Abstract
Extracellular superoxide dismutase (EC-SOD), also known as SOD3, is an antioxidant expressed at high levels in normal adult kidneys. Because oxidative stress contributes to a variety of kidney injuries, we hypothesized that EC-SOD may be protective in CKD progression. To study this hypothesis, we used a murine model of ADR nephropathy characterized by albuminuria and renal dysfunction. We found that levels of EC-SOD diminished throughout the course of disease progression and were associated with increased levels of NADPH oxidase and oxidative stress markers. EC-SOD null mice were sensitized to ADR injury, as evidenced by increases in albuminuria, serum creatinine, histologic damage, and oxidative stress. The absence of EC-SOD led to increased levels of NADPH oxidase and an increase in β-catenin signaling, which has been shown to be pathologic in a variety of kidney injuries. Exposure of EC-SOD null mice to either chronic angiotensin II infusion or to daily albumin injections also caused increased proteinuria. In contrast, EC-SOD null mice subjected to nonproteinuric CKD induced by unilateral ureteral obstruction exhibited no differences compared with wild-type mice. Finally, we also found a decrease in EC-SOD in human CKD biopsy samples, similar to our findings in mice. Therefore, we conclude that EC-SOD is protective in CKDs characterized by proteinuria.
Keywords: proteinuria, podocyte, oxidative stress, kidney disease
The treatment of proteinuric CKD remains a significant challenge. Proteinuria has been strongly associated with CKD development, and higher levels of proteinuria generally lead to faster progression and worsened prognosis. As such, a primary focus of nephrology care is to decrease renal damage by reducing proteinuria with renin-angiotensin system blockade.1 However, the protection is not complete, with therapies tending to decrease proteinuria, rather than completely abrogate it.2
Oxidative stress, defined as an imbalance favoring reactive oxygen species (ROS) over antioxidant protective mechanisms, has been implicated in the development and progression of CKD.3,4 There is an abundance of evidence for increased oxidative stress in proteinuric renal disease in both humans and animal models.5–8 Reduction of oxidative stress via antioxidants and other means is beneficial in a variety of kidney diseases.6,7,9 Elucidation of the exact mechanisms leading to imbalanced ROS/antioxidant balance could lead to the development of novel therapies to halt proteinuric CKD.
Extracellular superoxide dismutase (EC-SOD), also known as SOD3, is an antioxidant enzyme expressed in abundance in the kidney.10 There are three mammalian SODs, with copper-zinc SOD (Cu/Zn SOD, or SOD1) and manganese SOD (MnSOD, or SOD2) localized exclusively in the intracellular environment. On the other hand, EC-SOD exists primarily in the extracellular space, and would therefore be expected to have a special role in tissue homeostasis in that compartment.11 The SODs catalyze a dismutation reaction that scavenges the superoxide free radical, an ROS generated in excess in renal disease largely via upregulation of NADPH oxidases or via a leak from the mitochondrial electron transport chain.4,12,13 EC-SOD catalyzes the dismutation reaction at diffusion limited rates, making it an effective mechanism for removing superoxide in extracellular locations.11
EC-SOD has been previously shown to be important in disease states,14 although studies in the kidney have been relatively limited. Schneider and colleagues found that EC-SOD null mice were sensitized to increased oxidative stress and renal cast formation in AKI after ischemia/reperfusion injury compared with wild-type (WT) mice.15 Another study initially found that diabetic nephropathy in animals is accompanied by a loss of Cu/Zn SOD and EC-SOD,16 but subsequent investigations failed to show any worsening of disease in EC-SOD null mice.17 To our knowledge, there are no studies showing an effect of EC-SOD on proteinuric CKD.
To examine the role of EC-SOD in proteinuria, we utilized the murine model of ADR (ADR) nephropathy. In this model, a single injection of ADR leads to significant glomerular damage that recapitulates the human disease of FSGS. Massive proteinuria is followed by renal fibrosis and overall renal dysfunction.18 We found that levels of EC-SOD were suppressed in the course of this disease. Furthermore, we demonstrate that the absence of EC-SOD leads to increased proteinuria. Two other models of proteinuria using either continuous angiotensin II infusion or with repeated albumin injections showed similar results, validating our hypothesis. On the contrary, in an interstitial fibrosis model not characterized by proteinuria, we could not find an effect of EC-SOD on disease progression, implying a specific role for this antioxidant in proteinuric kidney disease.
Results
ADR Leads to Increased Oxidative Stress in the Kidney
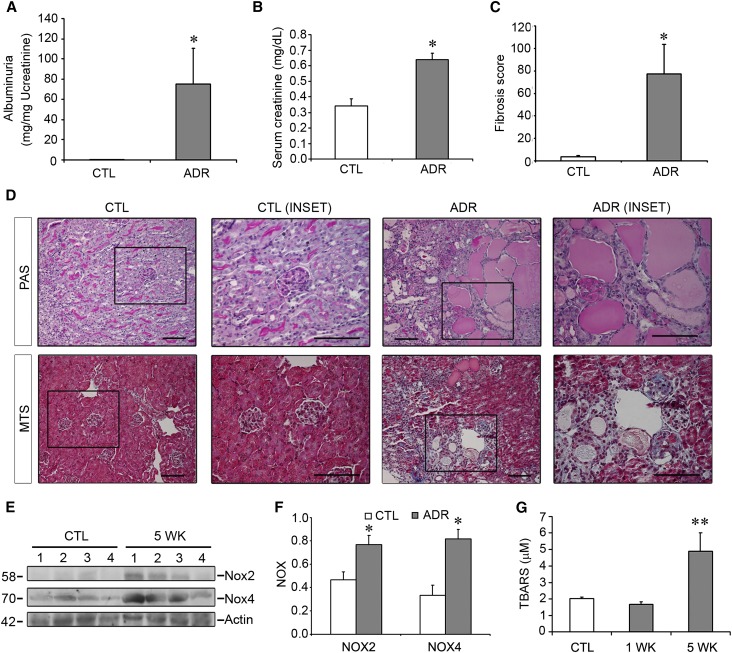
To determine whether EC-SOD plays a role in proteinuric CKD, we subjected BALB/c mice to ADR injury. A single intravenous injection of ADR (10 mg/kg) was performed. After 5 weeks, the mice experienced significant weight loss compared with controls (26.10±0.328 g in controls versus 22.26±1.538 g in the ADR group), and this was associated with significant renal dysfunction as evidenced by the development of massive albuminuria and increases in serum creatinine (Figure 1, A and B). This was accompanied by tubular injury and renal fibrosis (Figure 1, C and D).
Figure 1.
Mice treated with ADR suffer from significant proteinuria and fibrosis that is associated with increased oxidative stress. BALB/c mice are treated with vehicle or ADR (10 mg/kg) and euthanized at 5 weeks after injection. (A–C) Albuminuria (A), serum creatinine (B), and renal fibrosis (C) are all increased in ADR mice compared with controls. (D) PAS stains (upper panels) reveal tubular dilation with massive proteinaceous casts in ADR mice only, whereas MTS stains (lower panels) reveal interstitial fibrosis and glomerulosclerosis. (E) The NADPH oxidase isoforms Nox2 and Nox4 are both highly upregulated after ADR. Numbers indicate individual mice. (F) Quantitative densitometric analysis of blots. (G) TBARS assay showing increased levels of this oxidative stress marker 5 weeks after ADR. *P<0.05 compared with controls at the same time point; **P<0.05 compared with both control and 1 week time points. CTL, control; PAS, Periodic acid–Schiff; MTS, Masson’s trichrome stain. Bar, 100 μm.
Development of nephropathy was also accompanied by increases in oxidative stress. We first evaluated NADPH oxidase levels, focusing on two isoforms, Nox2 and Nox4, that are expressed at high levels in the mouse kidney.19 As shown in Figure 1, E and F, both Nox2 and Nox4 were markedly and significantly upregulated in injured kidneys after ADR exposure. NADPH oxidase upregulation would lead to increased generation of the superoxide free radical. As a result, overall renal oxidative stress was increased as demonstrated by higher levels of thiobarbituric acid reactive substances (TBARS), a marker for oxidative stress (Figure 1G).
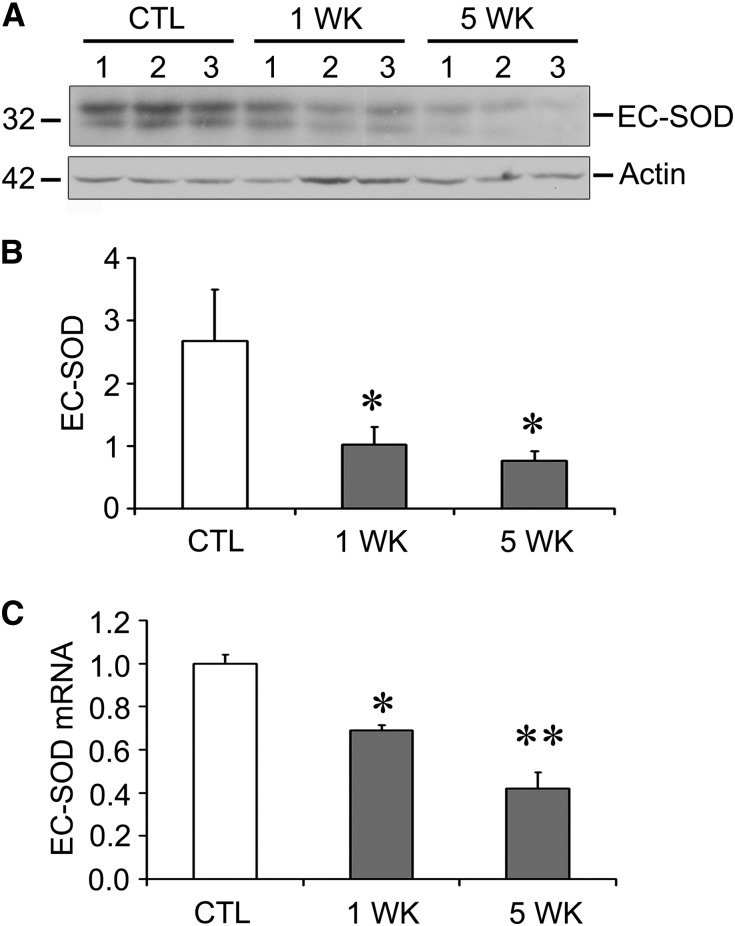
We next investigated whether there was an effect on superoxide scavenging by evaluating EC-SOD levels. We found that both protein (Figure 2, A and B) and mRNA levels (Figure 2C) were downregulated after ADR exposure in mice. Loss of this protective antioxidant, along with upregulation of Nox2 and Nox4, may lead to an imbalance leading to an excess of superoxide and oxidative stress in this model.
Figure 2.
EC-SOD levels are decreased after ADR injection. (A) EC-SOD protein levels are decreased at 1 and 5 weeks after ADR exposure. Numbers indicate individual mice. (B) Quantitative data for A, normalized to actin as a loading control. (C) EC-SOD mRNA levels are also decreased at the same time points. *P<0.05 compared with the control group; **P<0.05 compared with both the control and 1 week ADR groups. CTL, control.
EC-SOD Null Mice Are Sensitized to ADR-Induced Kidney Injury
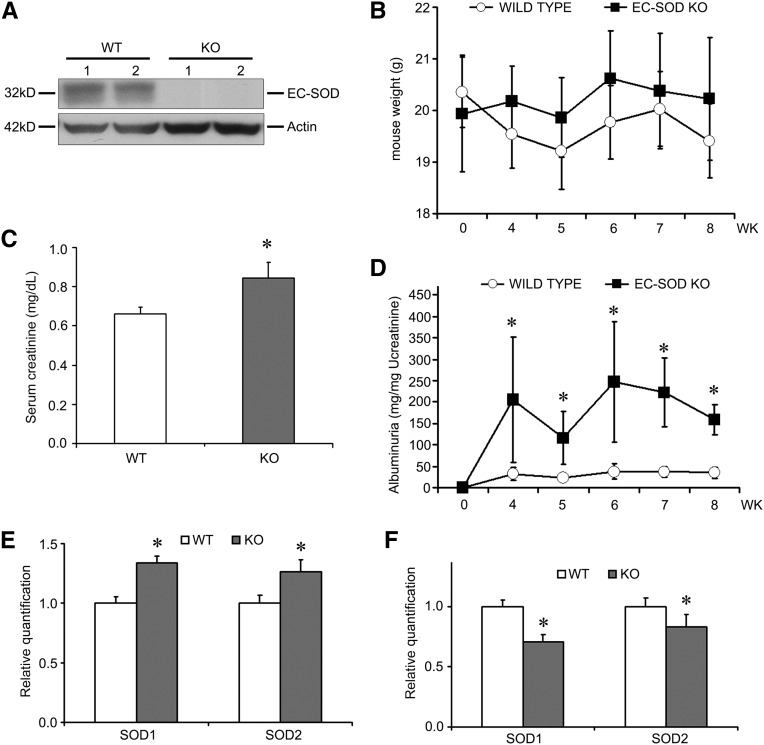
To more specifically evaluate the role of EC-SOD, we exposed EC-SOD knockout (KO) mice to ADR injury. These mice globally lack the expression of EC-SOD (Figure 3A), but have no renal dysfunction under normal conditions. These mice are bred on the C57BL/6 background strain, which is relatively resistant to ADR, requiring higher doses of ADR (18 mg/kg) and longer injury development time (8 weeks). Although body weight did not significantly differ between KO and WT mice in this injury (Figure 3B), we nonetheless found that serum creatinine was significantly increased in the KO compared with WT mice (Figure 3C). Even more impressive was a significant increase in albuminuria that was detected at several time points after ADR injection (Figure 3D). To determine whether there were compensatory changes in other SODs, we examined expression levels of SOD1 (Cu/Zn SOD) and SOD2 (MnSOD), the two intracellular isoforms. At baseline, the mRNA levels of both SODs were slightly upregulated in the EC-SOD KO mice (Figure 3E). Levels of SOD1 and SOD2 were decreased in WT mice treated with ADR compared with sham mice (0.552±0.273 versus 1.000±0.103 for SOD1, and 0.492±0.141 versus 1.000±0.032 for SOD2; both significant with P <0.05), and were further decreased in the KO compared with WT mice, albeit only by a small additional amount (Figure 3F). Collectively, these findings are consistent with an increased sensitivity to proteinuric renal injury specifically in the EC-SOD null mouse.
Figure 3.
EC-SOD KO mice are sensitized to ADR injury. (A) Kidney homogenates demonstrate that EC-SOD KO mice indeed lack EC-SOD. Numbers indicate individual mice. (B) KO and WT mice are treated with ADR (18 mg/kg) and weights are monitored over 8 weeks. There is no significant difference between the groups at any time point. (C) Serum creatinine measured at 8 weeks is increased in KO compared with WT mice. (D) Albuminuria is markedly elevated at numerous time points in the KO compared with the WT mice. (E) mRNA levels of SOD1 and SOD2 are assessed in untreated mice, showing a slight upregulation of both isoforms. (F) In ADR-treated mice, there is a small downregulation of both isoforms. *P<0.05 compared with the WT mice at the same time point.
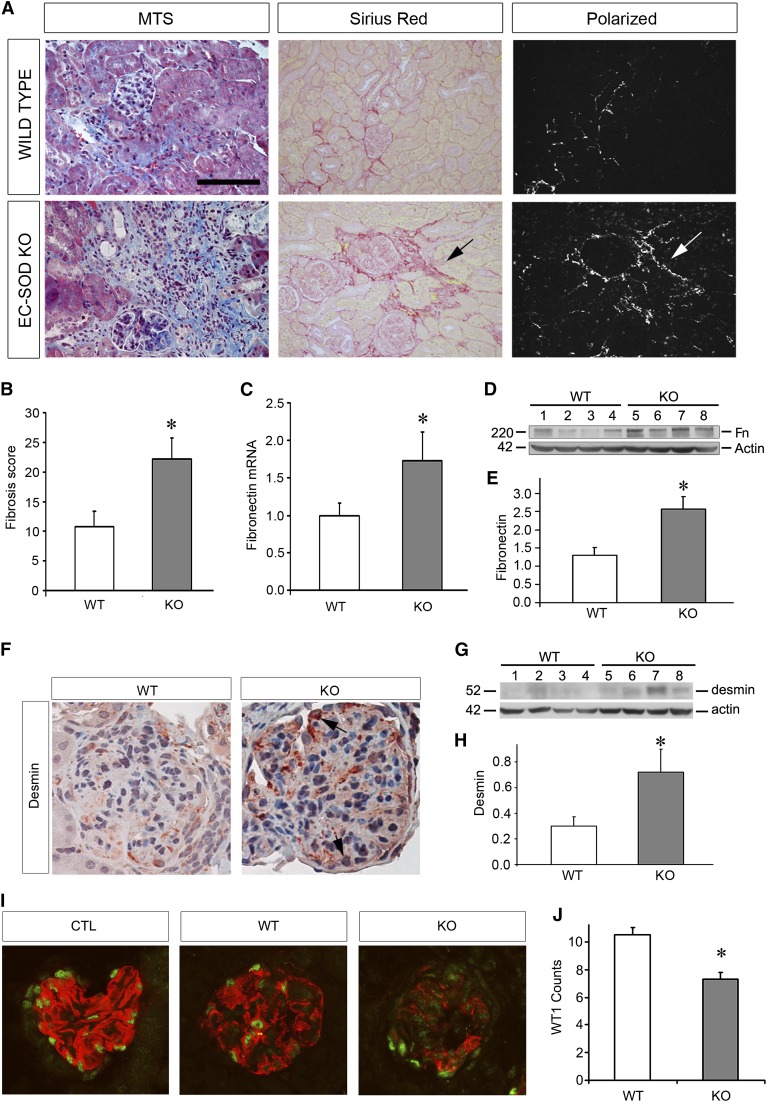
We then evaluated for markers of renal fibrosis, the sine qua non of CKD. As shown in Figure 4, A and B, EC-SOD KO mice had increased levels of fibrosis as determined by Masson’s trichrome and Sirius red staining at 8 weeks after ADR exposure. Fibronectin mRNA and protein levels were also increased in KO compared with WT mice (Figure 4, C–E). Desmin, a mesenchymal marker for podocyte injury, was found to be upregulated in KO mice compared with WT mice (Figure 4, F–H) and colocalized with Wilms Tumor 1 (WT1)- and nephrin-positive areas on immunofluorescence (Supplemental Figure 1). Therefore, KO mice had increased markers of chronic injury in addition to higher proteinuria levels compared with WT mice.
Figure 4.
ADR-treated EC-SOD KO mice develop greater fibrosis and glomerulosclerosis compared with WT mice. (A) MTS shows increased fibrosis (blue staining) in KO at 8 weeks postinjection. Sirius red staining shows similar results (red staining in normal light microscopy, with birefringence under polarized light, arrows). (B) Histologic scoring of fibrosis showing increased damage in KO mice. (C–E) Fibronectin mRNA levels (C) and protein levels (D) with quantitation (E) are increased in KO compared with WT mice. (F–H) Immunohistochemistry (F) and Western blotting (G with quantitation in H) for the mesenchymal marker desmin reveals increased levels in the KO group. Arrows indicate specific podocytes that are positive for desmin. (I) Representative micrograph of immunofluorescence double staining for nephrin (red) and WT1 (green), demonstrating that both proteins are decreased in ADR-treated mice, with the KO mice having greater decreases compared with WT mice. (J) Quantitation of WT1-positive cells per glomerulus is presented. *P<0.05 compared with the WT group. MTS, Masson’s trichrome stain; CTL, control. Bar, 100 μm.
In addition, we assessed levels of nephrin and WT1 as indicators of podocyte injury. Our results demonstrate disrupted nephrin in ADR-treated mice compared with untreated control mice, and this disruption was more pronounced in the KO compared with the WT mice (Figure 4I). Furthermore, ADR led to a depletion of WT1, a podocyte protein necessary for normal functioning and maintenance of podocyte differentiation.20 This decrease was more accentuated in the KO compared with the WT mice (Figure 4J). This indicates substantial podocyte injury after ADR injury that is accentuated in the KO mice.
EC-SOD KO Mice Have Increased Levels of Oxidative Stress
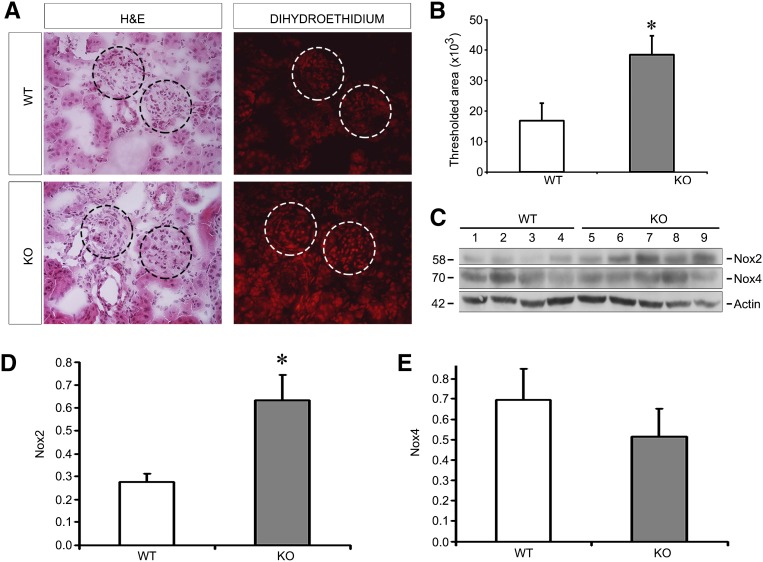
In order to determine levels of oxidative stress in EC-SOD KO mice, we subjected frozen kidney sections to dihydroethidium (DHE) staining, which is specific for superoxide free radicals. KO mice consistently had increased levels of DHE compared with WT mice both in glomeruli and in tubules (Figure 5, A and B). Because we identified increases in NADPH oxidases in ADR injury (Figure 1), we evaluated levels of Nox2 and Nox4 in KO versus WT kidneys after ADR. We found that while the Nox4 isoform was not different between KO and WT, the Nox2 isoform was indeed significantly increased in the KO compared with WT mice (Figure 5, C–E). Therefore, the increase in DHE staining (and superoxide production) may be due to both lack of EC-SOD, as well as increases in the Nox2 isoform of NADPH oxidase.
Figure 5.
Oxidative stress is increased in EC-SOD KO compared with WT mice treated with ADR. (A) DHE staining, which is specific for the superoxide free radical, is increased in KO versus WT mice. Circles outline glomeruli in these adjacent serial sections stained with H&E and with DHE. (B) Quantitation of positively stained area is shown. (C) Western blots for Nox2 and Nox4 reveal that Nox2 is higher in KO mice after ADR. (D and E) Quantitative data are shown in D and E normalizing to actin as a loading control. *P<0.05 compared with the WT group. DHE, dihydroethidium; H&E, hematoxylin and eosin.
β-Catenin Is Increased in EC-SOD KO Mice
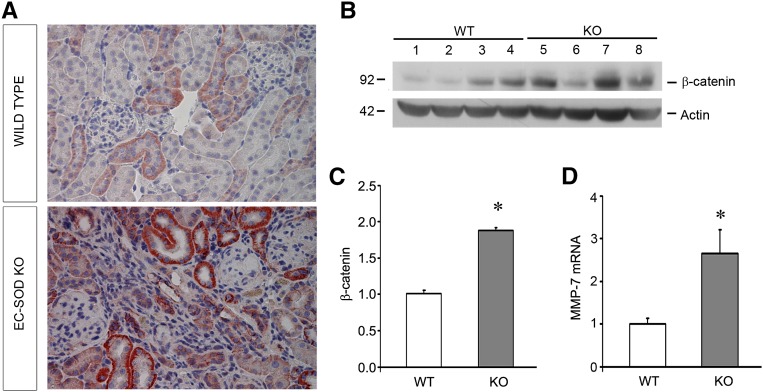
Because Wnt/β-catenin signaling is highly upregulated and pathologic in the development of both podocyte injury and renal fibrosis,21,22 we investigated this pathway in our model. As shown in Figure 6, A–C, total β-catenin levels were increased in EC-SOD null mice compared with WT mice after ADR injury. Immunohistochemical staining for β-catenin confirmed upregulation of this protein (Figure 6A). Consistent with these findings, the downstream target of β-catenin signaling, matrix metalloproteinase-7, was upregulated in the KO compared with WT mice (Figure 6D). This demonstrates that increased β-catenin activity underlies worsened ADR injury in KO mice.
Figure 6.
ADR-treated EC-SOD KO mice have increased pathologic β-catenin activation compared with WT mice. (A) Immunohistochemistry for β-catenin protein (red staining) in kidney sections shows increased levels in KO compared with WT mice. (B) Western blot for β-catenin. (C) Quantitative data for B, normalized to actin as a loading control. (D) Quantitative real-time RT-PCR analyses of renal matrix metalloproteinase-7 (MMP-7) mRNA expression in WT and KO mice. *P<0.05 compared with the WT group.
EC-SOD KO Mice Are Sensitized to Proteinuric, Rather Than Nonproteinuric, Renal Disease
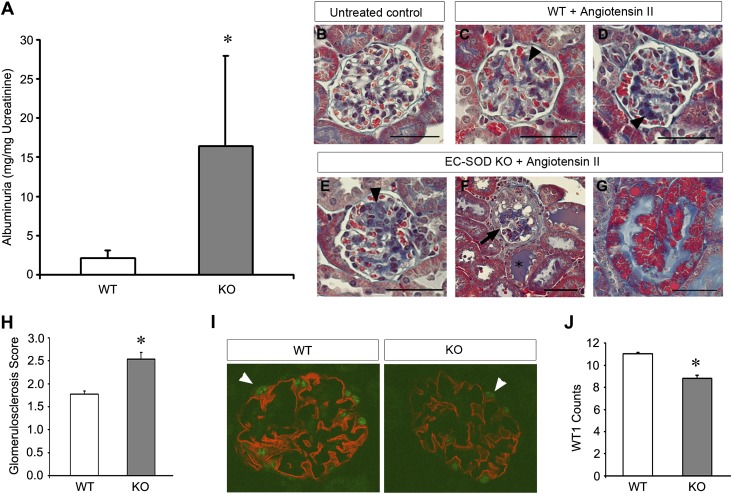
To determine whether protective effects of EC-SOD are specific to proteinuric disease, or more generalizable to all CKD, we subjected KO mice to additional models of renal injury. First, we tested a proteinuric model induced by combined unilateral nephrectomy and continuous infusion of angiotensin II (1.5 mg/kg per day) by osmotic minipump. Consistent with our results in ADR injury, we found that angiotensin II induced proteinuria to a significantly greater degree in KO compared with WT mice (Figure 7A). Histologic examination confirmed the marked increase in urinary protein excretion and demonstrated worsened glomerular injury, proteinaceous casts, and tubular protein reabsorption droplets (Figure 7, B–H). Finally, both nephrin staining and the number of glomerular cells staining positively for WT1 were decreased in KO compared with WT mice (Figure 7, I and J).
Figure 7.
EC-SOD KO mice are sensitized to angiotensin II–induced proteinuric renal injury. Mice undergo unilateral nephrectomy and are then treated with 1.5 mg/kg per day of angiotensin II via an osmotic minipump and euthanized after 4 weeks. (A–G) Albuminuria is significantly increased in KO mice compared with similarly treated WT mice. Trichrome staining shows glomerulosclerosis (arrowheads in C–E) in angiotensin II mice compared with untreated controls (B). The sclerosis lesions are further increased in the KO mice (E) and, in some cases, show heavily damaged glomeruli (arrow in F), increased cast formation (asterisk in F), and protein reabsorption droplets in some tubules (G). (H) Quantitation of the extent of glomerulosclerosis reveals greater glomerular damage in KO mice. (I) Representative micrograph of immunofluorescence double staining for WT1 (green) and nephrin (red). Arrowheads indicate WT1-positive cells. (J) Quantification of the WT1-positive cells per glomerulus are presented. *P<0.05 compared with the WT group. Bar, 50 μm.
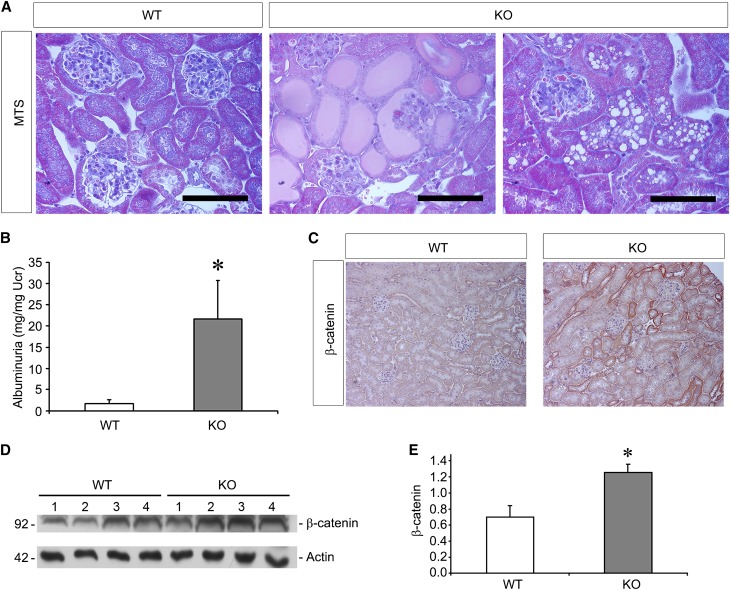
Second, we exposed KO mice to an albumin overload model of proteinuria. In this previously described model,23 mice are injected with BSA, which leads to overflow proteinuria in the urine. Histologically, the WT mice treated with BSA had very mild, if any, glomerulosclerosis. On the other hand, EC-SOD KO mice had significantly more glomerular damage as well as albuminuria as shown by the presence of proteinaceous casts or by tubular protein reabsorption droplets (Figure 8A). Quantitation of albuminuria did indeed show increased levels in the KO compared with WT mice (Figure 8B). Meanwhile, whereas β-catenin was only slightly elevated in these mice overall, it was significantly higher in the KO compared with the WT mice (Figure 8, C–E).
Figure 8.
EC-SOD KO mice are sensitized to an albumin overload model of proteinuria. Mice undergo sequential injections of BSA as described in the Concise Methods, leading to the development of significant proteinuria. (A) MTS demonstrates very little glomerular or tubular injury in response to BSA injections in WT mice. In KO mice, there is evidence of glomerular collapse and glomerulosclerosis, proteinaceous casts within tubules, and protein reabsorption droplets in tubular epithelia. (B) Levels of proteinuria are significantly increased in the KO mice compared with WT mice. (C–E) Assessment of β-catenin levels by immunohistochemistry (red staining) and Western blotting shows increased levels in the KO compared with WT mice. *P<0.05 compared with the WT group. MTS, Masson’s trichrome stain. Bar, 100 μm.
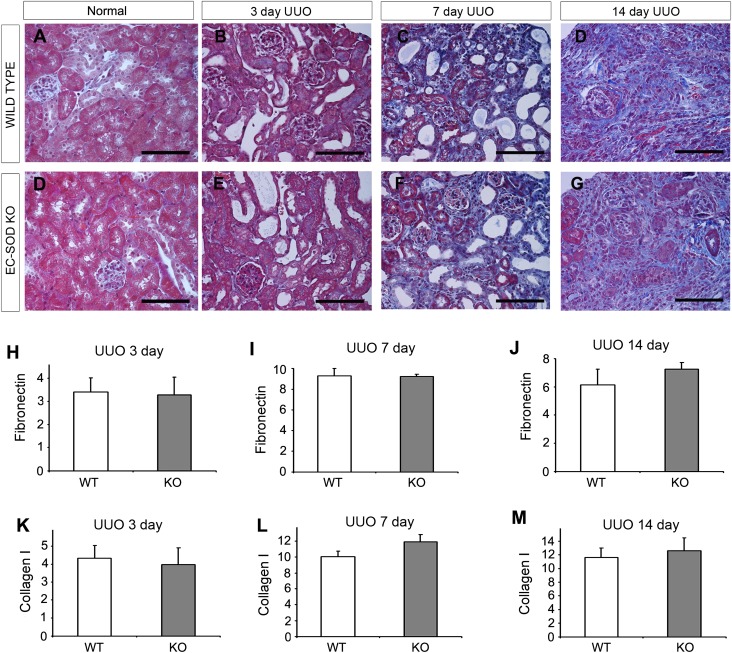
We also subjected KO mice to unilateral ureteral obstruction (UUO), which is a classic model of nonproteinuric tubulointerstitial disease.22 Although we examined several time points (3, 7, and 14 days after ureteral ligation), we were unable to find a discernable difference in fibrosis between KO and WT mice in fibrosis parameters, either by histology (Figure 9, A–G) or RNA analysis (Figure 9, H–M). We concluded that EC-SOD plays a role in proteinuric, rather than nonproteinuric, CKD.
Figure 9.
EC-SOD KO mice do not differ from WT mice in a model of nonproteinuric renal injury induced by urinary obstruction. Mice are subjected to UUO injury via the ligation of one ureter. (A–G) Normal/sham mice have the same surgeries without the actual ligation (A and D). Tubular dilation is seen at 3 days (B and E), with increasing fibrosis at 7 and 14 days postligation (C and F, and D and G, respectively), using a trichrome stain. However, histologic damage is not appreciably different between WT and KO mice at any examined time point. (H–M) Furthermore, levels of the extracellular matrix proteins fibronectin (H–J) and collagen (K–M) are not significantly different between the groups. Bar, 100 μm.
EC-SOD Is Depleted from Human CKD Kidneys
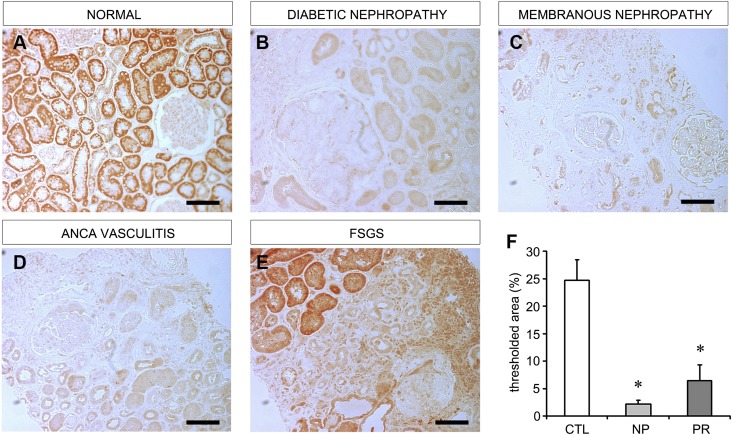
In order to examine the relevance of our findings in human renal disease, we examined human tissue sections for EC-SOD. As shown in Figure 10A, normal kidneys had high expression of EC-SOD, primarily in tubules. In fibrotic proteinuric CKD such as diabetic and membranous nephropathy, we found that EC-SOD was significantly depleted (Figure 10, B and C), just as it was in the ADR model. In nonproteinuric CKD such as that caused by ANCA-related vasculitis, we also found a similar depletion of EC-SOD (Figure 10D). It appeared that diseased areas of the kidney were consistently associated with decreased EC-SOD levels, and areas of transition between normal tissue with normal EC-SOD levels and diseased tissue with diminished EC-SOD levels could be identified (Figure 10E), supporting a loss of EC-SOD with increasing disease severity. Quantitation of EC-SOD staining in the entire renal biopsy did verify an overall loss of this antioxidant in human CKD regardless of proteinuric status (Figure 10F, Supplemental Table 1). This suggests that, in accordance with our animal data, EC-SOD is depleted in human CKD, and suggests that EC-SOD could play a protective role in the kidney.
Figure 10.
EC-SOD levels are decreased in human CKD tissue biopsies. Immunohistochemistry for human EC-SOD is performed on normal control tissues as well as renal biopsy specimens from patients with CKD. (A) Normal renal tissue showing abundant EC-SOD staining (red color). (B and C) EC-SOD staining is markedly decreased in proteinuric CKD due to diabetic and membranous nephropathy. Note the complete absence of staining in fibrotic areas. (D) EC-SOD staining is also diminished in nonproteinuric CKD due to c-ANCA vasculitis. (E) Areas of transition from normal tissue to fibrotic tissue are identified and show strong staining in normal tubules and absence of staining in fibrotic/diseased areas. (F) Quantitation of EC-SOD expressed as percent of total tissue area with positive EC-SOD staining (control tissue, n=3; nonproteinuric CKD, n=5; proteinuric CKD, n=9). CTL, control; NP, nonproteinuric CKD; PR, proteinuric CKD. Bar, 100 μm.
Discussion
Oxidative stress has long been hypothesized to participate in the development of kidney disease. In particular, recent evidence has shown that overproduction of the superoxide free radical is responsible for much of the renal dysfunction and injury.7,12,24,25 However, evidence regarding the role of the superoxide-scavenging antioxidant enzyme EC-SOD has been inconsistent. Whereas one study found that EC-SOD ameliorates ischemic AKI,15 another study failed to find an effect in a proteinuric diabetic nephropathy model.17 Although the latter study casts doubt on the importance of EC-SOD, it was unclear whether the result is generalizable to all forms of proteinuric CKD.
Our results indicate that EC-SOD is protective in at least three different models of proteinuria. First, we show that the presence of EC-SOD ameliorates ADR injury, a glomerular injury model that recapitulates human FSGS. EC-SOD decreased oxidative stress, NADPH oxidase activity, and pathologic β-catenin signaling. Second, EC-SOD decreased the proteinuria resulting from either continuous angiotensin II infusion or an albumin overload model. This argues that the protective effect of EC-SOD is not specific to the ADR injury model alone. However, protection by EC-SOD in mice did appear to be dependent on the presence of proteinuria, because we were completely unable to demonstrate any difference in injury when comparing KO and WT mice in the nonproteinuric UUO model. A specific role for oxidative stress in glomerular disease has been supported by other authors, who show that podocytes are particularly sensitive to oxidative stress.12
We examined the potential mechanisms by which EC-SOD may protect against ADR injury by examining NADPH oxidase expression and β-catenin signaling. In WT mice, we found an upregulation of both Nox2 and Nox4 NADPH oxidase isoforms in the kidneys of affected mice. To our knowledge, this is the first description of NADPH oxidase upregulation in ADR nephropathy. In EC-SOD null mice, the increase in Nox2 was further accentuated. Nox2 is expressed primarily in phagocytic cells, but also in podocytes and vascular smooth muscle cells.19 Nox2 has been described to be upregulated in proteinuric CKD, including diabetic nephropathy.26,27 Therefore, in ADR nephropathy, the kidney receives a “double hit” favoring excessive superoxide, because the protective antioxidant EC-SOD is lost while the ROS-generating NADPH oxidase is upregulated. This imbalance is further accentuated in EC-SOD KO mice.
In addition, we examined Wnt/β-catenin, an evolutionarily conserved signaling pathway, which is known to be pathologic in CKD.22,28 It has been shown that activation of Wnt/β-catenin leads to podocyte dysfunction and albuminuria in the ADR model as well as in human FSGS and diabetic nephropathy.21 In addition, Wnt/β-catenin blockade could reduce myofibroblast activation and the development of fibrosis.22,29 We found that EC-SOD null mice had increased levels of β-catenin signaling, which strongly suggests that this is at least one mechanism underlying the protective effect of EC-SOD.
It is currently unclear how loss of EC-SOD leads to increased Nox2/Nox4 expression or β-catenin activity. Because NADPH oxidases and β-catenin are increased in injury, a simple explanation may be that the increased glomerular injury and proteinuria in the KO mice leads to further upregulation of these pathologic mediators. In particular, angiotensin II is known to upregulate NADPH oxidase subunits and is commonly upregulated in renal injuries.30 In addition, it was previously demonstrated that superoxide itself leads to the upregulation of NADPH oxidase in the renal cortex,31 and that EC-SOD KO mice have increased NADPH activity and subunit expression.32 The loss of EC-SOD could therefore increase superoxide, leading to a feed-forward mechanism in which NADPH oxidase is further upregulated. Interestingly, β-catenin activity can be increased by ROS generated by NADPH oxidases, suggesting a potential link between these proteins that could explain our findings.33,34
To our knowledge, this is the first demonstration of a protective role for EC-SOD in proteinuric renal disease. It has previously been shown that intracellular SOD is protective against diabetic nephropathy,25 although the extracellular isoform was not vital to progression in this disease model.17 However, the high expression of EC-SOD in the kidney suggests that it plays a key role in maintaining homeostasis.10 In light of our data, it is now clear that extracellular scavenging of superoxide is necessary in models of proteinuric CKD induced by exposure to ADR, angiotensin II, and albumin overload.
The apparent discrepancy between the lack of effect of EC-SOD in diabetic nephropathy and our current results could be explained by the location of superoxide generation. As a negatively charged molecule, superoxide does not cross cellular membranes, so it has effects in the same intracellular or extracellular compartment in which it is generated. In diabetic nephropathy, one major mechanism of injury is the ligation of advanced glycation end products (AGEs) to their receptor, which ultimately leads to increased intracellular oxidative stress.35 On the other hand, NADPH oxidases can be inserted into plasma membranes, leading to extracellular superoxide generation.36 As we now demonstrate that ADR upregulates NADPH oxidase, it is possible that this leads to superoxide generation in the extracellular space, where it is accessible to scavenging by EC-SOD. Furthermore, it is important to note that the earlier diabetic study utilized a mouse that was genetically susceptible to diabetes but is somewhat resistant to the development of diabetic nephropathy.17 It is possible that the genetic factors underlying this phenotype could have masked any potential effects of EC-SOD.
It is also important to assess compensatory changes in other SODs in our experiments. We demonstrate that although there is a small increase in both cytoplasmic (SOD1) and mitochondrial (SOD2) SODs in our EC-SOD KO mice at baseline, there is lower expression in KO compared with WT mice after ADR treatment. The magnitude of this change is small (approximately 20%–30% as measured by quantitative real-time PCR), compared with the global and absolute ablation of EC-SOD. However, we cannot rule out contributions of these isoforms to the disease phenotypes described above, and their effect must be assessed in future studies.
Another question we sought to answer was whether EC-SOD had similar effects on interstitial fibrosis in the absence of proteinuria. It is well known that glomerular proteinuria can lead to tubular injury and ultimately fibrosis.1,37 We therefore examined the UUO model, in which interstitial fibrosis occurs in the absence of proteinuria. In spite of examination of a comprehensive series of time points after injury initiation, we did not find an effect of EC-SOD on fibrosis development, as assessed by either histology or generation of extracellular matrix components. This strongly suggests that EC-SOD primarily affects fibrosis development indirectly via modulation of proteinuria, rather than by a direct effect on interstitial fibrosis development.
In humans, it appears that the role of EC-SOD may be more ubiquitous. We did assess CKD tissue biopsies and found that EC-SOD was depleted in both proteinuric and nonproteinuric disease. This suggests nonspecific downregulation in various CKD etiologies. Although these data only show an association to human disease, it is reasonable to suggest that loss of EC-SOD may leave the kidney prone to further injury by oxidative stress. These findings also suggest a more universal role for this antioxidant in human proteinuric and nonproteinuric renal diseases compared with our animal models.
In conclusion, we find that EC-SOD is vital for renal protection against proteinuric renal injury in vivo. The mechanism by which EC-SOD offers protection is via inhibition of NADPH oxidase upregulation, as well as through downregulation of pathologic β-catenin signaling. In proteinuric renal diseases such as FSGS, EC-SOD may be a viable therapeutic target.
Concise Methods
Animals and Treatment Protocol
All animal studies were performed in accordance with the recommendations in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. For WT experiments, male BALB/c mice (aged 6–8 weeks) were purchased from Harlan Laboratories (Indianapolis, IN) and housed in the University of Pittsburgh Medical Center animal facility. Mice were subjected to a single intravenous tail-vein injection of ADR (10 mg/kg, doxorubicin hydrochloride; Sigma-Aldrich, St. Louis, MO). Urine was collected at various time points. Mice were euthanized at 1, 3, and 5 weeks, at which point blood and kidney tissues were collected.
EC-SOD null mice were bred from a colony originally generated by Carlsson et al.38 The background for these EC-SOD null mice is the C57BL/6 strain, which is relatively resistant to ADR injury. To induce ADR nephropathy, these resistant mice were injected once with 18 mg/kg of ADR and allowed to develop injury over an 8-week time period after injection. Urine, blood, and tissue samples were obtained.
Angiotensin II–induced proteinuria was performed in WT and EC-SOD null mice. Mice were first subjected to unilateral nephrectomy at day −7. On day 0, a subcutaneous osmotic minipump (model 2004; Alzet, Cupertino, CA) was implanted to deliver a constant infusion of angiotensin II (1.5 mg/kg per day; Sigma-Aldrich). Urine was collected and assessed for albumin content. Mice were euthanized 4 weeks after pump placement.
For the albumin overload model, we injected mice with increasing amounts of endotoxin-free BSA (Sigma-Aldrich) as previously described.23 Briefly, mice were subjected to unilateral nephrectomy 5 days before BSA injections. Mice were intraperitoneally injected with BSA dissolved in sterile saline (0.33 mg/ml) at 2, 4, 6, 8, and 10 mg/g body wt on consecutive days (days 1–5). Mice were then “rested” for 2 days, with daily injections resuming at the 10 mg/g dosage on days 8–10. Urine was collected on day 10, and mice were euthanized at this time as well.
To induce UUO, we followed previously described protocols.22 Briefly, the left kidney was exposed under anesthesia and the ureter ligated proximally with 4-0 silk suture. The right kidney was left unharmed and mice were euthanized at 3, 7, and 14 days after the surgery.
Biochemical Measurements
Serum and urine creatinine were measured with a Bioassay Systems kit (Hayward, CA). Similarly, we measured urinary albumin excretion using an ELISA-based kit from Bethyl Laboratories (Montgomery, TX). Urine albumin was normalized to urine creatinine to obtain units of measure that were milligrams of albumin per milligram of creatinine.
Western Blot Analyses
Western blot analyses were performed as previously described.39 Briefly, total kidney homogenates were prepared using radioimmunoprecipitation assay buffer supplemented with protease inhibitor cocktails. Total levels of protein were determined with the BCA protein assay kit (Sigma-Aldrich). Kidney homogenates were subjected to SDS-PAGE and blotted onto nitrocellulose membranes (GE Healthcare Life Sciences, Pittsburgh, PA). After blocking, blots were incubated overnight with primary antibody and then incubated with appropriate secondary antibodies. Signal was detected with Supersignal West Pico substrate reagents (Thermo Fisher Scientific, Rockford, IL). The primary antibodies used were as follows: anti-actin (MAB1501; Chemicon, Billerica, MA), anti–EC-SOD (AF4817; R&D Systems, Minneapolis, MN), anti-desmin (D1033, Sigma-Aldrich), anti-fibronectin (F3648; Sigma-Aldrich), anti–β-catenin (610154; BD Biosciences, San Jose, CA), anti-Nox2 (611414, BD Biosciences), and anti-Nox4 (sc-30141; Santa Cruz Biotechnology, Dallas, TX). Densitometry obtained with ImageJ software (NIH, Bethesda, MD) was utilized for quantitative data on band intensity compared with the loading control.
DHE Staining
Freshly isolated kidneys were frozen in optimal cutting temperature medium. Immediately after sectioning (5 µm), the slides were incubated with 10 μM DHE (Sigma-Aldrich) in PBS for 30 minutes. Results were visualized using an Olympus Fluoview 500 confocal microscope (Olympus, Center Valley, PA). Staining intensity was measured with MetaMorph software (Molecular Devices, Sunnyvale, CA).
TBARS Assay
TBARS were measured as a marker for oxidative stress using a kit (Cayman Chemical, Ann Arbor, MI).
Quantitative Real-Time RT-PCR
RNA was isolated from kidney homogenates using Trizol reagent (Life Technologies, Grand Island, NY). After first-strand cDNA synthesis from equivalent starting RNA material for each sample, quantitative real-time RT-PCR was performed using specific primers and a StepOne PCR instrument from Life Technologies/Applied Biosystems (Grand Island, NY). An annealing temperature of 60°C was used. The primer sequences are as follows: EC-SOD, forward ATCCCACAAGCCCCTAGTCT and reverse GTGCTATGGGGACAGGAAGA; fibronectin, forward CGAGGTGACAGAGACCACAA and reverse CTGGAGTCAAGCCAGACACA; collagen I, forward ATCTCCTGGTGCTGATGGAC and reverse ACCTTGTTTGCCAGGTTCAC; collagen III, forward AGGCAACAGTGGTTCTCCTG and reverse GACCTCGTGCTCCAGTTAGC; and matrix metalloproteinase-7, forward TAGGCGGAGATGCTCACTTT and reverse TTCTGAATGCCTGCAATGTC.
Histologic Assessment
Kidney tissue was fixed by standard protocols and embedded in paraffin. Sections (3 µm) were deparaffinized and stained with Periodic acid–Schiff or Masson’s trichrome staining reagents (both from Sigma-Aldrich) according to kit instructions. Separate deparaffined sections were stained with Sirius red (Sigma-Aldrich) for 2 hours before washes in acetic acid and mounting. Sirius red results were visualized with light microscopy with and without polarization.
The fibrosis score was obtained by scoring randomly selected fields with a grid overlay. Grid squares containing fibrotic tissue were counted and divided by total number of squares. The glomerulosclerosis score was obtained by scoring in a blinded fashion 20 randomly selected glomeruli per animal on the basis of sclerosis involvement compared with the total area of the glomerulus. The scoring system is as follows: 0=no damage; 1=1%–25%; 2=26%–50%; 3=51%–75%; and 4=76%–100% (or globally sclerotic). The numbers were averaged for each mouse, and then for each treatment group for determining significance.
Immunofluorescence Staining
Frozen tissue sections in optimal cutting temperature medium were cut to 5 μm thickness. After fixation with paraformaldehyde and blocking, sections were stained with antibody against WT1 (sc-192; Santa Cruz Biotechnology) and nephrin (2OR-NP002; Fitzgerald Industries International, Acton, MA) as previously described20 or to desmin (D1033; Sigma-Aldrich) and followed by appropriate Cy2- and Cy3-labeled secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA), respectively. The number of WT1-positive cells was performed on 20 randomly selected glomeruli per animal and presented as average count per glomeruli.
Immunohistochemistry
Paraffin-embedded mouse kidney tissues were subjected to immunohistochemical analysis with antibody specific to β-catenin (ab15180; Abcam, Inc., Cambridge MA) or desmin (D1033; Sigma-Aldrich). Briefly, slides were deparaffinized, with inactivation of endogenous peroxidases and blocking, followed by incubation with primary antibody overnight. Subsequent incubation with biotinylated secondary antibody (Jackson ImmunoResearch Laboratories), ABC Reagent, and AEC Reagent (Vector Laboratories, Burlingame, CA) were used to visualize staining.
Human kidney specimens were obtained from diagnostic renal biopsies performed at the University of Pittsburgh Medical Center. Tissues were formalin fixed and paraffin embedded. Tissue sections were cut at a thickness of 3 μm. Diagnoses were made by a renal pathologist (S.I.B.). Control tissue (n=3) was non-neoplastic renal tissue from patients undergoing nephrectomy for renal malignancy. CKD tissue consisted of five cases of nonproteinuric disease (c-ANCA vasculitis, arteriolar nephrosclerosis, and three cases of interstitial nephritis) and nine proteinuric cases (two amyloid, three membranous nephropathy, and one each of diabetic nephropathy, FSGS, lupus, and fibrillary GN). Immunohistochemistry was performed as described above except for use of an antibody specific for human EC-SOD (ADI-SOD-106; Enzo Life Sciences, Farmingdale, NY). To quantitate staining intensity, the entire core biopsy was photographed with sequential images and thresholded for positive staining on MetaMorph software. The areas of positive staining were divided by the total area of tissue per image to get a percent thresholded area. For the controls, 10 random images were taken and subjected to the same analysis.
Statistical Analyses
Statistical comparisons were made using a t test or one-way ANOVA for comparisons between two and three groups, respectively. P<0.05 was the threshold used for significance. SigmaStat software (Systat Software, San Jose, CA) was used for all analyses.
Disclosures
None.
Supplementary Material
Acknowledgments
Expertise in some surgical and histological techniques was obtained via the 2013 Vanderbilt O’Brien Center Mouse Kidney Injury Workshop.
This research was supported by the American Heart Association (Fellow-to-Faculty Award FTF 16990086) and the NIH (Grants DK064005, DK091239, and T32-DK061296). Core facilities in the University of Pittsburgh Center for Biologic Imaging as well as the Pittsburgh Center for Kidney Research (supported by NIH Grant P30-DK079307) were utilized in the generation of data for this work.
Parts of this work were presented in abstract form at the 2012 American Society of Nephrology Annual Meeting in San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014060613/-/DCSupplemental.
References
- 1.Ruggenenti P, Cravedi P, Remuzzi G: Mechanisms and treatment of CKD. J Am Soc Nephrol 23: 1917–1928, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Lambers Heerspink HJ, de Borst MH, Bakker SJ, Navis GJ: Improving the efficacy of RAAS blockade in patients with chronic kidney disease. Nat Rev Nephrol 9: 112–121, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Liu Y: Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Declèves AE, Sharma K: Novel targets of antifibrotic and anti-inflammatory treatment in CKD. Nat Rev Nephrol 10: 257–267, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Túri S, Németh I, Torkos A, Sághy L, Varga I, Matkovics B, Nagy J: Oxidative stress and antioxidant defense mechanism in glomerular diseases. Free Radic Biol Med 22: 161–168, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M: Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol 14[Suppl 3]: S250–S253, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Kinugasa S, Tojo A, Sakai T, Tsumura H, Takahashi M, Hirata Y, Fujita T: Selective albuminuria via podocyte albumin transport in puromycin nephrotic rats is attenuated by an inhibitor of NADPH oxidase. Kidney Int 80: 1328–1338, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Daehn I, Casalena G, Zhang T, Shi S, Fenninger F, Barasch N, Yu L, D’Agati V, Schlondorff D, Kriz W, Haraldsson B, Bottinger EP: Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest 124: 1608–1621, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizuguchi Y, Chen J, Seshan SV, Poppas DP, Szeto HH, Felsen D: A novel cell-permeable antioxidant peptide decreases renal tubular apoptosis and damage in unilateral ureteral obstruction. Am J Physiol Renal Physiol 295: F1545–F1553, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folz RJ, Guan J, Seldin MF, Oury TD, Enghild JJ, Crapo JD: Mouse extracellular superoxide dismutase: Primary structure, tissue-specific gene expression, chromosomal localization, and lung in situ hybridization. Am J Respir Cell Mol Biol 17: 393–403, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Fattman CL, Schaefer LM, Oury TD: Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med 35: 236–256, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Zhou LL, Hou FF, Wang GB, Yang F, Xie D, Wang YP, Tian JW: Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int 76: 1148–1160, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Tan RJ, Fattman CL, Watkins SC, Oury TD: Redistribution of pulmonary EC-SOD after exposure to asbestos. J Appl Physiol (1985) 97: 2006–2013, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Schneider MP, Sullivan JC, Wach PF, Boesen EI, Yamamoto T, Fukai T, Harrison DG, Pollock DM, Pollock JS: Protective role of extracellular superoxide dismutase in renal ischemia/reperfusion injury. Kidney Int 78: 374–381, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita H, Fujishima H, Chida S, Takahashi K, Qi Z, Kanetsuna Y, Breyer MD, Harris RC, Yamada Y, Takahashi T: Reduction of renal superoxide dismutase in progressive diabetic nephropathy. J Am Soc Nephrol 20: 1303–1313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita H, Fujishima H, Takahashi K, Sato T, Shimizu T, Morii T, Shimizu T, Shirasawa T, Qi Z, Breyer MD, Harris RC, Yamada Y, Takahashi T: SOD1, but not SOD3, deficiency accelerates diabetic renal injury in C57BL/6-Ins2(Akita) diabetic mice. Metabolism 61: 1714–1724, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan RJ, Zhou L, Zhou D, Lin L, Liu Y: Endothelin receptor a blockade is an ineffective treatment for ADR nephropathy. PLoS ONE 8: e79963, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill PS, Wilcox CS: NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, Li Y, He W, Zhou D, Tan RJ, Nie J, Hou FF, Liu Y: Mutual antagonism of Wilms’ Tumor 1 and β-catenin dictates podocyte health and disease [published online ahead of print July 28, 2014]. J Am Soc Nephrol 10.1681/ASN.2013101067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y: Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20: 1997–2008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eddy AA, Kim H, López-Guisa J, Oda T, Soloway PD: Interstitial fibrosis in mice with overload proteinuria: Deficiency of TIMP-1 is not protective. Kidney Int 58: 618–628, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL: NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol 21: 93–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeRubertis FR, Craven PA, Melhem MF: Acceleration of diabetic renal injury in the superoxide dismutase knockout mouse: Effects of tempol. Metabolism 56: 1256–1264, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS: Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int 67: 1890–1898, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, Loibner H, Janzek E, Schuster M, Penninger JM, Herzenberg AM, Kassiri Z, Scholey JW: Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes 59: 529–538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He W, Kang YS, Dai C, Liu Y: Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y: Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol 22: 1642–1653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsuyama M: NOX/NADPH oxidase, the superoxide-generating enzyme: its transcriptional regulation and physiological roles. J Pharmacol Sci 114: 134–146, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS: Angiotensin-induced defects in renal oxygenation: Role of oxidative stress. Am J Physiol Heart Circ Physiol 288: H22–H28, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill PS, Aslam S, Wang X, Ji H, Sandberg K, Jose P, Wilcox CS: Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension 48: 934–941, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Funato Y, Michiue T, Asashima M, Miki H: The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol 8: 501–508, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Kajla S, Mondol AS, Nagasawa A, Zhang Y, Kato M, Matsuno K, Yabe-Nishimura C, Kamata T: A crucial role for Nox 1 in redox-dependent regulation of Wnt-β-catenin signaling. FASEB J 26: 2049–2059, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Coughlan MT, Mibus AL, Forbes JM: Oxidative stress and advanced glycation in diabetic nephropathy. Ann N Y Acad Sci 1126: 190–193, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Leto TL, Morand S, Hurt D, Ueyama T: Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal 11: 2607–2619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorriz JL, Martinez-Castelao A: Proteinuria: Detection and role in native renal disease progression. Transplant Rev (Orlando) 26: 3–13, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Carlsson LM, Jonsson J, Edlund T, Marklund SL: Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci U S A 92: 6264–6268, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou D, Tan RJ, Zhou L, Li Y, Liu Y: Kidney tubular β-catenin signaling controls interstitial fibroblast fate via epithelial-mesenchymal communication. Sci Rep 3: 1878, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.