Abstract
Hexokinase 1 (HK1) and pyruvate kinase M2 (PKM2) are two key regulators in glycosis and oncogenic markers in cancers. In the present study, we investigated the expression profile by Western blotting and immunohistochemistry and determined their prognostic values in the gastric cancer. Expression of HK1 and PKM2 was remarkably increased in gastric cancer tissues and was significantly associated lymphatic metastasis and advanced TNM staging. In the COX regression model, HK1 and TNM stage were analyzed as adverse prognostic indicators in gastric cancer. Furthermore, patients with HK1 expression showed remarkable shorter survival duration in both lymphatic metastasis cohort and advanced staging cohort. Our results suggest that overexpression of PKM2 and HK1, especially the latter, significantly associates with lymphatic metastasis, advanced clinical staging and unfavorable prognosis in gastric cancer.
Keywords: Gastric cancer, HK1, PKM2
Introduction
Gastric cancer (GC) is one of the most common malignant tumors of digestive system and accounts for 7.8 percent of human cancers worldwide [1]. Incidence of GC in the Eastern Asia including China, Japan and Korea is several-fold higher than other low incidence areas including western countries and Africa. In China the morbidity of GC is on the rise in the past decades and has become the third leading cause of cancer mortality [2]. Early detection of GC is quite difficult and most patients are at advanced stages when firstly diagnosed.
Clinically, TNM classification of malignant tumors remains the most extensively applied staging system and assists clinicians to determine appropriated treatment and prognosis of cancer patients. Besides TNM staging, there still exists a lot of nonanatomic prognostic markers [3] and survival rate varies even in GC patients at the same TNM stage. Therefore, the development of new prognostic and predictive markers becomes quite urgent to provide an overall assessment of GC patients and might be potential drug targets for molecular therapies in the future.
Like normal mammalian cells, metabolism is a critical event to maintain growth and other numerous biological functions in cancer cells. Glycolysis, especially aerobic glycolysis, has drawn more and more attention and become a new target in caner researches [4-7]. In the present study, we screened the expression of a panel of glycolysis related key enzymes and aimed to evaluate the clinical significance of these glycolytic enzymes in staging and prognosis in GCs.
Patients and methods
Case selection
The population for this study consisted of 124 patients with primary gastric adenocarcinomas undergone surgery at the 401 hospital from 2002 to 2005. Non-epithelial derived gastric tumors, such as neuroendocrine tumors, lymphoma and sarcomas, were excluded from this study. All patients had never been pre-treated by adjuvant therapies before surgery. All these cases have matched non-neoplastic gastric mucosa obtained from resection margins. Clinical data were collected and the survival of these patients was calculated from the date of surgery to the date of death, or from the date of surgery to the date known to be alive. This study was ethically approved by the Ethics Committee of 401 hospital.
Tissue microarray and immunohistochemistry
Hematoxylin and eosin stained sections were reviewed by two pathologists and the corresponding paraffin blocks were retrieved. The tissue microarray was prepared using 1.5 mm tissue cores of gastric cancer tissue and matched normal epithelium that was placed into a recipient paraffin block. Then 4 μm sections of tissue microarray were mounted on the APES-coated slides. Expression of HK-1, PKM2 and PFK-2 was detected by immunohistochemistry on these sections after deparaffinization and rehydration. Antigen retrieval by microwave with 0.01 M citrate buffer (pH 6.0) was applied to unmask the antigen. Primary antibodies anti-HK1 antibody (dilution: 1:100, YT2265, ImmunoWay), anti-PFKB (dilution: 1:100, YT3685, ImmunoWay), and anti-PKM2 (dilution: 1:100, EPR10138 (B), EPITOMICS) were diluted in antibody dilution buffer and applied to the tissue slides overnight at 4°C. Counterstaining was performed with hematoxylin. Each section was scored according to the intensity of labelling, from 0 (no staining) to 3 (strong staining).
Western blot analysis
Proteins from 2 pairs of gastric cancer tissues were prepared for Western blot analyses. Standard Western blotting analysis was performed using a rabbit antibody against human HK1 (1:1000), PFKB (1:1000), PKM2 (1:1000) and an anti-rabbit IgG antibody, which was a horseradish peroxidase-linked F(ab’)2 fragments obtained from a donkey (Amersham). Protein samples were loaded equally and were monitored by probing the housekeeping gene, β-actin (1:10000, C4, Santa Cruz).
Data analysis
All statistical analyses were conducted using the SPSS 16.0 statistical software program. Categorical data were analyzed using X2 tests. The Kaplan-Meier method was used to estimate survival rates and the Cox proportional hazards model for multivariate survival analysis was used to assess predictors related to survival. Correlation between expressions of various proteins was evaluated using Pearson’s correlation coefficient. A two-sided P<0.05 was defined as statistically significant.
Results
Clinicopathologic findings
Among the 124 cases in this study, eighty-four were male and forty were female. The median age was 59.5 years. The size of neoplasm less than 6 cm was observed in 100 patients and over 6 cm in 24 patients. Clinically, 60 patients were classified into TNM stage I and II and 64 patients were classified into more advanced stage III and IV.
Pathologically, 80 cases were moderate to well/moderate-differentiated and 44 cases were poorly differentiated. The median survival time was 38 months, ranging from 1 to 108 months.
HK1, PFKB, and PKM2 expression in patients with gastric cancer by western blotting analysis
We detected the expression profile of HK1, PFKB, and PKM2 in tumor specimens and matched non-cancerous tissues by Western blotting assay. As shown in Figure 1, HK1 and PKM2 were up-regulated in cancer tissues, compared with normal tissues. However, no difference of PFKB expression was observed between these two kinds of tissues.
Figure 1.
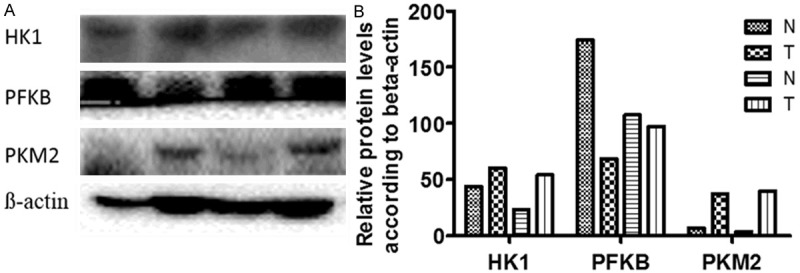
Expression of HK1, PFKB, and PKM2 in gastric cancer by Western blotting analysis. (A) Western blotting analysis of two paired primary GC specimens (T) and normal epithelial specimens (N); (B) Relative protein expression in the specimens vs β-actin from (A) analyzed by Image J.
Evaluation of HK1, PFKB, and PKM2 expression in gastric cancer by immunohistochemistry
We further evaluated the proportions and locations of HK1, PFKB, and PKM2 expression in gastric cancer cells by tissue microarray and immunohistochemistry. HK1, PFKB, and PKM2 were positively stained in the cytoplasm of cells. All the noncancerous epithelium showed weak staining of HK1 and PKM2 and moderate positive staining of PFKB, while cancer cells showed moderate to strong staining of HK1 and PKM2, but weak to moderate staining of PFKB (Figure 2A-C). Statistical analysis revealed that HK1 and PKM2 were significantly increased in cancer specimens than that in non-cancerous tissues, while PFKB showed the opposite result (Figure 2D).
Figure 2.
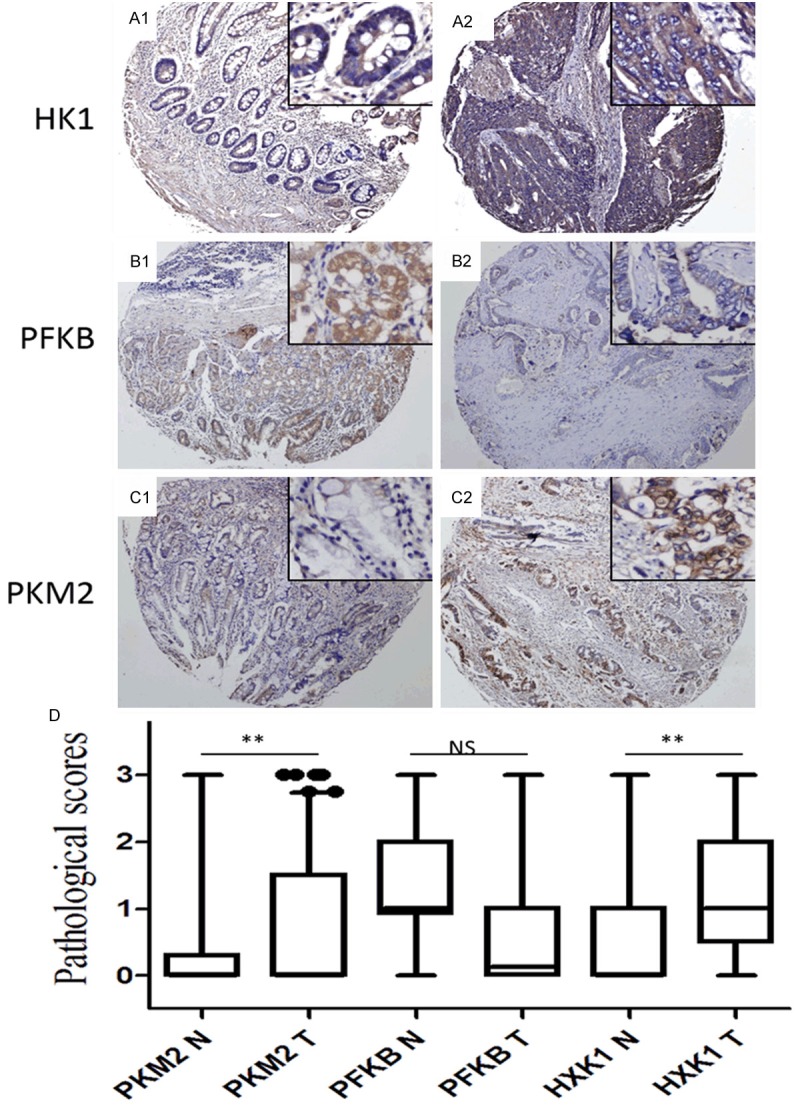
Expression of HK1, PFKB, and PKM2 in gastric cancer by immunohistochemistry. (A1-C1) Normal epithelium showed weak or negative expression of HK1 (A1), PFKB (B1), and PKM2 (C1). (A2-C2) Representative positive staining of HK1 (A2), PFKB (B2), and PKM2 (C2) in GC. (A2-C2) Graphical representation of the differences of HK1 (A2), PFKB (B2), and PKM2 (C2) staining in nonneoplastic (N) and cancer tissues (T). Original magnification: 100× for large pictures; 200× for large pictures. (D) Graphical representation of the intensity of HK1, PFKB, and PKM2 in nonneoplastic (N) and cancer tissues (T). **P<0.01.
HK1, PFKB, and PKM2 expression correlates with tumor grade and the disease stage in gastric cancer
Table 1 presented the correlation of HK1, PFKB, and PKM2 expression with clinicopathologic variables. The ratio of HK1, PFKB, and PKM2 positive staining in tumors was 66%, 35%, and 38%, respectively. HK1 overexpression was significantly associated with lymph node metastasis and advanced disease stage. Up-regulation of PKM2 significantly correlated with both nodal metastasis and advanced TNM stage. However, we observed that PFKB expression was preferentially associated with well histological differentiation but interestingly it was also correlated with early T stage.
Table 1.
PKM2, PFKB and HK1 expression correlated with pathologic parameters in gastric cancers
| Pathological factors | N | PKM2 positive | PFKB positive | HK1 positive | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n | % | P | n | % | P | n | % | P | ||
| Age | ||||||||||
| ≤60 y | 62 | 21 | 33.9 | 0.355 | 20 | 32.3 | 0.571 | 37 | 59.7 | 0.129 |
| >60 y | 62 | 26 | 41.9 | 23 | 37.1 | 45 | 72.6 | |||
| Gender | ||||||||||
| Male | 84 | 33 | 39.3 | 0.646 | 33 | 39.3 | 0.118 | 57 | 67.9 | 0.556 |
| Female | 40 | 14 | 35.0 | 10 | 25.0 | 25 | 62.5 | |||
| Size | ||||||||||
| ≤6 cm | 100 | 34 | 34.0 | 0.067 | 37 | 37.0 | 0.267 | 64 | 64.0 | 0.307 |
| 6 cm | 24 | 13 | 54.2 | 6 | 25.0 | 18 | 75.0 | |||
| Nerve invasion | ||||||||||
| + | 108 | 39 | 36.1 | 0.285 | 38 | 35.2 | 0.758 | 68 | 63.0 | 0.053 |
| - | 16 | 8 | 50.0 | 5 | 31.3 | 14 | 87.5 | |||
| T stage | ||||||||||
| T1/T2 | 46 | 8 | 17.4 | <0.001 | 22 | 47.8 | 0.018 | 26 | 56.5 | 0.083 |
| T3/T4 | 78 | 39 | 50.0 | 21 | 26.9 | 56 | 71.8 | |||
| Nodal metastasis | ||||||||||
| - | 48 | 11 | 22.9 | 0.006 | 19 | 39.6 | 0.362 | 25 | 52.1 | 0.009 |
| + | 76 | 36 | 47.4 | 24 | 31.6 | 57 | 75.0 | |||
| Differentiation | ||||||||||
| High/moderate | 80 | 29 | 36.3 | 0.609 | 33 | 41.3 | 0.038 | 49 | 61.3 | 0.122 |
| Poor/undifferentiated | 44 | 18 | 40.9 | 10 | 22.7 | 33 | 75.0 | |||
| TNM stage | ||||||||||
| I/II | 60 | 12 | 20.0 | <0.001 | 22 | 36.7 | 0.652 | 33 | 55.0 | 0.011 |
| III/IV | 64 | 35 | 54.7 | 21 | 32.8 | 49 | 76.6 | |||
| Total | 124 | 47 | 37.9 | 43 | 34.7 | 0.652 | 82 | 66.1 | ||
HK1 and PKM2 expression indicated poor outcome in gastric cancers
Survival analysis showed that overexpression of HK1 and PKM2, not PFKB, was associated with decreased median survival durations. Specifically, patients with HK1 overexpression had median survival duration of 49.9 months, whereas patients with HK1 absent expression had median survival duration of 94.9 months (P<0.001; Figure 3A). Similarly, patients with PKM2 overexpression had median survival duration of 42.6 months, whereas patients without PKM2 expression had median survival duration of 80.7 months (P<0.001; Figure 3B). Furthermore, multivariate analysis using the Cox proportional hazards model showed that HK1 expression was an independent prognostic factor with stronger significance (P=0.01) even than TNM staging (P=0.029) for patients with gastric cancer (Table 2).
Figure 3.
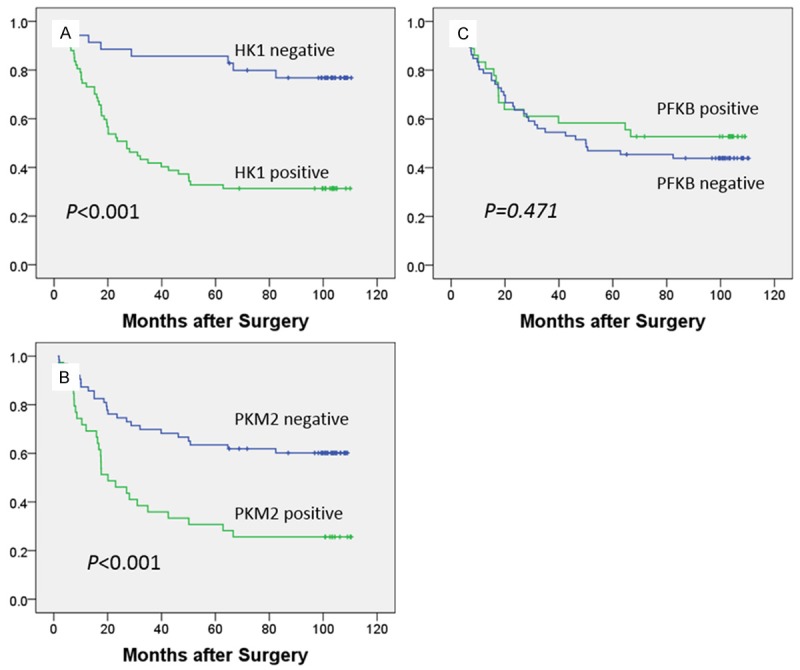
Kaplan-Meier curves of survival in patients with gastric cancer patients. A. Patients with HK1 overexpression had a median survival duration of 49.9 months, whereas patients with HK1 absent/low expression had a median survival duration of 94.9 months (P<.001). B. Survival durations were significantly longer in patients with low expression of PKM2 (median survival, 80.7 mo) than in those with high expression (median survival, 42.6 mo; P<0.001). C. No significant difference was observed between patients with high PFKB expression (median survival, 67.3 mo) and those with low expression (median survival, 65.0 mo; P=0.727).
Table 2.
COX regression model of GC patients
| B | SE | Wald | Sig. | Exp (B) | 95.0% CI for Exp (B) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower | Upper | ||||||
| Tumor size (≤6 cm vs. >6 cm) | -.334 | .322 | 1.073 | .300 | .716 | .381 | 1.346 |
| T stage (T1/2 vs. T3/4) | -.471 | .526 | .804 | .370 | .624 | .223 | 1.749 |
| N stage (N0 vs. N1-3) | -.281 | .603 | .218 | .641 | .755 | .231 | 2.462 |
| Differentiation (High/moderate vs. Poor/undifferentiated) | -.092 | .303 | .092 | .762 | .912 | .504 | 1.652 |
| TNM stage (I/II vs. III/IV) | -1.490 | .684 | 4.741 | .029 | .225 | .059 | .862 |
| PKM2 (positive vs. negative) | .209 | .308 | .459 | .498 | 1.232 | .674 | 2.254 |
| HXK1 (positive vs. negative) | -1.403 | .415 | 11.458 | .001 | .246 | .109 | .554 |
HK1 expression was significantly associated with lymph node metastasis and advanced TNM staging
As shown in Table 1, HK1 overexpression was significantly associated with lymph metastasis and advanced TNM stage. We stratified the survival data in this study based on lymph node metastasis and TNM stage. In the group of patients without nodal metastasis, HK1 expression showed no difference on the life time (HK1 positive vs. negative: 82.9 months vs. 99.3 months, P=0.356). However, in the group with nodal metastasis, HK1 positivity was remarkably associated with poor prognosis (HK1 positive vs. negative: 31.1 months vs. 89.9 months, P<0.01) (Figure S1). Likewise, similar results were found in the cohort stratified by TNM stage. Only in the advanced staging cohort (stage III/IV), HK1 expression revealed significant difference on the survival of gastric cancer patients (HK1 positive vs. negative: 23 months vs. 79 months, P=0.003), whereas no difference of survival time was observed between patients with various HK1 expression status in the stage I/II cohort (HK1 positive vs. negative: 101 months vs. 85 months, P=0.153) (Figure S2).
Discussion
Cancer cells require high metabolism to sustain their active proliferation, motility and other actively biological events that demand a large amount of energy, mostly in the form of adenosine triphosphate (ATP). Unlike normal mammalian cells, cancer cells are always in the microenvironment of hypoxia due to their outgrowth over oxygen supply. Thus, it is quite understandable that cancer cells prefer to utilize the glycolysis for energy generation. However, the Nobel Laureate and German biochemist, Otto Warburg, found that cancer cells, albeit far less efficiently, are dependent more on aerobic glycolysis than oxidative phosphorylation even in the presence of oxygen, which was then called as ‘Warburg effect’ [8]. With the ongoing researches on aerobic glycolysis or ‘Warburg effect’, more and more scientists believe that it could be a fundamental feature of cancer cells and upregulation of aerobic glycolysis becomes crucial in the progression of cancers [9-12]. Moreover, it is postulated that abnormalities of gene functions might be responsible for the induction of aerobic glycolysis. In the present study we examined the expression level of three critical enzymes (hexokinase 1/HK1, phosphofructokinase-B/PFKB, pyruvate kinase M2/PKM2) of aerobic glycolysis as well as their correlations with clinical parameters and survival in GCs.
HK-1, as a member of the hexokinase isoenzymes, is ubiquitously expressed in cells and mainly channels glucose into the glycolytic pathway by phosphorylation of the substrate. HK-1 was formally considered as a regulator in the metabolic diseases such as the type 2 diabetes mellitus [13-15] and hyperinsulinism [16]. Recently HK-1 has been found to block TNF-induced apoptosis in the mitochondria [17] as an anti-apoptotic factor. HK-2, another member of hexokinase family, has been noted to function critically in human cancers [18-20]. Remarkably increased expression of the HK1 protein was observed in gastric cancer and was significantly associated with nodal metastasis and advanced TNM stages. And survival analysis further showed that HK1 overexpression was an independently negative indicator for the prognosis of GCs. By stratified survival data we noted that HK1 revealed its prognostic significance mainly in the cohort of patients with nodal metastasis (31.1 months versus 89.9 months). Similarly, in the cohort of patients in advanced TNM stages (III/IV), HK1 positive patients lived far shortly than HK1 negative patients (23 months versus 79 months). Our findings manifest that HX1 is a strongly negative indicator for GC patients and suggest for the first time that it could be a promising marker in predicting the future of GC patients at advanced stages.
PKM2, the M2 splice isoform of pyruvate kinase, works as an initiation of aerobic glycolysis that determines whether the glucose is channeled into the lactate-producing pathway. It is noted that PKM2 is highly expressed in cancer cells, which indicates that an active aerobic glycolysis occurs and regulates numerous cell functions in these cells, such as proliferation, mobility and drug resistance [21-27]. Lim and colleagues [28] demonstrated that PKM2 is a poor prognostic marker for signet ring cell gastric cancer. Likewise, we found that PKM2 overexpression was negatively related with survival and it could be a negative indicator for the survival of GC patients but of less significance than HK1.
PFKB belongs to the ribokinase family and produces fructose 2,6-bisphosphate that activates PFKA, a molecule that in turn regulates aerobic glycolysis in cancer cells. Our study demonstrated that unlike HK1 and PKM2, PFKB acts as a relatively positive role in GCs. It was highly expressed in well differentiated and was associated with early TNM stage (I/II). However no significant difference was observed between PFKB expression and overall survival, indicating that PFKB might play a much less significant role than HK1 and PKM2 in the progression of GC.
In conclusions, this study revealed that overexpression of two key enzymes, HK1 and PKM2, in the aerobic glycolysis was associated with nodal metastasis, advanced TNM stages and poor prognosis. Meaningfully, HK1 could be a promising predictor in addition to traditional TNM staging.
Acknowledgements
We thank Drs. Xintai Zhao and Shaoan Fan at the the Research Base of Molecular Diagnosis Technology for Tumor Peronalized Medicine, Developmental Center for Medical Sciences and Technology, The Ministry of Health, P. R. China, for advice on data analyses and technical assistance. This paper is partly supported by supported by the Shanghai medical key specialty (ZK2012A26) and National Natural Science Foundation of China (81572856).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Lauwers GY, Carneiro F, Graham DY, Curado MP, editors. Gastric carcinoma. 4th edition. Lyon: International Agency for Research on Cancer (IARC); 2010. [Google Scholar]
- 2.Bu Z, Ji J. A current view of gastric cancer in China. Transl Gastrointest Cancer. 2013;2:1–4. [Google Scholar]
- 3.Sobin LH. TNM: principles, history, and relation to other prognostic factors. Cancer. 2001;91(Suppl 8):1589–1592. doi: 10.1002/1097-0142(20010415)91:8+<1589::aid-cncr1170>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Shuch B, Linehan WM, Srinivasan R. Aerobic glycolysis: a novel target in kidney cancer. Expert Rev Anticancer Ther. 2013;13:711–719. doi: 10.1586/era.13.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivenzon-Segal D, Margalit R, Degani H. Glycolysis as a metabolic marker in orthotopic breast cancer, monitored by in vivo (13)C MRS. Am J Physiol Endocrinol Metab. 2002;283:E623–630. doi: 10.1152/ajpendo.00050.2002. [DOI] [PubMed] [Google Scholar]
- 6.Marin-Hernandez A, Gallardo-Perez JC, Rodriguez-Enriquez S, Encalada R, Moreno-Sanchez R, Saavedra E. Modeling cancer glycolysis. Biochim Biophys Acta. 2011;1807:755–767. doi: 10.1016/j.bbabio.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Gatenby RA, Gillies RJ. Glycolysis in cancer: a potential target for therapy. Int J Biochem Cell Biol. 2007;39:1358–1366. doi: 10.1016/j.biocel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 9.Garber K. Energy boost: the Warburg effect returns in a new theory of cancer. J Natl Cancer Inst. 2004;96:1805–1806. doi: 10.1093/jnci/96.24.1805. [DOI] [PubMed] [Google Scholar]
- 10.Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Wang X, Zhang J, Lam EK, Shin VY, Cheng AS, Yu J, Chan FK, Sung JJ, Jin HC. Warburg effect revisited: an epigenetic link between glycolysis and gastric carcinogenesis. Oncogene. 2010;29:442–450. doi: 10.1038/onc.2009.332. [DOI] [PubMed] [Google Scholar]
- 13.Pare G, Chasman DI, Parker AN, Nathan DM, Miletich JP, Zee RY, Ridker PM. Novel association of HK1 with glycated hemoglobin in a non-diabetic population: a genome-wide evaluation of 14,618 participants in the Women's Genome Health Study. PLoS Genet. 2008;4:e1000312. doi: 10.1371/journal.pgen.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnefond A, Vaxillaire M, Labrune Y, Lecoeur C, Chèvre JC, Bouatia-Naji N, Cauchi S, Balkau B, Marre M, Tichet J, Riveline JP, Hadjadj S, Gallois Y, Czernichow S, Hercberg S, Kaakinen M, Wiesner S, Charpentier G, Lévy-Marchal C, Elliott P, Jarvelin MR, Horber F, Dina C, Pedersen O, Sladek R, Meyre D, Froguel P. Genetic variant in HK1 is associated with a proanemic state and A1C but not other glycemic control-related traits. Diabetes. 2009;58:2687–2697. doi: 10.2337/db09-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gjesing AP, Nielsen AA, Brandslund I, Christensen C, Sandbaek A, Jorgensen T, Witte D, Bonnefond A, Froguel P, Hansen T, Pedersen O. Studies of a genetic variant in HK1 in relation to quantitative metabolic traits and to the prevalence of type 2 diabetes. BMC Med Genet. 2011;12:99. doi: 10.1186/1471-2350-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinney SE, Ganapathy K, Bradfield J, Stokes D, Sasson A, Mackiewicz K, Boodhansingh K, Hughes N, Becker S, Givler S, Macmullen C, Monos D, Ganguly A, Hakonarson H, Stanley CA. Dominant form of congenital hyperinsulinism maps to HK1 region on 10q. Horm Res Paediatr. 2013;80:18–27. doi: 10.1159/000351943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schindler A, Foley E. Hexokinase 1 blocks apoptotic signals at the mitochondria. Cell Signal. 2013;25:2685–2692. doi: 10.1016/j.cellsig.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 19.Peschiaroli A, Giacobbe A, Formosa A, Markert EK, Bongiorno-Borbone L, Levine AJ, Candi E, D’Alessandro A, Zolla L, Finazzi Agro A, Melino G. miR-143 regulates hexokinase 2 expression in cancer cells. Oncogene. 2013;32:797–802. doi: 10.1038/onc.2012.100. [DOI] [PubMed] [Google Scholar]
- 20.Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, Chandel N, Laakso M, Muller WJ, Allen EL, Jha AK, Smolen GA, Clasquin MF, Robey RB, Hay N. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Le H. Dual roles of PKM2 in cancer metabolism. Acta Biochim Biophys Sin (Shanghai) 2013;45:27–35. doi: 10.1093/abbs/gms106. [DOI] [PubMed] [Google Scholar]
- 22.Wong N, Yan J, Ojo D, De Melo J, Cutz JC, Tang D. Changes in PKM2 associate with prostate cancer progression. Cancer Invest. 2014;32:330–338. doi: 10.3109/07357907.2014.919306. [DOI] [PubMed] [Google Scholar]
- 23.Wong N, Ojo D, Yan J, Tang D. PKM2 contributes to cancer metabolism. Cancer Lett. 2015;356:184–91. doi: 10.1016/j.canlet.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 24.Papadaki C, Sfakianaki M, Lagoudaki E, Giagkas G, Ioannidis G, Trypaki M, Tsakalaki E, Voutsina A, Koutsopoulos A, Mavroudis D, Georgoulias V, Souglakos J. PKM2 as a biomarker for chemosensitivity to front-line platinum-based chemotherapy in patients with metastatic non-small-cell lung cancer. Br J Cancer. 2014;111:1757–64. doi: 10.1038/bjc.2014.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Shi Y, Liu S, Cao Y, Wang X, Tao Y. PKM2: the thread linking energy metabolism reprogramming with epigenetics in cancer. Int J Mol Sci. 2014;15:11435–11445. doi: 10.3390/ijms150711435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong N, De Melo J, Tang D. PKM2, a Central Point of Regulation in Cancer Metabolism. Int J Cell Biol. 2013;2013:242513. doi: 10.1155/2013/242513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo W, Semenza GL. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol Metab. 2012;23:560–566. doi: 10.1016/j.tem.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim JY, Yoon SO, Seol SY, Hong SW, Kim JW, Choi SH, Cho JY. Overexpression of the M2 isoform of pyruvate kinase is an adverse prognostic factor for signet ring cell gastric cancer. World J Gastroenterol. 2012;18:4037–4043. doi: 10.3748/wjg.v18.i30.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


