Abstract
Background
Although warfarin is indicated to prevent ischemic strokes in most patients with atrial fibrillation (AF), evidence supporting its use in hemodialysis is limited. Our aim was to examine outcomes after warfarin initiation, relative to no warfarin use, following incident AF in a large cohort of hemodialysis patients who had comprehensive prescription drug coverage through Medicare Part D.
Study Design
Retrospective observational cohort study.
Setting & Participants
Patients in the US Renal Data System undergoing maintenance hemodialysis who were newly diagnosed with AF in 2007-2011, with Medicare Part D coverage, who had no recorded history of warfarin use.
Predictor
Warfarin initiation, identified by a filled prescription within 30 days of the AF event.
Outcomes
Death, ischemic stroke, hemorrhagic stroke, severe gastrointestinal bleeding, and composite outcomes.
Measurements
HRs estimated by applying Cox regression to an inverse probability of treatment-and-censoring-weighted cohort.
Results
Among 12,284 patients with newly diagnosed AF, 1838 (15%) initiated warfarin within 30 days; however, ~70% discontinued its use within one year. In intention-to-treat analyses, warfarin use was marginally associated with a reduced risk of ischemic stroke (HR, 0.68; 95% CI, 0.47-0.99), but not with any of the other outcomes. In as-treated analyses, warfarin use was associated with reduced mortality (HR, 0.84; 95% CI, 0.73-0.97).
Limitations
Short observation period, limited number of non-fatal events, limited generalizability of results to more affluent patients.
Conclusions
In hemodialysis patients with incident AF, warfarin use was marginally associated with a reduced risk of ischemic stroke, and there was a signal towards reduced mortality in as-treated analyses. These results support clinical equipoise regarding the use of warfarin in HD patients and underscore the need for randomized trials to fill this evidence gap.
Keywords: dialysis, end-stage renal disease (ESRD), hemodialysis, atrial fibrillation (AF), cardiac arrhythmia, warfarin, oral anticoagulation, drug safety, ischemic stroke, hemorrhagic stroke, bleeding, prevention, mortality
Atrial fibrillation (AF), the most common cardiac arrhythmia, is estimated to affect more than 2.7 million Americans.(1) Lower estimated glomerular filtration rate (eGFR) and higher albuminuria, key measures of kidney function, are strong independent risk factors for incident AF.(2) In older patients with end-stage renal disease (ESRD) initiating hemodialysis at age 67 years or older, the incidence of AF has been estimated at 148 per 1,000 person-years,(3) compared with 28 per 1,000 person-years in the general Medicare population.(4)
One of the most dreaded consequences of AF is ischemic stroke. Based on clear evidence from randomized trials, international guidelines recommend the use of oral anticoagulation in most patients with AF.(5) However, patients with advanced kidney disease, including those with ESRD, were systematically excluded from these trials. In the absence of randomized trials, there is considerable uncertainty about whether the benefits of oral anticoagulation in AF extend to patients with ESRD. Several observational studies examining the effectiveness and safety of oral anticoagulation in ESRD have yielded conflicting results,(6-9) leading the KDIGO (Kidney Disease: Improving Global Outcomes) expert panel to no longer universally recommend anticoagulation for primary and secondary stroke prevention in ESRD.(10)
We conducted the following study to examine outcomes after warfarin initiation, relative to no warfarin use, following newly diagnosed AF in a large cohort of hemodialysis patients who had comprehensive prescription drug coverage through Medicare Part D.
Methods
Study Population
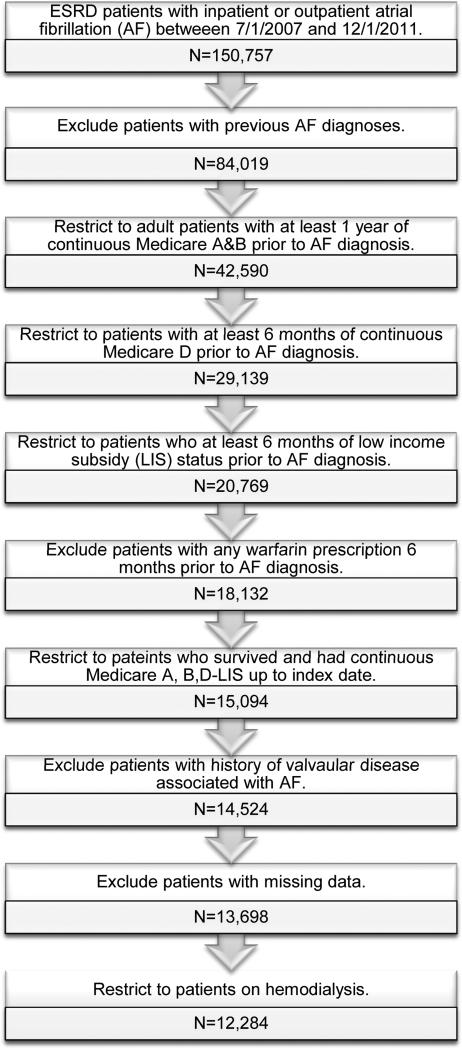
From the US Renal Data System (USRDS), we identified all hemodialysis patients who had a new diagnosis of AF in July 2007–December 2011, based on one inpatient or two outpatient diagnosis codes within 30 days of each other indicating AF or atrial flutter (International Classification of Diseases, 9th Revision [ICD-9] codes 427.3×; Figure 1). We excluded those with a history of valvular disease associated with AF (Table S1, available as online supplementary material).(11) For patients diagnosed with AF from an inpatient code, we excluded patients whose hospitalization exceeded 30 days (including transfers to a skilled nursing facility) or who died within 30 days of discharge. For patients diagnosed with AF from two outpatient codes, patients were required to survive 30 days from the first diagnosis.
Figure 1.
Study population selection from the US Renal Data System. We identified a cohort of adult patients on hemodialysis who were newly diagnosed with atrial fibrillation (AF) in 2007-2011 and who participated in a low-income subsidy program of Medicare Part D. Index date – the 30th day after discharge from the first hospitalization with an AF diagnosis or the 30th day after a first outpatient AF diagnosis. ESRD – end-stage renal disease.
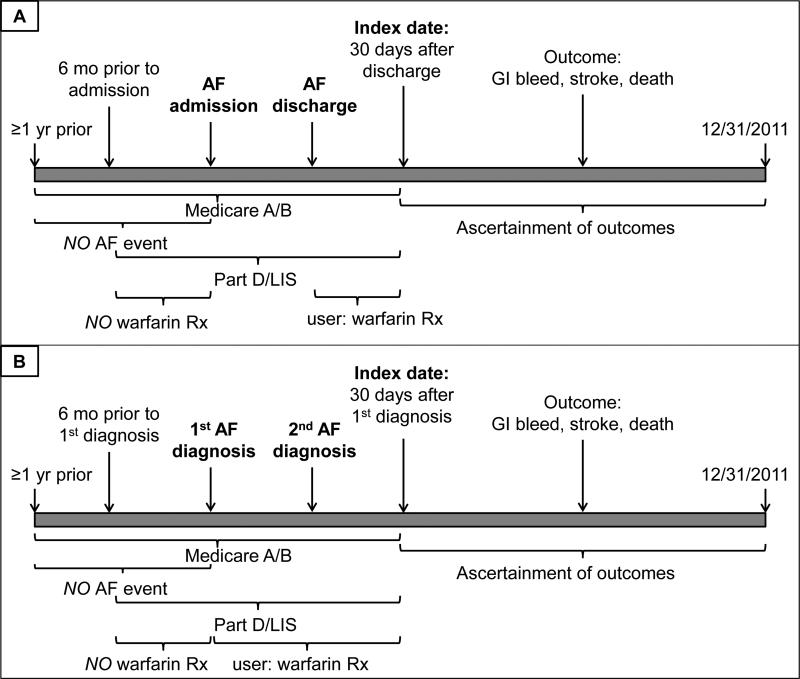
We further required the following additional conditions: uninterrupted Medicare Parts A and B coverage for at least one year prior to the first AF diagnosis code; at least 6 months of uninterrupted Medicare Part D coverage with a low-income subsidy prior to the AF diagnosis with at least one prescription filled as an indication of active use of the prescription drug benefit. We excluded patients with any filled prescription for warfarin during this time. The index date for all analyses was the 30th day after hospital discharge or the 30th day after the first outpatient AF diagnosis (Figure 2). All patients had to have continuous Medicare Parts A+B+D (low-income subsidy) coverage until the index date.
Figure 2.
Timeline of enrollment criteria, ascertainment of exposure, covariates, and outcomes for patients on maintenance hemodialysis newly diagnosed with atrial fibrillation as inpatients (Panel A) or outpatients (Panel B) between 7/1/2007 and 12/1/2011. AF – atrial fibrillation. Patients were required to have continuous Medicare A and B coverage for at least one year prior to either the AF admission or the 1st outpatient AF diagnosis until 30 days after discharge or the 1st outpatient diagnosis. They also were required to have continuous enrollment in a low-income subsidy program of Medicare Part D for at least 6 months prior to admission/1st outpatient diagnosis until 30 days after discharge/1st outpatient diagnosis. Patients with any AF in at least the year prior to admission/1st outpatient diagnosis were excluded, as were patients with any warfarin prescription in the 6 months prior to admission/1st outpatient diagnosis. Patients were considered users if they filled a prescription for warfarin in the 30 days after discharge/1st outpatient diagnosis. Outcomes were ascertained starting 30 days after discharge/1st outpatient diagnosis (index date) until 12/31/2011.
From the 12,684 patients on hemodialysis, 400 (3.2%) were missing the medical evidence form, or information on race or census division. Three percent were warfarin non-users and 3.9% users. Given the small percentage of observations missing, we performed a complete case analysis with the 12,284 patients with complete information available.
Outcomes
We examined death from any cause, cardiovascular death, and stroke-specific death. Non-fatal outcomes of interest were ischemic stroke, hemorrhagic stroke, and severe gastrointestinal bleeding (events requiring hospitalization or with gastrointestinal bleeding as reported cause of death). These were ascertained from validated claims-based algorithms (Table S2). In addition to individual outcomes, we also studied a composite end point of death, hemorrhagic or ischemic stroke or gastrointestinal bleeding. For the outcomes of death from any cause and cardiovascular death, patients were censored at end of study, January 1, 2012. For all other outcomes, patients were censored for end of study or loss of Medicare Parts A and B coverage.
Warfarin Use
The exposure of interest was initiation of warfarin within 30 days after discharge from the hospitalization or the first outpatient encounter during which AF was diagnosed (Figure 2). The number of days supplied of warfarin was ascertained from Medicare Part D claims. For analyses using an approach that corresponds to an intention-to-treat (ITT) analysis, patients were categorized as warfarin users if they filled a single prescription for warfarin during the initial 30-day period. “As-treated” (AT) analyses considered patients exposed for 60 days after the recorded supply from their previously filled prescription was exhausted (“refill grace period”). If patients failed to fill a subsequent prescription during this 60-day grace period, the follow-up time was censored. As a sensitivity analysis, we used a 30-day grace period. This rule censors warfarin users earlier than the 60-day ruled applied in the primary AT analyses and likely further reduces exposure misclassification. In AT analyses, follow-up for non-users was censored if a warfarin prescription was filled.
Patient Characteristics
We ascertained demographics, comorbidities, and indicators of health services utilization, from the Medical Evidence Report (form CMS-2728) and all available Medicare claims data predating the index date. Details about these algorithms have been published previously.(12, 13) We calculated the HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly) score, which estimates the one-year risk of major bleeding in patients with AF who are anti-coagulated, and the CHADS2 (congestive heart failure, hypertension, age 75 years or older, diabetes mellitus, prior stroke or transient ischemic attack) score, which estimates the risk for stroke in AF patients.(14, 15) Baseline medication use was ascertained from the 6 months of Medicare part D claims data predating the index date.
Statistical Analysis
We tabulated the characteristics of warfarin users and non-users using percentages and means ± standard deviations or medians (interquartile ranges) and compared the two groups using standardized differences.(16)
Our primary approach used inverse probability of treatment and censoring-weighted (IPTW) analysis (Item S1),(17) which mitigates selection bias by observed characteristics between warfarin users and non-users as well as by informative censoring between groups. Weights were computed from the treatment a patient received as well as from the censoring observed. To this end, two separate multivariable logistic regression models were applied that included the variables listed in Table 1 to estimate the propensity for 1) warfarin use and 2) censoring for loss of Medicare Parts A and B (ITT analysis) and loss of Medicare Part D, loss of low income status, or treatment switching (AT analysis).(18) For each model, we computed stabilized weights defined as the inverse of the estimated propensity multiplied by a constant equal to the observed proportion of patients being treated (in propensity model 1) or the observed proportion of patients being censored (in propensity model 2). Stabilization does not affect the point estimate but reduces the variability of the IPTW weights. However, some stabilized weights might still be too large (small) and, therefore, these were truncated and reset to the value 10 (0.1).(18, 19) Final weights were computed as the product of the stabilized and trimmed weight for treatment and the stabilized and trimmed weight for censoring. If necessary, final weights were also trimmed.(18)
Table 1.
Characteristics of patients on hemodialysis with newly diagnosed atrial fibrillation.
| Full cohort | IPTW Cohort | |||||
|---|---|---|---|---|---|---|
| Variable | Non-users (n=10,446) | Warfarin users (n=1838) | Std. diff., % | Non-users | Warfarin users | Std. diff., % |
| Demographics | ||||||
| Age, y | 62.1 ±13.6 | 61.2 ± 12.4 | 6.3 | 61.9 ±13.6 | 61.8 ±12.2 | 1.1 |
| Female sex | 51.3 | 50.3 | 1.9 | 51.2 | 53.0 | 3.7 |
| Race | ||||||
| White | 48.9 | 53.0 | 8.4 | 49.5 | 49.1 | 0.8 |
| Black | 43.2 | 41.7 | 3.0 | 43.0 | 44.0 | 2.0 |
| Other | 7.9 | 5.2 | 10.9 | 7.5 | 6.9 | 2.2 |
| Hispanic ethnicity | 18.4 | 17.8 | 1.5 | 18.3 | 17.7 | 1.5 |
| Dialysis vintage, y | 4 [2-7] | 4 [3-7] | 3.2 | 4 [2-7] | 4 [2-7] | 0.4 |
| Geographic locationa | ||||||
| Pacific | 16.0 | 12.0 | 11.8 | 15.4 | 14.0 | 4.1 |
| East South Central | 7.9 | 7.5 | 1.6 | 7.9 | 7.6 | 0.8 |
| West South Central | 15.8 | 12.7 | 9.0 | 15.4 | 15.6 | 0.7 |
| Mountain | 3.8 | 2.9 | 5.0 | 3.6 | 3.8 | 0.8 |
| New England | 2.8 | 3.2 | 2.2 | 2.8 | 2.9 | 0.7 |
| South Atlantic | 22.3 | 24.4 | 5.1 | 22.6 | 23.6 | 2.4 |
| West North Central | 4.6 | 5.8 | 5.3 | 4.8 | 4.8 | 0 |
| East North Central | 14.4 | 18.2 | 10.3 | 15.0 | 14.4 | 1.8 |
| Middle Atlantic | 12.3 | 13.3 | 3.1 | 12.5 | 13.2 | 2.3 |
| Reported comorbidities | ||||||
| Alcohol dependence | 3.4 | 1.5 | 12.4 | 3.1 | 2.7 | 2.2 |
| Arrhythmia | 26.8 | 24.5 | 5.1 | 26.4 | 25.6 | 1.8 |
| Cancer | 9.2 | 7.1 | 7.6 | 8.9 | 9.2 | 1.1 |
| Cerebrovascular disease | 26.8 | 22.0 | 11.2 | 26.1 | 27.2 | 2.5 |
| Coronary artery disease | 42.6 | 37.0 | 11.5 | 41.8 | 43.2 | 2.9 |
| Diabetes mellitus | 70.8 | 69.1 | 3.7 | 70.6 | 72.6 | 4.6 |
| Gastrointestinal bleeding | 6.0 | 2.2 | 19.3 | 5.4 | 6.8 | 5.8 |
| Heart failure | 68.3 | 67.3 | 2.1 | 68.2 | 69.3 | 2.5 |
| Hypertension | 98.6 | 97.2 | 9.6 | 98.4 | 98.5 | 0.5 |
| Inability to ambulate | 4.0 | 2.9 | 5.6 | 3.8 | 3.4 | 2.4 |
| Inability to transfer | 1.3 | 0.8 | 5 | 1.3 | 1.2 | 0.6 |
| Liver disease | 18.4 | 14.3 | 11.4 | 17.8 | 17.6 | 0.5 |
| Peptic ulcer disease | 5.1 | 2.7 | 12.2 | 4.7 | 6.4 | 7.1 |
| Peripheral artery disease | 39.9 | 35.1 | 9.9 | 39.3 | 41.3 | 4.1 |
| Pulmonary disease | 41.3 | 37.7 | 7.4 | 40.9 | 43.5 | 5.3 |
| Tobacco use | 16.4 | 13.1 | 9.4 | 15.9 | 17.2 | 3.5 |
| Valvular disease | 32.7 | 34.5 | 3.8 | 33.0 | 33.9 | 1.9 |
| CHADS2 scoreb ≥2 | 91.1 | 90.2 | 3.3 | 90.9 | 92.0 | 4.1 |
| HAS-BLED scorec ≥3 | 70.4 | 63.3 | 15 | 69.3 | 70.9 | 3.4 |
| Indicators of health services utilization | ||||||
| Days hospitalized in year before AF | 12 [5-22] | 9 [4-17] | 28.7 | 11 [5-21] | 11 [5-23]) | 9.7 |
| Admitted to skilled nursing facility in year before AF | 20.2 | 8.8 | 32.8 | 18.5 | 20.9 | 5.9 |
| Hospitalized in 30 d postdischarge for AF or 1st outpatient diagnosis | 32.5 | 33.1 | 1.1 | 32.7 | 34.2 | 3.3 |
| Days hospitalized in 30 d postdischarge for AF or post 1st outpatient diagnosis | 0 [0-4] | 0 [0-4] | 0.6 | 0 [0-4] | 0 [0-4] | 2.1 |
| Peripheral access surgery or revision in 6 mo prior to index date | 39.3 | 35.2 | 8.4 | 38.7 | 38.6 | 0.1 |
| Baseline medication use | ||||||
| Anticoagulantd | 0.5 | 1.8 | 11.9 | 0.7 | 0.7 | 0.6 |
| Antiplatelet agente | 23.0 | 21.5 | 3.6 | 22.8 | 24.1 | 3.0 |
| β blocker | 60.6 | 63.3 | 5.6 | 61.0 | 60.6 | 0.8 |
| Calcium channel blocker | 49.3 | 49.2 | 0.2 | 49.3 | 49.6 | 0.7 |
| Calcium acetate | 29.5 | 27.8 | 3.7 | 29.2 | 29.6 | 0.9 |
| Central acting agonistf | 23.6 | 20.9 | 6.4 | 23.2 | 23.3 | 0.4 |
| Diuretic | 17.1 | 17.5 | 0.9 | 17.2 | 17.5 | 0.8 |
| Lipid lowering agent, nonstating | 8.1 | 9.0 | 3.0 | 8.3 | 9.1 | 2.9 |
| Nitrate | 20.0 | 18.8 | 3.0 | 19.8 | 21.5 | 4.2 |
| NSAID | 8.1 | 7.3 | 3.0 | 8.0 | 7.8 | 1.0 |
| PPI or H2-blocker | 49.2 | 46.2 | 5.9 | 48.7 | 50.3 | 3.1 |
| Sevelamer | 43.7 | 45.1 | 2.7 | 44.0 | 44.3 | 0.7 |
| Statin | 37.7 | 38.9 | 2.5 | 37.9 | 38.6 | 1.5 |
| AF characteristics | ||||||
| Year AF diagnosed | ||||||
| 2007 | 10.3 | 9.2 | 3.7 | 10.2 | 10.8 | 1.9 |
| 2008 | 20.4 | 21.4 | 2.5 | 20.5 | 19.5 | 2.4 |
| 2009 | 21.5 | 22.8 | 3.2 | 21.7 | 23.4 | 4.0 |
| 2010 | 22.9 | 23.8 | 2.2 | 23.0 | 21.9 | 2.7 |
| 2011 | 24.9 | 22.7 | 5.1 | 24.6 | 24.4 | 0.4 |
| Diagnosed as outpatient | 11.7 | 16.5 | 14.0 | 12.4 | 12.4 | 0.1 |
| Discharged home after hospitalizationh | 69.3 | 75.1 | 13.1 | 70.1 | 68.8 | 2.8 |
| Length of stay,h d | 6 [3-11] | 6 [3-10] | 9.9 | 6 [3-11] | 6 [3-11] | 0.1 |
AF atrial fibrillation; IPTW inverse probability of treatment and censoring-weighted; NSAID nonsteroidal antiinflammatory drug; PPI, proton pump inhibitor; Std. Diff. standardized difference.
Note: Values for categorical variables are given as percentages; values for continuous variables, as mean ± standard deviation or median [interquartile range]. IPTW cohorts are generally considered to be balanced if the standardized differences for the characteristics are <10%.(16)
Facilities were categorized into one of nine U.S. Census Bureau divisions based on their state.(35)
CHADS2 score for stroke risk stratification in patients with AF: 1 point each for heart failure, hypertension, age ≥75 years, diabetes mellitus; 2 points each for prior stroke or transient ischemic attack.(36)
HAS-BLED score to assess 1-year risk of major bleeding in patients with AF: 1 point each for hypertension, kidney disease, liver disease, stroke, major bleeding history, age ≥65 use of antiplatelet drug or NSAID, and alcohol use. 1 point is also given for labile international normalized ratio; however, international normalized ratio measurements were not available in our data.(14)
Includes apixaban, ardeparin, dabigatran, dalteparin, danaparoid, enoxaparin, fondaparinux, pentosan, phenindione, rivaroxaban, and tinzaparin.
Includes cilostazol, clopidogrel, dipyridamole, prasugrel, ticagrelor, and ticlopidine.
Includes clonidine, guanabenz, guanfacine, and methyldopa.
Includes cholestyramine, clofibrate, colesevelam, colestipol, ezetimibe, fenofibrate, gemfibrozil, icosapent ethyl, lomitapide mesylate, mipomersen sodium, niacin, nicotinyl alcohol,
For outpatients = 0.
We performed several sensitivity analyses. We used IPTW to conduct AT analyses in which we reduced the grace period to 30 (vs. 60) days after the drug supply ran out. We also repeated all analyses using propensity score matched (Item S1), instead of IPTW, cohorts. One-on-one matching was conducted using the “psmatch2” program in Stata 12 (StataCorp LP) with a maximum caliper width of 0.01.(20)
All outcomes analyses were conducted using Cox regression analysis with robust standard errors for the IPTW analysis and stratified by the matched pair for the propensity score analysis. In the final models, we adjusted for any covariates that remained unbalanced. Departures from the proportionality assumption were visually identified from plots of scaled Schoenfeld residuals. All hazard ratios (HRs) were accompanied by their corresponding 95% confidence interval (CI). Heterogeneity by age (younger than 67 years or 67 years and older), history of stroke, and median length of hospital stay was assessed using multiplicative interaction terms.
Analyses were performed using SAS software, version 9.3 (SAS Institute Inc; www.sas.com) and Stata, version 12 (www.stata.com). The institutional review boards of Stanford University and Baylor College of Medicine approved the study.
Results
Patient Characteristics
We identified 12,284 patients undergoing hemodialysis who were newly diagnosed with AF, 15% of whom initiated warfarin (Figure 1). Users and non-users differed only slightly, and most of these differences were related to risk factors for bleeding (Table 1). Notably, 90% of both groups met the CHADS2 criteria for oral anti-coagulation for patients with AF (score ≥2), although a larger proportion of non-users (70% vs. 63%) had a HAS-BLED score ≥3, a situation in which anti-coagulation is not recommended in the general population. After weighting the cohort by their inverse probability of treatment with warfarin,(21) all observed characteristics were balanced between users and non-users (Table 1).
Association of Warfarin Initiation With Outcomes
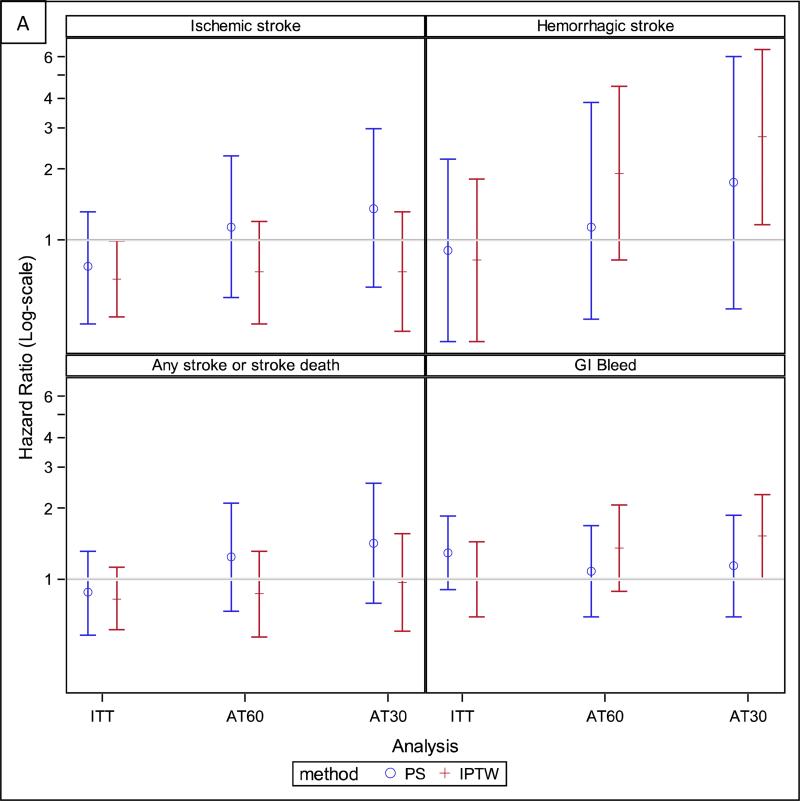
In the ITT analysis, we observed 5,427 deaths over 16,617 person-years of follow-up, for a mortality rate of 33 deaths per 100 person-years. We observed 13.7 cardiovascular deaths, 3.2 ischemic strokes, 1.3 hemorrhagic strokes, and 5.9 severe gastrointestinal bleeds per 100 person-years. The rate of the composite outcome was similar to that of all-cause mortality, 38 events per 100 person-years. The risk of ischemic stroke was lower for warfarin users than non-users (HR, 0.68; 95% CI, 0.47-0.99), but not different for any of the other outcomes (Table 2, Figure 3). Age (<67 vs ≥67 years), history of stroke, and median length of stay during the index hospitalization did not modify any of the associations.
Table 2.
Number of events, follow-up time, incidence rates, and hazard ratios for all study outcomes based on an inverse probability of treatment weighted population of approximately 15% warfarin users and 85% non-users using full cohort of 12,284 hemodialysis patients.
| Outcome | Analysis | Exposure group | No. of events | Follow-up, y* | IR, per 100 person-y | HR (95% CI) | |
|---|---|---|---|---|---|---|---|
| All-cause mortality | ITT | Warfarin | 832 | 1.34 ± 1.10; 1.01 | 33.0 | 1.01 (0.92-1.11) | |
| No warfarin | 4595 | 1.35 ± 1.14 | 1.01 | 32.5 | |||
| AT60a | Warfarin | 375 | 0.60 ± 0.63 | 0.36 | 31.7 | 0.84 (0.73-0.97) | |
| No warfarin | 4005 | 1.19 ± 1.11 | 0.84 | 32.1 | |||
| Cardiovascular mortality | ITT | Warfarin | 349 | 1.34 ± 1.10 | 1.01 | 13.8 | 1.00 (0.86-1.17) |
| No warfarin | 1940 | 1.35 ± 1.14 | 1.01 | 13.7 | |||
| AT60a | Warfarin | 184 | 0.60 ± 0.65 | 0.36 | 15.6 | 0.87 (0.70-1.07) | |
| No warfarin | 2071 | 1.22 ± 1.13 | 0.85 | 16.4 | |||
| Ischemic stroke | ITT | Warfarin | 63 | 1.42 ± 1.19 | 1.07 | 2.3 | 0.68 (0.47-0.99) |
| No warfarin | 503 | 1.40 ± 1.21 | 1.01 | 3.4 | |||
| AT60a | Warfarin | 32 | 0.61 ± 0.67 | 0.36 | 2.7 | 0.73 (0.44-1.20) | |
| No warfarin | 497 | 1.30 ± 1.20 | 0.89 | 3.7 | |||
| Hemorrhagic stroke | ITT | Warfarin | 29 | 1.46 ± 1.20 | 1.09 | 1.0 | 0.82 (0.37-1.81) |
| No warfarin | 192 | 1.44 ± 1.23 | 1.05 | 1.3 | |||
| AT60a | Warfarin | 26 | 0.61 ± 0.68 | 0.35 | 2.1 | 1.92 (0.82-4.48) | |
| No warfarin | 172 | 1.34 ± 1.23 | 0.94 | 1.2 | |||
| Any stroke or stroke death | ITT | Warfarin | 116 | 1.40 ± 1.17 | 1.06 | 4.4 | 0.83 (0.61-1.12) |
| No warfarin | 765 | 1.38 ± 1.19 | 1.00 | 5.3 | |||
| AT60a | Warfarin | 61 | 0.65 ± 0.69 | 0.40 | 5.0 | 0.87 (0.57-1.32) | |
| No warfarin | 650 | 1.19 ± 1.12 | 0.80 | 5.2 | |||
| Gastrointestinal bleeding | ITT | Warfarin | 153 | 1.36 ± 1.15 | 0.97 | 5.9 | 1.00 (0.69-1.44) |
| No warfarin | 833 | 1.36 ± 1.19 | 0.98 | 5.9 | |||
| AT60a | Warfarin | 108 | 0.60 ± 0.65 | 0.34 | 9.0 | 1.36 (0.89-2.07) | |
| No warfarin | 782 | 1.25 ± 1.18 | 0.86 | 6.0 | |||
| Composite outcomeb | ITT | Warfarin | 890 | 1.23 ± 1.06 | 0.91 | 38.8 | 1.01 (0.93-1.11) |
| No warfarin | 4961 | 1.25 ± 1.10 | 0.91 | 38.1 | |||
| ATa | Warfarin | 427 | 0.61 ± 0.65 | 0.37 | 37.7 | 0.89 (0.78-1.02) | |
| No warfarin | 4219 | 1.08 ± 1.03 | 0.75 | 37.1 | |||
AT60 – as-treated analysis where patients were censored 60 days after their drug supply ran out; CI – confidence interval, HR, hazard ratio; ITT – intention to treat,
Values given as mean ± standard deviation; median.
Stratified Cox by year when atrial fibrillation was diagnosed.
Composite outcome includes any stroke or stroke death, gastrointestinal bleeding, and all-cause mortality.
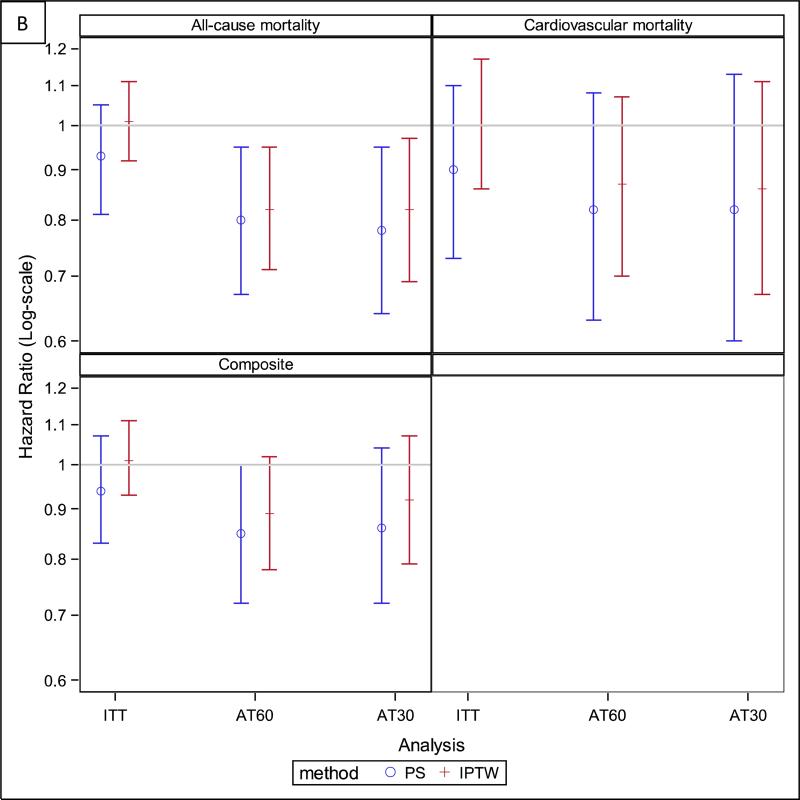
Figure 3.
Hazard ratios for all study outcomes for both the primary analyses [based on the full inverse probability of treatment and censoring-weighted (IPTW) cohort] and the sensitivity analyses [based on the propensity score (PS) matched cohort]. Panel A: Stroke and bleeding outcomes. Panel B: Mortality and composite outcome. AT60 – as treated analysis where patients were censored 60 days after their drug supply ran out, AT30 – as treated analysis where patients were censored 30 days after their drug supply ran out, ITT – intention to treat.
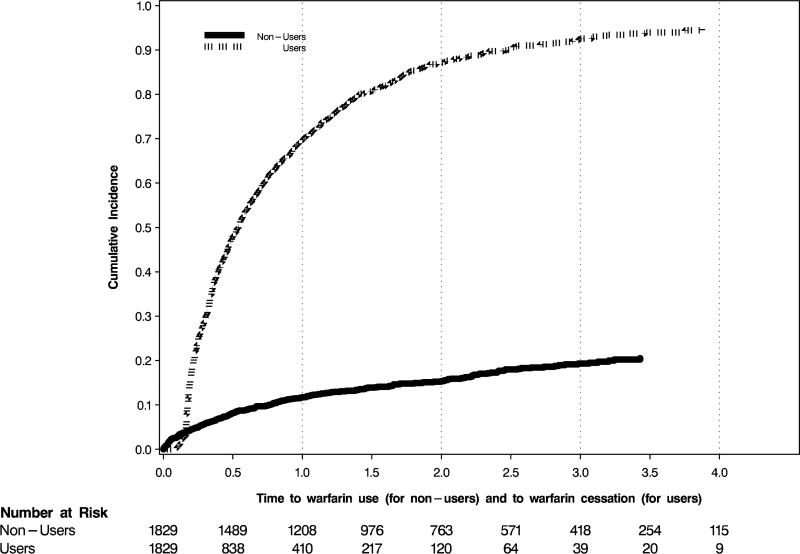
In the AT analyses, the follow-up time for warfarin-users was only about half that of warfarin non-users (Table 2), because warfarin users discontinued the drug at a high rate; 69.7% (95% CI, 67.4%-72.0%) of users were off-drug one year after initiation (Figure 4). In contrast, only about 10.8% (95% CI, 10.1%-11.4%) of non-users started using warfarin within one year of their AF diagnosis. The HRs for all of the outcomes were non-significant in AT analyses, except for the association with all-cause mortality, which indicated a lower mortality for warfarin users (HR, 0.84; 95% CI, 0.73-0.97; Table 2, Figure 3).
Figure 4.
Time to warfarin cessation for users and time to warfarin use for nonusers, in years. Warfarin users, those who filled a first prescription within 30 days of the index atrial fibrillation event, were considered to have discontinued warfarin use if they had not refilled their prescription within 60 days after their most recent supply expired. For example, if someone received a 30-day supply of warfarin, they had 90 days from their previous filled prescription to refill it.
Results were not materially changed in sensitivity analyses conducted on a propensity score matched cohort of 1829 warfarin users and 1829 non-users, although the association with a lower risk of ischemic stroke in the ITT analysis was no longer statistically significant (Figure 3, Table S3, Figure S1). Sensitivity analyses performed on the IPTW cohort that varied the length of the grace period in the AT analyses from 60 to 30 days after the drug supply ran out were also similar to the results from the primary analyses, except that there was now a significantly increased risk of hemorrhagic stroke and gastrointestinal bleeding (HRs of 2.74 [95% CI, 1.16-6.44] and 1.52 [95% CI, 1.00-2.29], respectively; Figure 3, Table S4).
Discussion
The benefits of oral anticoagulation for AF have been demonstrated in a number of randomized trials;(22) however, patients with ESRD were excluded from these studies. Therefore, whether the benefits of oral anticoagulation extend to patients undergoing hemodialysis is unclear,(23-25) as they have a substantially higher risk of stroke compared to the general population, but also a higher risk of bleeding(13, 23-26). This clinical equipoise was reflected in a survey of Canadian nephrologists, where 72% were unsure about whether to initiate warfarin in hemodialysis patients with AF(27), and in the particularly low rate of warfarin use in hemodialysis patients with prevalent AF where one quarter were on warfarin.(28) In our study of a large cohort of patients on hemodialysis with newly-diagnosed AF, we found that only 15% of patients initiated warfarin use within 30 days of the index AF event; only roughly 11% of patients without an initially filled warfarin prescription initiated treatment between 30 days and one year.
While null in ITT analyses, in all of the AT analyses warfarin use versus non-use showed a statistically significant trend towards lower risk of death. It is possible that the decreased risk for all-cause mortality for warfarin users in the AT analyses is due to the drug decreasing the risk of other fatal thromboembolic events. This is consistent with the trend towards a lower risk of cardiovascular mortality that we observed in all of our analyses, though the pattern did not reach statistical significance. Alternatively, it is possible that patients discontinued warfarin due to poor control of their international normalized ratio (INR) and those who continued were the ones whose INR was within the target range and who therefore derived benefit from oral anticoagulation. We are unable to substantiate this hypothesis in our data, absent INR measurements. More generally, our data illustrate the difficulty of maintaining oral anticoagulation in patients on maintenance dialysis, with more than two-thirds discontinuing anticoagulation treatment within one year.
We found a trend towards decreased risk of ischemic stroke in warfarin users, although it was only statistically significant in the IPTW ITT analysis. While AT analyses yielded similar estimates, the number of events during eligible follow-up was cut in half, which led to wider confidence limits. Regarding the risk of hemorrhagic stroke, confidence limits were also wide due to the small number of events; however, sensitivity analyses that reduced follow-up to 30 days from expiration of warfarin supply did show significantly and sizably (2.7-fold) increased risks of hemorrhagic stroke, likely due to reduced exposure misclassification. Our results are in contrast with several observational studies demonstrating higher risk of ischemic stroke associated with warfarin use. For example, in a cohort of incident US dialysis patients with prevalent AF by Chan et al., warfarin use (versus non-use) was associated with a nearly two-fold higher risk of ischemic (HR, 1.81; 95% CI, 1.12-2.92) or hemorrhagic strokes (HR, 2.22; 95% CI, 1.01-4.91).(6) Warfarin use was also associated with higher risks of any stroke in hemodialysis patients older than 75 years (HR, 2.17; 95% CI, 1.04-4.53) in a study by Wizemann et al. in the Dialysis Outcomes and Practice Patterns Study (DOPPS)(7) and with hemorrhagic stroke (HR, 2.38; 95% CI, 1.15-4.96), but not ischemic stroke (HR, 0.92; 95% CI, 0.61-1.37), in a propensity-score matched cohort of older patients initiating hemodialysis in the Northeastern United States (8) Notably, the Chan et al. and Wizemann et al. studies focused on patients with prevalent, rather than incident, AF. Another key difference among these three studies and our study is that the average age of patients in these studies ranged from 65-72 years, while our cohort was considerably younger, with an average age of 62 years. Although we did not find modification of the effect of warfarin by age as was described by Wizemann et al.,(7) our study had limited power to identify effect modification despite its relatively large size. A recent study from Canada of 1626 dialysis patients (hemo- and peritoneal dialysis) also did not detect any association between warfarin use and ischemic stroke (HR, 1.14; 95% CI, 0.78-1.67).(9) Another recent study focusing more broadly on kidney function, AF, and stroke reported secondary findings of a protective association between warfarin use (vs. non-use) and stroke or systemic thromboembolism in 901 Danish patients with ESRD (HR, 0.44; 95% CI, 0.26-0.74).(29) However, the study was not specifically designed to answer this question and covariate adjustment was not timed to the initiation of warfarin, which makes it difficult to evaluate the validity of that association. Similarly, a Swedish study of survivors of acute myocardial infarction with AF found that in the subgroup of patients with an eGFR ≤15 mL/min/1.73m2, those prescribed warfarin at discharge had a lower risk of a composite outcome of death, myocardial infarction, and ischemic stroke, though it did not reach statistical significance (HR, 0.80; 95% CI, 0.63-1.02).(30) This last study did not specify how many of these patients were actually on dialysis, though.
We did not find a solid link between warfarin use and gastrointestinal bleeding; an association was only identified in 30-day censored AT analyses (HR, 1.52; 95% CI, 1.00-2.29). Few studies have investigated the association between warfarin use and gastrointestinal bleeding or bleeding death in hemodialysis patients. A single-center study of 255 hemodialysis patients found that warfarin use increased the adjusted risk of major bleeding more than 3-fold, although the result was not statistically significant (HR, 3.59; 95% CI, 0.95-13.60).(31) Another study in patients with incident AF did not find an association between warfarin use and the risk of gastrointestinal bleeding (HR, 0.90; 95% CI, 0.60-1.35) or mortality from any cause (HR, 1.05; 95% CI, 0.83-1.33).(8) The recent Swedish study of myocardial infarction survivors with AF similarly found no association between the drug and bleeding in those with eGFR ≤15 mL.min/1.73m2 (HR, 0.73; 95% CI, 0.27-2.00).(30) By contrast, one study from a large dialysis provider ascertained warfarin, clopidogrel, and aspirin use during any point within the first 90 days after initiating dialysis in one of their clinics and found that, compared to use of neither of these medications, patients receiving warfarin had 27% (95% CI, 18%-37%) higher mortality from any cause. Similarly, clopidogrel users’ mortality was found to be 24% (95% CI, 13%-35%) higher.(32) Most recently, a Canadian study found that in a cohort of patients with newly diagnosed AF who had recently received dialysis, warfarin use was associated with a 44% (95% CI, 13%-85%) higher risk for bleeding.(9) However, in these last two studies, no information was available on the specific indication for warfarin, or on the prior duration of its use.
Our study has several strengths. We used a cohort derived from the most recent data available through the USRDS that included patients diagnosed as outpatients; to date, studies of warfarin use in incident AF patients on dialysis have only included those who were hospitalized with AF.(8, 29) We analyzed a cohort of incident AF patients who were new users of warfarin. The most important threat to validity of observational comparative effectiveness research is confounding by indication, where patients receiving a drug are inherently different from those not receiving it.(33) To avoid this bias, we first created an inception cohort in that we identified the clinical situation in which a decision to treat arose, which was when physicians first made note of a diagnosis of AF. Second, we pursued a new user design by excluding all patients with a recorded history of warfarin use. Inclusion of prevalent users means studying individuals who tolerate the drug and are adherent to it, which tends to select healthier patients and induce bias.(34) After such cohort selection, multivariable adjustment, propensity matching, or inverse probability of treatment weighting can then analytically control for observed differences in patient characteristics. However, residual confounding by unobserved characteristics that differ between groups remains a concern. One argument supporting the notion that we effectively controlled confounding for indication at baseline comes from the null association between warfarin use and all-cause mortality in ITT analyses with an estimate close to one. This argument is built on the short continuation of initiated warfarin in exposed patients, which leads to the majority of person time among warfarin initiators in the ITT analysis misclassified and truly unexposed. Indeed, about 70% of warfarin users had discontinued the drug by one year, despite a 60-day grace period allowed for non-adherence. Conversely, a certain proportion of unexposed patients initiate warfarin at a later point in time—11% by one and 18% by three years—which creates exposure misclassification in the non-user group as well. Therefore, an association close to one (especially under the null hypothesis of no warfarin effect) is indicative that the two exposure groups were indeed similar in prognosis at baseline. In other words, if warfarin initiators were substantially healthier than non-users at baseline, once IPTW/matched on observed characteristics, one would expect the HR for mortality to be significantly less than one, which was not the case. This provides reassurance that our results are not being driven by selection bias. Finally, due to the mandate to Medicare for the coverage of patients with ESRD regardless of their age, we were able to study a relatively younger population than in previous investigations.
Our study has limitations that merit discussion. Our time-to-event analysis was limited by the short observation period, with a median length of follow-up of only about a year. Whereas ITT analyses were hampered by substantial treatment cross-over, AT analyses may be subject to post-baseline selection forces (“healthy user” and “sick stopper” phenomena) that induce bias. We also observed a limited number of non-fatal events, which limited our power to detect a significant difference in the rates of these outcomes. We were also unable to ascertain instances of warfarin-induced calciphylaxis, a rare but serious adverse effect. We ascertained AF and comorbidities using administrative data, so were unable to assess the severity of these conditions. Furthermore, we could not adjust for the use of aspirin or INR as they were not recorded in this dataset. Finally, our population was restricted to patients who participated in a low-income subsidy program for Medicare Part D, which limits the generalizability of the results to more affluent patients.
In conclusion, in patients on hemodialysis with newly diagnosed AF, warfarin was initiated only in 15% of patients, 70% of whom discontinued its use within one year. Warfarin use was marginally associated with a lower risk of ischemic stroke, but not with the risks of death, hemorrhagic stroke, or gastrointestinal bleeding in ITT analyses. However, in AT analyses, warfarin use was nominally associated with reduced mortality. This adds to the small, but already conflicting, body of literature on the safety of warfarin use in dialysis patients with AF, and thus supports the need for further studies to answer this question.
Supplementary Material
Acknowledgements
Data reported herein were supplied by the USRDS. Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
Support: This work was supported by grants F32DK096765 (Dr Shen), K23DK095914 (Dr Chang), and R21DK077336 and R01DK095024 (Dr Winkelmayer) from the NIDDK, Bethesda, MD. The Stanford Nephrology fellowship program was supported by grant T32DK007357. The manuscript was reviewed and approved for publication by an officer of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr Shen was also supported by the Clinical Scientist in Nephrology Fellowship from the American Kidney Fund, the Satellite Dialysis Clinical Investigator Award from the National Kidney Foundation, grant KL2TR000122 from the National Institutes of Health/National Center for Advancing Translational Science, and an anonymous donor. Dr Winkelmayer received salary and research support from the endowed Gordon A. Cain Chair in Nephrology at Baylor College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
N section: Because an author of this article is an editor for AJKD, the peer-review and decision-making processes were handled entirely by an Associate Editor (Csaba P. Kovesdy, MD) who served as Acting Editor-in-Chief. Details of the journal's procedures for potential editor conflicts are given in the Information for Authors & Editorial Policies.
Financial Disclosure: Dr Winkelmayer reports having served as a scientific advisor to Amgen, Astra-Zeneca, and Bayer, and on data safety monitoring boards for Medtronic. Dr Turakhia reports serving as a consultant to Precision Health Economics, Medtronic, and St. Jude Medical. The other authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: MEM-R, JIS, WCW; data acquisition: WCW; data analysis/interpretation: TIC, CRL, MEM-R, JIS, MPT, WCW; statistical analysis: MEM-R; supervision or mentorship: WCW. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. WCW takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso A, Lopez FL, Matsushita K, et al. Chronic Kidney Disease Is Associated With the Incidence of Atrial Fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2011;123:2946–2953. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein BA, Arce CM, Hlatky MA, Turakhia M. Setoguchi S and Winkelmayer WC: Trends in the incidence of atrial fibrillation in older patients initiating dialysis in the United States. Circulation. 2012;126:2293–2301. doi: 10.1161/CIRCULATIONAHA.112.099606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among medicare beneficiaries, 1993-2007. Circulation Cardiovascular quality and outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 6.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–2233. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77:1098–1106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 8.Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and Safety of Warfarin Initiation in Older Hemodialysis Patients with Incident Atrial Fibrillation. Clin J Am Soc Nephrol. 2011;6:2662–2668. doi: 10.2215/CJN.04550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah M, Avgil Tsadok M, Jackevicius CA, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129:1196–1203. doi: 10.1161/CIRCULATIONAHA.113.004777. [DOI] [PubMed] [Google Scholar]
- 10.Herzog CA, Asinger RW, Berger AK, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 11.January CT, Wann LS, Alpert JS, et al. AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2014:2014. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Chang TI, Shilane D, Kazi DS, Montez-Rath ME, Hlatky MA, Winkelmayer WC. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol. 2012;23:2042–2049. doi: 10.1681/ASN.2012060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JY, Lee TC, Montez-Rath ME, et al. Trends in acute nonvariceal upper gastrointestinal bleeding in dialysis patients. J Am Soc Nephrol. 2012;23:495–506. doi: 10.1681/ASN.2011070658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? Jama. 2003;290:2685–2692. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 16.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology (Cambridge, Mass. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. American journal of epidemiology. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychological methods. 2010;15:234–249. doi: 10.1037/a0019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leuven E, Sianesi B. [1/10/2014];PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Available at http://ideas.repec.org/c/boc/bocode/s432001.html. 2003.
- 21.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. Journal of clinical epidemiology. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 22.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 23.Shen JI, Turakhia MP, Winkelmayer WC. Anticoagulation for atrial fibrillation in patients on dialysis: are the benefits worth the risks? Current opinion in nephrology and hypertension. 2012;21:600–606. doi: 10.1097/MNH.0b013e32835856fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nimmo C, Wright M, Goldsmith D. Management of atrial fibrillation in chronic kidney disease: double trouble. American heart journal. 2013;166:230–239. doi: 10.1016/j.ahj.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Clase CM, Holden RM, Sood MM, et al. Should patients with advanced chronic kidney disease and atrial fibrillation receive chronic anticoagulation? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27:3719–3724. doi: 10.1093/ndt/gfs346. [DOI] [PubMed] [Google Scholar]
- 26.Seliger SL, Gillen DL, Longstreth WT, Jr., Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64:603–609. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 27.Juma S, Thomson BK, Lok CE, Clase CM, Blake PG, Moist L. Warfarin use in hemodialysis patients with atrial fibrillation: decisions based on uncertainty. BMC nephrology. 2013;14:174. doi: 10.1186/1471-2369-14-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkelmayer WC, Liu J, Patrick AR, Setoguchi S, Choudhry NK. Prevalence of atrial fibrillation and warfarin use in older patients receiving hemodialysis. Journal of nephrology. 2012;25:341–353. doi: 10.5301/jn.5000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. The New England journal of medicine. 2012;367:625–635. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 30.Carrero JJ, Evans M, Szummer K, et al. Warfarin, kidney dysfunction, and outcomes following acute myocardial infarction in patients with atrial fibrillation. JAMA. 2014;311:919–928. doi: 10.1001/jama.2014.1334. [DOI] [PubMed] [Google Scholar]
- 31.Holden RM, Harman GJ, Wang M, Holland D, Day AG. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:105–110. doi: 10.2215/CJN.01810407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Anticoagulant and antiplatelet usage associates with mortality among hemodialysis patients. J Am Soc Nephrol. 2009;20:872–881. doi: 10.1681/ASN.2008080824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang TI, Winkelmayer WC. Comparative effectiveness research: what is it and why do we need it in nephrology? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27:2156–2161. doi: 10.1093/ndt/gfs154. [DOI] [PubMed] [Google Scholar]
- 34.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. American journal of epidemiology. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Census Bureau GD Census Bureau Regions and Divisions with State FIPS Codes [Google Scholar]
- 36.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.