Abstract
Objective
To investigate the relationship between time spent in non-exercise and exercise physical activity and severity of motor functions in Parkinson disease (PD).
Background
Increasing motor impairments of PD incline many patients to a sedentary lifestyle. We investigated the relationship between duration of both non-exercise and exercise physical activity over a 4-week period using the Community Health Activities Model Program for Seniors (CHAMPS) questionnaire and severity of clinical motor symptoms in PD. We accounted for the magnitude of nigrostriatal degeneration.
Methods
Cross-sectional study. PD subjects, n=48 (40M); 69.4±7.4 (56–84) years old; 8.4±4.2 (2.5–20) years motor disease duration, mean UPDRS motor score 27.5 ± 10.3 (7–53) and mean MMSE score 28.4 ± 1.9 (22–30) underwent [11C]dihydrotetrabenazine (DTBZ) PET imaging to assess nigrostriatal denervation and completed the CHAMPS questionnaire and clinical assessment.
Results
Bivariate correlations showed an inverse relationship between motor UPDRS severity scores and duration of non-exercise physical activity (R= −0.37, P=0.0099) but not with duration of exercise physical activity (R= −0.05, P= 0.76) over 4 weeks. Multiple regression analysis using UPDRS motor score as outcome variable demonstrated a significant regressor effect for duration of non-exercise physical activity (F=6.15, P=0.017) while accounting for effects of nigrostriatal degeneration (F=4.93, P=0.032), levodopa-equivalent dose (LED; F=1.07, P=0.31), age (F=4.37, P=0.043) and duration of disease (F=1.46, P=0.23; total model (F=5.76, P=0.0004).
Conclusions
Non-exercise physical activity is a correlate of motor symptom severity in PD independent of the magnitude of nigrostriatal degeneration. Non-exercise physical activity may have positive effects on functional performance in PD.
Keywords: dopamine, exercise, Parkinson disease, physical activity, PET, sedentariness
Introduction
There is growing evidence of an association between physical inactivity and negative outcomes in patients with PD, including impaired activities of daily living and gait instability [1, 2]. Axial motor dysfunctions in Parkinson disease (PD) [3] – which are generally the least responsive to dopaminergic therapy - incline patients towards a sedentary lifestyle [4], with a resulting increased risk for the negative consequences of physical inactivity [5]. The Sydney Multicenter Study of PD, for example, found that dopamine non-responsive problems dominate 15 years after initial assessments and include frequent falls, which occurs in 81% of the patients [6]. Patients with more severe postural and gait difficulties (PIGD) problems may also develop 'fear-of-falling,' and become even more sedentary [3]. This may contribute to decreases in muscle strength and 'deconditioned' postural reflexes, further exacerbating the motor decline caused by the disease process itself [7, 8]. A vicious cycle of worsening parkinsonism and increasingly sedentary behavior may explain decreasing physical activity in advanced PD. This vicious cycle raises a 'chicken and egg' mechanistic question where it is generally assumed that declining physical activity levels reflect the effects of increasingly severe nigrostriatal losses as an increasingly limiting factor in motor capacity and physical activity. It is also plausible that lack of physical activity worsens motor symptom severity in PD independent of the degree of nigrostriatal degeneration. The latter hypothesis is in keeping with an emerging body of literature in exercise physiology that both levels of general activity and levels of vigorous exercise have independent effects on health outcomes [5, 9]. A person meeting recommended weekly exercise guidelines may still be at risk of negative health effects if the remainder of the week consists of sedentary behavior [5].
Understanding to what degree physical activity (as determined by the hourly durations of vigorous exercise and non-exercise physical activity) is associated with motor symptom severity in PD independent of the degree of nigrostriatal degeneration requires objective assessment of the integrity of dopaminergic nerve terminals in the living brain of patient with PD. Positron emission tomography (PET) allows measurement of nigrostriatal dopaminergic nerve terminal density. PET imaging using vesicular monoamine transporter, type 2 (VMAT2) ligands quantifies nigrostriatal nerve terminal reductions in PD subjects reflecting neurodegeneration of dopaminergic neurons used to assess disease severity objectively [10, 11].
The aim of this cross-sectional study was to investigate the relationship between duration of time spent in vigorous exercise and non-exercise physical activity and severity of motor functions in PD while accounting for the degree of nigrostriatal degeneration using VMAT2 brain PET imaging. We performed a within-group analysis of PD subjects to test our hypothesis that the degree of physical activity (defined in our study as the combination of vigorous exercise and non-exercise physical activity) influences the expression of clinical motor symptom severity independent of the degree of nigrostriatal dopaminergic denervation. This study addresses a gap in our knowledge as it addresses the effects of physical activity and clinical expression of motor symptoms in PD while taking into account the quantitative relationship between parkinsonian motor symptoms and loss of dopaminergic nerve terminal using an objective in vivo biomarker of the defining neurodegeneration of PD.
Methods
Subjects and clinical test battery
This cross-sectional study involved 48 PD subjects (40 males, 8 females), mean age 69.4±7.4 years (SD; range 56–84), mean Mini-Mental State Examination (MMSE) score of 28.4±1.9 (22–30) and mean duration of disease of 8.4±4.2 years (7–53) who participated in an imaging biomarker study of mobility impairments in PD and who completed the CHAMPS physical activity questionnaire at the time of study enrollment and imaging procedures. Subjects met the UK PD Society Brain Bank clinical diagnostic criteria. Abnormal striatal [11C]DTBZ (DTBZ) PET findings were consistent with the diagnosis of PD in all subjects. No subjects had a history of a large artery stroke or other significant intracranial disease. Most subjects had moderate severity of disease: 1 patient in modified Hoehn & Yahr (HY) stage 1, 1 in stage 1.5, 6 in stage 2, 22 in stage 2.5, 14 in stage 3, 3 in stage 4 and 1 in stage 5. The mean HY stage was 2.7±0.7. All subjects were treated with dopaminergic agents. Thirty-four subjects with PD were taking a combination of dopamine agonist and carbidopa-levodopa medications, 13 were using carbidopa-levodopa alone, 1 was taking dopamine agonist alone. All subjects completed the Unified PD Rating Scale (UPDRS). Mean LED was 973.0±491.5 mg. Subjects on dopaminergic drugs were examined in the morning after withholding dopaminergic drugs overnight. Mean motor UPDRS score was 27.7±10.3 (7–53). UPDRS motor scores were divided into sub-scores for tremor (UPDRS items 20 and 21), rigidity (item 22), distal appendicular bradykinesia (items 23–26 and 31), and axial symptoms (items 27–30).
The CHAMPS physical activity questionnaire was completed to assess levels of non-exercise and exercise physical activity levels [12]. The survey is designed specifically to be utilized by older adults, and has established reliability, sensitivity, and construct validity [12]. This questionnaire provides data on the duration, in hours per week, of various physical activities over a 4-week timeframe. Physical activity here comprises of a number of listed activities that older individuals and less active patients would be more likely to participate in, encompassing a combination of varying levels of vigorous physical activity (exercise), in addition to daily routine activities and recreational activities with physical components (non-exercise physical activity). Use of the CHAMPS questionnaire allows greater sensitivity to measure modest physical activity in this population that would be missed by physical activity scales focusing on more vigorous activities. Total physical activity levels were calculated, based on the standard scoring formula, as summed scores of CHAMPS questionnaire items 7, 9, 10, 14–16, and 19–40. Exercise activities, defined as more vigorous physical activity levels, were summed from items 7, 14–16, 19, 21, 23–26, 29–33, 36–38 and 40. Non-exercise physical activity levels were calculated by determining the difference between total and exercise physical activity scores. Non-exercise and exercise physical activity scores reflect total duration of physical activity in hours during a 4-week period. Supplemental table 1 provides a listing of the individual demographic, clinical and CHAMPS activity data in the subjects.
The study was approved by the Institutional Review Boards of the University of Michigan and Ann Arbor VAMC. Written informed consent was obtained from all subjects prior to any research procedures.
Imaging techniques
All subjects underwent brain MRI and [11C]DTBZ vesicular monoamine transporter type 2 (VMAT2) PET except for a single subject where the PET scan failed because of technical reasons. [11C]DTBZ was prepared as described previously [13]. A bolus/infusion protocol was used for [11C]DTBZ (15 mCi) in 60 minutes [14]. [11C]DTBZ PET imaging was performed the morning after withholding dopaminergic medications overnight. The procedure was explained to the patients and the PET technologist ensured that they were laying properly and comfortably on the camera table to minimize movement. MRI was performed on a 3 Tesla Philips Achieva system (Philips, Best, The Netherlands) and PET imaging was performed in 3D imaging mode with an ECAT Exact HR+ tomograph (Siemens Molecular Imaging, Inc., Knoxville, TN) as reported previously [15].
Analysis
All image frames were spatially coregistered within subjects with a rigid-body transformation to reduce the effects of subject motion during the imaging session [16]. Interactive Data Language image analysis software (Research Systems, Inc., Boulder, CO) was used to manually trace volumes of interest (VOI) on MRI images to include caudate nucleus, and putamen of each hemisphere. Total neocortical VOI were defined using semi-automated threshold delineation of the cortical gray matter signal on the MRI scan [15].
[11C]DTBZ distribution volume ratios were estimated using the Logan plot graphical analysis method - a non-invasive kinetic modeling technique to measure PET binding using a reference region [17] - with the striatal time activity curves as the input function and the total neocortex as reference tissue, a reference region overall low in VMAT2 binding sites [10, 18], with the assumption that the non-displaceable distribution is uniform across the brain at equilibrium [14], as reported previously [15]. [11C]DTBZ PET imaging estimates of striatal binding provide robust correlates of nigral neuronal counts in primates [11].
Data analysis was performed on a de-identified data set. Furthermore, the clinical assessment, administration of the CHAMPS clinical questionnaire and analysis of the PET imaging data were performed by different examiners or analysts. Furthermore, the data analyst performing the MRI VOI manual tracing was blinded to the study aims and hypotheses.
Bivariate correlation coefficients were computed for the relationship between physical activity levels and UPDRS motor scores. Multiple regression was performed to determine the relationship between physical activity levels and UPDRS motor scores while controlling for nigrostriatal VMAT2, age and duration of disease covariates in the PD subjects. The VMAT2 regressor is a prerequisite for the specific testing of our primary hypotheses as detailed in the Introduction. Age and duration of disease covariates are standard covariates in this type of analysis as inclusion of age will be a proxy marker for general age-associated changes in the brain other than PD, and duration of disease will be a proxy marker for possible extra-nigral changes in the PD brain that become more prevalent with longer duration of disease. LED was included as a covariate in the model to account for possible dopaminergic medication effects. Shapiro-Wilk normality tests for the residuals of the variables were performed. Holm-Bonferrroni adjustment for multiple testing was performed. Analyses were performed using SAS version 9.2, SAS institute, Cary, North Carolina).
Results
PD subjects participated in non-exercise physical activities on average for 9.8±6.3 hours (range 0–26 hours) and in exercise physical activities for 8.5±7.2 hours (range 0–30 hr) during a 4 week period. Bivariate correlations showed that increased motor UPDRS severity scores were associated with decreased durations of non-exercise physical activity (R= −0.37, P=0.0099 within Holm-Bonferroni correction); however, there was no significant association of UPDRS motor score with duration of exercise physical activity (R= −0.05, ns). Multiple regression analysis using UPDRS motor score as the outcome variable demonstrated a significant regressor effect for duration of non-exercise physical activity (F=6.15, P=0.017, parameter estimate, PE=−0.51, standard error, SE=0.21) while accounting for effects of nigrostriatal degeneration (F=4.93, P=0.032; PE=−10.1, SE=4.6), levodopa-equivalent dose (LED; F=1.07, P=0.31; PE=0.00285, SE=0.00275), age (F=4.37, P=0.043; PE=0.38, SE=0.18) and duration of disease (F=1.46, P=0.23; PE=0.39, SE=0.33; total model: R2=0.41, adjusted R2=0.34, F(5,41)=5.76, P=0.0004).
Post hoc analysis
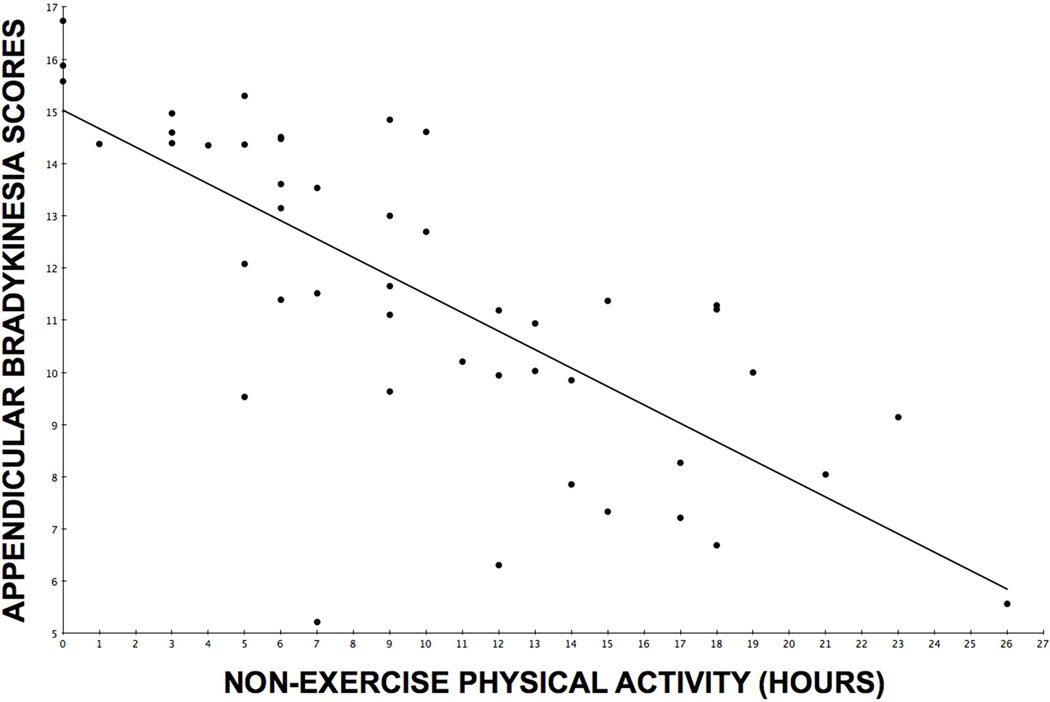
A post hoc analysis was performed to determine whether the inverse relationship between non-exercise physical activity and UPDRS motor scores was selectively associated with specific UPDRS motor subscores. Partial Pearson correlation coefficients (correcting for striatal VMAT2 binding) demonstrated significant inverse correlations between duration of non-exercise physical activity and distal appendicular bradykinesia (R=−0.47, P=0.0012, within Holm-Bonferroni correction) and axial (R=−0.39, P=0.0081, within Holm-Bonferroni correction) subscores, but not with rigidity (R=−0.27, ns) or tremor (R=0.01, ns) subscores. Figure 1 shows a scatter plot of the distribution of duration of non-exercise physical activity and covariate-adjusted distal appendicular bradykinesia scores.
Figure 1.
Scatter plot of the distribution of duration of non-exercise physical activity and covariate-adjusted distal appendicular bradykinesia scores.
Discussion
Our findings show that more severe parkinsonism is associated with lower non-exercise physical activity levels in PD. A plausible explanation is that declining physical activity levels reflect the effects of increasingly severe nigrostriatal losses as an increasingly limiting factor in motor capacity and physical activity. Our data, however, indicate that lower levels of non-exercise physical activity are associated with more severe motor symptom ratings in PD even when nigrostriatal losses are taken into account. One possibility is that declining non-exercise physical activity levels are influenced, at least in part, by non-dopaminergic system pathologies. Another non-exclusive possibility is that increasingly sedentary behavior influences motor features of PD. This latter interpretation is consistent with prior studies showing significant benefits of exercise on motor features of PD [19, 20].
An unexpected finding was that, unlike our a priori hypothesis that more intense exercise activity would be the strongest inverse correlate to motor feature severity in our subjects, non-exercise physical activity was actually the best predictor of UPDRS motor scores. It is conceivable that this dissociation may reflect fundamental pathophysiologic features of PD. The basal ganglia are crucial for efficient performance of habitual motor sequences and it is a truism that many PD patients can temporarily correct motor deficits in PD by during conscious performance of motor acts. Exercise activity may involve volitional mechanisms of motor control that to some degree circumvent the basic motor deficits of basal ganglia dysfunction in PD. In this scenario, exercise performance functions would not necessarily be related to PD motor deficits.
Our data add to a growing body of literature indicating that both intense physical activity and general inactivity have independent and significant impacts on health outcomes [5, 9], in this case motor outcomes in PD. The independent negative effect of sedentariness may be a possible explanation why recent exercise trials in PD, despite improved test performance, did not ultimately demonstrate improvements in disability and quality of life in PD [19, 20]. In other words, a person who participates in an exercise program may still be at risk of negative health effects if the gross remainder of the time consists of sedentary behavior [5].
It is plausible that our assessment of non-exercise physical activity may be a reverse proxy measure of sedentary behavior. Longer duration of non-exercise physical activity may have led to a significant improvement in motor impairments, rather than more vigorous exercise activity having an ameliorating effect on PD motor impairments. Therefore, it is conceivable that the critical relationships between exercise and motor outcomes in other PD observational studies may actually reflect the protective effects of (unmeasured) non-exercise physical activity rather than exercise itself.
It should be noted that there are many processes contributing to inactivity, such as poor peer support [21], lack of motivation, social stigma, suboptimal spousal support, and barriers to exercise [22]. It has also been suggested that muscle weakness may be a cardinal motor feature of PD [23] and weakness and lack of physical activity may possibly trigger manifestation of classic PD motor features [24].
One potential limitation of this study is its reliance on data obtained from a self-reported physical activity questionnaire. Subjective reporting of self-assessed physical activity through questionnaires may be not as reliable as objective sensor-based technology. It has been anecdotally (but frequently) reported that older subjects value being perceived as active people, and as a result may over-report vigorous physical activity. Older individuals with PD also have higher rates of memory and cognitive problems, which could make accurate recall of activity less reliable. As this study does not allow for independent measuring of the accuracy of self-reported physical activity, one future direction could be more objective or sensor-based monitoring of activity, such as actigraphy [25]. It should also be noted that the CHAMPS questionnaire does not capture well seasonal variations in physical activities that are pertinent for subjects residing in four-season climates.
Another consideration is that the survey questions themselves, particularly those involving non-exercise physical activity, may not encompass the full range of activity subjects may participate in during the span of several weeks; in our study, subjects did not have the option to add in information on activities not covered by our questionnaire, and thus may underreport the overall amount of physical activities that they are involved in. Future studies may aim to sample a wider breath of non-exercise physical activities, allowing for both the quantification of time spent on each activity as well as level of intensity. This will need to be balanced with the risk of making a questionnaire overly complicated for an older population that must complete it without requiring assistance.
A limitation of the study is the cross-sectional study design and lack of prospective assessment how the degree or lack of physical activity may modify the clinical course of the disease. Another limitation of the study is that we did not assess the cardiovascular or physiological fitness level in the patients.
We found that non-exercise physical activity levels are associated with decreased typical dopamine-responsive symptoms (such as distal appendicular bradykinesia). Pathophysiologically, this notion is supported by a number of studies suggesting exercise optimizes dopaminergic signaling and dopamine efficiency [4, 26]. The improvement of dopamine-responsive motor symptoms is also supported by animal models of PD [27], which have shown some recovery of nigrostriatal nerve terminals following exercise [28].
Our results, however, also suggest that non-exercise physical activity improves axial motor impairments, such as gait and balance difficulties, that tend to be less dopamine-responsive. Mobility limitations may be due to either PD-specific pathophysiology (i.e., nigrostriatal degeneration) or to cumulative multifactorial age-related changes in cardiopulmonary, musculoskeletal and peripheral nervous systems changes. One explanation for the association between non-exercise physical activity levels and axial motor impairments may be that sedentary behavior contributes to a patient’s general deconditioning and frailty, as opposed to a specific augmentation of a PD-specific neuronal system. Alternatively, non-exercise physical activity is by its nature focused on repetition of common tasks that patients experience in day-to-day life (separate from the unique demands of moderate or vigorous exercise) that may help maintain gait and balance skills through less strenuous daily activities. In this way, it is possible that non-exercise physical activity help to augment the postural reflexes whose gradual loss is invoked in PD patients who go on to develop PIGD symptoms. In this respect, non-exercise physical activities may improve habit-formation-maintenance via recruitment of non-dopaminergic systems. These findings reinforce the potential benefit of non-exercise physical activity in PD, as it facilitates improvement of motor features that are otherwise relatively refractory to dopaminergic therapy. Our findings did not show a selective association with ratings of tremor, suggesting the tremor expression may not be modulated by physical activity levels.
We conclude that non-exercise physical activity may influence motor symptom severity in PD, independent of nigrostriatal degeneration. It should also be noted that a lifestyle intervention program [29] increased the amount of weekly outdoor physical activity in PD [30]. However, our understanding of how to increase physical activity in PD remains incomplete. Active "Stand-up, sit-less, move-more" intervention strategies deserve further studies to reduce the sedentary lifestyle and improve patient functionality in PD.
Supplementary Material
Highlights.
Non-exercise physical activity is associated with better motor scores in PD.
This effect was independent from degree of nigrostriatal denervation.
Inactivity and vigorous exercise appear to have independent effects on health outcomes in PD.
Acknowledgements
The authors thank all patients for their time commitment and research assistants, PET technologists, cyclotron operators, and chemists, for their assistance with the study. This work was supported by the Department of Veterans Affairs [grant number I01 RX000317]; the Michael J. Fox Foundation; and the NIH [grant numbers P01 NS015655 and RO1 NS070856].
Dr. Muller has research support from the NIH, Michael J. Fox Foundation and the Department of Veteran Affairs. Dr. Kotagal has research support from the NIH. Dr. Koeppe receives research support from NIH, the American Academy of Neurology Clinical Research Training Fellowship, and the Blue Cross Blue Shield of Michigan Foundation. Dr. Scott receives Editorial Royalties from Wiley, is an owner of SynFast Consulting, LLC, and has received research funding from the University of Michigan, GE Healthcare, Bristol-Myers Squibb, Bayer Pharma AG, Eli Lilly, and Molecular Imaging Research. Dr. Albin serves on the editorial boards of Neurology, Experimental Neurology, and Neurobiology of Disease. He receives grant support from the National Institutes of Health, CHDI, and the Michael J. Fox Foundation. Dr. Albin serves on the Data Safety and Monitoring Boards of the PRIDE-HD, ISIS 443139-CS1 and LEGATO trials. Dr. Frey has research support from the NIH, GE Healthcare and AVID Radiopharmaceuticals (Eli Lilly subsidiary). Dr. Frey also serves as a consultant to AVID Radiopharmaceuticals, MIMVista, Inc, Bayer-Schering and GE healthcare. He also holds equity (common stock) in GE, Bristol-Myers, Merck and Novo-Nordisk. Dr. Bohnen has research support from the NIH, Department of Veteran Affairs, and the Michael J. Fox Foundation.
Abbreviations
- CHAMPS
Community Health Activities Model Program for Seniors questionnaire
- DTBZ
dihydrotetrabenazine
- LED
Levodopa Equivalent Dose
- PD
Parkinson disease
- PET
positron emission tomography
- UPDRS
Unified Parkinson's Disease Rating Scale
- VMAT2
vesicular monoamine transporter type 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest relevant to this work. Dr. Snider has no conflict of interest relevant to this work.
References
- 1.van Nimwegen M, Speelman AD, Hofman-van Rossum EJ, Overeem S, Deeg DJ, Borm GF, van der Horst MH, Bloem BR, Munneke M. Physical inactivity in Parkinson's disease. J Neurol. 2011;258:2214–2221. doi: 10.1007/s00415-011-6097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis T, Motl RW. Physical activity behavior change in persons with neurologic disorders: overview and examples from Parkinson disease and multiple sclerosis. J Neurol Phys Ther. 2013;37:85–90. doi: 10.1097/NPT.0b013e31829157c0. [DOI] [PubMed] [Google Scholar]
- 3.Bloem BR, van Vugt JP, Beckley DJ. Postural instability and falls in Parkinson's disease. Adv Neurol. 2001;87:209–223. [PubMed] [Google Scholar]
- 4.Speelman AD, van de Warrenburg BP, van Nimwegen M, Petzinger GM, Munneke M, Bloem BR. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol. 2011;7:528–534. doi: 10.1038/nrneurol.2011.107. [DOI] [PubMed] [Google Scholar]
- 5.Dunlop D, Song J, Arnston E, Semanik P, Lee J, Chang R, Hootman JM. Sedentary Time in U.S. Older Adults Associated With Disability in Activities of Daily Living Independent of Physical Activity. J Phys Act Health. 2014 doi: 10.1123/jpah.2013-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson's disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20:190–199. doi: 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson's disease. Arch Phys Med Rehabil. 2003;84:1109–1117. doi: 10.1016/s0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 8.Boelen M. The role of rehabilitative modalities and exercise in Parkinson's disease. Dis Mon. 2007;53:259–264. doi: 10.1016/j.disamonth.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Ekblom-Bak E, Ekblom B, Vikstrom M, de Faire U, Hellenius ML. The importance of non-exercise physical activity for cardiovascular health and longevity. Br J Sports Med. 2014;48:233–238. doi: 10.1136/bjsports-2012-092038. [DOI] [PubMed] [Google Scholar]
- 10.Frey KA, Koeppe RA, Kilbourn MR. Imaging the vesicular monoamine transporter. Adv Neurol. 2001;86:237–247. [PubMed] [Google Scholar]
- 11.Brown CA, Karimi MK, Tian L, Flores H, Su Y, Tabbal SD, Loftin SK, Moerlein SM, Perlmutter JS. Validation of midbrain positron emission tomography measures for nigrostriatal neurons in macaques. Ann Neurol. 2013;74:602–610. doi: 10.1002/ana.23939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Shao X, Hoareau R, Hockley BG, Tluczek LJ, Henderson BD, Padgett HC, Scott PJ. Highlighting the Versatility of the Tracerlab Synthesis Modules. Part 1: Fully Automated Production of [F]Labelled Radiopharmaceuticals using a Tracerlab FX(FN) J Labelled Comp Radiopharm. 2011;54:292–307. doi: 10.1002/jlcr.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koeppe RA, Frey KA, Kuhl DE, Kilbourn MR. Assessment of extrastriatal vesicular monoamine transporter binding site density using stereoisomers of [11C]dihydrotetrabenazine. J Cereb Blood Flow Metab. 1999;19:1376–1384. doi: 10.1097/00004647-199912000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Bohnen NI, Mueller MLTM, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, Albin RL, Frey KA. Heterogeneity of cholinergic denervation in Parkinson disease. J Cereb Blood Flow Metab. 2012;32:1609–1617. doi: 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minoshima S, Koeppe RA, Fessler JA, Mintun MA, Berger KL, Taylor SF, Kuhl DE. Integrated and automated data analysis method for neuronal activation studying using O15 water PET. In: Uemura K, Lassen NA, Jones T, Kanno I, editors. Quantification of brain function to tracer kinetics and image analysis in brain PET. Tokyo: Excerpta Medica; 1993. pp. 409–418. [Google Scholar]
- 17.Logan J, Fowler AH, Volkow ND, Wang G-J, Ding Y-S, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Vander Borght TM, Kilbourn MR, Koeppe RA, DaSilva JN, Carey JE, Kuhl DE, Frey KA. In vivo imaging of the brain vesicular monoamine transporter. J Nucl Med. 1995;36:2252–2260. [PubMed] [Google Scholar]
- 19.Corcos DM, Robichaud JA, David FJ, Leurgans SE, Vaillancourt DE, Poon C, Rafferty MR, Kohrt WM, Comella CL. A two-year randomized controlled trial of progressive resistance exercise for Parkinson's disease. Mov Disord. 2013 doi: 10.1002/mds.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shulman LM, Katzel LI, Ivey FM, Sorkin JD, Favors K, Anderson KE, Smith BA, Reich SG, Weiner WJ, Macko RF. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol. 2013;70:183–190. doi: 10.1001/jamaneurol.2013.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch MA, Sanjak M, Englert D, Iyer S, Quinlan MM. Parkinson patients as partners in care. Parkinsonism Relat Disord. 2014;20(Suppl 1):S174–S179. doi: 10.1016/S1353-8020(13)70041-5. [DOI] [PubMed] [Google Scholar]
- 22.van der Eijk M, Faber MJ, Aarts JW, Kremer JA, Munneke M, Bloem BR. Using online health communities to deliver patient-centered care to people with chronic conditions. Journal of medical Internet research. 2013;15:e115. doi: 10.2196/jmir.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koller W, Kase S. Muscle strength testing in Parkinson's disease. Eur Neurol. 1986;25:130–133. doi: 10.1159/000115998. [DOI] [PubMed] [Google Scholar]
- 24.Falvo MJ, Schilling BK, Earhart GM. Parkinson's disease and resistive exercise: rationale, review, and recommendations. Mov Disord. 2008;23:1–11. doi: 10.1002/mds.21690. [DOI] [PubMed] [Google Scholar]
- 25.van Nimwegen M, Speelman AD, Overeem S, van de Warrenburg BP, Smulders K, Dontje ML, Borm GF, Backx FJ, Bloem BR, Munneke M, ParkFit Study G. Promotion of physical activity and fitness in sedentary patients with Parkinson's disease: randomised controlled trial. BMJ. 2013;346:f576. doi: 10.1136/bmj.f576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol. 2013;12:716–726. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neurosci. 2003:119. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 28.Zigmond MJ, Cameron JL, Leak RK, Mirnics K, Russell VA, Smeyne RJ, Smith AD. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat Disord. 2009;15(Suppl 3):S42–S45. doi: 10.1016/S1353-8020(09)70778-3. [DOI] [PubMed] [Google Scholar]
- 29.Speelman AD, van Nimwegen M, Bloem BR, Munneke M. Evaluation of implementation of the ParkFit program: A multifaceted intervention aimed to promote physical activity in patients with Parkinson's disease. Physiotherapy. 2014;100:134–141. doi: 10.1016/j.physio.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 30.van der Kolk NM, van Nimwegen M, Speelman AD, Munneke M, Backx FJ, Donders R, Post B, Overeem S, Bloem BR. A personalized coaching program increases outdoor activities and physical fitness in sedentary Parkinson patients; a post-hoc analysis of the ParkFit trial. Parkinsonism Relat Disord. 2014;20:1442–1444. doi: 10.1016/j.parkreldis.2014.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.