB) approval, #L14-085), following various surgeries. Epidermal keratinocytes were isolated from these tissues and grown in DMEM/F12 medium supplemented with 10% FBS, 1% glutamine, epidermal growth factor (10 ng/ml) containing 10000 U/L penicillin and 50 mg/L streptomycin (Hannen et al., 2011; Slominski et al., 2015;Tiala et al., 2007). Briefly, after removing the dermis and connective tissue, the sample was chopped into small pieces, incubated with 0.25% trypsin for 1-2h at 37 °C, and the epidermis was separated from the membrane using needles to release basal keratinocytes. The cell suspension was centrifuged at 1000 × g for 5 min at room temperature, the pellet containing keratinocytes was resuspended in DMEM/F12 growth medium and seeded in 60 × 15 mm tissue culture flasks, in which media were changed every alternate day. When plates were 70-80% confluent, primary keratinocytes were utilized for experiments.
2.4. Extraction of RNA from skin tissues and semi-quantitative RT-PCR
Total RNA was extracted from primary cultures of isolated epidermal keratinocytes of elderly individuals using Trizol reagent (GIBCO-BRL, Grand Island, NY) (Manna et al., 2013; Manna et al., 2014). Extractions of total RNA from formalin fixed, paraffin-embedded, human skin tissues (IRB# L12-084) were carried out with optimized procedures (Hennig et al., 2010; Slominski et al., 2013a; Zhang et al., 2010). Briefly, skin tissues of various ages (14-86 years) and body locations, obtained from the Dermatology clinic at TTUHSC, were deparaffinized using xylene and then incubated in ethanol. Following aspiration of the ethanol, tissue samples were resuspended in 500 μl of RNA lysis buffer (10 nmol/L Tris-HCl, pH 8.0, 0.1 mmol/L EDTA, 2% SDS, pH 7.3) containing 50 μl of 60 mg/ml proteinase K, and incubated overnight at 60-65 °C. Total RNA was purified using two sequential extraction procedures with phenol:chloroform (70:30), and precipitated with isopropanol containing 1/10 volume of 3 mol/L sodium acetate (pH 5.2) and 20 mg/ml of carrier glycogen at −20 °C for 1-2 h. The RNA pellet was washed in 75% ethanol, treated with DNase (10 μg/ml) at 37 °C for 30 min and purified.
A semi-quantitative RT-PCR procedure was employed for amplifying StAR and L19 cDNAs, utilizing the following primer pairs; StAR sense, 5’-GACCTTGAAAGGCTCAGGAAGAAC-3’, StAR antisense, 5’-TAGCTGAAGATGGACAGACTTGC-3’, L19 sense, 5’-GAAATCGCCAATGCCAACTC-3’, and L19 antisense, 5’-TCTTAGACCTGCGAGCCTCA-3’, under optimized conditions (Manna et al., 1999; Manna et al., 2013; Manna et al., 2014). Briefly, RT and PCR were run consecutively in the same assay, which included [α32P]-dCTP (PerkinElmer) in the dNTP mixture. The molecular sizes of StAR (980 bp) and L19 (405 bp) were determined on 1.2% agarose gels, which were vacuum dried and exposed to X-ray films (Phenix Research Products, Candler, NC). Levels of StAR and L19 signals were quantified using an image analyzer (Quantity One Software).
2.5. Silencing of RXRα
Knockdown of RXRα in different cells was performed with small interfering RNA (siRNA) using Lipofectamine 2000 (Invitrogen) under optimized conditions (Manna et al., 2013; Manna et al., 2014; Tu et al., 2014). Silencer negative control and human RXRα (sense, 5’-AGGACUGCCUGAUUGACAAtt-3’; antisense, 5’-UUGUCAAUCAGGCAGUCCUtg-3’) siRNAs were obtained as annealed oligos from Ambion (Austin, TX). Cells were transfected with RXRα siRNA at 100 nM. Following 36-48h of transfections, cells were utilized in treatments.
2.6. Electrophoretic mobility shift assays (EMSAs)
EMSA experiments were performed using H295R and HaCaT nuclear extracts (NEs) that were prepared following the procedures described previously (Manna et al., 2004; Manna and Stocco, 2007; Manna et al., 2009c; Manna et al., 2014). The sense strands of the oligonucleotide sequences used were: LXR-RXR/RAR, 5’-GGTGACCCCTGCTTTCCC-3’ (Manna et al., 2014) and RARE-DR5, GGAGGGTTCACCGAAAGTTCACTCGCA (de The et al., 1990). The 5’-GG overhangs in the double-stranded oligonucleotides were end-labeled with [α32P]-dCTP (Perkin-Elmer) using Klenow (Promega) fill-in reaction, and protein:DNA binding assays were performed (Manna et al., 2011; Manna et al., 2014). Briefly, NE (10-15 μg) was incubated for 15-20 min at room temperature in a 25 μl reaction buffer (25 mM Tris-HCl, 1 mM EDTA, 4% Ficoll, 10 mM dithiothreitol, 2 μg poly dIdC, 40 ng/μl BSA, and 12 mM MgCl2, pH 7.9) before the addition of a 32P-labeled probe either alone or in the presence of unlabeled oligonucleotide. When Abs were used, the reactions were carried out for an additional 45-60 min on ice. Reactions were then subjected to electrophoresis on 5% PAGE gels in 0.5 × TBE buffer (90 mM Tris-borate, 2 mM EDTA, pH 8.3). The gels were dried, exposed to X-ray film (Phenix Research Products), and protein:DNA complexes were analyzed.
2.7. Chromatin immunoprecipitation (ChIP) assay
ChIP assays were carried out using a kit (Upstate/Chemicon, Temecula, CA) following the manufacturer’s instructions, as described previously (Manna et al., 2007; Manna et al., 2011; Manna et al., 2014). Briefly, following treatments, H295R and HaCaT cells were incubated with 1% formaldehyde for 10 min at 37 °C to crosslink DNA and its associated proteins. Cells were washed, scraped, collected in lysis buffer and sonicated for 9-10 cycles of 6 sec pulses using a Tekmar Sonic Disruptor (Fisher Scientific, Pittsburgh, PA). The supernatant containing chromatin was cleared with a protein A agarose/salmon sperm DNA 50% slurry for 30 min at 4 °C with agitation. After centrifugation, the supernatant was immunoprecipitated with 4 μg of Abs specific to RARα and RXRα for ~16h at 4 °C, followed by incubation with Protein A agarose/salmon sperm DNA for an additional 1h. After washing, protein-DNA complexes were eluted with freshly made elution buffer (1% SDS, 0.1 M NaHCO3), and the eluate containing 5M NaCl was incubated at 65 °C for 4h to reverse the formaldehyde cross-linking. The resulting samples were treated with 0.5 M EDTA, 1 M Tris-HCl (pH 6.5) and 10 mg/ml proteinase K for 1h at 45 °C, and the purified DNA samples were used for PCR using [α32P]-dCTP in the dNTP mixture. PCR was performed with ~100 ng of DNA and the mouse StAR promoter (forward, 5’-GTCTACTTTAGAGAAGCTAT-3’ (bases −255/−227), and reverse, 5’-GAAGGCTGTGCATCATCACTTGAG-3’ (bases −62/−39), as described recently (Manna et al., 2014). PCR products were determined on 2% agarose gels, which were vacuum dried, exposed to X-ray film for 1-3h, and the resulting signals were analyzed.
2.8. Statistical analysis
All experiments were repeated at least three times. Statistical analysis was performed by ANOVA using Statview (Abacus Concepts Inc., Berkeley, CA) followed by Fisher’s protected least significant differences test. Data presented are the mean ± SE, and p < 0.05 was considered statistically significant.
3. Results
3.1. Assessment of the role of retinoids in StAR expression and steroidogenesis in classical (gonadal and adrenal) and non-classical (glial and epidermal) steroidogenic cells
The involvement of retinoids in (Bu)2cAMP and/or type I/II PKA analog stimulated StAR expression and steroid biosynthesis has been recently demonstrated in MA-10 Leydig cells (Manna et al., 2014). The importance of retinoid signaling in the regulation of steroidogenic response was further assessed using a variety of clonal and primary cell culture models (Fig. 1). KK-1 (A) and H295R (B) cells treated with atRA (10 μM; (Manna et al., 2014)), for 6h, enhanced StAR protein (2.7 ± 0.4 and 2.9 ± 0.3 fold) and progesterone (6 ± 1.1 and 8 ± 1.3 fold) production, over their respective basal levels. Additionally, treatment of A172 (C) and HaCaT (D) cells with atRA, for 24h, resulted in increases in StAR protein (2.4 ± 0.3 and 2.6 ± 0.2 fold) and pregnenolone synthesis (5 ± 0.9 and 7 ± 1.2 fold), respectively. A172 and HaCaT cells were treated in the presence of SU-10603 (20 μM) and cyanoketone (5 μM) for blocking the pregnenolone metabolism (Karri et al., 2007). Phosphorylation of StAR at Ser194 was undetected in control and atRA treated cells. A suboptimal concentration of (Bu)2cAMP (0.1 mM) displayed little to no effects on StAR and steroid levels in these cells. Addition of (Bu)2cAMP to the atRA incubations significantly enhanced (p < 0.01) StAR, P-StAR, and steroid levels in all of the cell types tested. CYP11A1 protein levels were unaltered in KK-1 and H295R cells in response to atRA, but moderately increased (p < 0.05) in A172 and HaCaT keratinocytes, suggesting retinoid-responsive steroid biosynthesis involves differential regulation of this enzyme in classical vs. non-classical steroidogenic tissues.
Fig. 1.
Effect of atRA on (Bu)2cAMP stimulated StAR, P-StAR, CYP11A1, and steroid levels in KK-1, H295R, and A172 cells, and in HaCaT keratinocytes. These cells were treated without (Basal) or with atRA (10 μM), (Bu)2cAMP (0.1 mM), or their combination, for either 6h (A and B) or 24h (C and D), as indicated, and subjected to cellular protein preparation. A172 and HaCaT cells were treated in the presence of SU-10603 (20 μM) and cyanoketone (5 μM). Representative immunoblots illustrate StAR, P-StAR, and CYP11A1 levels using 20-30 μg (A and B) or 60-70 μg (C and D) of total protein. Immunoblots shown are representative of 4-6 independent experiments. β-actin expression was assessed for loading controls. Accumulation of progesterone (A and B; bottom panels) and pregnenolone (C and D; bottom panels) in the media was determined from different groups and expressed as ng/mg protein. Data represent the mean ± SE of 4 independent experiments. Different letters above the bars indicate that these groups differ significantly from each other at least at p < 0.05. Note the different scales on the graphs.
3.2. Overexpression and silencing of RARα and/or RXRα on StAR gene transcription and steroidogenesis
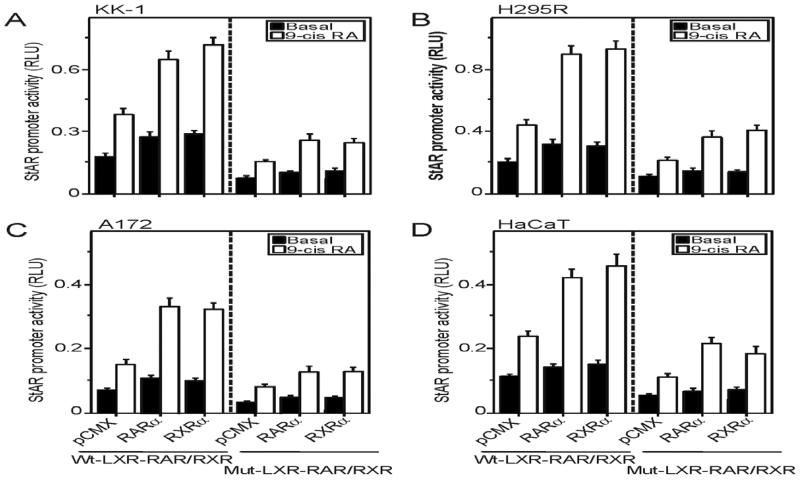
The significance of retinoid-induced StAR expression and steroid biosynthesis was next evaluated in conjunction with RARα and RXRα in different steroidogenic cells. Utilization of these isoforms was based on previous findings (Clagett-Dame and Knutson, 2011; Manna et al., 2014). As illustrated in Fig. 2, KK-1 (A), H295R (B), A172 (C), and HaCaT (D) cells transfected with either pCMX-RARα (RARα) or pCMX-RXRα (RXRα) expression plasmid, within the context of the −254/−1 bp StAR-Luc containing wild type LXR-RXR/RAR (Wt-LXR-RXR/RAR), resulted in 2-3 fold increases in StAR promoter activity in response to 10 μM 9-cis RA, over the responses seen in mock-transfected (pCMX) cells. Conversely, cells transfected with the −254/−1 bp StAR-Luc containing mutations in the LXR-RXR/RAR motif (Mut-LXR-RXR/RAR) decreased basal luciferase responses by 40-66% and coordinately repressed 9-cis RA-induced StAR reporter activity, demonstrating the importance of this putative element in retinoid mediated StAR gene transcription.
Fig. 2.
Overexpression of RARα and RXRα in 9-cis RA induced StAR promoter activity. KK-1 (A), H295R (B), A172 (C), and HaCaT (C) cells were transfected with pCMX, pCMX-RARα (RARα), and pCMX-RXRα (RXRα), within the context of either wild type −254/−1 bp StAR-Luc (Wt-LXR-RXR/RAR) or mutant (Mut-LXR-RXR/RAR) plasmid, as indicated, in the presence of pRL-SV40. Following 36h of transfection, cells were treated without (Basal) or with 9-cis RA (10 μM), for either an additional 6h (A and B) or 24h (C and D). Luciferase activity in the cell lysates was determined and expressed as StAR promoter activity, RLU (luciferase/Renilla). Data represent the mean ± SE of 3-5 independent experiments.
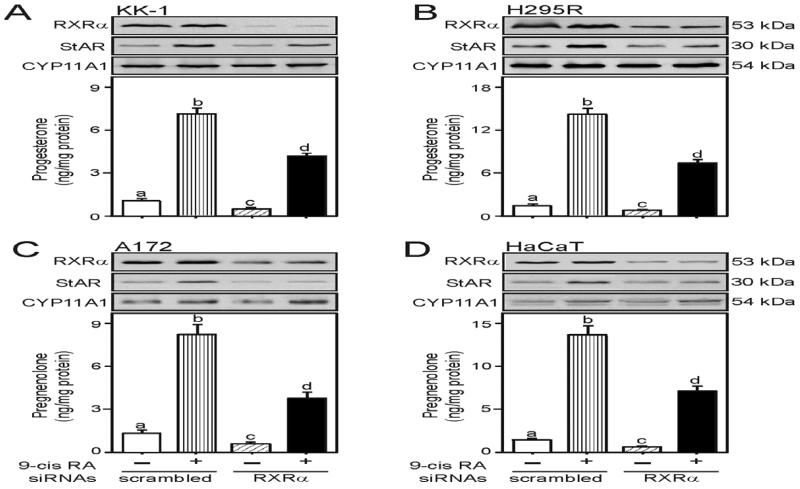
In additional studies, transfection of KK-1 (A), H295R (B), A172 (C), and HaCaT (D) cells with 100 nM of specific human RXRα siRNA resulted in 72-86% decreases in endogenous RXRα protein levels in these cells when compared with scrambled siRNAs (Fig. 3). Treatment with 9-cis RA had no apparent effects in RXRα protein levels in all of these cell lines tested. Depletion of RXRα attenuated basal and 9-cis RA-induced StAR and steroid levels between 43 and 68%. Expression of the CYP11A1 protein was unaltered, ruling out nonspecific silencing. Altogether, these results suggest that retinoid signaling enhances StAR protein and steroid biosynthesis by activating transcription of the StAR gene in granulosa, adrenal, glial and epidermal cells.
Fig. 3.
Silencing of RXRα in 9-cis RA induced StAR protein and steroid levels. KK-1 (A), H295R (B), A172 (C), and HaCaT (D) cells were transfected with either 100 nM of negative control (scrambled) or specific human RXRα (RXRα) siRNA, as indicated. Following 36-48h of transfection, cells were treated without or with 9-cis RA (10 μM) for either an additional 6h (A and B) or 24h (C and D), and subjected to cellular protein preparation. Representative immunoblots illustrate expression of RXRα, StAR, and CYP11A1 in different treatment groups. Immunoblots are representative of 4-6 independent experiments. Accumulation of progesterone (A and B; bottom panels) and pregnenolone (C and D; bottom panels) in the media was determined and expressed as ng/mg protein (n = 4, ± SE). Different letters above the bars indicate that these groups differ significantly from each other at least at p < 0.05. Note the different scales on the graphs.
3.3. Functional relevance of the LXR-RXR/RAR element in StAR gene expression
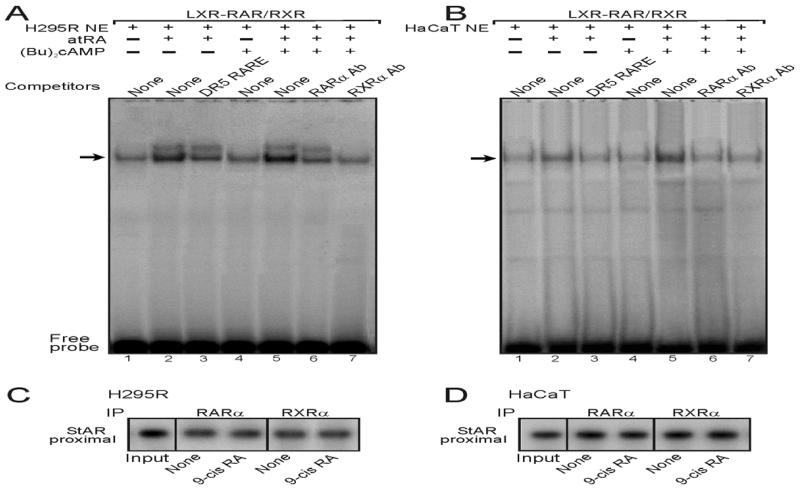
To better understand the involvement of the LXR-RXR/RAR heterodimeric motif in retinoid responsive StAR gene transcription, protein:DNA binding studies were carried out using EMSAs. The results presented in Fig. 4A show that a 32P-labeled oligonucleotide probe corresponding to the LXR-RXR/RAR region (a DR4-like sequence; (Manna et al., 2014)) in the mouse StAR promoter resulted in a major protein:DNA complex with H295R NE (lanes 1-7). Protein:DNA binding was increased with atRA (10 μM, 6h) treated NE (compare lanes 1 and 2), which was competitively inhibited with an unlabeled DR5 RARE consensus (de The et al., 1990) sequence (lane 3). NE obtained from (Bu)2cAMP (0.1 mM) stimulated H295R cells displayed no effect on protein:DNA binding (lane 4). The combined treatments with atRA and (Bu)2cAMP further elevated protein:DNA complex (lane 5), and the binding was affected by both RARα (lane 6) and RXRα (lane 7) Abs. Protein:DNA binding was markedly inhibited with RXRα Ab, suggesting NE binding to the LXR-RXR/RAR motif are largely RXR members. The LXR-RXR/RAR element was previously found to bind in vitro transcribed/translated RARα and RXRα proteins in EMSAs, as well as MA-10 NE, and protein:DNA binding was affected by its unlabeled and mutant sequences (Manna et al., 2014). Additionally, a 32P-labeled LXR-RXR/RAR probe displayed qualitatively similar results with HaCaT NE (Fig. 4B, lanes 1-7) in a retinoid response manner, suggesting the ability of this motif to bind the RAR and RXR family proteins.
Fig. 4.
Binding of the LXR-RXR/RAR motif in the StAR promoter to H295R and HaCaT NE in EMSAs, and association of RARα and RXRα with the StAR promoter by ChIP analyses. NE (10-15 μg) obtained from control, atRA (10 μM), (Bu)2cAMP (0.1 mM), and atRA plus (Bu)2cAMP treated H295R (A) and HaCaT (B) cells were incubated with the 32P-labeled LXR-RXR/RAR probe. A major protein:DNA complex was observed with the LXR-RXR/RAR probe and H295R (A) and HaCaT (B) NE (lanes 1-7 in both cases). Protein:DNA binding was challenged with unlabeled DR5 RARE consensus sequence (lane 3) and, RARα (lane 6) and RXRα (lane 7) Abs (A and B). Cold competitor was used at 100-fold molar excess. Migration of free probes is shown in panels A and B. Data are representative of 3-4 independent experiments. ChIP assays were carried out as described under Materials and Methods (C and D). Crosslinked sheared chromatin obtained from control (None) and 9-cis RA treated groups was immunoprecipitated (IP) without or with anti-RARα and anti-RXRα Abs. Recovered chromatin was subjected to PCR analysis using primers specific to the proximal −255/−39 bp region of the StAR promoter, as specified under Materials and Methods. Representative autoradiograms illustrate the association of RARα and RXRα with the StAR promoter. Data shown are representative of 3 independent experiments.
To obtain more insight into these mechanisms, ChIP analyses were performed. As illustrated in Fig. 4, H295R (C) and HaCaT (D) cells treated with 9-cis RA (10 μM), which activates both RARs and RXRs, did not affect the association of RARα and RXRα isoforms with the proximal StAR promoter when compared with untreated controls. Association of these RAR and RXR isoforms was observed with neither distal StAR promoter nor IgG (data not shown), observations in agreement with our recent findings (Manna et al., 2014). Taken together, these results indicate that regulation of retinoid mediated StAR gene transcription is predominantly mediated by the LXR-RXR/RAR heterodimeric motif.
3.4. Expression of StAR mRNA in aging and diseased skin tissues, and the influence of retinoid signaling on the steroidogenic response in aged epidermal keratinocytes
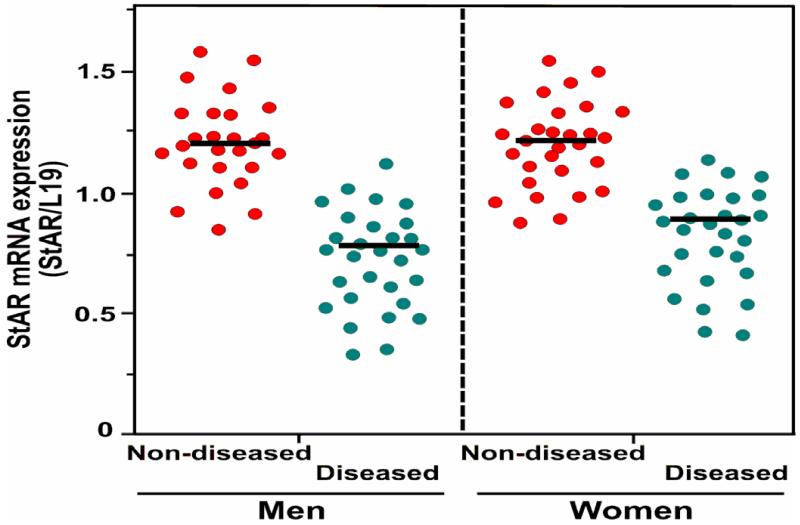
Aberrant skin cholesterol synthesis, resulting in a global reduction in steroids, is associated with many skin disorders (Elias et al., 2011; Slominski et al., 2013a; Slominski et al., 2013b); thus, it was of interest to study cutaneous steroidogenesis. The results summarized on a scatter plot revealed an inverse correlation between StAR mRNA expression and age (Fig. 5). StAR mRNA levels were decreased in skin tissues of elderly men and women (37-86 years) when compared with younger (14-35 years) individuals, suggesting an attenuation of steroid biosynthesis in aging skin of both sexes.
Fig. 5.
Expression of StAR mRNA in men and women skin tissues. De-identified, formalin fixed, paraffin-embedded skin specimens of various ages (14-86 years) and body locations were obtained from the Dermatology clinic at TTUHSC. Total RNA from these skin specimens was extracted and purified using the procedures described under Materials and Methods. StAR mRNA levels in different samples were determined by a semi-quantitative RT-PCR. A total of 106 skin specimens were analyzed and were made up of the following samples: elderly (37-86 years; 25 men and 24 women) and young (14-35 years; 26 men and 31 women).
The levels of StAR mRNA were also reduced in many inflammatory skin diseases, including psoriasis, intertrigo, eczema, and atopic dermatitis, and warts (human papillomavirus etiology), when compared to anatomic site-, age- and sex-matched non-diseased samples (Fig. 6). These data indicate that steroid biosynthesis is deceased in these skin disorders.
Fig. 6.
Relative levels of StAR mRNA in various inflammatory skin diseases. De-identified, formalin fixed, paraffin-embedded diseased and non-diseased men and women skin specimens were obtained from the Dermatology clinic at TTUHSC. Total RNA from these skin tissues was extracted and purified using procedures as described in the legend of Fig. 5 and under Materials and Methods. StAR mRNA levels in these skin specimens (a total of 87 samples were analyzed; 46 men and 41 women) were determined by a semi-quantitative RT-PCR.
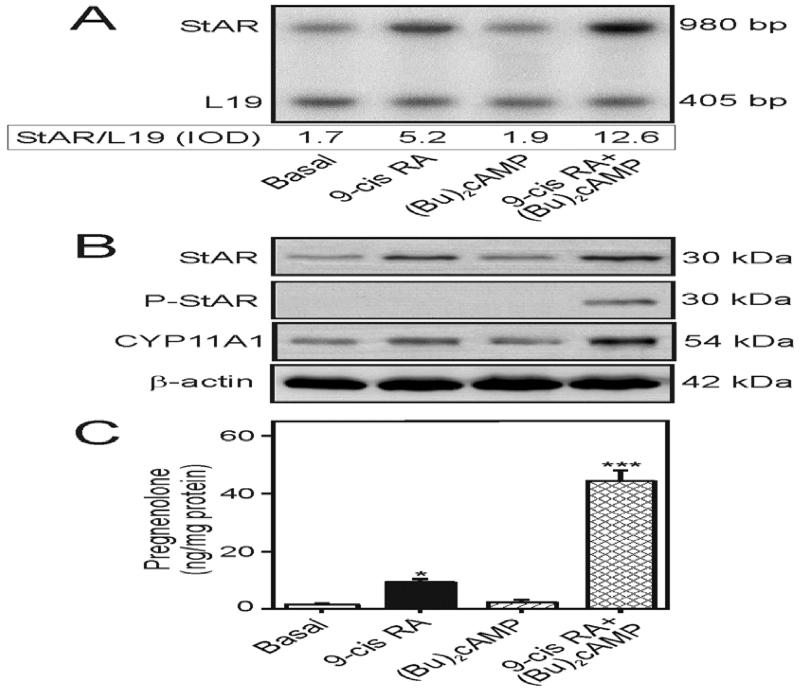
In additional studies, the hypothesis that retinoid signaling enhances the steroidogenic response in epidermal keratinocytes, and thereby can rescue steroid dependent functions in maintaining skin biological systems, was examined. Primary cultures of isolated epidermal keratinocytes of elderly men treated with 9-cis RA (10 μM), for 24h, in the presence of SU-10603 and cyanoketone, demonstrated increases in StAR mRNA (3.1 ± 0.4 fold), StAR protein (2.5 ± 0.3 fold), and pregnenolone (6 ± 1.2 fold) levels, over their respective basal values (Fig. 7). P-StAR was undetected in response to 9-cis RA. Whereas (Bu)2cAMP had no effects on these parameters, it markedly enhanced 9-cis RA-induced StAR, P-StAR and pregnenolone levels. Studies, albeit limited, on isolated epidermal keratinocytes from elderly women treated with 9-cis RA reflect qualitatively similar phenomena on StAR and pregnenolone levels (data not shown). These results indicate that retinoid signaling is capable of restoring steroid biosynthesis in aged skin keratinocytes that may play an important role in epidermal homeostasis.
Fig. 7.
Effect of 9-cis RA on (Bu)2cAMP-stimulated StAR mRNA, StAR protein, P-StAR, and pregnenolone levels in isolated epidermal keratinocytes. De-identified fresh skin tissues from elderly men (64-83 years; 5 different specimens) were obtained from the Dermatology clinic at TTUHSC upon surgery. Epidermal keratinocytes were isolated from these aged skin tissues following the procedures described under Materials and Methods. Primary cultures of isolated keratinocytes were treated without (Basal) or with 9-cis RA (10 μM), (Bu)2cAMP (0.1 mM), or their combination, for 24h, as indicated, in the presence of SU-10603 (20 μM) and cyanoketone (5 μM). Following treatments, cells were processed for either total RNA extraction or cellular protein preparation. A representative autoradiogram illustrates expression of StAR mRNA in different treatment groups using a semi-quantitative RT-PCR (A). Integrated optical density (IOD) values of each StAR band was quantified and normalized with the corresponding L19 bands and presented below the autoradiogram. Representative immunoblots illustrate StAR, P-StAR, and CYP11A1 levels in different treatment groups using 60-70 μg of total protein in each lane (B). Autoradiogram and immunoblots are representative of 3-5 independent experiments. β-actin expression was assessed as a loading control. Accumulation of pregnenolone in the media was determined from different treatment groups and expressed as ng/mg protein C). Data represent the mean ± SE of 4 independent experiments. *, p < 0.05; ***, p < 0.01; vs. basal.
4. Discussion
A hallmark of healthy physiological function is the ability to sense, respond to, and control innumerable processes, which require several regulatory systems to interact with one another. Complex and delicate endocrine changes affecting the structure and function of a multitude of organs, occur as life progresses from adulthood into senescence. This aging process results in a decline in various hormones and, as a consequence, affects many physiological functions (Chahal and Drake, 2007; Huhtaniemi and Forti, 2011; Makrantonaki and Zouboulis, 2007; Manor and Lipsitz, 2013). The occurrence of hormone deficiencies (or endocrinosenescence) is constituted to be an important cause of human senescence. Endocrinosenescence includes growth hormone/insulin-like growth factor-1 axis (somatopause), hypothalamic-pituitary gonadal axis (hypogonadism), testosterone (andropause), estradiol (menopause), and dehydroepiandrosterone (adrenopause) (Huhtaniemi and Forti, 2011; Janovick et al., 2013; Manor and Lipsitz, 2013; Shaw et al., 2009; Traub and Santoro, 2010; Veldhuis, 2013). The manifestations of these deficiencies include, but are not limited to, inefficient hypothalamic-pituitary-thyroidal-adrenal-gonadal (HPTAG) axes, decreased steroid biosynthesis, sexual dysfunction, depression, osteoporosis, increased risk of cardiovascular disease, and skin disorders. Preservation of hormonal balance is the key to proper functioning of various physiological activities during the course of healthy aging. A large body of epidemiological evidence indicates that eating a diet rich in vitamins has numerous health benefits, as well as protective effects on the development of diseases, and results in increased longevity (Hammar and Ostgren, 2013; Park et al., 2012; Thomas, 2006). One such category of vitamins, vitamin A (retinol) and its derivatives, notably RAs (collectively referred to as retinoids), have antioxidant properties, and play unique modulatory and integrative roles across multiple metabolic and physiological processes (Bikle, 2012; Clagett-Dame and Knutson, 2011; Das et al., 2014; Manna et al., 2014). The present findings expand our understanding of the action of retinoids by elucidating the events in which retinoid signaling up-regulates steroid biosynthesis in gonadal, adrenocortical, glial and epidermal cells, namely by increasing StAR expression, and that retinoids can reverse the decline in steroid biosynthesis in aged epidermal keratinocytes through this same mechanism.
Our current data demonstrate that RAs enhanced StAR expression and steroid levels, without altering StAR phosphorylation, in classical and non-classical steroidogenic cells. The activation of cAMP/PKA signaling, by a suboptimal dose of (Bu)2cAMP (0.1 mM), markedly elevated not only retinoid-induced StAR expression, but also its phosphorylation, concomitant with increased steroid production, demonstrating that a low level of PKA activity is critical in the modulation of steroidogenesis (Stocco et al., 2005; Manna et al., 2006; Manna et al., 2009a; Manna et al., 2013). The magnitude of induction on the steroidogenic response mediated by retinoids and (Bu)2cAMP is similar to that achieved with a maximally stimulating dose of (Bu)2cAMP (1.0 mM) (Manna et al., 2014; Slominski et al., 2015). Noteworthy, however, that steroidogenesis in glial and epidermal cells through retinoid signaling is quite modest when compared to cAMP/PKA stimulated StAR expression and steroid synthesis in gonadal and adrenal cells. As well, in contrast to the rapid induction of cAMP-responsive steroidogenesis in classical endocrine tissues, elevation in the steroidogenic potential requires a considerably longer period of time in non-classical target tissues. The latter event reinforces the notion that the chronic effect of retinoid-mediated steroidogenesis involves increased transcription/translation of the CYP11A1 enzyme (Wickenheisser et al., 2005). Hence, it is conceivable that regulation of retinoid dependent StAR expression and steroid production in gonadal and adrenal vs. glial and epidermal cells involve different mechanisms. We have reported that steroid biosynthesis in response to retinoids is influenced by hormone-sensitive lipase (HSL) in gonadal and adrenal cells (Manna et al., 2013; Manna et al., 2014); as such, the involvement of HSL in controlling retinoid-regulated steroidogenesis in non-classical steroidogenic tissues cannot be excluded. Nonetheless, it is worth mentioning (based on our preliminary data) that retinoid signaling increases StAR and LHβ mRNA levels in mouse pituitary gonadotrope LβT2 cells (Turgeon et al., 1996) (Manna PR. et al., unpublished observations). While there is yet no information demonstrating that the pituitary gland secretes steroid(s), this gland expresses cytochrome P450 17α-hydroxylase/C17, 20-lyase, 3β-HSD, and cytochrome P450 aromatase and has been proposed to synthesize androgens (Do Rego et al., 2007; Galmiche et al., 2006).
The contribution of retinoid signaling in the regulation of the steroidogenic response was elucidated by overexpression and silencing studies, utilizing RARα and/or RXRα, the functionally predominant retinoid isoforms in steroidogenic tissues (Chung et al., 2004; Clagett-Dame and Knutson, 2011; Manna et al., 2014; Mark et al., 2006). The present results document that an increase in either RARα or RXRα levels resulted in elevated retinoid responsive StAR gene transcription in KK-1, H295R, A172, and HaCaT cells, observations that are in agreement with our recent findings in MA-10 Leydig cells (Manna et al., 2014). In contrast, substantial knockdown of endogenous RXRα protein resulted in ~50% decreases in StAR expression and steroid biosynthesis, suggesting other isoforms may play permissive roles in steroidogenesis. These results are reminiscent of previous findings that demonstrated that disruption of RARα, RARγ, RXRα, and RXRβ isoforms exhibits abnormalities in gonadal and adrenal steroidogenic functions (Chung and Wolgemuth, 2004; Clagett-Dame and Knutson, 2011; Mark et al., 2006).
Regulation of StAR gene transcription has been shown to be coordinated by multiple trans-regulatory factors, which bind directly or indirectly to sequence-specific DNA elements located within the 5’-flanking ~250 bp region (that is highly conserved among different species) of the mouse StAR promoter, including an LXR-RXR/RAR motif (Clem et al., 2005; Cummins et al., 2006; Manna et al., 2003; Manna et al., 2009b; Manna et al., 2009c; Manna et al., 2014; Manna and Stocco, 2007). Transcriptional synergy requires the simultaneous interaction of multiple transcription factors with CREB binding protein (CBP) and its homolog p300 (CBP/p300) or additional relevant co-activators (Vo and Goodman, 2001; Manna and Stocco, 2007; Manna et al., 2014). In the present study, the functional relevance of the LXR-RXR/RAR element was assessed by different approaches demonstrating that alteration/inhibition of RARα and RXRα markedly affected the steroidogenic response. In addition, EMSA and ChIP analyses revealed the specificity of the LXR-RXR/RAR heterodimeric motif in retinoid-mediated regulation of StAR gene transcription. However, association of both RARα and RXRα was unaffected by 9-cis RA, suggesting promoter occupancy involves post-translational modification of these receptors. We have recently reported on the activation of CREB signaling in retinoid responsive steroidogenesis as well as recruitment of P-CREB (and not CREB) and CBP to the StAR promoter (Manna et al., 2014). CBP/p300 act as integrators among diverse signaling pathways (Vo and Goodman, 2001; Manna et al., 2009a; Manna and Stocco, 2007) and it is likely that retinoids phosphorylate Fos/Jun, CCAAT-enhancer binding protein β, and GATA-4 (which have been demonstrated to be involved in StAR gene expression) at different Ser and Thr residues and recruit CBP/p300 in retinoid regulated transcription of the StAR gene (Clem et al., 2005; Hiroi et al., 2004; Manna et al., 2009a; Manna and Stocco, 2008; Yivgi-Ohana et al., 2009). Studies have demonstrated that retinoid activity is influenced by phosphorylation of RAR and RXR at several Ser/Thr residues by cdk7/cyclin H, which is associated with the general transcription factor TFIIH and MAPKs (Bastien et al., 2002; Gianni et al., 2003; Macoritto et al., 2008). This suggests that retinoids phosphorylate several Ser and Thr residues via receptor-dependent and receptor-independent events, underlying the signaling cross talk between nuclear and cell-surface receptors. It should also be noted that RXR/RAR heterodimerizes with several factors including LXRs, peroxisome proliferator-activated receptors, vitamin D receptors and thyroid hormone receptors which bind to hexameric half-sites (Chambon, 2005; Kliewer et al., 1992; Lefebvre et al., 2010). Thus, these heterodimers can recognize the LXR-RXR/RAR motif in the StAR promoter and result in a large array of combinatorial actions that underlie the pleiotropic effects of retinoids in transcriptional regulation of the StAR gene.
Age-related changes affect the functional properties of all endocrine glands with many being so intertwined that a reduction in function in one gland can adversely affect the biological activities of others (Chahal and Drake, 2007; Makrantonaki and Zouboulis, 2007; Manor and Lipsitz, 2013). A failure in HPG axes results in hypogonadism, a common phenomenon in aging, which affects both testicular and ovarian functions (Huhtaniemi and Forti, 2011; Janovick et al., 2013; Traub and Santoro, 2010). Endocrinosenescence, involving the HPTAG axis, also affects feedback regulatory mechanisms and causes an array of physiological abnormalities. An intriguing aspect of the present study is that retinoids, in particular RAs, are capable of restoring/enhancing StAR expression and steroid biosynthesis in various target tissues including aged epidermal keratinocytes. This implies that retinoid signaling is capable of reversing the declines in steroid dependent age-associated impaired biological activities. Our present data also provide evidence that expression of StAR mRNA decreased not only in skin tissues of elderly men and women but also in several inflammatory skin diseases, suggesting that steroid biosynthesis is frequently attenuated and/or dysregulated during skin aging and in many skin disorders. These results are in support of previous findings that demonstrated that StAR expression is decreased or absent in a number of skin diseases (Hannen et al., 2011; Slominski et al., 2013a; Slominski et al., 2015; Suomela et al., 2009). In a recent report (Manna PR et al., 2015), we have demonstrated that retinoid signaling effectively enhances cholesterol efflux in mouse macrophages, an event is tightly connected with LXR pathways, especially the induction of the ATP-binding cassette transporter A1 that is a key regulatory molecule in cellular lipid transport and atherosclerosis. It was also found that mouse macrophages express relatively higher levels of RARα than that of RXRα, implicating that the majority of retinoid responsive macrophage cholesterol efflux is effected by RARs. Studies have demonstrated that removal of excess cholesterol from macrophage foam cells is critical in limiting plaque stability and progression of atherosclerosis (Ning et al., 2009; Taylor et al., 2010; Tazoe et al., 2008). Therefore, retinoid mediated macrophage cholesterol efflux may have important implications in regressing atherosclerotic cardiovascular disease.
Taken together, retinoid signaling plays a vital role in the up-regulation of StAR expression and steroid biosynthesis in gonadal, adrenal, glial, and epidermal cells. Retinoid mediated regulation of the steroidogenic response, in vivo, may influence multiple effects that serve to i) restore cholesterol/steroid coupled physiological activities, ii) reverse defects linked with vitamin A deficiency including reproductive capacity, iii) ameliorate abnormalities connected with hypogonadism, iv) alleviate problems associated with skin complications and diseases, v) regress/stabilize atherosclerotic cardiovascular disease. It is tempting to speculate that retinoid signaling is capable of preventing and/or restoring various deficiencies that occur as a result of the attenuation of cholesterol/steroid requiring physiological processes. These processes are especially paramount for geriatric populations to live longer and healthier.
Acknowledgements
The authors would like to thank Drs. Ronald M. Evans (Howard Hughes Medical Institute, The Salk Institute for Biological Studies, La Jolla, CA) and D.J. Mangelsdorf (UT Southwestern Medical Center, Dallas, TX) for the generous gifts of RARα and RXRα expression plasmids. We also thank Dr. Pamela Mellon (University of California San Diego, La Jolla, CA) for providing us with LβT2 pituitary gonadotrope cells. The assistance of the Clinical Research Institute at TTUHSC is greatly appreciated. This investigation was supported in part by NIH grants HD-17481, CA155223, the Robert A. Welch Foundation Grant B1-0028, and with funds from Department of Dermatology.
Abbreviations
- RA
retinoic acid
- Retinoids
RA and its derivatives
- atRA
all-trans RA
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- LXR
liver X receptor
- PKA
protein kinase A
- (Bu)2cAMP
dibutyryl adenosine 3’,5’ cyclic monophosphate
- StAR
steroidogenic acute regulatory protein
- CYP11A1
cytochrome P450scc
- siRNA
small interfering RNA
- EMSA
electrophoretic mobility shift assay
- NE
nuclear extract
- ChIP
chromatin immunoprecipitation
- RT-PCR
reverse transcription polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bastien J, Adam-Stitah S, Plassat JL, Chambon P, Rochette-Egly C. The phosphorylation site located in the A region of retinoic X receptor alpha is required for the antiproliferative effect of retinoic acid (RA) and the activation of RA target genes in F9 cells. J. Biol. Chem. 2002;277:28683–28689. doi: 10.1074/jbc.M203623200. [DOI] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012;13:3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose HS, Whittal RM, Baldwin MA, Miller WL. The active form of the steroidogenic acute regulatory protein, StAR, appears to be a molten globule. Proc. Natl. Acad. Sci. USA. 1999;96:7250–7255. doi: 10.1073/pnas.96.13.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal HS, Drake WM. The endocrine system and ageing. J Pathol. 2007;211:173–80. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- Chambon P. The nuclear receptor superfamily: a personal retrospect on the first two decades. Mol. Endocrinol. 2005;19:1418–1428. doi: 10.1210/me.2005-0125. [DOI] [PubMed] [Google Scholar]
- Chung SS, Sung W, Wang X, Wolgemuth DJ. Retinoic acid receptor alpha is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Dev. Dyn. 2004;230:754–766. doi: 10.1002/dvdy.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SS, Wolgemuth DJ. Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet. Genome. Res. 2004;105:189–202. doi: 10.1159/000078189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J. Cell Biochem. 2011;112:1499–1505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3:385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Pezzi V, Stocco DM, Rainey WE. The steroidogenic acute regulatory protein is induced by angiotensin II and K+ in H295R adrenocortical cells. Mol. Cell. Endocrinol. 1995;115:215–219. doi: 10.1016/0303-7207(95)03683-0. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J. Biol. Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- Clem BF, Hudson EA, Clark BJ. Cyclic adenosine 3′,5′-monophosphate (cAMP) enhances cAMP-responsive element binding (CREB) protein phosphorylation and phospho-CREB interaction with the mouse steroidogenic acute regulatory protein gene promoter. Endocrinology. 2005;146:1348–1356. doi: 10.1210/en.2004-0761. [DOI] [PubMed] [Google Scholar]
- Cummins CL, Volle DH, Zhang Y, McDonald JG, Sion B, Lefrancois-Martinez AM, Caira F, Veyssiere G, Mangelsdorf DJ, Lobaccaro JM. Liver X receptors regulate adrenal cholesterol balance. J. Clin. Invest. 2006;116:1902–1912. doi: 10.1172/JCI28400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das BC, Thapa P, Karki R, Das S, Mahapatra S, Liu TC, Torregroza I, Wallace DP, Kambhampati S, Van Veldhuizen P, Verma A, Ray SK, Evans T. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2014;22:673–683. doi: 10.1016/j.bmc.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Syapin PJ. Ethanol increases nuclear factor-kappa B activity in human astroglial cells. Neurosci. Lett. 2004;371:128–132. doi: 10.1016/j.neulet.2004.08.051. [DOI] [PubMed] [Google Scholar]
- de The H, Vivanco-Ruiz MM, Tiollais P, Stunnenberg H, Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990;343:177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- Do Rego JL, Tremblay Y, Luu-The V, Repetto E, Castel H, Vallarino M, Belanger A, Pelletier G, Vaudry H. Immunohistochemical localization and biological activity of the steroidogenic enzyme cytochrome P450 17alpha-hydroxylase/C17, 20-lyase (P450C17) in the frog brain and pituitary. J. Neurochem. 2007;100:251–268. doi: 10.1111/j.1471-4159.2006.04209.x. [DOI] [PubMed] [Google Scholar]
- Elias PM, Crumrine D, Paller A, Rodriguez-Martin M, Williams ML. Pathogenesis of the cutaneous phenotype in inherited disorders of cholesterol metabolism: Therapeutic implications for topical treatment of these disorders. Dermatoendocrinol. 2011;3:100–106. doi: 10.4161/derm.3.2.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmiche G, Richard N, Corvaisier S, Kottler ML. The expression of aromatase in gonadotropes is regulated by estradiol and gonadotropin-releasing hormone in a manner that differs from the regulation of luteinizing hormone. Endocrinology. 2006;147:4234–4244. doi: 10.1210/en.2005-1650. [DOI] [PubMed] [Google Scholar]
- Gianni M, Tarrade A, Nigro EA, Garattini E, Rochette-Egly C. The AF-1 and AF-2 domains of RAR gamma 2 and RXR alpha cooperate for triggering the transactivation and the degradation of RAR gamma 2/RXR alpha heterodimers. J. Biol. Chem. 2003;278:34458–24466. doi: 10.1074/jbc.M304952200. [DOI] [PubMed] [Google Scholar]
- Hammar M, Ostgren CJ. Healthy aging and age-adjusted nutrition and physical fitness. Best. Pract. Res. Clin. Obstet. Gynaecol. 2013;27:741–752. doi: 10.1016/j.bpobgyn.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Hannen RF, Michael AE, Jaulim A, Bhogal R, Burrin JM, Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem. Biophys. Res. Commun. 2011;404:62–67. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]
- Hennig G, Gehrmann M, Stropp U, Brauch H, Fritz P, Eichelbaum M, Schwab M, Schroth W. Automated extraction of DNA and RNA from a single formalin-fixed paraffin-embedded tissue section for analysis of both single-nucleotide polymorphisms and mRNA expression. Clin. Chem. 2010;56:1845–1853. doi: 10.1373/clinchem.2010.151233. [DOI] [PubMed] [Google Scholar]
- Hiroi H, Christenson LK, Strauss JF., 3rd. Regulation of transcription of the steroidogenic acute regulatory protein (StAR) gene: temporal and spatial changes in transcription factor binding and histone modification. Mol Cell Endocrinol. 2004;215:119–26. doi: 10.1016/j.mce.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I, Forti G. Male late-onset hypogonadism: pathogenesis, diagnosis and treatment. Nat. Rev. Urol. 2011;8:335–344. doi: 10.1038/nrurol.2011.47. [DOI] [PubMed] [Google Scholar]
- Inoue T, Miki Y, Abe K, Hatori M, Hosaka M, Kariya Y, Kakuo S, Fujimura T, Hachiya A, Honma S, Aiba S, Sasano H. Sex steroid synthesis in human skin in situ: The roles of aromatase and steroidogenic acute regulatory protein in the homeostasis of human skin. Mol. Cell. Endocrinol. 2012;362:19–28. doi: 10.1016/j.mce.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, Evans RM. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc. Natl. Acad. Sci. USA. 2006;103:3902–397. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janovick JA, Stewart MD, Jacob D, Martin LD, Deng JM, Stewart CA, Wang Y, Cornea A, Chavali L, Lopez S, Mitalipov S, Kang E, Lee HS, Manna PR, Stocco DM, Behringer RR, Conn PM. Restoration of testis function in hypogonadotropic hypogonadal mice harboring a misfolded GnRHR mutant by pharmacoperone drug therapy. Proc. Natl. Acad. Sci. USA. 2013;110:21030–21035. doi: 10.1073/pnas.1315194110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y, King SR, Khan SA, Stocco DM. Involvement of protein kinase C and cyclic adenosine 3′,5′-monophosphate-dependent kinase in steroidogenic acute regulatory protein expression and steroid biosynthesis in Leydig cells. Biol. Reprod. 2005;73:244–255. doi: 10.1095/biolreprod.104.037721. [DOI] [PubMed] [Google Scholar]
- Karri S, Dertien JS, Stocco DM, Syapin PJ. Steroidogenic acute regulatory protein expression and pregnenolone synthesis in rat astrocyte cultures. J. Neuroendocrinol. 2007;19:860–869. doi: 10.1111/j.1365-2826.2007.01600.x. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992;355:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P, Benomar Y, Staels B. Retinoid X receptors: common heterodimerization partners with distinct functions. Trends Endocrinol. Metab. 2010;21:676–683. doi: 10.1016/j.tem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Martin PJ, Flajollet S, Dedieu S, Billaut X, Lefebvre B. Transcriptional activities of retinoic acid receptors. Vitam. Horm. 2005;70:199–264. doi: 10.1016/S0083-6729(05)70007-8. [DOI] [PubMed] [Google Scholar]
- Li H, Clagett-Dame M. Vitamin A deficiency blocks the initiation of meiosis of germ cells in the developing rat ovary in vivo. Biol. Reprod. 2009;81:996–1001. doi: 10.1095/biolreprod.109.078808. [DOI] [PubMed] [Google Scholar]
- Macoritto M, Nguyen-Yamamoto L, Huang DC, Samuel S, Yang XF, Wang TT, White JH, Kremer R. Phosphorylation of the human retinoid X receptor alpha at serine 260 impairs coactivator(s) recruitment and induces hormone resistance to multiple ligands. J. Biol. Chem. 2008;283:4943–4956. doi: 10.1074/jbc.M707517200. [DOI] [PubMed] [Google Scholar]
- Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann. N. Y. Acad. Sci. 2007;1119:40–50. doi: 10.1196/annals.1404.027. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990;345:224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Manna PR, Chandrala SP, King SR, Jo Y, Counis R, Huhtaniemi IT, Stocco DM. Molecular mechanisms of insulin-like growth factor-I mediated regulation of the steroidogenic acute regulatory protein in mouse leydig cells. Mol. Endocrinol. 2006;20:362–378. doi: 10.1210/me.2004-0526. [DOI] [PubMed] [Google Scholar]
- Manna PR, Cohen-Tannoudji J, Counis R, Garner CW, Huhtaniemi I, Kraemer FB, Stocco DM. Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: its role in the regulation of the steroidogenic acute regulatory protein. J. Biol. Chem. 2013;288:8505–8518. doi: 10.1074/jbc.M112.417873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Stocco DM. Role of basic leucine zipper proteins in transcriptional regulation of the steroidogenic acute regulatory protein gene. Mol. Cell. Endocrinol. 2009a;302:1–11. doi: 10.1016/j.mce.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Jo Y, Stocco DM. Role of dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene 1 in protein kinase A- and protein kinase C-mediated regulation of the steroidogenic acute regulatory protein expression in mouse Leydig tumor cells: mechanism of action. Endocrinology. 2009c;150:187–199. doi: 10.1210/en.2008-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, Zeleznik AJ, Stocco DM. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol. Endocrinol. 2002;16:184–199. doi: 10.1210/mend.16.1.0759. [DOI] [PubMed] [Google Scholar]
- Manna PR, Eubank DW, Stocco DM. Assessment of the role of activator protein-1 on transcription of the mouse steroidogenic acute regulatory protein gene. Mol. Endocrinol. 2004;18:558–573. doi: 10.1210/me.2003-0223. [DOI] [PubMed] [Google Scholar]
- Manna PR, Huhtaniemi IT, Stocco DM. Mechanisms of protein kinase C signaling in the modulation of 3′,5′-cyclic adenosine monophosphate-mediated steroidogenesis in mouse gonadal cells. Endocrinology. 2009b;150:3308–3317. doi: 10.1210/en.2008-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Sennoune SR, Martinez-Zaguilan R, Slominski AT, Pruitt K. Regulation of retinoid mediated cholesterol efflux involves liver X receptor activation in mouse macrophages. Biochem. Biophys. Res. Commun. 2015 doi: 10.1016/j.bbrc.2015.06.150. doi: 10.1016/j.bbrc.2015.06.150. [DOI] [PubMed] [Google Scholar]
- Manna PR, Slominski AT, King SR, Stetson CL, Stocco DM. Synergistic activation of steroidogenic acute regulatory protein expression and steroid biosynthesis by retinoids: involvement of cAMP/PKA signaling. Endocrinology. 2014;155:576–591. doi: 10.1210/en.2013-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Soh JW, Stocco DM. The involvement of specific PKC isoenzymes in phorbol ester-mediated regulation of steroidogenic acute regulatory protein expression and steroid synthesis in mouse Leydig cells. Endocrinology. 2011;152:313–325. doi: 10.1210/en.2010-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Stocco DM. Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2005;5:93–108. doi: 10.2174/1568008053174714. [DOI] [PubMed] [Google Scholar]
- Manna PR, Stocco DM. Crosstalk of CREB and Fos/Jun on a single cis-element: transcriptional repression of the steroidogenic acute regulatory protein gene. J. Mol. Endocrinol. 2007;39:261–277. doi: 10.1677/JME-07-0065. [DOI] [PubMed] [Google Scholar]
- Manna PR, Stocco DM. The role of JUN in the regulation of PRKCC-mediated STAR expression and steroidogenesis in mouse Leydig cells. J. Mol. Endocrinol. 2008;41:329–341. doi: 10.1677/JME-08-0077. [DOI] [PubMed] [Google Scholar]
- Manna PR, Tena-Sempere M, Huhtaniemi IT. Molecular mechanisms of thyroid hormone-stimulated steroidogenesis in mouse Leydig tumor cells. Involvement of the steroidogenic acute regulatory (StAR) protein. J Biol. Chem. 1999;274:5909–5918. doi: 10.1074/jbc.274.9.5909. [DOI] [PubMed] [Google Scholar]
- Manna PR, Wang XJ, Stocco DM. Involvement of multiple transcription factors in the regulation of steroidogenic acute regulatory protein gene expression. Steroids. 2003;68:1125–1134. doi: 10.1016/j.steroids.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Manor B, Lipsitz LA. Physiologic complexity and aging: implications for physical function and rehabilitation. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;45:287–293. doi: 10.1016/j.pnpbp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu. Rev. Pharmacol. Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- Mihaly J, Gamlieli A, Worm M, Ruhl R. Decreased retinoid concentration and retinoid signalling pathways in human atopic dermatitis. Exp. Dermatol. 2011;20:326–330. doi: 10.1111/j.1600-0625.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J. Lipid Res. 2011;52:2111–2135. doi: 10.1194/jlr.R016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Y, Bai Q, Lu H, Li X, Pandak WM, Zhao F, Chen S, Ren S, Yin L. Overexpression of mitochondrial cholesterol delivery protein, StAR, decreases intracellular lipids and inflammatory factors secretion in macrophages. Atherosclerosis. 2009;204:114–120. doi: 10.1016/j.atherosclerosis.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CR, Mello CV. Significance of vitamin A to brain function, behavior and learning. Mol. Nutr. Food Res. 2010;54:489–495. doi: 10.1002/mnfr.200900246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Yamada M. Vitamin A and Alzheimer’s disease. Geriatr Gerontol Int. 2012;12:180–8. doi: 10.1111/j.1447-0594.2011.00786.x. [DOI] [PubMed] [Google Scholar]
- Park LK, Friso S, Choi SW. Nutritional influences on epigenetics and age-related disease. Proc. Nutr. Soc. 2012;71:75–83. doi: 10.1017/S0029665111003302. [DOI] [PubMed] [Google Scholar]
- Shaw ND, Srouji SS, Histed SN, McCurnin KE, Hall JE. Aging attenuates the pituitary response to gonadotropin-releasing hormone. J. Clin. Endocrinol. Metab. 2009;94:3259–3264. doi: 10.1210/jc.2009-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr. Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, Li W, Janjetovic Z, Postlethwaite A, Zouboulis CC, Tuckey RC. Steroidogenesis in the skin: implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013a;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Exp. Dermatol. 2014;23:369–374. doi: 10.1111/exd.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015 doi: 10.1016/j.steroids.2015.04.006. doi: 10.1016/j.steroids.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Semak I, Zbytek B, Pisarchik A, Li W, Zjawiony J, Tuckey RC. Cytochromes P450 and Skin Cancer: Role of Local Endocrine Pathways. Anticancer Agents Med. Chem. 2013b;14:77–96. doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol. Endocrinol. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- Suomela S, Elomaa O, Skoog T, Ala-aho R, Jeskanen L, Parssinen J, Latonen L, Grenman R, Kere J, Kahari VM, Saarialho-Kere U. CCHCR1 is up-regulated in skin cancer and associated with EGFR expression. PLoS One. 2009;4:e6030. doi: 10.1371/journal.pone.0006030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Borthwick F, Bartholomew C, Graham A. Overexpression of steroidogenic acute regulatory protein increases macrophage cholesterol efflux to apolipoprotein AI. Cardiovasc. Res. 2010;86:526–534. doi: 10.1093/cvr/cvq015. [DOI] [PubMed] [Google Scholar]
- Tazoe F, Yagyu H, Okazaki H, Igarashi M, Eto K, Nagashima S, Inaba T, Shimano H, Osuga J, Ishibashi S. Induction of ABCA1 by overexpression of hormone-sensitive lipase in macrophages. Biochem. Biophys. Res. Commun. 2008;376:111–115. doi: 10.1016/j.bbrc.2008.08.101. [DOI] [PubMed] [Google Scholar]
- Thomas DR. Vitamins in aging, health, and longevity. Clin. Interv. Aging. 2006;1:81–91. doi: 10.2147/ciia.2006.1.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiala I, Suomela S, Huuhtanen J, Wakkinen J, Holtta-Vuori M, Kainu K, Ranta S, Turpeinen U, Hamalainen E, Jiao H, Karvonen SL, Ikonen E, Kere J, Saarialho-Kere U, Elomaa O. The CCHCR1 (HCR) gene is relevant for skin steroidogenesis and downregulated in cultured psoriatic keratinocytes. J. Mol. Med. (Berl) 2007;85:589–601. doi: 10.1007/s00109-006-0155-0. [DOI] [PubMed] [Google Scholar]
- Traub ML, Santoro N. Reproductive aging and its consequences for general health. Ann. N. Y. Acad. Sci. 2010;1204:179–187. doi: 10.1111/j.1749-6632.2010.05521.x. [DOI] [PubMed] [Google Scholar]
- Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM, Selvaraj V. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J. Biol. Chem. 2014;289:27444–27454. doi: 10.1074/jbc.M114.578286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon JL, Kimura Y, Waring DW, Mellon PL. Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol. Endocrinol. 1996;10:439–450. doi: 10.1210/mend.10.4.8721988. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD. Changes in pituitary function with ageing and implications for patient care. Nat. Rev. Endocrinol. 2013;9:205–215. doi: 10.1038/nrendo.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147:96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- Wickenheisser JK, Nelson-DeGrave VL, Hendricks KL, Legro RS, Strauss JF, 3rd, McAllister JM. Retinoids and retinol differentially regulate steroid biosynthesis in ovarian theca cells isolated from normal cycling women and women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2005;90:4858–4865. doi: 10.1210/jc.2005-0330. [DOI] [PubMed] [Google Scholar]
- Yivgi-Ohana N, Sher N, Melamed-Book N, Eimerl S, Koler M, Manna PR, Stocco DM, Orly J. Transcription of steroidogenic acute regulatory protein in the rodent ovary and placenta: alternative modes of cyclic adenosine 3′, 5′-monophosphate dependent and independent regulation. Endocrinology. 2009;150:977–989. doi: 10.1210/en.2008-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang ZM, Liu HY, Bai Y, Wei S, Li Y, Wang M, Chen J, Zhou QH. Application of RT-PCR in formalin-fixed and paraffin-embedded lung cancer tissues. Acta Pharmacol. Sin. 2010;31:111–117. doi: 10.1038/aps.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouboulis CC. The skin as an endocrine organ. Dermatoendocrinol. 2009;1:250–252. doi: 10.4161/derm.1.5.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]