Abstract
The association between serum zinc level and preeclampsia (PE) remains controversial. A systematic literature search was performed in PubMed, Web of Science and Embase for relevant available articles. The articles were limited to those in English from January 1990 to April 2015. Observational studies evaluating the association between serum zinc level and PE were included. The I2 was used to assess heterogeneity and the random effect model (REM) was adopted as the pooling method. The pooled standard mean difference (SMD) with 95% confidence interval (CI) was used to estimate the association between serum zinc level and PE. Seventeen observational studies were included. Compared with healthy pregnancy controls, PE patients have lower serum zinc level in 14 studies about total PE (SMD (95% CI): −0.587 (−0.963, −0.212), Z = 3.06, p for Z = 0.002; I2 = 88.4%, p for I2 < 0.0001). In subgroup analysis, a lower serum zinc level in PE patients compared with healthy pregnancy controls was observed in studies conducted in Asia, studies with zinc level measured in serum, and studies involving fasting participants. The SMD did not differ significantly between studies with healthy pregnancy controls matched by individual age (yes or no), and by individual gestational age (yes or no), respectively. Results from this meta-analysis indicate that serum zinc level in PE patients is significantly lower than that in healthy pregnancy controls. A moderate amount of zinc supplementation during pregnancy is advocated to reduce the incidence of PE.
Keywords: zinc, Zn, preeclampsia, meta-analysis
1. Introduction
Preeclampsia (PE) is a progressive, multisystemic disorder developing after 20 weeks of gestation in women with previously normal blood pressure. PE is a syndrome defined by hypertension (blood pressure of 140 mmHg systolic or higher or 90 mmHg diastolic or higher) and proteinuria [1]. The incidence of PE in pregnancies ranges from 2% to 8% in the world [2,3,4]. The World Health Organization reported that PE is a major reason of mother and fetus morbidity and mortality [5]. The complications of PE are the third leading cause of pregnancy-related deaths [6,7].
PE is caused by multiple factors, and some studies have indicated that PE is associated with an imbalance of increased lipid peroxides (LPO) and decreased antioxidants [8,9]. As an antioxidant trace metal, zinc deficiency may cause increasing lipid peroxidation [10]. Many studies attempted to explore the relationship between the changes of serum zinc level in pregnant women and PE, but the results were conflicting. Some studies had discovered significantly lower levels of serum zinc in PE patients than in the control group [11,12,13]. However, other studies found mean serum level of zinc was significantly higher in PE patients than in healthy pregnancy controls [14,15,16]. Meanwhile, some studies found that the serum zinc concentrations were not significantly different between the PE patients and the healthy pregnancy controls [17,18,19]. Therefore, we performed this meta-analysis to assess the relationship between serum zinc level and PE.
2. Materials and Methods
We referred to Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines for reporting of meta analyses [20].
2.1. Literature Search and Selection
We performed a systematic literature search from January 1990 to April 2015 using the databases of PubMed, Web of Science and Embase literature databases. The following search terms “zinc”, “Zn” and “preeclampsia” were used to search the English related articles without other restrictions. Moreover, we also reviewed the references of the included studies and review articles to identify additional studies which were not captured by our database searches.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) Observational study designs; (2) Diagnosis of PE patients was in accordance with the criteria of the American College of Obstetricians and Gynecologists (ACOG) [1]; (3) The blood sample was venous blood and the time of blood collection was medium or late pregnancy; (4) Data of the serum zinc level was available in the results and the data were presented as mean ± standard deviation (SD); (5) Serum zinc level were detected by using atomic absorption spectrometry; (6) The controls were healthy pregnancy controls. We excluded the studies if the data of serum zinc level was higher than 10 times of normalhigh limit [21]. If the units of measurement were not given, the study was also excluded.
All identified studies were carefully reviewed independently by two investigators to determine whether an individual study was eligible for inclusion criteria in this meta-analysis.
2.3. Data Extraction
The following data were extracted: the mean ± SD on serum zinc level, first author’s name, publication year, country where the study was performed, mean age, mean gestational age, sample size, whether the participants were fasting or not, the matching of potential confounders and other information. The data of different groups according to the illness severity were also extracted. If the standard error mean (SEM) of zinc level was given in the study, the SD is calculated by the following formula: SEM = SD/. Because the underlying units of measurement varied among studies, all units were converted to μmol/L [21].
Data were extracted independently by two investigators with disagreements resolved through discussion. The Newcastle-Ottawa quality assessment scale was used to assess the study quality [22].
2.4. Statistical Analysis
All statistical analyses were performed using standard mean difference (SMD) with 95% CI to assess the strength of association between serum zinc level and PE. The SMD is the ratio of the mean difference to the pooled standard deviation. The I2 was used to assess heterogeneity and the random effect model (REM) was adopted to calculate the pooled SMD. Meta-regression was performed to assess the potentially important covariates that might exert substantial impacts on between-study heterogeneity.
An influence analysis was performed with one study removed at a time to assess whether the results could be affected markedly by a single study. Small-study effect was investigated with funnel plot and Egger test. All statistical analyses were performed with Stata 12.0 (Stata Corporation, College Station, TX, USA). All reported probabilities (p values) were two-sided with p < 0.05 considered statistically significant.
3. Results
3.1. Characteristics of Studies
The detailed steps of our literature search were shown in Figure 1. We identified 17 relevant articles [11,12,13,17,23,24,25,26,27,28,29,30,31,32,33,34,35] in this meta-analysis. There were 3 case-control studies [11,33,34] and 14 cross-sectional studies. The pooled subjects included a total of 725 healthy pregnancy controls and 700 PE cases. Thirteen articles [11,12,13,23,24,25,26,27,28,29,30,31,32] reported the results for total PE (without information for disease severity), and 3 articles [33,34,35] reported the results for mild PE and severe PE, and 1 article [17] reported the results for total PE, mild PE and severe PE. The study quality ranged from 7 stars (5 articles) to 8 stars (12 articles) (Table S1). The characteristics of included articles are shown in Table 1.
Figure 1.
Flow diagram of the literature search.
Table 1.
Characteristics of 17 including studies.
| Author [Ref.] (Year) |
Country Continent |
Study Design | Group | n | Mean Level of Serum Zinc (μmol/L) | SD (μmol/L) | p | Sample Type | Fasting | Age (Years, Mean ± SD) | Gestational Age (Weeks, Mean ± SD) | Match of Potential Confounders |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarwar, M.S.; [11] (2013) |
Bangladesh (Asia) |
case-control study | Control PE |
58 50 |
15.08 11.85 |
3.54 5.38 |
p < 0.001 | serum | 8 h fasting condition | 25.76 ± 0.73 25.46 ± 0.85 |
36.79 ± 0.27 35.32 ± 0.37 |
matching for gestational period |
| Rafeeinia, A.; [17] (2014) |
Iran (Asia) |
cross-sectional study | Control total PE control mild PE sever PE |
50 50 50 35 15 |
11.23 10.92 11.08 10.62 12.00 |
5.08 4.00 0.62 0.62 1.23 |
p = 0.76 p = 0.71 |
serum | overnight fast | 27.10 ± 4.6 26.50 ± 3.9 27.18 ± 4.6 27.0 ± 4.1 25.40 ± 3.5 |
31.50 ± 3.60 30.80 ± 3.30 26.41 ± 5.0 23.82 ± 4.60 28.02 ± 7.90 |
|
| Fenzl, V.; [24] (2013) |
Croatia (Europe) |
cross-sectional study | Control PE |
37 30 |
8.85 9.23 |
1.43 1.43 |
NS | serum | overnight fast | 30.8 31.2 |
37.42 36.55 |
|
| Farzin, L.; [13] (2012) |
Iran (Asia) |
cross-sectional study | Control PE |
60 60 |
15.48 11.77 |
3.10 2.71 |
p < 0.001 | serum | yes | 26.66 ± 3.72 27.43 ± 3.91 |
35.27 ± 1.20 35.48 ± 1.14 |
matched for age, gestational age, anthropometrics and socioeconomic status |
| Adam, B.; [29] (2001) |
Turkey (Asia) |
cross-sectional study | Control PE |
20 20 |
5.25 4.82 |
0.68 0.72 |
NS | plasma | no | 27 ± 6.8 29 ± 8 |
37 ± 3.9 35 ± 4 |
matched for age, gestational age |
| Ilhan, N.; [12] (2002) |
Turkey (Asia) |
cross-sectional study | Control PE |
30 21 |
19.26 12.76 |
3.73 4.45 |
p < 0.001 | plasma | overnight fast | 19–31 | 31–38 | |
| Kolusari, A.; [28] (2008) |
Turkey (Asia) |
cross-sectional study | Control PE |
48 47 |
0.20 0.16 |
0.06 0.07 |
NS | serum | overnight fast | 27.92 ± 4.25 27.91 ± 5.21 |
35.41 ± 1.62 34.87 ± 2.34 |
|
| Atamer, Y.; [27] (2005) |
Turkey (Asia) |
cross-sectional study | Control PE |
28 32 |
16.71 12.18 |
3.06 2.77 |
NS | serum | overnight fast | 25.85 ± 3.36 27.00 ± 3.89 |
36.53 ± 3.15 35.68 ± 2.94 |
|
| Borella, P.; [30] (1990) |
Italy (Europe) |
cross-sectional study | Control PE |
35 24 |
9.60 10.49 |
2.29 2.28 |
NS | plasma | yes | 29–40 | ||
| Akhtar, S.; [31] (2011) |
Bangladesh (Asia) |
cross-sectional study | Control PE |
30 60 |
17.74 13.88 |
1.03 2.42 |
p < 0.001 | serum | no | 25.20 ± 4.85 25.11 ± 5.66 |
31.53 ± 3.90 32.35 ± 3.53 |
ageand gestational period matched |
| Akinloye, O.; [23] (2010) |
Nigeria (Africa) |
cross-sectional study | Control PE |
40 49 |
9.40 8.60 |
0.80 1.40 |
p < 0.05 | serum | no | age-matched | ||
| Ahsan, T.; [25] (2013) |
Bangladesh (Asia) |
cross-sectional study | Control PE |
27 44 |
15.00 16.00 |
2.00 2.00 |
p = 0.560 | serum | no | 24.11 ± 4.93 26.05 ± 5.41 |
36.23 ± 2.64 35.60 ± 3.85 |
demographically well matched |
| Rathore, S.; [26] (2011) |
India (Asia) |
cross-sectional study | Control PE |
47 14 |
8.85 7.57 |
3.32 2.74 |
NS | serum | no | 19–35 | age-matched | |
| Ugwuja, E.I.; [32] (2010) |
Nigeria (Africa) |
cross-sectional study | Control PE |
40 40 |
10.87 9.97 |
10.30 9.74 |
p = 0.686 | plasma | no | 27.55 ± 4.23 29.45 ± 3.70 |
21.40 ± 3.22 | matched for age, gestational age, parity, anthropometrics andsocioeconomic status |
| Gupta, S.; [33] (2014) |
India (Asia) |
case-control study | Control mild PE sever PE |
75 47 18 |
10.63 10.46 9.28 |
1.82 2.05 1.63 |
NS p < 0.01 |
plasma | no | |||
| Araujo Brito, J.; [34] (2013) |
Brazil (America) |
case-control study | Control mild PE sever PE |
50 20 24 |
7.43 7.69 5.97 |
1.28 1.45 1.26 |
NS p < 0.05 |
plasma | fasting for at least 12 h | 24.13 ± 6.43 27.00 ± 6.59 |
39.17 ± 1.76 36.30 ± 3.01 |
|
| Jain, S.; [35] (2010) |
India (Asia) |
cross-sectional study | Control mild PE sever PE |
50 25 25 |
15.64 12.72 12.04 |
2.40 1.70 1.40 |
p < 0.05 p < 0.05 |
serum | no | 23.92 ± 3.42 23.04 ± 3.76 22.96 ± 3.81 |
33.62 ± 7.83 34.92 ± 3.54 35.08 ± 3.60 |
age-matched |
Abbreviations: SD: standard deviation; PE: preeclampsia; NS: nosignificant.
3.2. Serum or Plasma Zinc Level and PE
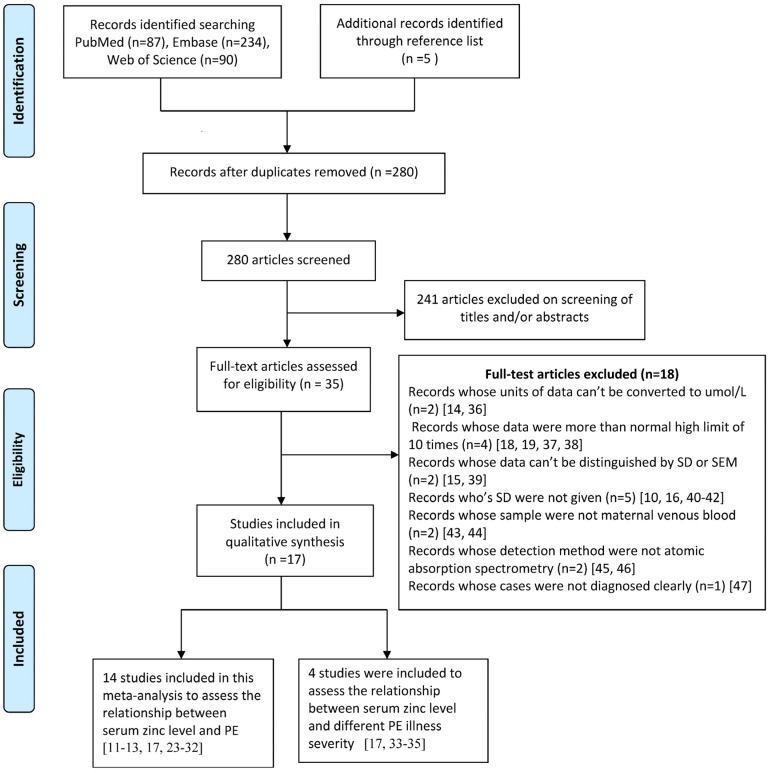
Fourteen articles reported the results for total PE compared with healthy pregnancy controls, PE patients have lower serum zinc level (SMD (95% CI): −0.587 (−0.963, −0.212), Z = 3.06, p for Z = 0.002; I2 = 88.4%; p for I2 < 0.0001) (Figure 2).
Figure 2.
Forest plot of standard mean difference (SMD) with corresponding 95% confidence interval (CI) of studies on zinc levels in total preeclampsia (PE) and healthy pregnancy controls. The size of grey box is positively proportional to the weight assigned to each study, and horizontal lines represent the 95% CIs.
In subgroup analysis, the pooled SMD for studies conducted in Asia was −0.812 (95% CI: (−1.263, −0.362), p for Z < 0.0001). The pooled SMD was −0.636 (95% CI: (−1.131, −0.141), p for Z = 0.012) in studies involving fasting participants. When we stratified studies by different types of sample, the pooled SMD was −0.637 (95% CI: (−1.080, −0.195), p for Z = 0.005) in studies with zinc level measured in serum. In stratified analysis by status of healthy pregnancy controls matched by individual age, the pooled SMD was −0.634 (95% CI: (−1.213, −0.056), p for Z = 0.032). The pooled SMD was −0.678 (95% CI: (−1.325, −0.030), p for Z = 0.040) in stratified analysis by status of healthy pregnancy controls matched by individual gestational age. The results of subgroup analysis are shown in Table 2.
Table 2.
Subgroup analyses of zinc level and preeclampsia (PE).
| Subgroup | Number of Studies | SMD (95% CI) | Test of SMD = 0 | Heterogeneity | Article Included | ||
|---|---|---|---|---|---|---|---|
| Z | p for Z | I2 | p for I2 | ||||
| Continent | |||||||
| Asia Europe Africa |
10 2 2 |
−0.812 (−1.263, −0.362) 0.323 (−0.033, 0.678) −0.389 (−0.971, 0.194) |
3.54 1.78 1.31 |
0.0001 0.075 0.191 |
88.4 0.0 72.2 |
0.0001 0.734 0.058 |
[11,12,13,17,25,26,27,28,29,31] [24,30] [23,32] |
| Sample type | |||||||
| plasma serum |
4 10 |
−0.460 (−1.246, 0.325) −0.637 (−1.080, −0.195) |
1.15 2.82 |
0.251 0.005 |
87.7 89.4 |
0.0001 0.0001 |
[12,29,30,32] [11,13,17,23,24,25,26,27,28,31] |
| Fasting status | |||||||
| yes no |
8 6 |
−0.636 (−1.131, −0.141) −0.522 (−1.159, 0.115) |
2.52 1.61 |
0.012 0.108 |
89.2 89.4 |
0.0001 0.0001 |
[11,12,13,17,24,27,28,30] [23,25,26,29,31,32] |
| Individual age match | |||||||
| yes no |
7 7 |
−0.634 (−1.213, −0.056) −0.540 (−1.060, −0.019) |
2.15 2.03 |
0.032 0.042 |
89.9 88.2 |
0.0001 0.0001 |
[13,23,25,26,29,31,32] [11,12,17,24,27,28,30] |
| Individual gestational age match | |||||||
| yes no |
6 8 |
−0.678 (−1.325, −0.030) −0.516 (−0.983, −0.050) |
2.05 2.17 |
0.040 0.030 |
91.5 86.0 |
0.0001 0.0001 |
[11,13,25,29,31,32] [12,17,23,24,26,27,28,30] |
Abbreviations: SMD: standard mean difference; CI: confidence interval.
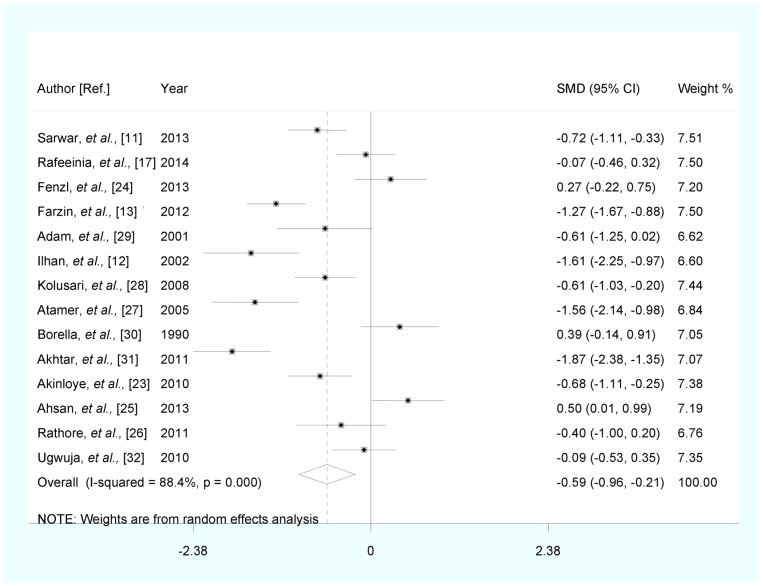
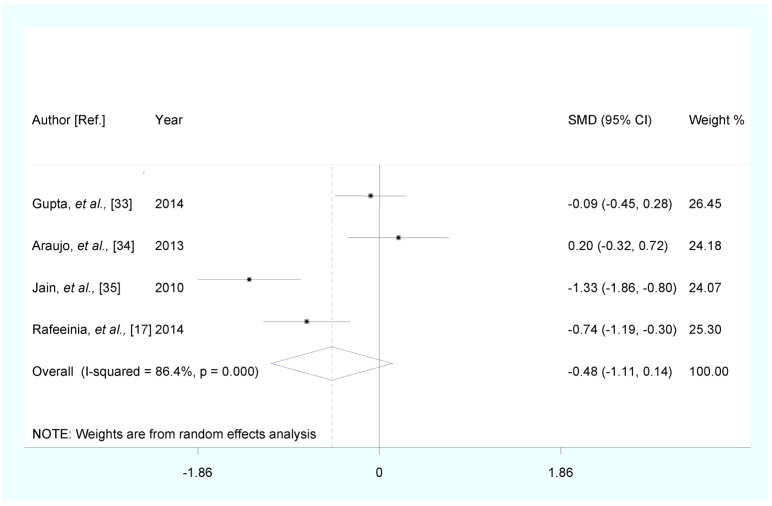
Stratified analysis by PE disease severity showed that, compared with healthy pregnancy controls, the pooled SMD was −0.484 (95% CI: (−1.105, 0.137), Z = 1.53, p for Z = 0.126; I2 = 86.4%; p for I2 < 0.0001) for mild PE, and −0.618 (95% CI: (−1.750, 0.515), Z = 1.07, p for Z = 0.285; I2 = 94.1%; p for I2 < 0.0001) for severe PE (Figure 3 and Figure 4).
Figure 3.
Forest plot of standard mean difference (SMD) with corresponding 95% CI of studies on zinc levels in mild PE and healthy pregnancy controls. The size of greybox is positively proportional to the weight assigned to each study, and horizontal lines represent the 95% CIs.
Figure 4.
Forest plot of standard mean difference (SMD) with corresponding 95% confidence interval (CI) of studies on zinc levels in severe preeclampsia (PE) and healthy pregnancy controls. The size of grey box is positively proportional to the weight assigned to each study, and horizontal lines represent the 95% CIs.
3.3. Meta-Regression
Strong evidence of heterogeneity among studies was found (Figure 2). However, the P values from univariate meta-regression analysis with the covariates of publication year, continent, sample type, fasting status of participants, individual age match, individual gestational age match and quality assessment were 0.930, 0.216, 0.710, 0.790, 0.832, 0.718 and 0.874, respectively. The results showed that no above-mentioned covariates conferred significant impact on between-study heterogeneity. The results of meta-regression are shown in Table S2.
3.4. Influence Analysis and Small-Study Effect Evaluation
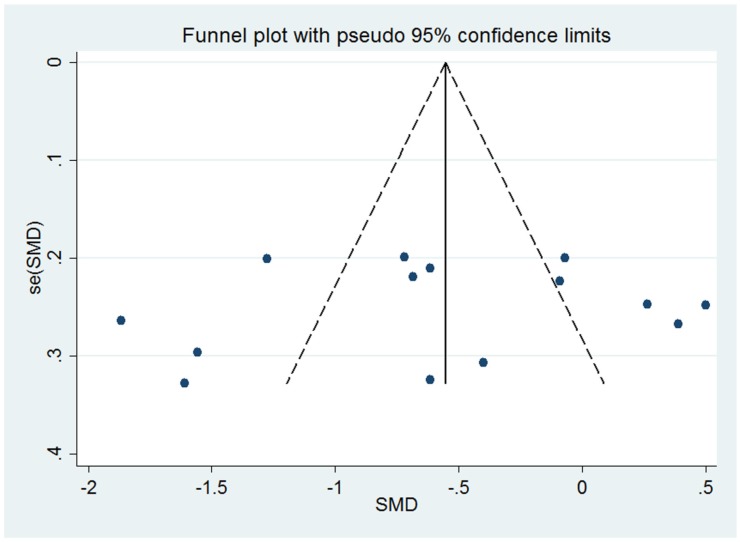
In influence analyses, we excluded 1 study at a time to assess the stability of the results. There was no significant change in the pooled SMD on excluding any of the studies (SMD lied between −0.671 and −0.488). This means no individual study had an excessive influence on the pooled effect between serum zinc level and PE. The visual inspection of the funnel plot was symmetrical (Figure 5). The Egger test showed no evidence of significant small-study effect for the analysis between serum zinc level and PE for all included studies (p = 0.621).
Figure 5.
Funnel plot for the analysis of serum zinc level and preeclampsia (PE).
We also conducted the above-mentioned analysis with weighted mean difference (WMD). The results of pooled WMD were consistent with those of SMD. The details of pooled WMD are shown in Supplementary Table S3.
4. Discussion
Our meta-analysis contained 17 articles, including 725 healthy pregnancy controls and 700 PE patients. The result of 14 articles about total PE identified that serum zinc level in PE patients was significantly lower than that in healthy pregnancy controls. In subgroup analysis, the lower serum zinc level in PE patients compared with healthy pregnancy controls was observed in studies conducted in Asia, studies with zinc level measured in serum, and studies involving fasting participants. The SMD did not differ significantly between studies with healthy pregnancy controls matched by age (yes or no), and by gestational age (yes or no), respectively. The serum zinc levels were lower in the PE patients compared with healthy pregnant controls in studies conducted in Europe and Africa, studies with zinc level measured in plasma, and studies involving participants without fasting, but the results were not statistically significant. Our meta-analysis didn’t find significant difference between serum zinc level and mild PE or severe PE, which might be caused by limited number of included studies.
The mechanisms underlying the association between serum zinc level and PE are still not fully understood. One underlying explanation for our findings is that zinc can alleviate oxidative stress by increasing antioxidants or serving as essential substrates or cofactors for the adequate activation of antioxidant enzymes, such as superoxide dismutase (SOD) [11,48,49]. Zinc is a cofactor of the antioxidant enzyme SOD [49], thus its deficiency may lead to the decrease of SOD, which was associated with impairment of the cell antioxidant capacity and oxidant/antioxidant balance [10].
Cu-Zn SOD, the cytosolic form of enzyme, provide important antioxidant defense [50]. The deficiency of zinc has a negative effect on Cu-Zn SOD enzyme system [51]. Impaired Cu-Zn SOD activity contributes to the oxidative damage in the body which may worsen several disease states [52]. The main way that cells counteract free radical damage is by the increased expression of Cu-Zn SOD [34,53,54]. The decreased of Cu-Zn SOD activity may affect the scavenging of free radical and led to oxidative stress and lipid peroxidation [53,54]. Oxidative stress can induce apoptosis [55]. Exaggerated apoptosis may prevent supply of syncytiotrophoblast, promotesyncytial degeneration and release inflammatory mediators into the maternal circulation [56]. This would impair the placentation process and finally to diffuse maternal endothelial cell dysfunction [57]. All these may lead to the development of PE. Furthermore, Yousef et al. found that zinc deficiency can cause an increase in lipid peroxidation [58]. Zinc deficiency may cause the imbalance between lipid peroxides (LPO) and antioxidants by above mentioned ways, which might promote the occurrence and development of PE.
Between-study heterogeneity was found in our meta-analysis between serum zinc level and PE. We carried out meta-regression but did not find the covariates of publication year, continent, sample type, fasting status, age match and gestational age match as the important contributors to the between-study heterogeneity. Therefore, we speculated that the potential contributors to the conflicting results could be: (1) the included studies were different in blood sample handling methods and preservation methods; (2) the potential confounders adjusted in each study were diverse.
As a meta-analysis of published studies, our study has several strengths. First, the large numbers of participants allowed a much greater possibility of reaching reasonable conclusions and conducting subgroup analysis. Second, all included studies had accounted for potential confounders such as age and gestational age and additional factors, which can reduce the effects of confounding factors. Third, we adopted random effect model to calculate the pooled SMD between serum zinc level and PE; therefore, the results were more reasonable and convincing. Fourth, the Newcastle-Ottawa quality assessment scale was used to assess thestudy quality, and all studies met a quality score of 7 stars or more. The results indicated that the quality of original articles was generally good. Fifth, the physiological decrease of serum zinc level may occur during pregnancy; then original articles which we included in this meta-analysis chose healthy pregnancy women as controls. Sixth, serum zinc level was significantly lower in PE patients compared with healthy pregnancy controls in studies involving fasting participants. Fasting conditions can accurate reflect the metabolism of zinc. Seventh, the results of pooled SMD were consistent with those of pooled WMD, suggesting the results of our meta-analysis were credible.
However, our study also has limitations. First, although the detection methods of serum zinc level were atomic absorption method, the testing instruments and testing conditions varied among studies; this may influence the detection results. Second, our meta-analysis didn’t find significant difference between serum zinc level and mild PE (n = 4) or severe PE (n = 4), which might be caused by the limited number of included studies and the limited sample size. Further research is needed to confirm the relation between serum zinc level and the disease severity of PE. Third, we were unable to explore the dose-response relationship between serum zinc level and PE because of the limitation of the data.
5. Conclusions
In summary, results from this meta-analysis showed that serum zinc level in PE patients was significantly lower than that in healthy pregnancy women. A moderate amount of zinc supplementation may reduce the incidence of PE, which needs to be confirmed.
Acknowledgments
The conduct of this study was not funded.
Supplementary Files
Author Contributions
All authors contributed to the inception of the research question and study design. Yue Ma contributed to the study selection, data synthesis and data analysis, and manuscript composition. Xiaoli Shen contributed to the study selection, quality assessment, and records review. Dongfeng Zhang was responsible for the integrity of this work and contributed to the study design, final study selection and manuscript review. All authors contributed to drafting the manuscript and have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.ACOG Committee on Obstetric Practice Diagnosis and management of preeclampsia and eclampsia. Int. J. Gynecol. Obstet. 2002;1:67–75. [PubMed] [Google Scholar]
- 2.Walker J.J. Pre-eclampsia. Lancet. 2000;356:1260–1265. doi: 10.1016/S0140-6736(00)02800-2. [DOI] [PubMed] [Google Scholar]
- 3.Sibai B., Dekker G., Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)71003-5. [DOI] [PubMed] [Google Scholar]
- 4.Ghulmiyyah L., Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 2012;36:56–59. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Make Every Mother and Child Count. World Health Organization; Geneva, Switzerland: 2005. [Google Scholar]
- 6.Wagner L.K. Diagnosis and management of preeclampsia. Am. Fam. Phys. 2004;70:2317–2324. [PubMed] [Google Scholar]
- 7.Mackay A.P., Berg C.J., Atrash H.K. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet. Gynecol. 2001;97:533–538. doi: 10.1016/S0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 8.Ziaei S., Bonab K.M., Kazemnejad A. Serum lipid levels at 28–32 weeks gestation and hypertensive disorders. Hypertens. Pregnancy. 2006;25:3–10. doi: 10.1080/10641950500543756. [DOI] [PubMed] [Google Scholar]
- 9.Rumiris D., Purwosunu Y., Wibowo N., Farina A., Sekizawa A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertens. Pregnancy. 2006;25:241–253. doi: 10.1080/10641950600913016. [DOI] [PubMed] [Google Scholar]
- 10.Kumru S., Aydin S., Simsek M., Sahin K., Yaman M., Ay G. Comparison of serum copper, zinc, calcium, and magnesium levels in pre-eclamptic and healthy pregnant women. Biol. Trace Elem. Res. 2003;94:105–112. doi: 10.1385/BTER:94:2:105. [DOI] [PubMed] [Google Scholar]
- 11.Sarwar M.S., Ahmed S., Ullah M.S., Kabir H., Rahman G.K., Hasnat A., Islam M.S. Comparative study of serum zinc, copper, manganese, and iron in preeclamptic pregnant women. Biol. Trace Elem. Res. 2013;154:14–20. doi: 10.1007/s12011-013-9721-9. [DOI] [PubMed] [Google Scholar]
- 12.Ilhan N., Simsek M. The changes of trace elements, malondialdehyde levels and superoxide dismutase activities in pregnancy with or without preeclampsia. Clin. Biochem. 2002;35:393–397. doi: 10.1016/S0009-9120(02)00336-3. [DOI] [PubMed] [Google Scholar]
- 13.Farzin L., Sajadi F. Comparison of serum trace element levels in patients with or without pre-eclampsia. J. Res. Med. Sci. 2012;17:938–941. [PMC free article] [PubMed] [Google Scholar]
- 14.Harma M., Kocyigit A. Correlation between maternal plasma homocysteine and zinc levels in preeclamptic women. Biol. Trace Elem. Res. 2005;104:97–105. doi: 10.1385/BTER:104:2:097. [DOI] [PubMed] [Google Scholar]
- 15.Katz O., Paz-Tal O., Lazer T., Aricha-Tamir B., Mazor M., Wiznitzer A., Sheiner E. Severe pre-eclampsia is associated with abnormal trace elements concentrations in maternal and fetal blood. J. Matern. Fetal Neonatal Med. 2012;25:1127–1130. doi: 10.3109/14767058.2011.624221. [DOI] [PubMed] [Google Scholar]
- 16.Mahomed K., Williams M.A., Woelk G.B., Mudzamiri S., Madzime S., King I.B., Bankson D.D. Leukocyte selenium, zinc and copper concentration in preeclamptic and normotensive pregnant women. Biol. Trace Elem. Res. 2000;75:107–118. doi: 10.1385/BTER:75:1-3:107. [DOI] [PubMed] [Google Scholar]
- 17.Rafeeinia A., Tabandeh A., Khajeniazi S., Marjani A.J. Serum copper, zinc and lipid peroxidation in pregnant women with preeclampsia in Gorgan. Open Biochem. J. 2014;8:83–88. doi: 10.2174/1874091X01408010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golmohammad Lou S., Amirabi A., Yazdian M., Pashapour N. Evaluation of serum calcium, magnesium, copper, and zinc levels in women with pre-eclampsia. Iran. J. Med. Sci. 2008;33:235–238. [Google Scholar]
- 19.Vigeh M., Yokoyama K., Ramezanzadeh F., Dahaghin M., Sakai T., Morita Y., Kitamura F., Sato H., Kobayashi Y. Lead and other trace metals in preeclampsia: A case-control study in Tehran. Iran. Environ. Res. 2006;100:268–275. doi: 10.1016/j.envres.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviewsandmeta-analyses: The PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Young D.S. Implementation of SI unitsforclinical laboratory data: Style specifications and conversion tables. J. Nutr. Biochem. 1990;1:599–613. doi: 10.1016/0955-2863(90)90050-U. [DOI] [PubMed] [Google Scholar]
- 22.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [(accessed on 5 August 2015)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 23.Akinloye O., Oyewale O.J., Oguntibeju O.O. Evaluation of trace elements in pregnant women with pre-eclampsia. Afr. J. Biotechnol. 2010;9:5196–5202. [Google Scholar]
- 24.Fenzl V., Flegar-Mestric Z., Perkov S., Andrišić L., Tatzber F., Žarković N., Duić Ž. Trace elements and oxidative stress in hypertensive disorders of pregnancy. Arch. Gynecol. Obstet. 2013;287:19–24. doi: 10.1007/s00404-012-2502-4. [DOI] [PubMed] [Google Scholar]
- 25.Ahsan T., Banu S., Nahar Q., Ahsan M., Khan M.N., Islam S.N. Serum trace elements levels in preeclampsia and eclampsia: Correlation with the pregnancy disorder. Biol. Trace Elem. Res. 2013;152:327–332. doi: 10.1007/s12011-013-9637-4. [DOI] [PubMed] [Google Scholar]
- 26.Rathore S., Gupta A., Batra H.S., Rathore R. Comparative study of trace elements and serum ceruloplasmin level in normal and pre-eclamptic pregnancies with their cord blood. Biol. Trace Elem. Res. 2011;22:207–210. [Google Scholar]
- 27.Atamer Y., Kocyigit Y., Yokus B., Atamer A., Erden A.C. Lipid peroxidation, antioxidant defense, status of trace metals and leptin levels in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005;119:60–66. doi: 10.1016/j.ejogrb.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 28.Kolusari A., Kurdoqlu M., Yildizhan R., Adali E., Edirne T., Cebi A., Demir H., Yoruk I.H. Catalase activity, serum trace element and heavy metal concentrations, and vitamin A, D and E levels in pre-eclampsia. J. Int. Med. Res. 2008;36:1335–1341. doi: 10.1177/147323000803600622. [DOI] [PubMed] [Google Scholar]
- 29.Adam B., Malatyalioqle E., Alvur M., Talu C. Magnesium, zinc and iron levels in pre-eclampsia. J. Matern. Fetal Med. 2001;10:246–250. doi: 10.1080/jmf.10.4.246.250-14. [DOI] [PubMed] [Google Scholar]
- 30.Borella P., Szilagyi A., Than G., Csaba I., Giardino A., Facchinetti F. Maternal plasma concentrations of magnesium, calcium, zinc and copper in normal and pathological pregnancies. Sci. Total Environ. 1990;99:67–76. doi: 10.1016/0048-9697(90)90212-D. [DOI] [PubMed] [Google Scholar]
- 31.Akhtar S., Begum S., Ferdousi S. Calcium and zinc deficiency in preeclamptic women. J. Bangladesh Soc. Physiol. 2011;6:94–99. doi: 10.3329/jbsp.v6i2.9758. [DOI] [Google Scholar]
- 32.Ugwuja E.I., Ejikeme B.N., Ugwu N.C., Obeka N.C., Akubugwo E.I., Obidoa O. Comparison of plasma copper, iron and zinc levels in hypertensive and non-hypertensive pregnant women in Abakaliki, south Eastern Nigeria. Pak. J. Nutr. 2010;9:1136–1140. doi: 10.3923/pjn.2010.1136.1140. [DOI] [Google Scholar]
- 33.Gupta S., Jain N.P., Avasthi K., Wander G.S. Plasma and erythrocyte zinc in pre-eclampsia and its correlation with foetal outcome. J. Assoc. Physicians India. 2014;62:306–310. [PubMed] [Google Scholar]
- 34.Araujo Brito J., do Nascimento Marreiro D., Moita Neto J.M., Michelle Costa e Silva D., Gonçalves de Sousa Almondes K., Valadares Neto Jde D., do Nascimento Nogueira N. Enzyme activity of superoxide dismutase and zincemia in women with preeclampsia. Nutr. Hosp. 2013;28:486–490. doi: 10.3305/nh.2013.28.2.6179. [DOI] [PubMed] [Google Scholar]
- 35.Jain S., Sharma P., Kulshreshtha S., Mohan G., Singh S. The role of calcium, magnesium, and zinc in pre-eclampsia. Biol. Trace Elem. Res. 2010;133:162–170. doi: 10.1007/s12011-009-8423-9. [DOI] [PubMed] [Google Scholar]
- 36.Atarod Z., Roohanizadeh H., Saberi M., Hashemi S.A., Fazli M. Circulating levels of homocysteine, zinc, iron and copper in pregnant women with pre-eclampsia. HealthMed. 2012;6:3329–3332. [Google Scholar]
- 37.Vafaei H., Dalili M., Hashemi S.A. Serum concentration of calcium, magnesium and zinc in normotensive versus preeclampsia pregnant women: A descriptive study in women of Kerman province of Iran. Iran. J. Reprod. Med. 2015;13:23–26. [PMC free article] [PubMed] [Google Scholar]
- 38.Bahadoran P., Zendehdel M., Movahedian A., Zahraee R.H. The relationship between serum zinc level and preeclampsia. Iran. J. Nurs. Midwifery Res. 2010;15:120–124. [PMC free article] [PubMed] [Google Scholar]
- 39.Rezende V.B., Barbosa F., Jr., Palei A.C., Cavalli R.C., Tanus-Santos J.E., Sandrim V.C. Correlations among antiangiogenic factors and trace elements in hypertensive disorders of pregnancy. J. Trace Elem. Med. Biol. 2015;29:130–135. doi: 10.1016/j.jtemb.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Mistry H.D., Gill C.A., Kurlak L.O., Seed P.T., Hesketh J.E., Méplan C., Schomburg L., Chappell L.C., Morgan L., Poston L., et al. Association between maternal micronutrient status, oxidative stress, and common genetic variants in antioxidant enzymes at 15 weeks’ gestation in nulliparous women who subsequently develop preeclampsia. Free Radic. Biol. Med. 2002;33:40–47. doi: 10.1016/j.freeradbiomed.2014.10.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Díaz E., Halhali A., Luna C., Díaz L., Avila E., Larrea F. Newborn birth weight correlates with placental zinc, umbilical insulin-like growth factor I, and leptin levels in preeclampsia. Arch. Med. Res. 2002;33:40–47. doi: 10.1016/S0188-4409(01)00364-2. [DOI] [PubMed] [Google Scholar]
- 42.Abo-Elmatty D.M., Badawy E.A., Hussein J.S., Elela S.A., Megahed H.A. Role of heme oxygenase, leptin, coenzyme Q10 and trace elements in pre-eclamptic women. Indian J. Clin. Biochem. 2012;27:379–384. doi: 10.1007/s12291-012-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Negi R., Pande D., Karki K., Kumar A., Khanna R.S., Khanna H.D. Trace elements and antioxidant enzymes associated with oxidative stress in the pre-eclamptic/eclamptic mothers during fetal circulation. Clin. Nutr. 2012;31:946–950. doi: 10.1016/j.clnu.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Açikgoz S., Harma M., Harma M., Mungan G., Can M., Demirtas S. Comparison of angiotensin-converting enzyme, malonaldehyde, zinc, and copper levels in preeclampsia. Biol. Trace Elem. Res. 2006;113:1–8. doi: 10.1385/BTER:113:1:1. [DOI] [PubMed] [Google Scholar]
- 45.Al-Jameil N., Tabassum H., Al-Mayouf H., Aljohar H.I., Alenzi N.D., Hijazy S.M., Khan F.A. Analysis of serum trace elements-copper, manganese and zinc in preeclamptic pregnant women by inductively coupled plasma optical emission spectrometry: A prospective case controlled study in Riyadh, Saudi Arabia. Int. J. Clin. Exp. Pathol. 2014;7:1900–1910. [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J., Kim Y.J., Lee R., Moon J.H., Jo I. Serum levels of zinc, calcium, and iron are associated with the risk of preeclampsia in pregnant women. Nutr. Res. 2012;32:764–769. doi: 10.1016/j.nutres.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Magri J., Sammut M., Savona-Ventura C. Lead and other metals in gestational hypertension. Int. J. Gynecol. Obstet. 2003;83:29–36. doi: 10.1016/S0020-7292(03)00212-1. [DOI] [PubMed] [Google Scholar]
- 48.Roberts J.M., Balk J.L., Bodnar L.M., Belizán J.M., Bergel E., Martinez A. Nutrient involvement in preeclampsia. J. Nutr. 2003;133:1684S–1692S. doi: 10.1093/jn/133.5.1684S. [DOI] [PubMed] [Google Scholar]
- 49.Powell S.R. The antioxidant properties of zinc. J. Nutr. 2000;130:1447s–1454s. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 50.Ali Akbar S., Nicolaides K.H., Brown P.R. Measurement of Cu/Zn SOD in placenta, cultured cells, various fetal tissues, decidua and semen by ELISA. J. Obstet. Gynaecol. 1998;18:331–335. doi: 10.1080/01443619867056. [DOI] [PubMed] [Google Scholar]
- 51.Sun J.Y., Jing M.Y., Weng X.Y., Fu L.J., Xu Z.R., Zi N.T., Wang J.F. Effects of dietary zinc levels on the activities of enzymes, weights of organs, and the concentrations of zinc and copper in growing rats. Biol. Trace Elem. Res. 2005;107:153–165. doi: 10.1385/BTER:107:2:153. [DOI] [PubMed] [Google Scholar]
- 52.Schuessel K., Schäfer S., Bayer T.A., Czech C., Pradier L., Müller-Spahn F., Müller W.E., Eckert A. Impaired Cu/Zn-SOD activity contributes to increased oxidative damage in APP transgenic mice. Neurobiol. Dis. 2005;18:89–99. doi: 10.1016/j.nbd.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Llurba E., Gratacós E., Martín-Gallán P., Cabero L., Dominguez C. A comprehensive study of oxidative stress and antioxidant status in preeclampsia and normal pregnancy. Free Radic. Biol. Med. 2004;37:557–570. doi: 10.1016/j.freeradbiomed.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 54.Sharma J.B., Sharma A., Bahadur A., Vimala N., Satyam A., Mittal S. Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. Int. J. Gynaecol. Obstet. 2006;94:23–27. doi: 10.1016/j.ijgo.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 55.Payne C.M., Bernstein C., Bernstein H. Apoptosis overview emphasizing the role of oxidative stress, DNA damage and signal-transduction pathways. Leuk. Lymphoma. 1995;19:43–93. doi: 10.3109/10428199509059662. [DOI] [PubMed] [Google Scholar]
- 56.Sharp A.N., Heazell A.E., Crocker I.P., Mor G. Placental apoptosis in health and disease. Am. J. Reprod. Immunol. 2010;64:159–169. doi: 10.1111/j.1600-0897.2010.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jauniaux E., Poston L., Burton G.J. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum. Reprod. Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yousef M.I., El-Hendy H.A., El-Demerdash F.M., Elagamy E.I. Dietary zinc deficiency induced-changes in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology. 2002;175:223–234. doi: 10.1016/S0300-483X(02)00049-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.