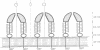Abstract
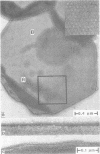
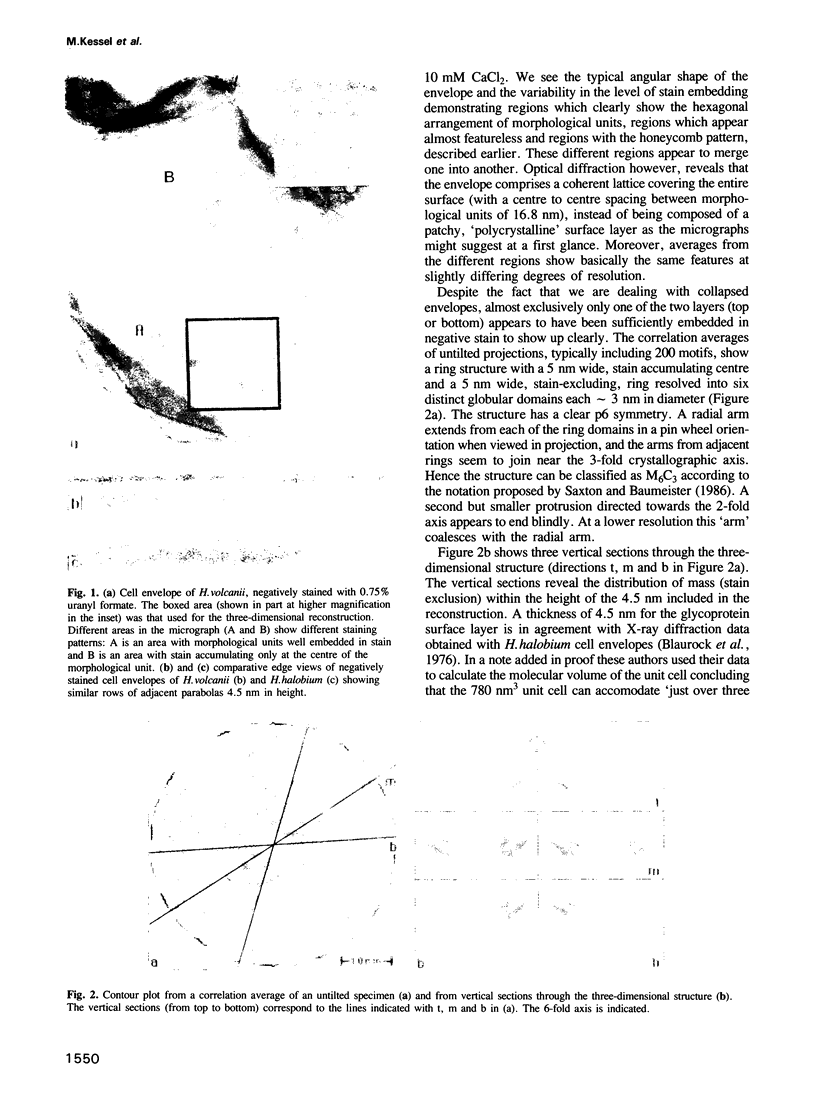
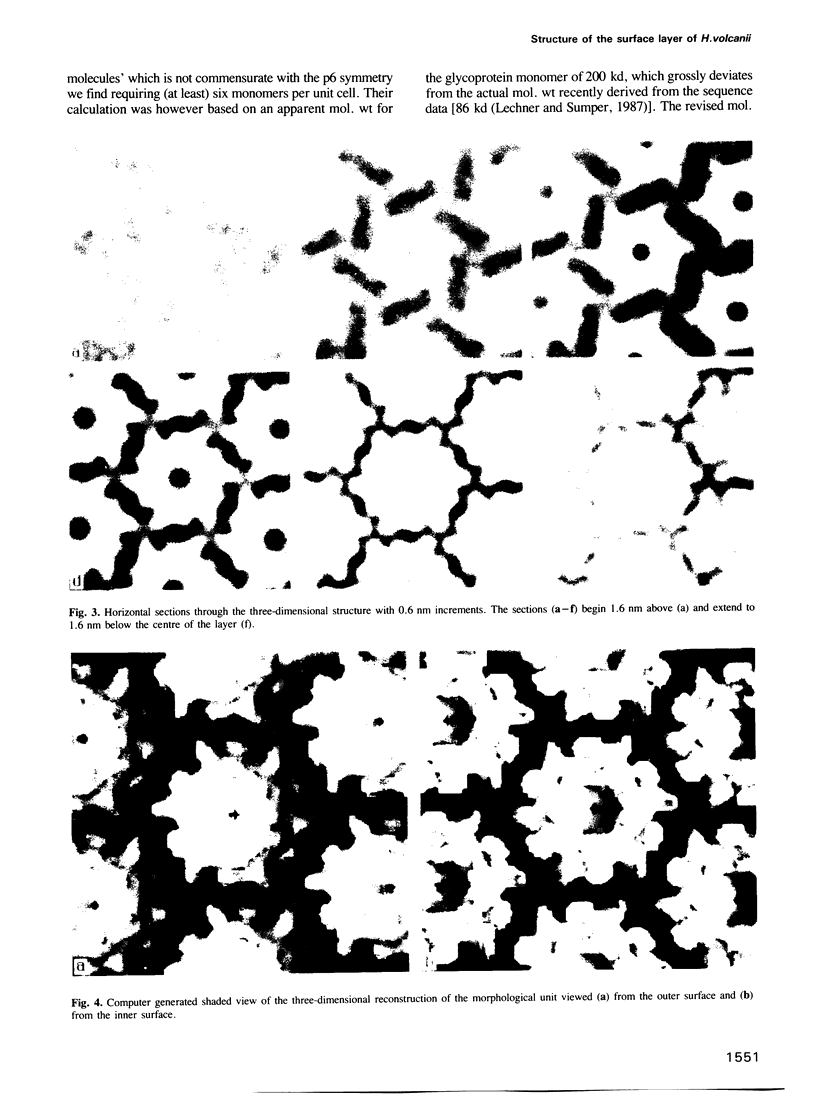
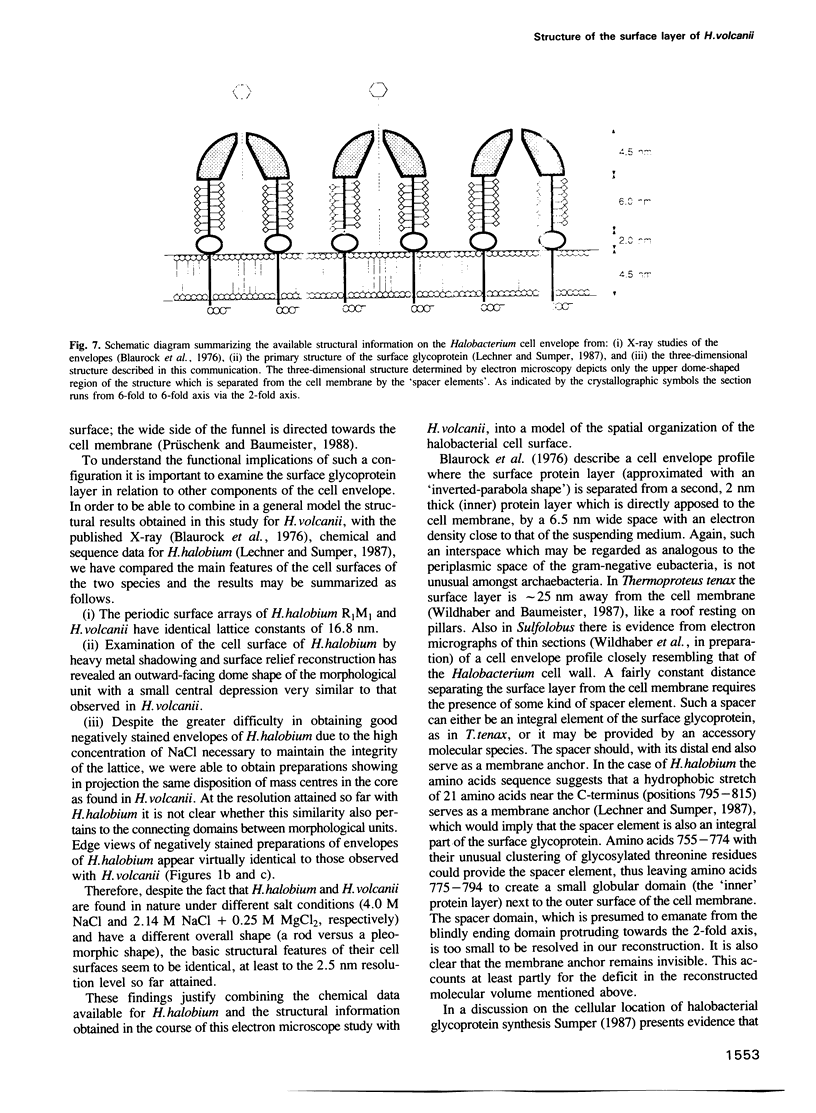
A three-dimensional reconstruction from electron micrographs of negatively stained cell envelopes of Halobacterium volcanii has revealed the structure of the surface glycoprotein to a resolution of 2 nm. The glycoprotein is arranged on a p6 lattice with a lattice constant of 16.8 nm. It forms 4.5 nm high, dome-shaped, morphological complexes with a narrow pore at the apex opening into a `funnel' towards the cell membrane. The polarity of the structure was derived from freeze-etching experiments and `edge' views. Six radial protrusions emanate from each morphological complex and join around the 3-fold axis to provide lateral connectivity. Using the primary structure of the surface glycoprotein of the closely related species Halobacterium halobium (Lechner and Sumper, 1987) and the cell envelope profile from a previous X-ray analysis of the same species (Blaurock et al., 1976) we have integrated our reconstruction into a model of halobacterial cell envelope.
Keywords: electron microscopy, halobacterial cell wall, glycoprotein, three-dimensional reconstruction
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaurock A. E., Stoeckenius W., Oesterhelt D., Scherfhof G. L. Structure of the cell envelope of Halobacterium halobium. J Cell Biol. 1976 Oct;71(1):1–22. doi: 10.1083/jcb.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aoust J. Y., Kushner D. J. The regular hexagonal surface layer of Halobacterium cutirubrum: a honeycomb network. Can J Microbiol. 1972 Nov;18(11):1767–1768. doi: 10.1139/m72-273. [DOI] [PubMed] [Google Scholar]
- HOUWINK A. L. Flagella, gas vacuoles and cell-wall structure in Halobacterium halobium; an electron microscope study. J Gen Microbiol. 1956 Aug;15(1):146–150. doi: 10.1099/00221287-15-1-146. [DOI] [PubMed] [Google Scholar]
- Hegerl R., Altbauer A. The "EM" program system. Ultramicroscopy. 1982;9(1-2):109–116. doi: 10.1016/0304-3991(82)90233-9. [DOI] [PubMed] [Google Scholar]
- Kirk R. G., Ginzburg M. Ultrastructure of two species of halobacterium. J Ultrastruct Res. 1972 Oct;41(1):80–94. doi: 10.1016/s0022-5320(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Lechner J., Sumper M. The primary structure of a procaryotic glycoprotein. Cloning and sequencing of the cell surface glycoprotein gene of halobacteria. J Biol Chem. 1987 Jul 15;262(20):9724–9729. [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976 Apr 10;251(7):2005–2014. [PubMed] [Google Scholar]
- Mullakhanbhai M. F., Larsen H. Halobacterium volcanii spec. nov., a Dead Sea halobacterium with a moderate salt requirement. Arch Microbiol. 1975 Aug 28;104(3):207–214. doi: 10.1007/BF00447326. [DOI] [PubMed] [Google Scholar]
- Paul G., Wieland F. Sequence of the halobacterial glycosaminoglycan. J Biol Chem. 1987 Jul 15;262(20):9587–9593. [PubMed] [Google Scholar]
- Robertson J. D., Schreil W., Reedy M. Halobacterium halobium. I. A thin-sectioning electron-microscopic study. J Ultrastruct Res. 1982 Aug;80(2):148–162. doi: 10.1016/s0022-5320(82)90014-4. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W., Hahn M. Three-dimensional reconstruction of imperfect two-dimensional crystals. Ultramicroscopy. 1984;13(1-2):57–70. doi: 10.1016/0304-3991(84)90057-3. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. Principles of organization in S layers. J Mol Biol. 1986 Jan 20;187(2):251–253. doi: 10.1016/0022-2836(86)90232-9. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Steensland H., Larsen H. A study of the cell envelope of the halobacteria. J Gen Microbiol. 1969 Mar;55(3):325–336. doi: 10.1099/00221287-55-3-325. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Rowen R. A morphological study of Halobacterium halobium and its lysis in media of low salt concentration. J Cell Biol. 1967 Jul;34(1):365–393. doi: 10.1083/jcb.34.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumper M. Halobacterial glycoprotein biosynthesis. Biochim Biophys Acta. 1987 Apr 27;906(1):69–79. doi: 10.1016/0304-4157(87)90005-0. [DOI] [PubMed] [Google Scholar]
- Usukura J., Yamada E., Tokunaga F., Yoshizawa T. Ultrastructure of purple membrane and cell wall of Halobacterium halobium. J Ultrastruct Res. 1980 Feb;70(2):204–219. doi: 10.1016/s0022-5320(80)80006-2. [DOI] [PubMed] [Google Scholar]
- Wieland F., Dompert W., Bernhardt G., Sumper M. Halobacterial glycoprotein saccharides contain covalently linked sulphate. FEBS Lett. 1980 Oct 20;120(1):110–114. doi: 10.1016/0014-5793(80)81058-1. [DOI] [PubMed] [Google Scholar]
- Wildhaber I., Baumeister W. The cell envelope of Thermoproteus tenax: three-dimensional structure of the surface layer and its role in shape maintenance. EMBO J. 1987 May;6(5):1475–1480. doi: 10.1002/j.1460-2075.1987.tb02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]