Abstract
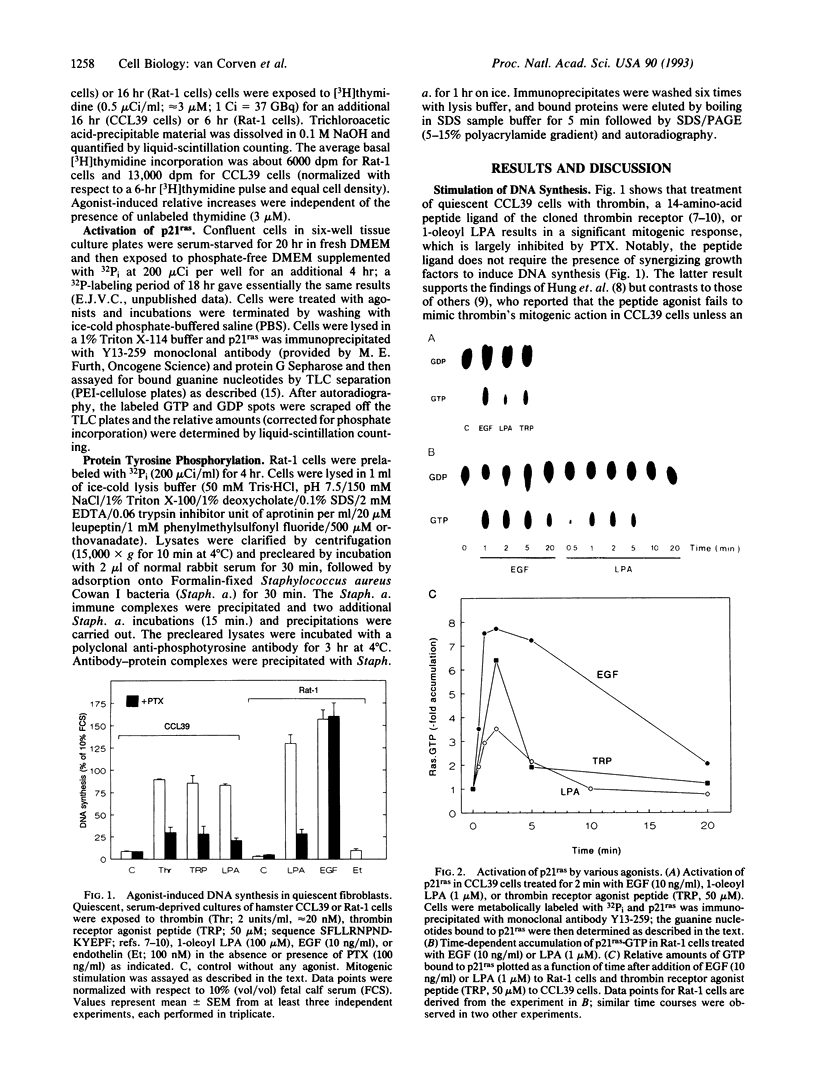
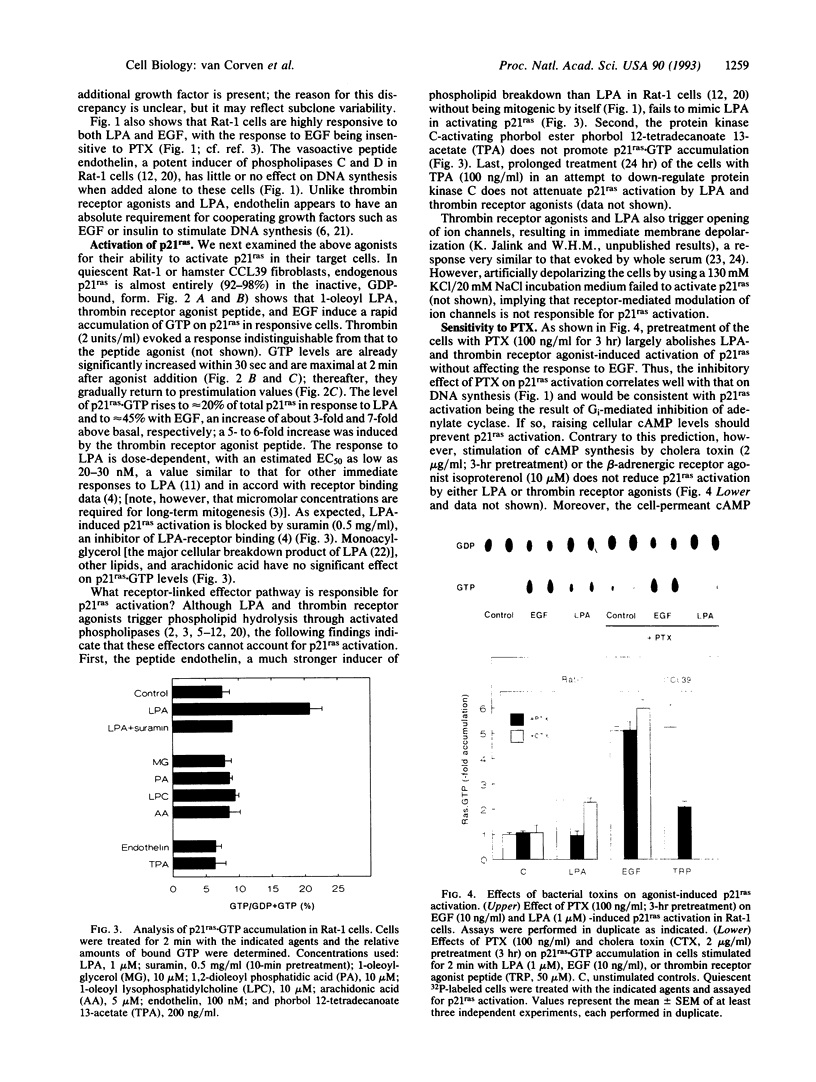
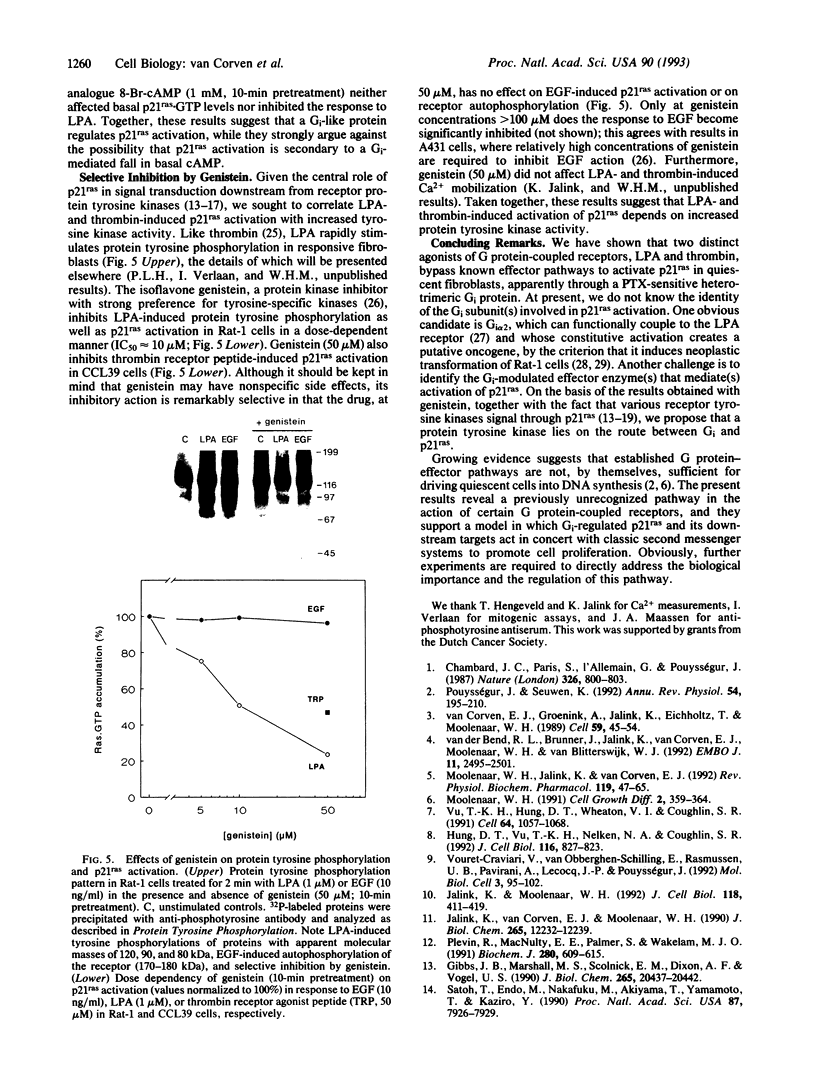
Some agonists of G protein-coupled receptors, such as thrombin and lysophosphatidic acid (LPA), can promote cell proliferation via a pertussis toxin (PTX)-sensitive signaling pathway. While these agonists stimulate phospholipase C and inhibit adenylate cyclase, it appears that other, as-yet-unidentified, effector pathways are required for mitogenesis. Here we report that LPA and a thrombin receptor agonist peptide rapidly activate the protooncogene product p21ras in quiescent fibroblasts. This activation is inhibited by PTX and yet not attributable to known PTX-sensitive G protein pathways, including stimulation of phospholipases, inhibition of adenylate cyclase, or modulation of ion channels. LPA- and peptide-induced p21ras activation is inhibited by the tyrosine kinase inhibitor genistein, at doses that do not affect epidermal growth factor-induced p21ras activation. Thus, a heterotrimeric G protein of the Gi subfamily regulates activation of p21ras by LPA and thrombin, possibly through an intermediary tyrosine kinase. This pathway may critically participate in mitogenic signaling downstream from certain G protein-coupled receptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ogawara H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Littlewood C. J. Endothelin stimulates DNA synthesis in Swiss 3T3 cells. Synergy with polypeptide growth factors. Biochem J. 1989 Nov 1;263(3):977–980. doi: 10.1042/bj2630977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering B. M., Medema R. H., Maassen J. A., van de Wetering M. L., van der Eb A. J., McCormick F., Bos J. L. Insulin stimulation of gene expression mediated by p21ras activation. EMBO J. 1991 May;10(5):1103–1109. doi: 10.1002/j.1460-2075.1991.tb08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Enomoto H., Yamashita S., Usa T., Namba H., Ohtsuru A., Villadolid M. C., Tsukazaki T., Matsumoto T., Iwasaki K. Autocrine/paracrine function of parathyroid hormone-related peptide in rat osteoblast-like cells. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1261–1269. doi: 10.1006/bbrc.1993.1353. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Marshall M. S., Scolnick E. M., Dixon R. A., Vogel U. S. Modulation of guanine nucleotides bound to Ras in NIH3T3 cells by oncogenes, growth factors, and the GTPase activating protein (GAP). J Biol Chem. 1990 Nov 25;265(33):20437–20442. [PubMed] [Google Scholar]
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990 Aug 10;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- Hung D. T., Vu T. H., Nelken N. A., Coughlin S. R. Thrombin-induced events in non-platelet cells are mediated by the unique proteolytic mechanism established for the cloned platelet thrombin receptor. J Cell Biol. 1992 Feb;116(3):827–832. doi: 10.1083/jcb.116.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K., Moolenaar W. H. Thrombin receptor activation causes rapid neural cell rounding and neurite retraction independent of classic second messengers. J Cell Biol. 1992 Jul;118(2):411–419. doi: 10.1083/jcb.118.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K., van Corven E. J., Moolenaar W. H. Lysophosphatidic acid, but not phosphatidic acid, is a potent Ca2(+)-mobilizing stimulus for fibroblasts. Evidence for an extracellular site of action. J Biol Chem. 1990 Jul 25;265(21):12232–12239. [PubMed] [Google Scholar]
- L'Allemain G., Pouyssegur J., Weber M. J. p42/mitogen-activated protein kinase as a converging target for different growth factor signaling pathways: use of pertussis toxin as a discrimination factor. Cell Regul. 1991 Aug;2(8):675–684. doi: 10.1091/mbc.2.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R. Two G protein oncogenes in human endocrine tumors. Science. 1990 Aug 10;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H. G-protein-coupled receptors, phosphoinositide hydrolysis, and cell proliferation. Cell Growth Differ. 1991 Jul;2(7):359–364. [PubMed] [Google Scholar]
- Moolenaar W. H., Jalink K., van Corven E. J. Lysophosphatidic acid: a bioactive phospholipid with growth factor-like properties. Rev Physiol Biochem Pharmacol. 1992;119:47–65. doi: 10.1007/3540551921_3. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Yarden Y., de Laat S. W., Schlessinger J. Epidermal growth factor induces electrically silent Na+ influx in human fibroblasts. J Biol Chem. 1982 Jul 25;257(14):8502–8506. [PubMed] [Google Scholar]
- Pace A. M., Wong Y. H., Bourne H. R. A mutant alpha subunit of Gi2 induces neoplastic transformation of Rat-1 cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7031–7035. doi: 10.1073/pnas.88.16.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevin R., MacNulty E. E., Palmer S., Wakelam M. J. Differences in the regulation of endothelin-1- and lysophosphatidic-acid-stimulated Ins(1,4,5)P3 formation in rat-1 fibroblasts. Biochem J. 1991 Dec 15;280(Pt 3):609–615. doi: 10.1042/bj2800609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Seuwen K. Transmembrane receptors and intracellular pathways that control cell proliferation. Annu Rev Physiol. 1992;54:195–210. doi: 10.1146/annurev.ph.54.030192.001211. [DOI] [PubMed] [Google Scholar]
- Qiu M. S., Green S. H. NGF and EGF rapidly activate p21ras in PC12 cells by distinct, convergent pathways involving tyrosine phosphorylation. Neuron. 1991 Dec;7(6):937–946. doi: 10.1016/0896-6273(91)90339-2. [DOI] [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakafuku M., Akiyama T., Yamamoto T., Kaziro Y. Accumulation of p21ras.GTP in response to stimulation with epidermal growth factor and oncogene products with tyrosine kinase activity. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7926–7929. doi: 10.1073/pnas.87.20.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouret-Craviari V., Van Obberghen-Schilling E., Rasmussen U. B., Pavirani A., Lecocq J. P., Pouysségur J. Synthetic alpha-thrombin receptor peptides activate G protein-coupled signaling pathways but are unable to induce mitogenesis. Mol Biol Cell. 1992 Jan;3(1):95–102. doi: 10.1091/mbc.3.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Wong Y. H., Federman A., Pace A. M., Zachary I., Evans T., Pouysségur J., Bourne H. R. Mutant alpha subunits of Gi2 inhibit cyclic AMP accumulation. Nature. 1991 May 2;351(6321):63–65. doi: 10.1038/351063a0. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- van der Bend R. L., Brunner J., Jalink K., van Corven E. J., Moolenaar W. H., van Blitterswijk W. J. Identification of a putative membrane receptor for the bioactive phospholipid, lysophosphatidic acid. EMBO J. 1992 Jul;11(7):2495–2501. doi: 10.1002/j.1460-2075.1992.tb05314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bend R. L., de Widt J., van Corven E. J., Moolenaar W. H., van Blitterswijk W. J. Metabolic conversion of the biologically active phospholipid, lysophosphatidic acid, in fibroblasts. Biochim Biophys Acta. 1992 Apr 8;1125(1):110–112. doi: 10.1016/0005-2760(92)90163-p. [DOI] [PubMed] [Google Scholar]
- van der Bend R. L., de Widt J., van Corven E. J., Moolenaar W. H., van Blitterswijk W. J. The biologically active phospholipid, lysophosphatidic acid, induces phosphatidylcholine breakdown in fibroblasts via activation of phospholipase D. Comparison with the response to endothelin. Biochem J. 1992 Jul 1;285(Pt 1):235–240. doi: 10.1042/bj2850235. [DOI] [PMC free article] [PubMed] [Google Scholar]