Abstract
Computer-assisted behavioral treatments hold promise for enhancing access to and reducing costs of treatments for substance use disorders. This study assessed the efficacy of a computer-assisted version of an efficacious, multicomponent treatment for cannabis use disorders (CUD), i.e., motivational enhancement therapy, cognitive-behavioral therapy, and abstinence-based contingency-management (MET/CBT/CM). An initial cost comparison was also performed. Seventy-five adult participants, 59% African Americans, seeking treatment for CUD received either, MET only (BRIEF), therapist-delivered MET/CBT/CM (THERAPIST), or computer-delivered MET/CBT/CM (COMPUTER). During treatment, the THERAPIST and COMPUTER conditions engendered longer durations of continuous cannabis abstinence than BRIEF (p < .05), but did not differ from each other. Abstinence rates and reduction in days of use over time were maintained in COMPUTER at least as well as in THERAPIST. COMPUTER averaged approximately $130 (p < .05) less per case than THERAPIST in therapist costs, which offset most of the costs of CM. Results add to promising findings that illustrate potential for computer-assisted delivery methods to enhance access to evidence-based care, reduce costs, and possibly improve outcomes. The observed maintenance effects and the cost findings require replication in larger clinical trials.
Behavioral treatments for Cannabis Use Disorders (CUD) show efficacy outcomes akin to those achieved with treatment for other types of substance use disorders (Budney, 2007; Danovitch & Gorelick, 2012; Denis, Lavie, Fatseas, & Auriacombe, 2006; Dutra et al., 2008). Arguably, the approach demonstrating the best abstinence outcomes is a multicomponent intervention that includes motivational enhancement therapy (MET), cognitive-behavioral therapy (CBT), and abstinence-based contingency-management (CM) (Budney, Higgins, Radonovich, & Novy, 2000; Budney, Moore, Rocha, & Higgins, 2006; Carroll et al., 2012; Kadden, Litt, Kabela-Cormier, & Petry, 2007; Litt, Kadden, & Petry, 2013). In this model, the data consistently indicate that CM appears most important for engendering abstinence during treatment, and that MET/CBT may enhance maintenance of this CM effect (Budney et al., 2006; Kadden et al., 2007). Cost-effective interventions for CUD are needed because an estimated 17.5% of substance abuse treatment admissions designate cannabis as their primary substance of abuse (third only to alcohol and all forms of opiates) (SAMHSA, 2014). Some are concerned that this need may further increase depending on changing cannabis laws and how effectively regulations can control reductions in price, increased access, and development of more potent and attractive cannabis products (Kilmer, 2014).
Addressing four limitations of this intervention might foster progress towards transportation of its use to the broader community. First, availability is low as few treatment programs provide MET/CBT or CM (Carroll, 2014; McGovern, Fox, Xie, & Drake, 2004). Second, the resources needed to train staff and achieve fidelity of MET/CBT and CM treatment delivery are not widely available, and high caseloads and turnover rates increase the difficulty of maintaining quality. Third, the cost, particularly of CM, but also of MET/CBT is considered high, i.e., therapist time, drug testing, and incentives (Kirby, Benishek, Dugosh, & Kerwin, 2006). Last, travel to clinics poses difficulty for those in rural areas and those with limited resources.
Technology-delivered interventions have the potential to improve access, ensure fidelity of treatment, reduce costs associated with training and delivery, and perhaps enhance potency (Marsch, Carroll, & Kiluk, 2014). Such interventions are being developed for an expanding array of mental health problems including substance use (Copeland & Martin, 2004; Marsch & Dallery, 2012; Moore, Fazzino, Garnet, Cutter, & Barry, 2011; Newman, Szkodny, Llera, & Przeworski, 2011a, 2011b; Taylor & Luce, 2003). Applications have included adjunct video demonstrations of CBT that teach and model coping skills (Carroll et al., 2008; Carroll et al., 2009; Carroll et al., 2014), and computer-delivered motivational and skills-based interventions for cigarette smokers, problem drinkers, opioid dependent outpatients, and HIV risk reduction (Bickel, Marsch, Buchhalter, & Badger, 2008; Campbell et al., 2014; Hester & Delaney, 1997; Hester, Delaney, & Campbell, 2012; Marsch & Bickel, 2004; Marsch, Carroll, et al., 2014; Marsch et al., 2011; Ondersma, Svikis, & Schuster, 2007). Of note, two of these studies combined computer-delivered evidence-based therapies with CM and demonstrated their efficacy, but neither demonstrated that the computer intervention was efficacious over and above the CM component.
Most related to the present study, computer-delivered MET/CBT (without CM) for those with cannabis, alcohol, and co-morbid depression problems produced significant cannabis reductions that were at least comparable to therapist-delivered MET/CBT (Kay-Lambkin, Baker, Kelly, & Lewin, 2011; Kay-Lambkin, Baker, Lewin, & Carr, 2009). Similarly, an initial quasi-experimental trial of the MET/CBT/CM intervention tested in the present study did not observe outcome differences between computer-assisted and therapist-delivered MET/CBT during the active treatment period (Budney et al., 2011). Acceptability of this intervention was high, consumer ratings were positive, and the therapist time per case was greatly reduced. However, post-treatment data were not collected and a comparison treatment was not included. Given that prior studies comparing CM alone with CM plus MET/CBT reported comparable effects on abstinence during treatment (Budney et al., 2006; Kadden et al., 2007), one should not be surprised that computerized MET/CBT sessions combined with CM produced during treatment outcomes equivalent to therapist delivered MET/CBT with CM. Whether or not computer-delivered MET/CBT can contribute to the post-treatment maintenance of abstinence as well as the therapist-delivered MET/CBT observed in prior studies (Budney et al., 2006; Kadden et al., 2007) remains a critical issue in need of testing.
The present randomized study recruited a sample of participants seeking treatment for CUD, and sought to replicate and extend prior findings with a primary focus on post-treatment maintenance of outcomes observed with MET/CBT when combined with CM, and a secondary aim on estimating the cost of delivery. A brief MET only condition (BRIEF) provided a comparison treatment to assess the efficacy of computer-assisted (COMPUTER) and therapist-delivered (THERAPIST) MET/CBT/CM interventions. If, as hypothesized, both MET/CBT/CM interventions engender superior outcomes than BRIEF, and the COMPUTER version either does not differ from or produces better outcomes than the THERAPIST version at a reduced cost, these findings would have important implications for enhancing access to MET/CBT/CM.
Materials and Methods
Participants and Inclusion Criteria
The study was conducted in compliance with the IRB of the University of Arkansas for Medical Sciences and registered with clinicaltrials.gov (NCT00594659). Participants seeking treatment for CUD from 2010–2012 were recruited via media advertisements, posted flyers, and mailings to health service professionals. Nine participants, 4 in the THERAPIST and COMPUTER conditions and 1 in BRIEF, were referred by probation, court, or employment assistance programs. Inclusion criteria were 1) ≥ 18 years, 2) current DSM-IV diagnosis of cannabis abuse or dependence, and 3) cannabis use on at least 50 of the previous 90 days. Exclusion criteria included dependence on alcohol or other drugs except nicotine, psychological distress in need of immediate treatment, legal status that might result in imminent incarceration, plans to move out of the area in the next 12 months, or not being fluent in English.
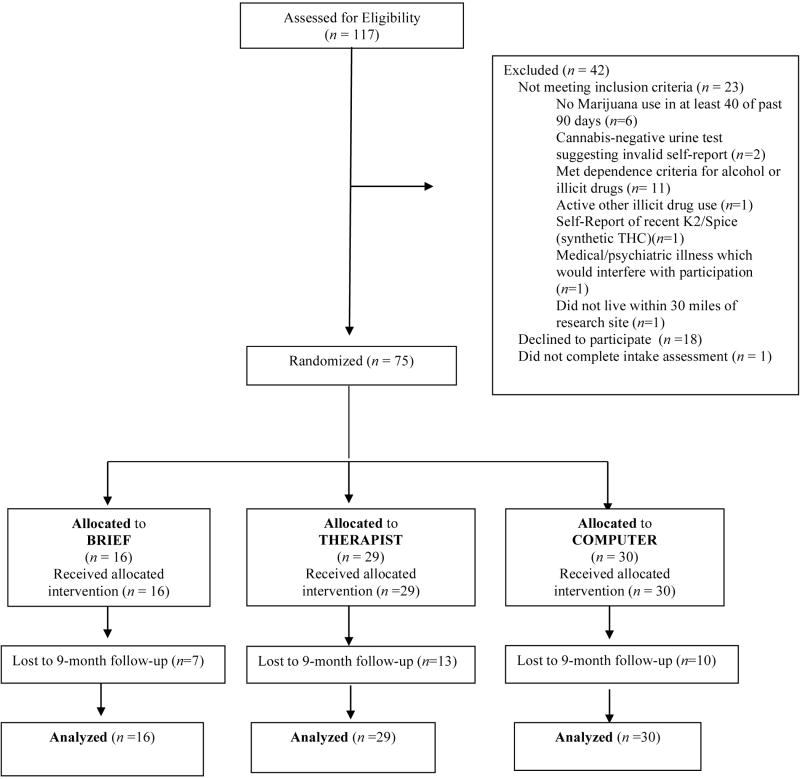
Of the 117 adults assessed, 23 did not meet inclusion criteria, 18 refused treatment, and one did not complete the intake (Figure 1). Seventy-five adults, aged 18 to 55 years (M = 35.9, SD = 10.5) enrolled in the trial. Participants were 59% African American and 57% male. Table 1 presents participant characteristics by condition.
Figure 1.
Clinical Trial Consort Diagram
Table 1.
Participant Characteristics at Intake
| COMPUTER (n=30) |
THERAPIST (n=29) |
BRIEF (n=16) |
p valuea | |
|---|---|---|---|---|
| Age | 34.87 (11.07) | 34.72 (11.06) | 35.06 (8.95) | 0.98 |
| Male (%) | 53% | 59% | 56% | 0.95 |
| Race | 0.74 | |||
| Black | 67% | 52% | 56% | |
| Caucasian | 30% | 45% | 38% | |
| Other | 3% | 3% | 6% | |
| Days of Cannabis Use (past 90 days) | 78.0 (17.1) | 81.2 (13.8) | 74.8 (17.7) | 0.56 |
| Age of first used MJ | 15.27 (2.97) | 15.00 (2.82) | 14.25 (2.74) | 0.53 |
| % Blunt User | 63% | 45% | 50% | 0.35 |
| Amount (oz) cannabis used per week (past 90 days) | 0.47 (0.31) | 0.54 (0.38) | 0.53 (0.27) | 0.68 |
| Money spent on cannabis per month (past 90 days) | 154.67 (138.53) | 233.62 (227.41) | 184.38 (116.60) | 0.27 |
| Hours per day high when smoked past 90 days | 7.60 (4.69) | 7.55 (4.65) | 4.81 (3.33) | 0.11 |
| Readiness to Change score | 3.37 (0.37) | 3.38 (0.43) | 3.43 (0.43) | 0.92 |
| Cigarette Use (past 90 days) | 53% | 55% | 56% | 0.99 |
Kruskal-Wallis and Fisher Exact tests were used to compare continuous and categorical variables across the three conditions.
Measures
Assessments
Assessments were performed at intake, end of treatment, and 3 and 9 months post treatment. The Psychoactive Substance Use Disorders section of the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1995) provided substance use diagnoses. A modified version of Form 90-I (Tonigan, Miller, & Brown, 1997) assessed substance use history. Daily information on substance use for the 90 days preceding each assessment was obtained using the timeline follow- back (TLFB) procedure (Sobell & Sobell, 1992). The Marijuana Problems Scale assessed negative consequences associated with cannabis use (Stephens et al., 2000; Marijuana Treatment Project Research Group, 2004). The Marijuana Self-efficacy Scale measured confidence in avoiding use in situations involving negative affect, social discomfort, and presence of others using marijuana (Litt, Kadden, & Stephens, 2005). The Coping Strategies Scale measured use of skills taught in CBT (Litt, Kadden, & Tennen, 2012).
Urine Toxicology
Urine specimens were collected under staff observation twice weekly throughout treatment and at each assessment. Specimens were immediately tested for 11-nor-delta-9-THC9-carboxylic acid (THCCOOH), the primary cannabis metabolite. Tests for creatinine (indicator of dilution) and other indicators of adulteration were performed. If specimens were deemed invalid, participants were asked to provide another specimen within 24 hours. Failure to submit a specimen was treated as a cannabis-positive result.
Cost Estimation
An initial cost analysis of services provided was performed from the perspective of the clinic following guidelines for cost-effectiveness analysis (Luce, Manning, Siegel, & Lipscomb, 1996). Similar to prior studies (McCrone et al., 2004; Olmstead, Ostrow, & Carroll, 2010), we used a resource costing approach where services involved in treatment were categorized and recorded by trained research assistants. Services included the providing of therapy, urinalysis, and incentive costs. Our focus was on the service costs because these costs account for 75% to 88% of the total costs of operating drug abuse treatment centers (French, Roebuck, and McLellan, 2004). We did not consider the costs of developing the software or initial training of therapists as these are sunk costs that would not be incurred in implementing the intervention. Given the small sample size of this initial efficacy trial, other costs, such as licensing costs for computer software, supervisor costs for ongoing training, supervision and hiring of therapists, and office space, which are important considerations in cost-effectiveness calculations, were not included in this cost analysis because these would vary dramatically depending on the caseload and staffing structure of a community treatment center.
Services were translated into cost estimates by multiplying participant utilization of each service by the average cost of providing the service. Therapy utilization time was coded in minutes (verified via timestamps of videotaped sessions). Missing time values (n=15) for sessions attended (total = 216) were imputed based on the mean time spent in that specific session by the participants whose data were available. Therapist session costs for each case were calculated as the per minute wage rate of the therapist including fringe benefits multiplied by the total number of minutes spent with the therapist for each participant. In addition, for each session attended, 20 minutes of therapist time were added for administrative costs (estimation for: preparation time, progress notes, time in weekly supervision, miscellaneous clinical contacts). Note that this calculation results in disproportional results across treatment conditions for time spent in session and total therapist cost because some therapist sessions tended to be longer (MET sessions) and others shorter (support sessions in COMPUTER), but administration time was allotted at 20 minutes regardless of length.
Cost of urinalysis services included staff time to administer each test (10 minutes) and cost of testing supplies (total cost per test was approximately $5.74). Cost per participant equaled the number of tests performed multiplied by the per test supply cost, added to the total staff time cost calculated by multiplying minutes spent (# of tests × 10 minutes) by the per minute staff wage and fringe rate.
Incentive costs were actual earnings per participant, plus estimated cost of staff time to purchase and administer incentives (retail gift certificates bought in bulk). The monthly staff time estimate was 5 hours per month total for all treatment conditions (all participants earned incentives). Total staff time spent for all participants was calculated by multiplying the monthly time by 24 (duration of trial was 24 months). Total time was multiplied by the per hour staff wage/fringe rate to get total cost of purchasing incentives, which were then allocated to each participant in proportion to their incentive earnings.
Finally, we calculated the additional cost of a computer for the COMPUTER condition, at approximately $6 per person assuming a $1,000 computer with a useful life of five years. Alternative assumptions about computer cost ($700) and useful life (4 years) produce a similar estimate for the cost of computer per participant ($5.83).
Treatment Procedures
Participants were randomly assigned to one of three treatment programs: 1) BRIEF; 2) THERAPIST; or 3) COMPUTER. BRIEF served as the comparison condition to assess the efficacy of the two MET/CBT/CM conditions. Assignment was performed on a 2 to 2 to 1 ratio (THERAPIST (n=29), COMPUTER (n=30) and BRIEF (n=16), respectively) because low rates of and little variance in abstinence outcomes with MET have been observed in prior trials (Budney et al., 2000; Marijuana Treatment Project Research Group, 2004; Stephens, Roffman, & Curtin, 2000), and this reduced the number of participants that would receive what was expected to be a weaker intervention. Baseline characteristics that could influence outcome (i.e., abstinence prior to treatment initiation, tobacco smoking status, and gender) were balanced using minimum-likelihood allocation (Aickin, 1982).
BRIEF
BRIEF involved two, individual counseling sessions similar to the intervention used in prior CUD studies (Budney et al., 2000; Marijuana Treatment Project Research Group, 2004; Stephens et al., 2000). These 60–75 minute sessions were scheduled for Weeks 1 and 5. Session 1 involved discussion of a personalized feedback report, cannabis use, and change plans using a motivational interviewing style. Session 2 continued this process and reviewed efforts to reduce cannabis use and plans for adjustments in strategy. BRIEF also included a 12-week incentive program that encouraged attendance at sessions and substance testing appointments. A $5.00 incentive was provided for attending each session and providing urine specimens independent of test results. Earnings were exchangeable for gift cards or certificates.
THERAPIST
THERAPIST involved 9 sessions of individual counseling (Budney et al., 2011; Steinberg et al., 2005). During Sessions 1 and 2, participants received MET as described above. During Sessions 3–9, CBT sessions were provided that included lifestyle goal setting/case management, treatment contracting, functional analysis training, and a set of core coping skills focused on cannabis use. THERAPIST also included an abstinence-based CM program (described below).
COMPUTER
COMPUTER participants received the same 9-session MET/CBT intervention via an internet-delivered program developed in collaboration with HealthSim, Inc. (Budney et al., 2011). This program is an adaptation of the Therapeutic Education System that has been evaluated in multiple substance use disorder clinical trials including a recent NIDA Clinical Trials Network multisite study (Campbell et al., 2014). Clinic visits followed the same weekly schedule as for THERAPIST. Participants completed a MET/CBT session module on the computer at each visit (~20–45 minutes each). Staff were available at each session to assist and ensure that participants were not intoxicated or experiencing any crises. Participants were also assigned a therapist and scheduled for three supportive sessions. The initial therapist session (~ 30 min) occurred immediately prior to the first computer session. The therapist prompted discussion of circumstances for entering treatment, oriented participants to the intervention, provided details on the incentive program, and discussed the plan for therapist contact. The two other sessions (~15 min) occurred during weeks 4 and 12. These involved discussion of progress, issues with the internet program, need for referral or clinical assistance, and encouragement to use their program remotely. Of note, only 5 of 30 participants accessed the program remotely (range: 1–5 times).
Abstinence-based Incentives (CM)
Participants in THERAPIST and COMPUTER earned abstinence-based incentives exchangeable for gift cards based on results of twice weekly urinalysis. Weeks 1 and 2 were considered a washout period because most participants would test positive for cannabis during this time due to the half-life of its urine metabolites. During the washout participants received $5.00 for provision of each specimen, independent of results. During weeks 3–12, incentives were earned contingent on specimens that tested negative for THCCOOH. An escalating schedule of reinforcement began with a value of $1.50 and increased by $1.50 with each subsequent negative test (Budney et al., 2011; Higgins et al., 1991). A $10 bonus was earned for each week of negative specimens. A cannabis-positive specimen resulted in the incentive being reset to the initial value, from which it could escalate again according to the same schedule. Participants remaining continuously abstinent could earn $435. COMPUTER participants received information about their incentive earnings each time they logged on the computer for a session. THERAPIST participants received this information from their therapist at each session. The therapist initiated a brief check in about amount earned each week, potential earnings in the future, and a brief discussion about how the participant might spend their earnings. At the second weekly urinalysis visit that did not involve an MET/CBT session, research staff informed all participants of the test results and incentive earnings. At any visit, participants could request to spend their earnings.
Therapist Training and Treatment Fidelity
One Masters level (6 years experience) and one licensed substance use counselor (16 years experience) conducted the MET/CBT sessions. An additional therapist (PCB) provided treatment to one participant because one therapist resigned prior to study completion. Therapists attended a 2-day workshop conducted by an MET/CBT expert (DW) prior to the study, and then received ongoing training and weekly supervision for highly experienced personnel (DW, AJB, and PCB). Sessions were videotaped and approximately one session every two weeks was reviewed (by DW, PCB or AJB) using the Motivational Interviewing Treatment Integrity and the Yale Adherence and Competence System (Carroll et al., 2000; Moyers, Martin, Manuel, Hendrickson, & Miller, 2005) to monitor fidelity and provide feedback.
Data Analysis
At baseline, non-parametric approaches including Wilcoxon, Kruskal-Wallis, and Fisher’s exact tests were used to compare categorical and continuous participant characteristic variables to ensure the efficacy of the randomization. Outcome analyses used an intent-to-treat approach. Cannabis abstinence outcomes were compared first within the treatment phase and then across follow-up time points. A bias-corrected and accelerated (BCA) bootstrapping approach (Efron & Tibshirani, 1993) was used to calculate 95% confidence intervals of the mean differences between the conditions on the longest duration of continuous abstinence (LDA) achieved during treatment. This method corrects bias from asymmetric distributions; for LDA, the distributions were right-skewed. Point prevalence abstinence rates at the end of treatment were compared across conditions as were abstinence rates at each follow-up (see below).
A piecewise mixed effects model with logit link and first-order auto-regressive correlation structure was used to model the trajectories of point prevalence cannabis abstinence assuming three pieces: intake to end of treatment (i.e., during treatment effect), end of treatment to 3 months (i.e., initial relapse rate), and 3 months to 9 months (i.e., relapse over time). The reference group for the initial analysis for each piece of the model was the BRIEF condition. The slope was estimated for BRIEF for each piece, and contrasts were estimated testing differences in the slopes for the other two conditions relative to BRIEF. The first piece of the model provided a test of whether the THERAPIST and COMPUTER engendered greater rates of abstinence than BRIEF during treatment. The second piece compared the rate of relapse immediately postreatment (first 3 months), and the third piece compared relapse effects over time. An additional contrast was used to estimate differences between the THERAPIST and COMPUTER conditions to compare abstinence outcomes on each piece of the model. For secondary outcomes, similar piecewise mixed effects models were estimated. PROC GLIMMIX and PROC MIXED were used for binary abstinence and continuous variable analyses respectively (SAS/STAT® 9.2 User’s Guide).
Missing Data
During treatment, all participants provided at least 1 urine specimen and details of their session attendance, and the number of specimens provided during the treatment period is included in Table 2. During treatment, missing urine specimens were imputed as positive to provide a conservative estimate of outcome. Among those who did not provide at least half of the scheduled specimens, the mean percentage of positive results among the specimens they did provide was 93%, while the mean percentage of positive results among those who provided at least half of the specimens was 32%, which represents a statistically significant difference (p<0.001). These data in addition to extensive outreach procedures and a flexible schedule for specimen collection strongly suggest that the great majority of missing specimens were indicators of recent use (Budney et al., 2011; Budney et al., 2006). At the end of treatment, 67%, 73%, and 69% provided urine specimens in the COMPUTER, THERAPIST, and BRIEF treatment arms, respectively. At the 9-month follow-up, the percentage providing urine specimens were 67%, 55%, and 56%. No significant differences among conditions were observed for the number or distribution of missing urine specimens during treatment or for the percentage of participants who provided follow-up assessments (Table 2). For secondary outcomes, all cases and data were included and analyzed assuming data were missing at random. For days of cannabis use, data were included only if the participant provided at least 65 days of data for the assessed 90-day periods; else their data were considered missing.
Table 2.
Retention and Cannabis Outcomes
| COMPUTER (n=30) | THERAPIST (n=29) | BRIEF (n=16) | statistic | p value | |
|---|---|---|---|---|---|
| Number of MET/CBT sessions attendeda (delivered by computer or therapist) | 4.8 (3.4) | 5.3 (3.4) | 1.4 (0.5) | t = 0.58 | .57 |
| Number of supportive therapist sessions | 2.0 (0.9) | — | — | ||
| Treatment Participation (number of specimens submitted) | |||||
| <3 | 27% | 17% | 31% | X2=7.76 | .26 |
| ≥3 & ≤6 | 20% | 28% | 13% | ||
| >6 & ≤12 | 13% | 7% | 31% | ||
| >12 | 40% | 48% | 25% | ||
| Mean weeks of continuous abstinencec | 2.8 (4.2) | 3.6 (4.4) | 0.8 (2.0) | ||
| THERAPIST vs BRIEF | 5.54 (3.39, 7.93) | < .05 | |||
| COMPUTER vs BRIEF | 4.07 (1.40, 6.99) | < .05 | |||
| THERAPIST vs COMPUTER | 1.47 (–1.91, 4.87) | ns | |||
| Participants available at follow-ups | |||||
| End of Treatment / 9 monthsd | 67% / 67% | 73% / 55% | 69% / 56% | ns/ns | |
| Participants abstinent (end of treatment) | 47% | 45% | 12.5% | ||
| THERAPIST vs BRIEF | X2 = 4.85 | < .05 | |||
| COMPUTER vs BRIEF | X2 = 5.37 | < .05 | |||
| THERAPIST vs COMPUTER | X2 = 0.02 | .89 | |||
| % days cannabis used (past 90 days)e | |||||
| End of Treatment | 26% | 23% | 40% | ||
| THERAPIST vs BRIEF | t= 1.55 | .12 | |||
| COMPUTER vs BRIEF | t = 1.29 | .20 | |||
| THERAPIST vs COMPUTER | t = 0.33 | .75 |
comparison of COMPUTER and THERAPIST;
mean (SD): Wilcoxon X2
arithmetic means (SD); results from BCA bootstrap: estimated mean difference between groups (95% CI)
no differences between conditions
estimated means from piecewise linear mixed model
Result
Participants
Participants across the three conditions did not differ significantly on any demographic or substance use measures (Table 1). Participants were 44% women, 37% White and 59% African American. Most used cannabis almost daily [M=78.5 (SD=16.0) days out of the last 90] and had long histories of cannabis use [mean age of initiation = 15.0 years (SD=2.9)]. Ninety-seven percent met DSM-IV criteria for cannabis dependence and 3% for abuse.
Participation and Retention
Treatment participation and retention as indicated by attending urinalysis visits did not differ across conditions (Table 2). The mean number of MET/CBT sessions attended also did not differ significantly between THERAPIST and COMPUTER. The proportion of post-treatment assessments obtained did not differ significantly by condition at any assessment (total means: end of treatment (ETX)=69%; 9 months=60%).
Therapist Effects
Number of sessions attended did not differ between therapists (n=2) across BRIEF, THERAPIST and COMPUTER conditions (Kruskal-Wallis X2 = 0.40, p=.53; X2 = 0.13, p=.72; X2 = 0.002, p=.96, respectively). Comparison of LDA by therapist within conditions also revealed no significant effect of therapist (Kruskal-Wallis X2 = 0.08, p=.78; X2 = 1.90, p=.17; X2 = 0.20, p=.66, respectively).
Cannabis Abstinence
Table 2 presents the cannabis abstinence outcomes achieved during treatment. Paired comparisons of confidence intervals of the mean differences in LDA showed that THERAPIST and COMPUTER engendered significantly greater LDA than BRIEF (p’s < .05) (effect sizes .71 and .55, respectively), and no difference was observed between the THERAPIST and COMPUTER. Significantly more participants in THERAPIST (44.8%) and COMPUTER (46.7%) than in BRIEF (12.5%) were abstinent at the end of treatment as indicated by provision of cannabis negative urine specimens during the last week of treatment or at the scheduled end of treatment follow-up (p’s < .05), and no significant difference was observed between THERAPIST and COMPUTER.
Table 3 and Figure 2 show the results of the piecewise abstinence models. The THERAPIST and COMPUTER conditions showed significantly greater increases in abstinence from intake to the end of treatment than BRIEF, which did not increase significantly. The observed THERAPIST and COMPUTER increases did not differ from each other. At 3 months post-treatment, the COMPUTER condition had a significantly higher rate of abstinence than the BRIEF condition. During the immediate 3-month post-treatment period and months 3–9 post-treatment period, non-significant decreases in abstinence were observed across all conditions with no significant differences observed. Figure 2 illustrates that abstinence rates at each assessment were at least as high as in the COMPUTER condition as in the THERAPIST condition.
Table 3.
Piecewise analyses for primary and secondary outcome variables over time
| Time Period | Abstinencea B (se) |
t | % Days Use B (se) |
t | MPSb B (se) |
t | Self-Efficacyc B (se) |
t | Copingd B (se) |
t |
|---|---|---|---|---|---|---|---|---|---|---|
| Intake to ETX: | ||||||||||
| BRIEF | 0.66(1.07) | 0.6 | −0.48(0.09) | −5.5*** | −4.89(1.79) | −2.7** | 0.25(0.39) | 0.6 | 0.24(0.18) | 1.3 |
| vs. THERAPIST | 2.45(1.10) | 2.2* | −0.16(0.11) | −1.6 | −1.26(2.15) | −0.6 | 0.97(0.46) | 2.1* | 0.31(0.22) | 1.4 |
| vs. COMPUTER | 2.28(1.12) | 2.0* | −0.14(0.10) | −1.3 | −2.44(2.23) | −1.1 | 1.04(0.48) | 2.2* | 0.39(0.23) | 1.7 |
| THERAPIST vs. COMPUTER | 0.17 (0.75) | 0.2 | −0.03 (0.09) | −0.3 | 1.18 (1.85) | 0.6 | −0.06 (0.40) | −0.1 | −0.08 (0.19) | −0.5 |
| ETX to 3mo: | ||||||||||
| BRIEF | −1.02(1.40) | −0.7 | −0.09(0.10) | −0.9 | 3.68(2.16) | 1.7 | 0.61(0.49) | 1.3 | 0.12(0.22) | 0.6 |
| vs. THERAPIST | −0.34(1.5) | −0.2 | 0.09(0.12) | 0.7 | −6.23(2.66) | −2.4* | −1.21(0.58) | −2.1* | −0.39(0.27) | −1.4 |
| vs. COMPUTER | 0.51(1.54) | 0.3 | 0.16(0.12) | 1.3 | −2.47(2.66) | −0.9 | −1.00(0.58) | −1.7 | −0.40(0.27) | −1.5 |
| THERAPIST vs. COMPUTER | −0.85 (0.90) | −0.9 | −0.07(0.10) | −0.7 | −3.77 (2.19) | −1.7 | −0.23 (0.47) | −0.5 | 0.010 (0.22) | 0.1 |
| 3mo to 9mo: | ||||||||||
| BRIEF | −0.00(0.83) | −0.0 | 0.05(0.06) | 0.8 | −2.73(1.35) | 2.0* | −0.36(0.30) | −1.2 | −0.17(0.14) | −1.2 |
| vs. THERAPIST | −0.47(0.92) | −0.5 | 0.09(0.07) | 1.3 | 3.63(1.64) | 2.2* | 0.44(0.36) | 1.2 | 0.13(0.17) | 0.8 |
| vs. COMPUTER | −0.48(0.90) | −0.5 | −0.05(0.7) | 0.7 | 1.93(1.60) | 1.2 | 0.36(0.36) | 1.0 | −0.08(0.16) | −0.5 |
| THERAPIST vs. COMPUTER | 0.006 (0.54) | 0.01 | 0.14 (0.05) | 2.5* | 1.69 (1.26) | 1.4 | 0.072 (0.28) | 0.26 | 0.21 (0.13) | 1.6 |
p<.001
p<.01
p<.05
% of participants with cannabis−negative urine test at each assessment
Score on the Marijuana Problem Scale
Score on the Self-Efficacy Scale
Score on the Total Coping Strategy Scale
Figure 2.
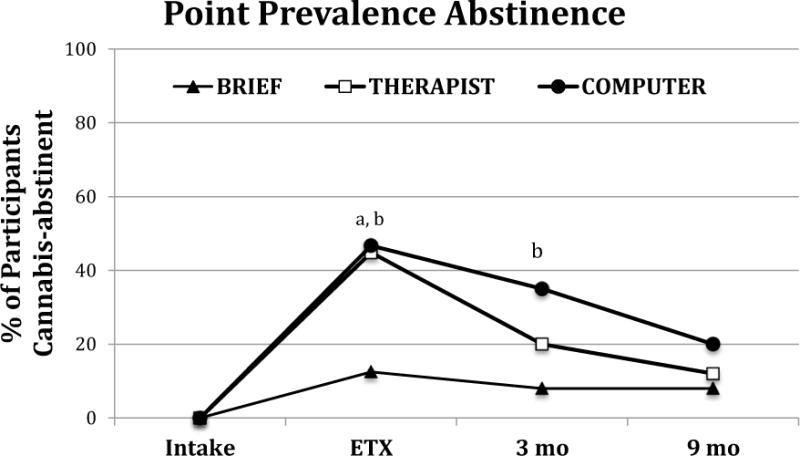
Percent of participants in each condition that provide a cannabis-negative urine specimen at each assessment time point from piecewise analysis. Missing specimens are considered cannabis-positive. ETX = end of treatment.
a: BRIEF vs. THERAPIST is significant (ETX: t(291)=2.22, p=0.03)
b: BRIEF vs. COMPUTER is significant (ETX: t(291)=2.03, p=0.04; 3M: t(291)=2.16, p=0.03)
Secondary Outcomes
The three-piece mixed models for percentage of days of cannabis indicated that all conditions showed a similar decline in use from intake to the end of treatment, and that all showed no significant change from the end of treatment to the 3-month follow up. From 3–9 months post-treatment, the THERAPIST condition showed a greater increase in days of use than the COMPUTER condition resulting in a significant difference between THERAPIST and COMPUTER conditions at 9 months (Table 3, Figure 3).
Figure 3.
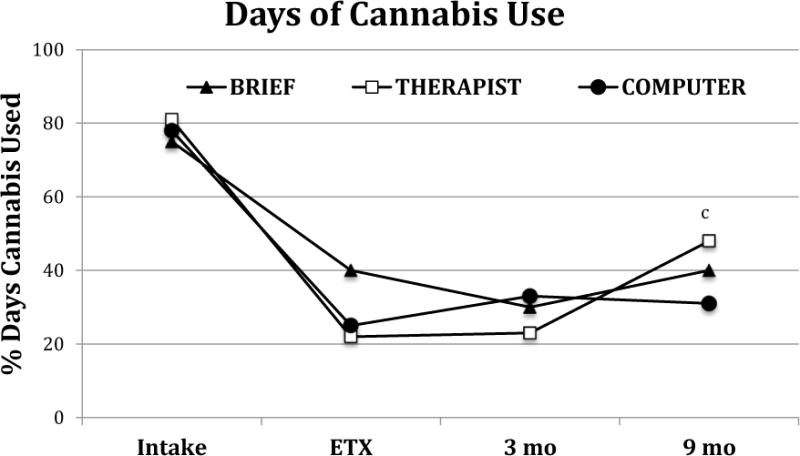
Estimated percent days of cannabis use (from timeline follow back assessments) during the 90 days prior to each assessment time point from piecewise analysis. ETX = end of treatment.
c: THERAPIST vs. COMPUTER is significant (9M: t(1.98)=2.02, p=0.04)
Marijuana Problems Scale scores showed similar declines from intake to the end of treatment for all conditions (Figure 4). No differences between THERAPIST and COMPUTER were observed during the follow-up periods. At 3 months, both THERAPIST and COMPUTER had lower mean scores than BRIEF. From end of treatment to the 3-months, THERAPIST showed a further decrease that significantly differed from changes observed in BRIEF, but from 3 to 9 BRIEF showed a significant decrease. At 9 months there were no treatment group differences.
Figure 4.
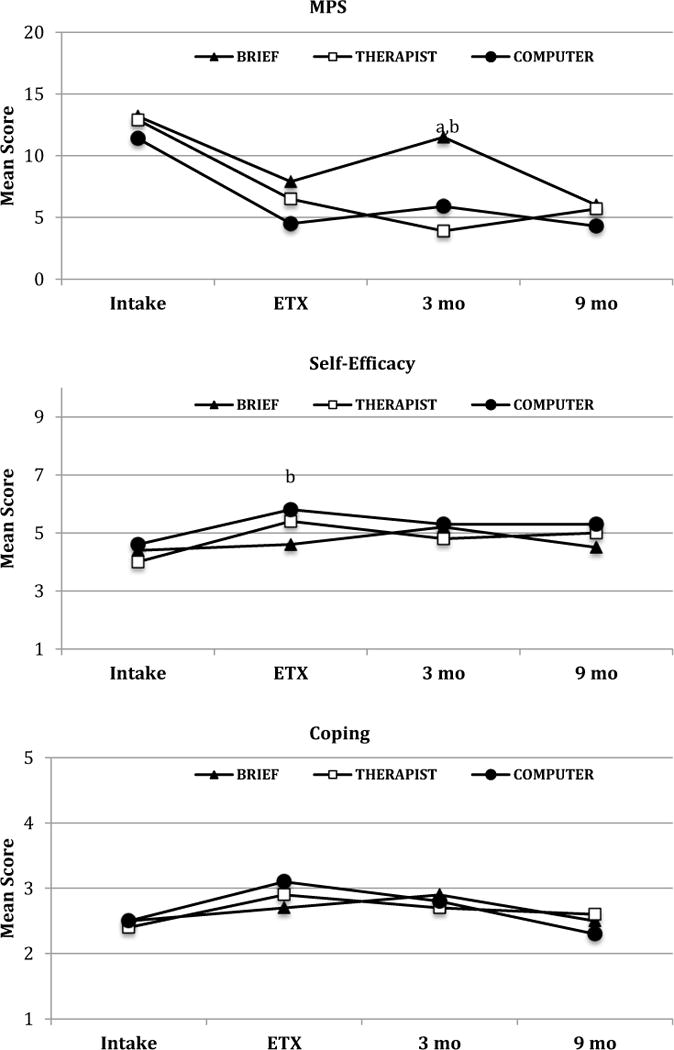
MPS, Self-efficacy, and Coping mean scores from piecewise analysis. MPS = Marijuana Problem Scale; ETX = end of treatment.
a: BRIEF vs. THERAPIST mean difference is significant (MPS 3M: t(173)=−2.82, p=0.005)
b: BRIEF vs. COMPUTER mean difference is significant (MPS 3M: t(173)=−2.13, p=0.03; Self-efficacy 3M: t(1.72)=2.03, p=0.04)
Self-Efficacy Scale scores showed similar and significantly greater improvements during treatment in the THERAPIST and COMPUTER conditions relative to BRIEF (Figure 4). THERAPIST and COMPUTER scores did not differ from each other during the post-treatment periods, however, the THERAPIST scores did decrease significantly more than BRIEF from end of treatment to the 3-month follow-up. No significant changes and no differences among conditions were observed for the Total Coping Strategies Scale score across all time periods (Figure 4).
Cost Comparisons
Cost estimate comparisons and test statistics are presented in Table 4. Mean (SD) overall costs per participant were: THERAPIST = $427.1 (335.7); COMPUTER = $251.8 (251.1); BRIEF = $171.1 (103.5). Significant differences in overall costs were observed between THERAPIST and the other two conditions (p’s < .05), with no difference found between BRIEF and COMPUTER. The greater cost of therapist time in the THERAPIST relative to COMPUTER condition ($164.6 (100.5) vs. $35.5 (14.8): p < .001), accounted for most of the difference in total cost ($170) between these two conditions. These mean savings in therapist cost ($129) approximate the CM incentive costs ($140) in the COMPUTER condition.
Table 4.
Service Utilization: Therapist, Urinalysis, and Incentive Costs per Participant
| BRIEF | THERAPIST | COMPUTER | |
|---|---|---|---|
| Therapy | |||
| Time in Session (minutes) | 86.2 (35.9) | 241.4 (147.0) | 37.7 (16.1) |
| * Therapist Costa | $54.4 (20.3) | $164.6 (100.5) | $35.5 (14.8) |
| Urinalysis | |||
| Number of Tests | 10.4 (7.6) | 14.6 (9.9) | 12.2 (9.3) |
| Staff Cost (10 min per test) | $26.1 (18.9) | $36.6 (24.8) | $30.6 (23.3) |
| * Total Test Costb | $59.9 (43.5) | $83.9 (56.9) | $70.2 (53.5) |
| Incentives | |||
| Amount Earned | $46.6 (38.0) | $146.6 (168.2) | $114.9 (158.3) |
| Staff Cost (purchasing) | $10.2 (8.3) | $32.1 (36.8) | $25.1 (34.6) |
| * Total Incentive Costc | $56.8 (46.2) | $178.7 (205.0) | $140.0 (192.9) |
| Computer Use ($) | – | – | 6.0 (0.0) |
|
| |||
| Total Cost ($)d | 171.1 (103.5) | 427.1 (335.7) | 251.8 (251.1) |
Therapist cost is mean time spent in all sessions per participant plus 20 minutes per session attended for administrative time multiplied by therapist wage/fringe. Total urinalysis test cost is the cost of the testing supplies plus wage/benefits for staff time (10 minutes per test) multiplied by the number of tests attended per participant. Total incentive cost is the total amount earned by each participant plus wage/fringe benefits for staff time to purchase and administer incentives (administration costs were 5 hours per month distributed proportionately based on incentive earnings across all participants). Paired t-tests were performed for therapist cost, urinalysis cost, incentive cost, and total cost.
THERAPIST > BRIEF, t = 4.31, p < .001; COMPUTER < BRIEF, t = −3.61, p < .001; COMPUTER < THERAPIST, t = −6.96, p < .001
THERAPIST > BRIEF, t =1.47, p =.151; COMPUTER > BRIEF, t =0.66, p =.513; COMPUTER < THERAPIST, t = −0.95, p =.344
THERAPIST > BRIEF, t =2.33, p =.024; COMPUTER > BRIEF, t=1.69, p =0.098 COMPUTER < THERAPIST, t = −0.75, p =.459
THERAPIST > BRIEF, t =2.96, p < .01; COMPUTER > BRIEF, t=1.23, p =.227 COMPUTER < THERAPIST, t =−2.28, p =.027
Discussion
This trial supports and extends findings from prior studies of computer-assisted treatments for substance use disorders. First, both MET/CBT/CM conditions achieved better abstinence outcomes than the BRIEF comparison condition, demonstrating that both were efficacious for CUD. Second, THERAPIST and COMPUTER engendered comparable cannabis outcomes during treatment, replicating a prior quasi-experimental trial that compared the same interventions (Budney et al., 2011), and supporting results from trials that compared similar interventions targeting opioid use (Bickel et al., 2008; Marsch, Guarino, et al., 2014) and cannabis or alcohol use in persons with depression (Kay-Lambkin et al., 2011; Kay-Lambkin et al., 2009). Third, rate of relapse, i.e., change in abstinence over the post-treatment period, did not differ among the three conditions, which supports the hypothesis that treatment effects on cannabis abstinence over time would not differ between the MET/CBT/CM conditions. This observation, if replicated, provides a particularly important contribution to the effectiveness literature because some, but not all, prior studies of therapist-delivered MET/CBT/CM have demonstrated that the MET/CBT component enhanced maintenance of post-treatment abstinence outcomes engendered with CM (Budney et al., 2006; Carroll et al., 2006; Carroll et al., 2012; Kadden et al., 2007; Rawson et al., 2006). The present study design, however, did not include a CM alone condition, therefore it did not directly test the efficacy of MET/CBT separate from CM either during or post-treatment, as done in prior studies. Nonetheless, demonstrating that using a computer rather than a therapist to deliver the MET/CBT in the MET/CBT/CM condition did not lead to worse outcomes over time offers potential and opportunity for advancing implementation of this previously demonstrated efficacious treatment model.
Prior to discussing implications, a few important limitations of this study warrant mention. First, this was a small, randomized trial, and missing data (urine specimens) both during and post-treatment assessment data was substantial. The impact of missing specimens particularly on LDA raises concern that this outcome measure might reflect retention more so than abstinence; however, given that both the THERAPIST and COMPUTER condition included incentives for providing negative specimens (motivation to attend if abstinent), retention did not differ among these conditions, and the observation that participants with larger amounts of missing data were much more likely to test positive for cannabis when they did provide a urine specimen, the LDA measure likely provides a valid indicator of abstinence for comparison. Because the post-treatment abstinence outcomes observed with the computer-assisted intervention reflect the first observation of maintenance effects comparable to therapist-delivered MET/CBT within a treatment model that included CM, cautious interpretation is warranted until replication in a larger trial with a more complete data set is obtained. Of note, two prior studies that did not include CM, reported a positive maintenance effect of computer-assisted CBT (Carroll et al., 2014; Kay-Lambkin et al., 2009), suggesting optimism that the present findings reflect a reliable maintenance effect.
Related to this limitation, the current trial used only two therapists, which limited the breadth of the comparison of the therapist and computer delivered interventions. The training, supervision, and monitoring procedures were designed to maintain a high level of treatment integrity, however, we did not quantify the quality of the MET/CBT. We employed community-based therapists rather than graduate students, fellows, or psychologists highly trained in MET/CBT to better represent the quality of therapy that might occur in a typical community setting. Anecdotally, based on tape review and supervision, the impression of the supervisors (DW, AJB, PCB) was that MET/CBT delivery might best be rated as “adequate”, in contrast to “very good” or “outstanding”. Hence, higher quality MET/CBT might result in more potent outcomes.
Another important limitation was the approach used to estimate comparative costs of the two MET/CBT/CM conditions. As our primary interest was in demonstrating the efficacy of COMPUTER relative to THERAPIST, we did not perform a formal cost-effectiveness evaluation because of the small scope of this trial. Instead, we focused on a simple cost-offset hypothesis to increase interest in implementation studies: would the savings in therapist costs per participant be sufficient to cover the cost of the incentives? The cost analysis relied on a clinic perspective and focused on service utilization using data from the clinical trial. We did not include costs of physical settings for the two interventions, as these were similar for both groups. We also did not include sunk costs (software development or therapist MET/CBT education and training), costs associated with software to deliver MET/CBT, therapist or computer replacement or maintenance (continuing education) costs, or costs from the patient perspective; note that some of these costs would vary greatly dependent on treatment clinic size (i.e., number of persons served). All such costs are important considerations for overall cost-effectiveness, and should be included in future larger scale comparative studies.
Notwithstanding these limitations, the observation that COMPUTER can produce comparable outcomes to THERAPIST at a potential cost savings in therapist time (estimated at $129 per case in this small study) has important implications for adoption of incentive-based CM programs (Budney et al., 2011; Carroll, 2014; Olmstead et al., 2010). Cost of MET/CBT and CM (incentives and their delivery) has been a primary barrier to dissemination to community clinics (Carroll, 2014). Based on our initial estimates, the potential reduced costs engendered with computer-assisted delivery could offset a substantial proportion of the costs associated with therapist implementation of CM and CBT, mitigating a major concern with broader implementation. Moreover, even lower cost incentive delivery models, such as using remotely loaded debit cards, can be explored to reduce administrative cost and burden associated with CM.
More generally, computer-assisted interventions like the COMPUTER can increase access to treatment by allowing more flexible scheduling of sessions because therapist and patient schedules do not need to be coordinated. From the clinic perspective, the computer does not go on vacation or require medical leave, and from the patient perspective, rescheduling due to job, family, or illness could be more readily accomplished with the computer than a therapist. For example, average time to complete MET/CBT sessions was lower in COMPUTER (therapist: 34.9–65.5 minutes/session; computer: 9.8–37.1 minutes/session). From a patient perspective, this time saving implies less time cost to the participants as well. Moreover, web-based programs have the potential to at least partially be delivered at remote sites (e.g., patient’s home), and thus transportation issues that limit access could be reduced. That said, it warrants mention that the intervention model tested here, and similar successful computer-assisted models, have included in-person contact with supportive staff, which appears important for obtaining good outcomes (Newman et al., 2011a, 2011b).
This study adds to promising findings demonstrating that computer- or web-based treatment adaptations have the potential to improve access to evidence-based care, to reduce costs, and to improve efficiency of delivery. Nonetheless, much work remains to advance the potential public health impact of such findings. Replications must confirm positive findings, and provide more comprehensive cost estimates, and test generality across diverse patient populations. Implementation studies must identify models of integration into diverse community settings. What appears clear at this time is that the myriad of possibilities for utilizing technological tools infuses new hope for enhancing services delivery and bettering the mental health of those with substance use and co-occurring disorders.
Acknowledgments
Funding for this study was provided by NIH-NIDA grants R01-DA023526 and T32-DA022981; the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. AJB and CS designed the study, wrote the protocols, and directed the study. JMT designed and analyzed the cost component of the study. PCB assisted with management and supervision of the study protocols. ES, ZI, and ZI performed the statistical analyses. DDW provided training and supervision to the therapist and feedback on the development of the computer modules. All authors contributed to the writing of the manuscript.
We thank Michael Grabinski of HealthSim, Inc., who assisted with the development of the computer software and provided technical assistance for the computer-assisted treatment. We also thank Patricia Costello who assisted with all aspects of the conduct of the study.
Footnotes
Conflict of Interest: All authors declare no conflicts of interest.
References
- Bickel WK, Marsch LA, Buchhalter AR, Badger GJ. Computerized behavior therapy for opioid-dependent outpatients: a randomized controlled trial. Experimental and Clinical Psychopharmacology. 2008;16(2):132–143. doi: 10.1037/1064-1297.16.2.132. doi: 2008-03846-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ. Marijuana dependence and its treatment. NIDA Science & Practice Perspectives. 2007;4(1):4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Fearer S, Walker DD, Stanger C, Thostensen J, Grabinski MJ, Bickel WK. An initial trial of a computerized behavioral intervention for cannabis use disorder. Drug Alcohol Dependence. 2011;115:74–79. doi: 10.1016/j.drugalcdep.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping-skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology. 2000;68:1051–1061. doi: 10.1037/0022-006X.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. Journal of Consulting and Clinical Psychology. 2006;74(2):307–316. doi: 10.1037/0022-006X.74.2.307. [DOI] [PubMed] [Google Scholar]
- Campbell AN, Nunes EV, Matthews AG, Stitzer M, Miele GM, Polsky D, Ghitza UE. Internet-delivered treatment for substance abuse: a multisite randomized controlled trial. American Journal of Psychiatry. 2014;171(6):683–690. doi: 10.1176/appi.ajp.2014.13081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM. Lost in translation? Moving contingency management and cognitive behavioral therapy into clinical practice. Annals of the New York Academy of Sciences. 2014;1327(1):94–111. doi: 10.1111/nyas.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Nuro KF, Rounsaville BJ. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. American Journal of Psychiatry. 2008;165(7):881–888. doi: 10.1176/appi.ajp.2008.07111835. doi: appi.ajp.2008.07111835 [pii]10.1176/appi.ajp.2008.07111835 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Rounsaville BJ. Enduring effects of a computer-assisted training program for cognitive behavioral therapy: a 6-month follow-up of CBT4CBT. Drug and Alcohol Dependence. 2009;100(1–2):178–181. doi: 10.1016/j.drugalcdep.2008.09.015. doi: S0376-8716(08)00360-8 [pii]10.1016/j.drugalcdep.2008.09.015 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Rounsaville BJ. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. Journal of Consulting and Clinical Psychology. 2006;74(5):955–966. doi: 10.1037/0022-006X.74.5.955. doi: 2006-13014-016 [pii]10.1037/0022-006X.74.5.955 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, Gordon MA, Portnoy GA, Marino DR, Ball SA. Computer-assisted delivery of cognitive-behavioral therapy: efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.2013.13070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Lapaglia DM, Peters EN, Easton CJ, Petry NM. Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less. Addiction. 2012;107(9):1650–1659. doi: 10.1111/j.1360-0443.2012.03877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Sifry RL, Nuro KF, Frankforter TL, Ball SA, Rounsaville BJ. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Copeland J, Martin G. Web-based interventions for substance use disorders: a qualitative review. Journal of Substance Abuse Treatment. 2004;26(2):109–116. doi: 10.1016/S0740-5472(03)00165-X. doi: 10.1016/S0740-5472(03)00165-XS074054720300165X [pii] [DOI] [PubMed] [Google Scholar]
- Danovitch I, Gorelick DA. State of the art treatments for cannabis dependence. Psychiatric Clinics of North America. 2012;35(2):309–326. doi: 10.1016/j.psc.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis C, Lavie E, Fatseas M, Auriacombe M. Psychotherapeutic interventions for cannabis abuse and/or dependence in outpatient settings. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD005336.pub2. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165(2):179–187. doi: 10.1176/appi.ajp.2007.06111851. doi: appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. CRC Monographs on Statistics and Applied Probability. New York: CRC Press/Chapman and Hall; 1993. An Introduction to the Bootstrap. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured clinical interview for DSM-IV axis I disorders–patient edition SCID I/P. New York, NY: Biometrics Research Department: New York State Psychiatric Institute; 1995. [Google Scholar]
- French MT, Roebuck MC, McLellan AT. Cost estimation when time and resources are limited: The brief DATCAP. Journal of Substance Abuse Treatment. 2004;27:187–193. doi: 10.1016/j.jsat.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Hester RK, Delaney HD. Behavioral Self-Control Program for Windows: results of a controlled clinical trial. Journal of Consulting and Clinical Psychology. 1997;65(4):686–693. doi: 10.1037//0022-006x.65.4.686. [DOI] [PubMed] [Google Scholar]
- Hester RK, Delaney HD, Campbell W. The college drinker’s check-up: outcomes of two randomized clinical trials of a computer-delivered intervention. Psychology of Addictive Behaviors. 2012;26(1):1–12. doi: 10.1037/a0024753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addictive Behaviors. 2007;32(6):1220–1236. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay-Lambkin FJ, Baker AL, Kelly B, Lewin TJ. Clinician-assisted computerised versus therapist-delivered treatment for depressive and addictive disorders: a randomised controlled trial. Medical Journal of Australia. 2011;195(3):S44–50. doi: 10.5694/j.1326-5377.2011.tb03265.x. [DOI] [PubMed] [Google Scholar]
- Kay-Lambkin FJ, Baker AL, Lewin TJ, Carr VJ. Computer-based psychological treatment for comorbid depression and problematic alcohol and/or cannabis use: a randomized controlled trial of clinical efficacy. Addiction. 2009;104(3):378–388. doi: 10.1111/j.1360-0443.2008.02444.x. doi: ADD2444 [pii]10.1111/j.1360-0443.2008.02444.x. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers’ beliefs and objections regarding contingency management: implications for dissemination. Drug and Alcohol Dependence. 2006;85(1):19–27. doi: 10.1016/j.drugalcdep.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kilmer B. Policy designs for cannabis legalization: starting with the eight Ps. American Journal of Drug and Alcohol Abuse. 2014;40(4):259–261. doi: 10.3109/00952990.2014.894047. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Petry NM. Behavioral treatment for marijuana dependence: Randomized trial of contingency management and self-efficacy enhancement. Addictive Behaviors. 2013;38(3):1764–1775. doi: 10.1016/j.addbeh.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Stephens RS. Coping and self-efficacy in marijuana treatment: results from the marijuana treatment project. Journal of Consulting and Clinical Psychology. 2005;73(6):1015–1025. doi: 10.1037/0022-006X.73.6.1015. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Tennen H. The nature of coping in treatment for marijuana dependence: latent structure and validation of the Coping Strategies Scale. Psychol Addict Behav. 2012;26(4):791–800. doi: 10.1037/a0026207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce B, Manning W, Siegel J, Lipscomb J. Estimating costs in cost-effectiveness analysis. In: Gold M, Siegel J, Russell LB, Weinstein MC, editors. Cost-Effectiveness inHealth and Medicine. New York, Oxford: Oxford University Press; 1996. pp. 176–213. [Google Scholar]
- Marijuana Treatment Project Research Group. Brief treatments for cannabis dependence: findings from a randomized multisite trial. Journal of Consulting and Clinical Psychology. 2004;72:455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK. Efficacy of computer-based HIV/AIDS education for injection drug users. American Journal of Health Behavior. 2004;28(4):316–327. doi: 10.5993/ajhb.28.4.3. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Carroll KM, Kiluk BD. Technology-based interventions for the treatment and recovery management of substance use disorders: A JSAT special issue. Journal of Substance Abuse Treatment. 2014;46(1):1–4. doi: 10.1016/j.jsat.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Dallery J. Advances in the psychosocial treatment of addiction: the role of technology in the delivery of evidence-based psychosocial treatment. Psychiatric Clinics of North America. 2012;35(2):481–493. doi: 10.1016/j.psc.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Grabinski MJ, Bickel WK, Desrosiers A, Guarino H, Muehlbach B, Acosta M. Computer-assisted HIV prevention for youth with substance use disorders. Substance Use and Misuse. 2011;46(1):46–56. doi: 10.3109/10826084.2011.521088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Guarino H, Acosta M, Aponte-Melendez Y, Cleland C, Grabinski M, Edwards J. Web-based behavioral treatment for substance use disorders as a partial replacement of standard methadone maintenance treatment. Journal of Substance Abuse Treatment. 2014;46(1):43–51. doi: 10.1016/j.jsat.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrone P, Knapp M, Proudfoot J, Ryden C, Cavanagh K, Shapiro DA, Tylee A. Cost-effectiveness of computerised cognitive-behavioural therapy for anxiety and depression in primary care: randomised controlled trial. British Journal of Psychiatry. 2004;185:55–62. doi: 10.1192/bjp.185.1.55. [DOI] [PubMed] [Google Scholar]
- McGovern MP, Fox TS, Xie H, Drake RE. A survey of clinical practices and readiness to adopt evidence-based practices: Dissemination research in an addiction treatment system. Journal of Substance Abuse Treatment. 2004;26(4):305–312. doi: 10.1016/j.jsat.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Moore BA, Fazzino T, Garnet B, Cutter CJ, Barry DT. Computer-based interventions for drug use disorders: a systematic review. Journal of Substance Abuse Treatment. 2011;40(3):215–223. doi: 10.1016/j.jsat.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. Journal of Substance Abuse Treatment. 2005;28(1):19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Newman MG, Szkodny LE, Llera SJ, Przeworski A. A review of technology-assisted self-help and minimal contact therapies for anxiety and depression: is human contact necessary for therapeutic efficacy? Clinical Psychology Review. 2011a;31(1):89–103. doi: 10.1016/j.cpr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Newman MG, Szkodny LE, Llera SJ, Przeworski A. A review of technology-assisted self-help and minimal contact therapies for drug and alcohol abuse and smoking addiction: is human contact necessary for therapeutic efficacy? Clinical Psychology Review. 2011b;31(1):178–186. doi: 10.1016/j.cpr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Olmstead TA, Ostrow CD, Carroll KM. Cost-effectiveness of computer-assisted training in cognitive-behavioral therapy as an adjunct to standard care for addiction. Drug and Alcohol Dependence. 2010;110(3):200–207. doi: 10.1016/j.drugalcdep.2010.02.022. doi: S0376-8716(10)00100-6 [pii]10.1016/j.drugalcdep.2010.02.022 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Schuster CR. Computer-based brief intervention a randomized trial with postpartum women. American Journal of Preventive Medicine. 2007;32(3):231–238. doi: 10.1016/j.amepre.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biological methods. Totowa, NJ: Human Press; 1992. pp. 41–72. [Google Scholar]
- Steinberg KL, Roffman R, Carroll KM, McRee B, Babor TF, Miller M, Stephens R. Brief counseling for marijuana dependence: A manual for treating adults 2005 [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000;68:898–908. [PubMed] [Google Scholar]
- SAMHSA. Treatment episode data set (TEDS): 2002–2012. National admissions to substance abuse treatment services. Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; 2014. [Google Scholar]
- Taylor CB, Luce KH. Computer- and internet-based psychotherapy interventions. Current Directions in Psychological Science. 2003;12(1):18–22. [Google Scholar]
- Tonigan JS, Miller WR, Brown JM. The reliability of Form 90: An instrument for assessing alcohol treatment outcome. Journal of Studies on Alcohol. 1997;58:358–364. doi: 10.15288/jsa.1997.58.358. [DOI] [PubMed] [Google Scholar]