A previously unidentified calcium-dependent mechanism contributes to light adaptation in mammalian rods.
Abstract
Sensory cells adjust their sensitivity to incoming signals, such as odor or light, in response to changes in background stimulation, thereby extending the range over which they operate. For instance, rod photoreceptors are extremely sensitive in darkness, so that they are able to detect individual photons, but remain responsive to visual stimuli under conditions of bright ambient light, which would be expected to saturate their response given the high gain of the rod transduction cascade in darkness. These photoreceptors regulate their sensitivity to light rapidly and reversibly in response to changes in ambient illumination, thereby avoiding saturation. Calcium ions (Ca2+) play a major role in mediating the rapid, subsecond adaptation to light, and the Ca2+-binding proteins GCAP1 and GCAP2 (or guanylyl cyclase–activating proteins [GCAPs]) have been identified as important mediators of the photoreceptor response to changes in intracellular Ca2+. However, mouse rods lacking both GCAP1 and GCAP2 (GCAP−/−) still show substantial light adaptation. Here, we determined the Ca2+ dependency of this residual light adaptation and, by combining pharmacological, genetic, and electrophysiological tools, showed that an unknown Ca2+-dependent mechanism contributes to light adaptation in GCAP−/− mouse rods. We found that mimicking the light-induced decrease in intracellular [Ca2+] accelerated recovery of the response to visual stimuli and caused a fourfold decrease of sensitivity in GCAP−/− rods. About half of this Ca2+-dependent regulation of sensitivity could be attributed to the recoverin-mediated pathway, whereas half of it was caused by the unknown mechanism. Furthermore, our data demonstrate that the feedback mechanisms regulating the sensitivity of mammalian rods on the second and subsecond time scales are all Ca2+ dependent and that, unlike salamander rods, Ca2+-independent background-induced acceleration of flash response kinetics is rather weak in mouse rods.
INTRODUCTION
Rod and cone photoreceptors adjust their sensitivity to light in response to changes in ambient illumination level, enabling vision over 10–log unit range of background light intensities. Rods can detect single photons in darkness, yet they remain functional in background lights, producing up to ∼104 visual pigment isomerizations s−1 per rod (Aguilar and Stiles, 1954; Naarendorp et al., 2010). This is enabled through light adaptation, which decreases the photoreceptors’ sensitivity and accelerates their response kinetics in response to increasing background light intensity, thus extending their operating range and avoiding saturation caused by the background light–driven activation. In amphibians, the feedback mechanisms regulating the gain of phototransduction appear to be mediated mainly by calcium ions (Nakatani and Yau, 1988; Fain et al., 1989). Ca2+ appears to play an important role also in mammalian rods, because genetic removal of guanylyl cyclase–activating proteins (GCAPs) compromises severely their light adaptation. However, contributions of Ca2+-dependent feedback mechanisms other than GCAPs, as well as of Ca2+-independent mechanisms to light adaptation in mammalian rods, remain unclear (Chen et al., 2010b).
Photon absorption by the visual pigment rhodopsin (R) transforms the pigment molecule to its active form R*, which can activate several G proteins (transducins). Active transducins can bind phosphodiesterase (PDE)6 to form a complex that hydrolyses cGMP. The subsequent decrease in cytoplasmic [cGMP] leads to the closure of CNG channels in the outer segment plasma membrane, reducing the inflow of Na+ and Ca2+. The continuing extrusion of Ca2+ by Na+/K+-Ca2+ exchangers results in lowering of the outer segment intracellular Ca2+ concentration ([Ca2+]i; Yau and Nakatani, 1984), which serves as a signal to several feedback mechanisms that extends the operating range of rods. The suggested Ca2+-feedback mechanisms shorten R* lifetime (Matthews et al., 2001; Chen et al., 2010a), accelerate cGMP synthesis by guanylyl cyclase (Koch and Stryer, 1988), and increase the CNG channel’s affinity to cGMP (Hsu and Molday, 1993). These feedback mechanisms are thought to be mediated through Ca2+-sensor proteins recoverin, GCAPs, and calmodulin, respectively. Of these, GCAPs play an important role in mammalian rod light adaptation. However, rods that do not express GCAPs can still regulate their sensitivity and phototransduction termination kinetics as a response to changes in background light intensity (Mendez et al., 2001; Burns et al., 2002). This residual adaptation appears not to be mediated by calmodulin (Chen et al., 2010b), but the role of recoverin is still controversial. Background light has been shown to accelerate response kinetics via recoverin (Chen et al., 2010a, 2012). However, the affinity of recoverin to Ca2+ seems to be too low compared with the physiological [Ca2+] range in rod outer segments (Chen et al., 1995; Klenchin et al., 1995; Woodruff et al., 2002), suggesting that Ca2+ feedback via recoverin may not be functional in physiological conditions. Moreover, deletion of recoverin does not affect the flash sensitivity of mouse rods (Makino et al., 2004; Chen et al., 2010b). Further, it has been demonstrated that the background light–triggered increase in the rates of both steady-state cGMP hydrolysis and synthesis together contribute significantly to sensitivity regulation of salamander rods in varying ambient illumination levels. Indeed, background light strongly modulates response kinetics and sensitivity of salamander rods as a result of increased cGMP hydrolysis rate, even when changes in [Ca2+]i have been prevented (Nikonov et al., 2000). It is not known how much these mechanisms modulate photoresponse kinetics and/or sensitivity of mammalian rods. Hence, the question remains: what are the mechanisms contributing to GCAP-independent light adaptation in mammalian rods, and are they Ca2+ dependent or not?
Our objective was to reveal the contribution of recoverin and other possible Ca2+-feedback mechanisms to mammalian rod light adaptation in the absence of the dominating effect of GCAPs. We found that exposing mouse rods lacking both GCAP1 and GCAP2 (GCAP−/−) to low [Ca2+] in darkness mimicked the effects of background light. Our experiments demonstrated significant GCAP-independent light adaptation. This was partly explained by the Ca2+-controlled recoverin pathway, demonstrating a direct Ca2+-dependent feedback through recoverin in mouse rods. However, some Ca2+-dependent light adaptation persisted in the absence of both GCAPs and recoverin. Our observation that photoresponse kinetics are only marginally modulated by this residual Ca2+-controlled mechanism suggests that it may not affect the rate of cGMP hydrolysis or synthesis. Our experimental results demonstrate that, in contrast to salamander rods, the Ca2+-independent mechanisms affect photoresponse kinetics only moderately in mouse rods. In summary, we identified a novel Ca2+-dependent light adaptation pathway that is operational in mammalian rod photoreceptors. This pathway appears to be almost exclusively mediated by Ca2+ and accounts for the residual fast sensitivity regulation in the absence of GCAPs and recoverin.
MATERIALS AND METHODS
Ethical approval
The use and handling of the animals were in accordance with the Finland Animal Welfare Act 1996 and guidelines of the Animal Experimentation Committee of University of Helsinki.
Transretinal electroretinography (ERG) experiments
WT mice, as well as GCAP−/− and Rv−/− mice (provided by J. Chen, University of Southern California, Los Angeles, CA; Mendez et al., 2001), were used in the experiments. The background strain of all mice was C57BL/6J. In addition, a double knockout (DKO; GCAP−/− Rv−/−) strain was produced by breeding the GCAP−/− and Rv−/− mice. Littermates from the GCAP+/− Rv+/− breeding pairs were used in experiments comparing GCAP−/− Rv+/+ and GCAP−/− Rv−/− mouse rod physiology.
The animals were sacrificed by CO2 inhalation and cervical dislocation, the eyes were enucleated and bisected along the equator, and the retinas were detached in cooled Ringer’s solution under dim red light. The isolated retina was placed in a specimen holder (Donner et al., 1988) with active recording area of 1.2-mm diameter. The upper (photoreceptor) side was superfused with a constant flow (∼3 ml/min) of Ringer’s solution. Experiments were conducted at 37°C in a medium containing (mM): 133.4 Na+, 3.3 K+, 2 Mg2+, 1 Ca2+, 142.7 Cl−, 10 glucose, 0.01 EDTA, and 12 HEPES, adjusted to pH 7.6 (at room temperature) with NaOH. 50 µM DL-AP4 and 50–100 µM BaCl2 were added to block synaptic transmission to second-order neurons, and the glial component was generated by K+ currents of Müller cells (Bolnick et al., 1979), respectively. In some experiments, 10 mM BaCl2 in contact with the proximal side of the retina was used instead of including barium in the perfusion, as described in Nymark et al. (2005). 0.72 mg/ml Leibovitz culture medium (L-15; Sigma-Aldrich) was added to improve the viability of the retina in all experiments. The temperature was controlled by a heat exchanger below the specimen holder and monitored with a thermistor in the bath close to the retina.
Recording and light stimulation
The transretinal potential was recorded with two Ag/AgCl pellet electrodes (EP2; World Precision Instruments): one in the subretinal space and the other in chloride solution connected to the perfusion Ringer’s solution through a porous plug. The DC signal was low-pass filtered (eight-pole Bessel; fc = 500 Hz) and sampled at 1,000 Hz with a voltage resolution of 0.25 µV. Light stimuli with homogeneous full-field illumination to the distal side of the retina were provided by a dual-beam optical system adapted from the setup used by Donner et al. (1988). In brief, 2-ms light flashes and/or longer light steps were generated with a 532-nm laser diode module (532 nm; ∼130 mW; IQ5C(532–100)L74; Power Technology, Inc.), a 633-nm HeNe laser (5 mW; 25 LHR 151; Melles Griot), and a Compur shutter for both laser paths, with the midpoint of the flash indicating the zero time for the recordings. The uniformity of the beam at the level of the retina was confirmed with a small aperture photodiode. The light intensity of each source was controlled separately with calibrated neutral density filters and wedges. The absolute intensity of the unattenuated laser beam (photons mm−2 s−1) incident on the retina was measured in each experiment with a calibrated photodiode (EG&G HUV-1000B; calibration by the National Standards Laboratory of Finland). The amount of isomerizations (R*) produced by the stimulating flash light in individual rods was calculated as described in Heikkinen et al. (2008).
Chemicals and pharmacological manipulations
All chemicals were purchased from Sigma-Aldrich. The low [Ca2+]-free (∼20 nM) solutions were prepared using EGTA, and the free [Ca2+] was calculated with an “EGTA calculator” (Portzehl et al., 1964) taking into account 2 mM [Mg2+] and 66 µM [Ca2+] (from 0.72 g/L L-15 supplement) present in our Ringer’s solution. pH was adjusted to 7.6 with NaOH.
Analysis
The Weber–Fechner relation commonly used to quantify the background light’s effect on rod sensitivity does not fit the light-adaptation data of GCAP−/− mouse rods. We used the following modified version, called here the Weber–Hill function,
| (1) |
where sF is flash sensitivity during background light, defined as dim-flash response amplitude divided by flash strength (µV R*−1); sF,D is the flash sensitivity in darkness; I is background light intensity (R* s−1); and n describes the slope of adaptation curve decay. In the standard Weber–Fechner function, n is 1 and larger n indicates narrower operating range of rods. The parameter I0 corresponds then to the sensitivity halving background light intensity.
We compared our light-adaptation data with two theoretical functions that describe how sensitivity would decay as a function of background light in the absence of any light adaptation. First, a traditional exponential saturation function,
| (2) |
where rsat is the amplitude of a saturated rod response, and ti is the integration time defined as the area of dim-flash response divided by its amplitude. The second function is the result of removing all feedback-regulation mechanisms from a phototransduction model (Chen et al., 2010b),
| (3) |
A phototransduction activation model (Lamb–Pugh [LP] model; Lamb and Pugh, 1992) was used to quantify the gain of the phototransduction activation. We fitted early parts of the negative-going leading edge of flash responses with a delayed Gaussian function,
| (4) |
where Φ is flash energy in R* per rod, td is a short delay, and A is the amplification constant describing the gain of the activation reactions in s−2.
RESULTS
Background light regulates rod sensitivity in the absence of both GCAPs and recoverin
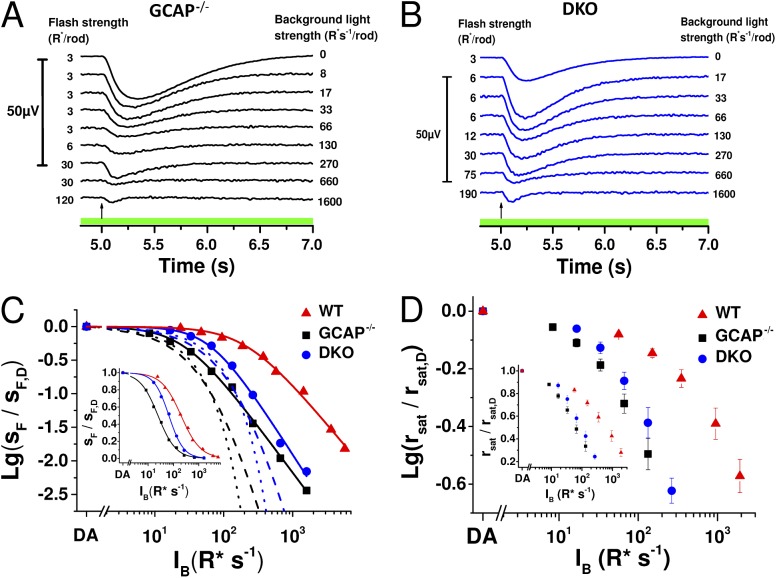
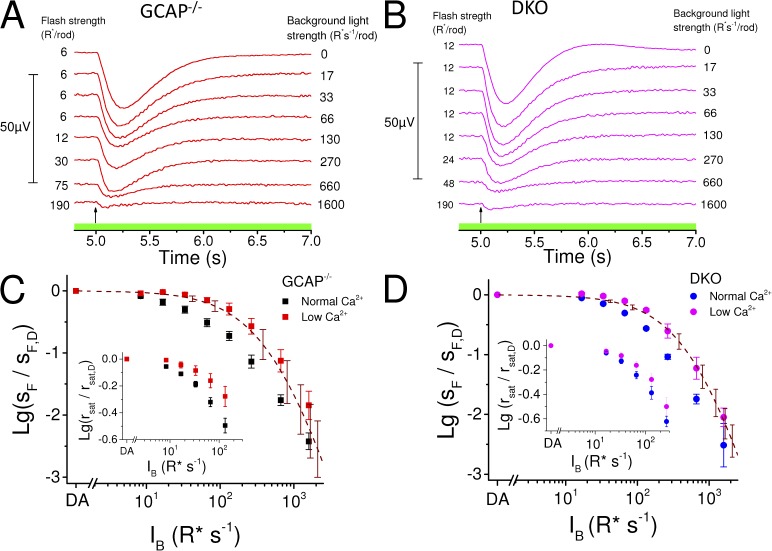
Previous studies have shown that background light modulates sensitivity and response kinetics also in GCAP−/− mouse rods, which lack a major mediator of the light-adaptation process (Chen et al., 2010b; Nymark et al., 2012). We studied the origins of this GCAP-independent light adaptation with transretinal ERG by recording dim-flash responses of GCAP−/− and GCAP−/− Rv−/− (DKO) mouse rods in darkness and during light steps of varying intensity (Fig. 1, A and B). Plotting the rod flash sensitivity (in µV R*−1) normalized to the sensitivity in darkness as a function of background light revealed that, as has been shown previously (Mendez et al., 2001), deleting GCAPs shifts the operating range of rods to significantly dimmer light and steepens the slope of the adaptation curve. Consistent with previous data (Makino et al., 2004), deletion of recoverin shifted the operating range of GCAP−/− rods to brighter light and further steepened the slope of their adaptation curve (Fig. 1 C and Table 1). The change in the slope of the adaptation curve is highlighted in the linear plot of the sensitivity data (Fig. 1 C, inset). The rightward shift of the adaptation curve by deletion of recoverin might seem surprising, as it indicates that DKO rods can actually function in brighter light than GCAP−/− rods. However, the shift is expected because removal of recoverin leads to a recoverin-free rhodopsin kinase that can maximally inactivate R*s and result in lower sensitivity and shorter integration time of the DKO rods (see Fig. 5). Hence, equal background light will cause weaker phototransduction activation in DKO as compared with GCAP−/− rods, effectively shifting the adaptation curve of the DKO mice to brighter backgrounds. However, steeper slope of the adaptation curve indicates that compared with GCAP−/− rods, the DKO mouse rods have a compressed range of background lights over which they can operate. The extent of adaptation mechanisms present can also be evaluated by comparing the experimental data to the theoretical decline of sensitivity in the absence of any adaptation mechanism except response compression caused by decreasing maximal response amplitude with increasing background light intensity. Dotted and dashed traces in Fig. 1 C show two functions modeling the expected decay of rod sensitivity in the absence of active feedback mechanisms (see Materials and methods for details). In these extreme situations without any light adaptation (except response compression), simple saturation of the phototransduction cascade caused by the background light activation predicts a steeper decay of sensitivity than that observed in our experimental data, even when either GCAPs or both GCAPs and recoverin have been removed from mouse rods. We also studied modulation of the steady-state CNG channel current between WT, GCAP−/−, and DKO rods by plotting the saturated response amplitude rsat as a function of background light intensity (Fig. 1 D). Similarly to sensitivity data, removal of GCAPs shifted the data points to dimmer background light intensities, whereas removal of recoverin in GCAP−/− mice caused a rightward shift to brighter backgrounds. These data demonstrate that both GCAPs and recoverin can regulate the steady-state CNG channel current during background light. Collectively, our results suggest that both the recoverin-dependent and currently unidentified recoverin-independent pathways modulate the operating range of mammalian rods in the absence of GCAP-mediated regulation of cGMP synthesis. In subsequent experiments, we aimed to determine whether these pathways are Ca2+ dependent and how much they contribute to regulation of the rod sensitivity and response kinetics.
Figure 1.
Deletion of recoverin narrows the operating range of GCAP−/− rods. Dim-flash responses (in microvolts; see scale bar on the left) recorded in darkness and during steps of light from isolated GCAP−/− (A; IB range: 8–1,600 R* s−1 per rod) and DKO (B; IB range: 17–1,600 R* s−1 per rod) mouse retinas. Flash strengths in darkness and under each background are indicated by numbers preceding each response, and the background light intensity is given on the right. Timing of the flash, 5 s after the background onset, is indicated by an arrow in each panel. (C) Sensitivity as a function of background light intensity (IB), normalized to sF,D, for a representative retina in GCAP−/− (black squares), DKO (blue circles), and WT (red triangles) mice. Smooth curves plot Eq. 1 with I0 = 27 R* s−1 per rod, n = 1.4; I0 = 65 R* s−1 per rod, n = 1.6; and I0 = 218 R* s−1 per rod, n = 1.2 in GCAP−/−, DKO, and WT mouse, respectively. Dotted and dashed lines plot Eqs. 2 and 3, respectively, for GCAP−/− (black) and for DKO (blue) mice. The inset shows the sensitivity data in linear scale and best-fitting functions (Eq. 1) with parameter values: I0 = 24 R* s−1 per rod, n = 1.2; I0 = 66 R* s−1 per rod, n = 1.5; and I0 = 199 R* s−1 per rod, n = 1.1 in GCAP−/−, DKO, and WT mouse, respectively. (D) Saturated response amplitude (rsat) normalized to the rsat in darkness as a function of IB in WT (red), GCAP−/− (black), and DKO (blue) mouse rods. Error bars represent mean ± SEM (n = 7).
Table 1.
Characteristics of WT, GCAP−/− and DKO mouse rods
| Parameter | Solution | WT (n = 4) | GCAP−/− (n = 9) | GCAP−/− Rv+/+ (n = 4) | DKO (n = 3) |
| rmax (µV) | 1 mM Ca2+ | 379 ± 98 | 165 ± 20 | 145 ± 27 (n = 7) | 156 ± 8 |
| Low Ca2+ | 556 ± 145 | 171 ± 23 | 154 ± 14 (n = 7) | 170 ± 12 | |
| SF,D (% R*−1) | 1 mM Ca2+ | 1.7 ± 0.6a | 4.5 ± 0.6a | 4.4 ± 0.7a,b | 2.8 ± 0.03a,b |
| Low Ca2+ | 0.3 ± 0.2a | 1.0 ± 0.2a | 1.4 ± 0.4a | 1.5 ± 0.2a | |
| tp (ms) | 1 mM Ca2+ | 156 ± 1a | 366 ± 9a | 369 ± 20a,c | 233 ± 7c |
| Low Ca2+ | 450 ± 40a | 267 ± 8a | 262 ± 19a | 231 ± 5 | |
| I0 (R*) | 1 mM Ca2+ | 181 ± 46/165 ± 43 | 28 ± 6a/39 ± 9a | 34 ± 9a/46 ± 11a,b | 65 ± 2d/68 ± 4b,d |
| Low Ca2+ | NA | 183 ± 46a/215 ± 54a | 131 ± 31a/187 ± 56a | 153 ± 20d/167 ± 33d | |
| n | 1 mM Ca2+ | 1.0 ± 0.1/1.1 ± 0.1 | 1.1 ± 0.05d/1.5 ± 0.1d | 1.1 ± 0.08d/1.6 ± 0.15d | 1.5 ± 0.03/1.7 ± 0.1d |
| Low Ca2+ | NA | 1.5 ± 0.1d/2.1 ± 0.2d | 1.4 ± 0.01d/2.0 ± 0.2d | 1.8 ± 0.3/2.0 ± 0.04d | |
| τD | 1 mM Ca2+ | 178 ± 21a | 231 ± 16 (n = 4) | 261 ± 19a (n = 7) | 200 ± 21 |
| ms | Low Ca2+ | 464 ± 54a | 191 ± 20 (n = 4) | 204 ± 11a (n = 7) | 200 ± 4 |
| A | 1 mM Ca2+ | 4.7 ± 1.2a | 7.9 ± 1.3 (n = 6) | 7.2 ± 1.4 (n = 7) | 11.3 ± 1 |
| s−2 | Low Ca2+ | 0.3 ± 0.1a | 6.2 ± 1.5 (n = 6) | 6.6 ± 1.3 (n = 7) | 9.8 ± 1 |
The GCAP−/− mice were divided into two groups: the GCAP−/− mice representing the original knockout strain, and the GCAP−/− Rv+/+ mice derived by crossing the GCAP−/− and Rv−/− strains. The latter were littermates to the DKO GCAP−/− Rv−/− mice. Parameter values for rods lacking GCAPs are given both in normal and low (∼20 nM) [Ca2+]. Parameters: rsat, saturated maximal rod response amplitude; SF,D, fractional dark-adapted sensitivity of rods to dim flashes, i.e., dim-flash response amplitude divided by rsat and flash strength in R*; tp, time from flash to the peak amplitude of a dim-flash response; I0 in R* s−1 per rod, sensitivity-halving background light intensity (see Eq. 1; values are given from fittings to linear/logarithmic data); n, slope of the modified Weber–Fechner function (see Eq. 1; values are given from fittings to linear/logarithmic data); τD, dominant time constant of saturated photoresponse recovery; A, amplification constant (Lamb and Pugh, 1992). Values are mean ± SEM, and the number of mice for each genotype is given in parentheses.
P < 0.005, paired Student’s t test is used to indicate statistical significance of low Ca2+ exposure in GCAP−/− and DKO mice.
P < 0.05, one-tailed Student’s t test is used to compare whether parameters between GCAP−/− Rv+/+ and DKO littermate mouse rods are different.
P = 0.003, one-tailed Student’s t test is used to compare whether parameters between GCAP−/− Rv+/+ and DKO littermate mouse rods are different.
P < 0.05, paired Student’s t test is used to indicate statistical significance of low Ca2+ exposure in GCAP−/− and DKO mice.
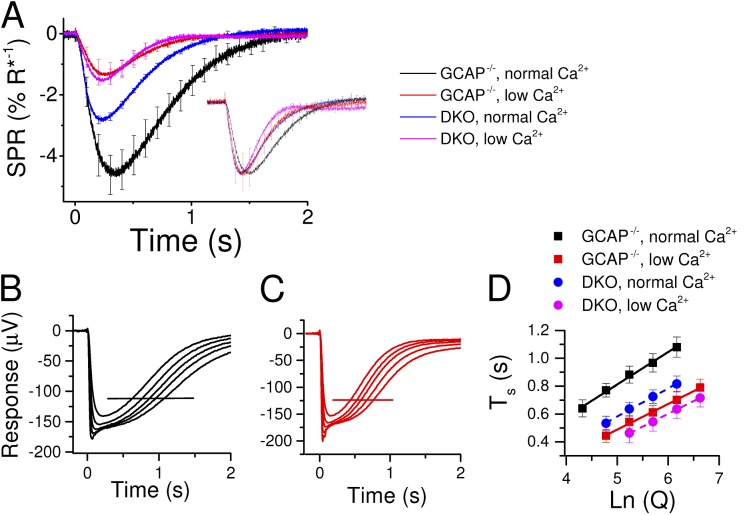
Figure 5.
Recoverin accounts for half of the desensitization caused by low Ca2+ exposure in GCAP−/− mouse rods and most changes in flash response kinetics. (A) Dim-flash responses (<20% of saturated response amplitude) normalized with the saturated response amplitude, and flash energy (in R* per rod) for GCAP−/− (n = 4) in normal (black) and low (red) Ca2+ solution, and for DKO (n = 3) mouse retinas in normal (blue) and low (magenta) Ca2+ solution. Inset shows the same responses normalized to their peak amplitudes. All data in A are mean ± SEM (B and C). Representative near-saturated and saturated responses recorded from dark-adapted GCAP−/− mouse retinas in normal (B; flash strength range: 990–6,300 R* per rod) and low (C; flash strength range: 630–4,000 R* per rod) Ca2+ solution. (D) Saturation times at 25% recovery as a function of flash strength in normal (black) and low (red) Ca2+ conditions from GCAP−/− (squares; n = 4) and in normal (blue) and low (magenta) Ca2+ conditions from DKO (circles; n = 4) mouse rod responses (mean ± SEM). The slopes of the fitted lines, τD, were 234 and 203 ms in normal [Ca2+] and 185 and 185 ms in low [Ca2+] for GCAP−/− and DKO mice, respectively. Error bars represent ± SEM.
Setting Ca2+-dependent feedback mechanisms to a steady level with low [Ca2+]o in WT and GCAP−/− rods
The role and significance of Ca2+-dependent feedback in light adaptation can in principle be studied by lowering outer segment Ca2+ concentration to mimic light-induced drop in [Ca2+]i. This can be achieved by reducing the extracellular Ca2+ concentration ([Ca2+]o) up to the level sufficient to essentially clamp the calcium-dependent mechanisms to their maximally light-adapted state. However, this method practically cannot be applied in the WT rods, as it leads to a highly increased [cGMP] in the rod outer segment as a result of the Ca2+-controlled acceleration of guanylyl cyclase activity via GCAPs. This, in turn, transiently yields a large CNG channel current that the cells cannot maintain, and eventually the light responses become very small (Yau et al., 1981). Exposure of rods to low [Ca2+]o is also accompanied with deceleration of light-response kinetics and large desensitization of photoreceptors that are consistent with elevated [cGMP] but at odds with the known effects of background light adaptation, such as moderate desensitization and acceleration of photoresponse termination (Lipton et al., 1977; Bastian and Fain, 1982; Matthews, 1995).
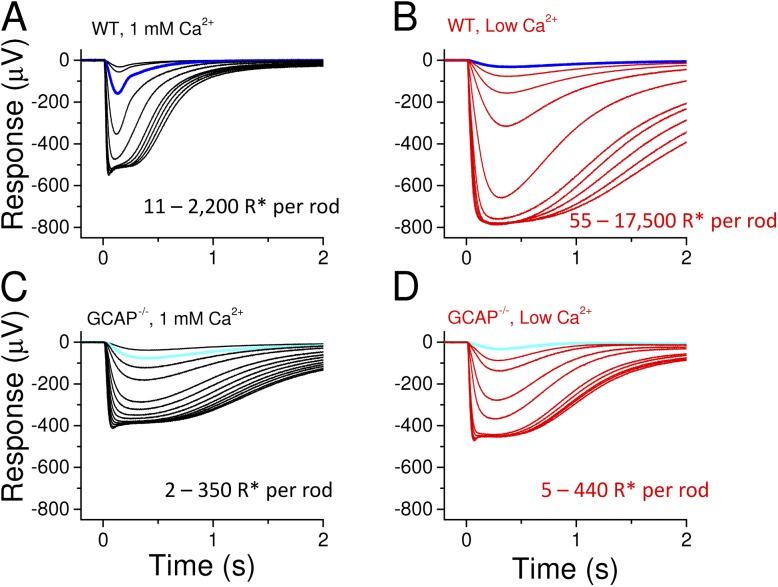
To overcome the problem of high cytoplasmic [cGMP] in low [Ca2+]o, we performed experiments on GCAP−/− mouse rods lacking the Ca2+ feedback on guanylyl cyclase activity. Based on previous biochemical and physiology experiments, these mice have normal cyclase activity and their saturated light response amplitudes are similar to WT rods, indicating that the steady-state Ca2+ levels are not affected by genetic removal of GCAPs (Mendez et al., 2001; Burns et al., 2002; Peshenko and Dizhoor, 2004; Nymark et al., 2012). We first compared the light responses of WT and GCAP−/− mouse rods under normal (1 mM) and low (∼20 nM) free [Ca2+]o. We chose a higher [Ca2+]o than the concentration used by Yau et al. (1981) to maintain stable response amplitudes. Yet this very low extracellular Ca2+ should be sufficiently low to reduce [Ca2+]i below the operating range of calcium-sensor proteins inside the rod outer segment, setting the rod phototransduction machinery to a steady state corresponding to the maximally light-adapted state in regard to the Ca2+-dependent feedback mechanisms. In WT mice, low Ca2+ exposure triggered an approximately fourfold increase of the saturated response amplitude (rsat) before gradually declining to a stable level, still about twice its value in normal Ca2+ (Fig. 2 A and Table 1). These dramatic increments of the photoresponse amplitude in low Ca2+ are not present in the GCAP−/− mouse rods, in which the maximal relative increase of rsat was much smaller, only ∼10% of that in WT mice (Fig. 2 B). Further, the steady-state rsat was somewhat larger but not statistically different in low Ca2+ than in normal Ca2+ in GCAP−/− or DKO mice (Table 1).
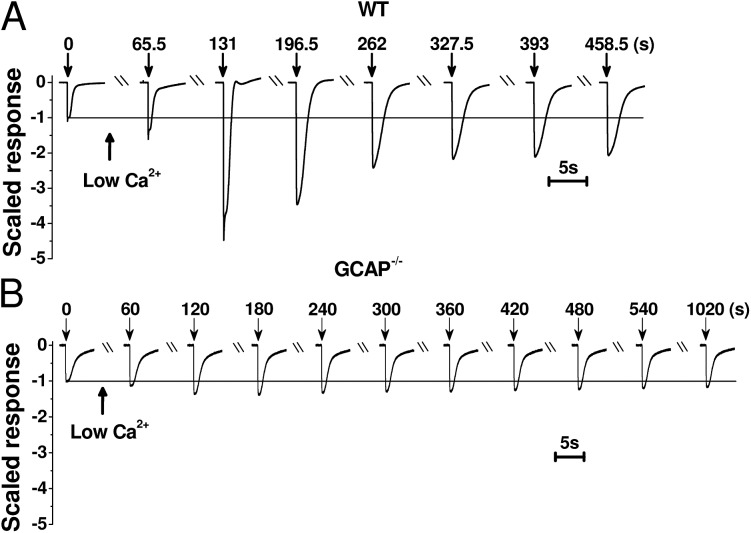
Figure 2.
Low Ca2+ exposure affects the photoresponse amplitudes dramatically in WT, but not in GCAP−/−, mice. Saturated rod responses recorded before and at different times during low Ca2+ exposure in representative isolated WT (A) and GCAP−/− (B) mouse retinas. Timing of the change to low Ca2+ solution and flashes are indicated by arrows together with the time (in seconds) from the first flash given just before the low Ca2+ exposure. Responses have been normalized to the amplitude in normal Ca2+ solution before the solution exchange.
Under steady-state conditions, about half an hour after the onset of low Ca2+ exposure, the saturated response amplitude in a representative WT retina remained ∼1.5-fold larger in low Ca2+ than in normal Ca2+ (Fig. 3, A and B). Furthermore, the fractional flash sensitivity was decreased by 10-fold, and the flash response kinetics were very slow, with the dim-flash response time-to-peak (tp) twice its value in normal Ca2+ conditions (see also Table 1). These results are consistent with earlier studies on amphibian rods (Lipton et al., 1977; Bastian and Fain, 1982; Matthews, 1995). Our transretinal recordings from GCAP−/− mice under standard conditions demonstrated slower response kinetics and higher sensitivity of rods lacking GCAPs as compared with WT mice (Fig. 3, A and C, and Table 1), in line with previous reports using a single-cell suction recording method (Mendez et al., 2001; Chen et al., 2010b; Nymark et al., 2012). In the original low Ca2+ experiments with WT mice, we measured larger maximum response amplitudes as compared with GCAP−/− or DKO mice (see Table 1), raising a possibility that, in contrast to previous reports, CNG channel current and steady-state Ca2+ levels would have been affected by deletion of GCAPs. To resolve this discrepancy, we performed a separate set of experiments from WT and GCAP−/− mice under normal Ca2+ perfusion. These experiments gave an rsat of 192 ± 31 µV (n = 12) and 156 ± 38 µV (n = 7) in WT and GCAP−/− rods, respectively. Although rsat also appeared somewhat larger in WT mice in these experiments, the difference was rather small and not statistically significant (P = 0.43). The sensitivity and kinetic parameters were similar between this and the original set of experiments both in WT and GCAP−/− mice. Low Ca2+ treatment of the GCAP−/− retina (Fig. 3 D) also decreased the rod sensitivity, but instead of decelerating response kinetics, it brought forward the typical hallmarks of light adaptation, including acceleration of flash responses (see Table 1). Furthermore, after the initial transient small increase of the rsat (see Fig. 2 B) by low Ca2+ exposure, the response amplitudes, kinetics, and sensitivity of GCAP−/− rods remained stable for at least 1 h (the longest period tested). We continued to investigate more carefully the effects of low Ca2+ exposure on the response properties and sensitivity regulation of GCAP−/− and DKO mouse rods.
Figure 3.
Low Ca2+ exposure decelerates the flash responses in WT rods but mimics the effects of light adaptation in the GCAP−/− rods. Representative rod response families to 2-ms flashes of light recorded from an isolated WT retina in normal (A) and low Ca2+ (B) solution. Flash strength ranges (R* per rod) are indicated in each panel. Responses to identical flash are shown in dark blue. Rod flash response families in normal (C) and low Ca2+ (D) solution recorded from a GCAP−/− mouse retina. Responses to identical flash are shown in light blue. Rods were allowed to reach a steady state after solution changes before the response families were recorded.
Ca2+ feedback via recoverin-dependent and -independent pathways accounts for the sensitivity regulation of GCAP−/− rods
We used the low Ca2+ method presented above to probe Ca2+ dependence of the light-adaptation mechanisms still present in GCAP−/− and DKO mouse rods (Fig. 1 C). Sensitivity data from GCAP−/− rods showed that the low Ca2+ exposure shifts the operating range of these cells significantly to higher background intensities (Fig. 4 C), with the sensitivity halving background light intensity (I0; Eq. 1) four- to sixfold larger in low Ca2+ than in normal Ca2+ conditions (Table 1). The steepness parameter of the adaptation curve (n in Eq. 1) increased in GCAP−/− rods by 30–40% when switched to low Ca2+ (Table 1), indicating compromised light-dependent feedback to the flash sensitivity. Low Ca2+ exposure also shifted the IB-rsat data to brighter backgrounds, demonstrating that lowered Ca2+ helps to prevent rod saturation under dimmer background lights in GCAP−/− mice (Fig. 4 C, inset). Similarly to GCAP−/− rods, the adaptation curve was right-shifted with an apparent increase of n during low Ca2+ exposure also in DKO mice (Table 1). Further, the suppression of rsat appeared to occur at brighter backgrounds in low Ca2+ than in normal Ca2+ in these mice (Fig. 4 D, inset). However, the effects of low Ca2+ exposure on both I0 and n were smaller in DKO than in GCAP−/− mice (Table 1). These results suggest that Ca2+ mediates both recoverin-dependent and -independent light-adaptation mechanisms in the GCAP−/− rods. The theoretical curves assuming no light adaptation (Eq. 3; Fig. 4, C and D, dashed red traces) under low Ca2+ conditions coincide well with the data of both the GCAP−/− and DKO rods, indicating that the sensitivity regulation of rods during steps of light is mediated exclusively by Ca2+-dependent mechanisms.
Figure 4.
Low Ca2+ exposure eliminates sensitivity regulation and minimizes the operating range of GCAP−/− and DKO mouse rods. Dim-flash responses (in microvolts; see scale bar on the left) in darkness and during steps of light in GCAP−/− (A; IB range: 17–1,600 R* s−1 per rod, indicated on the right side of each response) and DKO (B; IB range: 17–1,600 R* s−1 per rod, indicated on the right side of each response) mouse rods in low Ca2+ solution. Flash strengths ranged from 6 to 190 R* per rod and from 12 to 190 R* per rod in GCAP−/− and DKO mice, respectively, as indicated by the numbers preceding each response. Timing of the flash, 5 s after the background onset at t = 0 s, is indicated by an arrow. Average (±SEM) sensitivities normalized with sF,D as a function of background light intensity (IB) are plotted for four GCAP−/− (C) mouse retinas in normal (black marks) and low (red marks) Ca2+ solution and for three DKO (D) mouse retinas in normal (blue marks) and low (magenta marks) Ca2+ solution. Dashed lines plot Eq. 3 under low Ca2+ conditions for GCAP−/− (C) and DKO (D) rods (error bars represent mean ± SEM). Insets in C (GCAP−/−) and D (DKO) show rsat/rsat,dark as a function of IB in normal (black, GCAP−/−; blue, DKO) and low Ca2+ (red, GCAP−/−; magenta, DKO). Mean ± SEM (n = 7).
Role of recoverin-dependent and –independent Ca2+ pathways in modulating rod sensitivity and response kinetics of dark-adapted GCAP−/− mice
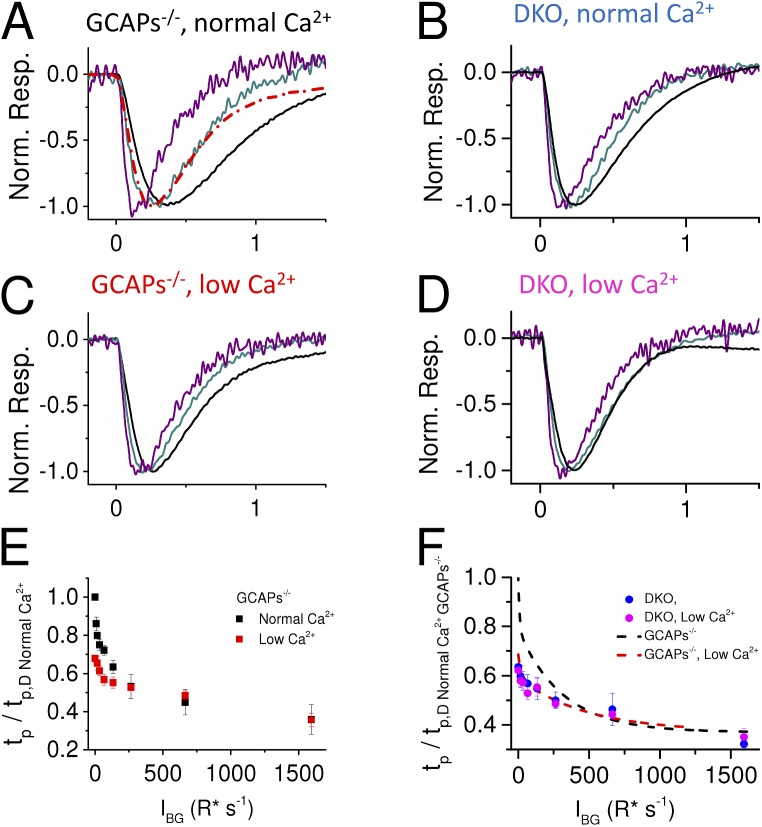
To dissect the role of recoverin-dependent and -independent Ca2+-feedback mechanisms on the response properties of GCAP−/− mouse rods, we analyzed how low Ca2+ exposure affected their sensitivity and flash response kinetics in darkness (Fig. 5). The estimated response to the absorption of a single photon, determined by normalizing a dim-flash response with the saturated response amplitude and flash strength (in R* per rod), decreased approximately fourfold and approximately twofold by low Ca2+ exposure in GCAP−/− and DKO mice, respectively (Fig. 5 A and Table 1). The difference in strains was in agreement with the approximately twofold reduction of the mean single-photon response amplitude resulting from recoverin deletion in GCAP−/− mice (Fig. 5 A, black and blue trace). The normalized dim-flash responses in the inset of Fig. 5 A highlight the acceleration of the dim-flash responses in the GCAP−/− mouse rods when treated with low Ca2+, with 30% mean decrease of tp (Table 1). In the absence of both GCAPs and Rv, the tp is no longer affected by low Ca2+ exposure, and only a minor acceleration of the response recovery phase can be observed when switched to low Ca2+ perfusion. In conclusion, these results indicate that the Ca2+-dependent recoverin-mediated pathway contributes to the observed acceleration of dim-flash responses as well as to about twofold desensitization of GCAP−/− mouse rods. However, the observation that lowered Ca2+ also desensitizes the DKO rods further supports the notion that some unknown Ca2+-mediated feedback mechanism can modulate the phototransduction gain, even in the absence of both GCAPs and recoverin.
The apparent gain reduction during low Ca2+ exposure can arise either from deceleration of phototransduction activation reactions or acceleration of shutoff reactions. To address the former possibility, we determined the gain of the activation reactions (amplification constant, A; LP model: Lamb and Pugh, 1992) of the GCAP−/− and DKO mouse rods under normal and low Ca2+ conditions. This analysis relies on a fitting of the phototransduction activation model to the early part of flash responses, before the response deactivation begins to take effect. The validity of the model is restricted only to a few first milliseconds of the responses in mouse rods, especially under low Ca2+ conditions when R* lifetime might be <20 ms (Lamb and Pugh, 1992; Gross and Burns, 2010). Careful use of the LP model to flash response families revealed a small but statistically insignificant reduction of the amplification constants by low Ca2+ exposure in both GCAP−/− and DKO mice (Table 1), suggesting that the observed desensitization stems from modulation of the response recovery.
One possible explanation for the accelerated rate of photoresponse recovery and decreased sensitivity in low Ca2+ is shortening of the lifetime of activated PDE (Chen et al., 2012). In salamanders, the lifetime of PDE* (corresponding to the rate-limiting time constant of saturated rod photoresponse recovery, τD) seems not to be modulated by light or changes in intracellular Ca2+ concentration (Nikonov et al., 1998). A more recent study, however, revealed that in mouse rods, τD is modulated by background light (Woodruff et al., 2008), and removal of recoverin seems to accelerate τD by ∼15–30% in mouse rods (Makino et al., 2004; Chen et al., 2012). Interestingly, the modulation of τD by background light appears to be mediated by rhodopsin kinase (GRK1) and recoverin, suggesting that Ca2+ feedback via recoverin might also have a direct role in the regulation of PDE* deactivation (Chen et al., 2012, 2015). To study directly whether the rate of PDE* deactivation is modulated by changes in [Ca2+]i, we determined τD in GCAP−/− rods in normal and in low Ca2+ perfusion. Representative GCAP−/− mouse rod responses to bright flashes are shown in Fig. 5 (B and C) in normal and low Ca2+, respectively. Fig. 5 D shows averaged saturation times at 25% recovery for four GCAP−/− (squares) retinas as a function of natural logarithm of flash strength. The dominant time constants determined as the slopes of the fitted straight lines were 234 ms in normal and 185 ms in low Ca2+ perfusion. Linear fittings to the data of individual experiments also revealed a statistically significant 20 ± 3% shortening of τD caused by low Ca2+ exposure (n = 11; P = 0.0005, two-tailed paired t test; see Table 1). Because the decrease in τD caused by low Ca2+ exposure is quantitatively close to the previously observed shortening of τD caused by recoverin deletion (Makino et al., 2004; Chen et al., 2012), we reasoned that the observed shortening of τD in low Ca2+ might be mediated by recoverin. To test this hypothesis, we determined the τD in normal and in low Ca2+ for DKO mouse rods (Fig. 5 D, circles). In these mice, τD was 200 ms, ∼23% smaller compared with their GCAP−/− Rv+/+ littermates (261 ms), and was not affected by low Ca2+ exposure (Table 1). In control experiments, we also observed a 23% shortening of the τD when recoverin was deleted in WT background mice (n = 4 for WT and 9 for Rv−/− mice; P = 0.02, one-tailed t test). Overall, the τD seems to be modulated through a pathway that is dependent on both recoverin and Ca2+. This feedback, however, cannot account for the much pronounced effects of low Ca2+ exposure on rod sensitivity and photoresponse kinetics in GCAP−/− mouse rods. Also, substantial vertical shifts of photoresponse saturation times of GCAP−/− and DKO rods take place when switched from normal to low Ca2+ (Fig. 5 D). These shifts exceed by far the small changes in τD and further indicate that phototransduction gain is modulated by both recoverin-dependent and -independent Ca2+-feedback mechanisms, which are mainly not directly targeting PDE (see Discussion).
Contribution of recoverin-dependent and -independent Ca2+-feedback mechanisms to light-induced acceleration of dim-flash response kinetics in GCAP−/− rods
One hallmark of light adaptation is the acceleration of flash response kinetics upon increased strength of background illumination. Although GCAP-dependent adaptation contributes to this phenomenon, a significant acceleration of flash response kinetics persists in GCAP−/− rods (Mendez et al., 2001; Chen et al., 2010b; Nymark et al., 2012). Background light also progressively accelerated the response shutoff kinetics of GCAP−/− rods in the standard perfusion in our experiments (Fig. 6 A). However, light-induced response acceleration was clearly attenuated under low Ca2+ perfusion (Fig. 6 C). The change in dim-flash response kinetics is quantified in Fig. 6 E by demonstrating the more pronounced acceleration of the dim-flash responses by background light in normal (black) than in low Ca2+ (red). Finally, we investigated how much the dim-flash response kinetics of mouse rods are modulated by background light in the absence of both GCAPs and recoverin. We found that photoresponse kinetics was somewhat accelerated as background light intensity increased under normal and low Ca2+ conditions (Fig. 6, B and D). However, differently from the GCAP−/− mice, low Ca2+ exposure did not affect the magnitude of acceleration in DKO mice (Fig. 6 F).
Figure 6.
Calcium-dependent, background-induced acceleration of the dim-flash response kinetics. Mean (n = 4 retinas) normalized dim-flash responses (<20% of maximum response amplitude during each background) in darkness (black) and during different background lights (IB 66 [dark cyan] and 664 [purple] R* s−1 per rod) in normal (A and B) and low (C and D) Ca2+ solution. The dark-adapted response from C is also plotted in A (dashed red) for comparison. All traces in A–D have been digitally low-pass filtered with cutoff at 30 Hz, which did not affect dim-flash response waveforms. (E and F) tp (mean ± SEM; n = 4) of dim-flash responses during background lights normalized to dark-adapted tp,D of GCAP−/− in normal Ca2+ as a function of background light intensity in normal (black) and low (red) Ca2+ for GCAP−/− (E) and in normal (blue) and low (magenta) Ca2+ conditions for DKO (F) mice. Dashed lines plot the data from E for comparison. Error bars represent ± SEM.
DISCUSSION
Contribution of different mechanisms to overall light adaptation has been studied previously in amphibian rods by manipulating outer segment [Ca2+]i (Koutalos et al., 1995a,b; Nikonov et al., 2000). These experiments have not been feasible with the more fragile and smaller mammalian rods. Here, we combined genetic, pharmacological, and electrophysiological tools to assess the contribution of recoverin and the residual Ca2+-dependent and -independent mechanisms to dark-adapted mouse rod response properties (sensitivity and kinetics), as well as to background light adaptation.
Mimicking light-induced decline in [Ca2+]i
Lowering extracellular [Ca2+] in darkness to reduce the [Ca2+]i can in principle provide a straightforward method to study the contribution of Ca2+-dependent feedback mechanisms to rod physiology. However, previous studies have demonstrated that lowering Ca2+ in darkness leads to a large but transient increase in the CNG channel current accompanied by permanent deceleration of flash responses and extensive desensitization of rods (Lipton et al., 1977; Yau et al., 1981; Bastian and Fain, 1982; Matthews, 1995). We have previously found that, by lowering extracellular Ca2+ concentration more moderately compared with the amphibian studies cited above, stable and relatively large transretinal ERG responses can be recorded from isolated WT mouse retinas at room temperature under perfusate containing ∼10−8 M [Ca2+]o (Vinberg and Koskelainen, 2010). However, consistent with the previous studies with amphibian photoreceptors, the response kinetics become slower and rods are desensitized >10-fold when [Ca2+]o is lowered <30 nM (Figs. 2 A and 3, A and B). These effects cannot be attributed to Ca2+-feedback mechanisms triggered by light that normally act to accelerate the shutoff of rod light responses. We hypothesized that preventing the excessive increase of [cGMP]i under low Ca2+ in darkness by using GCAP−/− mouse rods could alleviate the anomalous slowdown of flash responses. Indeed, the large transient increase of the saturated photoresponse amplitude was absent and the rod flash response recovery was accelerated instead of decelerated under low Ca2+ conditions with GCAP−/− mouse retinas (Figs. 2 B, 3, C and D, and 5 A). Although we do not know [Ca2+]i in the rod outer segment during our perfusion with a solution containing ∼20 nM of free [Ca2+]o, it seems most likely that the Na+/Ca2+-K+ exchange, which in physiological Ca2+ concentrations keeps the [Ca2+]i >3 log units lower than the [Ca2+]o, should drive the [Ca2+]i in our low Ca2+ conditions well below ∼50 nM, the level attained in bright light (Woodruff et al., 2002). However, long-term low Ca2+ exposure might trigger release of Ca2+ from internal stores preventing [Ca2+]i from dropping to extremely low levels (Molnar et al., 2012). Regardless of the possible effect of the internal stores, the low Ca2+ exposure should set [Ca2+]i at least to and probably well below that attained in bright light, so that any further decline of Ca2+ concentration in the outer segment caused by background light would not modulate phototransduction anymore. However, all Ca2+-sensor proteins (except GCAPs) are still present in the rod cells and probably mostly in Ca2+-free form. Consequently, GCAP−/− rod cells are “clamped” to a state that corresponds to a condition where the rods would be exposed to a very bright constant background light regarding the Ca2+-dependent adaptation mechanisms. Thus, our low Ca2+ method has an advantage over genetic removal of, for example, GCAPs or recoverin, where the effect of deletion depends on the mechanism in question. For example, removal of GCAPs in normal Ca2+ would essentially clamp cGMP synthesis by guanylyl cyclase approximately to its dark-adapted or minimum level, whereas removal of recoverin would clamp R* lifetime to its light-adapted or shortest value. Further, it is worth noting that both genetic and our low Ca2+ methods have limitations in addressing the normal physiological contributions of Ca2+-feedback mechanisms, as it is possible that under physiological conditions, Ca2+ would not drop low enough to, for example, result in 100% Ca2+-free recoverin (corresponding to our low Ca2+ treatment) or 100% recoverin-free rhodopsin kinase (corresponding to Rv−/− mice). Here, we used the low Ca2+ approach on GCAP−/− rods to investigate the contribution of Ca2+-dependent and -independent feedback mechanisms in mammalian rod light adaptation. In addition, we could reveal how reduced [Ca2+]i alone modulates response kinetics and sensitivity of rods in the absence of background light that has other Ca2+-independent effects on response properties. For example, steady light activates phototransduction, leading to increased hydrolysis rate of cGMP, which will decrease response amplitudes and sensitivity of rods, and accelerate their flash response shutoff kinetics (Nikonov et al., 2000). A minor shortcoming of our low Ca2+ approach is that it is feasible only in the absence of GCAPs (see above). It is possible that deletion of GCAPs has some indirect effects on the molecular or physiological properties of rods that might affect the physiological relevance of our conclusions. However, GCAP−/− (and Rv−/−) mouse rods have been extensively studied previously, and they appear to have normal expression of all the major phototransduction proteins (Mendez et al., 2001; Makino et al., 2004). Their maximal saturated response amplitudes as measured from single cells correspond very well to those of their WT littermates, indicating normal Ca2+ levels both in darkness and under bright-light conditions. Thus, we believe that the Ca2+-dependent or -independent adaptation mechanisms that we describe here in GCAP−/− or DKO mouse rods are also functional in WT rods.
Contribution of recoverin-mediated feedback to rod physiology
The role of recoverin in rod phototransduction and light adaptation has remained controversial. Earlier evidence from amphibian rods suggested that Ca2+ feedback strongly modulates R* lifetime, presumably via recoverin (Nikonov et al., 2000). Subsequent electrophysiological studies with recoverin knockout mice are somewhat contradictory. Although recoverin has been shown to participate in light-dependent acceleration of response termination in mouse rods, it does not seem to affect the light adaptation of WT rods (Makino et al., 2004; Chen et al., 2010a,b, 2012). Also, localization of recoverin primarily to the inner segment and synaptic terminal (even in dark-adapted retinas) and its more prominent role in synaptic transmission as compared with modulating the CNG channel current have raised questions as to the physiological importance of recoverin in the rod phototransduction (Sampath et al., 2005). Moreover, although biochemical evidence shows that recoverin can modulate phosphorylation of rhodopsin in a Ca2+-dependent manner, the affinity of Ca2+ to recoverin does not match the physiological range of [Ca2+]i (Kawamura, 1993; Chen et al., 1995; Klenchin et al., 1995). These apparent discrepancies might be caused by at least two reasons: (1) the GCAP-mediated feedback is the dominant factor in determining rod sensitivity both in dark- and light-adapted states, and therefore the feedback to R* inactivation is not clearly observable in WT mice; and/or (2) the recoverin-mediated regulation of photoresponse kinetics is Ca2+ independent. In this study, we addressed both of these possibilities.
Our results show that exposing dark-adapted rods lacking GCAPs, and thus calcium feedback on activation of guanylate cyclase, to low [Ca2+]o decreases their flash sensitivity about fourfold in the absence of GCAPs (Fig. 5 A). This gives the maximal flash sensitivity regulation achieved together by all the calcium-controlled mechanisms present in the GCAP−/− rods, and it seems reasonable to assume that this is also the upper limit of sensitivity regulation in WT mouse rods through Ca2+-controlled feedback mechanisms other than GCAPs. Our results demonstrate that deletion of recoverin in the GCAP−/− background removes about half of this Ca2+-dependent flash sensitivity regulation (Fig. 5 A). The result that the fractional sensitivities of the GCAP−/− Rv+/+ mice and their DKO littermates do not differ from each other in low [Ca2+]o (Table 1) suggests that Ca2+-free recoverin does not have any effect on rod sensitivity in darkness, and thus all the modulation of dark-adapted rod sensitivity via recoverin seems to be Ca2+ dependent.
In principle, it is possible that some compensatory mechanisms might alter the expression of phototransduction proteins in GCAP−/− rods, which would explain the differences of recoverin removal in WT and GCAP−/− background. However, gene expression data in GCAP−/− or Rv−/− mouse retinas do not indicate changes in the phototransduction protein expression (Mendez et al., 2001; Makino et al., 2004). Thus, we suggest that the effect of recoverin-mediated feedback is overrun by the fast synthesis of cGMP and its dynamic regulation so effectively in WT mice that its role is hard to distinguish in the presence of GCAPs (see also Gross et al., 2012).
It has become evident that the recovery of saturated rod photoresponses is rate-limited by PDE* inactivation catalyzed by RGS9 complex (Krispel et al., 2006). Earlier data with amphibian rods suggested that the rate-limiting time constant (τD) is not modulated by Ca2+ or background light (Lyubarsky et al., 1996). However, more recent results have demonstrated that τD actually is modulated by background light in mouse rods (Woodruff et al., 2008) via a recoverin- and GRK1-dependent pathway (Chen et al., 2012, 2015). In this study, we addressed the role of Ca2+ together with recoverin in τD modulation in mouse rods. We observed a subtle but statistically significant ∼20% decrease of τD by lowered Ca2+ in GCAP−/− mouse rods but not in DKO mouse rods. The decrease of τD in GCAP−/− mouse rods during low Ca2+ exposure was comparable to the previously published reduction of τD caused by removal of recoverin from WT mouse rods (Makino et al., 2004; Chen et al., 2012), and to the shortening of τD of 30 and 19% by removal of recoverin in WT and GCAP−/− background, respectively, observed in this study. Although our results are consistent with the idea that PDE* lifetime might be modulated by Ca2+ feedback via recoverin, the changes of τD by removal of recoverin or by low Ca2+ exposure are small considering the variability of τD values between different strains/groups of WT or GCAP−/− mice previously shown by others and by us here (e.g., Makino et al., 2004; Krispel et al., 2006; Woodruff et al., 2008). On the other hand, the vertical shifts of the “Pepperberg” plots caused by low Ca2+ exposure are notable: 340 and 180 ms in GCAP−/− and DKO mice, respectively (Fig. 5 D). If the difference between these vertical shifts (160 ms) is assumed to be caused purely by the shortening of R* lifetime by the Rv-mediated Ca2+ feedback (Eq. 1 in Gross et al., 2012), the recoverin pathway can maximally shorten the R* lifetime by about twofold. Collectively, our results here demonstrate that the recoverin-mediated Ca2+ feedback can account for almost all of the Ca2+-dependent dim-flash response kinetics acceleration (Figs. 5 A and 6 F) as well as half of the total Ca2+-dependent modulation of the saturation time (Fig. 5 D) and dark-adapted sensitivity (Fig. 5 A and Table 1) of GCAP−/− rods. Thus, although removal of recoverin does not seem to affect the operating range of WT rods in standard light-adaptation experiments, we show here that Rv-mediated Ca2+ feedback is functional in mouse rods and can explain the faster escape of rods from saturation observed by us here (Fig. 5 D) and by others previously (Makino et al., 2004). Our experiments do not discriminate directly the molecular pathway of recoverin action, and it is possible that recoverin could also have some other effects than shortening of R* lifetime. Indeed, comparing the leading edges of fractional responses of GCAP−/− and DKO rods (Fig. 5 A) between normal and low Ca2+ conditions gives an impression that the leading edge kinetics is significantly decelerated in GCAP−/− rods but not so much in DKO rods. Similar and even more pronounced changes in the leading edge slope have been shown previously for truncated salamander rods (Lagnado and Baylor, 1994) exposed to low Ca2+ conditions. The deceleration of the leading edge slope could actually mean that Ca2+ would control the activation reactions of phototransduction, which would be a completely novel phenomenon for which we do not have a molecular explanation. Another possibility is that the deactivation of phototransduction molecules already affects the early parts of the dim-flash response. These ideas were tested by fitting the LP activation model to responses (Lamb and Pugh, 1992). No significant change could be seen in the activation constant when changed from normal to low Ca2+ solution, either with GCAP−/− or DKO mice (Table 1). In mouse rods where the lifetime of R* is 40 ms in darkness (Gross and Burns, 2010) and probably approximately twofold shorter in background light (our Results and Chen et al., 2010a), the validity of the LP activation model would cover only a very small part of the leading edge for dim-flash responses, and the shutoff of the responses start particularly early. Thus, we believe that our low Ca2+ exposure modulates phototransduction deactivation rather than activation reactions. Because the only well-established recoverin-dependent pathway affects R* lifetime, we think that the most probable function for the recoverin-dependent Ca2+ feedback is to modulate R* inactivation, although other possibilities cannot be ruled out.
Mechanism for the residual Ca2+ feedback in the absence of GCAPs and recoverin
Although a significant proportion of the Ca2+ feedback in GCAP−/− rods could be explained by the Rv-mediated pathway, we found that some Ca2+-dependent sensitivity regulation remained even in the DKO mouse rods. One potential mechanism that has been suggested to improve photoreceptor’s light-adaptation capacity is modulation of the CNG channel’s affinity to cGMP through a Ca2+-dependent calmodulin pathway (Hsu and Molday, 1993; Nakatani et al., 1995). However, Chen et al. (2010b) showed that deletion of the binding site for calmodulin in the CNG channel β subunit did not significantly affect dark-adapted mouse rod’s photoresponse properties nor its ability to light adapt even in the GCAP−/− background. Instead, in recent studies, new Ca2+-dependent modulators of CNG channels have been found. Rebrik et al. (2012) reported that the sensitivity of channels to cGMP in striped bass cones is modulated by CNG modulin in a Ca2+-dependent manner. Subsequently, it was shown that the orthologue gene for the CNG modulin EML1 encodes a protein that modulates the sensitivity and light adaptation in zebrafish cones (Korenbrot et al., 2013). Our observation that the saturated response amplitudes of GCAP−/− mouse rods increased when switched to low Ca2+ perfusion (Fig. 2 B) would be consistent with an increased number of open CNG channels in low Ca2+, which could be caused by a higher affinity of channels to cGMP. However, we cannot rule out other possibilities such as a decrease in the spontaneous activity of PDE or an increase in single CNG channel conductance caused by reduced Ca2+ ion block of the CNG channels under our low Ca2+ conditions (Lamb and Matthews, 1988).
Possible Ca2+-independent light adaptation
So far, we have demonstrated that recoverin and some other Ca2+-feedback mechanism(s) contributes to mammalian rod phototransduction and light adaptation. The final question is whether some Ca2+-independent light-adaptation mechanism is also operational in mammalian rods. Comparison of the relative sensitivity of GCAP−/− and DKO mouse rods as a function of background intensity in low Ca2+ to the prediction of the theoretical model without phototransduction feedback (Eq. 3; see Fig. 4, C and D) suggests that mouse rod light adaptation is almost completely mediated by Ca2+. However, we still observed background light-induced acceleration of both GCAP−/− and DKO mouse rod responses even under our low Ca2+ conditions. This residual acceleration could be explained by increased steady-state activity of PDE, driven by the background-induced phototransduction activation that has been shown to significantly modulate the flash response kinetics of salamander rods (see Nikonov et al., 2000). However, we cannot rule out the role of Ca2+-independent feedback mechanisms in controlling, for example, bright flash response kinetics, adaptive potentiation of rod sensitivity (McKeown and Kraft, 2014), or slower light-adaptation mechanisms such as transducin, arrestin, or recoverin translocation between outer and inner segments (Brann and Cohen, 1987; Philp et al., 1987; Whelan and McGinnis, 1988; Sokolov et al., 2002; Strissel et al., 2005). For example, Mg2+ as well as phosphorylation of PDE may play a role in phototransduction (Janisch et al., 2009; Dizhoor et al., 2010; Azevedo and Rieke, 2011), although the physiological roles of these mechanisms remain unclear. In salamander rods, the light-induced Ca2+-independent increase in β significantly contributes to the kinetics of dim-flash responses and light adaptation (Nikonov et al., 2000). This acceleration occurs because the synthesis rate of cGMP remains constant, whereas increased cGMP hydrolysis lowers the steady-state cGMP concentration so that the same fractional change in cGMP and the CNG channel current can happen faster than in dark-adapted rods with higher absolute cGMP levels. In salamander rods under Ca2+-clamp conditions, a background light of ∼100 R* s−1 per rod shortens tp of a dim-flash response to 1.2 s from its value of 2.4 s in darkness (see Fig. 13 in Nikonov et al., 2000). Increase of background to 2,100 R* s−1 per rod further shortened tp to 0.8 s in salamander rods. Based on our experimental data under low Ca2+ conditions in GCAP−/− mouse rods, a background light of 100 R* s−1 would shorten tp to 0.22 s from its dark-adapted value of 0.27 s (∼18% decrease). Further shortening of tp to ∼70% of its dark-adapted value (in low Ca2+) occurs at ∼700 R* s−1 per rod, but after that the change of tp with increasing backgrounds appears to be small (see Fig. 6, E and F). In conclusion, the dynamic range of tp modulation by background light in the absence of Ca2+ feedbacks appears significantly narrower in mouse rods than previously reported in salamander.
Acknowledgments
We thank Dr. Vladimir Kefalov and Dr. Alexander Kolesnikov for their valuable comments on the manuscript.
This work was supported by the Academy of Finland (grants 111866 and 128081), the International Doctoral Program in Biomedical Engineering and Medical Physics, and Brain Research at Aalto University and University of Helsinki (BRAHE) consortium.
The authors declare no competing financial interests.
Angus C. Nairn served as editor.
Footnotes
Abbreviations used in this paper:
- DKO
- double knockout
- ERG
- electroretinography
- GCAP
- guanylyl cyclase–activating protein
- LP
- Lamb–Pugh
- PDE
- phosphodiesterase
References
- Aguilar M., and Stiles W.S.. 1954. Saturation of the rod mechanism of the retina at high levels of stimulation. Optica Acta: International Journal of Optics. 1:59–65. 10.1080/713818657 [DOI] [Google Scholar]
- Azevedo A.W., and Rieke F.. 2011. Experimental protocols alter phototransduction: The implications for retinal processing at visual threshold. J. Neurosci. 31:3670–3682. 10.1523/JNEUROSCI.4750-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian B.L., and Fain G.L.. 1982. The effects of low calcium and background light on the sensitivity of toad rods. J. Physiol. 330:307–329. 10.1113/jphysiol.1982.sp014343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick D.A., Walter A.E., and Sillman A.J.. 1979. Barium suppresses slow PIII in perfused bullfrog retina. Vision Res. 19:1117–1119. 10.1016/0042-6989(79)90006-3 [DOI] [PubMed] [Google Scholar]
- Brann M.R., and Cohen L.V.. 1987. Diurnal expression of transducin mRNA and translocation of transducin in rods of rat retina. Science. 235:585–587. 10.1126/science.3101175 [DOI] [PubMed] [Google Scholar]
- Burns M.E., Mendez A., Chen J., and Baylor D.A.. 2002. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 36:81–91. 10.1016/S0896-6273(02)00911-X [DOI] [PubMed] [Google Scholar]
- Chen C.K., Inglese J., Lefkowitz R.J., and Hurley J.B.. 1995. Ca2+-dependent interaction of recoverin with rhodopsin kinase. J. Biol. Chem. 270:18060–18066. 10.1074/jbc.270.30.18060 [DOI] [PubMed] [Google Scholar]
- Chen C.K., Woodruff M.L., Chen F.S., Chen D., and Fain G.L.. 2010a. Background light produces a recoverin-dependent modulation of activated-rhodopsin lifetime in mouse rods. J. Neurosci. 30:1213–1220. 10.1523/JNEUROSCI.4353-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Woodruff M.L., Wang T., Concepcion F.A., Tranchina D., and Fain G.L.. 2010b. Channel modulation and the mechanism of light adaptation in mouse rods. J. Neurosci. 30:16232–16240. 10.1523/JNEUROSCI.2868-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.K., Woodruff M.L., Chen F.S., Chen Y., Cilluffo M.C., Tranchina D., and Fain G.L.. 2012. Modulation of mouse rod response decay by rhodopsin kinase and recoverin. J. Neurosci. 32:15998–16006. 10.1523/JNEUROSCI.1639-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.K., Woodruff M.L., and Fain G.L.. 2015. Rhodopsin kinase and recoverin modulate phosphodiesterase during mouse photoreceptor light adaptation. J. Gen. Physiol. 145:213–224. 10.1085/jgp.201411273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizhoor A.M., Olshevskaya E.V., and Peshenko I.V.. 2010. Mg2+/Ca2+ cation binding cycle of guanylyl cyclase activating proteins (GCAPs): role in regulation of photoreceptor guanylyl cyclase. Mol. Cell. Biochem. 334:117–124. 10.1007/s11010-009-0328-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K., Hemilä S., and Koskelainen A.. 1988. Temperature-dependence of rod photoresponses from the aspartate-treated retina of the frog (Rana temporaria). Acta Physiol. Scand. 134:535–541. 10.1111/j.1365-201X.1988.tb10632.x [DOI] [PubMed] [Google Scholar]
- Fain G.L., Lamb T.D., Matthews H.R., and Murphy R.L.. 1989. Cytoplasmic calcium as the messenger for light adaptation in salamander rods. J. Physiol. 416:215–243. 10.1113/jphysiol.1989.sp017757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O.P., and Burns M.E.. 2010. Control of rhodopsin’s active lifetime by arrestin-1 expression in mammalian rods. J. Neurosci. 30:3450–3457. 10.1523/JNEUROSCI.5391-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O.P., Pugh E.N. Jr., and Burns M.E.. 2012. Calcium feedback to cGMP synthesis strongly attenuates single-photon responses driven by long rhodopsin lifetimes. Neuron. 76:370–382. 10.1016/j.neuron.2012.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen H., Nymark S., and Koskelainen A.. 2008. Mouse cone photoresponses obtained with electroretinogram from the isolated retina. Vision Res. 48:264–272. 10.1016/j.visres.2007.11.005 [DOI] [PubMed] [Google Scholar]
- Hsu Y.T., and Molday R.S.. 1993. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 361:76–79. 10.1038/361076a0 [DOI] [PubMed] [Google Scholar]
- Janisch K.M., Kasanuki J.M., Naumann M.C., Davis R.J., Lin C.S., Semple-Rowland S., and Tsang S.H.. 2009. Light-dependent phosphorylation of the gamma subunit of cGMP-phophodiesterase (PDE6gamma) at residue threonine 22 in intact photoreceptor neurons. Biochem. Biophys. Res. Commun. 390:1149–1153. 10.1016/j.bbrc.2009.10.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S. 1993. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 362:855–857. 10.1038/362855a0 [DOI] [PubMed] [Google Scholar]
- Klenchin V.A., Calvert P.D., and Bownds M.D.. 1995. Inhibition of rhodopsin kinase by recoverin. Further evidence for a negative feedback system in phototransduction. J. Biol. Chem. 270:16147–16152. 10.1074/jbc.270.27.16147 [DOI] [PubMed] [Google Scholar]
- Koch K.W., and Stryer L.. 1988. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 334:64–66. 10.1038/334064a0 [DOI] [PubMed] [Google Scholar]
- Korenbrot J.I., Mehta M., Tserentsoodol N., Postlethwait J.H., and Rebrik T.I.. 2013. EML1 (CNG-modulin) controls light sensitivity in darkness and under continuous illumination in zebrafish retinal cone photoreceptors. J. Neurosci. 33:17763–17776. 10.1523/JNEUROSCI.2659-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutalos Y., Nakatani K., Tamura T., and Yau K.W.. 1995a. Characterization of guanylate cyclase activity in single retinal rod outer segments. J. Gen. Physiol. 106:863–890. 10.1085/jgp.106.5.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutalos Y., Nakatani K., and Yau K.W.. 1995b. The cGMP-phosphodiesterase and its contribution to sensitivity regulation in retinal rods. J. Gen. Physiol. 106:891–921. 10.1085/jgp.106.5.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krispel C.M., Chen D., Melling N., Chen Y.J., Martemyanov K.A., Quillinan N., Arshavsky V.Y., Wensel T.G., Chen C.K., and Burns M.E.. 2006. RGS expression rate-limits recovery of rod photoresponses. Neuron. 51:409–416. 10.1016/j.neuron.2006.07.010 [DOI] [PubMed] [Google Scholar]
- Lagnado L., and Baylor D.A.. 1994. Calcium controls light-triggered formation of catalytically active rhodopsin. Nature. 367:273–277. 10.1038/367273a0 [DOI] [PubMed] [Google Scholar]
- Lamb T.D., and Matthews H.R.. 1988. External and internal actions in the response of salamander retinal rods to altered external calcium concentration. J. Physiol. 403:473–494. 10.1113/jphysiol.1988.sp017259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T.D., and Pugh E.N. Jr. 1992. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J. Physiol. 449:719–758. 10.1113/jphysiol.1992.sp019111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S.A., Ostroy S.E., and Dowling J.E.. 1977. Electrical and adaptive properties of rod photoreceptors in Bufo marinus. I. Effects of altered extracellular Ca2+ levels. J. Gen. Physiol. 70:747–770. 10.1085/jgp.70.6.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky A., Nikonov S., and Pugh E.N. Jr. 1996. The kinetics of inactivation of the rod phototransduction cascade with constant Ca2+i. J. Gen. Physiol. 107:19–34. 10.1085/jgp.107.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino C.L., Dodd R.L., Chen J., Burns M.E., Roca A., Simon M.I., and Baylor D.A.. 2004. Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. J. Gen. Physiol. 123:729–741. 10.1085/jgp.200308994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H.R. 1995. Effects of lowered cytoplasmic calcium concentration and light on the responses of salamander rod photoreceptors. J. Physiol. 484:267–286. 10.1113/jphysiol.1995.sp020664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H.R., Cornwall M.C., and Crouch R.K.. 2001. Prolongation of actions of Ca2+ early in phototransduction by 9-demethylretinal. J. Gen. Physiol. 118:377–390. 10.1085/jgp.118.4.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown A.S., and Kraft T.W.. 2014. Adaptive potentiation in rod photoreceptors after light exposure. J. Gen. Physiol. 143:733–743. 10.1085/jgp.201411163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez A., Burns M.E., Sokal I., Dizhoor A.M., Baehr W., Palczewski K., Baylor D.A., and Chen J.. 2001. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc. Natl. Acad. Sci. USA. 98:9948–9953. 10.1073/pnas.171308998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar T., Barabas P., Birnbaumer L., Punzo C., Kefalov V., and Križaj D.. 2012. Store-operated channels regulate intracellular calcium in mammalian rods. J. Physiol. 590:3465–3481. 10.1113/jphysiol.2012.234641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naarendorp F., Esdaille T.M., Banden S.M., Andrews-Labenski J., Gross O.P., and Pugh E.N. Jr. 2010. Dark light, rod saturation, and the absolute and incremental sensitivity of mouse cone vision. J. Neurosci. 30:12495–12507. 10.1523/JNEUROSCI.2186-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., and Yau K.W.. 1988. Calcium and light adaptation in retinal rods and cones. Nature. 334:69–71. 10.1038/334069a0 [DOI] [PubMed] [Google Scholar]
- Nakatani K., Koutalos Y., and Yau K.W.. 1995. Ca2+ modulation of the cGMP-gated channel of bullfrog retinal rod photoreceptors. J. Physiol. 484:69–76. 10.1113/jphysiol.1995.sp020648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov S., Engheta N., and Pugh E.N. Jr. 1998. Kinetics of recovery of the dark-adapted salamander rod photoresponse. J. Gen. Physiol. 111:7–37. 10.1085/jgp.111.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov S., Lamb T.D., and Pugh E.N. Jr. 2000. The role of steady phosphodiesterase activity in the kinetics and sensitivity of the light-adapted salamander rod photoresponse. J. Gen. Physiol. 116:795–824. 10.1085/jgp.116.6.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymark S., Heikkinen H., Haldin C., Donner K., and Koskelainen A.. 2005. Light responses and light adaptation in rat retinal rods at different temperatures. J. Physiol. 567:923–938. 10.1113/jphysiol.2005.090662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymark S., Frederiksen R., Woodruff M.L., Cornwall M.C., and Fain G.L.. 2012. Bleaching of mouse rods: microspectrophotometry and suction-electrode recording. J. Physiol. 590:2353–2364. 10.1113/jphysiol.2012.228627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peshenko I.V., and Dizhoor A.M.. 2004. Guanylyl cyclase-activating proteins (GCAPs) are Ca2+/Mg2+ sensors: Implications for photoreceptor guanylyl cyclase (RetGC) regulation in mammalian photoreceptors. J. Biol. Chem. 279:16903–16906. 10.1074/jbc.C400065200 [DOI] [PubMed] [Google Scholar]
- Philp N.J., Chang W., and Long K.. 1987. Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett. 225:127–132. 10.1016/0014-5793(87)81144-4 [DOI] [PubMed] [Google Scholar]
- Portzehl H., Caldwell P.C., and Rueegg J.C.. 1964. The dependence of contraction and relaxation of muscle fibres from the crab Maia squinado on the internal concentration of free calcium ions. Biochim. Biophys. Acta. 79:581–591. [DOI] [PubMed] [Google Scholar]
- Rebrik T.I., Botchkina I., Arshavsky V.Y., Craft C.M., and Korenbrot J.I.. 2012. CNG-modulin: A novel Ca-dependent modulator of ligand sensitivity in cone photoreceptor cGMP-gated ion channels. J. Neurosci. 32:3142–3153. 10.1523/JNEUROSCI.5518-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath A.P., Strissel K.J., Elias R., Arshavsky V.Y., McGinnis J.F., Chen J., Kawamura S., Rieke F., and Hurley J.B.. 2005. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron. 46:413–420. 10.1016/j.neuron.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Sokolov M., Lyubarsky A.L., Strissel K.J., Savchenko A.B., Govardovskii V.I., Pugh E.N. Jr., and Arshavsky V.Y.. 2002. Massive light-driven translocation of transducin between the two major compartments of rod cells: A novel mechanism of light adaptation. Neuron. 34:95–106. 10.1016/S0896-6273(02)00636-0 [DOI] [PubMed] [Google Scholar]
- Strissel K.J., Lishko P.V., Trieu L.H., Kennedy M.J., Hurley J.B., and Arshavsky V.Y.. 2005. Recoverin undergoes light-dependent intracellular translocation in rod photoreceptors. J. Biol. Chem. 280:29250–29255. 10.1074/jbc.M501789200 [DOI] [PubMed] [Google Scholar]
- Vinberg F., and Koskelainen A.. 2010. Calcium sets the physiological value of the dominant time constant of saturated mouse rod photoresponse recovery. PLoS ONE. 5:e13025 10.1371/journal.pone.0013025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan J.P., and McGinnis J.F.. 1988. Light-dependent subcellular movement of photoreceptor proteins. J. Neurosci. Res. 20:263–270. 10.1002/jnr.490200216 [DOI] [PubMed] [Google Scholar]
- Woodruff M.L., Sampath A.P., Matthews H.R., Krasnoperova N.V., Lem J., and Fain G.L.. 2002. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J. Physiol. 542:843–854. 10.1113/jphysiol.2001.013987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff M.L., Janisch K.M., Peshenko I.V., Dizhoor A.M., Tsang S.H., and Fain G.L.. 2008. Modulation of phosphodiesterase6 turnoff during background illumination in mouse rod photoreceptors. J. Neurosci. 28:2064–2074. 10.1523/JNEUROSCI.2973-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K.W., and Nakatani K.. 1984. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 311:661–663. 10.1038/311661a0 [DOI] [PubMed] [Google Scholar]
- Yau K.W., McNaughton P.A., and Hodgkin A.L.. 1981. Effect of ions on the light-sensitive current in retinal rods. Nature. 292:502–505. 10.1038/292502a0 [DOI] [PubMed] [Google Scholar]