Endogenous target mimecry of an miRNA affects nicotine biosynthesis.
Abstract
The interaction between noncoding endogenous target mimicry (eTM) and its corresponding microRNA (miRNA) is a newly discovered regulatory mechanism and plays pivotal roles in various biological processes in plants. Tobacco (Nicotiana tabacum) is a model plant for studying secondary metabolite alkaloids, of which nicotine accounts for approximately 90%. In this work, we identified four unique tobacco-specific miRNAs that were predicted to target key genes of the nicotine biosynthesis and catabolism pathways and an eTM, novel tobacco miRNA (nta)-eTMX27, for nta-miRX27 that targets QUINOLINATE PHOSPHORIBOSYLTRANSFERASE2 (QPT2) encoding a quinolinate phosphoribosyltransferase. The expression level of nta-miRX27 was significantly down-regulated, while that of QPT2 and nta-eTMX27 was significantly up-regulated after topping, and consequently, nicotine content increased in the topping-treated plants. The topping-induced down-regulation of nta-miRX27 and up-regulation of QPT2 were only observed in plants with a functional nta-eTMX27 but not in transgenic plants containing an RNA interference construct targeting nta-eTMX27. Our results demonstrated that enhanced nicotine biosynthesis in the topping-treated tobacco plants is achieved by nta-eTMX27-mediated inhibition of the expression and functions of nta-miRX27. To our knowledge, this is the first report about regulation of secondary metabolite biosynthesis by an miRNA-eTM regulatory module in plants.
MicroRNAs (miRNAs) are a class of small noncoding RNAs with a typical length of 20 to 22 nucleotides and have been shown to play important roles in development, signal transduction, and responses to biotic and abiotic stresses in plants (Phillips et al., 2007; Khraiwesh et al., 2012). MiRNAs are generated from single-strand RNA precursors (pre-miRNAs) with a stem-loop structure (Bartel, 2004). The pre-miRNA stem-loop structures are processed into miRNA/miRNA* duplexes by a protein complex including Dicer-like1 (a ribonuclease [RNase] III-like endoribonuclease). The mature miRNA is then loaded onto the Argonaute-containing RNA-induced silencing complex to cause target mRNA degradation or translational repression through complementary sequence binding (Naqvi et al., 2012). Most plant miRNAs have perfect or near-perfect complementarity with their targets (Axtell and Bowman, 2008; Mallory and Bouché, 2008); therefore, plant miRNA targets can usually be successfully predicted based on sequence complementarity between miRNAs and their targets (Rhoades et al., 2002; Sunkar and Zhu, 2004). A perfect base pairing between miRNAs and their targets at the ninth to 11th positions from the 5′ end of miRNAs is important for effective miRNA-mediated cleavage of targets (Jones-Rhoades et al., 2006; Pasquinelli, 2012).
In Arabidopsis (Arabidopsis thaliana), a long non-protein-coding mRNA gene, INDUCED BY PHOSPHATE STARVTION1 (IPS1), was found to be bound by miR399 with a three-nucleotide bulge in the middle of the miR399 binding site. This central mismatch disrupts crucial base pairing between miR399 and IPS1 and hence inhibits miR399-mediated cleavage of IPS1. This observation leads to the hypothesis that IPS1 functions as a noncleavable endogenous target mimicry (eTM) of miR399, which blocks the interaction between miR399 and its authentic targets by sequestering miR399 and arresting its cleavage activity (Franco-Zorrilla et al., 2007). Later, noncoding eTMs for diverse miRNAs have been identified in Arabidopsis, rice (Oryza sativa), and soybean (Glycine max), and some of them have been shown to regulate plant development by repressing miRNA function (Todesco et al., 2010; Ivashuta et al., 2011; Wu et al., 2013). These studies also suggested that eTMs are widespread in plant species. Recent studies based on transcriptome sequencing have demonstrated the widespread existence of long noncoding RNAs in plants (Li et al., 2014b; Zhang et al., 2014); however, functions of the majority of those long noncoding RNAs are largely unknown. It would not be surprising to find a portion of these long noncoding RNAs acting as eTMs. Additionally, short tandem target mimic (STTM) technology has been developed with an aim to investigate functions of small RNAs by blocking small RNA functions in plants and animals (Tang et al., 2012a; Yan et al., 2012). STTM is a powerful technology complementing the target mimic in plants and the miRNA sponge in animals.
Alkaloids are a major type of secondary metabolites. Nicotine accounts for approximately 90% of the total alkaloid content and serves as defensive compounds against herbivores in tobacco (Nicotiana tabacum; Saitoh et al., 1985; Baldwin et al., 2001). Meanwhile, nicotine is also the most important component in tobacco products due to its stimulatory and addictive effects. Nicotine is exclusively synthesized in tobacco roots and then transported to leaves through the xylem (Dewey and Xie, 2013). Nicotine alkaloids are accumulated in most Nicotiana species (Baldwin, 1999). The nicotine biosynthesis and catabolism pathways have been extensively studied (for review, see Dewey and Xie, 2013). Several key genes encoding enzymes of the nicotine biosynthesis pathway, such as quinolinate phosphoribosyltransferase (QPT), putrescine methyltransferase (PMT), N-methylputrescine oxidase, and nicotine N-demethylase (a cytochrome P450 monooxygenase [CYP82E4]), have been cloned and characterized (Conkling et al., 1990; Hibi et al., 1992; Siminszky et al., 2005; Katoh et al., 2006; Heim et al., 2007). QPT converts quinolinic acid to nicotinic acid mononucleotide and serves as the entry point into the pyridine nucleotide cycle that leads to the production of nicotinic acid and consequently nicotine (Dewey and Xie, 2013). QPT was first isolated from tobacco roots by Conkling et al. (1990) and later characterized by Song (1997) and Sinclair et al. (2000). QPT2 is strongly and exclusively expressed in root tissue and involved in nicotine biosynthesis, while its homolog QPT1 is nonresponsive to biotic and abiotic stresses and retains its original function in NAD production (Dewey and Xie, 2013). Transgenic tobacco lines with a low level of nicotine content have been developed by suppression of QPT2 using the antisense-mediated gene-silencing approach (Xie et al., 2004).
Recent reports have shown that miRNAs are involved in regulation of secondary-metabolite synthesis in plants. For example, miR163 can change the production profiles of secondary metabolite in Arabidopsis (Ng et al., 2011); miR393 redirects the secondary-metabolite productions via perturbing auxin signaling (Robert-Seilaniantz et al., 2011); and some miRNAs might be involved in biosynthesis of benzylisoquinoline alkaloids in opium poppy (Papaver somniferum; Boke et al., 2015). In tobacco, it has been shown that miR164 and its target NtNAC-R1, a unique NAC (for NAM, no apical meristem) transcription factor identified in tobacco, was down- and up-regulated in response to topping, respectively, and resulted in increase of lateral roots and nicotine contents (Fu et al., 2013). However, it is still unclear whether miRNAs are directly involved in regulation of the nicotine biosynthesis pathway in tobacco.
To determine the roles of miRNAs in nicotine biosynthesis, in this study, we first identified nicotine biosynthesis-related miRNAs in two small RNA populations generated from topping-treated tobacco roots using the newly available tobacco genome sequence as a reference (Sierro et al., 2014). We found four unique miRNAs that target several key genes of the nicotine biosynthesis and catabolism pathways. Further investigation on novel tobacco miRNA (nta)-miRX27, which targets QPT2, by overexpression and inhibition of the miRNA function confirmed that nta-miRX27 played an important role in regulation of tobacco plants in response to topping and nicotine accumulations. More interestingly, we identified an eTM (nta-eTMX27) for nta-miRX27 and proved that it can effectively inhibit the functions of nta-miRX27 and regulate nicotine biosynthesis in the topping-treated tobacco plants.
RESULTS
Identification of Nicotine Biosynthesis-Related miRNAs in Tobacco
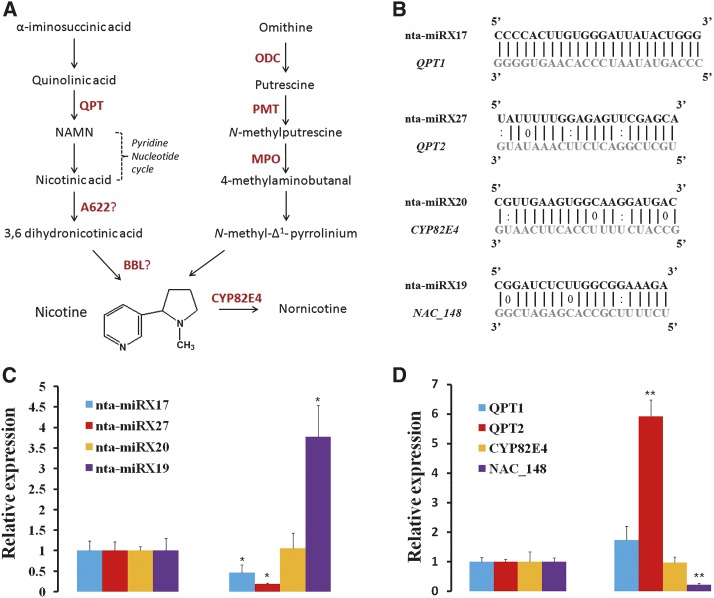
The recently available genome sequences of tobacco and its two progenitors provided an opportunity to identify miRNAs related to nicotine biosynthesis in tobacco. Based on the small-RNA data generated from roots of control and topping-treated tobacco plants by deep sequencing in our previous study (Tang et al., 2012b), we identified a number of unique miRNAs that have not been reported previously, including four that were predicted to target genes (QPT1, QPT2, PMT2, and CYP82E4) involved in nicotine biosynthesis (Fig. 1A; Supplemental Table S1). These four miRNAs seemed to be tobacco specific, as no homologous sequence was found in the miRBase database.
Figure 1.
Genes involved in nicotine biosynthesis in tobacco and expression changes of miRNAs and their nicotine-related targets at 48 h after topping. A, A schematic diagram of the nicotine biosynthesis pathway in tobacco (adapted from Dewey and Xie, 2013). A622, Isoflavone reductase-like protein; BBL, berberine bridge enzyme-like; MPO, N-methylputrescine oxidase; CYP82E4, nicotine N-demethylase; ODC, Orn decarboxylase. B, Alignment of nta-miRNAs and their targets. Base pairing between miRNA and its target are shown, in which a vertical line means a Watson-Crick pair, two dots represent a G-U pair, and 0 means a mismatch. C, RT-qPCR analyses of nta-miRX17, nta-miRX27, nta-miRX20, and nta-miRX19 at 48 h after topping. Mitochondrial 5S RNA served as an internal standard for expression normalization. D, RT-qPCR analyses of miRNA target genes (QPT1, QPT2, CYP83E4, and NAC_148) at 48 h after topping. Glyceraldehyde 3-phosphate dehydrogenase mRNA served as an internal standard for expression normalization. Three independent experiments, each consisting of three control and topping-treated plants, were carried out for quantification analyses, and representative results in one time are presented. The expression levels of miRNAs and their targets in plants without topping treatment (control) are arbitrarily set as 1. Error bars indicate sd. Student’s t tests were performed to compare differences of miRNAs and their targets between the control and the topping treatment. A single asterisk indicates a significant difference (P < 0.05), and double asterisks indicate a highly significant difference (P < 0.01) between the two paired samples.
Previous studies demonstrated that there was a significant increase in the activity of several nicotine biosynthetic enzymes in the tobacco roots at 24 to 48 h after topping (Dewey and Xie, 2013). To test the effects of topping on nicotine biosynthesis-related miRNAs and their target genes, we measured their expression changes, as well as NtNAC_148 (targeted by nta-miRX19), which has been shown to be down-regulated upon topping treatment (W. Wang and L. Fan, unpublished data), using samples collected at 48 h after topping. As expected, NAC_148 was down-regulated significantly after topping, which is most likely caused by up-regulation of nta-miRX19 (Fig. 1, B and C). The expression levels of nta-miRX17 and nta-miRX27 were down-regulated upon topping, and consequently, their targets QPT1 and QPT2 were up-regulated (Fig. 1, C and D). The negatively correlated relationship was striking for nta-miRX27 and QPT2 (Fig. 1, C and D). By contrast, topping seemed to have no effect on the expression of nta-miRX20, nta-miRX13, and their targets, CYP82E4 and PMT2, respectively. CYP82E4, which is responsible for nicotine to nornicotine demethylation (Dewey and Xie, 2013), has been shown to not be topping responsive. These results suggested that the nta-miRX27-QPT2 interaction may be important for regulation of nicotine biosynthesis in tobacco.
The reversely correlated expression relationship between nta-miRX27 and its target QPT2 suggests nta-miRX27-mediated cleavage of QPT2. Two binding sites of nta-miRX27 could be predicted on the QPT2 gene. The first one is located at exon number 2, and the second one is located at the intron between exon numbers 8 and 9 (based on AJ748263 deposited in the National Center for Biotechnology Information) or at exon number 8 (based on Fgenesh prediction; http://www.softberry.com). We used RNA ligase-mediated (RLM)-5′ RACE to confirm nta-miRX27-mediated cleavage of QPT2 but failed for both sites (Supplemental Fig. S1), although the first one was confirmed to be cleaved based on our previous degradome data (Supplemental Fig. S2)
Regulation of nta-miRX27 on Endogenous QPT2 Expression and Nicotine Accumulation
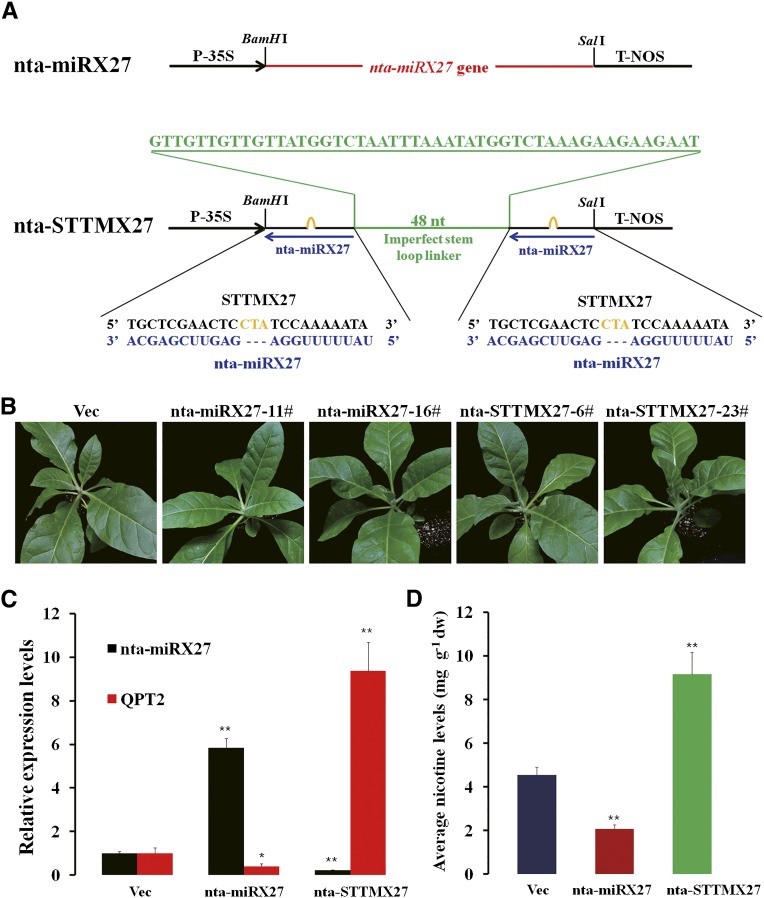
To investigate whether nta-miRX27 functions to repress expression of its target QPT2 and plays any role in nicotine production, we generated transgenic tobacco plants overexpressing or silencing nta-miRX27. The silencing transgenic plants (nta-STTMX27) were generated using the STTM strategy (Fig. 2A). The expression levels of nta-miRX27 in the overexpressing and silencing transgenic plants were confirmed to be significantly up- and down-regulated, respectively (Fig. 2C). The expression changes of nta-miRX27 in all transgenic plants did not cause observable phenotypic changes (Fig. 2B), consistent with the expected effects of nta-miRX27. The expression level of QPT2 was decreased by approximately 50% in the nta-miRX27 transgenic plants compared with the control plants, but a significantly increased level of QPT2 was observed in the nta-STTMX27 transgenic plants (Fig. 2C). Consistent with these expression changes of QPT2, decreased and increased nicotine contents were observed in the nta-miRX27 and nta-STTMX27 transgenic plants, respectively. The nicotine content in the leaves of the nta-STTMX27 and nta-miRX27 transgenic plants was 9.17 ± 1.29 mg g–1 and 2.17 ± 1.15 mg g–1, respectively (Fig. 2D). These results strongly suggested that nta-miRX27-mediated regulation of its target QPT2 plays an important role in nicotine biosynthesis in tobacco.
Figure 2.
nta-miRX27 repressed QPT2 expressions and reduced nicotine contents. A, Diagram of nta-miRX27 and nta-STTMX27 structures showing the design strategy. nta-miRX27 gene and two tandem target mimics (STTMX27) spaced by a 48-nucleotide (nt) imperfect stem-loop linker were cloned between a Cauliflower mosaic virus 35S promoter (P-35S) and nopaline synthase (NOS) terminator (T-NOS) to produce nta-miRX27 and nta-STTMX27 constructs, respectively. Red indicates the nta-miRX27 gene, green indicates the spacer region and the spacer sequence, blue indicates the mature nta-miRX27 sequences, and orange indicates the bulge sequences in the miRNA binding sites. B, Phenotypes of 30-d-old transgenic plants containing nta-miRX27 or nta-STTMX27 compared with that of plants transformed with a Vec. Two independent and representative transgenic lines overexpressing nta-miRX27 (nta-miRX27-11# and nta-miRX27-16#) and silencing nta-miRX27 (nta-STTMX27-6# and nta-STTMX27-23#) are shown. C, Relative expression levels of nta-miRX27 and its target gene QPT2 in nta-miRX27 and nta-STTMX27 transgenic plants compared with that of the Vec plants. Three individual plants per genotype were used in RT-qPCR, and bars represent sd of three replicates. D, Average nicotine contents in leaves sampled from the Vec, nta-miRX27, and nta-STTMX27 plants. Each mean value was derived from three independent experiments (n = 9). Error bars indicate the sd of three replicates. Significance tests were performed by the Student’s t test. A single asterisk indicates a significant difference (P < 0.05), and double asterisks indicate a highly significant difference (P < 0.01) between the two paired samples. dw, Dry weight.
Identification and Expression Analysis of eTMs for nta-miRX27
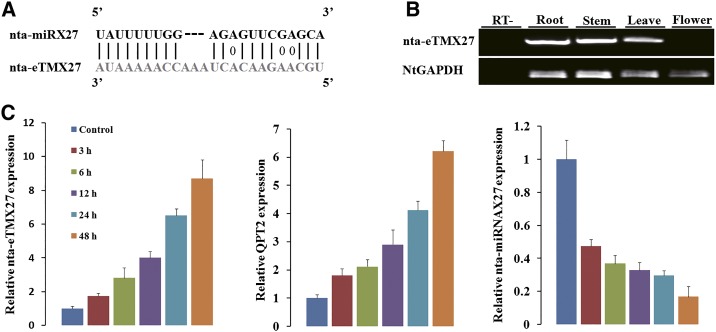
To determine potentially additional regulators related to the nat-miRX27-QPT2 module involved in regulation of nicotine biosynthesis, we performed eTM identification using various available transcripts, including those in-house assembled using available tobacco RNA sequencing data, and identified an eTM for nta-miRX27, which was named nta-eTMX27. The putative transcript of nta-eTMX27 was 1,213 bp long, with the largest predicted open reading frame being 144 bp in length. It was covered by several ESTs (FS399281, FS416548, and FG201217), and at least one EST (FG201217) covered the target mimic site of nta-eTMX27, although a few single-nucleotide polymorphisms were found between nta-eTMX27 and FG201217. The binding site of nta-miRX27 in nta-eTMX27 contains a three-nucleotide bulge between the ninth and 10th positions of nta-miRX27 (Fig. 3A). Moreover, according to the degradome data generated previously (Tang et al., 2012b), no degradomic read was mapped to the target mimic site of nta-eTMX27, supporting the notion that nta-eTMX27 may act as a decoy for nta-miRX27. Nta-eTMX27 was found to be expressed in root, stem, and leaf tissues but not in flowers (Fig. 3B).
Figure 3.
Topping-induced expression of endogenous nta-eTMX27 in tobacco root. A, The predicted base-pairing pattern between nta-miRX27 and its eTM nta-eTMX27. B. Semiquantitative RT-PCR analysis of expressions of nta-eTMX27 in four tissues. Tobacco glyceraldehyde 3-phosphate dehydrogenase gene (NtGAPDH) was used as control. RT– represents RT negative control, i.e. RT without reverse transcriptase. C, The relative transcription levels of nta-eTMX27, QPT2, and nta-miRX27 in tobacco root tissues measured at 3, 6, 12, 24, and 48 h after topping. Three independent experiments, each consisting of three control and topping-treated plants, were carried out for quantification analyses, and representative results in one time are presented. The expression level at the 0-h time point (control) was arbitrarily set as 1. Error bars indicate the sd.
To determine whether the predicted nta-eTMX27 plays a role in repressing miRNA function, its expression changes together with that of nta-miRX27 and its target QPT2 were analyzed in roots of the tobacco plants sampled at 3 to 48 h after the topping treatment (Fig. 3C). Apparently, the expression level of nta-eTMX27 increased steadily after the topping treatment and was approximately 9-fold higher than that of the control (0 h after topping) at 48 h after the topping treatment (Fig. 3C, first column). Similarly, the expression level of QPT2 was also up-regulated after topping (Fig. 3C, second column), while the expression level of nta-miRX27 was down-regulated during the same time period after the topping treatment (Fig. 3C, third column). These results indicate that topping treatment induces nta-eTMX27 expression, which leads to down-regulation of nta-miRX27 and an increase of QPT2 expression.
nta-eTMX27 Induced miRNA Degradation and Repressed miRNA Function
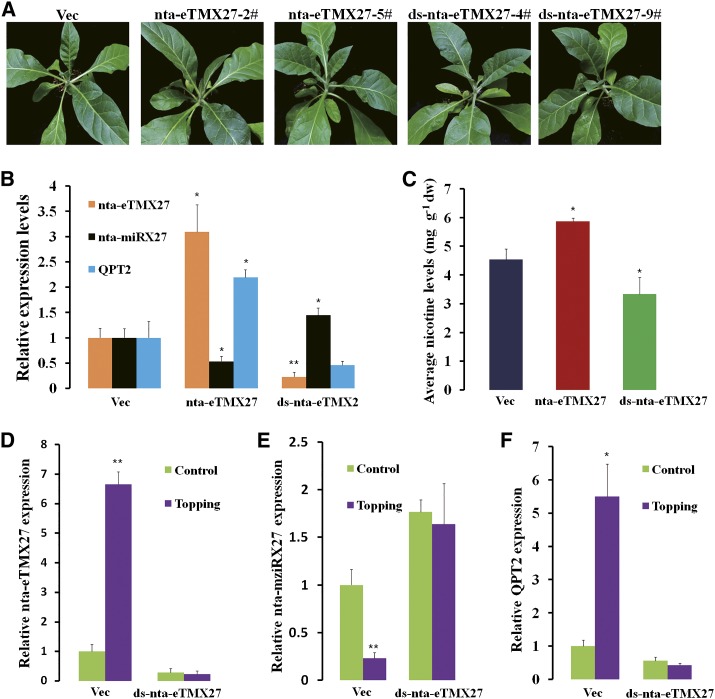
To confirm the function of nta-eTMX27, i.e. sequestration of nta-miRX27 away from its target QPT2, we generated transgenic tobacco plants overexpressing nta-eTMX27 or harboring a RNA interference (RNAi) construct (double-stranded [ds]-nta-eTMX27) targeting nta-eTMX27. Under the greenhouse conditions, all transgenic plants grew normally and did not show obvious altered phenotypes compared with the plants transformed with the empty vector (Vec; Fig. 4A). Real-time (RT) quantitative PCR (qPCR) assay showed that the expression level of nta-eTMX27 was approximately 3-fold higher in the nta-eTMX27 overexpressing lines than in the Vec lines, while the expression levels of nta-miRX27 and QPT2 were reduced by approximately 50% and increased by more than 2-fold, respectively (Fig. 4B). In the nta-eTMX27 RNAi transgenic plants (ds-nta-eTMX27), the expression level of nta-eTMX27 was reduced to approximately 23% of the level in the Vec plants. As a result, the expression levels of nta-miRX27 and QPT2 were increased and decreased, respectively (Fig. 4B). In addition, the nicotine contents in the nta-eTMX27 and ds-nta-eTMX27 transgenic lines were significantly increased and decreased, respectively (Fig. 4C). These results not only confirmed the regulatory module involving nta-miRX27, QPT2, and nta-eTMX27, but also indicate that the nicotine contents in tobacco plants can be manipulated by overexpression or silencing of nta-eTMX27.
Figure 4.
Functional analyses of nta-eTMX27. A, Phenotypes of nta-eTMX27 overexpression and silencing plants. Two independent and representative transgenic lines overexpressing nta-eTMX27 (nta-eTMX27-2# and nta-eTMX27-5#) and silencing nta-eTMX27 (ds-nta-eTMX27-4# and ds-nta-eTMX27-9#) are shown. B, Relative expression levels of nta-eTMX27, nta-miRX27, and QPT2 in nta-eTMX27-overexpressing and -silencing transgenic plants compared with that of the Vec plants. Three individual plants per genotype were used in RT-qPCR. Bars show sd. C. Average leaf nicotine contents of the Vec, nta-miRX27, and nta-STTMX27 lines. A single asterisk indicates a significant difference (P < 0.05) between the nta-miRX27 or nta-STTMX27 line and the Vec control. Error bars indicate the sd. dw, Dry weight. D, Expression levels of nta-eTMX27 in roots of transgenic plants transformed with the Vec or ds-nta-eTMX27 construct in response to topping. Samples were collected at 48 h after topping treatment (without topping as control). The expression level in the Vec plants without topping treatment was arbitrarily set as 1. Error bars indicate sd. Double asterisks indicate a significant difference (Student’s t test, P < 0.01) between the two samples. E, Relative expression levels of nta-eTMX27 in roots of transgenic plants transformed with the Vec or ds-nta-eTMX27 after topping treatment at 48 h. The expression level of the Vec plants with control treatment was arbitrarily set as 1. Error bars indicate the sd. F, Relative expression levels of QPT2 in the Vec and ds-nta-eTMX27 transgenic plants after topping treatment at 48 h. Error bars indicate sd. The expression level of the Vec plants with control treatment was arbitrarily set as 1. A single asterisk indicates a significant difference (Student’s t test, P < 0.05), and double asterisks indicate a highly significant difference (Student’s t test, P < 0.01) between the two paired samples.
The opposite functions of nta-miRX27 and nta-eTMX27 in regulating QPT2 transcript levels and nicotine biosynthesis led us to postulate that nta-eTMX27 may induce nta-miRX27 degradation and repress miRNA function in response to external stimuli, such as topping, during plant growth and development. To test this possibility, we compared the expression changes of nta-miRX27 and QPT2 in the transgenic plants containing the Vec or ds-nta-eTMX27 construct after the topping treatment. As expected, expression of nta-miRX27 was down-regulated, while expression of QPT2 and nta-eTMX27 was up-regulated in the Vec plants (Fig. 4, D–F); however, these changes were not observed in the ds-nta-eTMX27 transgenic plants (Fig. 4, D–F). These results indicate that nta-eTMX27 seems to be essential for topping-induced down-regulation of nta-miRX27 and that nta-eTMX27 may play a role in repressing the function of nta-miRX27 by degradation under stress conditions.
Evolution of nta-miRX27 and nta-eTMX27
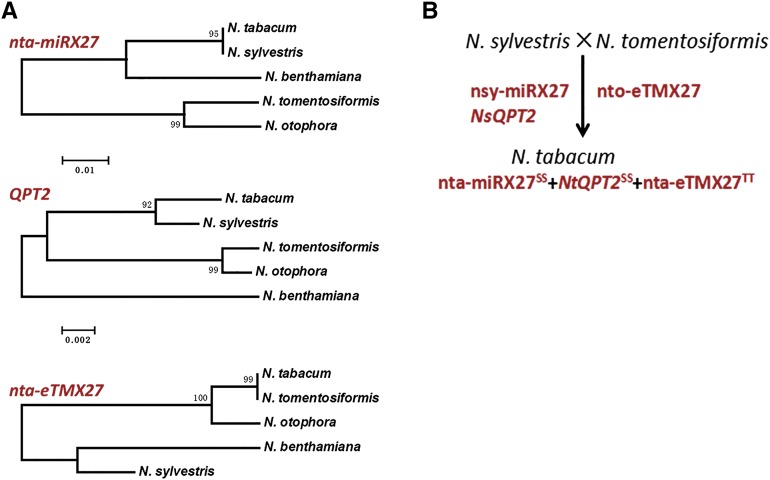
No ortholog of the primary transcript of nta-miRX27 or the nta-miRX27 mature sequence could be found in the current tomato genome (http://www.solgenomics.net/) and miRBase, suggesting that nta-miRX27 may be Nicotiana spp. specific. A near-identical sequence of nta-miRX27 was found in Nicotiana sylvestris but not in Nicotiana tomentosiformis (Fig. 5A), suggesting that nta-miRX27 was most likely derived from the progenitor N. sylvestris. Meanwhile, QPT2 of common tobacco also showed more sequence similarity, including the presence of the nta-miRX27 binding site, with its ortholog in N. sylvestris. By contrast, no miRNA and miRNA target site could be predicted in the ortholog of QPT2 and its surrounding region in N. tomentosiformis because of nucleotide mutations, implying that the nta-miRX27-QPT2 regulatory module might have been only evolved in the progenitor N. sylvestris but not in N. tomentosiformis. Interestingly, an almost identical sequence of nta-eTMX27 was found in N. tomentosiformis but not in N. sylvestris (Fig. 5A). Compared with common tobacco and N. tomentosiformis, N. sylvestris had one nucleotide substitution and an insertion/deletion in the target mimic site of the sequence corresponding to nta-eTMX27 (data not shown). Consequently, the nta-miRX27-nta-eTMX27 interaction could not be predicted in the N. sylvestris genome based on our current bioinformatic pipeline.
Figure 5.
Phylogenetic tree (A) and evolution model (B) of nta-miRX27, its target QPT2, and target mimic nta-eTMX27 in the Nicotiana genus. The nucleotide sequences of nta-miRX27, QPT2, and nta-eTMX27 from tobacco, N. sylvestris (nsy), N. benthamiana, N. otophora, and N. tomentosiformis (nto) were aligned using the neighbor-joining method with 1,000 replications.
DISCUSSION
The role of miRNAs in secondary metabolite biosynthesis has been demonstrated in plants (Ng et al., 2011; Robert-Seilaniantz et al., 2011), and potential regulation of alkaloid biosynthesis by miRNA in opium poppy has also been suggested (Boke et al., 2015). Target mimicry has emerged as a new mechanism regulating function of miRNAs, and a number of long noncoding RNAs acting as eTMs to block the biological function of miRNAs have been identified in plants (Franco-Zorrilla et al., 2007; Todesco et al., 2010; Ivashuta et al., 2011; Meng et al., 2012; Wu et al., 2013; Ye et al., 2014). In Arabidopsis, in addition to IPS1, the first validated functional eTM in plants, eTMs for miR160 and miR166 have also been shown to play an important role in regulation of plant development, which was possibly achieved by eTM-induced miRNA degradation (Wu et al., 2013). In this study, we showed evidence that topping could induce the expression changes of nicotine-related miRNAs and their targets. For example, the expression levels of nta-miRX27 and its target QPT2, one of the key genes of the nicotine biosynthesis pathway, were down- and up-regulated in the topping-treated tobacco plants, respectively. Using transgenic plants overexpressing or silencing nta-miRX27, we demonstrated that nta-miRX27 directly regulates expression of QPT2 and, consequently, the accumulation of nicotine. Furthermore, we identified a long noncoding RNA, i.e. nta-eTMX27, which contains a noncleavable nta-miRX27 binding site. Similar to QPT2, the expression of nta-eTMX27 was induced in response to topping. As reported previously in other plant species (Wu et al., 2013), topping-induced down-regulation of nta-miRX27 and up-regulation of QPT2 in tobacco observed in this study (Fig. 3C) might be a result of nta-miRX27 degradation caused by up-regulated nta-eTMX27. This notion was confirmed using transgenic plants containing a hairpin construct targeting nta-eTMX27, in which no topping-induced down-regulation of nta-miRX27 and up-regulation of QPT2 were observed. Our results demonstrated a crucial role of nta-eTMX27 in regulation of nicotine biosynthesis by acting as a decoy of nta-miRX27 to sequester and degrade nta-miRX27. To our knowledge, this is the first report showing that eTM played a role in biosynthesis of secondary metabolites in plants.
Two binding or cleavage sites of nta-miRX27 were predicted on the QPT2 gene with a predicted targeting score of 4.5 and 2.5, respectively. The first predicted binding site at exon number 2 was confirmed by degradome data (Supplemental Figs. S1 and S2); however, our further RLM-RACE experiments failed to confirm nta-miRX27-mediated cleavage at both sites. We repeated the RLM-RACE experiment a couple of times with different primers. For the second binding site, we used primers based on both the complementary DNA (cDNA) and the intron sequence downstream of the predicted binding site, which is located at an intron based on the QPT2 gene (AJ748263) deposited in the National Center for Biotechnology Information. For both sites, the RLM-RACE products were mapped to the flanking regions of the predicted cleavage sites of nta-miRX27 but not at the predicted cleavage sites (Supplemental Fig. S1). The distribution pattern of the 5′ ends of the RACE products suggests that the clones we sequenced are randomly degraded products, although similar distribution pattern of 5′ RACE products has been observed for some conserved but lowly expressed miRNAs (Shen et al., 2014; Chen et al., 2015). We cannot rule out the possibility that we failed to amplify and clone the right cleavage products due to technical issue, but it is most likely that the fragmented QPT2 resulting from nta-miRX27-mediated cleavage is very susceptible to degradation induced by RNA decay proteins such as nuclear exoribonucleases (5′-3′) and the exosome complex (3′-5′). In addition, we cannot exclude the possibility that nta-miRX27 targets unprocessed QPT2 in the nucleus, because the second predicted nta-miRX27 binding site is located at the intron of alternatively spliced QPT2. If that is the case, nta-miRX27 could be involved in regulation of QPT2 by interfering its splicing and/or mRNA maturation. Whatever the possible reason for the negative RACE result, the results observed in transgenic plants clearly suggest that the transcript level of QPT2 is regulated by nta-miRX27. Nevertheless, it is in our interest to perform further experiments to find out the mechanism underlying nta-miRX27-mediated regulation of QPT2.
Among various approaches for functional characterization of miRNAs, STTM technology has received more attention due to its high efficiency in suppressing miRNA functions using the target mimicry mechanism (Yan et al., 2012). In our work, overexpression of nta-STTMX27 could reduce the nta-miRX27 levels to approximately 20% of the control levels and produced transgenic lines with the highest contents of nicotine in this study. This result confirmed the efficiency of the technology and also suggested that nta-miRX27 played an important role in nicotine biosynthesis. Transgenic plants overexpressing and silencing nta-eTMX27 showed increased and reduced accumulation of nicotine, respectively, which was consistent with its function in repressing nta-miRX27 and facilitating expression of QPT2.
Common tobacco is a model plant organism for studying diverse fundamental biological processes, such as disease susceptibility and secondary metabolites. Tobacco has rich secondary metabolites (>4,000 chemical components) and prompted numerous studies on biologically active metabolic substances (Sierro et al., 2014). For example, the alkaloid biosynthesis pathway received a large amount of attention from scientists and has been well studied over the past 30 years (Dewey and Xie, 2013). A gene network including more than 10 nodes or protein-coding genes has been characterized for the pathway. Recent studies indicated that some transcription factors, such as NAC, ETS2 Repressor Factor, and MYC, might also be indirectly involved in alkaloid biosynthesis via regulating plant hormones (Zhang et al., 2012; Fu et al., 2013). In this study, an additional regulator, i.e. a noncoding RNA acting as an miRNA or miRNA decoy, was demonstrated to be directly involved in regulation of the key genes of the alkaloid biosynthesis pathway. We believe that more such noncoding genes will be identified for the pathway in the future. In this sense, our study opened a new channel for investigation of the genetic network related to alkaloid biosynthesis in plants.
Modern tobacco is a natural amphidiploid whose genomes originated from the hybridization of two wild progenitors, N. tomentosiformis and N. sylvestris (Dewey and Xie, 2013). It is a relatively new species but experienced a dramatic domestication selection after the hybridization of two progenitors. For example, over time, loss-of-function mutations in the major nicotine demethylase genes have been selected that enabled modern tobacco to accumulate nicotine rather than nornicotine (Gavilano et al., 2007). By contrast, the normal demethylase genes in the two progenitors made them accumulate a higher level of nornicotine content but not nicotine. According to our phylogenetic analysis results, we believe that the regulatory module of nta-miRX27-QPT2-nta-eTMX27 was only evolved in tobacco after the hybridization of its two progenitors, N. tomentosiformis and N. sylvestris, but not in the progenitors themselves (Fig. 5B). QPT2 plays a key role in biosynthesis of nicotine, one of the main agronomic traits in tobacco under artificial selection. Evolution of the nta-eTMX27-nta-miRX27-QPT2 regulatory module in modern tobacco could be driven by domestication and, subsequently, intensive genetic improvement that aimed to increase the nicotine content.
MATERIALS AND METHODS
Plant Materials and Treatment
All root samples were collected from tobacco (Nicotiana tabacum) ‘Hicks Broad.’ Tobacco plants were grown at 25°C and 65% humidity in a growth chamber with 16 h of light and 8 h of dark. At least three 60-d-old (days after seeding) plants were used for topping treatment, and plants with a similar size without topping treatment were used as control. After topping treatment, the plants were kept for another 3 to 48 h in the growth chamber before sample collection.
Identification of miRNAs, Their Targets, and eTMs
The tobacco small-RNA data set and the approach reported in our previous study (Tang et al., 2012b) were used to identify miRNAs based on the newly available tobacco reference genome (Sierro et al., 2014). Targets of miRNAs were predicted using the Web-based tool psRNATarget with the default settings (Dai and Zhao, 2011), and the tobacco degradomic data set was further used to confirm the predictions using the method in our previous study (Tang et al., 2012b). Genes, i.e. QPT1 (AJ748262), QPT2 (AJ748263), and CYP82E4 (KC120817), involved in nicotine biosynthesis were based on previous studies (Dewey and Xie, 2013; Sierro et al., 2014). NAC_148 (http://compsysbio.achs.virginia.edu/tobfac/; XM_009626731; Rushton et al., 2008), a NAC transcription factor that was responsive to topping in tobacco based on our unpublished results, was used as a positive control for the topping treatment in this study. Based on the publicly available EST and Plant Genome Database-assembled unique transcript databases and transcriptome (RNA sequencing) data of tobacco, we identified an eTM for nta-miRX27 in the intergenic region, which was named nta-eTMX27. The pipeline developed by our previous study (Ye et al., 2014) was used to predict eTMs for the miRNAs.
Phylogenetic Tree
Sequences of nta-miRX27 and its target QPT2 and eTM (nta-eTMX27) were used as queries to search for their orthologs in the available genome sequences of two progenitors, Nicotiana tomentosiformis and Nicotiana sylvestris, as well as two other Nicotiana species, Nicotiana benthamiana and Nicotiana otophora (Bombarely et al., 2012; Sierro et al., 2013, 2014). Phylogenetic trees were constructed using neighbor-joining method in MEGA6 with bootstrap 1,000 (Tamura et al., 2013).
Plasmid Constructs and Plant Transformation
The precursor sequence of nta-miRX27 was amplified from tobacco root DNA using a pair of primers containing BamHI and SalI site, respectively. To generate the nta-STTMX27-containing construct, a pair of back-to-back primers each containing a nta-miRX27 binding site with a central bulge, the 48-nucleotide oligonucleotide spacer sequence, and a BamHI or SalI restriction site (Supplemental Table S2) were used in PCR to generate a fragment containing two nta-miRX27 binding sites with the 48-nucleotide spacer in between. The fragment harboring nta-miRX27 or nta-STTMX27 was then subcloned into the binary vector pCHF3 between the BamHI and SalI sites and downstream of the Cauliflower mosaic virus 35S promoter to produce pCHF3-35S-nta-miRX27 and pCHF3-35S-nta-STTMX27, respectively.
The nta-eTMX27 sequence was amplified from tobacco root cDNA using a pair of primers, eTMX27-Full-F/eTMX27-Full-R, and the PCR products were subcloned into the binary vector pCHF3 to produce pCHF3-35S-nta-eTMX27. An RNAi construct containing an nta-eTMX27 inverted repeat sequence that was spaced by a soybean (Glycine max) intron was produced by overlapping PCR. The fragment of the nta-eTMX27 sense sequence was amplified with the eTMX27-A-F/eTMX27-intron-A-R primer pair and overlapped with the intron sequence amplified by eTMX27-intron-B-F/Intron-B-R primers. The overlapping product was cloned into pCHF3 between the SacI and BamHI sites to produce pCHF3-35S-nta-eTMX27-intron. The corresponding antisense nta-eTMX27 fragment was amplified with the primer pair eTMX27-C-F/eTMX27-C-R and subsequently cloned into pCHF3-35S-nta-eTMX27-intron between BamHI and SalI sites to produce the RNAi construct pCHF3-35S-dsNta-eTMX27. The Agrobacterium tumefaciens-mediated tobacco leaf disc transformation method was used to generate transgenic tobacco plants. Selection (on 200 μg mL–1 kanamycin media) of transformants and positive transgenic plants was performed as described previously (Li et al., 2014a). Primers used in PCR and RT-qPCR analyses are listed in Supplemental Table S2.
RT-qPCR Analysis
Total RNA was isolated from root samples of the control and topping-treated plants as well as various plant organ tissues using the Trizol reagent (Invitrogen). Three independent experiments, each consisting of at least three control and topping-treated plants, were used in quantification analyses. Expression levels of miRNA in root tissues were analyzed by RT-qPCR as described. Briefly, total RNA (1 μg) treated with RNase-free DNase I (Fermentas) was polyadenylated using Escherichia coli poly(A) polymerase (New England Biolabs). After phenol-chloroform extraction and ethanol precipitation, the RNAs were dissolved in RNase-free water. Reverse transcription was performed at 42°C for 30 min using a poly(T) adapter and Quantscript reverse transcriptase according to the manufacturer’s instructions (Tiangen). RT-qPCR reactions were performed using a LightCycler 480 RT PCR instrument (Roche) and SYBR Green I Master Kit using a forward primer complementary to the miRNA and a universal reverse primer (miRNA-qPCR-R) complementary to the poly(T) adapter. Mitochondrial 5S RNA was used as an internal control for data normalization.
Quantification of the target genes and eTM was also carried out using RT-qPCR using a LightCycler 480 RT PCR instrument. One microgram of DNase I-treated total RNA was used in generating the first strand cDNA using an oligo(dT) primer and a reverse transcription kit with genome DNAse (Tiangen, KR106). The expression levels of nta-eTMX27 in different plant tissues were analyzed by semiquantitative PCR. NtGAPDH was used as a control in both qPCR and semiquantitative PCR.
RLM-5′ RACE Analysis
Total RNA was extracted from topping-treated plants at 48 h using a Trizol reagent (Invitrogen) as recommended by the manufacturer followed by further purification with an RNeasy Plant Mini Kit (QIAGEN). The purified RNA was treated by DNase I (Thermo Scientific) to eliminate possible DNA contamination and extracted using a standard phenol-chloroform method followed by ethanol precipitation. 5′-RACE was performed using the DNase-treated RNA and the SMARTer RACE cDNA Amplification Kit (Clontech). Reverse primers QPT2-1R-new or QPT2-2R-new (for the first nta-miRX27 target site) and QPT2-3R or QPT2-4R (for the second nta-miRX27 target site) were used in the respective reverse transcription reactions. Reverse-transcribed cDNAs were then used in the first round PCR reactions using the corresponding primer used in reverse transcription (i.e. QPT2-2R-new for the first nta-miRX27 target site and QPT2-4R for the second nta-miRX27 target site) in combination with the Universal Primer A Mix that anneals to the 5′ adaptor. The nested PCR was performed according to the manufacturer’s instructions (Clontech) using a nested primer (i.e. QPT2-1R-new for the first nta-miRX27 target site and QPT2-3R for the second nta-miRX27 target site) in combination with the 5′-Nested Universal Primer. PCR products with expected size were gel purified and cloned to the pMD-18T vector (TakaRa). Clones with insert were sequenced using M13 primer. Sequencing results were analyzed using CLUSTALW (Thompson et al., 1994). Primers used in 5′ RACE are listed in Supplemental Table S2.
Nicotine Measurement
Fresh leaves were collected from the same positions of the topping-treated and control tobacco plants and dried at 105°C for 30 min and then 60°C for 3 d. Nicotine contents of the transgenic and control lines were measured in three biological samples, each with three technical replicates following the standard continuous flow protocol (YC/T160–2002) described by the State Tobacco Monopoly Administration of China.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. QPT2 gene annotation and validation of nta-miRX27-mediated cleavage of QPT2 by RLM-RACE.
Supplemental Figure S2. Validating nta-miRX27-mediated cleavage of the predicted target site using degradome data from roots of topping-treated tobacco plants.
Supplemental Table S1. Summary of novel miRNAs targeting the nicotine pathway genes.
Supplemental Table S2. Primers used in this study.
Supplementary Material
Glossary
- eTM
endogenous target mimicry
- miRNA
microRNA
- RNAi
RNA interference
- RNase
ribonuclease
- STTM
short tandem target mimic
- RLM
RNA ligase-mediated
- Vec
empty vector
- RT
real-time
- qPCR
quantitative PCR
- cDNA
complementary DNA
Footnotes
This work was supported by Yunnan Provincial Tobacco Company (grant no. 2011YN04 to L.F.), the National Natural Science Foundation of China (grant no. 31060046 to B.X.), and the Post-Doctoral Science Foundation of China (grant no. 2015M570514 to F.L.).
Articles can be viewed without a subscription.
References
- Axtell MJ, Bowman JL (2008) Evolution of plant microRNAs and their targets. Trends Plant Sci 13: 343–349 [DOI] [PubMed] [Google Scholar]
- Baldwin IT. (1999) Inducible nicotine production in native Nicotiana as an example of adaptive phenotypic plasticity. J Chem Ecol 25: 3–30 [Google Scholar]
- Baldwin IT, Halitschke R, Kessler A, Schittko U (2001) Merging molecular and ecological approaches in plant-insect interactions. Curr Opin Plant Biol 4: 351–358 [DOI] [PubMed] [Google Scholar]
- Bartel DP. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297 [DOI] [PubMed] [Google Scholar]
- Boke H, Ozhuner E, Turktas M, Parmaksiz I, Ozcan S, Unver T (2015) Regulation of the alkaloid biosynthesis by miRNA in opium poppy. Plant Biotechnol J 13: 409–420 [DOI] [PubMed] [Google Scholar]
- Bombarely A, Rosli HG, Vrebalov J, Moffett P, Mueller LA, Martin GB (2012) A draft genome sequence of Nicotiana benthamiana to enhance molecular plant-microbe biology research. Mol Plant Microbe Interact 25: 1523–1530 [DOI] [PubMed] [Google Scholar]
- Chen X, Xia J, Xia Z, Zhang H, Zeng C, Lu C, Zhang W, Wang W (2015) Potential functions of microRNAs in starch metabolism and development revealed by miRNA transcriptome profiling of cassava cultivars and their wild progenitor. BMC Plant Biol 15: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conkling MA, Cheng CL, Yamamoto YT, Goodman HM (1990) Isolation of transcriptionally regulated root-specific genes from tobacco. Plant Physiol 93: 1203–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Zhao PX (2011) psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res 39: W155–W159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey RE, Xie J (2013) Molecular genetics of alkaloid biosynthesis in Nicotiana tabacum. Phytochemistry 94: 10–27 [DOI] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, García JA, Paz-Ares J (2007) Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet 39: 1033–1037 [DOI] [PubMed] [Google Scholar]
- Fu Y, Guo H, Cheng Z, Wang R, Li G, Huo G, Liu W (2013) NtNAC-R1, a novel NAC transcription factor gene in tobacco roots, responds to mechanical damage of shoot meristem. Plant Physiol Biochem 69: 74–81 [DOI] [PubMed] [Google Scholar]
- Gavilano LB, Coleman NP, Bowen SW, Siminszky B, Siminszky B (2007) Functional analysis of nicotine demethylase genes reveals insights into the evolution of modern tobacco. J Biol Chem 282: 249–256 [DOI] [PubMed] [Google Scholar]
- Heim WG, Sykes KA, Hildreth SB, Sun J, Lu RH, Jelesko JG (2007) Cloning and characterization of a Nicotiana tabacum methylputrescine oxidase transcript. Phytochemistry 68: 454–463 [DOI] [PubMed] [Google Scholar]
- Hibi N, Fujita T, Hatano M, Hashimoto T, Yamada Y (1992) Putrescine N-methyltransferase in cultured roots of Hyoscyamus albus: n-butylamine as a potent inhibitor of the transferase both in vitro and in vivo. Plant Physiol 100: 826–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashuta S, Banks IR, Wiggins BE, Zhang Y, Ziegler TE, Roberts JK, Heck GR (2011) Regulation of gene expression in plants through miRNA inactivation. PLoS One 6: e21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAS and their regulatory roles in plants. Annu Rev Plant Biol 57: 19–53 [DOI] [PubMed] [Google Scholar]
- Katoh A, Uenohara K, Akita M, Hashimoto T (2006) Early steps in the biosynthesis of NAD in Arabidopsis start with aspartate and occur in the plastid. Plant Physiol 141: 851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khraiwesh B, Zhu JK, Zhu J (2012) Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim Biophys Acta 1819: 137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Huang C, Li Z, Zhou X (2014a) Suppression of RNA silencing by a plant DNA virus satellite requires a host calmodulin-like protein to repress RDR6 expression. PLoS Pathog 10: e1003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Eichten SR, Shimizu R, Petsch K, Yeh CT, Wu W, Chettoor AM, Givan SA, Cole RA, Fowler JE, et al. (2014b) Genome-wide discovery and characterization of maize long non-coding RNAs. Genome Biol 15: R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory AC, Bouché N (2008) MicroRNA-directed regulation: to cleave or not to cleave. Trends Plant Sci 13: 359–367 [DOI] [PubMed] [Google Scholar]
- Meng Y, Shao C, Wang H, Jin Y (2012) Target mimics: an embedded layer of microRNA-involved gene regulatory networks in plants. BMC Genomics 13: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi AR, Sarwat M, Hasan S, Roychodhury N (2012) Biogenesis, functions and fate of plant microRNAs. J Cell Physiol 227: 3163–3168 [DOI] [PubMed] [Google Scholar]
- Ng DW, Zhang C, Miller M, Palmer G, Whiteley M, Tholl D, Chen ZJ (2011) cis- and trans-Regulation of miR163 and target genes confers natural variation of secondary metabolites in two Arabidopsis species and their allopolyploids. Plant Cell 23: 1729–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli AE. (2012) MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet 13: 271–282 [DOI] [PubMed] [Google Scholar]
- Phillips JR, Dalmay T, Bartels D (2007) The role of small RNAs in abiotic stress. FEBS Lett 581: 3592–3597 [DOI] [PubMed] [Google Scholar]
- Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP (2002) Prediction of plant microRNA targets. Cell 110: 513–520 [DOI] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, MacLean D, Jikumaru Y, Hill L, Yamaguchi S, Kamiya Y, Jones JD (2011) The microRNA miR393 re-directs secondary metabolite biosynthesis away from camalexin and towards glucosinolates. Plant J 67: 218–231 [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Bokowiec MT, Han S, Zhang H, Brannock JF, Chen X, Laudeman TW, Timko MP (2008) Tobacco transcription factors: novel insights into transcriptional regulation in the Solanaceae. Plant Physiol 147: 280–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh F, Noma M, Kawashima N (1985) The alkaloid contents of sixty Nicotiana species. Phytochemistry 24: 477–480 [Google Scholar]
- Shen D, Suhrkamp I, Wang Y, Liu S, Menkhaus J, Verreet JA, Fan L, Cai D (2014) Identification and characterization of microRNAs in oilseed rape (Brassica napus) responsive to infection with the pathogenic fungus Verticillium longisporum using Brassica AA (Brassica rapa) and CC (Brassica oleracea) as reference genomes. New Phytol 204: 577–594 [DOI] [PubMed] [Google Scholar]
- Sierro N, Battey JN, Ouadi S, Bakaher N, Bovet L, Willig A, Goepfert S, Peitsch MC, Ivanov NV (2014) The tobacco genome sequence and its comparison with those of tomato and potato. Nat Commun 5: 3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierro N, Battey JN, Ouadi S, Bovet L, Goepfert S, Bakaher N, Peitsch MC, Ivanov NV (2013) Reference genomes and transcriptomes of Nicotiana sylvestris and Nicotiana tomentosiformis. Genome Biol 14: R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminszky B, Gavilano L, Bowen SW, Dewey RE (2005) Conversion of nicotine to nornicotine in Nicotiana tabacum is mediated by CYP82E4, a cytochrome P450 monooxygenase. Proc Natl Acad Sci USA 102: 14919–14924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair SJ, Murphy KJ, Birch CD, Hamill JD (2000) Molecular characterization of quinolinate phosphoribosyltransferase (QPRtase) in Nicotiana. Plant Mol Biol 44: 603–617 [DOI] [PubMed] [Google Scholar]
- Song W. (1997) Molecular characterizations of two tobacco root-specific genes: TobRB7 and NtQPT1. PhD thesis. North Carolina State University, Raleigh, NC [Google Scholar]
- Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16: 2001–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30: 2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Yan J, Gu Y, Qiao M, Fan R, Mao Y, Tang X (2012a) Construction of short tandem target mimic (STTM) to block the functions of plant and animal microRNAs. Methods 58: 118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Wang Y, Li Z, Gui Y, Xiao B, Xie J, Zhu QH, Fan L (2012b) Identification of wounding and topping responsive small RNAs in tobacco (Nicotiana tabacum). BMC Plant Biol 12: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todesco M, Rubio-Somoza I, Paz-Ares J, Weigel D (2010) A collection of target mimics for comprehensive analysis of microRNA function in Arabidopsis thaliana. PLoS Genet 6: e1001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Wang ZM, Wang M, Wang XJ (2013) Widespread long noncoding RNAs as endogenous target mimics for microRNAs in plants. Plant Physiol 161: 1875–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Song W, Maksymowicz W, Jin W, Cheah K, Chen W, Carnes C, Ke J, Conkling M (2004) Biotechnology: a tool for reduced risk tobacco products. The nicotine experience from test tube to cigarette pack. Rev. Adv. Tob. Sci. 30: 17–37 [Google Scholar]
- Yan J, Gu Y, Jia X, Kang W, Pan S, Tang X, Chen X, Tang G (2012) Effective small RNA destruction by the expression of a short tandem target mimic in Arabidopsis. Plant Cell 24: 415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye CY, Xu H, Shen E, Liu Y, Wang Y, Shen Y, Qiu J, Zhu QH, Fan L (2014) Genome-wide identification of non-coding RNAs interacted with microRNAs in soybean. Front Plant Sci 5: 743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HB, Bokowiec MT, Rushton PJ, Han SC, Timko MP (2012) Tobacco transcription factors NtMYC2a and NtMYC2b form nuclear complexes with the NtJAZ1 repressor and regulate multiple jasmonate-inducible steps in nicotine biosynthesis. Mol Plant 5: 73–84 [DOI] [PubMed] [Google Scholar]
- Zhang YC, Liao JY, Li ZY, Yu Y, Zhang JP, Li QF, Qu LH, Shu WS, Chen YQ (2014) Genome-wide screening and functional analysis identify a large number of long noncoding RNAs involved in the sexual reproduction of rice. Genome Biol 15: 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.