Abstract
The systems which control the levels of the gluconeogenic enzymes in Saccharomyces cerevisiae have been bypassed to ascertain their physiological significance. The coding regions of the genes FBP1 and PCK1, which encode fructose-1,6-bisphosphatase and phosphoenolpyruvate carboxykinase, have been put under the control of the promoter of ADC1 (alcohol dehydrogenase I), a gene not repressed by glucose, and introduced into yeast in multicopy plasmids. The transformed yeast cells show high levels of the gluconeogenic enzymes during growth on glucose. Generation time and growth yield of yeast expressing either fructose-1,6-bisphosphatase or phosphoenolpyruvate carboxykinase are not significantly different from those of the wild-type strain. For a strain expressing both enzymes the increase in generation time is about 20% and the decrease in growth yield around 30%. The concentration of ATP is about 1.5 mM in the growing cells of the different strains. The extent of in vivo cycling was measured by 13C NMR in cell-free extracts from yeast growing on [6-13C]glucose. Cycling between fructose-6-phosphate and fructose-1,6-bisphosphate is < 2%, most likely due to the very strong inhibition of fructose-1,6-bisphosphatase by fructose 2,6-bisphosphate. Cycling between phosphoenolpyruvate and pyruvate is low, but a precise figure could not be obtained due to poor equilibration of label between carbons 2 and 3 of oxaloacetate.
Full text
PDF
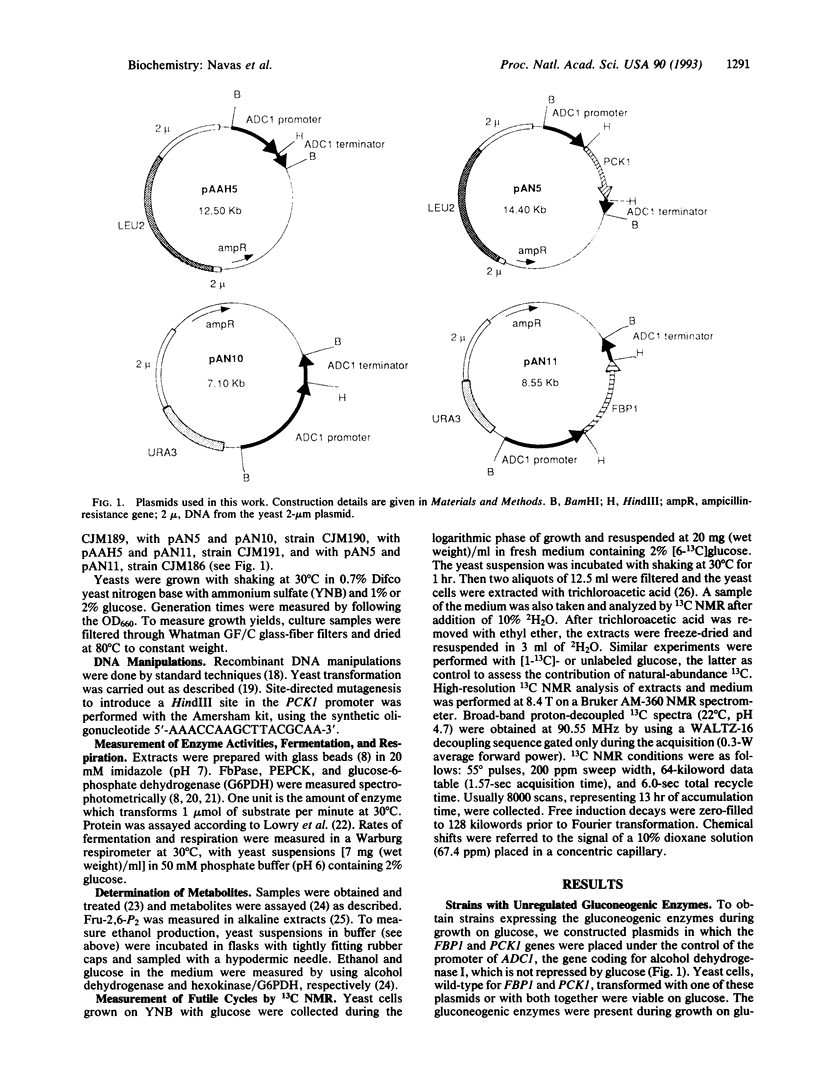
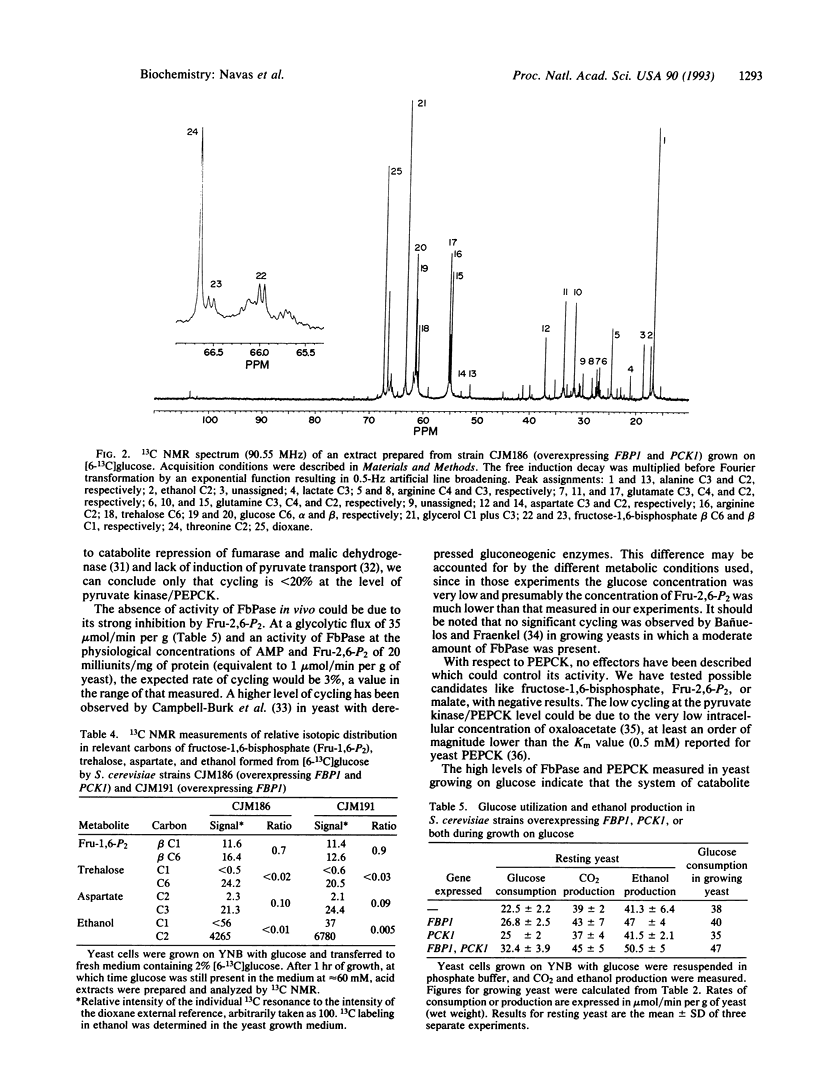

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammerer G. Expression of genes in yeast using the ADCI promoter. Methods Enzymol. 1983;101:192–201. doi: 10.1016/0076-6879(83)01014-9. [DOI] [PubMed] [Google Scholar]
- Aragón J. J., Sols A. Regulation of enzyme activity in the cell: effect of enzyme concentration. FASEB J. 1991 Nov;5(14):2945–2950. doi: 10.1096/fasebj.5.14.1752361. [DOI] [PubMed] [Google Scholar]
- Bartrons R., Van Schaftingen E., Vissers S., Hers H. G. The stimulation of yeast phosphofructokinase by fructose 2,6-bisphosphate. FEBS Lett. 1982 Jun 21;143(1):137–140. doi: 10.1016/0014-5793(82)80290-1. [DOI] [PubMed] [Google Scholar]
- Bañuelos M., Fraenkel D. G. Saccharomyces carlsbergensis fdp mutant and futile cycling of fructose 6-phosphate. Mol Cell Biol. 1982 Aug;2(8):921–929. doi: 10.1128/mcb.2.8.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañuelos M., Gancedo C. In situ study of the glycolytic pathway in Saccharomyces cerevisiae. Arch Microbiol. 1978 May 30;117(2):197–201. doi: 10.1007/BF00402308. [DOI] [PubMed] [Google Scholar]
- Campbell-Burk S. L., Shulman R. G. High-resolution NMR studies of Saccharomyces cerevisiae. Annu Rev Microbiol. 1987;41:595–616. doi: 10.1146/annurev.mi.41.100187.003115. [DOI] [PubMed] [Google Scholar]
- Campbell-Burk S. L., den Hollander J. A., Alger J. R., Shulman R. G. 31P NMR saturation-transfer and 13C NMR kinetic studies of glycolytic regulation during anaerobic and aerobic glycolysis. Biochemistry. 1987 Nov 17;26(23):7493–7500. doi: 10.1021/bi00397a044. [DOI] [PubMed] [Google Scholar]
- Chiang H. L., Schekman R. Regulated import and degradation of a cytosolic protein in the yeast vacuole. Nature. 1991 Mar 28;350(6316):313–318. doi: 10.1038/350313a0. [DOI] [PubMed] [Google Scholar]
- Clifton D., Fraenkel D. G. The gcr (glycolysis regulation) mutation of Saccharomyces cerevisiae. J Biol Chem. 1981 Dec 25;256(24):13074–13078. [PubMed] [Google Scholar]
- Cássio F., Leão C., van Uden N. Transport of lactate and other short-chain monocarboxylates in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol. 1987 Mar;53(3):509–513. doi: 10.1128/aem.53.3.509-513.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Torrontegui G., Palacián E., Losada M. Phosphoenolpyruvate carboxykinase in gluconeogenesis and its repression by hexoses. Biochem Biophys Res Commun. 1966 Jan 24;22(2):227–231. doi: 10.1016/0006-291x(66)90437-2. [DOI] [PubMed] [Google Scholar]
- Eraso P., Gancedo J. M. Catabolite repression in yeasts is not associated with low levels of cAMP. Eur J Biochem. 1984 May 15;141(1):195–198. doi: 10.1111/j.1432-1033.1984.tb08174.x. [DOI] [PubMed] [Google Scholar]
- Funayama S., Gancedo J. M., Gancedo C. Turnover of yeast fructose-bisphosphatase in different metabolic conditions. Eur J Biochem. 1980 Aug;109(1):61–66. doi: 10.1111/j.1432-1033.1980.tb04767.x. [DOI] [PubMed] [Google Scholar]
- Gancedo C., Gancedo J. M., Sols A. Metabolite repression of fructose 1,6-diphosphatase in yeast. Biochem Biophys Res Commun. 1967 Mar 9;26(5):528–531. doi: 10.1016/0006-291x(67)90096-4. [DOI] [PubMed] [Google Scholar]
- Gancedo C., Schwerzmann K. Inactivation by glucose of phosphoenolpyruvate carboxykinase from Saccharomyces cerevisiae. Arch Microbiol. 1976 Sep 1;109(3):221–225. doi: 10.1007/BF00446632. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Concentrations of intermediary metabolites in yeast. Biochimie. 1973;55(2):205–211. doi: 10.1016/s0300-9084(73)80393-1. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch Mikrobiol. 1971;76(2):132–138. doi: 10.1007/BF00411787. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Mazón M. J., Gancedo C. Kinetic differences between two interconvertible forms of fructose-1,6-bisphosphatase from Saccharomyces cerevisiae. Arch Biochem Biophys. 1982 Oct 15;218(2):478–482. doi: 10.1016/0003-9861(82)90370-8. [DOI] [PubMed] [Google Scholar]
- Haarasilta S., Oura E. On the activity and regulation of anaplerotic and gluconeogenetic enzymes during the growth process of baker's yeast. The biphasic growth. Eur J Biochem. 1975 Mar 3;52(1):1–7. doi: 10.1111/j.1432-1033.1975.tb03966.x. [DOI] [PubMed] [Google Scholar]
- Hess B., Haeckel R., Brand K. FDP-activation of yeast pyruvate kinase. Biochem Biophys Res Commun. 1966 Sep 22;24(6):824–831. doi: 10.1016/0006-291x(66)90322-6. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagunas R., Dominguez C., Busturia A., Sáez M. J. Mechanisms of appearance of the Pasteur effect in Saccharomyces cerevisiae: inactivation of sugar transport systems. J Bacteriol. 1982 Oct;152(1):19–25. doi: 10.1128/jb.152.1.19-25.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Müller H., Holzer H. Immunochemical studies on catabolite inactivation of phosphoenolpyruvate carboxykinase in Saccharomyces cerevisiae. J Biol Chem. 1981 Jan 25;256(2):723–727. [PubMed] [Google Scholar]
- Perea J., Gancedo C. Isolation and characterization of a mutant of Saccharomyces cerevisiae defective in phosphoenolpyruvate carboxykinase. Arch Microbiol. 1982 Aug;132(2):141–143. doi: 10.1007/BF00508719. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the activities of respiratory enzymes during the aerobic growth of yeast on different carbon sources. Biochem J. 1965 Oct;97(1):298–302. doi: 10.1042/bj0970298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. T., Hiller E., Mitsock L., Orr E. Characterization of the gene for fructose-1,6-bisphosphatase from Saccharomyces cerevisiae and Schizosaccharomyces pombe. Sequence, protein homology, and expression during growth on glucose. J Biol Chem. 1988 May 5;263(13):6051–6057. [PubMed] [Google Scholar]
- Sáez M. J., Lagunas R. Determination of intermediary metabolites in yeast. Critical examination of the effect of sampling conditions and recommendations for obtaining true levels. Mol Cell Biochem. 1976 Nov 30;13(2):73–78. doi: 10.1007/BF01837056. [DOI] [PubMed] [Google Scholar]
- Valdés-Hevia M. D., de la Guerra R., Gancedo C. Isolation and characterization of the gene encoding phosphoenolpyruvate carboxykinase from Saccharomyces cerevisiae. FEBS Lett. 1989 Dec 4;258(2):313–316. doi: 10.1016/0014-5793(89)81682-5. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- de la Guerra R., Valdés-Hevia M. D., Gancedo J. M. Regulation of yeast fructose-1,6-bisphosphatase in strains containing multicopy plasmids coding for this enzyme. FEBS Lett. 1988 Dec 19;242(1):149–152. doi: 10.1016/0014-5793(88)81004-4. [DOI] [PubMed] [Google Scholar]
- den Hollander J. A., Ugurbil K., Brown T. R., Bednar M., Redfield C., Shulman R. G. Studies of anaerobic and aerobic glycolysis in Saccharomyces cerevisiae. Biochemistry. 1986 Jan 14;25(1):203–211. doi: 10.1021/bi00349a029. [DOI] [PubMed] [Google Scholar]