Abstract
Complex regional pain syndrome (CRPS) is a chronic pain disorder that typically follows trauma or surgery. Suspected CRPS reported after vaccination with human papillomavirus (HPV) vaccines led to temporary suspension of proactive recommendation of HPV vaccination in Japan. We investigated the potential CRPS signal in relation to HPV-16/18-adjuvanted vaccine (Cervarix®) by database review of CRPS cases with independent expert confirmation; a disproportionality analysis and analyses of temporality; an observed versus expected analysis using published background incidence rates; systematic reviews of aggregate safety data, and a literature review.
The analysis included 17 case reports of CRPS: 10 from Japan (0.14/100,000 doses distributed) and seven from the United Kingdom (0.08/100,000). Five cases were considered by independent experts to be confirmed CRPS. Quantitative analyses did not suggest an association between CRPS and HPV-16/18-adjuvanted vaccine. Observed CRPS incidence after HPV-16/18 vaccination was statistically significantly below expected rates. Systematic database reviews using search terms varying in specificity and sensitivity did not identify new cases. No CRPS was reported during clinical development and no unexpected results found in the literature.
There is not sufficient evidence to suggest an increased risk of developing CRPS following vaccination with HPV-16/18-adjuvanted vaccine. Post-licensure safety surveillance confirms the acceptable benefit-risk of HPV-16/18 vaccination.
Keywords: Complex regional pain syndrome, Human papillomavirus vaccine, Safety, Chronic pain
Highlights
-
•
GSK investigated a potential safety signal after suspected CRPS cases were reported following HPV vaccination in Japan
-
•
Little is known of CRPS. We used database reviews and statistical methods to maximise case detection and reduce uncertainty
-
•
There is not sufficient evidence to suggest an increased risk of CRPS following HPV-16/18-adjuvanted vaccine
In 2013 the Japanese health authority temporarily suspended proactive recommendation of HPV vaccination after reports of a chronic pain condition (CRPS) in Japan. GSK, who manufactures one of the two available HPV vaccines (Cervarix®), investigated this potential safety issue by reviewing its safety database for all cases of CRPS ever reported after HPV vaccination, and by various statistical analyses. None of the reviews or analyses suggested a safety concern. The observed rate of CRPS after vaccination was lower than what might be expected by chance. At this time there is not enough evidence to suggest that Cervarix® causes CRPS.
1. Introduction
Complex Regional Pain Syndrome (CRPS) is a chronic pain disorder characterised by significant autonomic features, and typically develops in an extremity after acute tissue trauma (Merksey & Bogduk, 1994). Symptoms may include continuing pain, sensory abnormalities like allodynia and/or, hyperalgesia and/or hypoesthesia, along with a range of vasomotor (abnormalities in skin colour or temperature), sudomotor (abnormalities of sweating or oedema) and motor/trophic (abnormalities in hair, skin, nails, tremor or muscle weakness) signs or symptoms.
The estimated incidence of CRPS varies from 5.46 to 26.2 per 100,000 person-years, with an incidence in females of 8.57 to 40.4 per 100,000 person-years (De Mos et al., 2007; Sandroni et al., 2003). CRPS occurs slightly more often in the upper extremity and women are affected 3.4–4 times more often than men (De Mos et al., 2007). Diagnosis is based upon clinical criteria. The most commonly used diagnostic criteria are modifications to the original International Association for the Study of Pain (IASP) criteria according to Harden and Bruehl (Budapest diagnostic criteria) (Harden et al., 2010).
The pathophysiology of CRPS continues to be debated in the literature. Current understanding implicates peripheral, afferent, efferent and central mechanisms (Van Eijs et al., 2011). There are indications that auto-inflammatory and/or genetic predisposition (for example, polymorphisms of human leucocyte antigen (Kemler et al., 1999; Mailis & Wade, 1994; Van de Beek et al., 2003; Van Hilten et al., 2000; De Rooij et al., 2009a; Dirckx et al., 2015), TNF-alpha (Vaneker et al., 2002), or possibly angiotensin-converting enzyme gene (Hühne et al., 2004; Kimura et al., 2000) play a role in the pathophysiology (De Rooij et al., 2009b; Goebel & Blaes, 2013). Associations have been suggested between CRPS and exposure to some viruses and or bacteria, such as parvovirus B19, herpes simplex virus, Campylobacter jejuni, Borrelia burgdorferi, and spirochetes (Van de Vusse et al., 2001; Muneshige et al., 2003; Goebel et al., 2005; Sibanc & Lesnicar, 2002; Neumann et al., 1989), and to some vaccines (Jastaniah et al., 2003; Genc et al., 2005).
HPV-16/18-adjuvanted vaccine (Cervarix®, GSK Vaccines, Belgium) was first licenced in 2007 and is currently licenced in 134 countries for the prevention of persistent infection, pre-cancerous lesions, and cervical and other genital cancers caused by oncogenic HPV. In March 2013 the Japanese press reported the case of a 14-year-old girl who had been diagnosed with CRPS in 2011, with onset of symptoms hours after vaccination with HPV-16/18-adjuvanted vaccine in 2009. The case generated significant media reporting in Japan (Wilson et al., 2014). An anti-vaccines group publicly called into question the safety of HPV vaccination and the Japanese national immunisation programme. At meetings held in May and June 2013, the Japanese Ministry of Health, Labor and Welfare (MHLW) publicly concluded that they found no reason for concern, but temporarily suspended proactive recommendation of vaccination for both available HPV vaccines in Japan (Cervarix® and Gardasil™ [HPV-6/11/16/18 vaccine, Merck & Co.]) until more information was available. HPV vaccination remains part of the Japanese government-funded vaccine programme and physicians can continue vaccination after explanation of benefit-risk.
Here we report the results of analyses undertaken by GSK Vaccines to investigate the potential CRPS signal. These investigations comprise a review of individual case reports (including an independent evaluation by an external expert panel), quantitative analyses assessing potential associations, systematic reviews of aggregate safety data and a literature review. These data provide a comprehensive assessment of safety information pertaining to HPV-16/18-adjuvanted vaccine and CRPS, thereby facilitating informed decision-making by vaccine providers and recipients.
2. Methods
GSK Vaccines follows a systematic approach to safety assessment, which includes regular review of individual case reports and aggregate data based on database searches from adverse event (AE) reports received by the company (Angelo et al., 2014a). Reports of AEs after vaccination are received by GSK through spontaneous reporting via worldwide sources from healthcare professionals, regulatory authorities, members of the public, and literature sources. All spontaneously reported AEs are coded in GSK's central worldwide safety database using the International Conference on Harmonisation Medical Dictionary for Regulatory Activities (MedDRA) to the level of preferred term (PT) (Medical Dictionary for Regulatory Activities, 2015). For cases where important clinical details are lacking, attempts are made to contact the source for additional information.
2.1. Primary Analyses
2.1.1. Qualitative Analyses
All spontaneous reports with HPV-16/18-adjuvanted vaccine (or unspecified HPV vaccine) reported as suspect and containing the specific MedDRA PT ‘complex regional pain syndrome’ were identified from launch until the data lock point of 31 July 2013.
Identified cases were reviewed according to the proposed experimental IASP criteria (Harden et al., 1999) by a safety physician within GSK (JZ). Two independent external experts (FH and CM) were provided with the individual clinical narratives of identified cases for review using Budapest diagnostic criteria (Harden et al., 2010). The assessment of cases by GSK and the results of the quantitative analyses were only shared with the experts once their own separate assessments of individual cases were completed.
2.1.2. Quantitative Analyses
A disproportionality analysis provided information on the frequency of reporting of a vaccine-event pair relative to background reporting in the database (Van Holle et al., 2012; Karimi et al., 2013), aiming to detect higher than expected reporting of vaccine-event combinations. The analysis used an empirical Bayes data mining algorithm (Multi-Item Gamma Poisson Shrinker) which computed the adjusted relative reporting ratio and associated 90% confidence intervals (CI) stratifying for age, gender, calendar time period and region.
An analysis of temporality was performed to detect potential differences in the reported temporal time-to-onset distributions of CRPS post-HPV-16/18 vaccination, versus post-other injected GSK Vaccines. This analysis assumed that for other injected vaccines, the time-to-onset distribution of CRPS was dominated by reporting bias and noise. Thus, a different time-to-onset distribution post-HPV-16/18 vaccination could suggest a potential temporal association between HPV-16/18-adjuvanted vaccine and CRPS (Van Holle et al., 2012; Karimi et al., 2013). The time-to-onset distribution of HPV-16/18-adjuvanted vaccine-CRPS was tested with a two-sample Kolmogorov Smirnov test over a 30 day period post-immunisation.
An observed-versus-expected analysis used background incidence rates from the available published literature to calculate age-adjusted expected incidence rates with CI provided using a Monte-Carlo simulation. HPV-16/18-adjuvanted vaccine exposure was estimated based on the number of doses sold. There is no acknowledged risk period for CRPS after a precipitating event. While reports of possible CRPS after vaccination have occurred on the same day as the vaccination (Jastaniah et al., 2003; Genc et al., 2005), we considered a theoretical risk window of six days after vaccination. The observed-versus-expected analysis was conducted separately for Japan, the UK and worldwide.
2.2. Extended Analyses
-
(1)
There is little information on background incidence rates of CRPS and the proportion of cases that might be underreported is not known. The observed-versus-expected analysis was therefore extended by a set of simulations that considered a range of reporting rates and background incidence rates to establish underreporting magnitudes and incidence rates for which the observed number of CRPS cases can be considered as expected for Japan, the UK and worldwide.
-
(2)
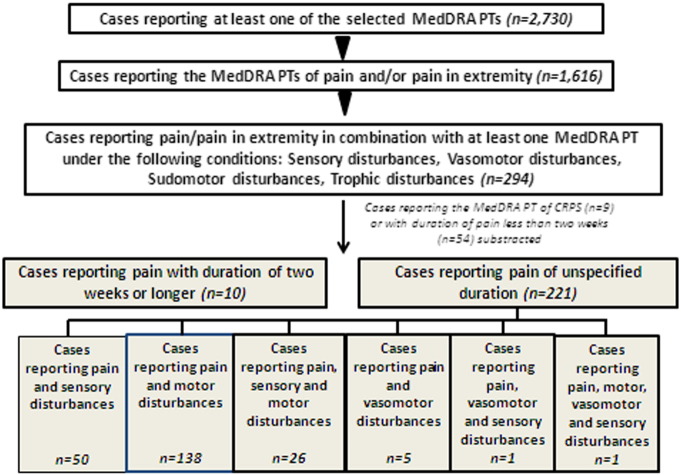
To investigate the possibility that CRPS after HPV-16/18 vaccination has occurred but gone unrecognised, an additional database search identified all spontaneous reports with MedDRA PTs describing signs and symptoms associated with CRPS mapped to Budapest diagnostic criteria (Harden et al., 2010) (Table 1). Reports were retrieved from launch until 31 December 2013 and were restricted to those in which an HPV vaccine had been administered. A decision tree was used to improve diagnostic precision: cases were categorised based on whether they included the MedDRA PTs of ‘pain’ or ‘pain in extremity’ in combination with another associated symptom, and whether the duration of pain was more than two weeks (Fig. 1).
-
(3)
As the MHLW conducted a similar search of locally reported cases but excluded cases where ‘pain’ was associated with other events, the company conducted a similar analysis. A broader evaluation of the safety data around pain (notably chronic pain) following HPV vaccination was undertaken. All cases under the MedDRA system organ classes (SOC) “musculoskeletal and connective tissue disorders” or “general disorders and administration site conditions” with a MedDRA PT of ‘pain’ were retrieved and analysed.
Table 1.
MedDRA terms used to identify potential unrecognised CRPS cases in the safety database.
| Proposed clinical diagnostic criteria for CRPS (Budapest criteria)(Harden et al., 2010)⁎ | MedDRA Preferred terms or low level terms |
|---|---|
| Pain: continuing pain disproportionate to vaccination | Pain; pain in extremity |
| Sensory: allodynia deep pressure pain, allodynia pain after movement, allodynia after light touch, hyperesthesia, hypoesthesia, hyperalgesia, hypoalgesia | Allodynia, hyperaesthesia, hypoaesthesia, sensory disturbance, skin burning sensation |
| Vasomotor: color change/difference, temperature difference | Skin discoloration, temperature difference of extremities |
| Sudomotor/edema: transpiration disturbance, edema | Oedema |
| Trophic: hair growth change, nail growth change, trophic skin disturbance | Hair growth abnormal, nail growth abnormal |
| Motor: limitation of movement, limitation of strength, dystonia, tremor, bradykinesia | Injection site movement impairment, injected limb mobility decreased, muscular weakness, dystonia, tremor, bradykinesia |
Pain should be continuous and disproportionate to the inciting event. In addition, for clinical diagnosis patients should have at least one symptom in each of the above categories and one sign in one category.
Fig. 1.
Schematic representation of search strategy and breakdown of selected cases.
The safety database was searched for cases reporting any of the selected MedDRA PTs (n = 2730). From this dataset, cases were selected reporting pain and/or pain in extremity (n = 1616) which was further narrowed down by identifying cases reporting a duration of ≥ 2 weeks or with unspecified event duration (n = 231). Cases reporting the MedDRA PT of CRPS (n = 9) were not further considered as they were evaluated separately. Most of the cases retained for further evaluation reported pain together with motor disturbances. Refer to boxes highlighted in grey for breakdown of cases according to the combination of events reported.
2.3. Role of the Funding Source
GlaxoSmithKline Biologicals SA collected and analysed the data. All authors had access to the data, responsibility for data interpretation, and the decision to submit for publication.
3. Results
At the time of the data lock point for this manuscript, there were 221,220 spontaneous adverse event reports in GSK's vaccines safety database, of which 18,391 were reported for HPV-16/18-adjuvanted vaccine. The 10 countries from where the most spontaneous event reports for HPV-16/18-adjuvanted vaccine originated were: Japan 32% of all reports, Italy 20%, Malaysia 16%, UK 11%, Netherlands 5%, Germany 2%, Korea 2%, Spain, Brazil and the US all 1%. Note that the reporting rate is influenced by the number of doses distributed in each country, as well as the robustness and efficiency of the pharmacovigilance systems and awareness of adverse event reporting tools in place.
3.1. Reports of CRPS
3.1.1. Analysis of CRPS Cases by GSK
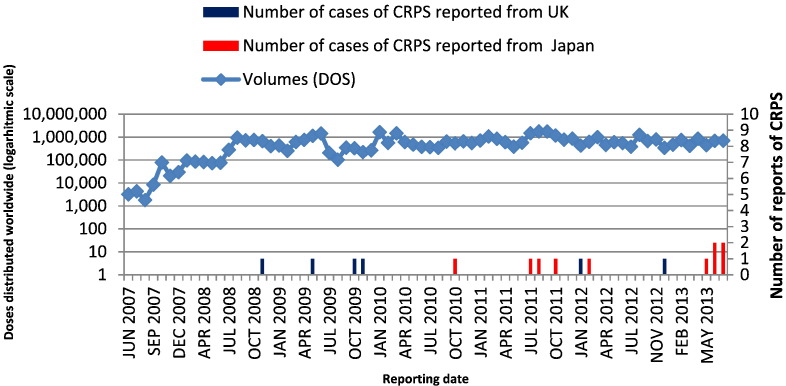
The first database search retrieved 17 case reports with the PT ‘complex regional pain syndrome’, with seven cases from the UK and 10 cases from Japan, corresponding to reporting rates of 0.08 and 0.14 cases per 100,000 doses distributed, respectively. The number of HPV-16/18-adjuvanted vaccine doses distributed worldwide each month has remained relatively stable since June 2008 (Fig. 2). Six CRPS cases (35% of all reports) were received after the initial case in Japan was reported in the media in March 2013 (Wilson et al., 2014).
Fig. 2.
Spontaneous reports of CRPS (n = 16*) and number of doses distributed each month since launch of HPV-16/18-adjuvanted vaccine.
*Following the initial analysis, one of the 17 originally identified CRPS cases was voided as the girl had received Gardasil™. Hence this case is not shown.
Note that HPV-16/18-adjuvanted vaccine was licenced in the UK two years before it was licenced in Japan.
The time between vaccination and the onset of symptoms ranged from immediately after vaccination to two years post-vaccination (Table 2). The diagnostic criteria for CRPS were met for 4/17 events: of these, one subject also received Japanese encephalitis vaccine before symptom onset, and one subject developed subacromal bursitis due to maladministration of the product. Seven cases were assessed as unconfirmed CRPS and six cases as unlikely to be CRPS based on the available information (Supplementary table 1).
Table 2.
Summary information of 17 reports of CRPS identified in GSK's safety database.
| Case details | N = 17 CRPS casesa | |
|---|---|---|
| Age (years) | Median (range) | 13 (12–46) |
| Report source n (%)b | Health care professional | 17 |
| Regulatory authority | 9 | |
| Other | 8 | |
| Time to onset | Range | Immediate to 2 years post-vaccination |
| Outcome n (%) | Resolved | 4 (23%) |
| Resolved with sequelae | 1 (6%) | |
| Unresolved | 3 (18%) | |
| Worse | 1 (6%) | |
| Unknown | 8 (47%) |
Cases identified by searching all spontaneous reports for cases containing the specific MedDRA preferred term CRPS from launch until 31 July 2013.
Some cases were reported by more than one source.
Results of quantitative analyses: few background CRPS incidence data were retrieved. These were limited to two countries: the US and the Netherlands (Van Holle et al., 2012; Karimi et al., 2013). The results of the disproportionality analysis did not suggest that the vaccine-event pair (HPV-16/18-adjuvanted vaccine and CRPS) was reported at a higher-than-expected rate relative to background reporting in the database (adjusted relative reporting stratified Empirical Bayes Geometric Mean of 1.018, 90% CI 0.703–1.435). The time-to-onset was known for seven HPV-16/18 cases with CRPS and for 13 cases retrieved for other injected vaccines. No significant difference in the time-to-onset distribution was observed (p-value 0.78).
Thirteen cases with a time-to-onset unknown or within six days after vaccination were included in the observed-versus-expected analysis (Table 3). Under the assumption that 100% of CRPS cases with time-to-onset within six days post-HPV-16/18 vaccination have been reported, the observed incidence rates were below expected rates, except under a worst case scenario for the UK and Japan, when all cases (confirmed/unconfirmed/unlikely) were included. Under these conditions the point estimates for the observed incidence rates were higher than the expected background incidence rates calculated from US data (95% CIs overlapped), but not using baseline data from the Netherlands (Table 3).
Table 3.
Observed versus expected analysis of CRPS cases after HPV-16/18 vaccination.
| Observed |
Expected-simulated age-adjusted incidence/100,000 person-years (95% CI) |
||||
|---|---|---|---|---|---|
| Number of cases | Incidence/100,000 person-years (95% CI) | Sandroni et al. (2003) United States | De Mos et al. (2007) The Netherlands | ||
| Worldwide | Confirmed CRPS with known time-to-onset | 3 | 0.4 (0.1; 1.3) | 2.8 (0.9; 5.7) | 16.4 (7.9; 26.4) |
| All cases: confirmed/unconfirmed/unlikely | 13 | 1.9 (1.0; 3.3) | 2.8 (0.9; 5.7) | 16.4 (7.9; 26.4) | |
| Japan | Confirmed CRPS with known time-to-onset | 1 | 0.9 (0.0; 4.8) | 3.1 (1.1; 6.0) | 16.9 (9.5; 26.0) |
| All cases: confirmed/unconfirmed/unlikely | 6 | 5.2 (1.9; 11.3) | 3.1 (1.1; 6.0) | 16.9 (9.5; 26.0) | |
| UK | Confirmed CRPS with known time-to-onset | 2 | 1.4 (0.2; 5.1) | 2.4 (0.3; 5.6) | 15.3 (6.5; 26.3) |
| All cases: confirmed/unconfirmed/unlikely | 7 | 4.9 (2.0; 10.2) | 2.4 (0.3; 5.6) | 15.3 (6.5; 26.3) | |
CI = confidence interval.
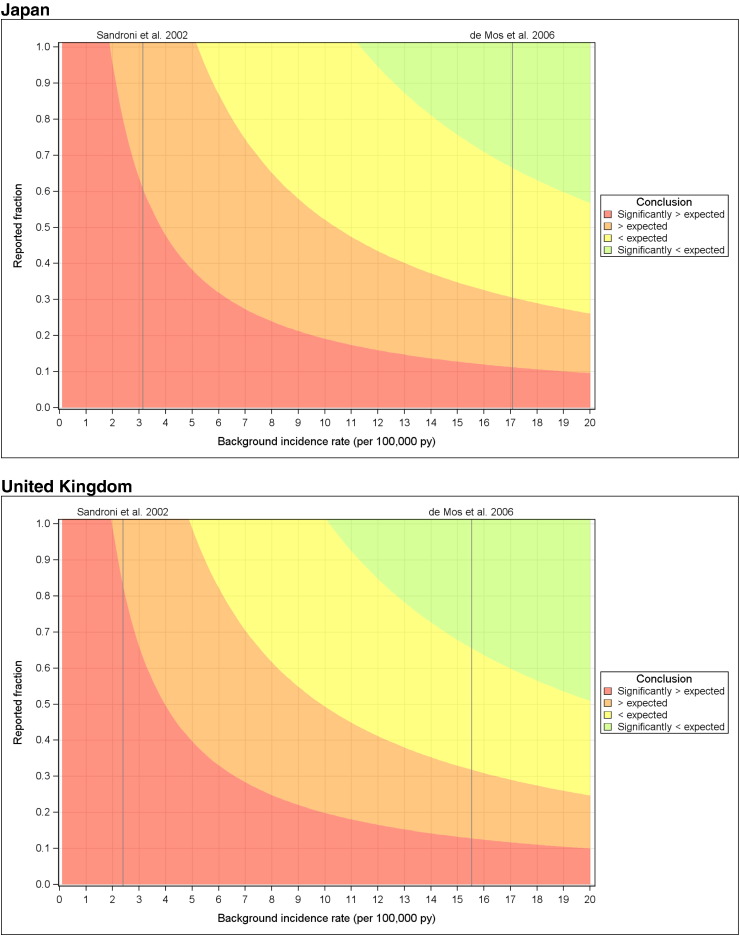
Fig. 3 shows results for the observed-versus-expected analysis using a simulated range of background incidence rates and underreporting. Considering a worst case safety scenario with inclusion of all cases (confirmed/unconfirmed/unlikely) the simulations indicated that for Japan, the observed rates would be significantly higher than expected rates under the assumption that less than 60% of cases were reported when comparing to US background incidence data, and that less than approximately 10% were reported when comparing to data from the Netherlands (Fig. 3). Results for the UK were 83% and 13%, respectively (Fig. 3).
Fig. 3.
Heat maps of the observed-to-expected analysis conclusion in the parameter plane defined by the background incidence rate and the under-reporting (two unknown parameters). Risk period of six days post HPV-16/18 immunisation and considering all cases irrespective of whether classified as confirmed/unconfirmed or unlikely to be CRPS.
3.1.2. Analysis of CRPS Cases by an Independent Expert Panel
Discrepancies noted between the experts' assessment and GSK's evaluation were discussed individually at a face-to-face meeting for clarification and consensus. The experts concurred in their independent analysis identifying five confirmed CRPS cases among the 17 reports (Supplementary table 1).
3.1.3. Reports Describing Signs/and Symptoms Associated With CRPS
A total of 2730 spontaneously reported cases reporting any, or a combination of MedDRA PTs describing signs/symptoms associated with CRPS (but excluding the specific PT ‘complex regional pain syndrome’) (Table 1) were retrieved from the GSK spontaneous report database. The decision tree to improve diagnostic precision led to identification of 231 potential cases (Fig. 1). For 221 cases the duration of pain was not specified. For 10 cases where the duration of pain was two weeks or more, the reported MedDRA PTs were hypoaesthesia (n = 4), injected limb mobility decreased (n = 4), muscular weakness (n = 5), sensory disturbance (n = 1) and skin discoloration (n = 1). Considering the specific features of pain reported by these subjects, none presented a clinical picture suggestive of a potential CRPS case.
The 221 cases without information on pain duration were individually assessed. The majority of cases reported MedDRA PTs indicative of motor disturbances with decreased mobility of the injected limb and muscular weakness: most cases reported injection site pain that prevented movement of the injected limb. The most frequently reported sensory AE was hypoaesthesia, with symptom onset, together with pain, soon after vaccination.
No cases indicative of unrecognised CRPS were identified. The nature of the cases and/or the information contained in the case reports did not add new information to the conclusions reached following review of 17 cases reporting the MedDRA PT of ‘complex regional pain syndrome’ itself.
Eleven additional reports of CRPS have been received from Japan since the data lock point of the previous analysis described above. None met the Budapest diagnostic criteria of CRPS as assessed by GSK (Supplementary table 2).
3.1.4. Reports Describing Chronic Pain
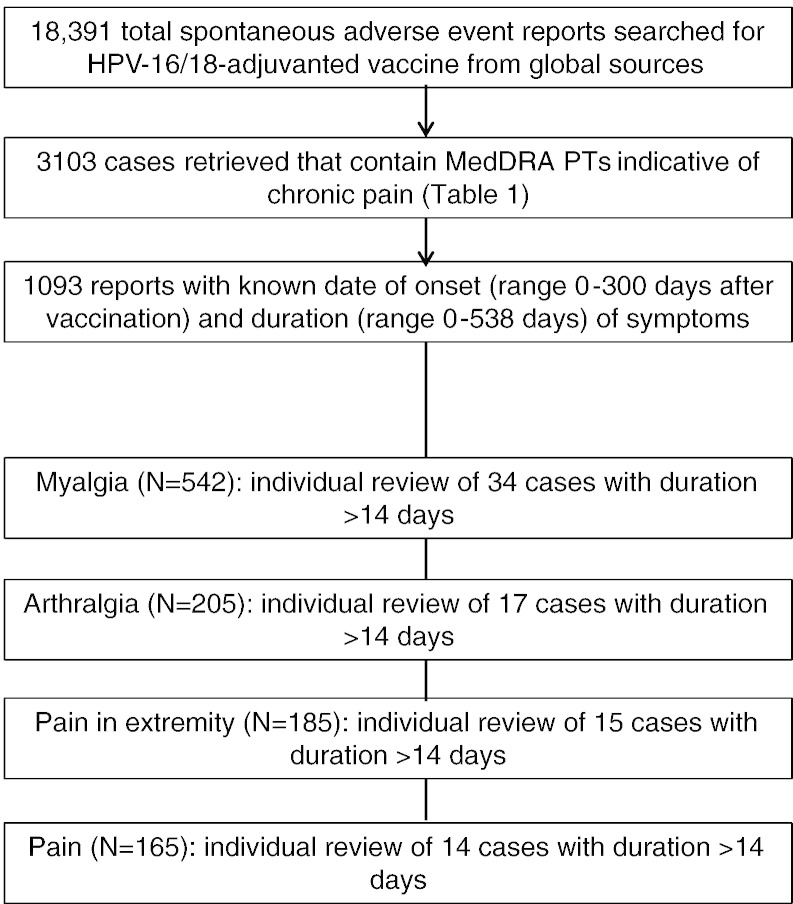
A total of 3103 spontaneously reported cases with HPV-16/18-adjuvanted vaccine or unspecified HPV vaccine as suspect vaccine were retrieved using the search criteria. The majority of reports came from Japan (31%), Italy (25%), Malaysia and UK (each 11%) and the Netherlands (7%), with 15% from other countries. The highest reporting rate was from Italy (38/100,000 doses distributed) and Malaysia and the Netherlands (each 15/100,000 doses) followed by Japan (14/100,000 doses distributed). The most frequently occurring PTs were myalgia (n = 1077 reports), pain (n = 912), pain in extremity (n = 650) and arthralgia (n = 538) (Fig.4). Because there is a theoretical risk that adjuvanted vaccines may trigger the onset of immune-mediated diseases in susceptible individuals, cases that contained a MedDRA PT indicating a potential immune-mediated disease (pIMD) were identified in this dataset (Tavares Da Silva et al., 2013). There were 59 cases with a pIMD covering eight SOCs and 27 different MedDRA PTs. Considering reports from Japan, no pattern or symptom cluster was apparent in comparison to global reports.
Fig. 4.
Flow digram illustrating the assessment of potential cases with chronic pain.
Of 3103 retrieved cases, the time-to-onset and duration of pain was known in 1093 (Fig.4). Individual review of the most frequently reported PTs with known time-to-onset and pain duration of at least two weeks did not show any consistent pattern in terms of the clinical picture, or a safety signal in association with HPV-16/18 vaccination.
The assessment of cases after exclusion of 1237 cases in which pain was associated with other diagnoses (as per MHLW criteria) was broadly consistent with the larger analysis.
3.1.5. CRPS Reported in Clinical Trials
A pooled safety analysis included data from all completed and ongoing studies in which HPV-16/18-adjuvanted vaccine had been administered to females from 9 years of age until the data lock point of 30 April 2011 (Angelo et al., 2014b). This analysis included 31,173 girls and women who received HPV-16/18-adjuvanted vaccine and 24,241 who received a control (placebo or comparator vaccine). No reports of CRPS were identified from clinical trials.
4. Discussion
Rigorous review of all potential CRPS cases reported to GSK since launch of HPV-16/18-adjuvanted vaccine identified five cases considered by independent experts to be confirmed CRPS. Considering all five cases globally, the reporting rate is 0.01 cases per 100,000 doses distributed. Quantitative analyses did not suggest a higher CRPS reporting rate after HPV-16/18-adjuvanted vaccine than after other vaccines, nor a temporal relationship with HPV-16/18-adjuvanted vaccine that differed from other vaccines. An observed-versus-expected analysis suggested that the observed incidence rate of CRPS after HPV-16/18-adjuvanted vaccine is not significantly higher than the expected rate for a range of plausible combinations of background incidence rate and reporting bias. Detailed systematic reviews of the central worldwide safety database using search terms varying in specificity and sensitivity did not add new information to the conclusions reached following review of cases reporting the MedDRA PT of CRPS. There was no report of CRPS in the overall clinical trial programme.
Regular review of spontaneous adverse event reports enables the detection of safety signals during the lifecycle of a vaccine. However, in most countries adverse event reporting is passive, and adverse event reporting rates are highly variable, influenced by awareness and ease of use of the adverse event reporting systems, cultural expectations, experience with the vaccine or with similar vaccines, characteristics and severity of the adverse event itself, the period of time which has elapsed since the vaccination procedure and onset of symptoms, and external factors such as media reports (Eberth et al., 2014). Indeed, increased reporting of suspected CRPS cases in Japan coincided with extensive media coverage of a CRPS case in Japan (Wilson et al., 2014). Aside from the limitations inherent in analysis of spontaneous adverse event reports, our analyses are limited by the condition itself: CRPS is an incompletely understood syndrome made up of a cluster of heterogeneous symptoms, many of which are non-specific. No diagnostic test is available, the list of differential diagnoses is extensive and there is little available information on background incidence rates in the literature. We conducted simulations to estimate how making assumptions on the underreporting and the background incidence rate of CRPS could affect the conclusions of the observed-versus-expected analysis. The results from these simulations allow experts to draw their own conclusions by applying, based on their knowledge, the most appropriate value for the two main sources of uncertainty affecting the observed-versus-expected analysis (Fig. 3). A constraint of these simulations is that they are limited to the two factors that were deemed as the main source of uncertainty (underreporting and background incidence). Other potential sources of uncertainty (e.g., the true risk window length) were not assessed.
Assessments of CRPS and HPV-16/18-adjuvanted vaccine have been conducted independently by regulatory agencies. In December 2012 the UK Medicines and Healthcare products Regulatory Agency (MHRA) published an observed-versus-expected analysis and a safety assessment report after 4 years of HPV-16/18-adjuvanted vaccine use in the UK (Medicines and Healthcare products Regulatory Agency (MHRA), 2012). The report concluded that “Reported cases of CRPS are consistent with chance and there is insufficient evidence of a causal association with Cervarix” (Medicines and Healthcare products Regulatory Agency (MHRA), 2012). The World Health Organization's Global Advisory Committee on Vaccine Safety could not ascertain a causal relationship of CRPS to HPV vaccination given lack of sufficient case information and inability to reach a definitive diagnosis in many cases (Global Advisory Committee on Vaccine Safety, 2013). Finally, in April 2014 after review of cumulative data, quantitative analyses and database searches provided by GSK, the European Medicines Agency concluded that the available data do not support a causal relationship between the use of HPV-16/18-adjuvanted vaccine and CRPS onset at this stage (EMA European Medicines Agency, 2014). CRPS remains under close surveillance.
We identified two case-series of CRPS after HPV vaccination in the literature. One article described peripheral sympathetic nerve dysfunction in 40 adolescent Japanese girls following immunisation with HPV vaccine (Kinoshita et al., 2014). According to the authors, four girls fulfilled the Japanese clinical diagnostic criteria of CRPS-1 (CRPS without known major nerve injury), and 14 fulfilled the Budapest diagnostic criteria (Harden et al., 2007). The paper describes five representative case reports. However, based on the case descriptions, none fulfil either of the CRPS diagnostic criteria (Japanese or Budapest); which raises the question of how the diagnostic criteria were applied. The authors also describe a postural orthostatic tachycardia syndrome (POTS), orthostatic hypotension (OH) and low plasma levels of noradrenaline in some patients. Whereas low levels of noradrenaline are described in CRPS, POTS and OH are not general findings in CRPS. Moreover, the sympathetic dysfunction is typically local and distal in one of the extremities and in no way general (Wasner, 2010), as seems to be the case with the girls described in the article.
The second article described five CRPS cases that the authors state fulfilled Budapest diagnostic criteria (Harden et al., 2007): four cases followed HPV vaccination (HPV-16/18-adjuvanted vaccine or HPV-6/11/16/18 vaccine ) and one followed diphtheria–tetanus–pertussis vaccine (Richards et al., 2012). In view of the different antigens administered, the authors proposed that the intramuscular injection was the event trigger, rather than the vaccine antigens themselves.
It remains unknown whether the development of CRPS could occur as an indirect effect of tissue trauma following injection of vaccine, or through an auto-inflammatory response, triggered by infection (or exposure to vaccine antigens) in susceptible individuals. The frequency of CRPS is so low compared to the frequency of infections, that the latter can be considered unlikely to play a pivotal etiological role. More obviously, the method of (injectable) vaccine administration is a procedure that initiates local tissue damage, which is acknowledged as a main trigger for a maladaptive and persistent response that continues after healing, which is pathophysiologic and characteristic for CRPS.
Altogether, based on the outcomes of this valuation, there is not sufficient evidence to suggest an increased risk of developing CRPS following vaccination with HPV-16/18-adjuvanted vaccine. Post-licensure safety surveillance confirms the acceptable benefit-risk of HPV vaccination in adolescent girls and adult women (Angelo et al., 2014a).
Trademarks
Cervarix is a registered trademark of the GSK group of companies. Gardasil is a registered trademark of Merck &Co.
Declaration of Interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: GA, OM, J-US, LVH, KV and JZ are employees of the GSK group of companies; GA, LVH and JZ additionally hold restricted shares in the GSK group of companies as part of their employee remuneration. FH declares receipt of financial compensation from GSK for consultancy previous to the study in question. CM declares the following remunerations made to her employer the University of the West of England, Bristol, UK: by GSK as a consultancy fee for reviewing and reporting on the data in this study; and outside the submitted work by Pfizer for delivery of a lecture series.
Details of Contributors
Maria-Genalin Angelo and Frank Huygen both contributed to conception and design of review, collection and assembling of data, performing and supervising the analysis, and interpretation of data. Candida McCabe was also involved with the conception and design of the review, and interpreting the data. Jens-Ulrich Stegmann contributed to the collection and assembling of data, performing and supervising the analysis, and interpreting the data. Olivia Mahaux and Lionel Van Holle both worked on the particular aspects of the conception and design of review, collection and assembling of data, performing and supervising the analysis, and interpretation of data that were concerned with the observed/expected analysis. Kristin Verschueren took part in the overall safety evaluations, literature searches and discussion and conclusion of results. Julia Zima was involved in the conception and design of the review, interpretation of data and medical review of the cases and confirmation of the diagnosis.
All authors accept full responsibility for the finished article, had access to any data, and participated in the decision to publish. All authors are “guarantors” in compliance with ICMJE 4th edition criterion.
The following are the supplementary data related to this article.
Overview of 17 case reports that included the MedDRA PT of CRPS from GSK's worldwide safety database.
Overview of 11 case reports that included the MedDRA PT of CRPS from GSK's worldwide safety database from 31 July 2013 to 31 December 2013.
Acknowledgements
The authors thank Felix Arellano who provided guidance and support in the overall functioning of vaccines' safety surveillance systems during his term at GSK Vaccines; Beverley Forsythe (GSK Vaccines) for providing guidance in conducting the GSK safety evaluation and Marta Lopez Fauqued (GSK Vaccines) for helping in data management. The authors thank all parents, health care professionals, regulatory authorities and others who support national safety surveillance systems through reporting of adverse events in post-marketing (passive) surveillance. Writing support services were provided by Joanne Wolter (independent medical writer, Brisbane, Australia); editing and publication co-ordinating services were provided by Veronique Delpire and Mandy Payne (Words & Science, Brussels, Belgium). All costs related to the development of this manuscript were met by GlaxoSmithKline Biologicals SA.
References
- Angelo M.-G., Zima J., Tavares Da Silva F., Baril L., Arellano F. Post-licensure safety surveillance for human papillomavirus-16/18-AS04-adjuvanted vaccine: more than 4 years of experience. Pharmacoepidemiol Drug Saf. 2014;23:456–465. doi: 10.1002/pds.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo M.-G., David M.-P., Zima J. Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol Drug Saf. 2014;23:466–479. doi: 10.1002/pds.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mos M., de Bruijn A.G.J., Huygen F.J.P.M., Dieleman J.P., Stricker B.H.C., Sturkenboom M.C.J.M. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- De Rooij A.M., Florencia Gosso M., Haasnoot G.W. HLA-B62 and HLA-DQ8 are associated with complex regional pain syndrome with fixed dystonia. Pain. 2009;145:82–85. doi: 10.1016/j.pain.2009.05.015. [DOI] [PubMed] [Google Scholar]
- De Rooij A.M., de Mos M., van Hilten J.J. Increased risk of complex regional pain syndrome in siblings of patients? J Pain Off J Am Pain Soc. 2009;10:1250–1255. doi: 10.1016/j.jpain.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Dirckx M., Schreurs M.W.J., de Mos M., Stronks D.L., Huygen F.J.P.M. The prevalence of autoantibodies in complex regional pain syndrome type I. Mediators Inflamm. 2015;2015:718201. doi: 10.1155/2015/718201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberth J.M., Kline K.N., Moskowitz D.A., Montealegre J.R., Scheurer M.E. The role of media and the Internet on vaccine adverse event reporting: a case study of human papillomavirus vaccination. J Adolesc Health Off Publ Soc Adolesc Med. 2014;54:289–295. doi: 10.1016/j.jadohealth.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA European Medicines Agency . PRAC recommendations on signals adopted at the PRAC meeting of 7–10 April 2014. Pharmacovigilance Risk Assessment Committee; 2014. [Google Scholar]
- Genc H., Karagoz A., Saracoglu M., Sert E., Erdem H.R. Complex regional pain syndrome type-I after rubella vaccine. Eur J Pain Lond Engl. 2005;9:517–520. doi: 10.1016/j.ejpain.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Global Advisory Committee on Vaccine Safety Relevé Épidémiologique Hebd Sect Hygiène Secrétariat Société Nations Wkly Epidemiol Rec Health Sect Secr Leag Nations. 2013;88:301–312. (12–13 June) [Google Scholar]
- Goebel A., Blaes F. Complex regional pain syndrome, prototype of a novel kind of autoimmune disease. Autoimmun Rev. 2013;12:682–686. doi: 10.1016/j.autrev.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Goebel A., Vogel H., Caneris O. Immune responses to campylobacter and serum autoantibodies in patients with complex regional pain syndrome. J Neuroimmunol. 2005;162:184–189. doi: 10.1016/j.jneuroim.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Harden R.N., Bruehl S., Galer B.S. Complex regional pain syndrome: are the IASP diagnostic criteria valid and sufficiently comprehensive? Pain. 1999;83:211–219. doi: 10.1016/s0304-3959(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Harden R.N., Bruehl S., Stanton-Hicks M., Wilson P.R. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med Malden Mass. 2007;8:326–331. doi: 10.1111/j.1526-4637.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- Harden R.N., Bruehl S., Perez R.S.G.M. Validation of proposed diagnostic criteria (the ‘Budapest criteria’) for complex regional pain syndrome. Pain. 2010;150:268–274. doi: 10.1016/j.pain.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hühne K., Leis S., Schmelz M., Rautenstrauss B., Birklein F. A polymorphic locus in the intron 16 of the human angiotensin-converting enzyme (ACE) gene is not correlated with complex regional pain syndrome I (CRPS I) Eur J Pain Lond Engl. 2004;8:221–225. doi: 10.1016/j.ejpain.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Jastaniah W.A., Dobson S., Lugsdin J.G., Petty R.E. Complex regional pain syndrome after hepatitis B vaccine. J Pediatr. 2003;143:802–804. doi: 10.1067/S0022-3476(03)00536-5. [DOI] [PubMed] [Google Scholar]
- Karimi G., Star K., Hägg S., Norén G.N. Time-to-onset in spontaneous reports: the possibility to detect the unexpected. Pharmacoepidemiol Drug Saf. 2013;22:556–557. doi: 10.1002/pds.3397. [DOI] [PubMed] [Google Scholar]
- Kemler M.A., van de Vusse A.C., van den Berg-Loonen E.M., Barendse G.A., van Kleef M., Weber W.E. HLA-DQ1 associated with reflex sympathetic dystrophy. Neurology. 1999;53:1350–1351. doi: 10.1212/wnl.53.6.1350. [DOI] [PubMed] [Google Scholar]
- Kimura T., Komatsu T., Hosoda R., Nishiwaki K., Shimida Y. Angiotensin-converting enzyme gene polymorphism in patients with neuropathic pain. In: Devor M., Rowbotham M., Wiesenfeld-Hallin Z., editors. Proceedings of the 9th World Congress on Pain. IASP Press; Seattle, WA; USA: 2000. pp. 471–476. [Google Scholar]
- Kinoshita T., Abe R.-T., Hineno A., Tsunekawa K., Nakane S., Ikeda S.-I. Peripheral sympathetic nerve dysfunction in adolescent Japanese girls following immunization with the human papillomavirus vaccine. Intern Med Tokyo Jpn. 2014;53:2185–2200. doi: 10.2169/internalmedicine.53.3133. [DOI] [PubMed] [Google Scholar]
- Mailis A., Wade J. Profile of Caucasian women with possible genetic predisposition to reflex sympathetic dystrophy: a pilot study. Clin J Pain. 1994;10:210–217. doi: 10.1097/00002508-199409000-00007. [DOI] [PubMed] [Google Scholar]
- Medical Dictionary for Regulatory Activities 2015. http://www.meddra.org/ published online June 8.
- Medicines and Healthcare products Regulatory Agency (MHRA) MHRA public assessment report Cervarix (HPV vaccine): update on UK safety experience at the end of 4 years use in the HPV routine immunisation programme. 2012. http://www.mhra.gov.uk/PrintPreview/DefaultSplashPP/CON023340?ResultCount=10&DynamicListQuery=&DynamicListSortBy=xCreationDate&DynamicListSortOrder=Desc&DynamicListTitle=&PageNumber=1&Title=Human%20papillomavirus%20(HPV)%20vaccine December. ( www.mhra.gov.uk, (accessed Jan 17, 2013))
- Merksey H., Bogduk N. IASP Press; Seattle, WA; USA: 1994. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. [Google Scholar]
- Muneshige H., Toda K., Kimura H., Asou T. Does a viral infection cause complex regional pain syndrome? Acupunct Electrother Res. 2003;28:183–192. doi: 10.3727/036012903815901660. [DOI] [PubMed] [Google Scholar]
- Neumann R.A., Aberer E., Stanek G. Evidence for spirochetal origin of Sudeck's atrophy (algodystrophy, reflex sympathetic dystrophy) Arch Orthop Trauma Surg. 1989;108:314–316. doi: 10.1007/BF00932322. [DOI] [PubMed] [Google Scholar]
- Richards S., Chalkiadis G., Lakshman R., Buttery J.P., Crawford N.W. Complex regional pain syndrome following immunisation. Arch Dis Child. 2012;97:913–915. doi: 10.1136/archdischild-2011-301307. [DOI] [PubMed] [Google Scholar]
- Sandroni P., Benrud-Larson L.M., McClelland R.L., Low P.A. Complex regional pain syndrome type I: incidence and prevalence in Olmsted County, a population-based study. Pain. 2003;103:199–207. doi: 10.1016/s0304-3959(03)00065-4. [DOI] [PubMed] [Google Scholar]
- Sibanc B., Lesnicar G. Complex regional pain syndrome and lyme borreliosis: two different diseases? Infection. 2002;30:396–399. doi: 10.1007/s15010-002-2006-4. [DOI] [PubMed] [Google Scholar]
- Tavares Da Silva F., De Keyser F., Lambert P.-H., Robinson W.H., Westhovens R., Sindic C. Optimal approaches to data collection and analysis of potential immune mediated disorders in clinical trials of new vaccines. Vaccine. 2013;31:1870–1876. doi: 10.1016/j.vaccine.2013.01.042. [DOI] [PubMed] [Google Scholar]
- Van de Beek W.-J.T., Roep B.O., van der Slik A.R., Giphart M.J., van Hilten B.J. Susceptibility loci for complex regional pain syndrome. Pain. 2003;103:93–97. doi: 10.1016/s0304-3959(02)00444-x. [DOI] [PubMed] [Google Scholar]
- Van de Vusse A.C., Goossens V.J., Kemler M.A., Weber W.E. Screening of patients with complex regional pain syndrome for antecedent infections. Clin J Pain. 2001;17:110–114. doi: 10.1097/00002508-200106000-00002. [DOI] [PubMed] [Google Scholar]
- Van Eijs F., Stanton-Hicks M., Van Zundert J. Evidence-based interventional pain medicine according to clinical diagnoses. 16. Complex regional pain syndrome. Pain Pract Off J World Inst Pain. 2011;11:70–87. doi: 10.1111/j.1533-2500.2010.00388.x. [DOI] [PubMed] [Google Scholar]
- Van Hilten J.J., van de Beek W.J., Roep B.O. Multifocal or generalized tonic dystonia of complex regional pain syndrome: a distinct clinical entity associated with HLA-DR13. Ann Neurol. 2000;48:113–116. doi: 10.1002/1531-8249(200007)48:1<113::aid-ana18>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- Van Holle L., Zeinoun Z., Bauchau V., Verstraeten T. Using time-to-onset for detecting safety signals in spontaneous reports of adverse events following immunization: a proof of concept study. Pharmacoepidemiol Drug Saf. 2012;21:603–610. doi: 10.1002/pds.3226. [DOI] [PubMed] [Google Scholar]
- Vaneker M., van der Laan L., Allebes W., Goris J. Genetic factors associated with complex regional pain syndrome 1: HLA DRB and TNF alpha promoter gene polymorphism. Disabil Med. 2002;2:69–74. [Google Scholar]
- Wasner G. Vasomotor disturbances in complex regional pain syndrome—a review. Pain Med Malden Mass. 2010;11:1267–1273. doi: 10.1111/j.1526-4637.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- Wilson R., Paterson P., Larson H. A Report of the CSIS Global Health Policy Centre. Cent Strateg Int Stud; 2014. The HPV vaccination in Japan. (published online May. http://csis.org/publication/hpv-vaccination-japan) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of 17 case reports that included the MedDRA PT of CRPS from GSK's worldwide safety database.
Overview of 11 case reports that included the MedDRA PT of CRPS from GSK's worldwide safety database from 31 July 2013 to 31 December 2013.