Abstract
Leukemia is the most frequent malignant disease affecting children. To date, the etiology of childhood leukemia remains largely unknown. Few risk factors (genetic susceptibility, infections, ionizing radiation, etc.) have been clearly identified, but they appear to explain only a small proportion of cases. Considerably more uncertain is the role of other environmental risk factors, such as indoor and outdoor air pollution. We sought to summarize and quantify the association between traffic-related air pollution and risk of childhood leukemia, and further examined results according to method of exposure assessment, study quality, leukemia subtype, time period and continent where studies took place. After a literature search yielded 6 ecologic and 20 case-control studies, we scored the studies based upon the Newcastle-Ottawa Scale. The studies assessed residential exposure to pollutants from motorized traffic by computing traffic density in the neighboring roads or vicinity to petrol stations, or by using measured or modeled nitrogen dioxide and benzene outdoor air levels. Because heterogeneity across studies was observed, random-effects summary odds ratios (OR) and 95% confidence intervals (CI) were reported. Whenever possible we additionally conducted stratified analyses comparing acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). Limiting the analysis to high-quality studies (Newcastle-Ottawa Scale ≥ 7), those using traffic density as the exposure assessment metric showed an increase in childhood leukemia risk in the highest exposure category (OR=1.07, 95% CI 0.93 – 1.24). However, we observed evidence of publication bias. Results for NO2 exposure and benzene showed an OR of 1.21 (95% CI 0.97 – 1.52) and 1.64 (95% CI 0.91 – 2.95) respectively. When stratifying by leukemia type, the results based upon NO2 were 1.21 (95% CI 1.04 – 1.41) for ALL and 1.06 (95% CI 0.51 – 2.21) for AML; based upon benzene were 1.09 (95% CI 0.67 – 1.77) for ALL and 2.28 (95% CI 1.09 – 4.75) for AML. Estimates were generally higher for exposures in the postnatal period compared to the prenatal period, and for European studies compared to North American studies. Overall, our results support a link between ambient exposure to traffic pollution and childhood leukemia risk, particularly due to benzene.
Keywords: meta-analysis, outdoor air pollution, childhood leukemia, traffic, epidemiology, benzene, nitrogen oxides
INTRODUCTION
Childhood leukemia is overall a rare occurrence, but it remains the most frequent malignancy affecting children under 15 years of age. The majority of these cases are acute lymphoblastic leukemia (ALL) that accounts for about 80% of leukemia cases, followed by acute myeloid leukemia (AML) that comprises about 15%. Chronic forms of leukemia are very rare in children [1].
To date, the etiology of childhood leukemia remains largely unknown. Few risk factors (genetic disorders, early infections, ionizing radiation, etc.) have been established, and these ones appear to explain only a small proportion of childhood leukemia. Additional potential environmental risk factors include pesticides, electromagnetic fields and air pollution, though some of them have been weakly and inconsistently associated with either major form of acute childhood leukemia [2, 3]. In particular, contaminants released by motorized traffic have been suggested to play a role in disease etiology by a few epidemiologic studies and particularly in most recent investigations, but this association is not clear and well defined. Two recent reviews were devoted to this issue, but both were restricted to studies using only traffic density as indicator of traffic exposure, the former including studies published up to July 2011 and founding a direct association between disease risk and exposure [4], the second updated until December 2013 [5]. This latter study found an overall weak excess risk of childhood leukemia in association with higher traffic density (summary OR 1.03, 95% CI 0.98 – 1.09), and the authors concluded that results did not support an association between traffic contaminants and the disease, due to the lack of so called statistically significant results. Furthermore, neither review conducted specific analyses for single pollutants, aiming at identifying specific associations with the contaminants more suspect to be involved in childhood leukemia etiology, namely benzene. Given that a number of the most recent studies utilized more sophisticated exposure assessment, an updated review and meta-analysis is warranted.
Traffic-related air pollution is a ubiquitous exposure. Consequently, the low levels of contaminants encountered in ambient air, although consistently associated with adverse health outcomes, frequently yield relatively weak effect estimates in epidemiologic studies [6-8]. When combined with crude exposure metrics, small sample sizes, and a reliance on traditional “statistical significance testing”, there is a likelihood of finding null associations. Thus we sought to capitalize upon the greater statistical power available in meta-analyses. Our aim was to critically summarize the existing epidemiologic evidence on the risk of childhood leukemia following long-term exposure to motorized traffic exhausts, attempting to update previous reviews on this issue and to use different proxies to estimate exposure to outdoor air contaminants from traffic.
METHODS
Identification of studies
We performed a systematic search in the PubMed-Medline database using the MeSH terms childhood leukemia, acute lymphoblastic leukemia, risk, air pollution, outdoor air pollution, traffic, traffic pollution, air pollutants, outdoor air pollutants, benzene, nitrogen dioxide, particulate matter up through 20 July, 2014. We included in the review all studies that assessed exposures to pollutants released by motorized traffic independently of the exposure window–prenatal or postnatal. Given that several of the studies, and the earlier review, identified the postnatal period as potentially more relevant in disease etiology, we hypothesized that postnatal exposures were of most interest. Thus, when more than one assessment time window was available in the same study, we selected the first of the following ones available: residence at diagnosis, the longest continual place of residence, maternal residence at child’s birth, maternal residence during pregnancy. The additional extracted data were study design, participants’ number and characteristics, subtypes of leukemia, statistical analyses and effect estimates. We listed and summarized the studies retrieved in Tables 1 and 2 for ecological and case-control studies respectively. Overall, we ascertained six ecological studies and twenty case-control studies which were suitable for inclusion in our review. We additionally conducted sensitivity analyses comparing results for studies which used these different residence addresses.
Table 1.
Characteristics of Ecological Studies on Air Pollution from Motorized Traffic and Childhood Leukemia.
| Reference | Region | Cases | Time frame | Type of cancera |
Age | Assessment methods | Main resultsa |
|---|---|---|---|---|---|---|---|
| Alexander et al. 1996 [15] |
United Kingdom |
438 | 1984-1989 | ALL | 0-14 1-7 |
Car ownership: number of cars per household within electoral wards (area unit) |
Inverse correlation between car ownership and total
ALL, which was also present in stratified analysis of ALL among children ages 1-7 years |
| Nordlinder et al. 1997 [16] |
Sweden | 1528 | 1975-1985 | ALL, AML, NHL, CML |
0-24 | Car density in km2: continuous scale and <5 vs. >20 cars/km2 |
In municipalities with more than 20
cars/km2 the incidence of AML was 5.5 (95% CI 4.4 – 6.8) as compared with 3.4 (1.9 – 5.7) cases per 1 million person-years in those with less than 5 cars/km2 (p=0.05) |
| Reynolds et al. 2002 [17] |
California | 7143 | 1988-1994 | All cancers, all leukemias, gliomas |
0-15 | Ambient air monitoring data and vehicle density (vehicles per square mile), road density (miles of roads per sq mi) and traffic density (vehicle miles traveled per day per sq mi) |
Traffic density indicators were strongly correlated
with measures of benzene and 1,3-butadiene, carbon monoxide and nitrogen dioxides and slightly with particulate matter. Rate ratios at the 90th percentiles of traffic density were 1.08 (95% CI 0.98 – 1.20) for all cancers, 1.15 (0.97 – 1.97) for leukemia and 1.14 (0.90 – 1.45) for gliomas. |
| Reynolds et al. 2003 [18] |
California | 6989 | 1988-1994 | All cancers, all leukemias, gliomas |
0-15 | 25 Hazardous air pollutant (HAPs) exposure by census tract modeled by US EPA and divided into percentiles |
Elevated rate ratios with increasing exposure levels
in tracts ranked highest for exposure to HAPs: for all leukemias they were 1.21 (95% CI 1.03 – 1.42) for of the combination of 25 HAPs grouped together and 1.32 (1.11 – 1.57) for point-source HAP exposure. |
| Whitworth et al. 2008 [19] |
Texas | 977 | 1995-2004 | All leukemias, ALL, AML, HL |
0-20 | Benzene and 1,3-butadiene exposure level: lowest vs. highest quartile US EPA ASPEN estimates of ambient air pollutants |
Elevated rate ratios for benzene were 1.37 (95% CI
1.05 – 1.78, p=0.019), 2.02 (1.03 – 3.96, p=0.153) and 1.24 (0.92 – 1.66, p=0.040) for all leukemias, AML and ALL respectively; for 1,3-butadiene rate ratios were 1.40 (1.07 – 1.81, p=0.013), 1.68 (0.84 – 3.35, p=0.064), and 1.32 (0.98-1.77, p=0.142) for all leukemias, AML and ALL respectively |
| Senkayi et al. 2014 [20] |
Texas | 2134 | 1995-2005 | All leukemias | 0-9 | Benzene modeled emissions from airports (aircraft exhaust and auxiliary power units) and roads |
Beta regression coefficients (β) showed strong
relations between benzene emissions from roads with β=0.497 (95% CI 0.358 – 0.637, p<0.001) and from airports with β=0.230 (0.149 – 0.311, p<0.001) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ANLL, acute non-lymphoblastic leukemia; NL, Hodgkin lymphoma; NHL, non-Hodgkin lymphomas; CML, chronic myeloid leukemia; CI, confidence intervals.
Table 2.
Characteristics of Case-Control Studies on Air Pollution from Motorized Traffic and Childhood Leukemia.
| Reference | Region | Cases/ Controls |
Time frame |
Type of cancera |
Age | Assessmentb | Methodsb | Address evaluated |
Case selection | Control selection | Matching variables |
Adjustment factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Savitz et al. 1989 [21] |
Colorado | 328/262 | 1976-1983 | All cancers, all leukemias (ALL, lymphoma), CNS tumors |
0-14 (0-4, 5-14) |
Traffic density in vehicles per day estimated in four different models |
Traffic density at the address of residence using street maps of Denver Standard Metropolitan Statistical Area: vehicles/day divided in two or three categories: <500 vs. ≥500 or <500, 500-4,999, ≥5,000, or <500, 500-9,999, ≥10,000 or <500 vs. ≥10,000. |
Residence at diagnosis |
Colorado Central Cancer Registry |
Community recruitment by random digit dial, data collection via telephone interview |
Sex, age and telephone exchange area |
Sex, age, year of diagnosis, type of residence, location at birth, mother’s age, father’s education and per capita income and wire configuration code at diagnosis |
| Feychting et al. 1998 [22] |
Sweden | 142/550 | 1960-1985 | All cancers, all leukemias, CNS tumors |
0-15 (0-4, 5-9, 10-15) |
NO2 exposure using continuous values and in two categorical models |
NO2 estimated levels through
continuous scale (1 μg/m3) and three categories, using 50th (≤39, 40-49, ≥50 μg/m3) and 75th percentiles (≤49, 50-79, ≥80 μg/m3) as referent respectively |
Residence lived for at least one year within 300 meters from power lines |
Swedish Cancer Registry linking medical records to verify diagnoses |
Community selection from same area |
Age, municipality and residence near the same high voltage power lines |
Magnetic fields and socioeconomic status |
| Harrison et al. 1999 [23] |
United Kingdom |
130/251 | 1990-1994 | All leukemias | 0-15 | Traffic density as measured by weighted distance from major roads and petrol stations alone and together |
Addresses within 100 m of major roads and/or petrol stations, using GIS (Geographic Information System) |
Residence at diagnosis |
West Midland Cancer Intelligence Units, using ICD-9 |
Solid tumor cases from the same Register as the cases |
None | None |
| Pearson et al. 2000 [24] |
Colorado | 320/259 | 1978-1983 | All cancers, all leukemias |
0-14 | Traffic density in vehicles per day estimated in: highest vs. lowest category: two different models. |
Traffic density estimated as Savitz et al. and with 750 foot distance- weighted traffic density using six categories: <500, 500-4,999, 5,000- 9,999, 10,000-14,999, 15,000-19,999, ≥20,000 |
Residence at diagnosis |
Colorado Central Cancer Registry |
Community selection by not- blinded telephone interviews with random digit dialing method. |
Sex, age, and telephone exchange area |
None |
| Raaschou- Nielsen et al. 2001 [25] |
Denmark | 1989/5506 | 1968-1991 | All cancers, all leukemias, lymphomas, CNS tumors |
0-15 | Traffic density as vehicles per day, and pollutant (benzene and NO2) exposure during childhood and pregnancy |
Traffic density estimated in vehicles/day using four categories: <500, 500-<5,000, 5,000-<10,000, ≥10,000. Pollutant exposure using a modified version of the Operational Street Pollution Model and 50th, 90th and 99th as cut-off points (data in 1,000 ppb- days) resulted in four categories for benzene (<3.8, 3.8-<10.4, 10.4-<27.0, ≥27.0) and for NO2 (<11.5, 11.5- <29.4, 29.4-<57.8, ≥57.8) |
Residence at diagnosis |
Danish Cancer Registry |
Danish Central Population Registry |
Sex, age, and calendar time |
Urban development, geographic region, type of residence, electromagnetic fields, mother’s age, and birth order |
| Reynolds et al. 2001 [36] |
California | 90/349 | 1988-1994 | All cancers, all leukemias, CNS tumors |
0-5 | Traffic density using average number of cars per day (ADT) |
ADT of road segments within a 550 foot radius from home address and divided into three categories: no ADT (reference), <75th and ≥75th percentiles |
Residence at birth |
California Cancer Registry |
Community selection from birth certificates |
Sex and birth year |
Sex, birth year, ethnicity, and median child family income |
| Langholz et al. 2002 [34] |
California | 212/202 | 1978-1984 | All leukemias | 0-10 | Traffic density as vehicles per day estimated in two models |
Traffic density at the longest lived
residence for all streets within 1500 foot radius, divided into three categories (<500, 500-9,999, ≥10,000) or in quintiles as cut-points (<2,301, 2,301-<5,997, 5,997- <13,264, 13,264-28,497, ≥28,497) |
Residence of longest duration |
Los Angeles Country Cancer Registry |
Community selection by not- blinded telephone interviews with random digit dialing method |
Sex and age | Wire-code |
| Crosignani et al. 2004 [26] |
Italy | 120/480 | 1978-1997 | All leukemias | 0-14 | Traffic density using distance from major roads and benzene exposure |
Distance from major roads (>150, 20-
150, <20 m). Benzene estimated exposure in three categories (<0.1, 0.1-10, >10 μg/m3) |
Residence at diagnosis |
Lombardy Cancer Registry |
Community selection by Health Services archives of Varese |
Sex and birth year |
Sex, age, and socioeconomic status |
| Steffen et al. 2004 [27] |
France | 280/285 | 1995-1999 | All leukemias (ALL, ANLL) |
0-14 | Traffic density: high traffic roads <50 meters Proximity of petrol station or repair garage |
Traffic density using heavy traffic road
within 50 m from residence (all roads types, secondary road or main street, primary road and motorway or similar road). Presence of petrol station or repair garage |
Residence at diagnosis |
Hospitals of Nancy, Lille, Lyon and Paris |
Hospital recruitment with face-to-face interviews to assess exposure |
Sex, age, ethnic origin, and hospital |
Sex, age, ethnic origin, and hospital |
| Reynolds et al. 2004 [37] |
California | 1928/3456 | 1988-1997 | All cancers, all leukemias, CNS tumors |
0-4 | Traffic density and road density |
Traffic density using combination of road length and vehicle traffic counts. Road density: summary of total road length (in miles per square) within 500-foot radius. For both 25th, 50th, 75th, and 90th percentiles were used as cut-points |
Residence at birth |
California Cancer Registry |
Community selection from birth certificates |
Sex and birth year |
Sex, birth year, and ethnicity |
| Von Behren at al. 2008 [28] |
California | 310/396 | 1995-2002 | ALL | 0-15 | Traffic density in total vehicle miles traveled per square mile |
Traffic density in total vehicle miles
traveled per square mile within 500- foot radius using 0th, 50th and 75th as cut-points (no roads, 1-38,499, 38,500-91,461, ≥91,462) |
Residence at birth, at diagnosis and the average lifetime |
Hospitals participating to Northern California Childhood Leukemia Study |
Community selection form birth certificates |
Sex, age, Hispanic ethnicity, and mother’s race |
Sex, age, Hispanic ethnicity, mother’s race, and household income category |
| Weng et al. 2008 [29] |
Taiwan | 308/308 | 1995-2005 | All leukemias | 0-14 | NO2 levels from 66 air monitoring stations |
NO2 levels divided in three categories:
<20.90, 20.99-25.34, 26.33-44.85 ppb |
Place of usual residence indicated in death certificate |
Death Certificates using ICD codes |
Death Certificates |
Sex, birth year, and death year |
Socioeconomic status |
| Brosselin et al. 2009 [30] |
France | 765/1681 | 2003-2004 | All leukemias (ALL, AML) |
0-15 | Petrol station and/or repair garage |
Presence of petrol stations or repair garages alone and together using never lived next to either as reference |
Residence at diagnosis |
French National Cancer Registry of Childhood Blood Malignancies |
Community recruitment with telephone interviews. Response rates for case/controls of 91% and 71% |
Sex, age | Sex, age and number of children under 15 years of age living in the household |
| Weng et al. 2009 [31] |
Taiwan | 729/729 | 1996-2006 | All leukemias | 0-14 | Petrol stations density (n°/km2): using three categories |
Petrol stations density estimated using summary of petrol station divided by municipality’s land area: ≤0.149, 0.150-0.395, 0.399-2.692 n°/km2 |
Place of usual residence indicated in death certificate |
Death Certificates using ICD codes |
Death Certificates |
Sex, birth year, and death year |
Socioeconomic status |
| Amigou et al. 2011 [32] |
France | 763/1681 | 2003-2004 | All leukemias (ALL, ANLL) |
0-14 | Traffic density estimated using three models and a composite of them |
Traffic density through proximity to main roads within area of 500 m using Navteq software function to characterize road classes on the basis of their importance and through density of heavy traffic roads within 500 m from the residence Traffic related NO2 levels |
Residence at diagnosis |
French National Cancer Registry of Childhood Blood Malignancies |
Community recruitment with telephone interviews. Response rates for case/controls of 91% and 71% |
Sex and age | Sex, age and socioeconomic status |
| Vinceti et al. 2012 [33] |
Italy | 83/332 | 1998-2009 | All leukemias (ALL, AML) |
0-14 (0-5, 5-14) |
Benzene and PM10
exposure |
Average and maximum hourly levels of benzene and PM10 estimated through the CALINE dispersion model and divided in quartiles: cut-points for average benzene (<0.10, 0.10-<0.25, 0.25-<0.50, ≥0.50 μg/m3) |
Residence at diagnosis |
Italian Association of Pediatric Hematology and Oncology Registry |
Community selection from Local Health Units of Modena and Reggio Emilia |
Sex, birth year, and province of residence |
Benzene and PM10
average and maximum hourly levels |
| Badaloni et al. 2013 [35] |
Italy | 747/1509 | 1998-2001 | All leukemias | 0-10 (0-4) |
Traffic density using two models and pollutants exposure (NO2, PM10, O3 and PM2,5) |
Traffic density using distance from main roads (≤50, 50-150, ≥150 m) and main roads length within area of 100 m. Pollutant exposures estimated using LUR model for NO2, PM10, O3 and PM2,5 divided in quartiles. |
Residence at birth |
Italian Association of Pediatric Hematology and Oncology Registry |
Community selection with face-to-face not- blinded interview. Response rates for case/controls of 91.4% and 69.2% |
Sex and age | Sex, age, region of residence and parental education level |
| Ghosh et al. 2013 [38] |
California | 1346/ 80658 |
1998-2008 | ALL, AML, bilateral RB |
0-5 | NO, NO2 and NOx
exposure |
Pollutant exposures estimated using LUR, unseasonalized and seasonalized models |
Residence at birth | California Cancer Registry | Community selection from birth certificates from mothers living in Los Angeles County |
Sex and birth year |
Sex, birth year, race/ethnicity, maternal education level, parity, prenatal care insurance type and neighborhood socioeconomic index |
| Heck et al. 2013 [39] |
California | 3590/ 80224 |
1998-2007 | All cancers, ALL, AML, NHL |
0-5 | Traffic density and CO, NOX and PM2,5 exposure estimated levels |
Traffic density in vehicles per day within 500 m of the residence. Pollutant exposures estimated using CALINE dispersion model for CO, NOX and PM2,5 |
Residence at birth |
California Cancer Registry |
Community selection from birth certificates |
Birth year | Birth year, maternal race/ethnicity, mother’s birth place and neighborhood socioeconomic index |
| Heck at al. 2014 [40] |
California | 69/2994 | 1990-2007 | ALL, AML | 0-6 | Air toxic exposure | Pollutant exposures (total PAHs, benzene, ethyl-benzene, 1,3 butadiene) taken from air monitors within 2 or 6 km of residence |
Residence at birth |
California Cancer Registry |
Community selection from birth certificates |
Birth year | Birth year, maternal race/ethnicity, mother’s birth place and neighborhood socioeconomic index |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ANLL, acute non-lymphoblastic leukemia; NHL, non-Hodgkin lymphomas; CNS, central nervous system; RB, retinoblastoma.
NO2, nitrogen dioxide, NO, nitric oxide; NOx, nitrogen oxides; PM, particulate matter; O3, ozone; CO, carbon monoxide; PAH, polycyclic aromatic hydrocarbons; CALINE, California line source dispersion model; LUR, land-use regression.
Quality Assessment of the Studies
To assess the overall quality and risk of bias of the case-control studies, we used the Newcastle-Ottawa Scale (NOS), originally developed to evaluate quality of non-randomized studies in order to support and strengthen the interpretation of meta-analytic results [9]. NOS assesses bias by assigning up to nine stars based upon a study’s data sources, population representativeness, control selection, control definition, case-control matching and variable adjustment (up to two stars), if exposure assessment is uniform, if response rates are similar between cases and controls, and if data collection methods allow blindness to case-control status. In sensitivity analyses, we restricted our meta-analysis to studies of low risk of bias with NOS ≥7, and we also repeated the entire analysis by removing the most influential study on the basis of its weight.
Meta-Analysis
We performed a meta-analysis of the results of the included studies. All but three of the included studies quantified the association between air pollutants and childhood leukemia by computing odds ratios (OR) of the disease in the highest exposure categories, and in our meta-analysis we estimated the summary ORs for all the meta-analyzed studies along with their 95% confidence intervals (CI). While meta-analyses have the advantage of improvements of statistical power and precision of the point estimates, which can yield better opportunities to identify real effects and settle scientific controversies, meta-analyses may also yield misleading results particularly when design, comparability and biases across the various studies are not carefully considered. Thus we sought to identify variation across studies (heterogeneity)[10] and since we found a moderate to high amount of heterogeneity across studies (I2 test greater than 50%), we chose to report results from the random effects model [11, 12]. We also performed subgroup analyses to identify the sources of this heterogeneity.
Since one of the sources of heterogeneity of studies is publication bias, where research with “statistically significant results” is more likely to be submitted and published than studies with null or non-significant results, combining only published works may lead to an overestimation of results. We therefore computed a funnel plot of the natural logarithm of ORs vs. the standard errors (SE) of ORs. In the absence of publication bias, it is presumed that the largest studies will be plotted near the mean, and smaller studies will be spread evenly on both sides of the mean, creating a roughly funnel-shaped distribution [13]. We used Stata 13.1 (Stata Corp. College Station, TX, 2013) to carry out all data analyses.
Exposure assessment methods
To assess exposure to traffic exhaust contaminants we chose four proxies. The first was traffic density, which is one of the oldest and most common indicators used in epidemiologic studies due to its relative ease and low cost to calculate [14]. Traffic density was expressed in most studies as the estimated number of vehicles per day in the closest roads to participants’ residences, or less frequently as distance from major roads, or a combination of this and vehicles per day. Another common indicator used was the density and proximity of repair garages and petrol stations to subjects’ residence, thus we calculated a summary OR of these studies. Other studies utilized measured or modeled levels of traffic-related air toxics such as benzene, 1,3-butadiene, nitric oxide (NO), nitrogen dioxide (NO2), nitrogen dioxides (NOx), ozone (O3), particulate matter (PM), or carbon monoxide (CO); however, since NO2 and benzene were the pollutants most frequently reported, we limited our analysis to these agents. In data analysis we considered the highest versus the lowest category independently of the exposure cut-points used, and when more than one regression model was reported we used only the fully adjusted one.
We carried out two additional meta-analyses of the disease risk associated with traffic density, by stratifying according to study region (Europe and North America) or to the exposure window assessment (at birth and at diagnosis).
RESULTS
Ecological Studies
The characteristics of ecologic studies on the relation between air pollution from motorized traffic and childhood leukemia incidence are summarized in Table 1. The two earliest studies were conducted in Europe, and they analyzed the association between the number of cars per household or km2 (“car density”) with childhood leukemia risk, but had contrasting results [15, 16]. Alexander et al. selected cases from the Registry of Hematopoietic Malignancies of England and Wales with diagnosis from 1984 to 1989 and found no increased risk between car ownership and the total childhood ALL cases within an electoral ward area, and an inverse correlation for ALL at ages 1-7 years. Nordlinder et al. used information from National Swedish Cancer Registry from the years 1975 to 1985 and found a linear positive correlation between the number of cars (“car density” in cars/km2) and gasoline deliveries (“gasoline density” in m3/km2). Furthermore, they examined the incidence rate according to type of leukemia and car density: their results suggested an association between AML and car density because in municipalities with more than 20 cars/km2 the incidence of AML was 5.5 (95% CI 4.4 – 6.8), as compared with 3.4 (95% CI 1.9 – 5.7) cases per 1 million person-years in municipalities with less than 5 cars/km2. They did not find any association with other leukemia types.
A third study [17] was conducted in California using cases from the California Cancer Registry with diagnosis from 1988 to 1994 and it compared air monitoring data and vehicles, road and traffic density. That study computed rate ratios for estimated traffic level as measured by spatial information on the density of neighborhood vehicles, surrounding roads and overall traffic. Results showed a correlation between traffic density indicator and measured levels of benzene, 1,3-butadiene, carbon monoxide and nitrogen dioxides, and a weaker association with particulate matter. Rate ratios associated with indicators of traffic density were 1.15 (95% CI 0.97 −1.37) for all leukemias, 1.14 (95% CI 0.94 – 1.39) for ALL and 1.01 (95% CI 0.69 – 1.58) for AML. A limited positive relation was also found for the two other indicators, vehicles and road density. A subsequent study by the same group assessed exposure to 25 hazardous air pollutants (HAPs) for all California census tracts using dispersion model performed by U.S. EPA [18]. In this study rate ratios were directly associated with exposure to HAPs, being for all leukemias 1.21 (95% CI 1.03 – 1.42) and 1.32 (95% CI 1.11 – 1.57) for combined of 25 HAPs and point-source HAP exposure, respectively.
A fifth study [19], conducted in Texas, showed an excess incidence of childhood leukemia in census tracts characterized by the highest benzene and 1,3-butadiene outdoor air levels, as estimated through a computer simulation model based on emissions data from ambient air monitors and other factors including meteorology. The results for the census tracts with the highest benzene levels showed an elevated rate ratio (RR) of 1.37 (95% CI 1.05 – 1.78) for all leukemias, with a RR of 2.02 (95% CI 1.03 – 3.96) for AML and 1.24 (95% CI 0.92 – 1.66) for ALL; with the highest 1,3-butadiene levels the observed RRs were 1.40 (95% CI 1.07 – 1.81), 1.68 (95% CI 0.84 – 3.35), and 1.32 (95% CI 0.98 – 1.77) for all leukemias, AML and ALL respectively. Finally, Senkayi et al. examined the association between childhood leukemia cases and airport benzene emissions in Texas and found that census block areas with the high standardized incidence ratios for leukemia were closer to airports than block areas with low ratios. They also developed a regression model to estimate the incidence of childhood leukemia based on county-wide benzene emissions, including those from aircraft exhaust, aircraft auxiliary power units as well as automobile exhaust and highlighted that emissions were a good predictor of leukemia incidence in Texas [20].
Case-control studies
The included studies are shown in Table 2 and encompassed over 11,000 cases and 98,000 controls allocated worldwide with time of diagnosis ranging from 1960 to 2009. Fifteen studies included children aged 0-14/15 [21-33], or 0-10 [34, 35], and four of them also included stratified analyses splitting into subgroups [21, 22, 33, 35]; five studies focused the analyses on children aged younger than six [36-40], and one of which conducted stratified analysis by age <1 versus age 1-4 [39]. A summary of the NOS scores we assigned to these studies is shown in Table 3. The median NOS value was 8, demonstrating that most studies were of good quality: the majority took data from national or regional cancer registries [21, 22, 24-26, 30, 32-40], while five studies used ICD codes to identify cases either from hospital registries [23, 27, 28] or from death certificates [29, 31]. Most studies used the same exposure assessment method for cases and controls, had a population-based design, attempted to control for potential confounders and/or included sex, age or date of birth in matching variables [21, 22, 24-26, 28-33, 35-40]. Exceptions were Harrison et al. in which controls were children with solid tumors [23] and Steffen et al. in which controls were hospital-based [27]; Harrison also did not match cases and controls and used only contingency tables to calculate ORs instead of a regression model.
Table 3.
Newcastle-Ottawa Scale score for Selected Case-Control Studies on Air Pollution from Motorized Traffic and Childhood Leukemia.
| Reference | Selection | Comparability | Exposure | Total |
|---|---|---|---|---|
| Savitz et al. 1989 [21] | 3 | 2 | 2 | 7 |
| Feychting et al. 1998 [22] | 4 | 2 | 3 | 9 |
| Harrison et al. 1999 [23] | 3 | 0 | 3 | 6 |
| Pearson et al. 2000 [24] | 3 | 2 | 2 | 7 |
| Raaschou Nielsen et al. 2001 [25] | 4 | 2 | 3 | 9 |
| Reynolds et al. 2001 [36] | 4 | 2 | 3 | 9 |
| Langholz et al. 2002 [34] | 3 | 2 | 1 | 6 |
| Crosignani et al. 2004 [26] | 4 | 2 | 3 | 9 |
| Steffen et al. 2004 [27] | 3 | 2 | 2 | 7 |
| Reynolds et al. 2004 [37] | 4 | 2 | 3 | 9 |
| Von Behren at al. 2008 [28] | 3 | 2 | 2 | 7 |
| Weng et al. 2008 [29] | 3 | 2 | 3 | 8 |
| Brosselin et al. 2009 [30] | 3 | 2 | 2 | 7 |
| Weng et al. 2009 [31] | 3 | 2 | 3 | 8 |
| Amigou et al. 2011 [32] | 3 | 2 | 2 | 7 |
| Vinceti et al. 2012 [33] | 4 | 2 | 3 | 9 |
| Badaloni et al. 2013 [35] | 3 | 2 | 1 | 6 |
| Ghosh et al. 2013 [38] | 4 | 2 | 3 | 9 |
| Heck et al. 2013 [39] | 4 | 2 | 3 | 9 |
| Heck et al. 2014 [40] | 4 | 2 | 3 | 9 |
Nine studies utilized only one method of exposure assessment [21, 22, 24, 28-31, 34, 36], while eleven studies used two or more [23, 25-27, 32, 33, 35, 37-40]. Seven studies used a buffer ranging from 500 to ~1640 feet (~150 to 500 meters) from home addresses [24, 28, 32, 34, 36, 37, 39], while another six considered only the crude distance to major roads [21, 23, 25-27, 35].
Most of the studies used residence at diagnosis [21, 23-27, 30, 32, 33] while six studies collected data from birth certificates and therefore used residence at birth [35-40]. Langholz et al. utilized the residence of longest duration [34], Weng et al. used the place of “usual residence” indicated on the death certificate [29, 31], and Feychting et al. used the residence lived for at least one year within 300 meters from power lines [22]. Only Von Behren et al. performed analyses using three locations: at birth, at diagnosis and the lifetime average that was calculated by summing the time-weighted (in months) traffic density at each address and dividing by the child’s age in months [28].
Additionally six studies investigated the relation between exposure to motorized traffic and leukemia risk according to the urban or rural status of residential address [22, 27, 29-32]. In these dichotomized analyses, two studies found a slightly stronger risk for children living in urban areas [22, 32]. Two studies performed an analysis restricted to children who never moved their residence: Savitz et al. found stronger association for children who never moved compared with those who varied their residence, while in the Badaloni et al. study results remained unchanged, except for NO2 exposure for which a slight dose-response effect emerged in this subgroup [21, 35].
Meta-analysis
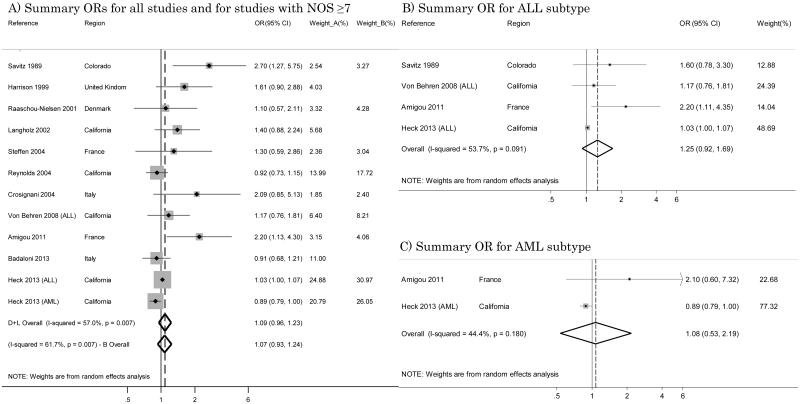
Thirteen studies used traffic density to assess air pollution exposure and leukemia risk. [21, 23-28, 32, 34-37, 39]. However, one work [36] was the pilot study, so we considered only the last one of the same author [37]; one study [24] was a re-analysis of previously published data [21], and we included only the earlier work because exposure assessment was more comparable with the other studies; and one assessed only ALL risk [28]. The summary OR from these eleven studies was 1.09 (95% CI 0.96 – 1.23), and results from the test for heterogeneity I2 was 57.0% (Figure 1-A). Restricting the analysis to studies with a low risk of bias (NOS score ≥7), we included 8 studies [21, 25-28, 32, 37, 39], which yielded an overall OR of 1.07 (95% CI 0.93 – 1.24) and a somewhat higher heterogeneity score (I2=61.7%) (Figure 1-A).
Figure 1.
Summary odds ratios (ORs) of childhood leukemia for studies with traffic density as the indicator of traffic exposure.
When we limited the meta-analysis to studies enrolling only the ALL subtype [21, 28, 32, 39], we estimated a summary OR of 1.25 (95% CI 0.92 – 1.69) with I2=53.7% (Figure 1-B). Only two studies [32, 39] focused on the AML subtype, and their results for traffic density were opposite with ORs of 2.10 (95% IC 0.60 – 7.32) and 0.89 (95% CI 0.79 – 1.00), respectively, and a summary OR of 1.08 (95% IC 0.53 – 2.19) with I2=44.4% (Figure 1-C).
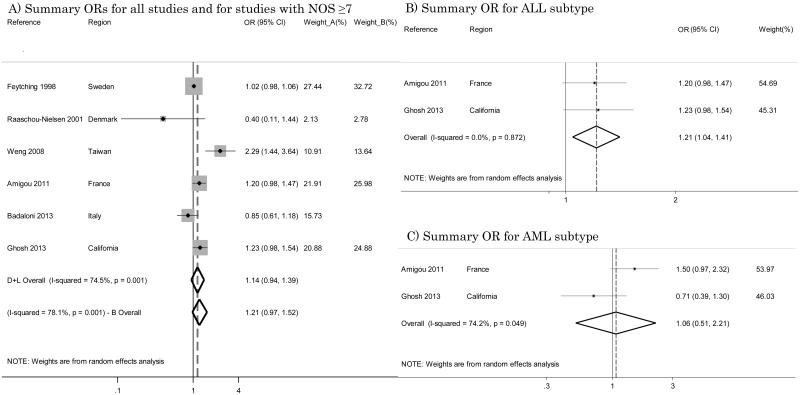
Six studies assessed exposure with NO2 [22, 25, 29, 32, 35, 38], and these were characterized by high heterogeneity (I2=74.5%); the summary OR was 1.14 (95% CI 0.94 – 1.39) (Figure 2-A). Restricting the analysis to studies with a high NOS score [22, 25, 29, 32, 38] resulted in an OR of 1.21 (95% CI 0.97 – 1.52) with I2=78.1% (Figure 2-A). Among these studies, only two performed a specific analysis for the ALL disease subtype [32, 38] yielding very consistent ORs of 1.20 (95% CI 0.98 – 1.47) and 1.23 (95% CI 0.98 – 1.54) respectively: their summary OR was 1.21 (95% CI 1.04 – 1.41) with I2=0% (Figure 2-B). For AML, there were conflicting results with ORs of 1.50 (95% CI 0.97 – 2.32) and 0.71 (95% CI 0.39 – 1.30) respectively and we estimated a summary OR of 1.06 (95% CI 0.51 – 2.21) with I2=74.2% (Figure 2-C).
Figure 2.
Summary odds ratios (ORs) of childhood leukemia for studies with NO2 as the indicator of traffic exposure.
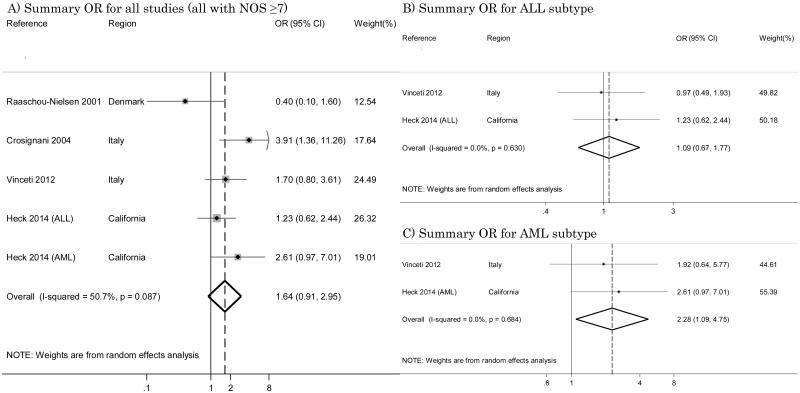
Four studies used a more sophisticated model that took into account benzene exposure levels [25, 26, 33, 40]. By pooling the results of these investigations, the summary OR was 1.64 (95% CI 0.91 – 2.95) with I2=50.7% (Figure 3-A). All of these investigations were characterized by a NOS score ≥7. Two studies considered single subtypes of leukemia [33, 40], reporting for ALL ORs of 0.97 (95% CI 0.49 – 1.93) and 1.23 (95% CI 0.62 – 2.43) respectively, and we estimated an overall OR of 1.09 (95% CI 0.67 – 1.77) with I2=0% (Figure 3-B); and for AML these studies estimated ORs of 1.92 (95% CI 0.64 – 5.78) and 2.61 (95% CI 0.97 – 6.99) respectively, and we calculated an overall OR of 2.28 (95% CI 1.09 – 4.75) with I2=0% (Figure 3-C).
Figure 3.
Summary odds ratios (ORs) of childhood leukemia for benzene analysis.
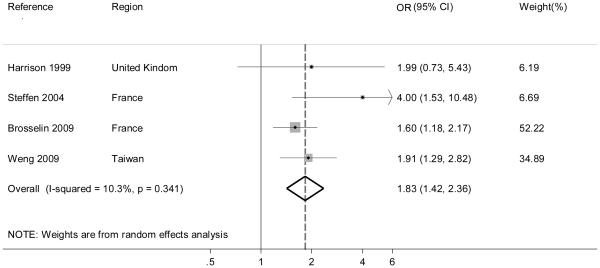
Four studies examined childhood leukemia in relation to residential proximity to repair garages or petrol stations [23, 27, 30, 31]. Their summary OR was 1.83 (95% IC 1.42 – 2.36) with I2=10.3% (Figure 4). All of these investigations were characterized by a NOS score ≥7.
Figure 4.
Summary odds ratio (OR) of childhood leukemia for petrol station/repair garage proximity.
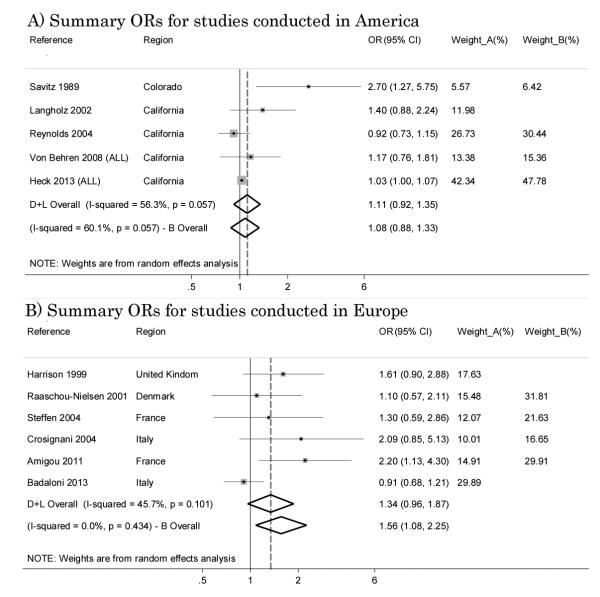
We also stratified the case-control studies which used traffic density as the indicator of exposure according to the continent where they had been conducted. We were only able to conduct pooled analyses for North America (U.S.A.) and Europe because only two studies were conducted in Asia [29, 31]. For the American region, data from Von Behren et al. and from Heck et al. included only ALL [28, 39]. In America five studies [21, 28, 34, 37, 39] and in Europe six studies [25-28, 32, 35] were included and in both four had NOS score ≥7: in America resulting ORs were 1.11 (95% CI 0.92 – 1.35) with I2=56.3% for all studies and 1.08 (95% CI 0.88 – 1.33) with I2=60.1% for studies with NOS score ≥7 (Figure 5-A). In Europe pooled ORs were 1.34 (95% CI 0.96 – 1.87) with I2=45.7% for all studies and 1.56 (95% CI 1.08 – 2.25) with I2=0% for studies with NOS≥7 respectively (Figure 5-B).
Figure 5.
Summary odds ratios (ORs) of childhood leukemia for studies with traffic density as the indicator of traffic exposure conducted in America and Europe.
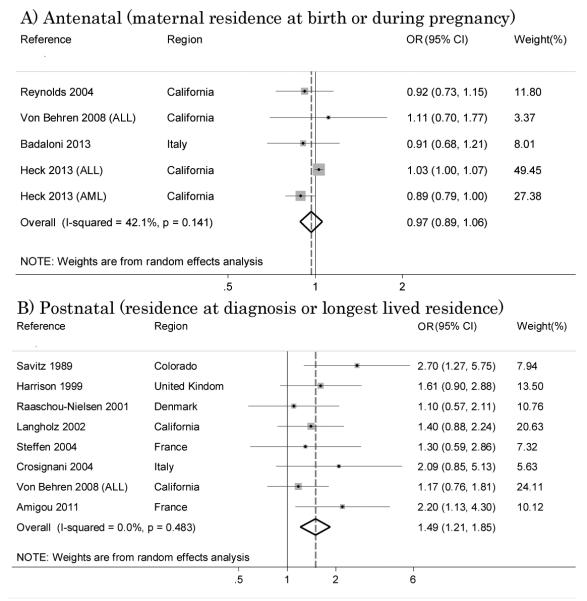
In order to evaluate the association between the exposure windows and disease risk, we performed stratified analyses for traffic density splitting studies into two subgroups: studies assessing exposure on the basis of either child’s residence at diagnosis [21, 23, 25-28, 32, 34] or child’s longest place of residence [34], yielding an OR of 1.61 (95% CI 1.26 – 2.06) with I2=0% (Figure 6-A). Studies which used the maternal residence at birth [28, 35, 37, 39] for exposure assessment showed null results with summary OR of 0.97 (95% CI 0.89 – 1.06) with I2=42.1% (Figure 6-B).
Figure 6.
Relation between traffic density and disease risk: effect of exposure window.
We performed a sensitivity analysis by removing the most influential study from all the above analyses, to confirm that relative independence from single studies of the summary estimates. The results of such analysis were substantially comparable to those obtained by including all eligible studies (data not shown).
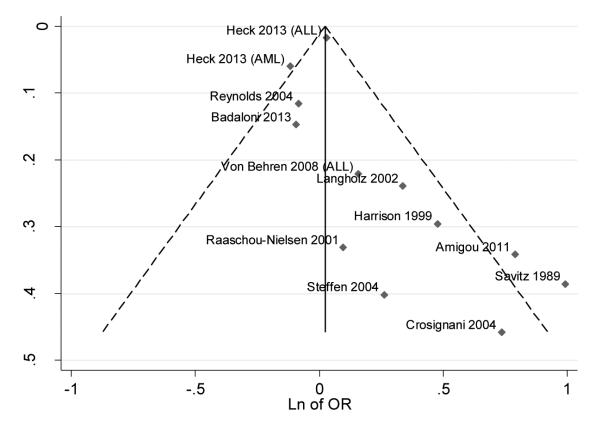
We finally created different funnel plots, using the different exposure assessment methods, to explore the possibility of publication bias, but results were generally similar regardless of the exposure assessment methods. Figure 7 shows the funnel plot for studies using traffic density indicator, as these studies account for a largest number of published studies. Funnel plots generally showed an asymmetric distribution, supporting the likely occurrence of publication bias [13].
Figure 7.
Funnel plot with pseudo 95% confidence limits of studies with traffic density as the indicator of traffic exposure
DISCUSSION
Outdoor air pollution, particularly arising from traffic exhaust, has been investigated for its possible association with childhood acute leukemia, due to its potential for carcinogenicity according to epidemiologic [33, 41] and laboratory evidence [42]. Motor vehicle emissions release contaminants such as PM, NO2, benzene, and polycyclic aromatic hydrocarbons, and IARC recently classified diesel engine exhaust as carcinogenic for humans (Group 1) and gasoline exhaust as probably carcinogenic (Group 2A) [43-45]. Our results showed excesses in childhood leukemias with traffic pollution. Given the universal nature of the exposure, however, the attributable risk may be substantial.
A relation between outdoor air pollution from traffic exhausts and childhood acute leukemia is supported by biological plausibility [45-47]. Benzene in particular is viewed as hematotoxic and a leukemogen through the induction of several mechanisms which involve benzene metabolites and may induce DNA double-strand breaks [48, 49], and children might be more susceptible to these effects [50-52]. In particular, the benzene metabolite benzoquinone has been associated with DNA damage including sister chromatid exchanges and oxidative damage, and benzene metabolites interfere with DNA binding resulting in DNA breakage, chromosomal aberrations, and chromosomal translocations similar to those seen in leukemia [53, 54]. Benzene may also inhibit topoisomerase II, an enzyme which may relieve the torsional stress that occurs in DNA during replication and transcription [55], and induce PTEN (Phosphatase and tensin homolog) methylation thus suppressing PTEN-mRNA expression which is involved in benzene-induced hematotoxicity [56]. Transplacental benzene exposure can induce hematopoietic malignancies in mice [57], and benzene and its metabolites may adversely affect the immune system and expression of cytokines and chemokines [58], further supporting the biological plausibility of its carcinogenicity.
In addition, NO2, particulate matter and other pollutants from motorized traffic and their mixtures have potential for causing cancer due to their toxic effects [43, 45, 59], including mutagenicity [2, 60, 61]. In particular, NO2 can induce DNA-single strand breaks in cultured animal cells and also in alveolar macrophages alone and in combination with ozone [62-64]. Particulate matter can interact with DNA either directly or after enzymatic transformation to induce DNA modifications that may be associated with increased frequencies of pollution-associated diseases, such as lung cancer [65]. It should be noted that NO2 and particulate matter may either be mutagens themselves or may simply be markers for overall traffic pollution exposure, and it is not known which agents in traffic pollution are most relevant for childhood leukemia, or if indeed the mixture is responsible.
Overall, our results suggest that traffic air pollutants increase risk of childhood leukemia, both among all leukemias as well as within the major subtypes (ALL and AML), and such findings are consistent across the different indicators of exposure (traffic density, contaminant levels, petrol stations), and study region. These results were confirmed across sensitivity analyses, and they appear to affirm the observations of Boothe et al. on a more limited number of studies based on traffic density [4]. Benzene exposure in particular appeared to be the traffic contaminant most strongly associated with leukemia risk, thus apparently mirroring for children the results already known in adults [43, 66, 67]. Parental occupational exposure also might be involved in childhood cancer [68, 69]: of interest is the recently reported association of increased leukemia risk among children whose mothers were occupationally exposed to benzene [70].
Our analysis suggests that the postnatal exposure window is more important than the prenatal one in increasing childhood leukemia risk, in accordance with the observations of Boothe et al. [4]. This finding appears inconsistent with the hypothesis that long-term exposure occurring across the antenatal period is more important than postnatal exposure in influencing childhood leukemia risk, and it suggests that induction and latent periods for this disease are likely to be short, also considering the low residential mobility of children at least in some populations [33]. Because hallmark genetic translocations are known to be present at birth for at least some leukemia subtypes [71, 72], under the “two-hit hypothesis” traffic pollution occurring postnatally might be the second of the two hits that is required for the development of leukemia.
Residential mobility is more common in US than in European populations [73] which may in part explain why the analyses which stratified by geographic region found higher effect estimates for Europe. US studies may suffer from more exposure misclassification if families moved frequently. Other explanations for the regional differences might include variation in pollutant mixtures as well as genetic variation [74-77]
An inherent limitation of our review was the use of different cut-points for the highest level of exposure across the various studies entered in the meta-analysis, as also noted in previous reviews [4, 5], a choice forced by data availability but which could have hampered detection of real and homogeneous risk patterns due to differences in exposure ranges. In fact, the different studies chose various metrics to define exposure to air toxics, such as a 1-unit increase in the interquartile range, an a priori defined category, or a percentile of exposure, and such different approaches will have influenced the risk estimates yielded by the various studies and therefore have similarly influenced our summary odds ratios. In the absence of additional data which could allow for a pooled analysis comparing specific exposure levels, the approach used in this and in prior reviews should nevertheless convey reliable information about the associations found in the various studies and allow their comparison.
Our review indicates that, among the different indicators investigated, benzene exposure appears to be the strongest predictor of disease risk, and this is fully consistent with the biological plausibility of such an association [46, 49]. This association was much higher than that found for NO2 or for traffic density, and was present in both two Italian studies based on modeled benzene air levels [26, 33] and for a large US study using measured benzene levels from air monitors [40], while the Danish study found no association [25]. The potential for a leukemogen effect of benzene in children indicates that this effect may occur even at ambient air levels lower than currently allowed limits, and it is consistent with the results of other recent epidemiologic studies investigating benzene exposure in other contexts [20, 47].
A systematic bias which may have affected almost all published studies incorporated in our review, and therefore also our summary odds ratios, is unmeasured confounding which could be one of the source of heterogeneity that we frequently found between included studies. However, the exclusion of this bias is very difficult, for a number of reasons. First, little information is available about the role of environmental factors in the etiology of childhood leukemia, so any adequate control of confounding is hampered by such lack of knowledge. Moreover, the studies which carefully collected information about potential confounders, such as socioeconomic status, pesticide use, exposure to ionizing and non-ionizing radiation, were also generally affected by selection bias due to families’ self-selection with regards to study participation and questionnaire completion; this causes a trade-off between adequate control of confounding and risk of selection bias. However, in some of the case-control studies which did not require active participation of the subjects and were therefore free from selection bias, the control for potential confounders such as socio-economic status and magnetic field exposure did not alter study results [33, 39, 40]. Finally, genetic factors were not generally taken into account in these studies, though there is little basis to consider them as confounders as there is no evidence they would be related to traffic exposure. Confounding may also have occurred due to lack of information on other pollutants from traffic or other correlated pollutants from other sources, which may be implicated in childhood leukemia etiology but have been rarely considered to date. This might be the case for selenium for ALL and for butadiene, ortho-xylene, and toluene for AML, since a recent large study carried out in California found direct associations with leukemia risk which are worth further investigation [40].
Another limitation of our estimates is their limited statistical precision as shown by the wide confidence intervals, due to the low number of studies particularly when single pollutants were taken into consideration. However, the latter studies appear to be much more adequate in estimating and assessing exposure to traffic pollutants as compared with traffic density. Traffic density is a rough proxy of exposure since it generally does not take into consideration type and speed of vehicles, fuel type, meteorological conditions, the influence of chemical reactions between specific emissions and other environmental agents, and finally the contribution of minor roads to air pollution from traffic. Perhaps as a consequence, traffic density tends to yield lower effect estimates in comparison to more refined exposure assessment methods [78], and indeed we observed lower summary ORs from traffic density than from other methodologies.
The occurrence and potential influence of publication bias is very difficult to assess. The funnel plot we computed suggested that publication bias occurred [79], so caution must be used in attempting to draw conclusions on the basis of the published studies and from this meta-analysis.
Overall, the study findings suggest that any public health, technological and policy measures leading to the reduction of release of contaminants from motorized traffic, and particularly but not exclusively of benzene, may likely contribute to a reduction of childhood leukemia incidence, though such effects are likely to be long-term and possibly enhanced or confined to children with specific genetic susceptibility. In general, the reduction of traffic through different measures such as the choice of less polluting fuels, technological improvements in emissions, and attempts to increase the distance of residential buildings from roads (particularly the most polluted ones) is clearly desirable for preventive medicine purposes [4], but our review suggests that childhood leukemia is among the additional diseases which are likely to be prevented to some extent by reducing exposure to air toxics from motorized traffic. A critical analysis of the studies included in the present meta-analysis also suggests the need to carry out further epidemiologic studies which examine leukemia cytogenetic subtypes and are conducted across regions with different pollution mixtures, to more precisely determine contributions to and relevant co-factors which increase risk. Ideally these studies should carefully avoid selection bias and particularly avoid voluntary study participation, but nevertheless should attempt to incorporate some control of potential confounders through external sources of ascertainment whenever possible. Such studies should also attempt to include the different contaminants in data analyses by using multivariate regression models, in an attempt to identify the specific traffic pollutants associated with childhood leukemia risk.
Acknowledgments
FUNDINGS
This study was supported by Associazione Sostegno Ematologia Oncologia Pediatrica (ASEOP) of Modena, Italy to Dr. Vinceti and by the US National Institute of Health (grants no. R21CA175959 and no. R03ES021643) to Dr. Heck.
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures 2014. Atlanta: 2014. [Google Scholar]
- 2.Buffler PA, Kwan ML, Reynolds P, Urayama KY. Environmental and genetic risk factors for childhood leukemia: appraising the evidence. Cancer Invest. 2005;23:60–75. [PubMed] [Google Scholar]
- 3.Linet MS. Etiology of childhood leukemia: environment, genes, controversies, and conundrums. Cancer Invest. 2005;23:99. [PubMed] [Google Scholar]
- 4.Boothe VL, Boehmer TK, Wendel AM, Yip FY. Residential traffic exposure and childhood leukemia: a systematic review and meta-analysis. Am J Prev Med. 2014;46:413–422. doi: 10.1016/j.amepre.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun XX, Zhang SS, Ma XL. No association between traffic density and risk of childhood leukemia: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:5229–5232. doi: 10.7314/apjcp.2014.15.13.5229. [DOI] [PubMed] [Google Scholar]
- 6.Pope CA, 3rd, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW., Jr Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 7.Schildcrout JS, Sheppard L, Lumley T, Slaughter JC, Koenig JQ, Shapiro GG. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol. 2006;164:505–517. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- 8.Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect. 2008;116:680–686. doi: 10.1289/ehp.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 10.Deeks JJ, Higgins JPT, Altman DG. Higgins JPT, Green S, editors. Chapter 9: Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions. 2011 Version 5.1.0. [updated March 2011]. The Cochrane Collaboration. http://handbook.cochrane.org.
- 11.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Altman DG, Sterne JAC. Higgins JPT, Green S, editors. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions. 2011 Version 5.1.0. [update March 2011]. The Cochrane Collaboration. http://handbook.cochrane.org.
- 13.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reungoat P, Chiron M, Momas I. Assessment of exposure to traffic pollution in epidemiological studies: a review. Rev Epidemiol Sante Publique. 2004;52:271–296. doi: 10.1016/s0398-7620(04)99052-9. [DOI] [PubMed] [Google Scholar]
- 15.Alexander FE, Leon DA, Cartwright RA. Isolation, car ownership, and small area variation in incidence of acute lymphoblastic leukaemia in children. Paediatr Perinat Epidemiol. 1996;10:411–417. doi: 10.1111/j.1365-3016.1996.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 16.Nordlinder R, Jarvholm B. Environmental exposure to gasoline and leukemia in children and young adults--an ecology study. Int Arch Occup Environ Health. 1997;70:57–60. doi: 10.1007/s004200050186. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A, Smith D. Traffic patterns and childhood cancer incidence rates in California, United States. Cancer Causes Control. 2002;13:665–673. doi: 10.1023/a:1019579430978. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A, Smith DF. Childhood cancer incidence rates and hazardous air pollutants in California: an exploratory analysis. Environ Health Perspect. 2003;111:663–668. doi: 10.1289/ehp.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitworth KW, Symanski E, Coker AL. Childhood lymphohematopoietic cancer incidence and hazardous air pollutants in southeast Texas, 1995-2004. Environ Health Perspect. 2008;116:1576–1580. doi: 10.1289/ehp.11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senkayi SN, Sattler ML, Rowe N, Chen VCP. Investigation of an association between childhood leukemia incidences and airports in Texas. Atmospheric Pollution Research. 2014;5:189–195. [Google Scholar]
- 21.Savitz DA, Feingold L. Association of childhood cancer with residential traffic density. Scand J Work Environ Health. 1989;15:360–363. doi: 10.5271/sjweh.1848. [DOI] [PubMed] [Google Scholar]
- 22.Feychting M, Svensson D, Ahlbom A. Exposure to motor vehicle exhaust and childhood cancer. Scand J Work Environ Health. 1998;24:8–11. doi: 10.5271/sjweh.272. [DOI] [PubMed] [Google Scholar]
- 23.Harrison RM, Leung PL, Somervaille L, Smith R, Gilman E. Analysis of incidence of childhood cancer in the West Midlands of the United Kingdom in relation to proximity to main roads and petrol stations. Occup Environ Med. 1999;56:774–780. doi: 10.1136/oem.56.11.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson RL, Wachtel H, Ebi KL. Distance-weighted traffic density in proximity to a home is a risk factor for leukemia and other childhood cancers. J Air Waste Manag Assoc. 2000;50:175–180. doi: 10.1080/10473289.2000.10463998. [DOI] [PubMed] [Google Scholar]
- 25.Raaschou-Nielsen O, Hertel O, Thomsen BL, Olsen JH. Air pollution from traffic at the residence of children with cancer. Am J Epidemiol. 2001;153:433–443. doi: 10.1093/aje/153.5.433. [DOI] [PubMed] [Google Scholar]
- 26.Crosignani P, Tittarelli A, Borgini A, Codazzi T, Rovelli A, Porro E, Contiero P, Bianchi N, Tagliabue G, Fissi R, Rossitto F, Berrino F. Childhood leukemia and road traffic: A population-based case-control study. Int J Cancer. 2004;108:596–599. doi: 10.1002/ijc.11597. [DOI] [PubMed] [Google Scholar]
- 27.Steffen C, Auclerc MF, Auvrignon A, Baruchel A, Kebaili K, Lambilliotte A, Leverger G, Sommelet D, Vilmer E, Hemon D, Clavel J. Acute childhood leukaemia and environmental exposure to potential sources of benzene and other hydrocarbons; a case-control study. Occup Environ Med. 2004;61:773–778. doi: 10.1136/oem.2003.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Behren J, Reynolds P, Gunier RB, Rull RP, Hertz A, Urayama KY, Kronish D, Buffler PA. Residential traffic density and childhood leukemia risk. Cancer Epidemiol Biomarkers Prev. 2008;17:2298–2301. doi: 10.1158/1055-9965.EPI-08-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng HH, Tsai SS, Chen CC, Chiu HF, Wu TN, Yang CY. Childhood leukemia development and correlation with traffic air pollution in Taiwan using nitrogen dioxide as an air pollutant marker. J Toxicol Environ Health A. 2008;71:434–438. doi: 10.1080/15287390701839042. [DOI] [PubMed] [Google Scholar]
- 30.Brosselin P, Rudant J, Orsi L, Leverger G, Baruchel A, Bertrand Y, Nelken B, Robert A, Michel G, Margueritte G, Perel Y, Mechinaud F, Bordigoni P, Hemon D, Clavel J. Acute childhood leukaemia and residence next to petrol stations and automotive repair garages: the ESCALE study (SFCE) Occup Environ Med. 2009;66:598–606. doi: 10.1136/oem.2008.042432. [DOI] [PubMed] [Google Scholar]
- 31.Weng HH, Tsai SS, Chiu HF, Wu TN, Yang CY. Childhood leukemia and traffic air pollution in Taiwan: petrol station density as an indicator. J Toxicol Environ Health A. 2009;72:83–87. doi: 10.1080/15287390802477338. [DOI] [PubMed] [Google Scholar]
- 32.Amigou A, Sermage-Faure C, Orsi L, Leverger G, Baruchel A, Bertrand Y, Nelken B, Robert A, Michel G, Margueritte G, Perel Y, Mechinaud F, Bordigoni P, Hemon D, Clavel J. Road traffic and childhood leukemia: the ESCALE study (SFCE) Environ Health Perspect. 2011;119:566–572. doi: 10.1289/ehp.1002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinceti M, Rothman KJ, Crespi CM, Sterni A, Cherubini A, Guerra L, Maffeis G, Ferretti E, Fabbi S, Teggi S, Consonni D, De Girolamo G, Meggiato A, Palazzi G, Paolucci P, Malagoli C. Leukemia risk in children exposed to benzene and PM10 from vehicular traffic: a case-control study in an Italian population. Eur J Epidemiol. 2012;27:781–790. doi: 10.1007/s10654-012-9727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langholz B, Ebi KL, Thomas DC, Peters JM, London SJ. Traffic density and the risk of childhood leukemia in a Los Angeles case-control study. Ann Epidemiol. 2002;12:482–487. doi: 10.1016/s1047-2797(01)00317-9. [DOI] [PubMed] [Google Scholar]
- 35.Badaloni C, Ranucci A, Cesaroni G, Zanini G, Vienneau D, Al-Aidrous F, De Hoogh K, Magnani C, Forastiere F, Group SS Air pollution and childhood leukaemia: a nationwide case-control study in Italy. Occup Environ Med. 2013;70:876–883. doi: 10.1136/oemed-2013-101604. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds P, Elkin E, Scalf R, Von Behren J, Neutra RR. A case-control pilot study of traffic exposures and early childhood leukemia using a geographic information system. Bioelectromagnetics. 2001;(Suppl 5):S58–68. doi: 10.1002/1521-186x(2001)22:5+<::aid-bem1024>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A. Residential exposure to traffic in California and childhood cancer. Epidemiology. 2004;15:6–12. doi: 10.1097/01.ede.0000101749.28283.de. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh JK, Heck JE, Cockburn M, Su J, Jerrett M, Ritz B. Prenatal exposure to traffic-related air pollution and risk of early childhood cancers. Am J Epidemiol. 2013;178:1233–1239. doi: 10.1093/aje/kwt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heck JE, Wu J, Lombardi C, Qiu J, Meyers TJ, Wilhelm M, Cockburn M, Ritz B. Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ Health Perspect. 2013;121:1385–1391. doi: 10.1289/ehp.1306761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heck JE, Park AS, Qiu J, Cockburn M, Ritz B. Risk of leukemia in relation to exposure to ambient air toxics in pregnancy and early childhood. Int J Hyg Environ Health. 2014;217:662–668. doi: 10.1016/j.ijheh.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savitz DA, Andrews KW. Review of epidemiologic evidence on benzene and lymphatic and hematopoietic cancers. Am J Ind Med. 1997;31:287–295. doi: 10.1002/(sici)1097-0274(199703)31:3<287::aid-ajim4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 42.DeMarini DM. Genotoxicity biomarkers associated with exposure to traffic and near-road atmospheres: a review. Mutagenesis. 2013;28:485–505. doi: 10.1093/mutage/get042. [DOI] [PubMed] [Google Scholar]
- 43.IARC . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 100F. Lyon, France: 2012. A review of human carcinogens: chemical agents and related occupations; pp. 1–599. [PMC free article] [PubMed] [Google Scholar]
- 44.IARC . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 109. Lyon, France: 2014. Outdoor air pollution. in press. [PMC free article] [PubMed] [Google Scholar]
- 45.Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K, International Agency for Research on Cancer Monograph Working Group I The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14:1262–1263. doi: 10.1016/s1470-2045(13)70487-x. [DOI] [PubMed] [Google Scholar]
- 46.Pyatt D, Hays S. A review of the potential association between childhood leukemia and benzene. Chem Biol Interact. 2010;184:151–164. doi: 10.1016/j.cbi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 47.D'Andrea MA, Reddy GK. Health effects of benzene exposure among children following a flaring incident at the British Petroleum Refinery in Texas City. Pediatr Hematol Oncol. 2014;31:1–10. doi: 10.3109/08880018.2013.831511. [DOI] [PubMed] [Google Scholar]
- 48.Whysner J, Reddy MV, Ross PM, Mohan M, Lax EA. Genotoxicity of benzene and its metabolites. Mutat Res. 2004;566:99–130. doi: 10.1016/s1383-5742(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 49.Smith MT. Advances in understanding benzene health effects and susceptibility. Annu Rev Public Health. 2010;31:133–148. doi: 10.1146/annurev.publhealth.012809.103646. 132 p following 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ginsberg G, Hattis D, Sonawane B, Russ A, Banati P, Kozlak M, Smolenski S, Goble R. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci. 2002;66:185–200. doi: 10.1093/toxsci/66.2.185. [DOI] [PubMed] [Google Scholar]
- 51.Hattis D, Ginsberg G, Sonawane B, Smolenski S, Russ A, Kozlak M, Goble R. Differences in pharmacokinetics between children and adults--II. Children's variability in drug elimination half-lives and in some parameters needed for physiologically-based pharmacokinetic modeling. Risk Anal. 2003;23:117–142. doi: 10.1111/1539-6924.00295. [DOI] [PubMed] [Google Scholar]
- 52.Neri M, Ugolini D, Bonassi S, Fucic A, Holland N, Knudsen LE, Sram RJ, Ceppi M, Bocchini V, Merlo DF. Children's exposure to environmental pollutants and biomarkers of genetic damage. II. Results of a comprehensive literature search and meta-analysis. Mutat Res. 2006;612:14–39. doi: 10.1016/j.mrrev.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Snyder R. Recent developments in the understanding of benzene toxicity and leukemogenesis. Drug Chem Toxicol. 2000;23:13–25. doi: 10.1081/dct-100100099. [DOI] [PubMed] [Google Scholar]
- 54.Tung EW, Philbrook NA, Macdonald KD, Winn LM. DNA double-strand breaks and DNA recombination in benzene metabolite-induced genotoxicity. Toxicol Sci. 2012;126:569–577. doi: 10.1093/toxsci/kfs001. [DOI] [PubMed] [Google Scholar]
- 55.Mondrala S, Eastmond DA. Topoisomerase II inhibition by the bioactivated benzene metabolite hydroquinone involves multiple mechanisms. Chem Biol Interact. 2010;184:259–268. doi: 10.1016/j.cbi.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Zuo X, Bai W, Niu P, Tian L, Gao A. PTEN methylation involved in benzene-induced hematotoxicity. Exp Mol Pathol. 2014;96:300–306. doi: 10.1016/j.yexmp.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Badham HJ, LeBrun DP, Rutter A, Winn LM. Transplacental benzene exposure increases tumor incidence in mouse offspring: possible role of fetal benzene metabolism. Carcinogenesis. 2010;31:1142–1148. doi: 10.1093/carcin/bgq074. [DOI] [PubMed] [Google Scholar]
- 58.Minciullo PL, Navarra M, Calapai G, Gangemi S. Cytokine network involvement in subjects exposed to benzene. J Immunol Res. 2014;2014:937987. doi: 10.1155/2014/937987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151:362–367. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 60.Belpomme D, Irigaray P, Hardell L, Clapp R, Montagnier L, Epstein S, Sasco AJ. The multitude and diversity of environmental carcinogens. Environ Res. 2007;105:414–429. doi: 10.1016/j.envres.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 61.Koehler C, Thielen S, Ginzkey C, Hackenberg S, Scherzed A, Burghartz M, Paulus M, Hagen R, Kleinsasser NH. Nitrogen dioxide is genotoxic in urban concentrations. Inhal Toxicol. 2013;25:341–347. doi: 10.3109/08958378.2013.788104. [DOI] [PubMed] [Google Scholar]
- 62.Gorsdorf S, Appel KE, Engeholm C, Obe G. Nitrogen dioxide induces DNA single-strand breaks in cultured Chinese hamster cells. Carcinogenesis. 1990;11:37–41. doi: 10.1093/carcin/11.1.37. [DOI] [PubMed] [Google Scholar]
- 63.Arroyo PL, Hatch-Pigott V, Mower HF, Cooney RV. Mutagenicity of nitric oxide and its inhibition by antioxidants. Mutat Res. 1992;281:193–202. doi: 10.1016/0165-7992(92)90008-6. [DOI] [PubMed] [Google Scholar]
- 64.Bermudez E, Ferng SF, Castro CE, Mustafa MG. DNA strand breaks caused by exposure to ozone and nitrogen dioxide. Environ Res. 1999;81:72–80. doi: 10.1006/enrs.1999.3955. [DOI] [PubMed] [Google Scholar]
- 65.Traversi D, Cervella P, Gilli G. Evaluating the genotoxicity of urban PM2.5 using PCR-based methods in human lung cells and the Salmonella TA98 reverse test. Environ Sci Pollut Res Int. 2014 doi: 10.1007/s11356-014-3435-1. [DOI] [PubMed] [Google Scholar]
- 66.Snyder R. Leukemia and benzene. Int J Environ Res Public Health. 2012;9:2875–2893. doi: 10.3390/ijerph9082875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Irons RD, Kerzic PJ. Cytogenetics in benzene-associated myelodysplastic syndromes and acute myeloid leukemia: new insights into a disease continuum. Ann N Y Acad Sci. 2014;1310:84–88. doi: 10.1111/nyas.12336. [DOI] [PubMed] [Google Scholar]
- 68.Feingold L, Savitz DA, John EM. Use of a job-exposure matrix to evaluate parental occupation and childhood cancer. Cancer Causes Control. 1992;3:161–169. doi: 10.1007/BF00051656. [DOI] [PubMed] [Google Scholar]
- 69.Colt JS, Blair A. Parental occupational exposures and risk of childhood cancer. Environ Health Perspect. 1998;106(Suppl 3):909–925. doi: 10.1289/ehp.98106909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spycher BD, Lupatsch J, Huss A, Vermeulen R, Rischewski J, Schindera C, Spoerri A, Kuehni CE. Parental Occupational Exposure to Benzene and Risk of Childhood Cancer: a Census-Based Cohort Study. Proceedings of the 26th Conference of International Society for Environmental Epidemiology. Seattle. 2014 Aug;:24–28. [Google Scholar]
- 71.Greaves MF, Wiemels J. Origins of chromosome translocations in childhood leukaemia. Nat Rev Cancer. 2003;3:639–649. doi: 10.1038/nrc1164. [DOI] [PubMed] [Google Scholar]
- 72.Wiemels JL, Cazzaniga G, Daniotti M, Eden OB, Addison GM, Masera G, Saha V, Biondi A, Greaves MF. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354:1499–1503. doi: 10.1016/s0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- 73.Long L. Residential mobility differences among developed countries. Int Reg Sci Rev. 1991;14:133–147. doi: 10.1177/016001769101400202. [DOI] [PubMed] [Google Scholar]
- 74.Edwards YH, Potter J, Hopkinson DA. Human FAD-dependent NAD(P)H diaphorase. Biochem J. 1980;187:429–436. doi: 10.1042/bj1870429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith MT. Benzene, NQO1, and genetic susceptibility to cancer. Proc Natl Acad Sci U S A. 1999;96:7624–7626. doi: 10.1073/pnas.96.14.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li C, Liu Y, Wei S, Zhou Y. A meta-analysis of the association between NQO1 C609T variation and acute myeloid leukemia risk. Pediatr Blood Cancer. 2014;61:771–777. doi: 10.1002/pbc.24924. [DOI] [PubMed] [Google Scholar]
- 77.Li C, Zhou Y. Association between NQO1 C609T polymorphism and acute lymphoblastic leukemia risk: evidence from an updated meta-analysis based on 17 case-control studies. J Cancer Res Clin Oncol. 2014;140:873–881. doi: 10.1007/s00432-014-1595-5. [DOI] [PubMed] [Google Scholar]
- 78.Wu J, Wilhelm M, Chung J, Ritz B. Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environ Res. 2011;111:685–692. doi: 10.1016/j.envres.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]