Innate immunity is now known to impact tissue metabolic responses with members of the Toll-like receptor 2 and 4 (TLR 2/4) family implicated in dyslipidemia. However, the impact of other TLR proteins on metabolic regulation has remained poorly understood. In this issue of Diabetes, Strodthoff et al. (1) provide interesting new evidence that TLR3 regulates glucose metabolism though the modulation of insulin levels in both rodents and humans. These findings reveal a new area of investigation in regulating glucose metabolism beyond fatty acid modulation of TLR 2/4 that may have the potential to reveal novel therapeutic targets.
TLRs are critical to the regulation of innate immune responses. TLRs are transmembrane proteins that contain leucine-rich repeat domains capable of binding to conserved pathogen-associated molecular patterns (PAMPs). The PAMPS are comprised of bacterial, parasitic, or viral components, and the binding of these PAMPS to the extracellular domain of TLRs results in the activation of intracellular adaptor molecules. The two most common and studied downstream molecular adaptors are the myeloid differentiation factor 88 (MyD88) and Toll-interleukin-1 receptor domain-containing adapter-inducing interferon-β (TRIF). Almost all identified TLRs signal through MyD88 except for TLR3. In contrast, only TLR3 and TLR4 signal via TRIF (2). The activation of MyD88 and TRIF results in a signaling cascade that leads to the formation of proinflammatory mediators. TLR3 is able to recognize double-stranded RNA, which induces a TRIF signaling cascade that results in the activation of the inflammasome and subsequent interleukin-1β–mediated responses as well as the increased expression of type I interferons that aid in the control of viral pathogens (3,4). Although TLRs are classically associated with infectious insult, it is now clear that they are key regulators of inflammatory responses observed during metabolic diseases (5). There is evidence that TLRs are able to recognize excess lipids in the forms of free fatty acids and modified LDLs, mostly via TLR2 and/or TLR4 activation (6,7). The recognition of these lipids causes chronic low-level inflammation that contributes to disease progression of type 2 diabetes, metabolic syndrome, and cardiovascular disease. Recently, it has been suggested that TLR3 recognizes mRNA during tissue necrosis (8). Thus, TLR responses are critical not just to infectious disease but during sterile inflammation as well.
Immune system dysfunction has been recognized to contribute to metabolic disorders for some time. A key contributor to metabolic diseases is chronic inflammatory responses that alter insulin sensitivity, lipid metabolism, atherosclerosis progression, and adipose tissue angiogenesis. For instance, free fatty acids activate macrophages in adipose tissue that skew them toward inflammatory responses and inhibit effective angiogenesis of adipose tissue (9). However, metabolic pathways and immune responses are even more integrated than simple TLR recognition of excess lipid compounds. Cellular metabolic and immune responses are coordinated in such a manner as to ensure that immune cells have sufficient energy to carry out their cellular function (10). This is most evident and eloquently demonstrated in macrophages. Antipathogenic responses of macrophages require glycolysis, while tissue repair requires β-oxidation (11). TLR activation is sufficient to elicit glycolysis, while interleukin-4 receptor activation induces β-oxidation. Interestingly, this metabolic programming is not one direction, as enhancement of β-oxidation hampers pathogenic responses, and vice versa. Also, critical to metabolic disease is the regulation and responsiveness of cells to insulin. As metabolic disease progresses, cells become less responsive to insulin. Interestingly, there seems to be a counterregulatory component in the interaction between insulin and TLRs. Insulin is capable of reducing the expression of TLR1, -2, -4, -7, and -9 in human mononuclear cells (12). In turn, TLR activation makes macrophages resistant to insulin (13). TLR expression is not exclusive to immune cells; numerous other cells, including β-islet cells, express TLRs (14,15). This would suggest that TLR3 function is critical to β-cell activity and insulin production and response during type 2 diabetes. However, the contribution of TLR3 to type 2 diabetes has been enigmatic and the study by Strodthoff et al. (1) begins to illuminate the contribution of TLR3 signaling during type 2 diabetes.
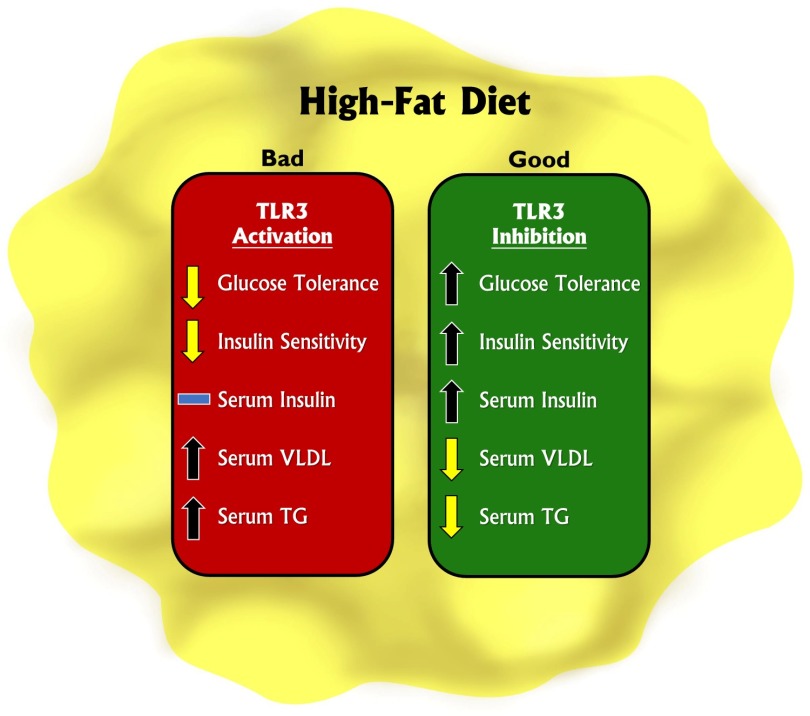
Major findings in the article by Strodthoff et al. (1) provide some of the most convincing evidence to date regarding the importance of TLR3 in regulating glucose metabolic responses. Using a mouse model genetically deficient for TLR3 combined with a high-fat diet (HFD), it was found that the loss of TLR3 augments glucose tolerance and increases circulating insulin levels after glucose stimulation. These responses were not associated with a change in whole-body insulin resistance or insulin sensitivity. Likewise, the loss of TLR3 resulted in decreased circulating VLDL associated with a decrease in circulating free fatty acids along with decreased hepatic hydroxymethylglutaryl-CoA reductase expression and triglyceride (TG) synthesis. Conversely, the activation of TLR3 in wild-type animals distinctly elicited opposite metabolic responses and increased TG levels, thereby closing the loop that TLR3 could possibly act as central metabolic regulator. TLR3 single nucleotide polymorphism analysis for a mutation predicted to blunt TLR3 function in two healthy cohorts found a significant association of this single nucleotide polymorphism with fasting insulin levels but not with glucose levels, providing clinical evidence for an important role of TLR3 as a metabolic regulator. Figure 1 illustrates key metabolic changes to HFD consumption in response to TLR3 activation or inhibition.
Figure 1.
Modulation of TLR3 activity alters metabolic activity in response to an HFD. TLR3 activation decreases glucose tolerance and insulin sensitivity, while increasing serum VLDL and TG. Conversely, inhibition of TLR3 increases glucose tolerance, insulin sensitivity, and serum insulin, while decreasing serum VLDL and TG.
While these novel data revealed a potential new role for TLR3 in metabolic regulation, many key questions require further study, such as: is TLR3 differentially expressed in various tissues during diabetes or prediabetes? Does TLR3 differentially affect metabolic signaling pathways (e.g., AKT or AMPK) in various tissues? What are the endogenous TLR3 ligands produced during HFD metabolic dysfunction and do they elicit TLR3-dependent alteration of glucose metabolism? Does the loss of TLR3 fundamentally affect β-cell function or does it simply reduce the overall level of inflammation mediating tissue damage? Would pharmacological disruption of TLR3 serve as a useful therapeutic target to manage metabolic dysfunction? Additionally, an important issue is whether modulation of TLR3 activity would adversely impact innate immune responses of the host. Nonetheless, this latest report by Strodthoff et al. (1) provides useful new information regarding specific roles of TLR3 activation and regulation of metabolic responses that further reinforce the dynamic relationship between innate immunity and diabetes, which just might reveal future therapeutic approaches.
Article Information
Funding. This work was supported by the National Institutes of Health (HL113303).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 3425.
References
- 1.Strodthoff D, Ma Z, Wirström T, et al. Toll-like receptor 3 influences glucose homeostasis and β-cell insulin secretion. Diabetes 2015;64:3425–3438 [DOI] [PubMed]
- 2.Bryant CE, Symmons M, Gay NJ. Toll-like receptor signalling through macromolecular protein complexes. Mol Immunol 2015;63:162–165 [DOI] [PubMed] [Google Scholar]
- 3.Pothlichet J, Meunier I, Davis BK, et al. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS Pathog 2013;9:e1003256 [DOI] [PMC free article] [PubMed]
- 4.Yamamoto M, Sato S, Mori K, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol 2002;169:6668–6672 [DOI] [PubMed] [Google Scholar]
- 5.Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J Clin Endocrinol Metab 2014;99:39–48 [DOI] [PubMed] [Google Scholar]
- 6.Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab 2011;300:E145–E154 [DOI] [PMC free article] [PubMed]
- 7.Wiesner P, Choi SH, Almazan F, et al. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ Res 2010;107:56–65 [DOI] [PMC free article] [PubMed]
- 8.Cavassani KA, Ishii M, Wen H, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med 2008;205:2609–2621 [DOI] [PMC free article] [PubMed]
- 9.Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol 2008;3:545–556 [DOI] [PMC free article] [PubMed]
- 10.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol 2014;32:609–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 2011;11:738–749 [DOI] [PMC free article] [PubMed]
- 12.Ghanim H, Mohanty P, Deopurkar R, et al. Acute modulation of Toll-like receptors by insulin. Diabetes Care 2008;31:1827–1831 [DOI] [PMC free article] [PubMed]
- 13.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010;72:219–246 [DOI] [PubMed] [Google Scholar]
- 14.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol 2002;168:554–561 [DOI] [PubMed] [Google Scholar]
- 15.Albert ML, Pearce SF, Francisco LM, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med 1998;188:1359–1368 [DOI] [PMC free article] [PubMed]