Abstract
Background
Clinical trials measuring the effect of an intervention on clinical outcomes are more influential than those investigating surrogate measures but are costly. We developed methods to reduce costs substantially by using existing data in primary care systems, to ask whether Helicobacter pylori eradication would reduce the incidence of hospitalisation for ulcer bleeding in aspirin users.
Methods
The Helicobacter Eradication Aspirin Trial (HEAT) is a National Institute of Health Research-funded, double-blind placebo controlled randomised trial of the effects of H. pylori eradication on subsequent ulcer bleeding in infected individuals taking aspirin daily, conducted in practices across the whole of England, Wales and Northern Ireland. A bespoke web-based trial management system developed for the trial (and housed within the secure NHS Data Network) communicates directly with the HEAT Toolkit software downloaded at participating practices, which issues queries searching entry criteria (≥ 60 years, on chronic aspirin ≤ 325 mg daily, not on anti-ulcer therapy or non-steroidal anti-inflammatory drugs) for GP review of eligibility. Trial participation is invited using a highly secure automated online mail management system. Interested patients are seen once for consent and breath testing. Those with a positive test are randomised to eradication treatment (lansoprazole, clarithromycin, metronidazole) or placebo, with drug sent by post. Events are tracked by upload of accumulating information in the GP database, patient contact, review of National Hospital Episode Statistics and Office of National Statistics data.
Results
HEAT is the largest Clinical Research Network-supported drug trial, with 115,660 invitation letters sent from 850 practices, 22,922 volunteers, and 3038 H. pylori positive patients randomised to active or placebo treatment after 2.5 years of recruitment. 178 practices have performed their first follow-up data search to identify 21 potential endpoints to date.
Discussion
HEAT is important medically, because aspirin is so widely used, and methodologically, as a successful trial would show that large-scale studies of important clinical outcomes can be conducted at a fraction of the cost of those conducted by industry, which in turn will help to ensure that trials of primarily medical rather than commercial interest can be conducted successfully in the UK.
Keywords: Aspirin, Helicobacter pylori, Clinical trial, Ulcer, Bleed
Highlights
-
•
HEAT, a large clinical trial investigating effects of H. pylori eradication on incidence of ulcer bleeding in aspirin users
-
•
HEAT methodology allows large-scale trials of important clinical outcomes to be conducted at low cost affordable to the NHS
-
•
As the largest CRN-supported drug study, HEAT has currently invited participation from over 115,000 patients countrywide
1. Introduction
1.1. Background and Rationale
Clinical trials measuring the effect of an intervention on clinical outcomes are more influential than those investigating intermediate surrogate measures. However, if the outcome occurs infrequently such studies need to be large, making them costly to conduct. Consequently, outcomes studies tend only to be done where there is a substantial funding source and commercial motivation, resulting in systematic bias in the information available to prescribers.
The development of cyclooxygenase (COX)-2 inhibitors generated robust outcomes data about these drugs compared to non-steroidal anti-inflammatory drugs (NSAIDs). Funds have not been available to conduct similar investigations into low dose aspirin, though prescribing volumes are higher (http://www.hscic.gov.uk/NHS information centre, n.d.) and the influence on peptic ulcer bleeding is now probably greater (Taha et al., 2007) than for NSAIDs. Moreover understanding how to prevent ulcer bleeding in patients on aspirin is likely to grow in importance with increasing recognition of the effectiveness of aspirin in preventing various cancers (Din et al., 2010a), as well as its benefits in coronary heart disease and cerebrovascular disease. Whilst analgesic doses of aspirin cause gastroduodenal damage this is much reduced with the lower doses prescribed for prevention of vascular disease.
The primary aim of the HEAT trial is to investigate the hypothesis that H. pylori eradication will reduce the incidence of ulcer bleeding in patients taking aspirin. We therefore investigated how we could conduct a large scale intervention trial into the prevention of ulcer bleeding in patients on aspirin within the resources available from funding bodies for academic studies.
1.2. Objectives and Trial Design
In some epidemiological studies, H. pylori increases the odds of ulcer bleeding in aspirin users, even in studies which show an opposite effect for non-aspirin NSAIDs (Stack et al., 2002; Lanas et al., 2002). In endoscopic studies of patients on low dose aspirin there is a fivefold increase in ulcer development in H. pylori positive compared to negative patients (Yeomans et al., 2005). Some studies of secondary prevention in patients at high risk of ulcer bleeding suggest that H. pylori eradication may substantially reduce the risk of further events (Chan et al., 2001; Lai et al., 2002).
HEAT brings together a number of approaches, methods and devices to streamline a large-scale interventional trial:
-
•
Recruitment in primary care using the MIQUEST (http://systems.hscic.gov.uk/data/miquest, n.d.) search tool that enables electronic scrutiny of records in any of the clinical systems used in UK general practice
-
•
Use of an automated Docmail (http://www.cfhdocmail.com/, n.d.) postal system that ensures invitations are received within 48 h of patient identification, whilst maintaining record confidentiality
-
•
Use of nurses available through the National Institute for Health Research (NIHR) Clinical Research Network (CRN) for the purposes of recruitment
-
•
Using the recruitment visit opportunistically to obtain health check data to patient and GP benefit, including blood pressure, Body Mass Index, alcohol consumption and smoking status
-
•
A dedicated central facility for H. pylori analysis
-
•
Despatch of treatment by post
-
•
Replacement of conventional follow-up visits by multiple methods for endpoint ascertainment: participant report, automated scrutiny of GP records, regular download of hospital episode statistics (HES) and mortality data, using the secure NHS N3 spine (http://n3.nhs.uk/technicalinformation/n3networkoverview.cfm, n.d.)
-
•
Validation of endpoints by the adjudication process used in the TARGET study (Schnitzer et al., 2004)
These methods were selected after validation in a pilot study (HEAT Trial Pilot Study Report, n.d.), and have several advantages over conventional approaches. They are streamlined, access an unselected population, enable predictable and scalable recruitment, and require minimal manual input of data which largely obviates error and the need for monitoring. The process is popular with GPs because it is minimally intrusive upon their time, and the recruitment process can also generate patient health data that returns to the GP record.
HEAT has three objectives:
-
1.
Medical: To test the hypothesis that a one week course of H. pylori eradication in patients using aspirin ≤ 325 mg daily will reduce the incidence of subsequent adjudicated peptic ulcer bleeding that results in hospitalisation
-
2.
Economic: To test the hypothesis that the intervention has a positive net monetary benefit
-
3.
Methodological: To establish a methodology for large simple outcomes studies using electronically extracted primary care follow-up data, to reduce costs to a level that enables outcomes studies of clinically important questions to be done without the need for industry support
2. Pilot Study
A total of 2525 participants from 9 participating practices in the East Midlands, South West and North East were invited to undergo H. pylori testing and eradication. Of those, 1198 (47.4%) replied and 944 (37.4%) volunteered to participate (897 aged 60 +). Positive response rates ranged from 32.6% to 48.8% with no obvious geographical differences. Of those who volunteered, 77 were ineligible at initial contact, and a small number were screen failures, leaving 825 who were enrolled in the study (87.4% of those initially responding positively). Of these, 184 were positive for H. pylori infection (22.3%) with 12 borderline results (1.5%). Approximately two months post-eradication all positive participants had a follow-up appointment for post-treatment breath testing. Participants and their GPs were notified of their current H. pylori status; those who remained positive were referred back to their GP. At follow-up, 177 (91.3%) of those with an initial positive breath test had a negative test indicating successful treatment.
In response to a questionnaire, 72% of participants in this non-randomised study said they would participate in a randomised trial in which only half the participants were treated during the trial. Results of the pilot study and logistical experience were used to inform the design of the main trial.
3. Main Trial
3.1. Methods
3.1.1. Trial Design
HEAT is a double-blind, placebo-controlled, parallel 1:1, randomised multi-centre trial of the effects of H. pylori eradication treatment vs placebo on subsequent ulcer bleeding in infected individuals taking aspirin ≤ 325 mg daily (http://www.controlled-trials.com/ISRCTN10134725/, n.d.). The protocol was designed by collaborators at the Universities of Nottingham, Southampton, Durham, Oxford and Birmingham, and the trial's sponsor is the University of Nottingham. This is currently believed to be the largest interventional drug trial taking place in the UK, aiming to contact 170,000 potential participants, consent and breath-test 33,000, and randomise 6600 who are H. pylori positive.
3.1.2. Governance & Ethics
HEAT has been approved by the East Midlands-Leicester Research Ethics Committee (Ref 11/EM/0434), and the Medicines and Healthcare products Regulatory Agency (MHRA) (CTA Ref 03057/0052/001-0001) and is conducted according to Good Clinical Practice and the Declaration of Helsinki. The trial was approved by the ethics committee and MHRA in December 2011, and there has since been one protocol amendment (to version 2.0, 3-Mar-14), approved for use in April 2014.
3.1.3. Setting
Recruitment takes place solely in primary care, in 145 (former) Primary Care Trusts (PCTs) spread across England, Wales and Northern Ireland. This is further to a recent expansion of the trial, from 67 PCTs initially identified in 2011. The trial has been adopted by 24 former Comprehensive Local Research Networks (CLRNs) across England, led by Trent CLRN. Principal Investigators (PIs) are based at 5 regional centres (Nottingham, Durham, Southampton, Oxford/Birmingham, Belfast). Each practice has a nominated Study Site Coordinator (SSC) acting under the guidance of the regional PI, who identifies potentially suitable patients.
3.1.4. Inclusion Criteria
Subjects are males or females ≥ 60 years of age, taking daily aspirin ≤ 325 mg daily (4 or more 28-day prescriptions in the last year). Subjects are excluded if currently prescribed anti-ulcer therapy (within the last 3 months), oral NSAIDs or any medication with a clinically significant interaction with the H. pylori eradication treatment. Subjects are not excluded if they have a previous record of H. pylori eradication therapy, as the success of this is not checked as standard practice in routine care.
3.1.5. Patient Contact
Patients meeting entry criteria are identified and eligibility is checked by the SSC. Trial participation is invited using Docmail (http://www.cfhdocmail.com/, n.d.), a highly secure online mail management system approved by the ethics committee. Each letter of invitation has a unique screening number and is accompanied by a Participant Information Sheet and a reply slip to the regional centre.
3.1.6. Patient Visit and Breath Test
All patients expressing an interest are contacted by telephone to allow them to ask questions, and invited to attend a screening visit at their GP practice. Recruitment is performed either by nurses employed by the regional centre or by suitably trained practice nurses. Trial eligibility is checked and written informed consent is obtained. Basic demographic and health data are recorded before 13C-urea breath testing for H. pylori (INFAI, (http://www.infai.com/, n.d.)).
A random preselected 10% of participants receive ‘Health-Related Quality of Life’ questionnaires (for economic analysis) and are re-breath-tested at the end of the trial to determine the eradication rate (this sample size gives a +/− 4.5% 95% confidence interval in the rate estimation), as are all participants experiencing a trial endpoint. All participants and their GPs are notified of their breath test results and those that are H. pylori positive also receive active or placebo eradication treatment. Patients with a borderline breath test result are not randomised, but their GP is informed of the result in case they feel further action is required.
3.1.7. Participant Flow Chart
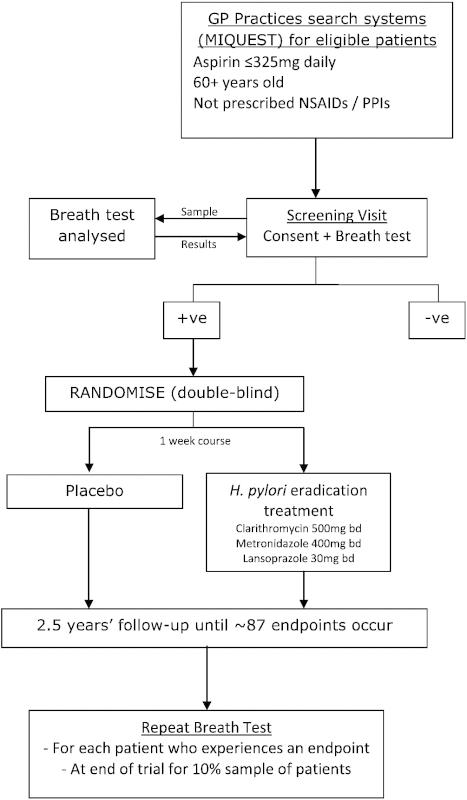
3.1.8. Software, Patient Selection & Confidentiality
All trial data are stored on a secure dedicated web server within the N3 NHS Private Data Network; access is restricted by user identifiers and secure passwords. All participant data is treated confidentially.
A bespoke HEAT web-based database and trial management system was developed for the trial by TCR Nottingham (http://tcrnottingham.com/, n.d.). This centralised database communicates directly with the HEAT Toolkit, a software that is downloaded at participating practices. Within the Toolkit, practices issue MIQUEST queries to their clinical computer system as per the inclusion/exclusion criteria, and import the responses back for GP manual review of eligibility. An electronic screening log is generated based on the GP-approved list of patients.
Prior to consent, only basic information is stored in the trial database: screening number, patient initials, year of birth, and encrypted NHS Number. Once a participant has consented to the trial, existing basic information is de-anonymised and relevant healthcare information is uploaded into the trial database directly from practice records.
3.1.9. Randomisation and Blinding
A randomisation schedule has been developed by the Nottingham Clinical Trials Unit (CTU) with separate randomisation sequences for each regional centre (Nottingham, Durham, Southampton, Oxford/Birmingham, Belfast), using permuted blocks of randomly varying size. In order to randomise a participant, a web-based randomisation system is used, which has undergone thorough validity testing by the CTU.
Participants and staff at all sites will be blinded to treatment allocation. Blinding is maintained as active and placebo treatment kits look identical. Emergency unblinding will be via a Clinical Trials Pharmacist at Nottingham University Hospitals NHS Trust.
3.1.10. Trial Treatment
Active treatment is seven days of lansoprazole 30 mg, clarithromycin 500 mg and metronidazole 400 mg given twice daily. This regimen was chosen to match the regimen used in the pilot study, so we are able to infer comparable eradication rates (this will also be checked by re-breath testing a 10% sample of patients at the end of the study, and any patient who experience an endpoint). This regimen is also reputed to achieve high eradication rates over a shorter treatment duration than other regimens (Huang and Hunt, 1999). The control group receives placebos to the same regimen. All medication was manufactured and packaged by Modepharma (http://www.modepharma.com/, n.d.).
Treatment is dispatched to the participants by post, including a form on which the participant can log dose timings and record any adverse effects.
3.1.11. Participant Compliance and Adverse Events
Participants receive a telephone call approximately 7 days following despatch of medication to check compliance and adverse effects. Because of the high eradication rates achieved in the pilot study, only a 10% sample will be retested to confirm successful treatment and indirectly act as a measure of adherence. This is also measured via patient self-reporting, as all patients are asked to complete a Treatment Record Form, stating the date and time they take each medication dose.
Serious adverse event data is collected by the GP practices for only 4 weeks from the start of treatment. This is because the trial is classified by the MHRA as the lowest risk trial of an investigational medicinal product (Type A study) (http://www.mhra.gov.uk/Howweregulate/Medicines/Licensingofmedicines/Clinicaltrials/Submittinganotificationforatrial/index.htm, n.d.), using established medications within their licensed indication. This means that, because the primary outcome is a safety one, events are not reported after this time unless the Chief Investigator thinks there are compelling reasons to implicate treatment in an event detected during subsequent interrogation of GP records.
3.1.12. Data Acquisition
Participant-reported adverse effects are entered on the trial database. Participants are asked to inform their Regional Centre of any hospitalisation but will also be contacted by letter on an annual basis. They also carry a trial participant ID card which asks hospitals to inform the Regional Centre of an admission.
GP records are interrogated every 6–12 months by repeated MIQUEST searches to detect any new Read codes indicating an endpoint, as well as current health and prescribing information.
Participants in this trial consent to their HES and the Office of National Statistics (ONS) Mortality records being accessed, and this will be downloaded regularly. This information will be matched to that provided by the MIQUEST search of GP practice records.
3.1.13. Primary and Secondary Outcomes
The primary outcome is hospitalisation due to peptic ulcer bleeding (only the first event per participant will be analysed), adjudicated by a blinded Adjudication Team as definite or probable. The three person GI Adjudication Team will adjudicate possible ulcer bleeding endpoints, as previously done in the TARGET study (Schnitzer et al., 2004).
Secondary endpoints include other causes of GI bleeding, cardiovascular outcomes (APTC endpoint, myocardial infarction, stroke), the incidence of detected uncomplicated ulcers, ulcer site (duodenal ulcer vs. gastric ulcer), dyspepsia, and the need for new proton pump inhibitor (PPI) prescriptions or other prescribed antiulcer/dyspepsia medication.
These data will be collected from electronic searches from GP practice records, HES and ONS data, and these methods of data acquisition were validated in the pilot study, and in previous trials (for example, (Avery et al., 2009)).
3.1.14. Sample Size
A total of 87 events are required to detect a hazard ratio of 0.5 comparing the intervention with the control arm, with a 5% two sided significance level and 90% power. Our power calculations are based on assumptions derived from event rates in published randomised clinical trials and observational studies. Based on an average of the rates of ulcer bleeding in control patients in RCTs and observational studies we assume a rate of 0.16% per annum, in patients not taking aspirin. If aspirin increases the bleeding rate 2.5 fold we would predict an ulcer bleeding rate in patients ≥ 60 years old on aspirin of 0.4% per annum. We assume approximately 25% of patients are H. pylori positive (as in our pilot study) and that infection increases the risk threefold. These assumptions translate into an ulcer bleeding rate of 0.8% per annum in the 25% of patients who are on aspirin and H. pylori positive. Assuming event rates of 4 per 1000 per year in the intervention arm and 8 per 1000 per year in the control arm then 7250 person-years are required per study arm to obtain this number of events and approximately 3300 participants will need to be randomised per study arm (6600 total) assuming an average follow-up of 2.3 years and allowing for 2% loss to follow-up per year.
3.1.15. Statistical Analysis
A Cox proportional hazards model will be used to analyse time to first episode of hospitalisation because of definite or probable peptic ulcer bleeding, to estimate a hazard ratio and 95% confidence interval comparing treatment arms, adjusted for regional centre. This primary intention to treat analysis will be supplemented by secondary analyses that take account of eradication treatment compliance, whether aspirin is continued and whether a PPI is started.
3.1.16. Health Economics
We will conduct an incremental economic analysis comparing treated participants with control participants. The analysis will compare resource use between the two arms, with key cost drivers for gastric bleed management being recorded. The economic analysis will be via Markov decision analysis models, with an assumed life expectancy of 14.0 years for men and 16.0 years for women aged 65 or more, with an indication for aspirin, based on results from the ACTION study (Clayton et al., 2005).
3.1.17. Post-Trial Care
GP care of participants continues as usual after randomisation. All participants will be told which arm they were in at the end of the trial, along with the trial results, to enable them and their GP to make an informed decision on whether or not eradication therapy for those on placebo would now be beneficial.
3.1.18. Monitoring and Audits
An important component of this trial is the attempt to develop low-cost, streamlined, simplified methodologies to bring outcomes studies within the affordability of academic investigators. New MHRA guidelines from 1 April 2011 are helpful to this aspiration. For Type A trials, a low-intensity approach to monitoring is advised, taking into account the trial risk assessment, and specifics of the trial design. As this is a low risk trial, with the majority of the study data captured via electronic methods, required monitoring surrounds just those participants who experience a trial endpoint.
3.2. Results
After approximately 2.5 years of recruitment, we have now sent out invitation letters to over 115,000 patients, have heard from almost 23,000 volunteers (~ 20% volunteering rate), and have consented 17,628 participants to date, 3038 of which have been positive for H. pylori (17% prevalence). Recruitment will continue until 87 adjudicated endpoints have occurred. We have run follow-up MIQUEST searches in 178 practices to date, and have identified 21 potential endpoints in H. pylori positive participants. Further details will now be collected to enable adjudication of these events.
3.3. Discussion
HEAT is an innovative trial, both medically and methodologically. If its hypothesis is supported, it will have identified a cost effective strategy that reduces the risk of the adverse events increasingly associated with the alternative strategy of PPI prophylaxis (Nealis and Howden, 2008). If there is specifically a reduction in the incidence of bleeding ulcers, the results of the trial will strengthen an explanatory paradigm that suggests that the principal effect of low dose aspirin is an anti-haemostatic rather than an erosive one, requiring H. pylori infection for the development of an ulcer that bleeds because of impaired haemostasis. Data that associate aspirin use more strongly with duodenal than gastric ulceration (Yeomans et al., 2005; Sostres et al., 2015) may support this paradigm and an analysis of ulcer site is part of the study plan. With the likely increase in aspirin use following the recognition of its apparent ability to prevent and slow cancer growth (Din et al., 2010b), H. pylori eradication could make a difference to the health of an increasing number of people, either by supporting a strategy of H. pylori eradication or, in the event of a negative result, PPI prophylaxis. Application of the trial's results may differ between countries where its incidence is declining (Calvet et al., 2013; Axon, 2014) versus those parts of the world where H. pylori prevalence remains high, as is peptic ulceration and its complications. Regardless of the trial's results, its methodological advances remain important for future low-cost outcomes studies.
Our pilot study and the first 30 months of the current trial show that assessment and recruitment of large numbers of patients in primary care is feasible. This is due to the relative ease of screening for patients using the MIQUEST search tool, with efficiency also enhanced by use of the automated postal system that ensures invitations are received by several hundred patients per practice within 48 h of their identification.
Endpoint identification via automated Read code searches is perhaps the most time- and cost-saving element of this trial, removing the requirement for face-to-face follow-up of 6600 randomised participants, and increasing the completeness of data recording, which is often lost with participant- and practice-reported outcomes.
The establishment of the NIHR Clinical Research Network has had a major influence in facilitating the trial, which in turn is arguably a model of the reach of clinical research that is possible under the auspices of the CRN. If successful the trial will show that large-scale studies of important clinical outcomes across the whole of medicine can be conducted at a fraction of the cost of those conducted by industry. This will in turn restore some balance in the generation of data for evidence-based medicine by increasing the influence of investigator driven assessment of clinical issues that are not of direct interest to funding bodies with substantial funds, such as the pharmaceutical industry.
4. Authors' Contributions
Miss Jennifer S. Dumbleton: paper drafting and finalising
Prof. Anthony J. Avery: manuscript review
Dr. Carol Coupland: manuscript review
Prof. FD Richard Hobbs: manuscript review
Prof. Denise Kendrick: manuscript review
Prof. Michael V. Moore: manuscript review
Mr. Clive Morris: manuscript review
Prof. Greg P. Rubin: manuscript review
Dr. Murray D. Smith: manuscript review
Dr. Diane J. Stevenson: paper drafting and finalising
Prof. Chris J. Hawkey: paper drafting and finalising
5. Role of Funding Source
National Institute for Health Research Health Technology Assessment (NIHR HTA) (09/55/52) Programme provided funding for the trial, and monitored and advised on the trial's progress. The trial is also supported by service support costs from participating research networks, and excess treatment costs from Clinical Commissioning Groups.
6. Conflicts of Interest
None to disclose.
7. Department of Health Disclaimer
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA Programme, NIHR, NHS or the Department of Health.
Acknowledgements
We would like to thank the NIHR HTA Programme for their continued support for this trial, along with all participating CRNs, CCGs and GP practices, without whom this trial would not be possible. We would also like to acknowledge and thank the help and support of TCR Nottingham, those involved in the pilot study, all the research nurses who are working on the trial, and the thousands of patients who have volunteered to date.
References
- Avery A., Rogers S., Cantrill J., Armstrong S., Elliott R., Howard R. Protocol for the PINCER trial: a cluster randomised trial comparing the effectiveness of a pharmacist-led IT-based intervention with simple feedback in reducing rates of clinically important errors in medicines management in general practices. Trials. 2009;10:28. doi: 10.1186/1745-6215-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axon A. Helicobacter pylori and public health. Helicobacter. 2014;19(Suppl. 1):68–73. doi: 10.1111/hel.12155. [DOI] [PubMed] [Google Scholar]
- Calvet X., Ramirez Lazaro M., Lehours P., Meggraud F. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter. 2013;18(Suppl. 1):5–11. doi: 10.1111/hel.12071. [DOI] [PubMed] [Google Scholar]
- Chan F.K., Chung S.C., Suen B.Y., Lee Y.T., Leung W.K., Leung V.K. Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. N. Engl. J. Med. 2001;344(13):967–973. doi: 10.1056/NEJM200103293441304. [DOI] [PubMed] [Google Scholar]
- Clayton T.C., Lubsen J., Pocock S.J., Voko Z., Kirwan B.A., Fox K.A.A., Poole-Wilson P.A., on behalf of the ACTION investigators Risk score for predicting death, myocardial infarction and stroke in patients with stable angina, based on a large randomised trial cohort of patients. BMJ. 2005;331:869. doi: 10.1136/bmj.38603.656076.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din F.V., Theodoratou E., Farrington S.M., Tenesa A., Barnetson R.A., Cetnarskyj R. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut. 2010;59(12):1670–1679. doi: 10.1136/gut.2009.203000. [DOI] [PubMed] [Google Scholar]
- Din F.V., Theodoratou E., Farrington S.M., Tenesa A., Barnetson R.A., Cetnarskyj R. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut. 2010;59(12):1670–1679. doi: 10.1136/gut.2009.203000. [DOI] [PubMed] [Google Scholar]
- HEAT Trial Pilot Study Report.
- http://n3.nhs.uk/technicalinformation/n3networkoverview.cfm (last accessed 17/09/14).
- http://systems.hscic.gov.uk/data/miquest (last accessed 17/09/14).
- http://tcrnottingham.com/ (last accessed 17/09/14).
- http://www.cfhdocmail.com/ (last accessed 17/09/14).
- http://www.controlled-trials.com/ISRCTN10134725/ (last accessed 17/09/14).
- http://www.hscic.gov.uk/ NHS information centre, prescription cost analysis. (last accessed 17/09/14).
- http://www.infai.com/ (last accessed 17/09/14).
- http://www.mhra.gov.uk/Howweregulate/Medicines/Licensingofmedicines/Clinicaltrials/Submittinganotificationforatrial/index.htm (last accessed 17/09/14).
- http://www.modepharma.com/ (last accessed 17/09/14).
- Huang J.-Q., Hunt R.H. The importance of clarithromycin dose in the management of Helicobacter pylori infection: a meta-analysis of triple therapies with a proton pump inhibitor, clarithromycin and amoxicillin or metronidazole. Aliment. Pharmacol. Ther. 1999;13:719–729. doi: 10.1046/j.1365-2036.1999.00530.x. [DOI] [PubMed] [Google Scholar]
- Lai K.C., Lam S.K., Chu K.M., Wong B.C., Hui W.M., Hu W.H. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N. Engl. J. Med. 2002;346(26):2033–2038. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- Lanas A., Fuentes J., Benito R., Serrano P., Bajador E., Sainz R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low-dose aspirin. Aliment. Pharmacol. Ther. 2002;16(4):779–786. doi: 10.1046/j.1365-2036.2002.01230.x. [DOI] [PubMed] [Google Scholar]
- Nealis T.B., Howden C.W. Is there a dark side to long-term proton pump inhibitor therapy? Am. J. Ther. 2008;15(6):536–542. doi: 10.1097/MJT.0b013e31817149bf. [DOI] [PubMed] [Google Scholar]
- Schnitzer T.J., Burmester G.R., Mysler E., Hochberg M.C., Doherty M., Ehrsam E., on behalf of the TARGET Study Group Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research & Gastrointestinal Event Trial (TARGET), reduction in ulcer complications Lancet. 2004;364(9434):665–674. doi: 10.1016/S0140-6736(04)16893-1. [DOI] [PubMed] [Google Scholar]
- Sostres C., Carrera-Lasfuentes P., Benito R., Roncales P., Arruebo M., Arroyo M.T. Peptic ulcer bleeding risk. The role of Helicobacter pylori infection in NSAID/low-dose aspirin users. Am. J. Gastroenterol. 2015;110:684–689. doi: 10.1038/ajg.2015.98. [DOI] [PubMed] [Google Scholar]
- Stack W.A., Atherton J.C., Hawkey G.M., Logan R.F., Hawkey C.J. Interactions between Helicobacter pylori and other risk factors for peptic ulcer bleeding. Aliment. Pharmacol. Ther. 2002;16(3):497–506. doi: 10.1046/j.1365-2036.2002.01197.x. [DOI] [PubMed] [Google Scholar]
- Taha A.S., Angerson W.J., Prasad R., McCloskey C., Blatchford O. Upper gastrointestinal bleeding and the changing use of COX-2 non-steroidal anti-inflammatory drugs and low-dose aspirin. Aliment. Pharmacol. Ther. 2007;26(8):1171–1178. doi: 10.1111/j.1365-2036.2007.03458.x. [DOI] [PubMed] [Google Scholar]
- Yeomans N.D., Lanas A.I., Talley N.J., Thomson A.B., Daneshjoo R., Eriksson B. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment. Pharmacol. Ther. 2005;22(9):795–801. doi: 10.1111/j.1365-2036.2005.02649.x. [DOI] [PubMed] [Google Scholar]


