Abstract
Background
High-quality human hepatocytes form the basis of drug safety and efficacy tests, cell-based therapies, and bridge-to-transplantation devices. Presently the only supply of cells derives from an inadequate pool of suboptimal disqualified donor livers. Here we evaluated whether machine perfusion could ameliorate ischemic injury that many of these livers experience prior to hepatocyte isolation.
Methods
Non-heparinized female Lewis rat livers were exposed to an hour of warm ischemia (34°C) and then perfused for 3 hours. Five different perfusion conditions that utilized the cell isolation apparatus were investigated, namely: (1) modified Williams Medium E and (2) Lifor, both with active oxygenation (95%O2/5%CO2), as well as (3) Lifor passively oxygenated with ambient air (21%O2/0.04%CO2), all at ambient temperatures (20±2°C). At hypothermic temperatures (5±1°C) and under passive oxygenation were (4) University of Wisconsin solution (UW) and (5) Vasosol. Negative and positive control groups comprised livers that had ischemia (WI) and livers that did not (Fresh) prior to cell isolation, respectively.
Results
Fresh livers yielded 32±9 million cells/g liver while an hour of ischemia reduced the cell yield to 1.6±0.6 million cells/g liver. Oxygenated Williams medium E and Lifor recovered yields of 39±11 and 31±2.3 million cells/g liver, respectively. The passively oxygenated groups produced 15±7 (Lifor), 13±7 (Vasosol), and 10±6 (UW) million cells/g liver. Oxygenated Williams Medium E was most effective at sustaining pH values, avoiding the accumulation of lactate, minimizing edematous weight gain and producing bile during perfusion.
Conclusions
Machine perfusion results in a dramatic increase in cell yields from livers that have had up to an hour of warm ischemia, but perfusate choice significantly impacts the extent of recovery. Oxygenated Williams Medium E at room temperature is superior to Lifor, UW and Vasosol, largely facilitated by its high oxygen content and low viscosity.
Keywords: Perfusion, UW, Williams E, Vasosol, Lifor, hepatocytes, viscosity, temperature, isolation
Introduction
Human hepatocytes are a particularly sought after cell type used in testing the safety and efficacy of drugs [1], cell transplantation [2, 3], and bridge-to-transplantation bioartificial assist devices [4, 5]. Hepatocytes are typically procured from human donor organs rejected for transplantation [6, 7]. Due to the poor quality and limited supply, disqualified organs are an inadequate source of cells. Techniques to enhance the viable cell volume of these organs would therefore be immensely valuable.
The vast majority of disqualified donor organs, particularly those from donors after cardiac death, are exposed to periods of warm ischemia that rapidly diminish organ viability. Reperfusion of these organs can ameliorate ischemic injury provided it is conducted in both a timely [8, 9] and non-injurious manner [10, 11]. The cell isolation apparatus is conveniently suited to this task since therapeutic perfusates can be circulated through the organs prior to initiating the cell isolation process. To determine the efficacy of such a preconditioning step, we investigated five different perfusion conditions that could be easily integrated into an existing cell isolation system (Table 1). In addition, since countless perfusate options exist, we also performed a data mining analysis of perfusate properties to weigh their relative importance in predicting cell yields.
TABLE 1.
| Contents (mmol/L)* | WE | Vasosol | UW |
|---|---|---|---|
| Adenine (free base) | 5.0 | ||
| Calcium Chloride (dihydrate) | 1.8 | 0.46 | |
| Dextrose (+) | 10.0 | ||
| Glutathione (reduced) | 1.6x10−4 | 3.0 | 3.0 |
| HEPES (free acid) | 10 | ||
| Hydroxyethyl starch (g/L) | 50 | 50 | |
| Magnesium gluconate (anhydrous) | 2.7 | ||
| Mannitol | 29 | ||
| Potassium Phosphate (monobasic) | 25 | 25 | |
| Ribose, D(−) | 5.0 | ||
| Sodium Gluconate | 80 | ||
| Sodium Hydroxide/Hydrochloric Acid | 18 | ||
| Lactiobionic acid | 100 | ||
| Magnesium Sulphate | 0.83 | 5.0 | |
| Raffinose (pentahydrate) | 30 | ||
| Adenosine | 5.0 | ||
| Allopurinol | 1.0 | ||
| Potassium Hydroxide | 100 | ||
| Cupric Sulphate (pentahydrate) | 4x10−7 | ||
| Iron Trinitrate (nonahydrate) | 2x10−7 | ||
| Manganese Chloride (tetrahydrate) | 5x10−7 | ||
| Potassium Chloride | 5.4 | ||
| Sodium Bicarbonate | 26 | ||
| Sodium Chloride | 116 | ||
| Sodium Phosphate (anhydrous) | 1.0 | ||
| Zinc Sulphate (heptahydrate) | 7x10−7 | ||
| Glucose | 11 | ||
| Methyl Linoleate | 9x10−6 | ||
| Pyruvic Acid | 0.28 | ||
| Amino acids (n=20) | 1.0 (A), 0.33 (C), 0.23 (D), 0.30 (E), 0.15 (F), 0.6705 (G), 0.10 (H), 0.38 (I), 0.48 (K), 0.57 (L), 0.10 (M), 0.15 (N), 0.26 (P), 2.0 (Q),0.29 (R), 0.10 (S), 0.34 (T), 0.43 (V), 0.05 (W), 0.28 (Y) | ||
| Vitamins (n=15) | 0.0003 (A), 0.01 (C), 0.003 (B1), 0.0003 (B2), 0.008 (B3), 0.002 (B5), 0.002 (B6), 0.002 (B7), 0.002 (B9), 0.00014 (B12), 0.0002 (D), 0.00002 (E), 0.00006 (K), 0.001 (choline chloride), 0.01 (myoinositol) | ||
|
| |||
| pH (26°C) | 7.4 | 7.4 | 7.4 |
| Osmolarity (mOsm) (26°C) | 320 | 300 | 320 |
| pO2 (mmHg) (26°C) | 159 | 159 | 159 |
Except where stated.
The first choice of perfusate was modified Williams Medium E (WE). This was based on the observation that livers exposed to an hour of warm ischemia yielded a meager 1.9 million hepatocytes/g liver but within 3 hours of perfusion with WE, the yield recovered to 39 million hepatocytes/g liver [12]. The treated cells were comparable both in number and function to cells from fresh, non-ischemic livers while untreated cells could not survive beyond 8 days of plate culture. Moreover, WE is a wholly artificial perfusate developed to operate at room temperatures (20°C–25°C). The perfusion system therefore need only comprise a single pump, perfusate reservoir/organ chamber, bubble trap, and oxygenator; the same circuitry used for the cell isolation process.
Lifor (Lifeblood Medical, Freehold, NJ), a proprietary solution designed for extended donor organ preservation that contains sugars, amino acids, salts, buffers, colloids, and lipid nanoparticles [13, 14], was selected as an alternative to WE. A study showed ischemic rat livers perfused with oxygenated (>400mmHg) Lifor at room temperature performed comparably to fresh livers during a subsequent normothermic reperfusion phase [15]. Additionally, Lifor oxygenated passively with ambient air (21%O2/0.03%CO2) at room temperature (26°C) and perfused at low flow rates through guinea pig hearts was found to be superior to perfusion with ViaSpan under the same conditions; only the Lifor-perfused hearts approached positive control performance during normothermic reperfusion [16]. Ambient temperature (20.8°C) and air oxygenation was also used in Lifor-perfusion of porcine kidneys [26]. Lifor reduced expression of inflammatory markers compared to kidneys perfused at 5.7°C with Vasosol [17], a perfusate becoming widely accepted in dynamic donor kidney preservation [18]. The potential to remove the need for an active oxygen supply would further simplify the perfusion system design while remaining suitable for cell isolation.
Though the studies above suggest inferior outcomes with cold-storage solutions, hypothermic machine perfusion (0°C–5°C) may also support the use of passive oxygenation by further reducing the metabolic requirements of donor organs. Ischemia recovery was therefore also evaluated with Vasosol, which had been used in the first clinical trials of machine-perfused extended criteria donor livers [19]. University of Wisconsin (UW) solution was selected as the final perfusate since it is one of the most commonly available organ preservation solutions used for static cold storage and has yet to be investigated in terms of its ability to improve hepatocyte yields from ischemic livers.
This paper demonstrates that ischemic livers can benefit from a range of MP conditions, increasing viable cell yields significantly within hours of treatment. Cell yields can be maximized by optimizing the variables of hepatic oxygenation, and perfusate viscosity.
Materials and Methods
Experimental Groups
Experiments were conducted on female Lewis rats (160g–180g) that were kept in accordance with United States National Research Council guidelines. The Subcommittee on Research Animal Care, Committee on Research, Massachusetts General Hospital, approved the experimental protocols. Animals were randomly divided into two non-perfusion control groups and five experimental perfusion groups, according to the perfusate and oxygenation conditions used (Table 2).
TABLE 2.
| Condition | n | Perfusion Temperature (°C) | Viscosity (m2/s) | Cell Yield (106/g liver) | Viability (%) | Weight Gain (%) |
|---|---|---|---|---|---|---|
| 1 Fresh (Static) | 6 | NA | NA | 32±8.6 | 92±4.0 | NA |
| 2 WI (Static) | 6 | NA | NA | 1.6±0.6* | 90±4.0 | 13±0.4 |
| 3 WE+O2 (MP) | 4 | 20 | 4.5x10−6 | 39±11 | 92±2.7 | 6.6±0.6† |
| 4 Lifor+O2 (MP) | 4 | 20 | 9.4x10−6 | 31±2.3 | 89±3.7 | 17±3.9 |
| 5 Lifor (MP) | 4 | 20 | 9.4x10−6 | 15±7.2** | 90±2.4 | 23±12 |
| 6 Vasosol (MP) | 4 | 5 | 1.5x10−5 | 13±7.0** | 90±0.6 | 40±1.3‡ |
| 7 UW (MP) | 4 | 5 | 1.4x10−5 | 9.6±6** | 93±2.5 | 26±11 |
p<0.05 compared to Fresh
p<0.05 compared to all other groups
p<0.05 compared to all other groups
Hepatectomy and Induction of Ischemia
Livers not exposed to ischemia (Fresh) were prepared directly for cell isolation as described by Seglen [20] and modified by Dunn [21]. Briefly, the animals were anesthetized (Forane, Deerfield, IL, USA) and a transverse abdominal incision was made to expose the liver, portal vein (PV), hepatic artery (HA), common bile duct (CBD), and inferior vena cava (IVC). The hepatic lobes were mobilized. 200 IU of heparin (APP, Schaumberg, IL, USA) were administered via cardiac puncture. The PV was cannulated with an 18G catheter (SurFlash, Terumo, Somerset, NJ) and then connected to the flow from the cell isolation system. The IVC was transected and the liver removed and placed into the organ chamber for the completion of the isolation process.
All other livers were exposed to ischemia. We used an ischemic model employed in several previous publications [8, 22, 23] including a publication that evaluated the function of the resulting hepatocytes [12]. Briefly, livers were excised according to the harvesting technique of Delrivière et. al. [24]. No heparin was used to pre-treat the animals, mimicking uncontrolled cardiac death. A transverse abdominal incision was made and the intestines retracted to expose the PV, the CBD, and the IVC. The CBD was cannulated (12 cm, 22 G polyethylene stent, Surflo, Terumo, Somerset, NJ) and the IVC freed from the right renal and adrenal veins. The PV was freed from the splenic and gastroduodenal veins. The liver was freed from any ligaments. The HA was ligated and the IVC clamped. Finally, the PV was clamped and the ischemic time started. The diaphragm was opened, the suprahepatic vena cava was transected, followed by the lumbar vessels, IVC, PV and HA. The liver was removed and weighed prior to being fully submerged in a 0.9% saline bath at 34°C for 60 minutes of warm ischemia. The PV was then stented open using a 16G-catheter cuff for attachment to the perfusion system. At the end of the warm ischemic insult, all livers were removed from the saline bath and weighed again. Livers in the warm ischemic (WI) group were placed into the organ chamber, connected to the cell isolation system and cell isolation was initiated. All other livers were first perfusion-treated for 3 hours.
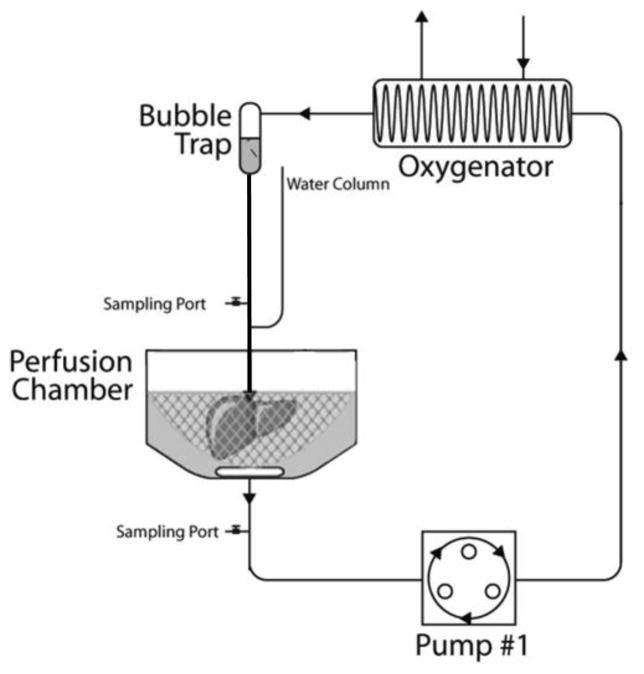
Perfusion System
The perfusion circuit and cell isolation circuit were one and the same system. During perfusion treatment, the circuit was closed (Figure 1). A peristaltic pump brought perfusate from the organ chamber to a membrane oxygenator that comprised 3 meters of oxygen-permeable silastic tubing. For passively oxygenated livers the silastic tubing was simply exposed to air, while for actively oxygenated livers, the tubing was placed in an enclosure through which a mixture of 95%O2/5%CO2 was continually flushed. The perfusate then passed through a bubble trap to an 18G catheter that connected to the portal cuff. For the first 20 minutes, the liver was flushed with non-recirculated perfusate. Portal pressure and flow rates were recorded every 5 minutes during this time using a simple manometer and digital pump readouts. At the end of the flush, the circuit was closed and beginning at t=0.5hrs, pressure and flow were recorded every half hour, and samples were taken for lactate analysis (Trinity Biotech, Jamestown, NY, USA), inflow and outflow pO2, and pH measurements (Rapidlab, Chiron Diagnostics, Norwood, MA). Bile was collected in a specimen tube and both it and the livers were weighed at the end of perfusion. Hypothermic machine perfusion was enabled by a jacketed cooling system which kept the livers at 5±1°C; this was disabled for room temperature perfusions (20±2°C). The cooling system was set to 37°C at the start of cell isolation. The perfusion was conducted in a sterile hood.
Figure 1.
The perfusion and cell isolation system comprised an organ chamber and reservoir, a pump, an oxygenator exposed to air or 95%O2/5%CO2, a bubble trap and a manometer. The system was jacketed for thermal regulation.
Perfusates
Initial perfusate volumes were 500ml in all groups. Lifor® Perfusion Medium (Lifeblood Medical, Inc. Adelphia, NJ) was supplemented with 500 units of heparin (APP, Schaumberg, IL). Vasosol® (PPS solution, Waters Medical Systems, LLC. Rochester MN) and WE (W1878, Sigma Aldrich, St. Louis, MO) were supplemented with 2 u/L insulin (28.85units/mg Humulin, Eli Lily, Indianapolis, IN), 100,000 u/L penicillin, 100mg/L streptomycin sulfate (Gibco, Invitrogen, Grand Island, NY), 0.292 g/L L-glutamine (Gibco), 10mg/L hydrocortisone (Solu-Cortef, Pharmacia & Upjohn, Kalamazoo, MI), and 1000 u/L heparin (APP, Schaumberg, IL). UW received no supplements.
Perfusate Viscosity
In the absence of available data, an estimation of perfusate viscosity was obtained using the method of capillary flow, which assumed the perfusates behaved as Newtonian fluids. Since the perfusates comprised water with a low concentration of solutes, Newtonian behavior is expected. The kinematic viscosity v of the perfusate was calculated as the ratio of the coefficient of viscosity μ to the perfusate density ρ. The perfusion system without the liver in place was utilized for this purpose. The pump established a pressure head h at the site of the manometer, a length l=10cm from the tip of the catheter, with a tubing radius of a=0.8mm. For a flow rate of Q:
| [1] |
| [2] |
A plot of htubing vs. Q (Supplemental Information) provides a linear correlation where the gradient m is the value of the coefficient of Q in Equation 2, such that v can be solved for:
| [3] |
Portal Pressure and Hepatic Resistance
The portal pressure was determined from the manometer htubing+liver, after correcting for the drop in pressure due to tubing, at a particular flow rate. The dynamic value of htubing was derived from the linear correlations found for each perfusate:
| [4] |
| [5] |
Hepatic resistance was estimated using a hydraulic analog of Ohm’s law, where resistance R is directly proportional to pressure P and inversely proportional to the flow rate Q (Rliver∝Pliver/Q) where Pliver is the product of ρ, g and hliver (Pliver=ρghliver). Rearranging and normalizing Equation 1 above with μ we get an estimate of the resistance of the liver as a function of its effective length l and radius a:
| [6] |
| [7] |
Hepatic Oxygen Uptake
The oxygen delivery rate to the liver (ODR (mlO2/min/g liver)) was estimated based on the dissolved oxygen in the perfusate calculated as a function of the flow rate (Q) and partial oxygen tension (pO2) (Equation 8). The oxygen binding coefficient (k) was assumed to be equivalent to Henry’s constant for oxygen dissolved in water at 5°C (k5°C=6.4x10−5 mlO2/mmHg/ml perfusate) and at 20°C (k20°C=4.1x10−5 mlO2/mmHg/ml perfusate). Measurements were taken immediately prior to entering the liver. Oxygen exiting the liver (OER) revealed the adequacy of the perfusate to oxygenate the liver; near-zero values implied oxygen starvation. OERs were determined from samples taken at the IVC (Equation 9). Oxygen uptake rate (OUR) was calculated as the difference between ODR and OER (Equation 10) and provided information on the metabolic state of the organ.
| [8] |
| [9] |
| [10] |
Hepatocyte Isolation
Towards the end of perfusion, the circuit was opened by disconnecting the pump inflow tubing from the perfusion chamber and placing it into 500ml of pre-warmed (37°C) oxygenated Krebs Ringer Buffer and Ethylenediamine tetraacetic acid (KRB+EDTA) solution. The pump was adjusted to a flow rate of approximately 17ml/min. The perfusion chamber was positioned on a catch basin to allow overflow to drain. When a few milliliters of KRB+EDTA remained, collagenase (type IV, C5138-1G) solution with KRB and CaCl2 was introduced into the KRB+EDTA container and allowed to flow until successful digestion was observed. The collagenase was freshly prepared at every isolation; the amount was adjusted depending on the specific activity of each collagenase batch to enable similar digestion rates between livers. The livers were then disconnected from the perfusion circuitry, placed into a petri dish on ice where approximately 10mL of sterile, cold KRB were added. The liver capsule was gently broken to release the cells which were then passed through a 250um filter followed by a 60um filter. The suspension was divided into 50 mL conical tubes and centrifuged at low speed (15g–21g, 4°C, no brake, 5 minutes). The supernatant was aspirated and the pellet re-suspended with 10ml KRB. An initial cell count and viability was performed. A volume of 24mL of cold Percoll solution (9 parts Percoll: 1 part 1.5M NaCl, pH 5–5.5) was used for every 25mL of cell suspension. Cells were added at a concentration of 5 million cells/mL and inverted five times before being centrifuged (49–58 g, 4°C, no brake, 10 minutes). The buffy coat and supernatant were discarded and the cells resuspended to 10mL in Dulbecco’s Modified Eagle Medium (DMEM) + 10% fetal bovine serum (FBS) + 100,000 u/L penicillin + 100mg/L streptomycin sulfate, after which a final count was performed using Trypan Blue exclusion. All materials used for the cell isolation were procured from Sigma Aldrich (St Louis, MO).
Statistical Analyses
Comparisons between groups was carried out using 2-way analysis of variance (ANOVA) at α=0.05, with Tukey-Kramer correction for multiple comparisons. Subsequent principal component analysis and partial least squares regression were conducted to determine the effect of process variables on response variables. Nine process variables provided data for multivariate analyses: viscosity, flow rate, pressure, resistance, pHin, pHout, pO2in, pO2out, OUR. Temperature was not selected as a process variable because each solution used here already has a prescribed optimal temperature range. The three response variables chosen were: cell yield, cell viability, and weight gain. All variables were mean-centered and unit-variance scaled for all statistical analyses.
Principal Component Analysis (PCA) and Multi-way PCA (MPCA)
Multivariate methodologies such as PCA have been proposed for the analysis of datasets with many correlated variables [25, 26]. By applying these methodologies to the data accrued here, guiding principles in perfusate design and optimization can be elicited without the need to experimentally evaluate all the permutations and combinations of the selected variables. PCA captures the correlation structure between the variables in data matrix X [(I x J), j = 1,…, J variables; i = 1,…, I independent samples] and forms a model plane with fewer R dimensions using only the R largest variance directions. R is chosen such that adding additional components to the model does not provide additional significant information. Instead of working with highly correlated collinear J variables in X, PCA yields fewer uncorrelated R projections (R<J), called scores T.
| [11] |
PCA is performed by singular value decomposition in the covariance of X and the loadings P (J x R) are derived. The R eigenvectors are the R highest variance directions. Scores T (I x R) are the new uncorrelated variable projections onto the newly-derived PCA plane. E is the residual matrix. Score biplots (t1 vs t2) reveal the similarities and clustering among the samples and also the outliers. Loading plots show the correlation among J variables and identify the variables that have a large influence on each model score vector t.
For statistical process monitoring (SPM) of dynamic datasets, PCA is extended to multiway-PCA (MPCA), which is used for the analysis of three dimensional data arrays X (I x J x K) where I independent processes are referred to as batches (i = 1,…, I). A multi-way PCA model is equivalent to an ordinary PCA model performed on a 2D matrix constructed by unfolding the three-way data array [27–32]. An (I x J x K) data array can be unfolded by preserving the batch direction I and augmenting the J variable measurements taken at each time point k (k = 1,…, K) side by side resulting in an I x JK matrix.
Partial Least Squares (PLS) and Multi-way PLS (MPLS)
Partial least squares or projections to latent structures (PLS) is a regression extension of PCA and is used to connect the information in two blocks of variables, namely the predictor block X and response block Y [33]. PLS finds the orthogonal directions that maximize the correlation between X and Y. An algorithm for Nonlinear Iterative Partial Least Squares (NIPALS) is provided in [32].
| [12] |
PLS is extended to multi-way PLS (MPLS) for batch processes. MPLS is equivalent to performing PLS where X predictor set is the unfolded batch data and Y is a 2D matrix of end-of-batch quality variables. The predictor set (X) consists of the 3-D dynamic data collected at each sampling time and unfolded into a I x JK matrix. The MPLS model reveals the similarities of the five perfusion media and explains the interrelationship between perfusion conditions and the perfusion outcomes in terms of cell yield, viability, and percent weight gain, and enables induction of the perfusion parameters for optimum perfusion outcome. Our MPLS model was built using a (13 x 9 x 6) X matrix. The matrix comprised 13 livers analyzed, since a Lifor and Vasosol liver were determined to be outliers and removed from the subsequent analyses, and 9 process variables measured at 6 different time points during perfusion. The Y response matrix is a (13 x 3) matrix that consists of the three perfusion response indicators: cell yield, viability, and weight gain. For the MPLS model the X matrix is unfolded into a (13 x 54) matrix.
Results
Cell Yields and Viabilities
Fresh liver isolations produced 32±8.6 million cells per gram liver (Table 2). After 60 minutes of warm ischemia however, cell yield was reduced 20-fold to 1.6±0.6 million cells per gram liver (p<0.05). Pre-treatment of ischemic livers with either WE+O2 or Lifor+O2 resulted in a full recovery of liver cell yields within 3 hours of perfusion. In the groups without active oxygenation, cell yield was considerably less. Lifor recovered only 50% of the cells (15±7.2 million cells per gram liver, p<0.05), which was not significantly different from yields by livers perfused with Vasosol (13±7.0) and UW (9.6±6.0) (p=0.6). As a consequence of Percoll treatment, cell viabilities were consistently high, ranging from 89±3.7 % (Lifor+O2) to 93±2.5 % (UW).
Hepatic Oxygenation
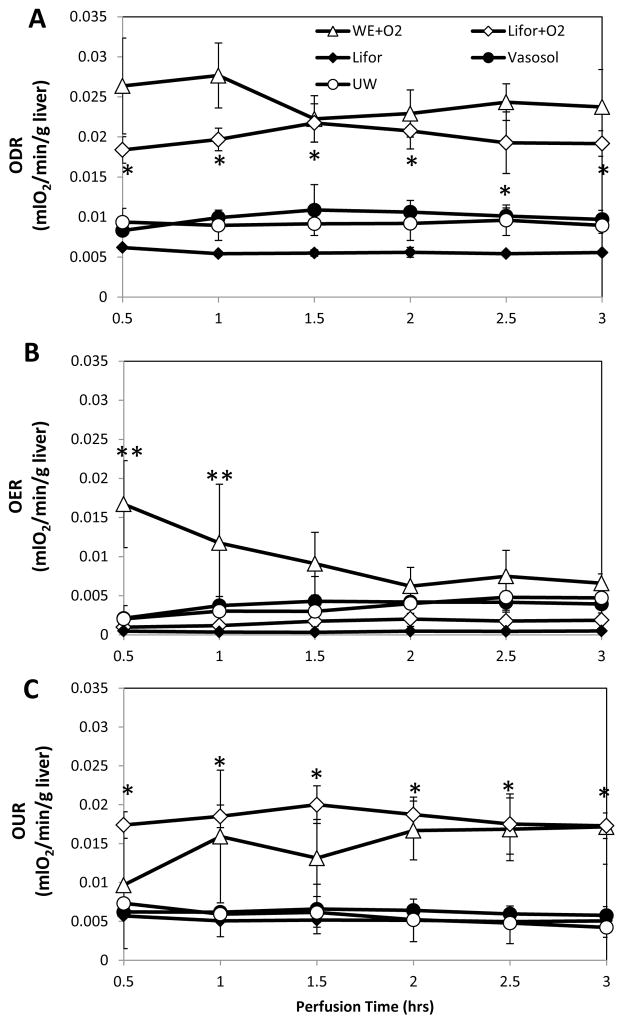
Oxygen delivery rates, illustrating the effectiveness of the oxygenators, were stable for all groups. A comparison of the Lifor groups shows that for comparable flow rates, passive oxygenation produced an average pO2 of 151±8 mmHg and an ODR of 0.006±0.0003 mlO2/min/g liver, which was significantly lower (p<0.005) than active oxygenation with a pO2 of 418±28 mmHg and an ODR of 0.02±0.001 mlO2/min/g liver. OER, a useful metric of whether the livers were being adequately oxygenated, was close to zero throughout perfusion of Lifor livers with an average of 0.0004±0.0000 mlO2/min/g liver and an average efflux pO2 of 8.3±1.2 mmHg (Figure 2B). Lifor+O2 livers had an average OER of 0.0016±0.004 mlO2/min/g liver and an average efflux pO2 of 34±11 mmHg. OERs in all other livers were significantly (p<0.05) greater than Lifor livers: Vasosol pO2 levels were 59±24 mmHg, UW pO2 levels were 56±23 mmHg and WE+O2 pO2 levels were 111±10 mmHg, demonstrating that not all the oxygen available to them had been utilized. Although ODRs of WE+O2 and Lifor+O2 were similar, OERs in the WE+O2 group were significantly elevated during the first 1.5 hours of perfusion by contrast demonstrating poor initial oxygen extraction. OER values gradually declined over time so that by t=2hrs the differences with Lifor+O2 livers were no longer significant (p=0.1). OURs, the difference between ODRs and OERs, demonstrated that Lifor livers were oxygen starved since they consumed an average of 0.005±0.0002 mlO2/min/g liver, 3.5 times less than Lifor+O2 livers (Figure 2C). By using the van’t Hoff prediction that an approximately 2.5-fold decline in metabolism occurs for every 10°C decline in temperature, which was empirically evaluated in perfused livers by Fujita et. al. [34, 35], we see that the livers perfused in this study fall within the expected ranges. Using the hepatic oxygen consumption from a similar study of normothermic MP as the maximum value (100%) [36], livers perfused at room temperatures and near-freezing temperatures consumed approximately 34% and 9% of the maximum, respectively. This is more than the 25% and 5% predicted by van’t Hoff’s equation, and closer to Fujita et. al’s findings of 32% and 12%, respectively [35]. The livers therefore appear to be functioning at metabolic rates appropriate for the perfusion temperature.
Figure 2.
Livers were oxygenated actively with a 95%O2/5%CO2 mixture or passively with ambient air (20%O2/0.03%CO2). (A) Oxygenator efficacy was demonstrated through a constant oxygen delivery rate (ODR) measured at the inflow to the portal vein as a function of the perfusate flow rate and gas composition. (B) The rate of oxygen exiting the liver (OER), measured in the vena cava, depicted the amount of oxygen remaining in the perfusate after passing through the liver. (C) The difference in oxygen delivery rate (ODR) and exit rate (OER) described the oxygen uptake rate by the liver (OUR), which declined with temperature and oxygen availability. Data are expressed as mean ± s.d. *Greater than passive oxygenation (p<0.05). **Greater than all other groups (p<0.05).
Perfusate Viscosity
The estimated kinematic viscosity differed substantially between the perfusates (Table 2). At their respective operating temperatures, WE was found to be the least viscous. Compared to WE, Lifor was approximately twice as viscous, and UW and Vasosol approximately three times as viscous. A strong inverse correlation between viscosity and cell yield was observed (Pearson 0.967, p=0.03); passively oxygenated Lifor results were excluded from this calculation to remove the oxygenation bias since only this group was observed to be oxygen deprived, which clearly impacted cell yields.
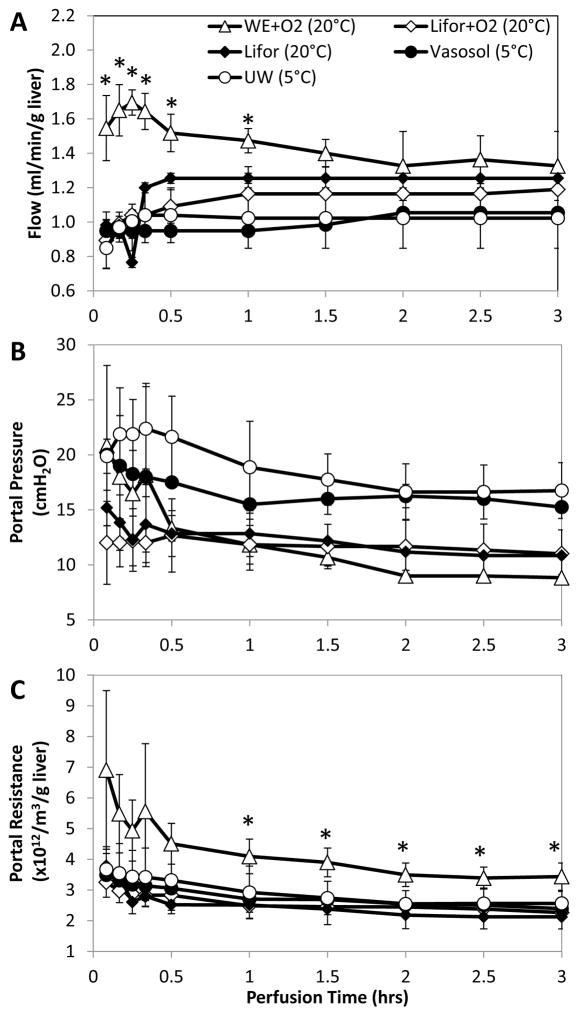
Hepatic Portal Pressure, Flow and Resistance
Within the first 0.5hr of perfusion portal pressures and flow rates stabilized (Figure 3A&B). As perfusate viscosity increased, portal pressures increased and flow rates decreased. WE, with the lowest viscosity, approached rat physiological flow rates of 1.8 ml/min/g liver [37], averaging 1.4±0.08 ml/min/g liver with portal pressures averaging 10±1.8 cmH2O. Lower average flow rates of 1.2±0.03 ml/min/g liver in the higher-viscosity Lifor perfusate produced similar average portal pressures of 12±0.5 cmH2O. Vasosol and UW, with the highest viscosities, resulted in significantly higher average portal pressures of 16±0.8 cmH2O and 18±2.0 cmH2O respectively when average flow rates of 1.0±0.05 ml/min/g liver and 1.0±0.01 ml/min/g liver respectively were used to ensure adequate oxygenation. Portal resistance (Figure 3C), calculated to account for differences in perfusate viscosity, was observed to be similar amongst all groups, except in WE+O2 livers, which had significantly higher resistances than all other groups (p<0.05).
Figure 3.
Perfusate viscosity played an influential role in fine-tuning the balance between adequate flow rates and low portal pressures. As perfusate viscosities increased, (A) lower flow rates produced (B) higher portal pressures. (C) Hepatic resistance, which accounted for differences in perfusate viscosity, was not significantly elevated across groups with the exception of WE livers. Data are expressed as mean ± s.d.
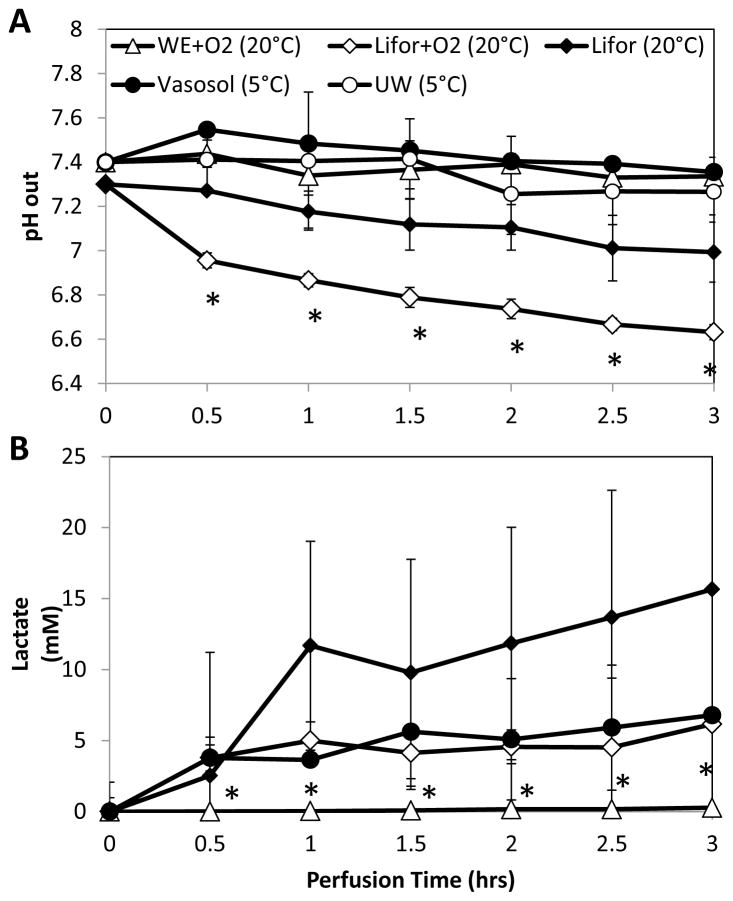
pH
Livers perfused with Lifor produced significant declines in pH values over the course of perfusion, measured at the IVC (Figure 4A). The starting pH value in Lifor+O2 was 7.0 and declined to 6.7 at the end of perfusion. Lifor livers started at a pH of 7.3 and ended with a pH of 7.0. WE+O2, UW and Vasosol livers began at a pH of 7.4 and remained above 7.3 however, while WE+O2 and UW livers had stable pH values, Vasosol livers showed a gradual but steady decline.
Figure 4.
pH and lactate trends during perfusion described perfusate pH buffering capabilities and the extent of aerobic metabolism. (A) Except for WE+O2 and UW, pH values tended to decline over the course of perfusion, but only Lifor-perfused livers exceeded physiological range (7.2–7.332). (B) Lactate values increased above physiological levels (1–1.2mM) in all groups except WE+O2. *Less than all other groups (p<0.05). Data are expressed as mean ± s.d.
Lactate Production
WE+O2 livers produced negligible amounts of lactate during perfusion (0.1 mM/hr) (Figure 4B). Lifor livers produced significantly more lactate than WE+O2 livers; by the end of 3 hours of perfusion Lifor perfusate had a concentration of 15 mM compared to 0.27 mM in WE+O2 (p<0.01). While each Lifor liver produced lactate at a similar rate during perfusion, a wide range in the amount of lactate released upon the initiation of perfusion was observed. Vasosol and Lifor+O2 livers released a comparable amount of lactate to Lifor livers during the first half hour of perfusion. Lifor+O2 livers produced 0.6 mM/hr of lactate during the remainder of the perfusion, while Vasosol livers produced 1.2 mM/hr. UW levels were not measured. There was no significant correlation in lactate production with the decline in pH levels (Pearson −0.65, p=0.35).
Bile Production
A measurable amount of bile was produced only by WE-perfused livers which amounted to 0.1±0.05g in 3 hours of perfusion.
Hepatic Weight Gain
After an hour of warm ischemia, livers gained 13±0.4% in weight compared to their fresh weight values (Table 2). Perfusion with WE reduced weight gain to 6.6±0.6% of their initial weights. All other perfusions exacerbated weight gain with Vasosol increasing it the most to 40±1.3%. Weight gain showed a strong positive correlation with viscosity (Pearson 0.92, p=0.027), but only a weak negative correlation with cell yield (Pearson −0.83, p=0.08).
Multivariate Statistical Analyses
i. Analysis of the effect of perfusion medium on the response variables Cell Yield, Viability, and Weight Gain
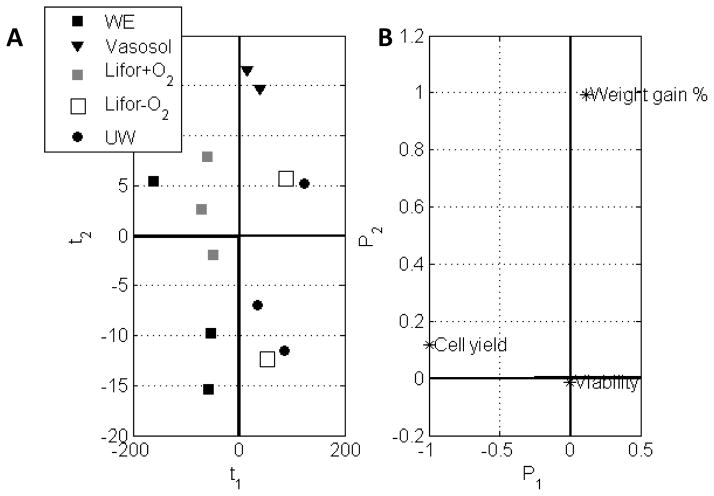
A PCA model on a (13 x 3) response matrix with 2 principal components (R=2) explains 99.8% of the variability in the data. The first PC (P1), which describes mostly the cell yield variable, explains 98.7% of the data variability and is subsequently given more importance in evaluation of the score and loading plots. The second PC (P2), which describes mostly the % weight gain variable, explains a much smaller portion of the total variation. The (t1 vs t2) score biplot (Figure 5A) and (P1 vs P2) loading plot (Figure 5B) together show that WE and Lifor+O2 perfusates tend to yield more cells than other media (t1<0) while Vasosol solution causes the largest weight gain (t2>0). The loading plot also shows that the cell viability (0,0) is independent of the different media used.
Figure 5.
The effect of perfusion medium on the response cell yield, viability, and percent weight gain are expressed in PCA score and loading plots. (A) Score biplots cluster groups primarily by cell yield and weight gain. (B) The loading plot illustrates the greatest degree of variability is explained by cell yield and weight gain, with little contribution from cell viability.
ii. Analysis based on the process variables to determine the perfusion media conditions that influence the response variables
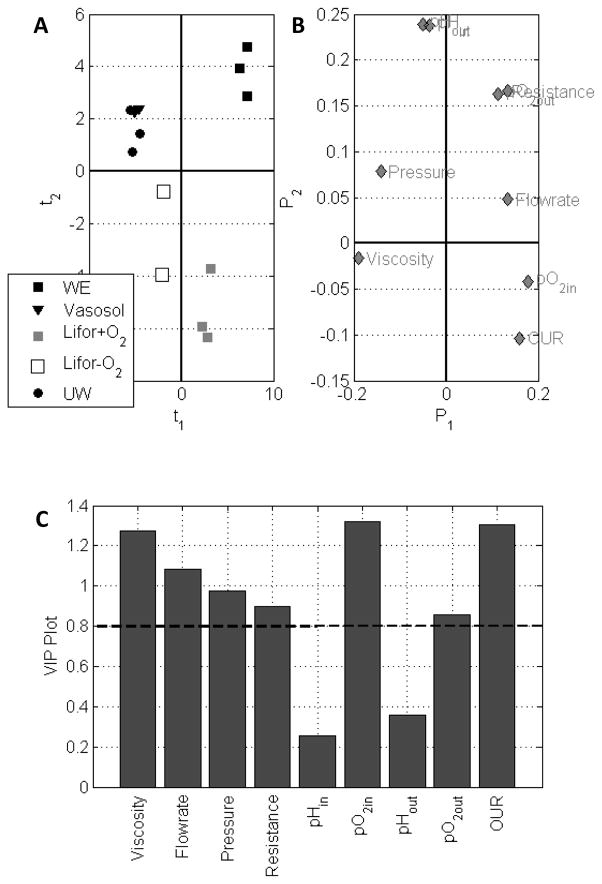
An MPLS model with five principal components (R=5) explained 94.9% of the total variation in process variables (X (13x54)) and 74.5% of the variation among the perfusion response variables (Y (13x3)). The MPLS (t1 vs t2) score biplot shows a distinct clustering of livers, separating UW and Vasosol from Lifor, Lifor+O2, and WE+O2 livers (Figure 6A). The loading plot shows that livers perfused with Vasosol and UW are likely clustered together because of similarly elevated portal pressures, in addition to their higher viscosities (P1<0) (Figure 6B). WE+O2 livers are distinguished by their higher portal resistances, oxygen trends, and flow rates. Lifor livers differ from the other groups by significantly lower pH values while Lifor+O2 perfused livers fall in the fourth quadrant, distinct from Lifor, because of higher OUR and pO2in values. Variable importance on projection (VIP) plot represents the contribution of each of the process variables in X in fitting the MPLS model (Figure 6C). VIP is a weighted sum of squares of the PLS weights taking into account the amount of explained Y-variance in each principal component dimension. Wold et al. considers a value greater than 0.8 to be significant for the VIP [38]. The variables that have major influence on the model response, in order of decreasing importance, and hence the separation among different perfusion media are pO2in, OUR, viscosity, flow rate, pressure, resistance, and pO2out.
Figure 6.
MPLS models analyze the process variables with regards to the perfusates used. (A) Score biplots cluster groups illustrating similarities between UW and Vasosol based on viscosity, but differences between Lifor and Lifor+O2 based on oxygenation. (B) The loading plot and (C) VIP plot with a cutoff of 0.8 illustrates that viscosity, pressure, resistance and oxygen delivery rates have a major influence on the model response.
iii. MPLS determines how the process variables impact the response variables
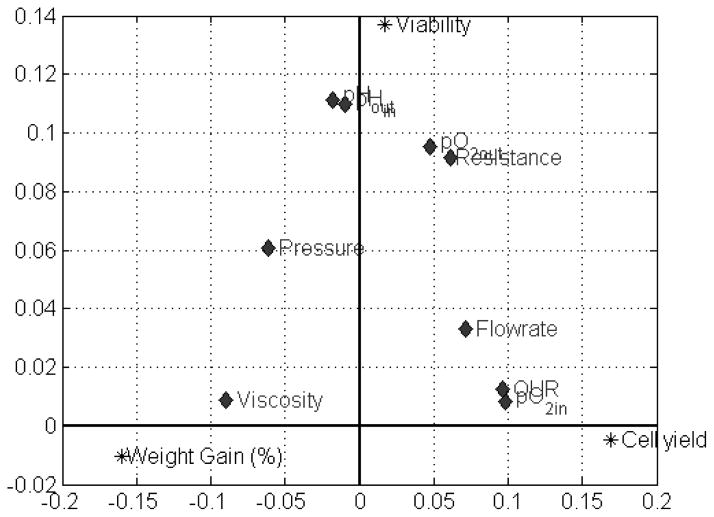
The MPLS model reveals both the similarities between the five perfusion groups and explains the interrelationship between perfusion conditions and the perfusion outcomes in terms of cell yield, viability, and percent weight gain (Figure 7). This enables induction of the perfusion parameters for optimum perfusion outcome. For instance, higher cell yields are favored by high oxygen delivery rates (higher flow rates and pO2in), oxygen uptake rates, and low viscosities. Weight gain is exacerbated by high viscosities and portal pressures and reduced when flow rates are high and there is no oxygen starvation. Highest cell viability appears to be promoted by having a stable pH.
Figure 7.
Combined analysis of response and process variables enables the effects of perfusion media properties on perfusion outcomes to be evaluated. Cell yield is increased when media has a high oxygen delivery rate but low viscosity and portal pressure, while the antithesis results in high weight gain. Stable pH and the absence of oxygen starvation ensure high cell viabilities.
Discussion
Numerous MP designs are in existence today, with operating conditions ranging from normothermic (37°C) to hypothermic (0°C–5°C) temperatures, and perfusates ranging from artificial salt-based buffers to whole-blood derivatives [39]. Here we show that while the liver tolerates and benefits from a wide range of conditions, MP design considerations, such as perfusate viscosity, oxygenation, temperature, and composition, can dramatically influence cell yields.
Optimizing WE perfusions
WE proved most effective at recovering hepatocytes from ischemic livers, which have been shown to be comparable to non-ischemic livers in both quantity and quality [12]. WE also sustained normal pH values, avoided the accumulation of lactate, produced bile and minimized edematous weight gain. High flow rates in the beginning of perfusion, found to be useful in clearing clots, appeared to be associated with increased vascular resistances in the portal vein compared to other groups. The addition of a vasodilator or thrombolytic to reduce hepatic resistance and ensure optimal oxygen uptake rates early in perfusion may therefore be beneficial.
Optimizing Lifor perfusions
In a similar study by Olschewski et. al. [15] livers were exposed to an hour of warm ischemia and perfused with oxygenated Lifor at 21°C for 6 hours. The authors used a comparable flow rate of 1 ml/min/g liver and oxygen consumption was 0.02 ml/min/g liver in both studies. Although we observed oxygen exit rates that were not as low as passively oxygenated Lifor livers, the delivery rate may not have been high enough based on the large amounts of lactate produced suggestive of anaerobic metabolism. We measured 6 mM of lactate at 3 hours and Olschewski et. al. measured approximately 5 mM at 4 hours; this was twice the concentration that they measured at 2 hours, but signs of reaching a plateau were seen by 6 hours. Bile production was also extremely low (2 μL/g liver after 6 hours); approximately 100 times less than what was produced during a subsequent 1 hr of normothermic reperfusion. The similarities in results between our studies corroborate the effect of Lifor on hepatic performance. A reduction in Lifor viscosity may improve perfusion, which could address the incline in lactate observed in Lifor+O2 livers, and an increase in pH buffering capacity could mitigate the extensive decline in pH, both of which may result in a more stable perfusate for cell isolation purposes. However, the complete retardation of bile production suggests that, despite achieving high viability post-Percoll, the quality of the cells produced compared to WE+O2 [12] must also be evaluated. Passive oxygenation of Lifor resulted in oxygen starvation with even higher lactate production and the recovery of only 50% of the total possible cell yield. This is therefore an inappropriate simplification of the perfusion system.
Optimizing Vasosol and UW perfusions
Vasosol and UW, high-potassium “extracellular-like” fluids, are designed for use at low temperatures. However, low temperatures have several cumulative effects on reducing cell yield. Perfusate viscosity is increased at low temperatures and, as seen from the data here, the higher the perfusate viscosity, the lower the flow rates must be in order to maintain portal pressures within a physiological range. Since pO2,out values exceeded those of oxygen-starved Lifor livers, suggesting the livers were not using all the oxygen available to them, flow rates could have been lowered further. Slower flow rates still could have been facilitated by active oxygenation. This would serve to reduce both damage to the vascular endothelium and edematous weight gain [40]. The caveat of extremely slow flow rates is potentially inferior flushing and therefore permanent ischemia at the microcirculatory level [41, 42]. The elevated lactate levels compared to WE+O2 livers suggest that this may already have been a concern in these livers. While active oxygenation and low viscosities have been shown to improve outcomes [43, 44], additional considerations in the use of hypothermic systems include reduced metabolic rates that may necessitate longer ischemic damage recovery times in MP, and a risk of cold-induced cell damage [15, 45, 46].
MP is a desirable intervention in the routine procurement and preservation of donor organs since it is beneficial to both healthy and unhealthy organs [12] and may enable the safe expansion of the donor pool by quantitatively evaluating marginal organ transplantability [47, 48]. Already an established technique in kidney preservation through justification of its cost-effectiveness [49], MP is being actively investigated in clinical trials for the heart, lungs and liver [50–53]. MP of disqualified donor organs for cell isolation purposes is a useful step toward this goal since the number of viable cells procured is an additional metric of the extent of organ recovery. The MP circuitry tested here demonstrates that it can be the same as that used for cell isolation purposes. For human-sized organs the primary difference in perfusion design is that a dual inflow system would be set up to also accommodate flow to the hepatic artery. This can be done either with two separate circuits [54] or by using a split in flow after the pump [55].
In conclusion, treatment of ischemically damaged donor livers with MP has the capacity to significantly increase viable cell yields. The liver appears capable of tolerating a range of MP conditions with positive outcomes, though the greatest viable cell yields occur when actively-oxygenated low-viscosity perfusates, particularly modified Williams Medium E, are used. Treatment of ischemic damage and cell isolation can be conducted with the same perfusion circuitry and subsequently MP should be considered as a preconditioning step in cell isolation protocols.
Supplementary Material
Acknowledgments
Funding from the US National Institutes of Health (R01DK59766, R01DK096075, R00DK080942), the Shriners Hospitals for Children, and Lifeblood Medical Inc. is gratefully acknowledged.
Abbreviations
- CBD
Common bile duct
- EDTA
Ethylenediamine tetraacetic acid
- HA
Hepatic artery
- IVC
Inferior vena cava
- KRB
Krebs Ringer Buffer
- MP
Machine Perfusion
- MPCA
Multi-way principal component analysis
- MPLS
Multi-way partial least squares
- ODR
Oxygen delivery rate
- OER
Oxygen exit rate
- OUR
Oxygen uptake rate
- PCA
Principal component analysis
- PLS
Partial least squares
- PV
Portal vein
- UW
University of Wisconsin solution
- WE
Williams Medium E
- WI
Warm ischemia
Footnotes
Disclosure Statement
- (WO2011140241) METHODS AND COMPOSITIONS FOR PRESERVING TISSUES AND ORGANS
- (WO2011002926) ISOLATED ADULT CELLS, ARTIFICIAL ORGANS, REHABILITATED ORGANS, RESEARCH ROOLS, ORGAN ENCASEMENTS, ORGAN PERFUSION SYSTEMS, AND METHODS FOR PREPARING AND UTILIZING THE SAME
No competing financial interests exist for the authors Candice Calhoun and François Berthiaume.
Supplemental Information: The height of the manometer level (h) plotted against corresponding flow rates (Q) in the absence of a liver enables calculation of the kinematic viscosity (v) of the perfusate from the slope of the curve.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gomez-Lechon MJ, Castell JV, Donato MT. Hepatocytes--the choice to investigate drug metabolism and toxicity in man: in vitro variability as a reflection of in vivo. Chem Biol Interact. 2007;168:30–50. doi: 10.1016/j.cbi.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Bonora-Centelles A, Donato MT, Lahoz A, Pareja E, Mir J, Castell JV, et al. Functional characterization of hepatocytes for cell transplantation: customized cell preparation for each receptor. Cell Transplantation. 2010;19:21–8. doi: 10.3727/096368909X474267. [DOI] [PubMed] [Google Scholar]
- 3.Dhawan A, Strom SC, Sokal E, Fox IJ. Human hepatocyte transplantation. In: Maurel P, editor. Hepatocytes. Vol. 640. Springer Science + Business Media; 2010. pp. 525–534. [DOI] [PubMed] [Google Scholar]
- 4.Nyberg SL. Bridging the Gap: Advances in Artificial Liver Support. Liver Transplantation. 2012;18:S10–S14. doi: 10.1002/lt.23506. [DOI] [PubMed] [Google Scholar]
- 5.Demetriou AA, Brown RS, Busuttil RW, Fair J, McGuire BM, Rosenthal P, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004 May;239:660–667. doi: 10.1097/01.sla.0000124298.74199.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhogal RH, Hodson J, Bartlett DC, Weston CJ, Curbishley SM, Haughton E, et al. Isolation of primary human hepatocytes from normal and diseased liver tissue: a one hundred liver experience. PLoS One. 2011;6:e18222. doi: 10.1371/journal.pone.0018222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes RD, Mitry RR, Dhawan A, Lehec SC, Girlanda R, Rela M, Heaton ND, Muiesan P. Isolation of hepatocytes from livers from non-heart-beating donors for cell transplantation. Liver Transpl. 2006;12:713–7. doi: 10.1002/lt.20732. [DOI] [PubMed] [Google Scholar]
- 8.Tolboom H, Pouw RE, Izamis ML, Milwid JM, Sharma N, Soto-Gutierrez A, et al. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion. Transplantation. 2009 Jan 27;87:170–7. doi: 10.1097/TP.0b013e318192df6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schön MR, Kollmar O, Wolf S, Schrem H, Matthes M, Akkoc N, et al. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surg. 2001;233:114–123. doi: 10.1097/00000658-200101000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Amara M, Yang SY, Tapuria N, Fuller B, Davidson B, Seifalian A, et al. Liver ischemia/reperfusion injury: processes in inflammatory networks - A review. Liver Transpl. 2010;16:1016–32. doi: 10.1002/lt.22117. [DOI] [PubMed] [Google Scholar]
- 11.Jaeschke H. Mechanisms of reperfusion injury after warm ischemia of the liver. J Hepatobiliary Pancreat Surg. 1998;5:402–8. doi: 10.1007/s005340050064. [DOI] [PubMed] [Google Scholar]
- 12.Izamis ML, Calhoun C, Uygun BE, Guzzardi MA, Price G, Luitje M, et al. Simple machine perfusion significantly enhances hepatocyte yields of ischemic and fresh rat livers. Cell Medicine. 2012;4:109–23. doi: 10.3727/215517912X658927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer J, Baker J, Shorr RGL. Composition for maintaining organ and cell viability. 7,220,538 B2. United States Patent US. 2007
- 14.Stowe D, Camara AKS, Heisner JS, Aldakkak M, Harder DR. Low-flow perfusion of guinea pig isolated hearts with 26C air-saturated Lifor solution for 20 hours preserves function and metabolism. J Heart Lung Transplant. 2008;27:1008–1015. doi: 10.1016/j.healun.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olschewski P, Gass P, Ariyakhagorn V, Jasse K, Hunold G, Menzel M, et al. The influence of storage temperature during machine perfusion on preservation quality of marginal donor livers. Cryobiology. 2010;60:337–43. doi: 10.1016/j.cryobiol.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Stowe DF, Camara AK, Heisner JS, Aldakkak M, Harder DR. Ten-hour preservation of guinea pig isolated hearts perfused at low flow with air-saturated Lifor solution at 26C: comparison to ViaSpan solution. Am J Physiol Heart Circ Physiol. 2007;293:H895–901. doi: 10.1152/ajpheart.00149.2007. [DOI] [PubMed] [Google Scholar]
- 17.Gage F, Leeser DB, Porterfield NK, Graybill JC, Gillern S, Hawksworth JS, et al. Room temperature pulsatile perfusion of renal allografts with Lifor compared with hypothermic machine pump solution. Transpl Proc. 2009;41:3571–3574. doi: 10.1016/j.transproceed.2009.06.228. [DOI] [PubMed] [Google Scholar]
- 18.Deng RG, G, Wang D, Tai Q, Wu L, Ju W, Zhu X, Guo Z, He X. Machine Perfusion versus Cold Storage of Kidneys Derived from Donation after Cardiac Death: A Meta-Analysis. PLoS ONE. 2013;8:e56368. doi: 10.1371/journal.pone.0056368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guarrera JV, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2009 Feb;10:372–81. doi: 10.1111/j.1600-6143.2009.02932.x. [DOI] [PubMed] [Google Scholar]
- 20.Seglen PO. Preparation of isolated rat liver cells. Method Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 21.Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Progr. 1991;7:237–45. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 22.Berendsen TA, Bruinsma BG, Lee J, D’Andrea V, Liu Q, Izamis ML, et al. A simplified subnormothermic machine perfusion model restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Tranplantation Research. 2012;1:6. doi: 10.1186/2047-1440-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolboom H, Izamis ML, Sharma N, Milwid JM, Uygun B, Berthiaume F, et al. Subnormothermic machine perfusion for recovery and preservation of ischemic rat liver grafts. J Surg Res. 2011;175 doi: 10.1016/j.jss.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delriviere L, Gibbs P, Kobayashi E, Goto S, Kamada N, Gianello P. Detailed modification technique for safer harvesting and preparation of liver graft in the rat. Microsurgery. 1996;17:690–696. doi: 10.1002/(SICI)1098-2752(1996)17:12<690::AID-MICR6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 25.Jackson JE. Principal components and factor analysis: Part I - Principal Components. J Quality Technol. 1980;12:201–213. [Google Scholar]
- 26.Tracy ND, Young JC, Mason RL. Multivariate control charts for individual observations. J Quality Contr. 1992;24:88–95. [Google Scholar]
- 27.Nomikos P, MacGregor JF. Monitoring Batch Processes Using Multi-way Principal Component Analysis. AIChE J. 1994;40:1361–1375. [Google Scholar]
- 28.Nomikos P, MacGregor JF. Multi-way partial least squares in monitoring batch processes. Chemometr Intell Lab. 1995;30:97–108. [Google Scholar]
- 29.Nomikos P, MacGregor JF. Multivariate SPC Charts For Monitoring Batch Processes. Technometrics. 1995;37:41–59. [Google Scholar]
- 30.Cinar A, Parulekar SJ, Undey C, Birol G. Batch Fermentation: Modeling, monitoring, and control. Marcel Dekker; 2003. [Google Scholar]
- 31.Undey C, Cinar A. Statistical monitoring of multi-stage, multi-phase batch processes. IEEE Contr Syst Mag. 2002;22:40–52. [Google Scholar]
- 32.Wold S, Geladi P, Esbensen K, OJ Multi-way principal components and PLS analysis. J Chemometr. 1987;1:41–56. [Google Scholar]
- 33.Eriksson L, Johansson E, Kettaneh-Wold N, Trygg J, Wikstrom C, Wold S. Multi- and Megavariate Data Analysis Part I: Basic Principles and Applications. Umetrics; 2006. [Google Scholar]
- 34.Blaxter KL. Energy metabolism in animals and man. Cambridge: Press Syndicate of the University of Cambridge; 1989. [Google Scholar]
- 35.HI, Fujita S, Nakamura K, Tanaka K, Ozawa K. Isolated perfusion of rat livers: effect of temperature on O2 consumption, enzyme release, energy store, and morphology. Nippon Geka Hokan. 1993;62:58–70. [PubMed] [Google Scholar]
- 36.Izamis ML, Tolboom H, Uygun K, Berthiaume F, Yarmush M. Resuscitation of ischemic donor livers with normothermic machine perfusion: A dynamic metabolic analysis of treatment in rats. PLoS ONE. 2013;8:e69758. doi: 10.1371/journal.pone.0069758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izamis ML, Sharma NS, Uygun B, Bieganski R, Saeidi N, Nahmias Y, et al. In situ metabolic flux analysis to quantify the liver metabolic response to experimental burn injury. Biotechnol Bioeng. 2010;108:839–52. doi: 10.1002/bit.22998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wold H. Parial Least Squares. 1985 [Google Scholar]
- 39.Izamis ML, Berendsen T, Uygun K, Yarmush ML. Addressing the Donor Liver Shortage with Machine Perfusion. Journal of Healthcare Engineering. 2012;3:279–98. [Google Scholar]
- 40.Fuller BJ, Lee CY. Hypothermic perfusion preservation: The future of organ preservation revisited? Cryobiology. 2007;54:129–145. doi: 10.1016/j.cryobiol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Pirenne J, Van Gelder F, Coosemans W, Aerts R, Gunson B, Koshiba T, Fourneau I, Mirza D, Van Steenbergen W, Fevery J, Nevens F, McMaster P. Type of donor aortic preservation solution and not cold ischemia time is a major determinant of biliary strictures after liver transplantation. Liver Transpl. 2001;7:540–5. doi: 10.1053/jlts.2001.24641. [DOI] [PubMed] [Google Scholar]
- 42.Bessems M, Doorschodt BM, Albers PS, van Vliet AK, van Gulik TM. Wash-out of the non-heart-beating donor liver: a comparison between ringer lactate, HTK, and polysol. Transpl Proc. 2005;37:395–8. doi: 10.1016/j.transproceed.2004.12.260. [DOI] [PubMed] [Google Scholar]
- 43.Luer B, Koetting M, Efferz P, Minor T. Role of oxygen during hypothermic machine perfusion preservation of the liver. Transpl Int. 2010;23:944–50. doi: 10.1111/j.1432-2277.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 44.de Rougemont O, Breitenstein S, Leskosek B, Weber A, Graf R, Clavien PA, Dutkowski P. One hour hypothermic oxygenated perfusion (HOPE) protects nonviable liver allografts donated after cardiac death. Ann Surg. 2009;250:674–83. doi: 10.1097/SLA.0b013e3181bcb1ee. [DOI] [PubMed] [Google Scholar]
- 45.Vairetti M, FA, Rizzo V, Boncompagni E, Carraro A, Grigneri E, Milanesi G, Barni S, Freitas I, Cillo U. Correlation between liver temperature employed during machine perfusion and reperfusion damage: role of Ca2+ Liver Transplant. 2008;14:494–503. doi: 10.1002/lt.21421. [DOI] [PubMed] [Google Scholar]
- 46.Fondevila C, HAJ, Maathuis MH, Munoz J, Taura P, Calatayud D, et al. Hypothermic oxygenated machine perfusion in porcine donation after circulatory determination of death liver transplant. Tranplantation. 2012;94:22–9. doi: 10.1097/TP.0b013e31825774d7. [DOI] [PubMed] [Google Scholar]
- 47.Perk S, Izamis ML, Tolboom H, Uygun B, Berthiaume F, Yarmush ML, et al. A metabolic index of ischemic injury for perfusion-recovery of cadaveric rat livers. PLoS ONE. 2011;6:e28518. doi: 10.1371/journal.pone.0028518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perk S, Izamis ML, Tolboom H, Uygun B, Yarmush ML, Uygun K. A fitness index for transplantation of machine-perfused cadaveric rat livers. BMC Res Notes. 2012;5 doi: 10.1186/1756-0500-5-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R. The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model. Health Technol Assess. 2009;13:1–156. doi: 10.3310/hta13380. [DOI] [PubMed] [Google Scholar]
- 50.Dutkowski P, Schlegel A, de Oliveira M, Müllhaupt B, Clavien PA. HOPE for Human Liver Grafts obtained from Donors after Cardiac Death. J Hepatol. 2013;60:765–72. doi: 10.1016/j.jhep.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 51.Wallinder A, Ricksten SE, Hansson C, Riise GC, Silverborn M, Liden H, et al. Transplantation of initially rejected donor lungs after ex vivo lung perfusion. J Thorac Cardiovasc Surg. 2012;144:1222–8. doi: 10.1016/j.jtcvs.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Cypel M, Yeung JC, Machuca T, Chen M, Singer LG, Yasufuku K, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg. 2012;144:1200–6. doi: 10.1016/j.jtcvs.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Collins MJ, Moainie SL, Griffith BP, Poston RS. Preserving and evaluating hearts with ex vivo machine perfusion: an avenue to improve early graft performance and expand the donor pool. Eur J Cardiothorac Surg. 2008;34:318–25. doi: 10.1016/j.ejcts.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruinsma BG, Yeh H, Ozer S, Martins PN, Farmer A, Wu W, et al. Subnormothermic Machine Perfusion for ex vivo Preservation and Recovery of the Human Liver for Transplantation. American Journal of Transplantation. 2014 doi: 10.1111/ajt.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Izamis ML, Efstathiades A, Keravnou C, Georgiadou S, Martins PN, Averkiou M. The effects of air embolism size and location on porcine hepatic microcirculation in machine perfusion. Liver Transpl. 2014 doi: 10.1002/lt.23838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.