Abstract
Alcohol addiction is a complex, uniquely human disease. Breaking addiction down into contributing endophenotypes enables its study in a variety of model systems. The Drosophila model system has been most often used to study alcohol sensitivity, tolerance, and physiological dependence. However, none of these endophenotypes can account for the near-permanent quality of the addicted state. It has been recently discussed that addictive drugs may hijack the learning-and-memory machinery to produce persistent behavioral changes. Learning and memory is amenable to experimental study, and provides us with a window into how alcohol affects higher-order mental functions that are likely to contribute compulsive drug use. Here, we review the Drosophila literature that links alcohol-related behaviors to learning and memory.
Introduction
Alcoholism is a serious health concern worldwide. In the United States, almost 4% of the population meet the criteria for alcohol addiction, and alcohol-related problems are estimated to cost more than 223 billion dollars per year [1, 2]. Unfortunately, the success rate of treatment is dismal. During the first year of treatment, two-thirds of individuals have bouts of heavy drinking [3], while the best three year average shows ~25% rate of recidivism [4]. Rational treatment of alcoholism is dependent on a clear understanding of the mechanics of alcohol addiction.
Addiction to alcohol involves changes that are understandable at the single cell level and also changes that are clearly emergent properties of complex networks of many neurons. In the clinical diagnosis of alcohol Dependence (a.k.a. alcohol addiction, alcoholism), an individual is expected to exhibit at least three of seven criteria [5]. Two criteria, tolerance and withdrawal symptoms, are clearly rooted in cellular adaptations to ethanol. The five remaining diagnostic attributes include compulsive ethanol consumption, obsessive desire for alcohol, spending too much time pursuing alcohol, neglecting social, recreational, or occupational activities, and continued alcohol use in spite of accumulating negative consequences. These latter five groups are clearly complex changes in behavior and are probably all emergent properties of a dysfunctional nervous system.
Behavioral responses to ethanol are highly conserved. In mammals and invertebrates, ethanol intoxication proceeds from stimulation to incoordination to sedation with increased dose. These can be followed by the appearance of functional-ethanol tolerance and physiological dependence. Ethanol tolerance is inducible ethanol resistance and in humans includes metabolic (pharmacokinetic) tolerance and functional (pharmacodynamic) tolerance. Functional tolerance of the nervous system is the earliest recognized neuronal plasticity change produced by ethanol. The cellular changes underlying functional tolerance have long been thought to overlap with the changes that produce withdrawal symptoms [6]. Symptoms of withdrawal are indicative of physiological dependence [7]. In Drosophila, a form of rapid ethanol tolerance and an ethanol withdrawal hyperexcitability phenotype have both been shown to share a common genetic basis - the involvement of the slo gene, which encodes the BK-type Ca2+-activated K+ channel [8].
The purpose of this review is to recap recent developments that demonstrate that the Drosophila model system and mammals share some of the higher-order ethanol responses that are linked to alcohol addiction. In general, genetic analysis in Drosophila is more advanced than in mammals. However, the primary value of this model system lies in the fact that Drosophila studies are exponentially cheaper and faster than genetic manipulation of mammals. Between Drosophila and mammals there is a strong and meaningful evolutionary concordance among the genes that underlie cellular activities of the nervous system. However, Drosophila and mammals show poor conservation of brain structures and neural circuitry. This suggests that the conservation of ethanol responses between Drosophila and humans arises because ethanol disrupts evolutionarily ancient attributes of neurons that are capable of adaptation.
Alcohol reward learning in the adult fly
It has been proposed that addiction is a type of pathological associative memory that is produced by the over-activation of a reward pathway [9]. The capacity to learn and remember are functionally and mechanistically conserved phenomena in the Animalia. The learning-addiction link in Drosophila is supported by a recent study in adult flies on the rewarding properties of ethanol. In this study (Figure 1), flies were able to form associations between an odor (CS) and an intoxicating level of ethanol vapor (US, internal ethanol ~6 mM). Following training, flies preferentially moved into a compartment that included the conditioned odor. This assay mimicked aspects of the conditioned place preference (CPP) assay commonly used to study rewarding drugs in rodents. Additionally, the expression of conditioned odor preference in Drosophila was dependent on dopamine signaling in the fly brain [10**]. The importance of dopamine signaling is a recurrent theme in addiction literature across many species. Here we also see another reccuring theme in the Drosophila alcohol literature—that the mushroom bodies, the brain structure most tightly associated with learning and memory in flies, is of critical importance in forming the memory of the association between an ethanol “reward” and a specific odor. Blocking mushroom-body signaling blocks the retrieval of this memory.
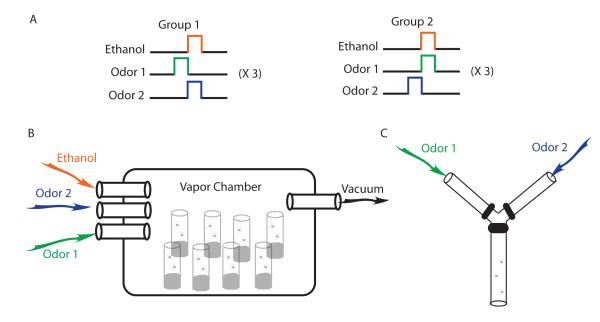
Figure 1.
Adult Drosophila can associate the rewarding aspects of ethanol intoxication with an odor. A) In group 1, flies are exposed to two odors, one of which is delivered in the presence of an intoxicating dose of ethanol vapor. In group 2, the odor that is paired with ethanol is switched. B) Simultaneous training of many vials of flies can be performed in a single vapor chamber. C) Twenty four hours following three training sessions, when placed at the base of a T- or Y-maze, flies will chose the odor paired with the ethanol over the unpaired odor [10**].
Shohat-Ophir et al. [11**] further examine the rewarding properties of ethanol by evaluating how ethanol reward relates to, and whether it is represented internally in the same way as, another natural reward (sex). In their study, reward appears to be encoded as elevated neuropeptide F (NPF) signaling. NPF is the fly homolog of mammalian neuropeptide Y, which has been linked to reward and ethanol behaviors in mammals [12, 13]. In flies, increased NPF signaling (produced by sexual satiation or transgenic overexpression) decreases the salience of an ethanol reward, while a deficit in NPF signaling (produced by sexual rejection or transgenic RNAi expression) increases the drive for other rewards, such as ethanol.
Alcohol-adapted larvae show cognitive alcohol dependence
In a recent study [14], we established the third instar larva of Drosophila melanogaster as an animal model for exploring the relationship between small doses of ethanol and associative learning. In this assay, an otherwise attractive odor (CS) is paired with a heat pulse (US) in three training trials over a 30 minute period. Untrained larvae will crawl to a spot of odorant in a petri dish. However, trained animals have learned to be repulsed by the odor and most of them avoid the odorant. It was shown that an internal ethanol concentration of ~7 mM ethanol, which did not affect heat sensitivity, odor sensitivity, or locomotion, would nevertheless disrupt this type of learning. These results are consistent with the idea that higher-order neural activites that are dependent on extensive neuronal interconnectivity, such as learning and memory, are more sensitive to the effects of ethanol than are the simpler neural functions underlying sensory input and motor activity.
Because we could isolate the effect of ethanol on learning and memory, we used this system to model cognitive ethanol tolerance and dependence [15**]. Drosophila larvae eat continuously, and they treat ethanol-laced food as palatable. As expected, when larvae consume 5% ethanol food for 1 hour, their capacity to learn plummets. However, chronically consuming ethanol food over a five-day period causes them to adapt to the point that the magnitude of learning is equivalent to that of ethanol-naive animals. This adaptation is chronic tolerance. In the ethanol-adapted larvae, it is the withholding of ethanol that impairs learning, while the capacity to learn is restored by ethanol reinstatement. Thus, chronic ethanol consumption has made the animals functionally dependent on ethanol for normal cognition. These effects occurred in larvae with internal ethanol concentrations equivalent to 0.05 to 0.08 BAC (10-17 mM). In a human, this level would be mildly intoxicating. Alcohol addiction is a disease of complex changes in behavior. The adaptations that affect larval learning might, in a human, contribute to cognitive changes that promote uncontrollable drinking. Thus, a mechanistic description of the changes produced by chronic ethanol in larvae is an important goal.
Mutations in learning and memory genes disrupt alcohol-related behaviors
Mutation studies have provided further evidence for a linkage between learning and memory genes and addiction. A long list of memory genes have been associated with alcohol sensitivity, functional tolerance, conditioned place preference, and drinking in flies (Table 1). One striking recent addition is a mutant allele of the Drosophila discs large 1 (dlg1) gene. The dlg1 gene encodes two proteins—DlgA and DlgS97. The human homolog of DlgA is the PSD-95 synaptic scaffolding protein and the DlgS97 product most closely resembles human SAP97. Maiya et al. [16**] identified a new mutant allele of dlg1, generated by P element mutagenesis, based on a reduced capacity of the mutant to display rapid ethanol tolerance. This allele, called dlg1intol, eliminates expression of the DlgS97 splice variant. The SAP97, NMDAR, and CASK proteins have all been shown to interact and to have roles in learning and memory, long-term potentiation (LTP), or long-term depression (LTD) [17, 18]. DlgS97 protein was shown to co-immunoprecipitate with the fly NMDA receptors and is also thought to bind the Caki/Camguk protein (homolog of human CASK). In flies, mutations in any one of these genes impede the production of ethanol rapid tolerance. This study also showed that mutant analysis of the role of DlgS97 in an ethanol response was predictive of the consequence of a reduction in SAP97 expression in mice. As for DlgS97 in flies, a loss of SAP97 expression in mice caused the mice to be unable to acquire rapid ethanol tolerance in a loss-of-righting-reflex assay.
Table 1.
Drosophila genes and their proteins that have been implicated in both alcohol-related behaviors and learning and memory. The abbreviations in the fourth column indicate whether the mutation alters ethanol sensitivity (S), tolerance, (T), conditioned place preference-like responses (CPP), and/or ethanol drinking (D)
| Gene | Protein | Function | Alcohol Phenotype |
|---|---|---|---|
| amncheapdate [29] | PACAP | cAMP Pathway | S |
| aru [30] | Eps8 | EGFR Pathway | S |
| dco [31] | PKA | Kinase | S |
| dlg1 [16] | PSD-95 & SAP97 | Synaptic Scaffolding | T |
| DopR [32] | Dopamine D1 Receptor | Dopamine Signaling | S |
| Egfr [33] | EGFR | EGFR Pathway | S |
| exbakrasavietz [34, 35] | Initiation Factor 5C | Translation Regulation | S, T, D |
| fas2 [36] | Fasciclin 2 | Cell Adhesion | S |
| homer [37] | Homer | Postsynaptic Scaffolding | S, T |
| KCNQ [38] | KCNQ | Synaptic Transmission | S, T |
| klgruslan [35] | Klg | Axon Guidance? | T |
| Nmdar1 [10] | dNR1 | Synaptic Transmission | CPP |
| npf [11, 39] | NPF | Neuropeptide Signaling | S, CPP |
| pummilord-1 [35] | Pum | Translation Regulation | T |
| pxbbaika [35] | Pxb | Axon Guidance? | T |
| RhoGAP18B [40] | RhoGAP18B | Rho GTPase Regulation | S |
| rhoiks [35] | Rho | Developmental | T |
| rut [29] | Adenylate Cylase | cAMP Pathway | S |
| sca [10] | Notch Pathway | Notch Pathway | CPP |
| scb, mys [41] | α, β Integrin | Cell Adhesion | T |
| Sir2 [42] | Sir2 | HDAC Activity | S, T |
| Syn [43] | Synapsin | Presynaptic Scaffolding | T |
| Tbh [44] | Octopamine | Octopamine Signaling | T |
| TH [10, 45] | Dopamine | Dopamine Signaling | S, CPP |
Thoughts and Conclusions
One particularly vexing aspect of addiction is the persistent nature of the disease. The addicted state persists beyond the period of functional tolerance, physiological dependence, and the manifestation of withdrawal symptoms that are precipitated by abstention. The idea that addictive drugs co-opt the learning-and-memory machinery to produce the long-lasting addictive state is attractive. Addiction has been proposed to represent maladaptive associative learning, in which the drug hyperactivates brain reward pathways and results in overlearning that rapidly transitions from mild associative conditioning to habit [9]. However, there exists a generally recognized contradiction. The negative effect of ethanol on learning has been well documented in both hippocampal and cortical LTP studies and behavioral assays [19, 20]. Therefore, how can ethanol result in overlearning to the point of pathology if acute or chronic ethanol intoxication results in a depression in the capacity for learning and memory?
Recently, Bernier et al. [21**] addressed this question in a study of the effects of ethanol on LTP in the mouse ventral tegmental area (VTA), a mammalian structure that is strongly implicated in drug addiction. This group used an LTP protocol that closely replicates the stimulation experienced during reward-based learning. They observed that chronic intermittent ethanol facilitates the inducibility of activity-dependent plasticity in the VTA. This type of change is considered an example of metaplasticity, which is a higher order modulation of the capacity for LTP. This novel response to ethanol might be a reflection of the novel LTP induction protocol employed, or it might mean that cellular learning in the VTA responds to ethanol in an manner opposite to other parts of the brain. The latter interpretation is supported by behavioral experiments showing that ethanol experience inhibits most forms of rodent associative learning but enhances cocaine CPP, which involves the VTA [21**, 22].
We propose that the reason ethanol responses are so tightly conserved between mammals and invertebrates is because the list of functionally relevant ethanol targets include some evolutionarily ancient cellular mechanisms. A recent addition to this list are the enzymes that modulate histone acetylation—a target that is linked to both functional tolerance and withdrawal in mammals [23] and one that could be an additional point of unification for ethanol responses and the learning-and-memory machinery. Over the last 10 years, the learning and memory field has accumulated substantial evidence that histone acetylation (and other epigenetic modifications) contribute to the formation of long-term memory [reviewed in 24]. It has been shown that different types of learning can produce different patterns of histone acetylation [25]. This is remarkable for a modification that was relatively recently considered generic and uninteresting. Ethanol exposure, in turn, has been shown to potently increase histone acetylation in the brain. While there is some disagreement concerning how the increase is produced, there is evidence that a metabolite of ethanol (probably acetate) is responsible [26-28].
Much of the recent alcoholism research in Drosophila has focused on the development and characterization of fly behavioral assays that are already well established in mammalian model systems. This is necessary because Drosophila has only more recently become an alcoholism model system. The conservation of behavioral responses to ethanol has to this point been impressively high. Not only are the adaptive responses of tolerance and dependence conserved but there is evidence of similar interactions between ethanol and the reward-and-learning mechanisms in flies and mammals. The novel genetic tools available in Drosophila will allow questions to be addressed in ways that are not possible or perhaps not practical with a mammalian model system. The diminutive fruit fly is becoming invaluable in the discovery of the mechanisms leading to alcohol addiction.
Highlights.
-Alcohol addiction may involve hijacking of the learning and memory machinery.
-Higher-order aspects of addiction have recently been modeled in Drosophila.
-The genes involved in learning and memory overlap with alcohol-related genes.
-Economical Drosophila genetics can contribute to higher-order addiction research.
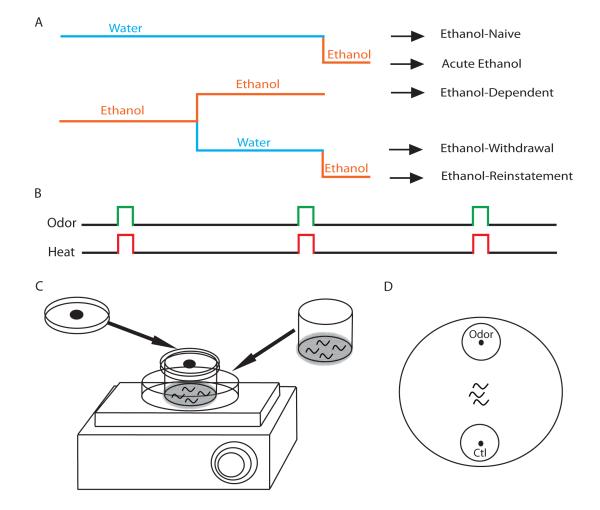
Figure 2.
Larval Drosophila that chronically feed on food containing ethanol become functionally dependent on the drug. A) The larval ethanol and control treatment schedule results in 5 separate groups. The control group is naive to ethanol. An acute ethanol group receives a 1 hour ethanol treatment. A chronically treated ethanol group receives ethanol continuously for 6 days. A withdrawal group receives a chronic ethanol treatment followed by a 6-hour ethanol abstention. The ethanol reinstatement group receives the withdrawal treatment followed by a subsequent 1-hour ethanol treatment. B) All of these groups were then trained with three rounds of 42°C heat shock-odor pairing to induce associative conditioning. C) Larvae are placed in a transfer chamber with a mesh bottom for training. The chamber is placed on a heated petri dish and covered with a plastic cap spotted with odor for an associative conditioning trial. D) Following the training, larvae were placed in the middle of an agar plate with the odor on one side and a control on the other to measure the level of attraction to the odor. Animals that have learned to associate the odor with the unpleasant heat treatment will avoid the odor zone, while animals that fail to learn will move into the odor zone [15**].
Figure 3.
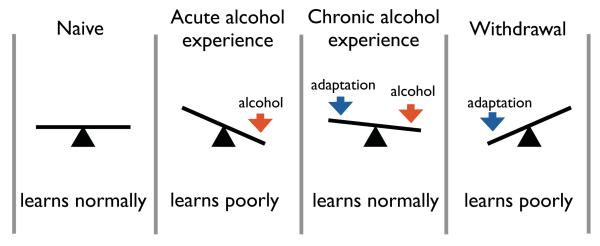
Interpretation of the alcohol-induced homeostatic adaptation of learning demonstrated with Drosophila larvae. Not only is learning an important process in the development of addiction, but the ability to learn adapts concomitantly with the progression of dependence. An acute alcohol exposure initially results in an impaired learning capability. With continued ethanol exposure however, homeostatic adaptations countering the intoxicating effects of alcohol result in a seemingly normal learning ability. The adaptations remain however and are no longer balanced when the drug is removed creating a withdrawal state in which learning is again impaired [15**].
Acknowledgments
We would like to thank Dr. Morikawa and members of the NSA laboratory for valuable insights while writing this manuscript. We also thank Dr. Sukant Khurana for assistance with graphical design and Jane Kirschman for copyediting.
Funding Sources: This work was supported by National Institute of Health Grant R01AA018037 to NSA and T32AA007471 awarded to BGR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 2.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211–220. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- 4.Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up. Alcohol Clin Exp Res. 2007;31:2036–2045. doi: 10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 2000:943. [Google Scholar]
- 6.Himmelsbach CK. The morphine abstinence syndrome, its nature and treatment. Ann Intern Med. 1941;15:829–843. [Google Scholar]
- 7.Koob GF, Le Moal M. Neurobiology of Addiction. Elsevier/Academic Press; Amsterdam ;Boston: 2006. What is addiction? [Google Scholar]
- 8.Ghezzi A, Krishnan HR, Atkinson NS. Susceptibility to ethanol withdrawal seizures is produced by BK channel gene expression. Addiction Biology. 2012 doi: 10.1111/j.1369-1600.2012.00465.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 10 **.Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. A Drosophila model for alcohol reward. Nat Neurosci. 2011;14:612–619. doi: 10.1038/nn.2805. **This work demonstrated that adult Drosophila can associate an olfactory cue with ethanol intoxication to produce a lasting increase in attraction for the ethanol-paired cue. Expression of the conditioned place preference-like response required dopaminergic signaling. A major conclusion of the paper is that ethanol intoxication is rewarding to flies.
- 11 **.Shohat-Ophir G, Kaun KR, Azanchi R, Heberlein U. Sexual deprivation increases ethanol intake in Drosophila. Science. 2012;335:1351–1355. doi: 10.1126/science.1215932. **Data support the hypothesis that ethanol intoxication activates signaling pathways that are used to register natural rewards such as sexual experience. NPF (homolog of mammalian NPY) signaling is thought to be a neural representation of reward. In males, sexual reward (mating) enhances NPF signaling which depresses ethanol consumption. Conversely, sexual deprivation produces a reward deficit in the form of decreased NPF signaling that results in increased drinking of ethanol food.
- 12.Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- 13.Josselyn SA, Beninger RJ. Neuropeptide Y: intraaccumbens injections produce a place preference that is blocked by cis-flupenthixol. Pharmacol Biochem Behav. 1993;46(3):543–552. doi: 10.1016/0091-3057(93)90542-2. [DOI] [PubMed] [Google Scholar]
- 14.Robinson BG, Khurana S, Pohl JB, Li WK, Ghezzi A, Cady AM, Najjar K, Hatch MM, Shah RR, Bhat A, et al. A low concentration of ethanol impairs learning but not motor and sensory behavior in Drosophila larvae. PLoS One. 2012:7e37394. doi: 10.1371/journal.pone.0037394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15 **.Robinson BG, Khurana S, Kuperman A, Atkinson NS. Neural Adaptation Leads to Cognitive Ethanol Dependence. Current Biology. 2012 doi: 10.1016/j.cub.2012.10.038. Epub ahead of print. **In this work, a learning and memory paradigm was used to demonstrate physiological ethanol dependence in Drosophila larvae. Acute ethanol consumption impeded learning while chronic ethanol consumption produced an adapted state in which the capacity to learn was normal only in the continued presence of ethanol. Ethanol abstinence impaired the capacity to learn.
- 16 **.Maiya R, Lee S, Berger KH, Kong EC, Slawson JB, Griffith LC, Takamiya K, Huganir RL, Margolis B, Heberlein U. DlgS97/SAP97, a Neuronal Isoform of Discs Large, Regulates Ethanol Tolerance. PLoS One. 2012:7e48967. doi: 10.1371/journal.pone.0048967. **In this study, the Drosophila homologue of mammalian PSD-95 and SAP97, is shown to be important for ethanol tolerance. This Drosophila protein, DlgS97, forms a complex with NMDA receptors that are also shown to be important for ethanol tolerance.
- 17.Hodge JJ, Mullasseril P, Griffith LC. Activity-dependent gating of CaMKII autonomous activity by Drosophila CASK. Neuron. 2006;51:327–337. doi: 10.1016/j.neuron.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Xu W. PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity. Curr Opin Neurobiol. 2011;21(2):306–312. doi: 10.1016/j.conb.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews DB, Silvers JR. The use of acute ethanol administration as a tool to investigate multiple memory systems. Neurobiol Learn Mem. 2004;82:299–308. doi: 10.1016/j.nlm.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 20.McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2011;61:1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21 **.Bernier BE, Whitaker LR, Morikawa H. Previous ethanol experience enhances synaptic plasticity of NMDA receptors in the ventral tegmental area. J Neurosci. 2011;31:5205–5212. doi: 10.1523/JNEUROSCI.5282-10.2011. **This paper proposes how ethanol could hijack a learning mechanism to produce addiction. In mice, repeated in vivo ethanol exposure resulted in a metaplastic increase in the capacity for NMDA-mediated long-term potentiation in mouse brain mesolimbic dopamine neurons. As a result, ethanol treatments increased the magnitude of cocaine-induced conditioned place preference. This work indicates that ethanol treatment may enhance the capacity for drug-related memories.
- 22.Hunt DL, Castillo PE. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol. 2012;22(3):496–508. doi: 10.1016/j.conb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starkman BG, Sakharkar AJ, Pandey SC. Epigenetics-beyond the genome in alcoholism. Alcohol Res. 2012;34:293–305. doi: 10.35946/arcr.v34.3.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day JJ, Sweatt JD. Cognitive neuroepigenetics: a role for epigenetic mechanisms in learning and memory. Neurobiol Learn Mem. 2011;96:2–12. doi: 10.1016/j.nlm.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38:62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soliman ML, Rosenberger TA. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol Cell Biochem. 2011;352:173–180. doi: 10.1007/s11010-011-0751-3. [DOI] [PubMed] [Google Scholar]
- 27.Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choudhury M, Shukla SD. Surrogate alcohols and their metabolites modify histone H3 acetylation: involvement of histone acetyl transferase and histone deacetylase. Alcohol Clin Exp Res. 2008;32:829–839. doi: 10.1111/j.1530-0277.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 29.Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- 30.Eddison M, Guarnieri DJ, Cheng L, Liu CH, Moffat KG, Davis G, Heberlein U. arouser reveals a role for synapse number in the regulation of ethanol sensitivity. Neuron. 2011;70:979–990. doi: 10.1016/j.neuron.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 31.Rodan AR, Kiger JAJ, Heberlein U. Functional dissection of neuroanatomical loci regulating ethanol sensitivity in Drosophila. J Neurosci. 2002;22:9490–9501. doi: 10.1523/JNEUROSCI.22-21-09490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong EC, Woo K, Li H, Lebestky T, Mayer N, Sniffen MR, Heberlein U, Bainton RJ, Hirsh J, Wolf FW. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS One. 2010:5e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corl AB, Berger KH, Shohat G Ophir, Gesch J, Simms JA, Bartlett SE, Heberlein U. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell. 2009;13:7949–960. doi: 10.1016/j.cell.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Devineni AV, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol. 2009;19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger KH, Kong EC, Dubnau J, Tully T, Moore MS, Heberlein U. Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol Clin Exp Res. 2008;32:895–908. doi: 10.1111/j.1530-0277.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Y, Endo K, Wu K, Rodan AR, Heberlein U, Davis RL. Drosophila fasciclin II is required for the formation of odor memories and for normal sensitivity to alcohol. Cell. 2001;105:757–768. doi: 10.1016/s0092-8674(01)00386-5. [DOI] [PubMed] [Google Scholar]
- 37.Urizar NL, Yang Z, Edenberg HJ, Davis RL. Drosophila homer is required in a small set of neurons including the ellipsoid body for normal ethanol sensitivity and tolerance. J Neurosci. 2007;27:4541–4551. doi: 10.1523/JNEUROSCI.0305-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavaliere S, Gillespie JM, Hodge JJ. KCNQ Channels Show Conserved Ethanol Block and Function in Ethanol Behaviour. PLoS One. 2012:7e50279. doi: 10.1371/journal.pone.0050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen T, Parrish CA, Xu D, Wu Q, Shen P. Drosophila neuropeptide F and its receptor, NPFR1, define a signaling pathway that acutely modulates alcohol sensitivity. Proc Natl Acad Sci U S A. 2005;102:2141–2146. doi: 10.1073/pnas.0406814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothenfluh A, Threlkeld RJ, Bainton RJ, Tsai LT, Lasek AW, Heberlein U. Distinct behavioral responses to ethanol are regulated by alternate RhoGAP18B isoforms. Cell. 2006;127:199–211. doi: 10.1016/j.cell.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Bhandari P, Kendler KS, Bettinger JC, Davies AG, Grotewiel M. An Assay for Evoked Locomotor Behavior in Drosophila Reveals a Role for Integrins in Ethanol Sensitivity and Rapid Ethanol Tolerance. Alcohol Clin Exp Res. 2009;33:1794–1805. doi: 10.1111/j.1530-0277.2009.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong EC, Allouche L, Chapot PA, Vranizan K, Moore MS, Heberlein U, Wolf FW. Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in Drosophila. Alcohol Clin Exp Res. 2010;34:302–316. doi: 10.1111/j.1530-0277.2009.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Godenschwege TA, Reisch D, Diegelmann S, Eberle K, Funk N, Heisenberg M, Hoppe V, Hoppe J, Klagges BR, Martin JR, et al. Flies lacking all synapsins are unexpectedly healthy but are impaired in complex behaviour. Eur J Neurosci. 2004;20:611–622. doi: 10.1111/j.1460-9568.2004.03527.x. [DOI] [PubMed] [Google Scholar]
- 44.Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28:261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 45.Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]