Abstract
A negative correlation between fertility and longevity has been documented in many species under a variety of conditions, but the association is not always observed,1 leading to heated discussion about the nature of the reproduction–longevity relationship.2 This debate is further fueled by the fact that no genes or molecules have been clearly shown to link the 2 traits. A recent study by Thondamal et al., in the nematode C. elegans has identified one potential link. The authors showed that the steroid signaling pathway, which regulates reproduction, is activated in response to dietary restriction (DR) and is in fact required for DR-induced lifespan extension.3 Steroid signaling mutants subjected to DR not only failed to undergo lifespan extension but also exhibited altered germline plasticity. Interestingly, the requirement for steroid signaling was bypassed when germline plasticity was restored, suggesting that the DR response is mediated, at least in part, by signals from the germline. In this commentary, I discuss the implications of these findings. Several theories of aging have proposed the existence of an energetic trade-off between reproduction and lifespan,4,5 but mechanistic details are lacking. I propose that revisiting and dissecting at the molecular level the link between reproduction, nutrition, and lifespan, will lead to a better understanding of the aging process and its connection to reproduction.
Keywords: longevity, reproduction, evolution, steroid signalling
Caenorhabditis elegans Is a Good Aging Model
Discussions at the most recent Nobel Week Dialog, which focused on aging, made it quite clear that despite important progress, we still lack a consensus on whether or how the aging process is regulated.2 Contrary to common belief, the aging process is very diverse across the tree of life,6 and selecting model organisms for the study of human aging is not straightforward. The nematode Caenorhabditis elegans is one of the most widely used and best characterized models in which to study human aging (Fig. 1). Indeed, under laboratory conditions, C. elegans hermaphrodites enjoy a long post-reproductive life, similar to that observed in human females. Of course, nematodes do not experience menopause and they retain the capacity to produce progeny later in life if they mate with the (relatively rare) males.7,8 Thus, in addition to being short-lived and genetically malleable, C. elegans is well suited to aging research because – at least in its hermaphrodite form – it actually experiences a life history that is not so different from that of humans.
Figure 1.
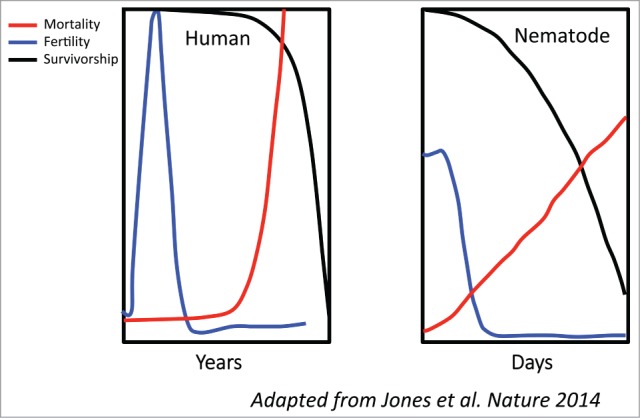
Caenorhabditis elegans is a good aging model. Although a recent report highlighted the great variety of aging across the tree of life, 6 C. elegans hermaphrodites have a long post-reproductive life, as do human females.
Long Life Is Unlikely to Be an Adaptive Trait
Studies of experimental animals and observations in humans have led to the identification of an array of genes, metabolic processes, and nutritional conditions that can increase lifespan. The first identified lifespan-enhancing intervention was downregulation of the insulin signaling pathway,9,10 which activates several stress response mechanisms, including antioxidant and autophagic responses.11,12 Similarly, downregulation of mitochondrial metabolism triggers the mitochondrial unfolded protein response, an event that may lead to lifespan extension.13-15 Ablation of the germline in worms and flies also extends the lifespan, in this case by altering fat metabolism, insulin signaling, and TOR signaling.16-19 However, unlike disruption of insulin signaling and mitochondrial metabolism, germline ablation has not yet been confirmed to extend lifespan in mammals. Finally, dietary restriction (DR), defined as reducing food intake without reaching malnutrition, has been shown to extend lifespan in a wide range of species20 via TOR and AMP kinase signaling and antioxidant and detoxification responses.21 Taken together, these observations have established that the lifespan of wild type animals, and probably that of humans too, can be substantially enhanced by numerous interventions. Although this suggests that the concept of anti-aging treatments is not so far-fetched, it does imply that many cellular processes resulting from millions of years of evolution actually limit the lifespan. If one assumes that evolution results in increased fitness, the implication is that lifespan enhancement must not contribute to fitness. Accordingly, any genetic or pharmacological intervention that extends lifespan may well have a negative effect on traits that do contribute to fitness, unless appropriate compensatory mechanisms are in place. Thus, it is imperative that we understand at the molecular level all consequences of lifespan-extending manipulations.
On the other hand, it is important to stress that the DR response does not require an active modification of the organism and it is conserved across species.20 Since most organisms have, in theory, evolved to withstand conditions of suboptimal food availability, it is reasonable to ask whether DR-associated lifespan extension is an adaptive response. One can imagine that such a response does not contribute to fitness per se but is either a remote consequence of other adaptive changes or a part of a (currently poorly understood) evolutionary strategy to promote species survival. The data presented in the report of Thondamal et al. begin to explore these possibilities.3
The Steroid Signaling Pathway Mediates Lifespan Extension Through Dietary Restriction
Compared with their well-fed counterparts, animals subjected to DR are healthier, remain healthy for a longer duration, and experience a significant delay in the onset of frailty, all of which contribute to their extended lifespan. In general, we know little about the strategies employed by a species to ensure their survival and propagation during periods of famine. For nematodes and a large number of other animals, development and growth can be arrested if harsh conditions are encountered before reaching reproductive maturity. Entry of C. elegans larvae into diapause (also called the Dauer stage) is regulated by nutrient-sensing pathways, such as the insulin and transforming growth factor β (TGFβ) signaling pathways, and by the steroid signaling pathway.22 Production of the steroid hormone dafachronic acid is curtailed when food is scarce, contributing to growth arrest.23 When feeding is reinitiated, the cytochrome P450 DAF-9 is activated and produces dafachronic acid, which in turn activates the nuclear hormone receptor DAF-12. This results in the resumption of growth and reproductive development.22 The events occurring in adult nematodes subjected to DR are much less clear. The recent study by Thondamal et al. showed that steroid signaling in adult C. elegans is also affected by food availability.3 However, 2 important differences were noted between the events in developing and adult animals. First, contrary to developing larvae, adults produce dafachronic acid during conditions of food scarcity. Second, the induction of daf-9 in adults does not depend on the presence or the activity of either the insulin or the TGFβ pathways. How daf-9 is activated by nutrient scarcity remains to be determined (Fig. 3). Induction of the steroid signaling pathway is required for DR-mediated lifespan extension; indeed, daf-9 mutants are incapable of increasing dafachronic acid production and do not live longer under DR conditions. However, addition of exogenous dafachronic acid restores lifespan extension to that of wildtype animals subjected to DR. The nuclear hormone receptor DAF-12, which mediates steroid signaling in developing wildtype animals22 (and in germline-less animals19), is not implicated in DR-induced lifespan extension. Rather, the closely related homolog NHR-8, involved in cholesterol homeostasis,24 is required. However, dafachronic acids do not directly transactivate NHR-8 in vitro23. Therefore, it is unclear how NHR-8 is activated under these conditions (Fig. 3). First, it is possible that NHR-8 is activated by dafachronic acid in vivo, and the failure to detect this in vitro is due to a requirement for missing co-activators. Second, it is possible that NHR-8 is activated by a currently unidentified dafachronic acid metabolite. A recent study demonstrated that several forms of dafachronic acid can activate DAF-1225, and this may also be true for NHR-8. Finally, NHR-8 may act downstream of dafachronic acid in an indirect manner. More work will be required to clarify this issue.
Figure 3.
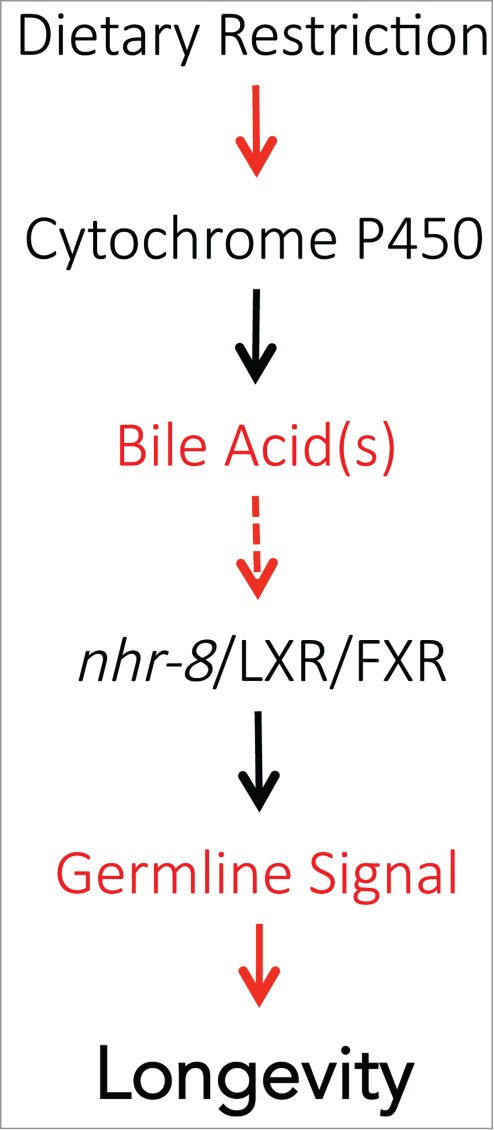
Hypothetical model based on the findings of Thondamal et al.3 Red arrows and text serve to highlight several outstanding questions: How is the key cytochrome P450 enzyme activated by DR?; which bile acid(s) serves as a ligand for NHR-8?; and what is the identity and mechanism of action of the signal(s) emitted by the germline that affects lifespan?
The Steroid Signaling Pathway Links the Germline to the Dietary Restriction-mediated Lifespan Extension
In contrast to its effect on lifespan in C. elegans, DR has a negative effect on fertility resulting from a delay in reproduction.21,26-28 In C. elegans fed ad libitum, egg laying ceases at day 4–6. However, animals subjected to DR display an reduced daily egg production but continue to lay eggs until day 9–12.26,29 A similar trend is observed in mammals when exposed to moderate DR and then returned to an ad libitum diet.27 These observations suggest that delaying reproduction in times of food scarcity is also conserved across species. Whether reproduction and lifespan are co-regulated in animals during DR conditions is not yet known. In 1977, Thomas Kirkwood proposed that the 2 processes are linked through energy constraints. This theory, known as the disposable soma theory of aging,4 proposes that energy resources are either dedicated to promoting reproduction or could be reallocated to maintain somatic tissues, leading naturally to lifespan extension. However, it is not clear how this theory might accommodate situations when food is limiting. One could predict that when energy stores are low, priority should be given to reproduction at the expense of lifespan. However, it is also possible that reproduction might be turned off under such conditions, because progeny survival would be compromised. In this case, the best strategy may be to delay reproduction until energy stores can be replenished.
Interestingly, Drosophila fed a DR diet supplemented with amino acids have the same reproductive capacity and lifespan as their well-fed counterparts, whereas supplementation with other nutrients has no effect on the typical DR phenotype of extended lifespan and reduced fecundity.1 These data suggest first that lifespan extension and reduced fertility is triggered by specific amino acid-associated signals rather than by energy limitation per se, and second, that reproduction and lifespan are not strictly coupled under DR (Fig. 2A).
Figure 2.
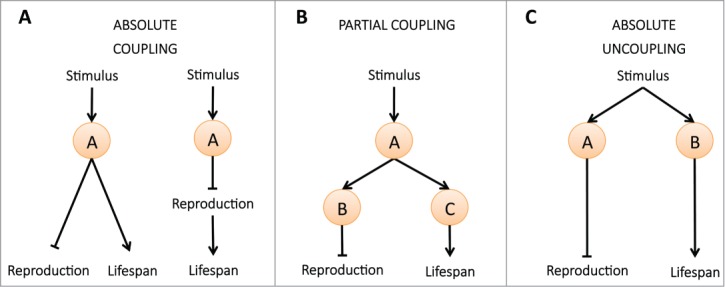
Three scenarios to explain the mechanistic link between fertility and longevity in response to nutritional changes. In (A), the traits are strictly linked, either sequentially or non-sequentially. In (B), the traits are partially linked, raising the possibility that fertility and lifespan may be affected independently but are also regulated by common mediators. In (C), the traits are strictly unlinked.
Food restriction is known to cause shrinkage of the germline in C. elegans.30,31 Thondamal et al. found that the DR-mediated loss of germline nuclei is abrogated in animals in which steroid signaling is disrupted by mutation of either daf-9 or nhr-8. This is not surprising, considering that TOR signaling is known to control germ cell proliferation upon nutrient limitation, but TOR mRNA levels in the steroid signaling mutants are unaffected by food deprivation.30 Intrigued by the dual capacity of the steroid signaling pathway to affect lifespan and germline plasticity in response to DR, Thondamal et al. searched for a potential link between the 2 phenotypes. Perturbation of the Notch and TGFβ signaling pathways is known to diminish the germ cell count in the distal gonad.32 Remarkably, disruption of these pathways in steroid mutants reduced the germ cell count in the distal gonad under ad libitum conditions and restored germline plasticity upon DR. Moreover, the animals also recovered their capacity to live longer upon DR. These findings suggest that the germline is involved in lifespan extension upon nutrient deprivation and additionally implicate steroid signaling as the link between these events.
Taken together, the study of Thondamal et al. in C. elegans3 and the study of Drosophila discussed above1 argue against a strict coupling between reproduction and lifespan under conditions of nutrient restriction (Fig. 2A) but instead argue for a partial coupling (Fig. 2B). The findings in C. elegans also exclude a complete uncoupling (Fig. 2C) because the 2 traits are clearly linked. This underscores the urgent need to identify, at the molecular level, all components of the pathways that couple reproduction and lifespan. Although steroid signaling is one component, many other molecules are undoubtedly involved and await identification.
Although Thondamal et al. were able to correlate the reduction in germ cell count in the proximal gonad with lifespan extension upon DR, the molecular identity of the hypothetical signal emitted by the germline to mediate the lifespan response remains to be determined (Fig. 3). Nevertheless, several studies have provided some important clues. First, the eat-2(ad1116) allele (which reduces pharyngeal pumping and imposes mechanical DR) extends the lifespan of wild type C. elegans but not of germline-ablated animals.26 Second, DR by bacterial dilution greatly extends the lifespan of glp-1(e2141ts) mutants, which are deficient in germline stem cell proliferation and contain only a few non-proliferative germline stem cells (our unpublished data). Assuming that the eat-2(ad1116) allele and bacterial dilution trigger a similar DR response, these data would suggest that the presence of germline stem cells (even in the non-proliferative state) is a key to lifespan extension upon DR. Finally, 2 recent studies found that mating of C. elegans hermaphrodites with males abolished their capacity to respond to DR.33,34 Mating also provoked a drastic shrinking of the hermaphrodites, including the gonad,34 effectively excluding the possibility that gonad shrinking alone explains lifespan extension though DR. Instead, the data suggest that the animals’ reproductive status plays a role in the decision to trigger the longevity response. More work will be required to gain a detailed understanding of how this is achieved and to identify the critical factors that transmit “reproductive messages” to the soma.
Conservation of the Molecular Components Linking the Reproductive and Longevity Responses to Dietary Restriction
Because the link between lifespan extension and low fertility is widely observed across the tree of life, it is reasonable to ask whether these phenotypes are also coordinately regulated in a conserved manner. One important test will be to determine whether DR lengthens the lives of sterile animals of various species. However, such experiments are not straightforward. The germline-derived signal(s) capable of triggering the somatic DR response would first need to be identified to ensure that the method of sterilization is capable of impinging on the production of the signal(s).
As mentioned above, the study by Thondamal et al. showed that coordination of the reproductive and lifespan phenotypes by steroid signaling involves NHR-8.3 This receptor is homologous to several mammalian sterol-sensing receptors, such as the farnesoid X receptor (FXR), vitamin D receptor (VDR), liver X receptor (LXR), constitutive androstane receptor (CAR), and pregnane X receptor (PXR). These receptors are capable of binding to a wide variety of cholesterol derivatives (oxysterols). Also, as discussed above, the NHR-8 ligand involved in the DR response is not known. The secretion of most bile acids is induced upon feeding in mammals, but some are overproduced upon fasting as well35 which makes it difficult to predict which steroid hormone might function analogously to dafachronic acid. However, it is interesting to note that FXR was recently described to affect autophagy,36,37 as does NHR-8.3
FGF21 is another candidate for linking reproduction and lifespan in mammals. This peptidic hormone is induced by fasting, and transgenic mice constitutively overexpressing FGF21 have extended lifespans and disturbed reproductive capabilities compared with wild type mice.38,39 No clear FGF21 homolog has been identified in the C. elegans genome, but it is interesting that the expression of FGF21 in mice is also controlled by sterol-sensing nuclear receptors that resemble NHR-8,40 supporting the possibility that FGF21 may link lifespan and reproduction in mice. In sum, many nutrient-regulated longevity and reproductive phenotypes are clearly conserved, and there are some intriguing clues suggesting that similar molecular mediators may be involved in different species. However, confirmation must await complete identification of the genes and molecules that link lifespan to reproduction in different species.
Conclusion
Accumulating evidence suggests that lifespan is affected by numerous proximal processes. Interference with normal control of proteostasis, mitochondrial metabolism, and fat metabolism, for example, all limit lifespan to some extent, and these are areas of intense interest in aging research. Less clear is whether there is a conserved intervention that can extend the lifespan across species. DR is currently the only non-pharmalogical intervention known to promote longevity in wild type animals. A full understanding of this response will be essential to design appropriate interventions that induce all proximal processes required to extend lifespan. In addition, elucidating the pathways that link lifespan to other adaptive traits, including reproduction, may pave the way to the design of interventions that extend lifespan without incurring biological costs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
I apologize to all authors whose work was not cited due to space limitation and thank all members of the Aguilaniu Lab, as well as Anne O’Rourke, for fruitful discussion and critical reading of this manuscript.
References
- 1. Grandison RC, Piper MDW, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 2009; 462:1061-4; PMID:19956092; http://dx.doi.org/ 10.1038/nature08619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nobel Week Dialogue [Internet] [cited 2015 Jan 5]; Available from: http://www.nobelweekdialogue.org/ [Google Scholar]
- 3. Thondamal M, Witting M, Schmitt-Kopplin P, Aguilaniu H. Steroid hormone signalling links reproduction to lifespan in dietary-restricted Caenorhabditis elegans. Nat Commun 2014; 5:4879; PMID:25209682; http://dx.doi.org/ 10.1038/ncomms5879 [DOI] [PubMed] [Google Scholar]
- 4. Kirkwood TB. Evolution of ageing. Nature 1977; 270:301-4; PMID:593350; http://dx.doi.org/ 10.1038/270301a0 [DOI] [PubMed] [Google Scholar]
- 5. Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution 1957; 11:398; http://dx.doi.org/ 10.2307/2406060 [DOI] [Google Scholar]
- 6. Jones OR, Scheuerlein A, Salguero-Gómez R, Camarda CG, Schaible R, Casper BB, Dahlgren JP, Ehrlén J, García MB, Menges ES, et al. . Diversity of ageing across the tree of life. Nature 2014; 505:169-73; PMID:24317695; http://dx.doi.org/ 10.1038/nature12789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pickett CL, Dietrich N, Chen J, Xiong C, Kornfeld K. Mated progeny production is a biomarker of aging in caenorhabditis elegans. G3 GenesGenomesGenetics 2013; 3:2219-32; http://dx.doi.org/full_text [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mendenhall AR, Wu D, Park S-K, Cypser JR, Tedesco PM, Link CD, Phillips PC, Johnson TE. Genetic dissection of late-life fertility in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci 2011; 66:842-54; PMID:21622982; http://dx.doi.org/ 10.1093/gerona/glr089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedman DB, Johnson TE. Three mutants that extend both mean and maximum life span of the nematode, Caenorhabditis elegans, define the age-1 gene. J Gerontol 1988; 43:B102-9; PMID:3385139; http://dx.doi.org/ 10.1093/geronj/43.4.B102 [DOI] [PubMed] [Google Scholar]
- 10. Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature 1993; 366:461-4; PMID:8247153; http://dx.doi.org/ 10.1038/366461a0 [DOI] [PubMed] [Google Scholar]
- 11. Meléndez A, Tallóczy Z, Seaman M, Eskelinen E-L, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 2003; 301:1387-91; http://dx.doi.org/ 10.1126/science.1087782 [DOI] [PubMed] [Google Scholar]
- 12. Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003; 424:277-83; PMID:12845331; http://dx.doi.org/ 10.1038/nature01789 [DOI] [PubMed] [Google Scholar]
- 13. Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 2011; 144:79-91; PMID:21215371; http://dx.doi.org/ 10.1016/j.cell.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo Y-S, Viswanathan M, Schoonjans K, et al. . The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 2013; 154:430-41; PMID:23870130; http://dx.doi.org/ 10.1016/j.cell.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bennett CF, Choi H, Kaeberlein M. Searching for the elusive mitochondrial longevity signal in C. elegans. Worm 2014; 3:e959404; http://dx.doi.org/ 10.4161/21624046.2014.959404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hansen M, Flatt T, Aguilaniu H. Reproduction, fat metabolism, and life span: what is the connection? Cell Metab 2013; 17:10-9; PMID:23312280; http://dx.doi.org/ 10.1016/j.cmet.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goudeau J, Bellemin S, Toselli-Mollereau E, Shamalnasab M, Chen Y, Aguilaniu H. Fatty acid desaturation links germ cell loss to longevity through NHR-80/HNF4 in C. elegans. PLoS Biol 2011; 9:e1000599; PMID:21423649; http://dx.doi.org/ 10.1371/journal.pbio.1000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lapierre LR, Gelino S, Meléndez A, Hansen M. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr Biol CB 2011; 21:1507-14; http://dx.doi.org/ 10.1016/j.cub.2011.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 1999; 399:362-6; PMID:10360574; http://dx.doi.org/ 10.1038/20694 [DOI] [PubMed] [Google Scholar]
- 20. Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev 2005; 126:913-22; PMID:15885745; http://dx.doi.org/ 10.1016/j.mad.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 21. Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem 2008; 77:727-54; PMID:18373439; http://dx.doi.org/ 10.1146/annurev.biochem.77.061206.171059 [DOI] [PubMed] [Google Scholar]
- 22. Antebi A. Steroid regulation of C. elegans diapause, developmental timing, and longevity. Curr Top Dev Biol 2013; 105:181-212; PMID:23962843; http://dx.doi.org/ 10.1016/B978-0-12-396968-2.00007-5 [DOI] [PubMed] [Google Scholar]
- 23. Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, et al. . Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell 2006; 124:1209-23; PMID:16529801; http://dx.doi.org/ 10.1016/j.cell.2006.01.037 [DOI] [PubMed] [Google Scholar]
- 24. Magner DB, Wollam J, Shen Y, Hoppe C, Li D, Latza C, Rottiers V, Hutter H, Antebi A. The NHR-8 Nuclear Receptor Regulates Cholesterol and Bile Acid Homeostasis in C. elegans. Cell Metab 2013; 18:212-24; PMID:23931753; http://dx.doi.org/ 10.1016/j.cmet.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mahanti P, Bose N, Bethke A, Judkins JC, Wollam J, Dumas KJ, Zimmerman AM, Campbell SL, Hu PJ, Antebi A, et al. . Comparative metabolomics reveals endogenous ligands of DAF-12, a nuclear hormone receptor, regulating C. elegans development and lifespan. Cell Metab 2014; 19:73-83; PMID:24411940; http://dx.doi.org/ 10.1016/j.cmet.2013.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crawford D, Libina N, Kenyon C. Caenorhabditis elegans integrates food and reproductive signals in lifespan determination. Aging Cell 2007; 6:715-21; PMID:17711560; http://dx.doi.org/ 10.1111/j.1474-9726.2007.00327.x [DOI] [PubMed] [Google Scholar]
- 27. Selesniemi K, Lee H-J, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell 2008; 7:622-9; PMID:18549458; http://dx.doi.org/ 10.1111/j.1474-9726.2008.00409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tilly JL, Sinclair DA. Germline energetics, aging, and female infertility. Cell Metab 2013; 17:838-50; PMID:23747243; http://dx.doi.org/ 10.1016/j.cmet.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Houthoofd K, Braeckman BP, Lenaerts I, Brys K, De Vreese A, Van Eygen S, Vanfleteren JR. Axenic growth up-regulates mass-specific metabolic rate, stress resistance, and extends life span in Caenorhabditis elegans. Exp Gerontol 2002; 37:1371-8; PMID:12559406; http://dx.doi.org/ 10.1016/S0531-5565(02)00173-0 [DOI] [PubMed] [Google Scholar]
- 30. Korta DZ, Tuck S, Hubbard EJA. S6K links cell fate, cell cycle and nutrient response in C. elegans germline stem/progenitor cells. Dev Camb Engl 2012; 139:859-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science 2009; 326:954-8; PMID:19713489; http://dx.doi.org/ 10.1126/science.1178343 [DOI] [PubMed] [Google Scholar]
- 32. Hubbard EJA, Korta DZ, Dalfó D. Physiological control of germline development. Adv Exp Med Biol 2013; 757:101-31; PMID:22872476; http://dx.doi.org/ 10.1007/978-1-4614-4015-4_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maures TJ, Booth LN, Benayoun BA, Izrayelit Y, Schroeder FC, Brunet A. Males shorten the life span of C. elegans hermaphrodites via secreted compounds. Science 2014; 343:541-4; PMID:24292626; http://dx.doi.org/ 10.1126/science.1244160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shi C, Murphy CT. Mating induces shrinking and death in Caenorhabditis mothers. Science 2014; 343:536-40; PMID:24356112; http://dx.doi.org/ 10.1126/science.1242958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Han J, Liu Y, Wang R, Yang J, Ling V, Borchers CH. Metabolic profiling of bile acids in human and mouse blood by LC-MS/MS in combination with phospholipid-depletion solid-phase extraction. Anal Chem 2015; 87:1127-36; PMID:25496250; http://dx.doi.org/ 10.1021/ac503816u [DOI] [PubMed] [Google Scholar]
- 36. Seok S, Fu T, Choi S-E, Li Y, Zhu R, Kumar S, Sun X, Yoon G, Kang Y, Zhong W, et al. . Transcriptional regulation of autophagy by an FXR-CREB axis. Nature 2014; 516:108-11; PMID:25383523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee JM, Wagner M, Xiao R, Kim KH, Feng D, Lazar MA, Moore DD. Nutrient-sensing nuclear receptors coordinate autophagy. Nature 2014; 516:112-5; PMID:25383539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Owen BM, Bookout AL, Ding X, Lin VY, Atkin SD, Gautron L, Kliewer SA, Mangelsdorf DJ. FGF21 contributes to neuroendocrine control of female reproduction. Nat Med 2013; 19:1153-6; PMID:3933983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, et al. . The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 2012; 1:e00065; PMID:23066506; http://dx.doi.org/ 10.7554/eLife.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Archer A, Venteclef N, Mode A, Pedrelli M, Gabbi C, Clément K, Parini P, Gustafsson J-Å, Korach-André M. Fasting-induced FGF21 is repressed by LXR activation via recruitment of an HDAC3 corepressor complex in mice. Mol Endocrinol Baltim Md 2012; 26:1980-90; http://dx.doi.org/ 10.1210/me.2012-1151 [DOI] [PMC free article] [PubMed] [Google Scholar]


